Abstract
Adding protein-bound polysaccharide K (PSK) to adjuvant chemotherapy with mitomycin and fluorouracil after gastrectomy for gastric cancer was demonstrated to improve survival in a previous study in Japan. However, the efficacy of PSK outside Japan and in combination with other adjuvant chemotherapeutic agents remains unclear. The aims of this study were to evaluate the efficacy of PSK.
We conducted a population-based historical cohort study using the National Health Insurance Research Database of Taiwan. We performed sensitivity analysis with propensity score matching to control for possible confounders. Patients who used PSK (PSK group) were matched at a 1:4 ratio to those who had never used PSK (control group) after adjusting for covariates including sex, age, urbanization, income and comorbidities. The primary outcome was overall survival. Multivariate hazard ratios from competing risk analysis were calculated by adjusting for demographic data and all confounding factors.
From 1999 to 2008, we identified 10,617 patients with gastric cancer received gastrectomy and adjuvant chemotherapy. 1295 patients used PSK (PSK group) and 5180 patients never used PSK (control group) were analyzed after propensity score matching. The median overall survival was 6.49 years (95% confidence interval [CI] 5.22–7.63) in the PSK group and 3.59 years (95% CI 3.38–3.80) in the control group. After adjusting for age, sex, urbanization, income, and comorbidities, adding PSK to adjuvant chemotherapy was the most significant prognostic factor for improved survival (hazard ratio 0.76, P < .0001).
Adjuvant chemotherapy combined with PSK significantly prolonged overall survival in gastric cancer patients after gastrectomy.
Keywords: adjuvant chemotherapy, gastric cancer, protein-bound polysaccharide K, survival analysis
1. Introduction
Gastric cancer is the fifth most commonly diagnosed cancer and the third leading cause of cancer deaths worldwide.[1] Except for early tumors limited to the mucosa, gastrectomy with regional lymph node dissection remains the standard treatment for locoregional disease. postoperative adjuvant chemotherapy also plays an important role in improving the prognosis, even though the best regimen has yet to be elucidated.[2]
Protein-bound polysaccharide K (PSK) isolated from the cultured mushroom Coriolus versicolor (Family Polyporaceae) is an oral biological response modifier which has been used as an immunochemotherapeutic agent in the treatment of several types of cancer for decades.[3–5] A randomized controlled trial in Japan demonstrated the benefit of adding PSK to adjuvant chemotherapy with mitomycin and fluorouracil in improving both 5-year disease-free and 5-year survival rates after curative gastrectomy.[6] Based on this positive result, the National Health Insurance (NHI) program in Taiwan has reimbursed the cost of PSK in combination with cytotoxic chemotherapy as adjuvant treatment for gastric cancer patients after curative surgery since 1995.[6] However, the clinical efficacy of PSK in Taiwan remains unclear, as only data from a single institute support the survival benefits of PSK in combination with adjuvant chemotherapy in routine clinical practice.[7] In addition to intravenous fluorouracil, the efficacy of combining PSK with oral fluoropyrimidines such as tegafur/gimeracil/oteracil (S-1) or uracil-ftorafur (UFT) in operated advanced gastric cancer patients has also been shown in retrospective studies[8–10] and in 1 small randomized control trial.[11] However, all of these studies were conducted in a single country (Japan), limited to combinations with fluoropyrimidines, conducted in a retrospective manner, and only enrolled a small number of patients. The treatment effect of the addition of PSK to other adjuvant chemotherapy backbones such as epirubicin, platinum or taxane-based regimens is unknown. Moreover, there are currently no on-going phase III randomized control studies to investigate this issue.
Real-world experience of PSK in combination with adjuvant chemotherapy would help to elucidate its efficacy in operated gastric cancer patients. Therefore, the aims of this study were to use data from the National Health Insurance Research Database (NHIRD) in Taiwan to analyze the overall survival benefits of PSK in combination with adjuvant chemotherapy in Taiwanese patients with advanced gastric cancer and analyze the prognostic factors for survival outcomes.
2. Methods
2.1. Data source
The NHI program was initiated by the Taiwanese government on March 1, 1995, and in December 2013 it was reported to cover 99.5% of all Taiwanese residents. The NHIRD includes details about ambulatory care, inpatient care, prescription data, medical procedures, and diagnostic coding data (using the International Classification of Diseases, ninth revision [ICD-9] classification system), providing a unique, inclusive, national perspective of patients in Taiwan. The data used in this study were derived from the NHIRD between January 1, 1997 and December 31, 2013.[12]
2.2. Study cohort
This study was approved by the Institutional Review Board of Chang Gung Memorial Hospital (201900031B0C501). We conducted this population-based historical cohort study to assess the effects of adding PSK to adjuvant chemotherapy in resected gastric cancer patients. The study included all patients older than 18 years who were newly diagnosed with gastric cancer (ICD-9 codes 151.x) between January 1,1997 and December 31,2008. We identified patients who underwent gastrectomy (ICD-9 procedure codes 43.5, 43.6, 43.7, 43.8, 43.81, 43.82, 43.89, 43.91, and 43.99)[13] and received any of the following chemotherapeutic drugs as adjuvant chemotherapy within 3 months from the date of surgery: epirubicin, cisplatin, carboplatin, oxaliplatin, fluorouracil, capecitabine, mitomycin-C, S-1, UFT, paclitaxel, or docetaxel. Patients who had synchronous malignancies or any other cancer history before the diagnosis of gastric cancer were excluded. Patients who received neoadjuvant chemotherapy were also excluded from the analysis because the disease and surgery conditions of such patients may be heterogeneous compared with other gastric patients. We defined the patients with at least 1 prescription for PSK as the PSK group, and the patients who had never received PSK as the control group. Each PSK group case was matched to 4 controls in the control group using propensity score matching.
