Abstract
To investigate the feasibility and efficacy of transcanal endoscopic treatment for congenital middle ear cholesteatoma in children.
Eleven children diagnosed with congenital middle ear cholesteatoma, who underwent total ear endoscopic surgery under general anesthesia, were included from the Huazhong University of Science and Technology Union Shenzhen Hospital between January 2016 and December 2020. We retrospectively analyzed their operation process and surgical complications through the surgical video; moreover, we compared the pre- and postoperative hearing outcomes.
One child underwent a planned second operation to reconstruct the ossicular chain. At 6 postoperative months, all 11 children underwent reexamination. There was no significant change and a significant decrease in the mean bone and air conduction hearing thresholds, respectively (P > .05 and P < .05); moreover, there was a significant reduction in the air–bone conduction difference (P < .05). Further, the air–bone conduction difference was reduced to >20 dB and >10 dB in 11 and 7 children, respectively. Follow-up of the children did not reveal sensorineural deafness, facial paralysis, and other serious complications; further, there were no cases of recurrence.
Transcanal endoscopic treatment for congenital middle ear cholesteatoma in children is feasible, minimally invasive, and functional.
Keywords: children, congenital middle ear cholesteatoma, ear endoscopic surgery
1. Introduction
In 1986, Levenson et al defined congenital cholesteatoma (CC) as a pearly white mass on the inner side of the normal tympanic membrane in patients without a history of otorrhea, tympanic membrane perforation, or ear surgery. It is currently widely recognized and is diagnosed based on Levenson’s revised diagnosis standard.[1] CC is clinically rare, accounting for 1% to 5% of cases of middle ear cholesteatoma.[2] However, the actual incidence rate of CC could be higher because some clinically suspected cases of CC were excluded due to secondary otorrhea and tympanic membrane perforation. The insidious symptoms of CC impede early diagnosis; moreover, they lead to missed clinical diagnosis and delayed clinical treatment. The treatment of congenital middle ear cholesteatoma (CMEC) in children is often delayed because they cannot promptly report the hearing loss (HL). Compared with CC, CMEC has a higher incidence and greater impact on normal life. It can affect the hearing of children and cause intracranial and extracranial complications, leading to serious consequences. Gülşen and Arici[3] reported that the graft success rates for ear endoscopic and microscopic surgeries were 93.7% and 91.4%, respectively, with no significant between-technique difference. However, the mean operative time was significantly shorter in the endoscopic group (37.2 ± 3.1 minutes) than in the microscopic group (52.9 ± 9.2 minutes). Endoscopic ear surgery can effectively control the CMEC recurrence rate. A prospective study reported that the incidence of cholesteatoma in facial recess was 25% and 20% through endoscopic and microscopic examination, respectively, whereas the corresponding incidence rates of sinus tympani cholesteatoma were 35% and 5%. This study aimed to discuss the advantages of otoendoscopy for CMEC treatment in children by describing the surgical procedures and outcomes in representative patients.
2. Methods
2.1. Case data collection
We retrospectively analyzed case data, surgical videos, and treatment outcomes of 13 children diagnosed with CMEC at the Huazhong University of Science and Technology Union Shenzhen Hospital from January 2016 to December 2020. Among them, 11 and 2 children underwent total ear endoscopic surgery and microsurgery, respectively. We recorded the following information: age, ear side, surgical procedures, and pre- and postoperative hearing results.
2.2. Examination method
All the patients underwent detailed taking of otolaryngological history, specialist examination, and otoendoscopy. Further, a high-resolution temporal bone computerized tomography (CT) scan was preoperatively performed. Pure-tone audiometry and auditory brainstem response audiometry were performed in children aged >6 years and <6 years (or with poor coordination), respectively, to examine air–bone conductance. All surgeries were performed by the same group of experienced ear surgeons.
Pure-tone audiometry was conducted in a soundproof room based on the national standard guidelines (GB/T16403-1996) using a Genemed Synthesis Inc audiometer. Tympanometry was performed using a Genemed Synthesis Inc Tympstar middle ear analyzer. The auditory brainstem response was measured based on international audiometry standards. Pre- and postoperative audiological tests were performed. The pure-tone threshold average and pre- and postoperative air–bone gap (ABG) were calculated at 500, 1000, 2000, and 4000 Hz. Outpatient and intraoperative examinations were performed using 3-mm 0° and 30° otoendoscopes purchased from STORZ.
2.3. Diagnosis and grading standards
The diagnostic criteria were as follows: a white mass behind the intact tympanic membrane, no history of otorrhea and tympanic membrane perforation, no history of ear surgery, no congenital external or middle ear malformation, no acute otitis media, and no mixed or sensorineural HL.
