Abstract
Diabetes mellitus (DM) was found to be more common in hepatitis C virus (HCV)-related cirrhotic males. However, the association between DM, or other extrahepatic manifestations (EHMs), and liver cirrhosis is still undetermined. We used a large-scale long-term study to analyze the cirrhosis risk of treatment-naïve HCV patients with EHMs as compared to those without.
In this retrospective nested case-control study, we identified 11 872 treatment-naïve patients with chronic HCV between 2001 and 2013 from Taiwan National Health Insurance Research Database and divided them into patients with (cases) and without cirrhosis (controls). All patients were followed up from the index month (exact month of diagnosis) to the end of 2013, death, or study outcome, whichever occurred first. The cases and controls were 1:6 propensity score matched for age, sex, and exact month of diagnosis; finally, 8078 patients (1154 with and 6924 without cirrhosis) were included in the analysis. The presence of coexisting EHMs and a new diagnosis of cirrhosis was analyzed. Adjusted hazard ratios (HRs) and cumulative incidence for cirrhosis were calculated in conditional Cox regression models after propensity score matching.
Patients with high-cirrhosis-risk EHMs, such as DM (HR: 1.72, 95% CI: 1.51–1.96, P < .001), HCD (HR: 1.45, 95% CI: 1.27–1.67, P < .007), CKD (HR: 1.21, 95% CI: 1.05–1.38, P < .001), hyperlipidemia (HR: 0.53, 95% CI: 0.46–0.60, P < .001), lichen planus (HR: 2.71, 95% CI: 1.56–4.72, P < .001), and palpable purpura (HR: 2.67, 95% CI: 2.13–3.35, P < .001) exhibited significantly higher risk of liver cirrhosis than those without. Cumulative incidence (P < .001) of liver cirrhosis by pairwise comparisons of multiple high-cirrhosis-risk EHMs, and that of lichen planus was the highest.
Our study provided direct estimates of specific HCV-associated EHM time trends of cirrhosis risk, with an upward trend in incidence. Lichen planus was at the top of the list of single-EHM comparisons, and the maximum combination of certain EHMs was the greatest risk factor across a different array of multi-EHM comparisons for liver cirrhosis development.
1. Introduction
Hepatitis C virus (HCV) infection is not only etiologically associated with developing end-stage liver disease, including cirrhosis, decompensated liver disease, and hepatocellular carcinoma, but is also associated with multiple systemic diseases beyond the liver; these are called extrahepatic manifestations (EHMs). EHMs include metabolic, cardiovascular, renal, autoimmune, lymphoproliferative, and neurologic conditions, reported to exist at a rate of 31% of HCV-infected individuals.[1–6] Considered as a major contributor to many systemic diseases, HCV is associated with extrahepatic morbidities. Chronic hepatitis C infection (CHC) has been linked to a 4 times higher risk of insulin resistance and type 2 diabetes mellitus (DM) than previously reported, with cardiovascular disease in 17% to 37% of patients,[7] and with increased risk for cerebrovascular mortalities and lymphoproliferative disorders, particularly non-Hodgkin B-cell lymphoma. A kidney is involved in 35% to 60% of patients with CHC-associated mixed cryoglobulinemia.[7] EHMs of HCV involve multiple organ systems, not just the liver, that are linked to a variety of comorbidities, leading to significantly increased mortality from nonliver-associated disorders.
Our research aimed to use a large-scale long-term study to analyze the cirrhosis risk of treatment-naïve HCV patients with EHMs as compared to those without.
2. Materials and Methods
2.1. Data source
Data for this analysis were retrieved from the Taiwan National Health Insurance (NHI) Research Database (NHIRD). The NHIRD includes the original medical data of 3000,000 randomly sampled NHI beneficiaries (from among roughly 27 million total) for the years 2000, 2005, and 2010. All diagnoses were coded according to the International Classification of Disease, Ninth Revision, Clinical Modification. Between January 1, 2000, and December 31, 2013, 16,743 eligible HCV patients were sampled from the 3000,000 insureds of the NHI program. The data used for analysis in this cohort study were acquired from Taiwan National Health Insurance Research Database (NHIRD). This database, which is controlled by the Taiwan National Health Research Institutes and the Department of Health, includes claims data for all medical and surgical treatment provided by the Taiwan National Health Insurance. Taiwan National Health Insurance is a compulsory health insurance program that started in 1995. It contains 99% of the total population of Taiwan based on the obligatory rule. By the end of 2013, this nationwide administrative claims database contained person-level facts of examinations, diagnoses, and medications for approximately 23.5 million persons. The computerized databases of the National Health Insurance Research Database include data regarding medical diseases recorded during inpatient care and outpatient visits. It also contains drugs prescribed under the NHI system. This research was approved by the Institutional Review Board of Show Chwan Memorial Hospital on October 5, 2015 (SCMH_IRB NO:1040905).
2.2. Study design
This study was designed as a retrospective event risk analytical cohort study. We enrolled consecutive patients with HCV from Taiwan National Health Insurance Research Database (NHIRD) from January 1, 2001, to December 31, 2013, and divided them into patients with cirrhosis (cases) and with cirrhosis (controls). We recorded the EHMs of interest, namely diabetes mellitus (DM), hypertensive cardiovascular disease (HCD), chronic kidney disease (CKD), hyperlipidemia, lichen planus, and palpable purpura, from the day of HCV diagnosis to cirrhosis occurrence in the cirrhosis group or a matched end of the follow-up in the control group.
2.3. Case identification
Patients with HCV were recognized using the diagnostic codes per the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). A patient was identified to have chronic HCV (ICD-9-CM codes: 070.41, 070.44, 070.51, 070.54, 070.70, 070.71, and V02.62) infection if there was ≥ 1 documented chronic HCV diagnosis that required hospitalization or ≥ 3 unrelated chronic HCV diagnoses at an outpatient clinic within 1 year. The month of the first diagnosis was used as the index month. Other comorbidities recorded in the NHIRD include HCD (ICD-9-CM codes: 401-405), CKD (ICD-9-CM codes), DM (ICD-9-CM codes), hyperlipidemia (ICD-9-CM code: 585), hyperthyroidism (ICD-9-CM code: 242), thyroiditis (ICD-9-CM code: 245), lichen planus (ICD-9-CM codes: 697), palpable purpura (ICD-9-CM codes: 680-709), autoimmune thyroiditis (ICD-9-CM code: 245.2), diabetic nephropathy (ICD-9-CM code: 250.42), necrolytic acral erythema (ICD-9-CM code: 695.89), myocardial infarction (ICD-9-CM code: 410), congestive heart failure (ICD-9-CM code: 428), peripheral arterial disease (ICD-9-CM code: 440), cerebral vascular disease (ICD-9-CM codes: 430-437), coronary artery disease (CAD, ICD-9-CM codes: 410-414), autoimmune hepatitis (ICD-9-CM code: 571.42), HIV (ICD-9-CM code: 42), hepatitis B virus infection (HBV, ICD-9-CM codes: 070.2, 070.3, and V02.61), alcoholic liver disease (ICD-9-CM code: 571.3), liver cirrhosis (ICD-9-CM codes: 571.2, 571.5, and 571.6), and hepatocellular carcinoma (HCC, ICD-9-CM code: 155). Each disease was considered if ≥ 3 diagnoses were made during hospitalization or at a clinic.