2.3. Endpoint
The primary outcome was overall survival. The date of gastrectomy was defined as the index date.
2.4. Covariates
Sociodemographic variables including age at the index date, income, and urbanization were retrieved from the NHIRD files. Potential confounding factors included baseline diabetes mellitus (ICD-9-CM code: 250), hypertension (ICD-9-CM codes: 401~405), liver cirrhosis (ICD-9-CM codes: 571.2, 571.5, or 571.6), smoking-related disorders (ICD-9-CM codes: 305.1, 491.2, 492.8, 496, 523.6, 959.84, 649.0, V15.82), alcohol-related diseases (ICD-9-CM codes: 265.2, 291, 303, 305.0, 357.5, 425.5, 571.0~571.3), and chronic kidney disease (ICD-9-CM code: 585) which were recorded in outpatient and inpatient claims records before the index date.[12,14,15] All of the patients used fluoropyrimidines as adjuvant chemotherapy, and they were divided into 4 groups according to the regimen. Patients who used an adjuvant mitomycin-c-containing regimen were defined as group 1 (mitomycin-based group), those who used an adjuvant epirubicin-containing regimen were defined as group 2 (epirubicin-based group), those who used an adjuvant platinum-based regimen (without mitomycin or epirubicin) were defined as group 3 (platinum-based group), and the others (such as fluoropyrimidines alone, the analogues or in combination with other) were defined as group 4 (others group).
2.5. Statistical analysis
The characteristics of the PSK or control groups at baseline were summarized and compared using the χ2 test. As the underlying comorbidities and baseline conditions were different between the 2 groups, we performed sensitivity analysis using propensity score matching to control for possible confounders including sex, age, residential area, income and comorbidities. The propensity score of using PSK was estimated using logistic regression analysis for the confounders. Using this score, patients in the PSK group were matched at a 1:4 ratio to the control group using an SAS macro (SAS Institute, Cary, NC).[16]
Overall survival was estimated using the Kaplan-Meier method for both groups, and comparisons were made using the log-rank test. Multivariate hazard ratios (HRs) from competing risk analysis were calculated by adjusting for the demographic data and all confounding factors. Statistical analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC). A P value < 0.05 was considered to indicate a statistically significant difference.
3. Results
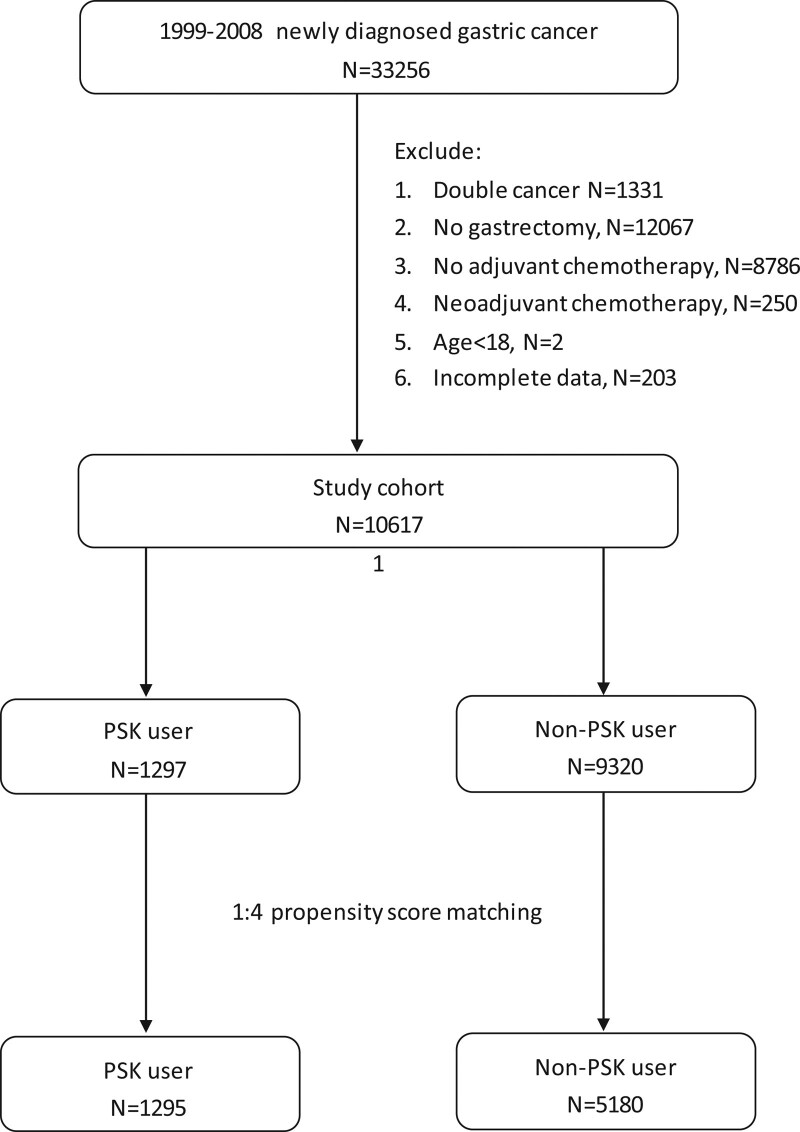
From 1999 to 2008, we identified 10,617 patients with gastric cancer who received gastrectomy and adjuvant chemotherapy, of whom 1295 patients who used PSK (PSK group) and 5180 patients who did not use PSK (control group) were enrolled using 1:4 propensity score matching after adjusting for covariates including sex, age, urbanization, income and comorbidities. The study flow chart is shown in Figure 1.
