Background:
Opioid-induced nausea and vomiting are common side effects of patient-controlled intravenous analgesia (PCIA). This study aimed to explore the inhibitory effect of a naloxone admixture on the incidence of sufentanil-induced postoperative nausea and vomiting (PONV).
Methods:
A total of 132 Uyghur American Society of Anesthesiologists I and II patients scheduled to undergo elective gynecological laparoscopic surgery were recruited; among these, 120 patients were enrolled and randomly allocated into 4 groups: patients receiving PCIA but no naloxone were included in the control group (group A); patients receiving PCIA with a low-dose naloxone admixture at 0.2 μg·kg−1·h−1 were included in group B; patients receiving PCIA with naloxone admixture at 0.4 μg·kg−1·h−1 were included in group C; patients receiving PCIA with naloxone admixture at 0.6 μg·kg−1·h−1 were included in group D. All patients were administered sufentanil at 0.04 kg−1·h−1, butorphanol at 2 kg−1·h−1, and dexmedetomidine at 0.08 kg−1·h−1 using a PCIA device within 2 days of surgery. The occurrence of nausea and vomiting, visual analogue scores for pain intensity, mean arterial pressure, heart rate, oxygen saturation, pruritus, lethargy, respiratory depression, etc, was recorded at 2, 8, 12, 24, and 48 hours postoperatively.
Results:
There was a significant difference in the PONV scores between the groups at 8, 12, and 24 hours after surgery (P < 0.01). At 8 and 12 hours, the score of group C/D was significantly lower than that of group A/B (P < 0.01). At 24 hours after surgery, the PONV score of group B/C/D was significantly lower than that of group A (P < 0.01). No significant difference was observed in the general data and visual analogue scores for postoperative pain between the 4 groups.
Conclusion:
Naloxone admixture administered at 0.4 to 0.6 μg·kg−1·h−1 can exert an effective inhibitory effect on the incidence and intensity of PONV in gynecological laparoscopic surgery.
Keywords: naloxone, patient-controlled intravenous analgesia, postoperative nausea and vomiting
1. Introduction
Postoperative analgesia can reduce complications and mortality by relieving pain.[1] Analgesia is an essential part of painless treatment and is an intrinsic requirement for enhanced recovery after surgery.[2] Patient-controlled intravenous analgesia (PCIA) with opioid analgesics is the most commonly used method for the treatment of moderate or severe postoperative pain. Regardless of the injection method, the utilization of opioids can produce numerous undesired side effects, including nausea and vomiting, pruritus, dizziness, drowsiness, urinary retention, constipation, dependence, tolerance, cognitive impairment, respiratory depression, and opioid-induced hyperalgesia.[3] Although these side effects are not life-threatening, unpleasant experiences may result in discomfort and decreased quality of life. Among these side effects, postoperative nausea and vomiting (PONV) is widespread, and frequently so debilitating that patients cannot tolerate with them even would rather be in pain. Amelioration or elimination of side effects, especially PONV, has increasingly become one of the trickiest challenges in acute pain management for anesthesiologists.
PONV is a common complication associated with all types of anesthesia (general, regional, or local) within 24 hours, which may give rise to wound dehiscence, electrolyte imbalances, dehydration, unpleasant experiences, delayed discharge time, additional medical interventions, and higher health care costs.[4,5] The incidence of PONV ranges from 30% to 50%. In particular, the incidence has reached 80% after gynecological laparoscopic surgery in high-risk patients.[6,7] Vomiting is a complex nerve reflex, and stimulation of these afferent pathways activates the sensation of vomiting through cholinergic (muscarine), dopaminergic, histamine, or 5-serotonergic receptors.[8] Despite advances in prevention and treatment, PONV is still a concern for many patients undergoing gynecological laparoscopic surgery.[6,9] The use of opioids, agonists for the classical mu, kappa, and delta opioid receptors, has sharply increased the incidence of PONV in a dose-dependent manner during postoperative pain management.[10–12]
Naloxone, a widely used opioid receptors antagonist, is mainly used to diagnose and alleviate respiratory depression involving opioid overdose or alcoholism. Some studies have suggested that the combined application of naloxone and opioids may reduce opioid-related side effects.[13–15] A combination of low-dose epidural naloxone at 0.25 μg·kg−1·h−1 with fentanyl is valid in attenuating undesired side effects, especially PONV, besides enhancing analgesia during the early postoperative period.[16] Intravenous infusion of naloxone at a low dose of 0.25 to 1.65 μg·kg−1·h−1 reduces PONV in children, with greater evidence of its effectiveness as a preventative strategy than in the treatment of existing pruritus.[17] Movafegh et al[18] also showed that an ultralow dose of naloxone infusion could reduce morphine consumption, as well as the incidence of opioid-induced PONV.