2.4. Exposure measurement and end point
We recorded the presence of liver cirrhosis—compensated (primary endpoint) or decompensated (secondary endpoint)—in case of 1 hospitalization or 3 independent outpatient clinic claims based on its diagnostic code within 1 year. The patients were followed up from the index month to the study outcome, end of enrollment, or December 31, 2013, whichever occurred first.
For the 6 EHMs of interest, the cumulative period for the EHMs was defined as the date of HCV diagnosis to that of cirrhosis diagnosis in the cirrhosis group and to the date of the matched end of the follow-up in the control group.
3. Statistical methods
3.1. Sampling Scheme
For propensity score matching for age and sex, we using nearest-neighbor matching with a caliper of 0.2 by using the R package MatchIt. Next, the cases and controls were propensity score matched also by the index month (exact month of diagnosis) to reduce confounding and selection bias. Adjusted hazard ratios (HRs) of cirrhosis were calculated using Cox regression models.[8]
The paired t test and McNemar test were used to compare patients’ baseline characteristics and EHMs after adjustment for age, sex, and index month. The HRs and 95% CIs of the outcomes of cirrhosis were calculated and compared between patients with and without EHMs after adjustment for age, sex, and index month.
3.2. Adjustment for baseline characteristics
We used multiple Cox regression to evaluate the associations between risk factors and outcomes after adjustment for characteristics that had significant differences at baseline. Cumulative incidence of outcomes was estimated using the modified Kaplan-Meier method. The Gray method was used to analyze between-group differences in incidence. Kaplan-Meier curves were used to evaluate the outcomes in different EHMs and were compared using a log-rank test. The data are presented as point estimates with 95% CIs. All HRs and the cumulative probability for competing mortality were adjusted using the R package “cmprsk” (version 3.4.2). Therefore, the EHM groups could be compared after adjustment for characteristics in baseline and competing mortality risk.
All tests were 2-sided, with P < .05 set as statistically significant. All statistical analyses were performed using SPSS version 24.0 (IBM® SPSS Statistics Inc., Chicago, Illinois, USA) and R Statistical Software (version 2.14.0; R Foundation for Statistical Computing, Vienna, Austria).
4. Results
4.1. Baseline characteristics of the study population
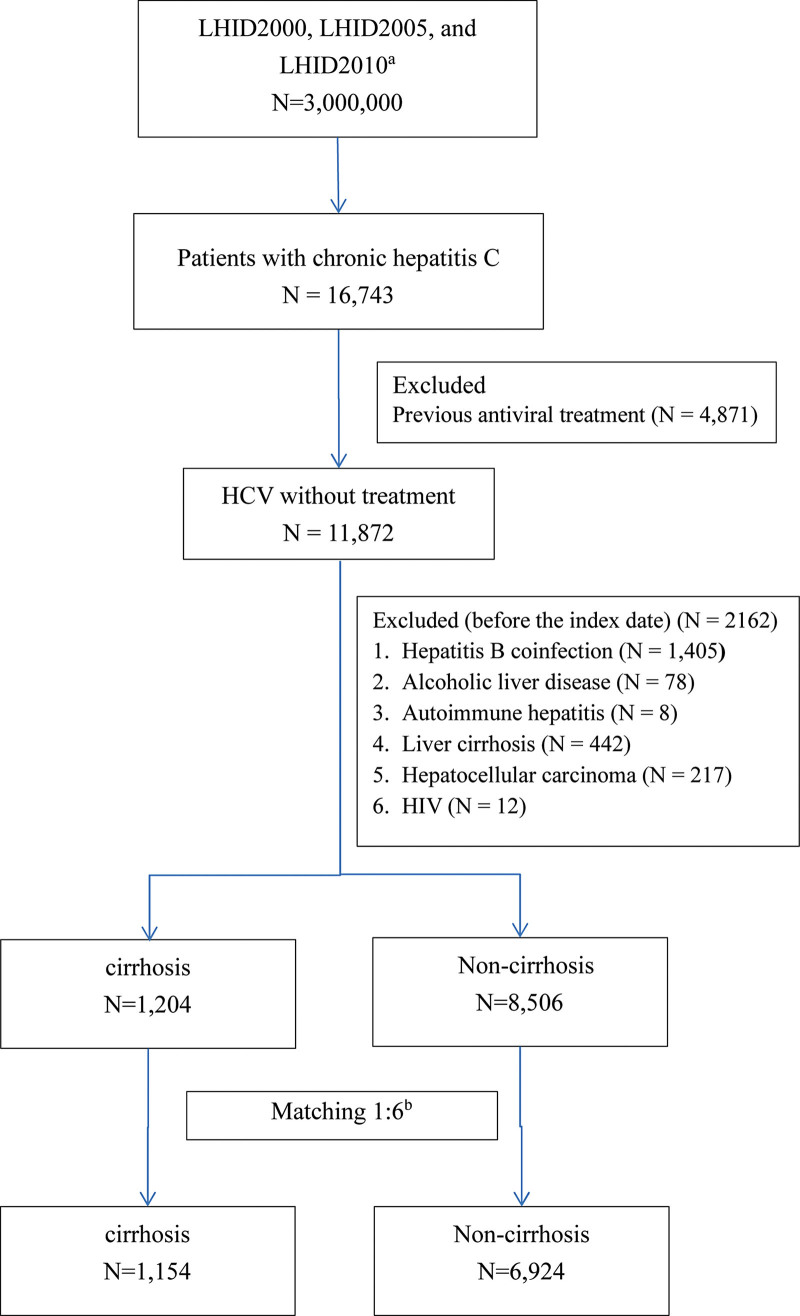
Of 16,743 patients with chronic HCV, we identified 11,872 treatment-naïve patients. We excluded 2162 patients because of HBV coinfection (n = 1405), other preexisting liver diseases (alcoholic liver disease, n = 86; autoimmune hepatitis, n = 8; liver cirrhosis, n = 442; and liver malignancies, n = 217), or HIV (n = 12). The remaining patients with cirrhosis (n = 1204) and without cirrhosis (n = 8506) were 1:6 propensity score matched for age, sex, and index month. Finally, 1154 patients with cirrhosis and 6924 matched controls were included in the analysis. Figure 1 presents the flowchart of patient identification and selection.
Figure 1.
Flowchart of identification and enrollment of the study subjects. a. The analytical data were collected from the 3 subsets of the NHIRD, the Longitudinal Health Insurance Database (LHID) 2000, 2005 and 2010 as data sources. b. The cirrhosis and noncirrhosis groups were matched 1:6 by age, sex, and year of HCV diagnosis. HCV: hepatitis C virus; HIV: human immunodeficiency virus; LHID: Longitudinal Health Insurance Databases.
The average follow-up periods were 3.3 ± 3.2 years in the cirrhotic group (n = 1154, mean age = 44.8 ± 20.7 years, male/female ratio of 541: 613) and 6.3 ± 3.9 years in the noncirrhotic group (n = 6924, mean age = 44.5 ± 20.4 years, male/female ratio of 3465: 3924).
Table 1 lists the demographic characteristics of treatment-naïve patients with chronic HCV with and without cirrhosis after propensity score matching. After propensity score matching, age and sex distributions were similar between the groups.