Figure 1.
Study flow chart. From 1999 to 2008, this study enrolled a total of 10,617 patients with gastric cancer who received gastrectomy and adjuvant chemotherapy. Among the cohort, 1295 patients used PSK, and 5180 patients who did not use PSK were matched as the control group using 1:4 propensity score matching after adjusting for covariates including sex, age, urbanization comorbidities.
There were no significant differences in basic characteristics between the 2 groups (Table 1). Overall, 45.9% of the patients used multi-agent combinations with either mitomycin, epirubicin or platinum, and the other 54.1% used fluoropyrimidine alone, its analogues or other cytotoxic chemotherapy combinations. A higher proportion of patients in the PSK group used a mitomycin-based regimen than the control group (39.2% vs 16.4%). Other regimens were more frequently used in the control group than the study group, including an epirubicin-based regimen (7% vs 3.6%), platinum-based regimen (19.7% vs 14.2%) and others (56.9% vs 42.9%). The mean follow-up duration of this study was 5.84 years (5.65 years in the PSK group and 6.61 years in the control group).
Table 1.
Basic characteristics of the study groups.
| Total (N = 6475) | PSK group (N = 1295) | Control group (N = 5180) | P value* | ||||
|---|---|---|---|---|---|---|---|
| Variables | n | % | n | % | n | % | |
| Sex | 0.620 | ||||||
| Female | 2252 | 34.8 | 458 | 35.4 | 1794 | 34.6 | |
| Male | 4223 | 65.2 | 837 | 64.6 | 3386 | 65.4 | |
| Age at surgery (yr) | 0.524 | ||||||
| 18–44 | 769 | 11.9 | 159 | 12.3 | 610 | 11.8 | |
| 45–59 | 1832 | 28.3 | 362 | 28.0 | 1470 | 28.4 | |
| 60–74 | 2754 | 42.5 | 566 | 43.7 | 2188 | 42.2 | |
| ≥75 | 1120 | 17.3 | 208 | 16.1 | 912 | 17.6 | |
| Mean (SD) | 61.6 (13.2) | 61.3 (13.2) | 61.7 (13.2) | 0.379 | |||
| Urbanization | 0.968 | ||||||
| Very high | 1911 | 29.5 | 385 | 29.7 | 1526 | 29.5 | |
| High | 2995 | 46.3 | 596 | 46.0 | 2399 | 46.3 | |
| Moderate | 1143 | 17.7 | 232 | 17.9 | 911 | 17.6 | |
| Low | 426 | 6.6 | 82 | 6.3 | 344 | 6.6 | |
| Income level (NTD†/per month) | 0.901 | ||||||
| 0 | 1042 | 16.1 | 201 | 15.5 | 841 | 16.2 | |
| 1–15,840 | 856 | 13.2 | 170 | 13.1 | 686 | 13.2 | |
| 15,841–25,000 | 3149 | 48.6 | 631 | 48.7 | 2518 | 48.6 | |
| ≥25,001 | 1428 | 22.1 | 293 | 22.6 | 1135 | 21.9 | |
| Comorbidities | |||||||
| Diabetes mellitus | 1026 | 15.9 | 203 | 15.7 | 823 | 15.9 | 0.852 |
| Hypertension | 2270 | 35.1 | 451 | 34.8 | 1819 | 35.1 | 0.845 |
| Alcoholism | 115 | 1.8 | 21 | 1.6 | 94 | 1.8 | 0.638 |
| Smoking-related disorders | 609 | 9.4 | 121 | 9.3 | 488 | 9.4 | 0.932 |
| Chronic renal failure | 100 | 1.5 | 19 | 1.5 | 81 | 1.6 | 0.801 |
| Liver cirrhosis | 210 | 3.2 | 44 | 3.4 | 166 | 3.2 | 0.725 |
| Chemotherapy regimens | <0.001 | ||||||
| Group 1 (mitomycin-based) | 1357 | 21.0 | 508 | 39.2 | 849 | 16.4 | |
| Group 2 (epirubicin-based) | 410 | 6.3 | 47 | 3.6 | 363 | 7.0 | |
| Group 3 (platinum-based) | 1204 | 18.6 | 184 | 14.2 | 1020 | 19.7 | |
| Group 4 (others) | 3504 | 54.1 | 556 | 42.9 | 2948 | 56.9 | |
Pearson chi-square test for categorical variables and t-test for continuous variables.