However, not all studies had the same opinion. Admixing low-dose naloxone with a PCIA solution increased opioid requirement and pain, following the increasing incidence of side effects.[19] A similar conclusion was obtained and confirmed by another study.[20] In another study, the combination of naloxone with morphine in normal saline did not decrease the incidence or severity of PONV.[21] The incidence of opioid-induced side effects in postoperative pediatric patients receiving PCIA was not eliminated when the infusion rate of naloxone was <1 μg·kg−1·h−1.[3] The main reason for reaching converse conclusions lies in the difference in opioid choice (morphine fentanyl, sufentanil, etc), the discriminate usage and dosage of naloxone, and the distinct observation indicators of side effects. Thus, there is no consistency and standard on this issue which encouraged us to evaluate the definite effects of a naloxone admixture on the incidence and intensity of sufentanil-induced PONV in gynecological laparoscopic surgery.
2. Methods
2.1. General information
This study was conducted in accordance with the Declaration of Helsinki of 1975, as revised in 2010, and was approved by the Ethics Review Committee of the 950 Hospital. Before the study, each patient’s consent was obtained. The inclusion criteria were as follows: American Society of Anesthesiologists physical status I to II, adult Uyghur women aged >18 years, and patients diagnosed with gynecologic benign disease undergoing laparoscopic surgery, general anesthesia, and postoperative PCIA. The exclusion criteria included refusal to participate in the trial, entering the intensive care unit after the surgery, reinsertion of the nasogastric tube, cognitive impairment, and receiving steroid or antiemetic treatment within 24 hours before the surgery.
2.2. Study design and setting
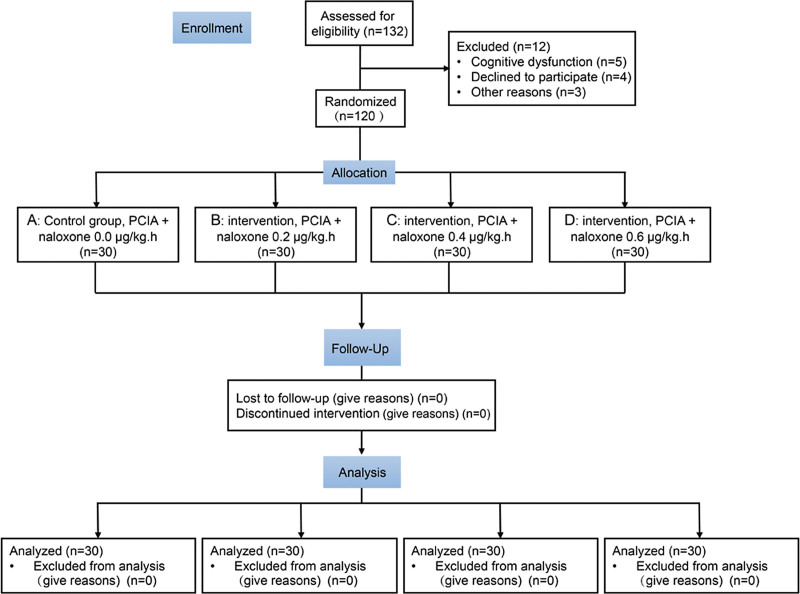
General anesthesia for all patients was achieved using midazolam 1 mg, propofol 1.5 mg/kg, sufentanil 0.6 μg/kg, and rocuronium bromide 0.6 mg/kg. Anesthesia was induced for endotracheal intubation by continuously pumping propofol and remifentanil to maintain the depth of anesthesia during the surgery. Intermittent intravenous rocuronium bromide was administered to maintain muscle relaxation. After the surgery, the endotracheal tube was pulled out awake, and PCIA was used for postoperative analgesia. The eligible patients were randomly divided into 4 groups by a computer (Fig. 1). Patients receiving PCIA but no naloxone were allocated to the control group (group A), and patients receiving PCIA with naloxone admixture at a rate of 0.2, 0.4, 0.6 μg·kg−1·h−1 were assigned to groups B, C, and D, respectively. The injection rate of the PCIA pump was 0.04 μg·kg−1·h−1 for sufentanil, 2 μg·kg−1·h−1 for butorphanol tartrate, and 0.08 μg·kg−1·h−1 for dexmedetomidine, with a total volume of 100 mL. Self-controlled analgesia was delivered at 0.5 mL each time with an interval of 15 minutes. Before the surgery, each patient was trained to understand the assessment scales used in this study and the methods of using the PCIA system.
Figure 1.
Flowchart of the present research. PCIA = patient-controlled intravenous analgesia.