Table 1.
Characteristics of untreated HCV-related cirrhotic and noncirrhotic patients among multiple extrahepatic manifestations after propensity score–matching.
| Cirrhosis(N = 1154) | Noncirrhosis(N = 6924) | P | ||||
|---|---|---|---|---|---|---|
| Age | 44.8 ± 20.7 | 44.5 ± 20.4 | .892 | |||
| Sex | Female | 613 | 53.1% | 3677 | 53.1% | .971 |
| Male | 541 | 46.9% | 3247 | 46.9% | ||
| HCD | No | 367 | 31.8% | 3095 | 44.7% | <.001 |
| Yes | 787 | 68.2% | 3829 | 55.3% | ||
| Hyperlipidemia | No | 792 | 68.6% | 4251 | 61.4% | <.001 |
| Yes | 362 | 31.4% | 2673 | 38.6% | ||
| Diabetes mellitus | No | 625 | 54.2% | 4736 | 68.4% | <.001 |
| Yes | 529 | 45.8% | 2188 | 31.6% | ||
| Chronic kidney disease | No | 822 | 71.2% | 5512 | 79.6% | <.001 |
| Yes | 332 | 28.8% | 1412 | 20.4% | ||
| Hyperthyroidism | No | 1113 | 96.4% | 6654 | 96.1% | .571 |
| Yes | 41 | 3.6% | 270 | 3.9% | ||
| Hypothyroidism | No | 1122 | 97.2% | 6765 | 97.7% | .690 |
| Yes | 32 | 2.8% | 159 | 2.3% | ||
| Thyroiditis | No | 1123 | 97.3% | 6696 | 96.7% | .302 |
| Yes | 31 | 2.7% | 228 | 3.3% | ||
| Non-Hodgkin lymphoma | No | 1150 | 99.7% | 6882 | 99.4% | .277 |
| Yes | 4 | 0.3% | 42 | 0.6% | ||
| Sicca syndrome | No | 1119 | 97.0% | 6682 | 96.5% | .486 |
| Yes | 35 | 3.0% | 242 | 3.5% | ||
| Mixed cryoglobulinemia | No | 1153 | 99.9% | 6924 | 100.0% | .309 |
| Yes | 1 | 0.1% | 0 | 0.0% | ||
| Depression | No | 1024 | 88.7% | 6162 | 89.0% | .794 |
| Yes | 130 | 11.3% | 762 | 11.0% | ||
| Lichen planus | No | 1141 | 98.9% | 6903 | 99.7% | <.001 |
| Yes | 13 | 1.1% | 21 | 0.3% | ||
| Autoimmune hemolytic anemia | No | 1153 | 99.9% | 6910 | 99.8% | 1.000 |
| Yes | 1 | 0.1% | 14 | 0.2% | ||
| Palpable purpura | No | 1072 | 92.9% | 6786 | 98.0% | <.001 |
| Yes | 82 | 7.1% | 138 | 2.0% | ||
| Autoimmune thyroiditis | No | 1150 | 99.7% | 6903 | 99.7% | .541 |
| Yes | 4 | 0.3% | 21 | 0.3% | ||
| Diabetic nephropathy | No | 1149 | 99.6% | 6882 | 99.4% | .806 |
| Yes | 5 | 0.4% | 42 | 0.6% | ||
| Necrolytic acral erythema | No | 1145 | 99.2% | 6876 | 99.3% | .745 |
| Yes | 9 | 0.8% | 48 | 0.7% | ||
| Myocardial infarction | No | 1111 | 96.3% | 6730 | 97.2% | .085 |
| Yes | 43 | 3.7% | 194 | 2.8% | ||
| Congestive heart failure | No | 692 | 60.0% | 4791 | 69.2% | <.001 |
| Yes | 462 | 40.0% | 2133 | 30.8% | ||
| Peripheral arterial disease | No | 1028 | 89.1% | 6218 | 89.8% | .455 |
| Yes | 126 | 10.9% | 706 | 10.2% | ||
| Cerebral vascular disease | No | 1034 | 89.6% | 6405 | 92.5% | .001 |
| Yes | 120 | 10.4% | 519 | 7.5% | ||
| CAD | No | 741 | 64.2% | 4902 | 70.8% | <.001 |
| Yes | 413 | 35.8% | 2022 | 29.2% | ||
P-values marked in bold indicate statistically significant differences between the groups.
Some comorbidities were significantly different between the case and control groups, including DM, HCD, CKD, hyperlipidemia, lichen planus, and palpable purpura (all P < .001); their proportion was significantly higher in patients with cirrhosis than those without (Table 1).
Table 2 presents the adjusted HRs of developing compensated and decompensated liver cirrhosis in patients with 18 EHMs: HCD, hyperlipidemia, DM, CKD, hypothyroidism, thyroiditis, non-Hodgkin lymphoma, Sjögren’s syndrome, mixed cryoglobulinemia, depression, lichen planus, autoimmune hemolytic anemia, palpable purpura, autoimmune thyroiditis, diabetic nephropathy, necrolytic acral erythema, connective tissue disease, and coronary artery disease.
Table 2.
Adjusted hazard ratio (HR) for occurrence of compensated and decompensated liver cirrhosis among multiple extrahepatic manifestations in untreated HCV patients after Cox regression analysis.