1 United States dollar (US$) = 32.3 New Taiwan Dollars (NTD) in 2008.
PSK = protein-bound polysaccharide K, SD = standard deviation.
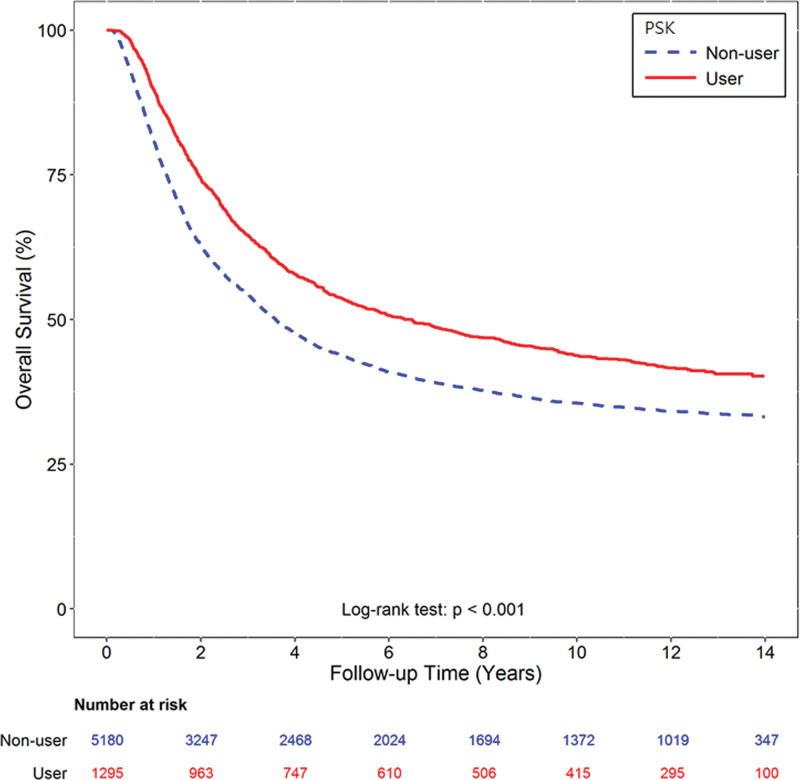
The median overall survival was 6.49 years (95% confidence interval [CI] 5.22–7.63) in the PSK group and 3.59 years (95% CI 3.38–3.80) in the control group. The PSK group had a significantly longer survival than the control group (HR 0.76, P < .001) (Fig. 2). After adjusting for age, sex, urbanization, income, comorbidities and chemotherapy regimen, the use of PSK was still significantly associated with a reduction in the risk of death (adjusted HR 0.77, P < .001). In prognostic factor analysis, age 75 years (adjusted HR 1.30, P < .001), diabetes mellitus (adjusted HR 1.19, P = .001), hypertension (adjusted HR 1.10, P = .010) and smoking-related disorders (adjusted HR 1.13, P = .026) were found to be significant poor prognostic factors (Table 2).
Figure 2.
Income and survival analysis. The Kaplan-Meier survival curves of overall survival according to those who did and did not receive PSK. The median overall survival was 6.49 years (95% CI 5.22–7.63) in the PSK group and 3.59 years (95% CI 3.38–3.80) in the control group (log-rank test: P < .001).
Table 2.
Analyses of prognostic factors.
| Crude | Adjusted† | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | HR | 95% CI | P value | HR | 95% CI | P value | |||
| Used PSK (reference: nonuser) | 0.76 | 0.70 | 0.83 | <0.001 | 0.77 | 0.71 | 0.83 | <0.001 | |
| Sex (reference: female) | |||||||||
| Male | 1.08 | 1.01 | 1.15 | 0.020 | 1.04 | 0.97 | 1.11 | 0.288 | |
| Age (yr) (reference: 18–44) | |||||||||
| 45–59 | 1.04 | 0.93 | 1.16 | 0.518 | 1.01 | 0.91 | 1.13 | 0.825 | |
| 60–74 | 1.07 | 0.97 | 1.19 | 0.178 | 1.00 | 0.89 | 1.11 | 0.958 | |
| ≥75 | 1.49 | 1.33 | 1.67 | <0.001 | 1.30 | 1.15 | 1.48 | <0.001 | |
| Urbanization (reference: low) | |||||||||
| Very high | 1.06 | 0.93 | 1.22 | 0.368 | 1.06 | 0.92 | 1.22 | 0.409 | |
| High | 1.09 | 0.96 | 1.24 | 0.187 | 1.08 | 0.95 | 1.24 | 0.233 | |
| Moderate | 1.02 | 0.89 | 1.17 | 0.792 | 1.03 | 0.89 | 1.19 | 0.690 | |
| Income level (NTD†, reference: 0) | |||||||||
| 1–15,840 | 1.12 | 1.00 | 1.24 | 0.048 | 1.13 | 1.01 | 1.26 | 0.037 | |
| 15,841–25,000 | 0.86 | 0.79 | 0.93 | <0.001 | 0.92 | 0.84 | 1.01 | 0.074 | |
| ≥25,001 | 0.88 | 0.80 | 0.98 | 0.015 | 0.96 | 0.87 | 1.07 | 0.497 | |
| Comorbidities (reference: without) | |||||||||
| Diabetes mellitus | 1.28 | 1.18 | 1.39 | <0.001 | 1.19 | 1.09 | 1.29 | <0.001 | |
| Hypertension | 1.23 | 1.15 | 1.31 | <0.001 | 1.10 | 1.02 | 1.18 | 0.010 | |
| Alcoholism | 1.19 | 0.96 | 1.49 | 0.117 | 1.12 | 0.89 | 1.40 | 0.340 | |
| Smoking-related disorders | 1.27 | 1.15 | 1.40 | <0.001 | 1.13 | 1.01 | 1.25 | 0.026 | |
| Chronic renal failure | 1.33 | 1.06 | 1.68 | 0.016 | 1.22 | 0.97 | 1.54 | 0.097 | |
| Liver cirrhosis | 1.19 | 1.01 | 1.41 | 0.035 | 1.11 | 0.94 | 1.31 | 0.232 | |
*Adjusted for age, sex, urbanization, income, comorbidities.