2.3. Data collection
During the follow-up period, we collected information on the basic vital signs (mean arterial pressure, heart rate, and oxygen saturation [SpO2]) at 2, 8, 12, 24, and 48 hours after surgery and recorded the patient’s nausea and vomiting score (PONV) and visual analogue score (VAS) at each time period, as well as the number of cases of itching, drowsiness, dizziness, hyperhidrosis, and respiratory depression (respiratory rate, <8 breaths/min or SpO2, <90%). The PONV scoring standards were as follows: no nausea or vomiting, 1 point; mild nausea, abdominal discomfort, but no vomiting, 2 points; obvious nausea and vomiting but no stomach contents vomiting, 3 points; severe vomiting, stomach contents vomiting that was difficult to control without drugs, 4 points. The VAS recording standards were as follows: according to the analog score scale, 0 points indicated no pain, while 10 points represented the most severe pain. VAS was independently assessed during activity and quiet time, which is denoted by Moving-VAS (M-VAS) and Quiet-VAS (Q-VAS), respectively. Simultaneously, postoperative complications were observed such as pruritus, drowsiness, dizziness, hyperhidrosis, and respiratory depression. Respiratory depression was defined as a respiratory rate of <8 breaths/min or SpO2 of <90%, which should be dealt with in time. If it persisted, PCIA could be stopped and switched to other analgesic methods, along with the termination of the experiment. If PCIA is unsatisfactory, non-opioid drugs can be appropriately added to enhance the analgesic effect. If it is ineffective, other analgesic methods should be adopted along with the termination of the experiment.
2.4. Statistics
The required sample size was estimated based on a 50% reported incidence of sufentanil-induced PONV in gynecological laparoscopic surgery. We suppose that naloxone admixture would reduce the incidence of PONV to 20% according to a pilot study, and a minimum of 30 patients per group was required. All data were processed with SPSS 25.0 or GraphPad Prism 8.0. One-way analysis of variance was used to analyze the data. If the variances were uniform, the Student’s t test was applied for pairwise comparison; otherwise, the rank-sum test was used. The χ2 test or Fisher exact test was used for count data. The Kruskal-Wallis test was used for the grade count data. Statistical significance was set at P < .05.
3. Results
3.1. Patients’ characteristics
In total, 132 patients were recruited between March 2021 and August 2021. Twelve patients were excluded because they did not meet the inclusion criteria; therefore, 120 patients were enrolled in this study (Fig. 1). The patient characteristics (American Society of Anesthesiologists classification, age, body mass index, and surgery time) in the 4 groups were not statistically different (Table 1).
Table 1.
Patient characteristics, ASA classification, and surgery time.
| Group | ASA classification | Age | BMI | Surgery time |
|---|---|---|---|---|
| A | 1.27 ± 0.082 | 37.30 ± 2.06 | 24.56 ± 0.46 | 1.74 ± 0.06 |
| B | 1.30 ± 0.085 | 37.37 ± 1.84 | 24.13 ± 0.41 | 1.69 ± 0.07 |
| C | 1.23 ± 0.079 | 39.30 ± 1.79 | 23.53 ± 0.33 | 1.61 ± 0.06 |
| D | 1.20 ± 0.074 | 36.70 ± 1.68 | 24.13 ± 0.34 | 1.64 ± 0.07 |
Values are expressed as mean ± standard error.
ASA = American Society of Anesthesiologists, BMI = body mass index.
3.2. Primary outcome
The incidence and intensity of PONV between each group were not different at 2 and 48 hours after surgery but were different at 8, 12, and 24 hours after surgery (Table 2). Among them, the assessed score of PONV in the C/D group was significantly lower than that of the A/B group at 8 and 12 hours after surgery, with a significant difference (P < 0.01). In addition, the PONV score of group A was still higher than that of group B/C/D at the 24th hour after surgery (P < 0.01). No PCIA was stopped for various reasons in either group.
Table 2.
Postoperative PONV score of patients within 48 h.
| Group | 2 h | 8 h | 12 h | 24 h | 48 h | |
|---|---|---|---|---|---|---|
| PONV (n = 30) | A | 58.05 | 72.00 | 76.03 | 75.05 | 62.92 |
| B | 63.05 | 69.42 | 66.88 | 58.57* | 57.22 | |
| C | 61.45 | 47.50* | 49.20* | 54.80* | 64.90 | |
| D | 59.45 | 53.08* | 49.88* | 53.58* | 56.97 | |
| P value | 0.939 | 0.002 | <0.001 | <0.001 | 0.301 |
The Kruskal-Wallis K independent test was used to assess statical significance among groups. Data are expressed as average rank.
PONV = postoperative nausea and vomiting.
P < .01.