| Univariate analysis | Multivariate analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| Overall cirrhosis | Compensated | Decompensated | Overall | |||||
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| HCD | 1.51 (1.09–2.09) | .012 | 1.51 (1.3–1.75) | <.001 | 1.12 (0.77–1.61) | .551 | 1.45 (1.27–1.67) | <0.001 |
| Hyperlipidemia | 0.50 (0.35–0.72) | <.001 | 0.54 (0.47–0.63) | <.001 | 0.32 (0.22–0.47) | <.001 | 0.53 (0.46–0.60) | <0.001 |
| Diabetes mellitus | 2.84 (2.08–3.87) | <.001 | 1.61 (1.4–1.85) | <.001 | 3.49 (2.47–4.95) | <.001 | 1.72 (1.51–1.96) | <0.001 |
| Chronic kidney disease | 1.94 (1.40–2.69) | <.001 | 1.18 (1.01–1.36) | .032 | 1.56 (1.10–2.22) | .013 | 1.21 (1.05–1.38) | 0.007 |
| Hypothyroidism | 0.44 (0.11–1.76) | .245 | 0.92 (0.63–1.34) | .656 | 0.46 (0.11–1.90) | .285 | 0.89 (0.62–1.29) | 0.535 |
| Thyroiditis | 0.17 (0.02–1.22) | .077 | 0.84 (0.56–1.25) | .381 | 0.26 (0.04–1.85) | .177 | 0.78 (0.53–1.16) | 0.217 |
| Non-Hodgkin lymphoma | 1.00 (0.14–7.14) | 1.000 | 0.38 (0.12–1.19) | .096 | 0.64 (0.09–4.67) | .662 | 0.45 (0.17–1.21) | 0.112 |
| Sjögren’s syndrome | 0.65 (0.24–1.75) | .395 | 0.88 (0.56–1.37) | .571 | 0.90 (0.26–3.11) | .870 | 0.86 (0.57–1.31) | 0.488 |
| Mixed cryoglobulinemia | 0.05 (0.00–>20) | .904 | 6.67 (0.9–49.19) | .063 | N/A | N/A | 6.15 (0.84–45.2) | 0.074 |
| Depression | 1.05 (0.65–1.69) | .850 | 0.94 (0.77–1.14) | .521 | 1.01 (0.62–1.64) | .972 | 0.97 (0.80–1.16) | 0.722 |
| Lichen planus | 1.99 (0.28–14.19) | .494 | 3.03 (1.7–5.39) | <.001 | 2.37 (0.33–17.2) | .393 | 2.71 (1.56–4.72) | <0.001 |
| Autoimmune hemolytic anemia | 0.05 (0.0–>20) | .735 | 0.49 (0.07–3.48) | .475 | 0.00 (0.00–>20) | .973 | 0.41 (0.06–2.92) | 0.373 |
| Palpable purpura | 5.78 (3.58–9.33) | <.001 | 2.44 (1.89–3.16) | <.001 | 5.93 (3.65–9.65) | <.001 | 2.67 (2.13–3.35) | <0.001 |
| Autoimmune thyroiditis | 0.05 (0–>20) | .653 | 1.86 (0.64–5.39) | .255 | 0.00 (0.00–>20) | .974 | 1.81 (0.62–5.22) | 0.276 |
| Diabetic nephropathy | 1.18 (0.17–8.45) | .867 | 0.54 (0.2–1.45) | .222 | 0.60 (0.08–4.34) | .611 | 0.56 (0.23–1.35) | 0.198 |
| Necrolytic acral erythema | 0.05 (0–>20) | .553 | 1.87 (0.97–3.61) | .063 | 0.00 (0.00–>20) | .964 | 1.55 (0.80–2.99) | 0.192 |
| Connective tissue disease | 0.64 (0.35–1.19) | .158 | 0.8 (0.61–1.05) | .115 | 0.64 (0.30–1.36) | .245 | 0.81 (0.63–1.05) | 0.107 |
| Coronary artery disease | 0.97 (0.69–1.36) | .851 | 1.17 (1.02–1.35) | .023 | 0.84 (0.59–1.21) | .346 | 1.11 (0.98–1.27) | 0.104 |
HCD = hypertensive cardiovascular disease, N/A = not applicable—Since cases were too few to arrive at a significant solution, coefficients did not converge.
The hazard ratio of cirrhosis for competing mortality was adjusted using the R package “cmprsk.”
The univariate analysis revealed that 5 EHMs were associated with a higher risk of cirrhosis: HCD, hyperlipidemia, DM, chronic kidney disease, lichen planus, and palpable purpura. We subdivided cirrhosis into compensated and decompensated for univariate analysis. The 5 EHMs were associated with a higher risk of cirrhosis in patients with compensated and decompensated cirrhosis.
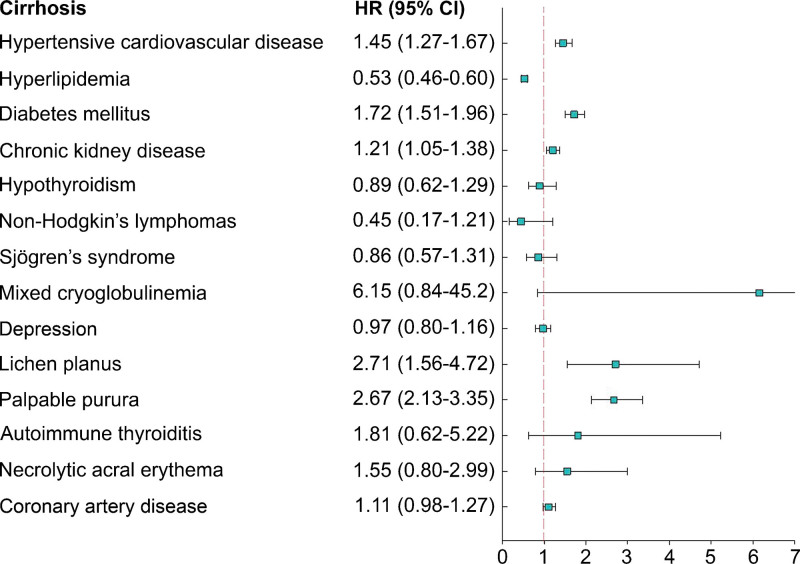
Multivariate analysis revealed that compared with patients without any of the 5 EHMs, those with any of 5 EHMs had significantly higher overall liver cirrhosis risks: DM (HR: 1.72, 95% CI: 1.51–1.96, P < .001), HCD (HR: 1.45, 95% CI: 1.27–1.67, P < .007), CKD (HR: 1.21, 95% CI: 1.05–1.38, P < .001), hyperlipidemia (HR: 0.53, 95% CI: 0.46–0.60, P < .001), lichen planus (HR: 2.71, 95% CI: 1.56–4.72, P < .001), and palpable purpura (HR: 2.67, 95% CI: 2.13–3.35, P < .001). The forest plot presented in Figure 2 clearly demonstrates the aforementioned trend.
Figure 2.
Forest plot showing a hazard ratio (HR) of liver cirrhosis among 14 different HCV-related EHMs in untreated HCV patients.
4.2. Occurrence of liver cirrhosis with 1 or multiple EHMs
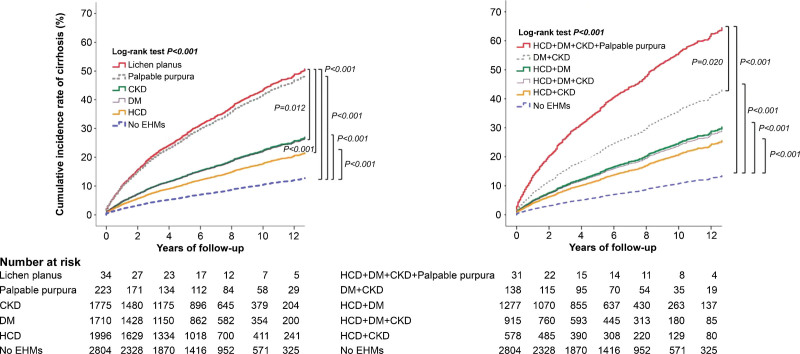
The left panel of Figure 3 shows the adjusted cumulative incidence of cirrhosis over time. The incidence rates (IR) of cirrhosis were highest in lichen planus than in other EHMs for the entire study period. Pairwise comparisons of multiple high-cirrhosis-risk EHMs ranked lichen planus first among all EHMs. The right panel of the attached figure shows IR trends for all study years in different combinations of high-cirrhosis-risk EHMs, in which the maximum combination of palpable purpura, CKD, DM, and HCD most likely placed patients at an increased cirrhosis risk.
Figure 3.
Cumulative incidence rates of liver cirrhosis over time among different single (left panel) and combined multiple high-cirrhosis-risk-related EHMs (right panel) in untreated HCV patients.