1 United States dollar (US$) = 32.3 New Taiwan Dollars (NTD) in 2008.
PSK = protein-bound polysaccharide K.
Subgroup analysis by chemotherapy regimen showed that the patients who received PSK with adjuvant mitomycin-based, platinum-based or fluoropyrimidines alone, fluoropyrimidine analogues and other chemotherapy combination regimens had significantly better overall survival than those who received only adjuvant chemotherapy (adjusted HRs 0.72 [P < .001], 0.74 [P = .002] and 0.75 [P < .001], respectively) (Table 3). Only the adjuvant epirubicin-based group showed a nonsignificant difference between receiving an epirubicin-based regimen in combination with or without PSK (adjusted HR 1.24, P = .234).
Table 3.
Subgroup analysis by chemotherapy regimen.
| Crude | Adjusted* | |||||||
|---|---|---|---|---|---|---|---|---|
| Variables | HR | 95% CI | P value | HR | 95% CI | P value | ||
| Chemotherapy regimen | ||||||||
| Group 1 (mitomycin-based) | ||||||||
| Used PSK (reference: nonuser) | 0.72 | 0.62 | 0.83 | <0.001 | 0.72 | 0.62 | 0.83 | <0.001 |
| Group 2 (epirubicin-based) | ||||||||
| Used PSK (reference: nonuser) | 1.22 | 0.86 | 1.72 | 0.268 | 1.24 | 0.87 | 1.77 | 0.234 |
| Group 3 (platinum-based) | ||||||||
| Used PSK (reference: nonuser) | 0.75 | 0.63 | 0.90 | 0.002 | 0.74 | 0.62 | 0.90 | 0.002 |
| Group 4 (others) | ||||||||
| Used PSK (reference: nonuser) | 0.77 | 0.68 | 0.87 | <0.001 | 0.75 | 0.66 | 0.85 | <0.001 |
Adjusted for age, sex, urbanization, income, and comorbidities.
CI = confidence interval, HR = hazard ratio, PSK = protein-bound polysaccharide K.
4. Discussion
Curative surgery with gastrectomy and D2 lymph node dissection is now the mainstay of treatment for locally advanced gastric cancer, however the survival outcome is disappointing due to recurrence and metastasis. Several different approaches have emerged and resulted in postoperative survival benefits. Adjuvant chemoradiation, perioperative adjuvant chemotherapy, and postoperative chemotherapy for locally advanced gastric cancer have shown efficacy in different patient populations. For example, the results of the Intergroup 0116 study, MAGIC and FNCLL/FCCD trials showed significant overall survival benefits with adjuvant chemoradiation therapy and perioperative adjuvant chemotherapy for gastric cancer.[17–19] However, these positive results were not widely demonstrated in Asian countries for nonD2 gastrectomy and gastroesophageal tumors and due to a poor treatment protocol completion rate (approximately 50% of patients were intolerant to postoperative chemotherapy). Furthermore, the subsequent ARTIST and EORTC 40954 studies failed to show survival benefits of adjuvant chemoradiotherapy for patients who had undergone D2 gastrectomy for gastric cancer.[20,21] The recent CRITICS trial reported better 5-year survival benefits with adjuvant chemotherapy than chemoradiotherapy in per-protocol analysis, however only about 60% of the enrolled patient completed the planned treatment.[22] Treatment toxicity and tolerability of adjuvant chemoradiotherapy were concerns, and adjuvant chemotherapy was considered to be a better treatment strategy for these operated patients. Despite the controversial results of meta-analysis studies of adjuvant chemotherapy, 2 pivotal phase III trials, the ACTS-GC and the CLASSIC trials, showed the efficacy of postoperative adjuvant chemotherapy after D2 gastrectomy.[22–25] Consequently, adjuvant chemotherapy after R0 resection with D2 lymph node dissection is now listed as standard treatment in guidelines globally.[26] Despite the increasing popularity of perioperative chemotherapy for gastric cancer in Western countries, adjuvant chemotherapy after radical surgery is the preferred approach in Taiwan and other Asian countries.