3.3. Secondary outcomes
VAS was applied to evaluate postoperative pain, and VAS data were reported at 2, 8, 12, 24, and 48 hours after surgery. The Q-VAS and M-VAS scores were assessed and recorded separately. The analysis indicated that the VAS recording was similar between the test and control groups at each time point (P > .05), as shown in Table 3.
Table 3.
Postoperative vital signs of each group.
| Item | Group | 2 h | 8 h | 12 h | 24 h | 48 h |
|---|---|---|---|---|---|---|
| MAP (mm Hg) | A | 79.53 ± 12.67 | 77.63 ± 13.26 | 75.12 ± 11.28 | 75.26 ± 10.51 | 72.75 ± 11.24 |
| B | 77.16 ± 10.73 | 79.36 ± 14.46 | 76.28 ± 13.21 | 74.78 ± 12.59 | 71.56 ± 10.25 | |
| C | 78.25 ± 13.26 | 78.21 ± 11.78 | 76.16 ± 11.28 | 73.76 ± 13.46 | 70.67 ± 13.23 | |
| D | 79.57 ± 12.56 | 77.26 ± 13.47 | 75.36 ± 10.29 | 74.37 ± 14.26 | 69.77 ± 10.12 | |
| HR (rpm) | A | 91.61 ± 13.32 | 83.65 ± 11.67 | 81.22 ± 10.79 | 75.21 ± 10.23 | 71.74 ± 9.89 |
| B | 93.72 ± 11.21 | 85.62 ± 12.79 | 80.25 ± 11.71 | 74.23 ± 11.37 | 70.27 ± 10.26 | |
| C | 89.84 ± 15.71 | 82.55 ± 14.27 | 79.23 ± 12.13 | 72.35 ± 11.86 | 72.56 ± 8.37 | |
| D | 92.88 ± 13.27 | 83.43 ± 11.82 | 78.46 ± 13.21 | 71.67 ± 12.77 | 71.79 ± 10.39 | |
| SpO2 (%) | A | 95.21 ± 4.27 | 96.45 ± 3.34 | 97.21 ± 2.31 | 98.21 ± 1.48 | 98.51 ± 1.23 |
| B | 96.23 ± 3.76 | 97.24 ± 2.21 | 96.48 ± 3.07 | 96.78 ± 1.70 | 97.20 ± 1.61 | |
| C | 95.56 ± 3.27 | 95.98 ± 2.86 | 96.21 ± 2.80 | 97.21 ± 2.01 | 97.67 ± 1.30 | |
| D | 96.34 ± 2.98 | 96.47 ± 3.11 | 97.11 ± 2.78 | 97.56 ± 1.81 | 98.01 ± 1.66 |
Values are expressed as mean ± standard division.
HR = heart rate, MAP = mean arterial pressure, SpO2 = oxygen saturation.
After the patients returned to the ward, they were examined at 2, 8, 12, 24, and 48 hours, and the mean arterial pressure, heart rate, and SpO2 were recorded at each time point (Table 4). The vital signs of the patients in the 4 groups were relatively stable, and no statistical difference was observed (P > .05). No serious adverse events affecting the patients were observed.
Table 4.
Postoperative VAS among groups.
| Item | Group | 2 h | 8 h | 12 h | 24 h | 48 h |
|---|---|---|---|---|---|---|
| Q-VAS | A | 66.13 | 66.55 | 69.70 | 57.32 | 60.50 |
| B | 56.63 | 59.93 | 68.52 | 61.77 | 56.97 | |
| C | 61.35 | 58.32 | 59.33 | 64.65 | 60.52 | |
| D | 57.88 | 57.20 | 53.45 | 58.27 | 64.03 | |
| P value | .679 | .695 | .354 | .795 | .853 | |
| M-VAS | A | 64.33 | 57.32 | 52.60 | 52.20 | 56.60 |
| B | 64.88 | 60.02 | 62.58 | 63.72 | 60.03 | |
| C | 53.53 | 54.47 | 54.63 | 55.05 | 56.50 | |
| D | 59.25 | 70.02 | 72.18 | 71.03 | 69.20 | |
| P value | .524 | .293 | .089 | .097 | .341 |
The Kruskal-Wallis K independent test was used to assess statical significance among groups. Data are expressed as average rank.
M-VAS = moving visual analog scale, Q-VAS = quiet-visual analog scale.
Postoperative complications, such as pruritus, drowsiness, dizziness, hyperhidrosis, and respiratory depression, were recorded at various time points in each group. The incidence of complications was analogous, and no statistical difference was observed (P > .05). No respiratory depression occurred, as shown in Table 5.
Table 5.