4.3. Stratified analyses according to patient subgroups
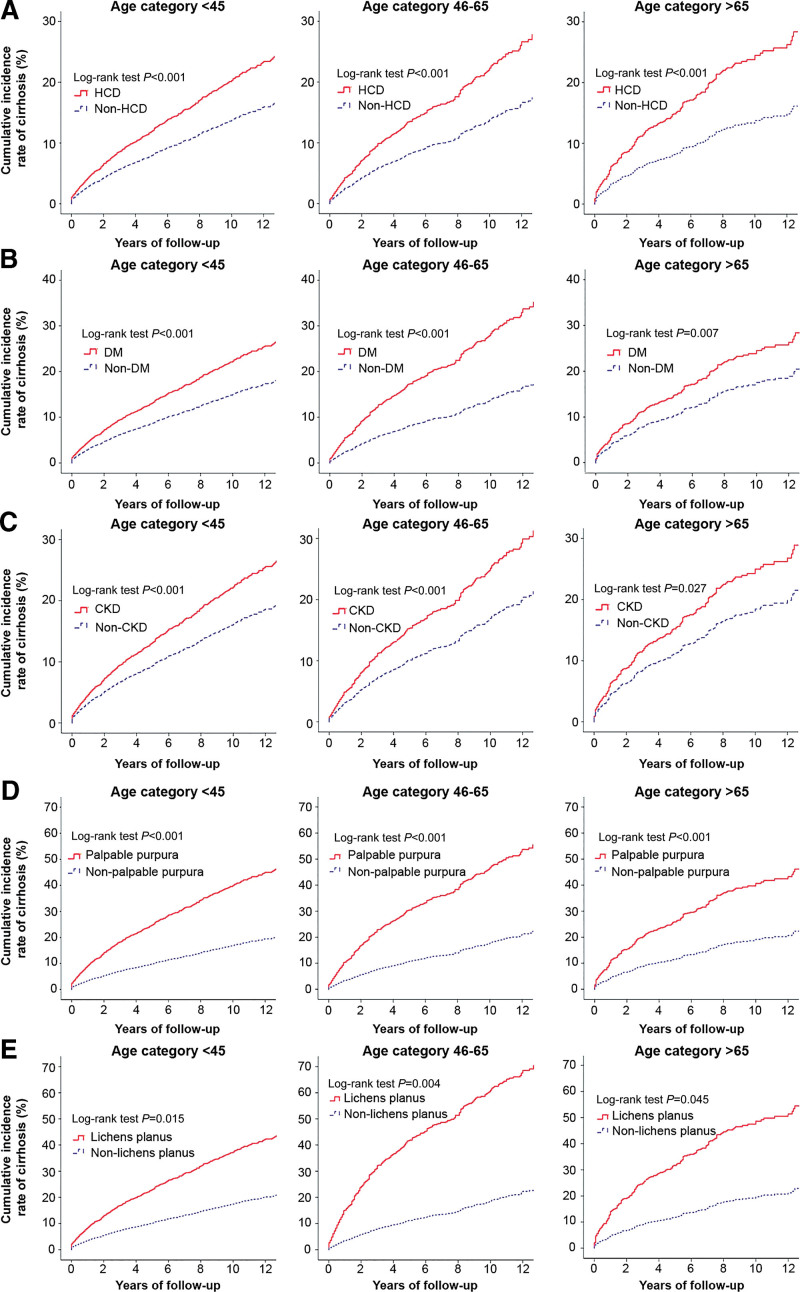
We determined 12-year cumulative IR of liver cirrhosis for patients with and without specific high-cirrhosis-risk EHMs after age and sex stratification.
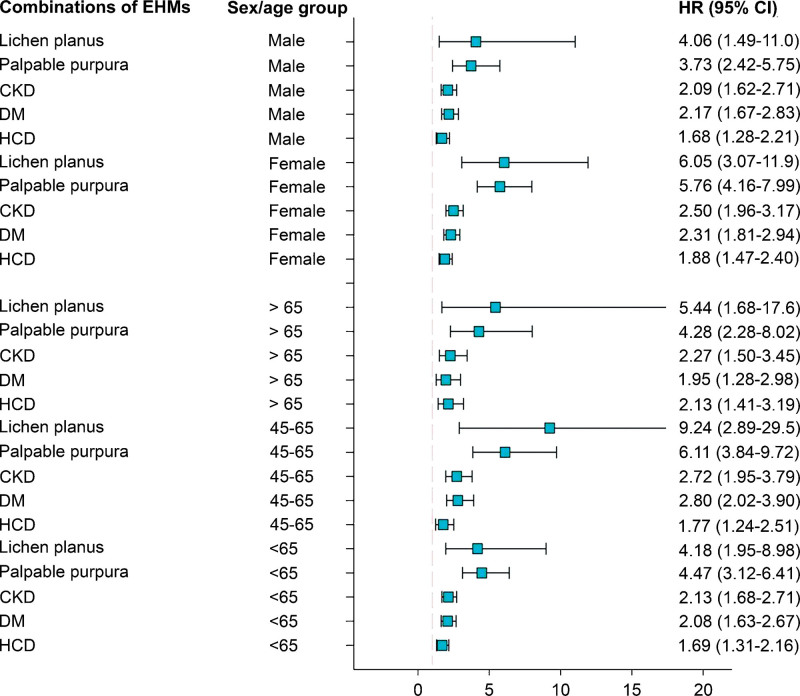
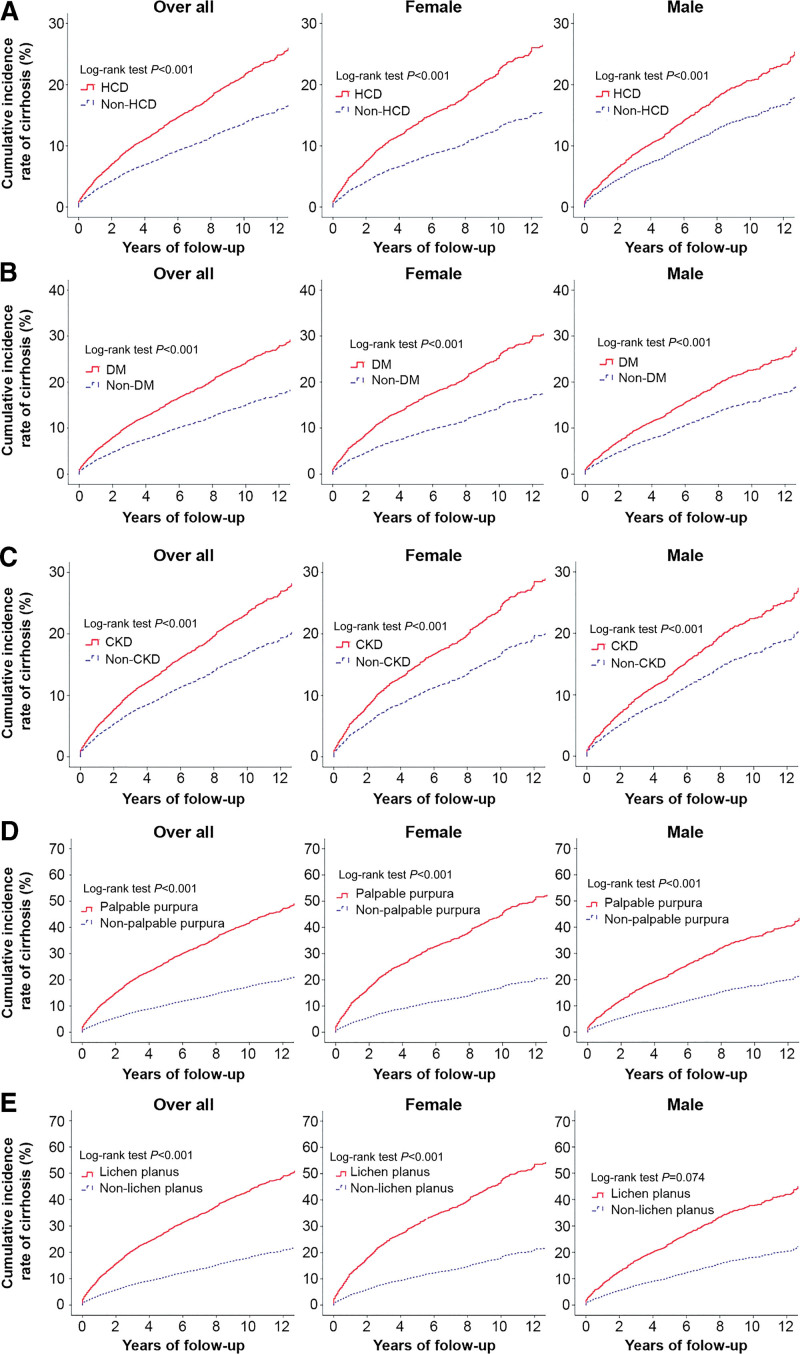
Figure 4 illustrates a forest plot of HRs in subgroup analyses of sex (men and women) and age (≤45 years, 45–65 years, ≥65 years); subgroup analysis was used to comparatively analyze the various categories of high-cirrhosis-risk EHMs, such as lichen planus, palpable purpura, CKD, DM, and HCD. Multivariate analyses stratified by sex and age revealed an increased risk of liver cirrhosis in patients with lichen planus, palpable purpura, CKD, DM, and HCD. We then assessed the association of occurrence of liver cirrhosis between EHMs after stratifying by age and sex. In all age groups, the 12-year cumulative IR of liver cirrhosis were significantly higher in patients with HCD (Fig. 5A), DM (Fig. 5B), CKD (Fig. 5C), palpable purpura (Fig. 5D), and lichen planus (Fig. 5E) than in those without the corresponding EHMs (all P < .001). Similarly, irrespective of sex stratification (overall participants, female, and male), the 12-year cumulative IR of liver cirrhosis were significantly higher in patients with HCD (Fig. 6A), DM (Fig. 6B), CKD (Fig. 6C), and palpable purpura (Fig. 6D) than in those without the corresponding EHMs (all P < .001), except for men with lichen planus (Fig. 6E, P < .074).
Figure 4.
Forest plot of hazard ratios in subgroup analyses comparing extrahepatic manifestations (EHMs) are shown on the left and hazard ratios (HR) and confidence intervals (CI) on the right.
Figure 5.
Stratified by patient subgroups of age and cumulative incidence of liver cirrhosis between presence and absence of high-cirrhosis-risk extrahepatic manifestations cohorts: (A) hypertensive cardiovascular disease (HCD), (B) diabetes mellitus (DM), (C) chronic kidney disease (CKD), (D) palpable purpura, and (E) lichen planus.
Figure 6.
Stratified by patient subgroups of gender and cumulative incidence of liver cirrhosis between presence and absence of high-cirrhosis-risk extrahepatic manifestations cohorts: (A) hypertensive cardiovascular disease (HCD), (B) diabetes mellitus (DM), (C) chronic kidney disease (CKD), (D) palpable purpura, and (E) lichen planus.
5. Discussion
5.1. Main findings
In this large, population-based long-term cohort of HCV-infected patients, we observed several specific HCV-associated EHM time trends of cirrhosis risk with an ongoing upward trend in incidence. This suggests that HCV-infected individuals will more likely have a progressively larger contribution to the overall emergence of cirrhosis and its related complications. The trends in the adjusted incidence of cirrhosis were not different between genders. The associations between HCV infection and the risk of cirrhosis were analyzed across strata of age and gender among all high-cirrhosis-risk EHMs, suggesting an insignificantly increased risk of cirrhosis in females and significantly increased risk of cirrhosis in elderly patients with HCD and DM.[9]
5.2. Comparison with other studies
The complexity of this effect for differing EHMs on liver disease progression is demonstrated by our study which reveals that the same prevalence of metabolic risk factors, such as DM, HCD, and CKD, was associated with a higher IR of liver cirrhosis. Interestingly, attention should focus on HCV infection-related skin manifestations, such as lichen planus and palpable purpura, which ranked first and second, respectively, among high-cirrhosis-risk EHMs in our study. Lichen planus is a chronic inflammatory disease of the skin and mucosa; it can affect the hair and nails and is characterized by pruritic papules.[10] These most often appear on the skin of extremities, face, scalp, nails, and mucosa of the gastrointestinal and genitourinary tracts. Cutaneous vasculitis of HCV-related MC, resulting in palpable purpura, is reported in 24% to 30% of cryoglobulin-positive patients.[11] It is secondary to small- and/or medium-vessel vasculitis with deposition of immune complexes in the small- and medium-sized dermal vessels.[12] Lichen planus occurs in various chronic liver diseases. Anti-HCV antibodies can be found in 10% to 40% of patients with lichen planus.