The patient-level meta-analysis by the GASTRIC Group in 2010 reported small absolute benefits in overall survival of 5.8% at 5 years and 7.4% at 10 years with postoperative fluorouracil-based adjuvant chemotherapy compared with surgery alone.[27] In addition, Iacovelli et al found that a combination of platinum and fluorouracil was the most commonly used adjuvant chemotherapy regimen in a systematic review and meta-analysis of published trials.[28] The novel adjuvant fluorouracil agent, S-1, has demonstrated overall survival benefits in resected gastric cancer patients, but it was not reimbursed by the Taiwan NHI program until 2016. In this study, fewer than 50% of the patients with resected gastric cancer received adjuvant combination chemotherapy regimens with mitomycin, epirubicin or platinum. Adjuvant fluoropyrimidine-based regimens or its analogues (such as UFT or capecitabine) are the most commonly used adjuvant chemotherapy regimens for resected gastric cancer patients in Taiwan. However, in the present study, we found a significant overall survival benefit with PSK in combination with adjuvant chemotherapy in the multivariate analysis. Only the epirubicin-based chemotherapy group showed a nonsignificant overall survival difference with PSK combination treatment. However, this result could be biased, as only 6.3% of all patients in this study received adjuvant epirubicin-based chemotherapy, and fewer than 50 patients were included in the PSK group. Interestingly, the Japanese JACCRO GC-07 trial showed that docetaxel combined with fluorouracil further reduced the risk of hematological and lymph node metastasis in stage II and III resected gastric cancer patients, and this benefit remained in more advanced stage IIIB and IIIC disease.[29] However, adjuvant docetaxel in Taiwan is not reimbursed by the NHI program for resected gastric cancer patients, and it is only used by physicians for patients with more advanced disease postoperatively.
PSK has been used as an additive in chemotherapy for gastric cancer in Asia for decades. The antitumor activity of PSK in vivo, in vitro and in various types of cancers has been demonstrated.[30] The mechanism of action of PSK has yet to be fully elucidated, however its role in immune modulation and as a biological response modifier have been proposed.[3] The benefit of adding immunotherapy to chemotherapy to treat gastric cancer in a metastatic or adjuvant setting has recently been demonstrated in 2 phase III trials.[31,32] In addition, combining PSK with adjuvant chemotherapy for gastric cancer has the additional benefit of not causing additional side effects in the postoperative setting. The role of PSK in immunotherapy or as a biological response modifier may improve the host-tumor microenvironment, and increase the ability of the host to defend itself from tumor progression.
One Japanese study by Tanaka et al reported adding PSK to adjuvant chemotherapy showed survival benefits only in pN3 and early recurrent patients.[33] But in this study, we clearly showed an overall survival benefit (6.49 vs 3.59 years) in all patients with gastric cancer using PSK in combination with chemotherapy after curative surgery. The major difference was only oral fluoropyrimidine agents were used in this Japanese study but intravenous multi-cytotoxic agents combinations were used as adjuvant treatment in this study. There are currently no robust biomarkers to tailor the immunotherapy, however programmed death-ligand 1 has been widely tested as a predictive biomarker. Hsu et al reported longer survival in patients negative for programmed death-ligand 1 treated with adjuvant PSK, and demonstrated significantly increased percentages of natural killer and natural killer T cells in this patient group.[7] In addition, the neutrophil to lymphocyte ratio was also evaluated as an indicator of the immunoenhancing effect of PSK in 1 Japanese study.[9] Clinically, we found that old age (75 years) and underlying diabetes mellitus, hypertension or smoking-related disorders were associated with poorer survival. Only PSK combined with adjuvant chemotherapy had a determinant effect on survival.
In this study, we used propensity score matching to overcome the heterogeneity of the patients’ characteristics. We found a 23% reduction in overall mortality in the resected gastric patients who used PSK in combination with adjuvant chemotherapy after adjusting for confounding factors. According to the regulations of the Taiwan NHI program, indications for adjuvant PSK must be reviewed and approved before it can be prescribed. Therefore, the NHIRD provided more reliable information and restricted PSK use in postgastrectomy patients. This government-powered NHI program also provided an exact date of death of patients and the overall survival was confirmed. The limitations of this study included the inevitable bias in national data base for patient selection, allocation and follow-up. The details of gastric cancer stage, the choice, dosage, treatment cycles and way of administration of chemotherapy were not possible to be elucidated. There was not detailed disease-free survival and the cause of death specific to gastric cancer were also not available. However, we provide real-world data with long-term follow-up, and demonstrated that the combination of PSK with adjuvant chemotherapy prolonged survival in gastric cancer patients treated with curative surgery.