Postoperative complications in each group within 48 h.
| Item | Group | 2 h | 8 h | 12 h | 24 h | 48 h |
|---|---|---|---|---|---|---|
| Itching | A | 1 | 2 | 1 | 1 | 0 |
| B | 2 | 1 | 1 | 1 | 1 | |
| C | 0 | 2 | 2 | 1 | 1 | |
| D | 1 | 2 | 1 | 1 | 0 | |
| P value | .901 | 1.000 | 1.000 | 1.000 | 1.000 | |
| Lethargy | A | 1 | 2 | 2 | 1 | 0 |
| B | 2 | 2 | 2 | 1 | 0 | |
| C | 3 | 4 | 2 | 2 | 1 | |
| D | 3 | 4 | 2 | 2 | 0 | |
| P value | .872 | .751 | 1.000 | 1.000 | 1.000 | |
| Dizziness | A | 5 | 4 | 2 | 2 | 2 |
| B | 4 | 4 | 3 | 3 | 2 | |
| C | 4 | 3 | 3 | 2 | 2 | |
| D | 5 | 3 | 2 | 2 | 1 | |
| P value | 1.000 | 1.000 | 1.000 | 1.000 | .733 | |
| Sweating | A | 1 | 1 | 2 | 1 | 1 |
| B | 1 | 2 | 1 | 0 | 0 | |
| C | 0 | 1 | 1 | 1 | 1 | |
| D | 2 | 2 | 2 | 1 | 0 | |
| P value | .901 | .855 | 1.000 | 1.000 | 1.000 |
The χ2 test was applied to assess the incidence of complications among groups in each time period. When the number of observation samples was <5, the Fisher exact test was employed. The P values were all >.05.
4. Discussion
This study illustrates that a naloxone admixture infusion at 0.4 to 0.6 μg·kg−1·h−1 opportunely decreases the incidence and intensity of sufentanil-induced side effects such as PONV as well as maintains a normal level of analgesia during gynecological laparoscopic surgery. Possibly due to the limited sample size, other side effects of opioids, including itching, drowsiness, dizziness, and hyperhidrosis, were not statistically significant among the groups.
Opioids are effective in pain management. However, the use of opioids may produce several adverse effects including nausea, vomiting, pruritus, and constipation. Previously, it has been reported that naloxone was used to prevent side effects caused by opioids, such as nausea, vomiting, constipation, and respiratory depression.[22–25] Regarding the prevention of PONV, the naloxone’s usage, dosage, using time, and types of compatible opioids may have contradictory conclusions.[3,18,21,26,27] No agreement or standard on this issue urged us to conduct this study to determine the definite effects of a naloxone admixture on the incidence and intensity of sufentanil-induced PONV.
PONV is a common and uncomfortable postoperative complication, with an overall incidence of 30% to 50%, which significantly increases patient suffering and prolongs hospital stay.[6] Therefore, there is an urgent need to seek methods or measures to reduce the incidence of PONV. The pathogenesis of PONV is relatively complicated, and the high-risk factors include patient characteristics (female, <50 years of age, nonsmoker, history of PONV, or motion sickness), anesthetic origin (method of anesthesia, anesthesia time, inhalation anesthetics, and use of opioids or neostigmine), and surgical factors (surgical site, surgical type such as gynecological and laparoscopic surgery, and length of surgery). The pathophysiological mechanism of PONV involves the transmission of various stress stimuli to the central nervous system, activation of the vagus from the peripheral nerve, reduced gastrointestinal motility, and receptor activation pathways of drug toxins.[28]
Routine use of opioids in perioperative pain management is an important contributor to PONV. In recent years, due to the development of minimally invasive surgery and the increasing emphasis on early activities and discharge, as well as the objective requirements for enhanced recovery after surgery, opioid-sparing strategy and some new antiemetics have been utilized, but a high incidence of PONV still exists. Clinically, PONV management is divided into drug and nondrug treatments. Drug treatment mainly involves the use of 5 hydroxytryptamine (5-HT3) receptor antagonists, antihistamines, neurokinin 1 (NK-1) receptor antagonists, anticholinergics, and dopamine receptor antagonists.[29] Nondrug prophylaxis involves reducing the fasting time, maintaining water and electrolyte balance, and decreasing the use of inhaled anesthetics. The pharmacological management of PONV should use a validated risk-scoring system, adjust the risk level of patients, adopt cost-effective methods for high-risk groups, and minimize the possibility of adverse side effects due to drug interactions during the perioperative period. For populations at high risk of PONV, the main preventive measures are local anesthesia or nerve block, intravenous anesthesia if possible, reducing the use of opioids and inhaled anesthetics, and ensuring an appropriate perioperative water supply. In addition, the use of preventive antiemetics, an opioid-sparing strategy combined with multimodal analgesia techniques, is also available.[29,30] Overall, the use of strategies to lower the baseline risk of PONV (decreasing water deprivation time, reducing opioid usage, and multimodal analgesia) will reduce the likelihood of patients developing PONV.