[13] It is assumed that the occurrence of lichen planus is immunologically mediated, but the exact mechanism is unknown. Several large epidemiologic studies published in recent years[12–18] have strengthened the link between HCV infection and the earlier mentioned skin manifestations. Our data revealed a striking association between dermatological EHMs and the subsequent development of liver cirrhosis in HCV patients, compared with other EHMs. Interestingly, the risk of developing liver cirrhosis increases as the combinations of coexisting EHMs increase in patients. Based on our further gender- and age-based subanalysis, HCV-infected patients with coexisting HCD, DM, CKD, and cutaneous palpable purpura were more likely to develop liver cirrhosis over time. Having both DM and HCV may be associated with a higher cirrhosis risk.[19–21] Patients with cirrhosis have a 2-fold higher prevalence of DM than those without cirrhosis. Analogously, Miyaaki et al found that DM was significantly associated with severe fibrosis.[21] Turner et al reported that HCV-infected Hispanics, who are obese and diabetic, have a far higher risk of developing advanced liver disease than other racial or ethnic groups.[22] Our study supports this notion. These findings highlight the need for concurrent HCV and DM therapies. Any chronic liver disease will eventually lead to liver insufficiency. Our reports also highlight the benefits of the population-based cohort model of our study, which was followed up and observed longitudinally for 12 years to assess relationships among long-term HCV exposure, multiple coexisting EHMs, and the outcome of liver cirrhosis development. This special interaction in our study model may be defined as the effect modification.[23] In our study, the effect of HCV exposure on cirrhosis outcome depends on a third variable, EHMs, which are considered as effect modifiers. The effect of HCV exposure and cirrhosis outcome is modified by the presence of coexisting EHMs. The research uses statistical methods to interpret clinical observations and achieve preventive or therapeutic aims. Our research is a large-scale observational database, which consists of a huge sample size of several hundred thousands of participants. Their collected healthcare data are often utilized in population-based studies. This is an indispensable method of surveying epidemiological research. However, the inherent limitations of healthcare databases may impede their use, highlighting the need to assess the feasibility and validity of studies that use this type of data.[24,25] Given the complexity of the clinical scenario, we used Cox proportional hazards models to estimate HRs, adjusting using propensity score weighting for demographic characteristics and EHMs. The encouraging results from long-term outcomes in our study helped to uncover major EHMs that can contribute to developing liver cirrhosis over time. Among these, EHMs, HCD, DM, CKD, lichen planus, and palpable purpura had a higher-than-average chance of being attributed to developing liver cirrhosis in HCV patients. It was also further observed that the more high-cirrhosis-risk EHMs HCV patients had the higher risk of developing liver cirrhosis over time, irrespective of gender and age. Moreover, based on Cox regression subanalysis investigating the effect of multiple EHMs upon the time liver cirrhosis occurs, the above results were mainly evident in female subjects and patients aged <45 years. These findings hold great promise in improving the evaluation of individual risk with an increase in the IR of cirrhosis and even decompensated cirrhosis among HCV-infected patients. It is noteworthy that skin disorders, including lichen planus and palpable purpura, are the top leading EHMs which had higher cumulative rates of liver cirrhosis. Ample evidence in the literature is available to implicate the close relationship between lichen planus and liver disease,[26–29] or even liver cirrhosis.[30–34] In a study from the Mayo Clinic, 24 patients with primary biliary cirrhosis and lichen planus were identified, 7 of whom had not been treated with penicillamine.[35] Chronic hepatitis C infection will progress to liver cirrhosis over time. Although the data are not conclusive, many reports have demonstrated that lichen planus is associated with liver cirrhosis.[36,37] Rebora et al reported that there are remarkably similar histologic and immunologic features between lichen planus and chronic active hepatitis, which showed that the lichenoid infiltrate in the dermis was similar to infiltration of T lymphocytes in the portal spaces, resulting in destruction of the normal architecture of the hepatic lobule.[38]