5. Conclusions
This study provides real-world evidence of overall survival benefits with PSK in combination with adjuvant chemotherapy for resected gastric cancer patients. After adjusting for all other confounding factors, the use of PSK with adjuvant chemotherapy was the most important factor for better survival. The proposed mechanism is that PSK may act as an immune modulator or biological response modifier to potentially change the patient-tumor microenvironment. Prospective studies regarding the tumor microenvironment and clinical trials of PSK in combination with modern immunotherapeutic agents are warranted.
Acknowledgments
We thank the Health Information and Epidemiology Laboratory at Chang Gung Memorial Hospital, Chiayi for the comments and assistance with the data analysis.
Author contributions
TY Wang: project development, manuscript writing
CY Chen: manuscript editing
TH Huang: data collection
YH Yang: project development, data analysis
KJ Chen: data analysis
WC Chou: manuscript editing
CH Lu: manuscript writing/editing.
Abbreviations:
- CI =
- confidence interval
- HR =
- hazard ratio
- ICD-9 =
- International Classification of Diseases, ninth Revision
- ICD-9-CM =
- The International Classification of Diseases, 9th Revision, Clinical Modification
- NHI =
- National Health Insurance
- NHIRD =
- National Health Insurance Research Database
- PSK =
- protein-bound polysaccharide K
- S-1 =
- tegafur/gimeracil/oteracil
- UFT =
- uracil-ftorafur
How to cite this article: Wang T-Y, Chen C-Y, Huang T-H, Yang Y-H, Chen K-J, Chou W-C, Lu C-H. Protein-bound polysaccharide K prolonged overall survival in gastric cancer patients from a non-Japanese Asian country who received gastrectomy and adjuvant chemotherapy. Medicine 2022;101:29(e29632).
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
The authors declare that they have no conflicts of interests.
Funding: This study was supported by a grant from Chang Gung Medical Foundation (CFRPG6J0031). The funding body had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Contributor Information
Ting-Yao Wang, Email: tywang.onco@gmail.com.
Chao-Yu Chen, Email: chenkojung@gmail.com.
Tzu-Hao Huang, Email: mr8916@cgmh.org.tw.
Yao-Hsu Yang, Email: r95841012@ntu.edu.tw.
Ko-Jung Chen, Email: chenkojung@gmail.com.
Wen-Chi Chou, Email: wenchi3992@yahoo.com.tw.
References
- [1].Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- [2].National comprehensive cancer network. gastric cancer (Version 2.2020). 2020. Available at: https://www.nccn.org/professionals/physician_gls/pdf/gastric.pdf [access date June 6, 2020].
- [3].Maehara Y, Tsujitani S, Saeki H, et al. Biological mechanism and clinical effect of protein-bound polysaccharide K (KRESTIN((R>: review of development and future perspectives. Surg Today. 2012;42:8–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ito G, Tanaka H, Ohira M, et al. Correlation between efficacy of PSK postoperative adjuvant immunochemotherapy for gastric cancer and expression of MHC class I. Exp Ther Med. 2012;3:925–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Sakamoto J, Teramukai S, Koike A, et al. Prognostic value of preoperative immunosuppressive acidic protein in patients with gastric carcinoma. Findings from three independent clinical trials. Tumor marker committee for the study group of immunochemotherapy with PSK for gastric cancer. Cancer. 1996;77:2206–12. [DOI] [PubMed] [Google Scholar]
- [6].Administration NHI. Provisions of the national health insurance drug payment. 2004. Available at: https://www.nhi.gov.tw/Resource/bulletin/650_0930051014 [access date June 6, 2020].
- [7].Hsu JT, Hsu CS, Le PH, et al. Immunochemotherapy benefits in gastric cancer patients stratified by programmed death-1 ligand-1. J Surg Res. 2017;211:30–8. [DOI] [PubMed] [Google Scholar]
- [8].Fukuchi M, Mochiki E, Ishiguro T, et al. Improved efficacy by addition of protein-bound polysaccharide K to adjuvant chemotherapy for advanced gastric cancer. Anticancer Res. 2016;36:4237–41. [PubMed] [Google Scholar]
- [9].Namikawa T, Fukudome I, Ogawa M, et al. Clinical efficacy of protein-bound polysaccharide K in patients with gastric cancer undergoing chemotherapy with an oral fluoropyrimidine (S-1). Eur J Surg Oncol. 2015;41:795–800. [DOI] [PubMed] [Google Scholar]
- [10].Yoshikawa K, Shimada M, Kurita N, et al. The effect of polysaccharide k with S-1 based chemotherapy in advanced gastric cancer. Hepatogastroenterology. 2013;60:1387–90. [DOI] [PubMed] [Google Scholar]
- [11].Ahn MS, Kang SY, Lee HW, et al. 5-Fluorouracil, mitomycin-c, and polysaccharide-k versus uracil-ftorafur and polysaccharide-K as adjuvant chemoimmunotherapy for patients with locally advanced gastric cancer with curative resection. Onkologie. 2013;36:421–6. [DOI] [PubMed] [Google Scholar]