Uyghur women, particularly the older women, are often nervous and anxious during hospitalization due to differences in language and culture, which virtually increases the incidence of PONV. This study was carried out at a secondary hospital in the Uyghur-populated area and focused on patients undergoing gynecological laparoscopic surgery who were at a high risk of PONV. In this study, we added a gradient dose of naloxone to a PCIA device to explore its possible role in reducing the incidence and intensity of PONV. Previous studies have shown that small doses of naloxone in the epidural space can effectively prevent side effects such as nausea and vomiting caused by opioids, without weakening or even enhancing analgesia.[18,19] However, similar studies have had opposing opinions. Studies have shown that continuous infusion of naloxone aggravates the pain and increases the use of opioids.[3,31] The main reasons for reaching converse conclusions are discussed above. In recent years, PCIA has been more widely used in postoperative analgesia, but there are few reports on whether the simultaneous use of low-dose naloxone in PCIA has a similar impact on reducing the incidence of PONV. Previous studies have focused on observing the incidence of PONV rather than the intensity of PONV, which is clinically more relevant.
Studies have shown that the use of naloxone at a concentration of 1 μg·kg−1·h−1 did not weaken the analgesic effect and increased the use of opioids.[3] In this study, we set up a control group (group A) and 3 experimental groups (low-dose naloxone gradients, groups B, C, and D). The results showed that the application of naloxone in groups C and D can reduce the incidence and intensity of PONV at 8 hours postoperatively, and the lower dose of naloxone in group B plays a delayed role at 12 hours postoperatively, confirming that a naloxone admixture ameliorates the side effects of PONV caused by opioids. Concurrently, we also analyzed the Q-VAS and M-VAS scores and found that naloxone admixture administered at a rate of 0.2 to 0.6 μg·kg−1·h−1 maintained a normal level of analgesia without increasing sufentanil consumption. Evidence suggests that the side effects associated with opioids result from the activation of excitatory mu receptors.[32] As mentioned above, naloxone, a nonselective opioid receptor antagonist, may reduce the incidence of side effects associated with opioids, such as itching, drowsiness, dizziness, hyperhidrosis, pruritus, and respiratory depression.[33–35] However, in this study, we found no statistically significant differences in these side effects between the groups. The underlying reason may be that the mechanisms of the various side effects caused by opioids are not exactly the same. The population study of previous literature was different from the present study, which involved a wide range of subjects, aged 6 to 18 years.[35] Second, the incidence of side effects such as itching, drowsiness, dizziness, hyperhidrosis, and respiratory depression in this study was extremely low or even absent in this study. Coupled with the low sample size and single-center research, this may produce some interference with the true results.
This study has several limitations. This was a single-center study conducted in a secondary hospital and lacked multicenter large-scale clinical trial validation. In addition, the mechanism by which naloxone antagonizes the side effects of opioids requires further investigation.
In conclusion, an admixture of low-dose naloxone at 0.4 to 0.6 μg·kg−1·h−1 can better ameliorate the symptoms of PONV caused by opioids in gynecological laparoscopic surgery earlier. The efficacy of naloxone in preventing opioid-induced PONV was identified in this study, suggesting that naloxone may be widely used in clinical practice and may significantly improve the quality of life of patients.
Author contributions
Siyi He designed and performed the study. Haihong Yang, Meiling Xu, Xuedong Gu, Yunju Rao, and Liang Gao collected and analyzed the data. Haihong Yang, Gu Gong, and Siyi He analyzed the results and wrote the manuscript. All authors read and approved the final manuscript.
Acknowledgments
Thanks to all the peer reviewers and editors for their valuable opinions and suggestions.
Abbreviations:
- ASA =
- American Society of Anesthesiologists
- PCIA =
- patient-controlled intravenous analgesia
- PONV =
- postoperative nausea and vomiting
- SpO2 =
- oxygen saturation
- VAS =
- visual analogue score
HY and XG contributed equally to this work.
How to cite this article: Yang H, Gu X, Xu M, Yang G, Rao Y, Gao L, Gong G, He S. Preventing nausea and vomiting after gynecological laparoscopic surgery by patient-controlled intravenous analgesia with a naloxone admixture: a randomized controlled trial. Medicine 2022;101:29(e29584).
This study was conducted after approval from the Ethics Review Committee of the 950 Hospital (2021-001; Kashi, China. Written informed consent was obtained from all patients before the study.
This study was supported by the National Natural Science Foundation of China (grant number: 81902847) and college-level funding (grant number: 2021-XZYG-B15).
The authors have no conflicts of interest to disclose.
All authors have given full consent for publication.
The data used to support the findings of this study are available from the corresponding author upon request.
Contributor Information
Haihong Yang, Email: yangguan001@163.com.