5.3. Study strengths
Depending on previous studies, our study has a much larger sample size, and we used a nationwide database that can provide considerable statistical power. A key strength of this study was the observation of the tangled interplay between multiple EHMs and cirrhosis risk in HCV infection using the concept of interaction or heterogeneity of effect. The patients were stratified by age and sex to derive the HRs of liver cirrhosis, and the variables of EHMs were included in the multivariable analyses. Lichen planus and palpable purpura were identified as EHMs that have a high HR for liver cirrhosis, particularly in women and patients aged 45 to 65 years. Notably, patients with a combination of palpable purpura, CKD, DM, and HCD had the highest cumulative risk of cirrhosis.
6. Limitations, Recommendations, and Future Directions
Our study has some limitations. First, this study had a retrospective cohort design; although we matched all the potential confounding factors among the study groups, selection or observational bias still exists. However, given that it is a large-scale and well-validated database, this study may control possible bias to depict a clinically important link. Second, although the NHIRD version used was not the updated version, the results of our analysis remain convincing in terms of a large-scale long-term cohort study. Third, the NHIRD lacks specific laboratory information, including that of liver function tests, HCV genotypes, and HCV RNA levels. Nonetheless, the conclusion of this study should be the same. Fourth, the data of other confounding factors, such as education level, socioeconomic status, dietary habits, and body mass index, are not included in the NHIRD. Fifth, the NHIRD does not have data on the duration of preexisting EHMs. Sixth, the observational design of the study precludes the determination of causality. Further studies should clarify the associations and underlying mechanisms.
7. Conclusion
Many specific HCV-associated EHMs demonstrate time trends of cirrhosis risk, with an upward trend in the incidence. As an important dermatological HCV-related EHM, lichen planus ranked first on the list of single-EHM comparisons. Moreover, the maximum combination of certain EHMs led to all other multi-EHM comparisons for the development of liver cirrhosis.
Author contributions
Conceptualization: Chun-Hsiang Wang, Yuan-Tsung Tseng
Data curation: Chun-Hsiang Wang, Yuan-Tsung Tseng
Formal analysis: Chun-Hsiang Wang, Yuan-Tsung Tseng
Methodology: Yuan-Tsung Tseng
Visualization: Chun-Hsiang Wang, Yuan-Tsung Tseng
Writing—original draft: Chun-Hsiang Wang, Shih-Fang Ou
Writing—review and editing: Chun-Hsiang Wang, Shih-Fang Ou, Yuan-Tsung Tseng
Abbreviations:
- CAD =
- coronary artery disease
- CI =
- confidence intervals
- CKD =
- chronic kidney disease
- DM =
- diabetes mellitus
- EHMs =
- extrahepatic manifestations
- HCD =
- hypertensive cardiovascular disease
- HCV =
- hepatitis C virus
- HRs =
- hazard ratios
- IR =
- incidence rates
- N/A =
- not applicable
- NHI =
- National Health Insurance
- NHIRD =
- National Health Insurance Research Database
- RNA =
- Ribonucleic acid
Author Shih-Fang Ou contributed equally to this work.
The data that support the findings of this study are available from a third party, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are available from the authors upon reasonable request and with permission of the third party.
This work was supported in part by the Tainan Municipal Hospital Research Grant (RD:109-14).
How to cite this article: Wang C-H, Ou S-F, Tseng Y-T. Long-term impact of certain coexisting extrahepatic unisystem and multisystem manifestations on trends in incidence of liver cirrhosis in treatment-naïve patients with chronic hepatitis C: a nested case-control study. Medicine 2022;101:29(e29697).
Contributor Information
Chun-Hsiang Wang, Email: chunhsiangwang1@gmail.com.
Shih-Fang Ou, Email: 2lm646@tmh.org.tw.