- [12].Chen CY, Yang YH, Lee CP, et al. Risk of depression following uterine cancer: a nationwide population-based study. Psychooncology. 2017;26:1770–6. [DOI] [PubMed] [Google Scholar]
- [13].Cheng KC, Liao KF, Lin CL, et al. Gastrectomy correlates with increased risk of pulmonary tuberculosis: a population-based cohort study in Taiwan. Medicine (Baltim). 2018;97:e11388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Yang YH, Chen WC, Tsan YT, et al. Statin use and the risk of cirrhosis development in patients with hepatitis C virus infection. J Hepatol. 2015;63:1111–7. [DOI] [PubMed] [Google Scholar]
- [15].Tsan YT, Lee CH, Ho WC, et al. Statins and the risk of hepatocellular carcinoma in patients with hepatitis C virus infection. J Clin Oncol. 2013;31:1514–21. [DOI] [PubMed] [Google Scholar]
- [16].LS a. Performing a 1:N case-control match on propensity score. In: Proceedings of the twenty-ninth annual SAS (Users Group International Conference. Cary NC: SAS Institute Inc; 2004. 165–29. Available at http://www2.sas.com/proceedings/sugi29/toc.html. [Google Scholar]
- [17].Smalley SR, Benedetti JK, Haller DG, et al. Updated analysis of SWOG-directed intergroup study 0116: a phase III trial of adjuvant radiochemotherapy versus observation after curative gastric cancer resection. J Clin Oncol. 2012;30:2327–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20. [DOI] [PubMed] [Google Scholar]
- [19].Ychou M, Boige V, Pignon JP, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol. 2011;29:1715–21. [DOI] [PubMed] [Google Scholar]
- [20].Lee J, Lim DH, Kim S, et al. Phase III trial comparing capecitabine plus cisplatin versus capecitabine plus cisplatin with concurrent capecitabine radiotherapy in completely resected gastric cancer with D2 lymph node dissection: the ARTIST trial. J Clin Oncol. 2012;30:268–73. [DOI] [PubMed] [Google Scholar]
- [21].Schuhmacher C, Gretschel S, Lordick F, et al. Neoadjuvant chemotherapy compared with surgery alone for locally advanced cancer of the stomach and cardia: European Organisation for Research and Treatment of Cancer Randomized Trial 40954. J Clin Oncol. 2010;28:5210–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].de Steur WO, van Amelsfoort RM, Hartgrink HH, et al. Adjuvant chemotherapy is superior to chemoradiation after D2 surgery for gastric cancer in the per-protocol analysis of the randomized CRITICS trial. Ann Oncol. 2021;32:360–7. [DOI] [PubMed] [Google Scholar]
- [23].Hermans J, Bonenkamp JJ, Boon MC, et al. Adjuvant therapy after curative resection for gastric cancer: meta-analysis of randomized trials. J Clin Oncol. 1993;11:1441–7. [DOI] [PubMed] [Google Scholar]
- [24].Earle CC, Maroun JA. Adjuvant chemotherapy after curative resection for gastric cancer in non-Asian patients: revisiting a meta-analysis of randomised trials. Eur J Cancer. 1999;35:1059–64. [DOI] [PubMed] [Google Scholar]
- [25].Sakuramoto S, Sasako M, Yamaguchi T, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007;357:1810–20. [DOI] [PubMed] [Google Scholar]
- [26].Bang YJ, Kim YW, Yang HK, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet. 2012;379:315–21. [DOI] [PubMed] [Google Scholar]
- [27].Kim IH. Current status of adjuvant chemotherapy for gastric cancer. World J Gastrointest Oncol. 2019;11:679–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Iacovelli R, Pietrantonio F, Maggi C, et al. Combination or single-agent chemotherapy as adjuvant treatment of gastric cancer: a systematic review and meta-analysis of published trials. Crit Rev Oncol Hematol. 2016;98:24–8. [DOI] [PubMed] [Google Scholar]
- [29].Yoshida K, Kodera Y, Kochi M, et al. Addition of docetaxel to oral fluoropyrimidine improves efficacy in patients with stage III gastric cancer: interim analysis of JACCRO GC-07, a randomized controlled trial. J Clin Oncol. 2019;37:1296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Sun C, Rosendahl AH, Wang XD, et al. Polysaccharide-K (PSK) in cancer—old story, new possibilities? Curr Med Chem. 2012;19:757–62. [DOI] [PubMed] [Google Scholar]
- [31].Moehler M, Shitara K, Garrido M, et al. LBA6_PR Nivolumab (nivo) plus chemotherapy (chemo) versus chemo as first-line (1L) treatment for advanced gastric cancer/gastroesophageal junction cancer (GC/GEJC)/esophageal adenocarcinoma (EAC): First results of the CheckMate 649 study. Ann Oncol. 2020;31:S1191. [Google Scholar]
- [32].Kelly RJ, Ajani JA, Kuzdzal J, et al. LBA9_PR Adjuvant nivolumab in resected esophageal or gastroesophageal junction cancer (EC/GEJC) following neoadjuvant chemoradiation therapy (CRT): First results of the CheckMate 577 study. Ann Oncol. 2020;31:S1193–S4. [Google Scholar]
- [33].Tanaka H, Muguruma K, Ohira M, et al. Impact of adjuvant immunochemotherapy using protein-bound polysaccharide-K on overall survival of patients with gastric cancer. Anticancer Res. 2012;32:3427–33. [PubMed] [Google Scholar]