Xuedong Gu, Email: guxd123@163.com.
Meiling Xu, Email: 153200561@qq.com.
Guan Yang, Email: yangguan001@163.com.
Yunju Rao, Email: 885163201@qq.com.
Liang Gao, Email: 103306594@qq.com.
Gu Gong, Email: gonggugg@163.com.
References
- [1].Barratt DT, Sia AT, Tan EC, et al. Innate immune and neuronal genetic markers are highly predictive of postoperative pain and morphine patient-controlled analgesia requirements in Indian but not Chinese or Malay hysterectomy patients. Pain Med. 2021;11:2648–2660. [DOI] [PubMed] [Google Scholar]
- [2].Andres J, Pogatzki-Zahn E, Huygen F, et al. Controlling acute pain to improve the quality of postoperative pain management: an update from the European Society of Regional Anesthesia meeting held in Maastricht (September 2016). Pain Manag. 2017;7:513–22. [DOI] [PubMed] [Google Scholar]
- [3].Monitto CL, Kost-Byerly S, White E, et al. The optimal dose of prophylactic intravenous naloxone in ameliorating opioid-induced side effects in children receiving intravenous patient-controlled analgesia morphine for moderate to severe pain: a dose finding study. Anesth Analg. 2011;113:834–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hill RP, Lubarsky DA, Phillips-Bute B, et al. Cost-effectiveness of prophylactic antiemetic therapy with ondansetron, droperidol, or placebo. Anesthesiology. 2000;92:958–67. [DOI] [PubMed] [Google Scholar]
- [5].Skledar SJ, Williams BA, Vallejo MC, et al. Eliminating postoperative nausea and vomiting in outpatient surgery with multimodal strategies including low doses of nonsedating, off-patent antiemetics: is “zero tolerance” achievable? ScientificWorldJournal. 2007;7:959–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Tao B, Liu K, Wang D, et al. Effect of intravenous oxycodone versus sufentanil on the incidence of postoperative nausea and vomiting in patients undergoing gynecological laparoscopic surgery. J Clin Pharmacol. 2019;59:1144–50. [DOI] [PubMed] [Google Scholar]
- [7].Zheng XZ, Cheng B, Luo J, et al. The characteristics and risk factors of the postoperative nausea and vomiting in female patients undergoing laparoscopic sleeve gastrectomy and laparoscopic gynecological surgeries: a propensity score matching analysis. Eur Rev Med Pharmacol Sci. 2021;25:182–9. [DOI] [PubMed] [Google Scholar]
- [8].Horn CC, Wallisch WJ, Homanics GE, et al. Pathophysiological and neurochemical mechanisms of postoperative nausea and vomiting. Eur J Pharmacol. 2014;722:55–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ames WA, Machovec K. An update on the management of PONV in a pediatric patient. Best Pract Res Clin Anaesthesiol. 2020;34:749–58. [DOI] [PubMed] [Google Scholar]
- [10].Wallden J, Halliday TA, Hultin MR, et al. PONV in bariatric surgery: time for opioid-free anaesthesia. Acta Anaesthesiol Scand. 2017;61:858. [DOI] [PubMed] [Google Scholar]
- [11].Jorgensen H, Wetterslev J, Moiniche S, et al. Epidural local anaesthetics versus opioid-based analgesic regimens on postoperative gastrointestinal paralysis, PONV and pain after abdominal surgery. Cochrane Database Syst Rev. 2000;4:CD001893. [DOI] [PubMed] [Google Scholar]
- [12].Roberts GW, Bekker TB, Carlsen HH, et al. Postoperative nausea and vomiting are strongly influenced by postoperative opioid use in a dose-related manner. Anesth Analg. 2005;101:1343–8. [DOI] [PubMed] [Google Scholar]
- [13].Frank JW, Levy C, Calcaterra SL, et al. Naloxone administration in US emergency departments, 2000-2011. J Med Toxicol. 2016;12:148–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Dunne RB. Prescribing naloxone for opioid overdose intervention. Pain Manag. 2018;8:197–208. [DOI] [PubMed] [Google Scholar]
- [15].Leppert W, Zajaczkowska R, Wordliczek J. The role of oxycodone/naloxone in the management of patients with pain and opioid-induced constipation. Expert Opin Pharmacother. 2019;20:511–22. [DOI] [PubMed] [Google Scholar]
- [16].Nimeeliya Z, Derlin T, Kundil Alungal SR, et al. Epidural naloxone attenuates fentanyl induced PONV in patients undergoing lower limb orthopaedic surgeries. a prospective randomized double-blind comparative study. Rom J Anaesth Intensive Care. 2020;27:23–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kjellberg F, Tramer MR. Pharmacological control of opioid-induced pruritus: a quantitative systematic review of randomized trials. Eur J Anaesthesiol. 2001;18:346–57. [DOI] [PubMed] [Google Scholar]