References
- [1].Kanwal F, Hoang T, Kramer JR, et al. Increasing prevalence of HCC and cirrhosis in patients with chronic hepatitis C virus infection. Gastroenterology. 2011;140:1182–1188.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Younossi ZM, Stepanova M, Nader F, et al. Associations of chronic hepatitis C with metabolic and cardiac outcomes. Aliment Pharmacol Ther. 2013;37:647–52. [DOI] [PubMed] [Google Scholar]
- [3].Petta S, Maida M, Macaluso FS, et al. Hepatitis C virus infection is associated with increased cardiovascular mortality: a meta-analysis of observational studies. Gastroenterology. 2016;150:145–155.e4; quiz e15. [DOI] [PubMed] [Google Scholar]
- [4].Park H, Chen C, Wang W, et al. Chronic hepatitis C virus (HCV) increases the risk of chronic kidney disease (CKD) while effective HCV treatment decreases the incidence of CKD. Hepatology. 2018;67:492–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Cacoub P, Nahon P, Layese R, et al. Prognostic value of viral eradication for major adverse cardiovascular events in hepatitis C cirrhotic patients. Am Heart J. 2018;198:4–17. [DOI] [PubMed] [Google Scholar]
- [6].Pol S, Vallet-Pichard A, Hermine O. Extrahepatic cancers and chronic HCV infection. Nat Rev Gastroenterol Hepatol. 2018;15:283–90. [DOI] [PubMed] [Google Scholar]
- [7].Gill K, Ghazinian H, Manch R, et al. Hepatitis C virus as a systemic disease: reaching beyond the liver. Hepatol Int. 2016;10:415–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lunceford JK, Davidian M. Stratification and weighting via the propensity score in estimation of causal treatment effects: a comparative study. Stat Med. 2004;23:2937–60. [DOI] [PubMed] [Google Scholar]
- [9].Greenland S, Morgenstern H. Ecological bias, confounding, and effect modification. Int J Epidemiol. 1989;18:269–74. [DOI] [PubMed] [Google Scholar]
- [10].Gumber SC, Chopra S. Hepatitis C: a multifaceted disease. Review of extrahepatic manifestations. Ann Intern Med. 1995;123:615–20. [DOI] [PubMed] [Google Scholar]
- [11].Cacoub P, Renou C, Rosenthal E, et al. Extrahepatic manifestations associated with hepatitis C virus infection. A prospective multicenter study of 321 patients. The GERMIVIC. Groupe d’Etude et de Recherche en Medecine Interne et Maladies Infectieuses sur le Virus de l’Hepatite C. Medicine. 2000;79:47–56. [DOI] [PubMed] [Google Scholar]
- [12].Sene D, Ghillani-Dalbin P, Thibault V, et al. “Longterm course of mixed cryoglobulinemia in patients infected with hepatitis C virus,”. J Rheumatol. 2004;31:2199–206. [PubMed] [Google Scholar]
- [13].Yarom N, Dagon N, Shinar E, et al. Association between hepatitis C virus infection and oral lichen planus in Israeli patients. Isr Med Assoc J. 2007;9:370–2. [PubMed] [Google Scholar]
- [14].Giuliani M, Lajolo C, Miani MC, et al. Hepatitis C virus chronic infection and oral lichen planus: an Italian case-control study. Eur J Gastroenterol Hepatol. 2007;19:647–52. [DOI] [PubMed] [Google Scholar]
- [15].Campisi G, Di Fede O, Craxi A, et al. Oral lichen planus, Hepatitis C virus, and HIV: no association in a cohort study from an area of high hepatitis C virus endemicity. J Am Acad Dermatol. 2004;51:364–70. [DOI] [PubMed] [Google Scholar]
- [16].Das A, Das J, Majumdar G, et al. No association between seropositivity for hepatitis C virus and lichen planus: a case control study. Indian J Dermatol Venereol Leprol. 2006;72:198–200. [DOI] [PubMed] [Google Scholar]
- [17].Prabhu S, Pavithran K, Sobhanadevi G. Lichen planus and hepatitis C virus (HCV)—is there an association? A serological study of 65 cases. Indian J Dermatol Venereol Leprol. 2002;68:273–4. [PubMed] [Google Scholar]
- [18].Rahnama Z, Esfandiarpour I, Farajzadeh S. The relationship between lichen planus and hepatitis C in dermatology outpatients in Kerman, Iran. Int J Dermatol. 2005;44:746–8. [DOI] [PubMed] [Google Scholar]
- [19].Desbois AC, Cacoub P. Diabetes mellitus, insulin resistance and hepatitis C virus infection: a contemporary review. World J Gastroenterol. 2017;23:1697–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Raff EJ, Kakati D, Bloomer JR, et al. Diabetes mellitus predicts occurrence of cirrhosis and hepatocellular cancer in alcoholic liver and non-alcoholic fatty liver diseases. J Clin Transl Hepatol. 2015;3:9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Miyaaki H, Ichikawa T, Taura N, et al. Predictive value of the fibrosis scores in patients with chronic hepatitis C associated with liver fibrosis and metabolic syndrome. Intern Med. 2011;50:1137–41. [DOI] [PubMed] [Google Scholar]
- [22].Turner BJ, Wang CP, Melhado TV, et al. Significant Increase in risk of fibrosis or cirrhosis at time of HCV diagnosis for hispanics with diabetes and obesity compared with other ethnic groups. Clin Gastroenterol Hepatol. 2019;17:1356–63. [DOI] [PubMed] [Google Scholar]
- [23].Corraini P, Olsen M, Pedersen L, et al. Effect modification, interaction and mediation: an overview of theoretical insights for clinical investigators. Clin Epidemiol. 2017;9:331–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Nicholls SG, Langan SM, Sørensen HT, et al. The RECORD reporting guidelines: meeting the methodological and ethical demands of transparency in research using routinely collected health data. Clin Epidemiol. 2016;8:389–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].van Staa TP, Goldacre B, Buchan I, et al. Big health data: the need to earn public trust. BMJ. 2016;354:i3636. [DOI] [PubMed] [Google Scholar]
- [26].Hassan JA, Saadiah S, Roslina AM, et al. The triad of Lichen planus, thymoma and liver cirrhosis-hepatoma. First reported case. CASE Malays J Sci. 2000;7:38–42. [PMC free article] [PubMed] [Google Scholar]
- [27].Rebora A. HCV and lichen planus: HCV and lichen planus. Hepat Mon. 2011;11:134–5. [PMC free article] [PubMed] [Google Scholar]
- [28].Da Nagao YD, Sata M, Suzuki H, et al. Effectiveness of glycyrrhizin for oral lichen planus in patients with chronic HCV Infection. J Gastroenterol. 1996;31:691–5. [DOI] [PubMed] [Google Scholar]
- [29].Italiano Studi G. Lichen planus and liver diseases: a multicentre case-control study. Gruppo Italiano Studi Epidemiologici in dermatologia (GISED). [Lichen planus and liver diseases: a multicentre case-control study. Gruppo Italiano Studi Epidemiologici in Dermatologia (GISED)]. BMJ. 1990;300:227–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Graham-Brown RA, Sarkany I. ILichen sclerosus et atrophicus. With primary biliary cirrhosis and lichen planus. Int J Dermatol. 1986;25:317. [DOI] [PubMed] [Google Scholar]
- [31].Powell FC, Rogers RS, 3rd, Dickson ER. Primary biliary cirrhosis and Lichen planus. J Am Acad Dermatol. 1983;9:540–5. [DOI] [PubMed] [Google Scholar]
- [32].Sowden JM, Cartwright PH, Green JR, et al. Isolated lichen planus of the nails associated with primary biliary cirrhosis. Br J Dermatol. 1989;121:659–62. [DOI] [PubMed] [Google Scholar]
- [33].Strauss RA, Fattore L, Soltani K. The association of mucocutaneous lichen planus and chronic liver disease. Oral Surg Oral Med Oral Pathol. 1989;68:406–10. [DOI] [PubMed] [Google Scholar]
- [34].Rebora AL. Lichen planus and the liver. Int J Dermatol. 1992;31:392–5. [DOI] [PubMed] [Google Scholar]
- [35].Powell FC, Rogers RS, Dickson ER. Lichen planus, primary biliary cirrhosis and penicillamine. Br J Dermatol. 1982;107:616. [DOI] [PubMed] [Google Scholar]
- [36].Ayala F, Balato N, Tranfaglia A, et al. Oral erosive lichen planus and chronic liver disease. J Am Acad Dermatol. 1986;14:139–40. [DOI] [PubMed] [Google Scholar]
- [37].Rebora A. Lichen planus and the liver. Lancet. 1981;10:805–6. [DOI] [PubMed] [Google Scholar]
- [38].Rebora A, Rongioletti F. Lichen planus and chronic active hepatitis. A retrospective survey. Acta Derm Venereol. 1984;64:52–6. [PubMed] [Google Scholar]