- [18].Movafegh A, Shoeibi G, Ansari M, et al. Naloxone infusion and post-hysterectomy morphine consumption: a double-blind, placebo-controlled study. Acta Anaesthesiol Scand. 2012;56:1241–9. [DOI] [PubMed] [Google Scholar]
- [19].Cepeda MS, Africano JM, Manrique AM, et al. The combination of low dose of naloxone and morphine in PCA does not decrease opioid requirements in the postoperative period. Pain. 2002;96:73–9. [DOI] [PubMed] [Google Scholar]
- [20].Bijur PE, Schechter C, Esses D, et al. Intravenous bolus of ultra-low-dose naloxone added to morphine does not enhance analgesia in emergency department patients. J Pain. 2006;7:75–81. [DOI] [PubMed] [Google Scholar]
- [21].West N, Ansermino JM, Carr RR, et al. A naloxone admixture to prevent opioid-induced pruritus in children: a randomized controlled trial. Can J Anaesth. 2015;62:891–900. [DOI] [PubMed] [Google Scholar]
- [22].Armenian P, Vo KT, Barr-Walker J, et al. Fentanyl, fentanyl analogs and novel synthetic opioids: a comprehensive review. Neuropharmacology. 2018;134:121–32. [DOI] [PubMed] [Google Scholar]
- [23].Strang J, McDonald R, Campbell G, et al. Take-home naloxone for the emergency interim management of opioid overdose: the public health application of an emergency medicine. Drugs. 2019;79:1395–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Baykara S, Alban K. The effects of buprenorphine/naloxone maintenance treatment on sexual dysfunction, sleep and weight in opioid use disorder patients. Psychiatry Res. 2019;272:450–3. [DOI] [PubMed] [Google Scholar]
- [25].Nee J, Zakari M, Sugarman MA, et al. Efficacy of treatments for opioid-induced constipation: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2018;16:1569–84. e1562. [DOI] [PubMed] [Google Scholar]
- [26].Ma K, Wu X, Chen Y, et al. Effect of multimodal intervention on postoperative nausea and vomiting in patients undergoing gynecological laparoscopy. J Int Med Res. 2019;47:2026–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].He F, Jiang Y, Li L. The effect of naloxone treatment on opioid-induced side effects: a meta-analysis of randomized and controlled trails. Medicine (Baltim). 2016;95:e4729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Elvir-Lazo OL, White PF, Yumul R, Cruz Eng H. Management strategies for the treatment and prevention of postoperative/postdischarge nausea and vomiting: an updated review. F1000Res. 2020;9:F1000 Faculty Rev-983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Fu C, Wu T, Shu Q, et al. Acupuncture therapy on postoperative nausea and vomiting in abdominal operation: a Bayesian network meta analysis. Medicine (Baltim). 2020;99:e20301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Fu CW, Shu Q, Jiao Y, et al. A comparison of noninvasive and invasive acupuncture in preventing postoperative nausea and vomiting: a protocol for systematic review and Bayesian network meta-analysis. Medicine (Baltim). 2020;99:e21544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Johnson EG, Nguyen J, Oyler D, et al. Naloxone continuous infusion for spinal cord protection in endovascular aortic surgery leads to higher opioid administration and more pain. J Cardiothorac Vasc Anesth. 2021;35:1143–8. [DOI] [PubMed] [Google Scholar]
- [32].Phan NQ, Blome C, Fritz F, et al. Assessment of pruritus intensity: prospective study on validity and reliability of the visual analogue scale, numerical rating scale and verbal rating scale in 471 patients with chronic pruritus. Acta Derm Venereol. 2012;92:502–7. [DOI] [PubMed] [Google Scholar]
- [33].Crain SM, Shen KF. Antagonists of excitatory opioid receptor functions enhance morphine’s analgesic potency and attenuate opioid tolerance/dependence liability. Pain. 2000;84:121–31. [DOI] [PubMed] [Google Scholar]
- [34].Cepeda MS, Alvarez H, Morales O, et al. Addition of ultralow dose naloxone to postoperative morphine PCA: unchanged analgesia and opioid requirement but decreased incidence of opioid side effects. Pain. 2004;107:41–6. [DOI] [PubMed] [Google Scholar]
- [35].Maxwell LG, Kaufmann SC, Bitzer S, et al. The effects of a small-dose naloxone infusion on opioid-induced side effects and analgesia in children and adolescents treated with intravenous patient-controlled analgesia: a double-blind, prospective, randomized, controlled study. Anesth Analg. 2005;100:953–8. [DOI] [PubMed] [Google Scholar]