Based on Potsic’s cholesteatoma standards,[4] we staged the lesions as follows: stage I, lesions only existing in a single quadrant; stage II, lesions involving multiple quadrants without invading the ossicular chain; stage III, lesions involving multiple quadrants that invade ossicular chain; and stage IV, lesions develop to the mastoid process.
2.4. Statistics
Statistical analyses were performed using SPSS 26.0 statistical software. Variables are expressed as X ± s, with between-group comparisons using an independent sample t test. Statistical significance was set at P < .05.
This study was complied based on the principles of the Declaration of Helsinki on Biomedical Research Involving Human Subjects and was approved by the Ethical Review Board of Huazhong University of Science and Technology Union Shenzhen Hospital (Nanshan Hospital). Written informed consent was obtained from each child’s parent or supervisor.
3. Results
3.1. Preoperative examination results
The mean age of the included patients was 7.91 ± 3.11 (3–14) years. There were 8 and 3 cases of the left and right ear, respectively. Moreover, 8 cases (72.7%) were hospitalized for conductive HL with different disease courses, whereas 3 cases (27.3%) did not pass the hearing screening in physical examination. All children had conductive HL, with an average ABG difference of 29.09 ± 8.46 dB HL. High-resolution temporal bone CT scan and otoendoscopy were preoperatively performed. Three (27.3%), 4 (36.4%), and 4 (36.4%) cases were Potsic stage I, stage II, and stage III, respectively. Five cases in the early Potsic stage were preschoolers (age <7 years) with intact ossicular chain and good prognosis. Four children with Potsic stage III were aged >8 years, with HL as the chief complaint. Table 1 presents details regarding the preoperative data.
Table 1.
Preoperative case data of 11 children.
| No. | Age, yr | Location of lesions (side, quadrant) | ABG (dB HL) | Tympanometry | Chief complaint | Potsic stage |
|---|---|---|---|---|---|---|
| 1 | 10 | Left, posterior attic + mesotympanum | 30 | B | Hearing loss | III |
| 2 | 6 | Right, mesotympanum | 32 | AS | Hearing loss | I |
| 3 | 3 | Left, anterior hypotympanum + posterior hypotympanum | 20 | B | Physical examination (–) | II |
| 4 | 8 | Right, posterior attic + posterior hypotympanum | 55 | C | Hearing loss | III |
| 5 | 7 | Left, posterior attic | 40 | B | Hearing loss | I |
| 6 | 14 | Left, epitympanum | 21 | C | Hearing loss | III |
| 7 | 4 | Left, epitympanum | 30 | B | Physical examination (-) | I |
| 8 | 11 | Left, posterior epitympanum | 25 | C | Hearing loss | II |
| 9 | 8 | Right, anterior epitympanum + posterior epitympanum | 35 | B | Hearing loss | II |
| 10 | 9 | Left, posterior attic + posterior epitympanum | 20 | C | Hearing loss | III |
| 11 | 7 | Left, posterior attic + posterior epitympanum | 25 | C | Physical examination (–) | II |
ABG = air–bone gap.
3.2. Surgical procedure and intraoperative results
The surgery duration was 60 to 90 minutes and blood loss was controlled within 5 mL. Table 2 shows the surgical procedures. Figure 1 shows the surgical procedures of a representative case. A rectangular flap incision was made in the external auditory canal 3 mm from the tympanic annulus. A STORZ curette or electric drill was used if there was a bulge in the inferior and posterior wall of the external auditory canal. The tympanic annulus was raised to maintain the integrity of the chorda tympani nerve. After stripping the cholesteatoma cyst, the eustachian tube, the entrance of the tympanic sinus, facial recess, tympanic sinus, and anterior tympanic crypt were explored using a 30° otoendoscope to guarantee no cholesteatoma epithelial residue. Further, hearing reconstruction was performed. The external auditory meatus was filled with antibiotic NasoPore without pressure dressing after external auditory meatus-tympanic membrane flap reduction. Finally, the surgery was completed.
Table 2.
Intraoperative findings in 11 children patients.
| No. | Location of lesions | Cavity condition | Hearing reconstruction |
|---|---|---|---|
| 1 | Left, posterior attic + mesotympanum | Long process of incus is damaged, nerve of tympanic cord is severed, tympanic mucosa is smooth, stapes is intact | Implantation of 1.0-mm PORP |
| 2 | Right, mesotympanum | Tympanic mucosa is smooth, ossicular chain is intact | – |
| 3 | Left, anterior hypotympanum + posterior hypotympanum | Tympanic mucosa is smooth, ossicular chain is intact | – |
| 4 | Right, posterior attic + posterior hypotympanum | Long process of incus is damaged, tympanum mucosa is smooth, stapes superstructure is absent, soleplate moves well | Implantation of 4.5-mm TORP |
| 5 | Left, posterior attic | Tympanic mucosa is smooth, ossicular chain is intact | – |
| 6 | Left, epitympanum | Long process of incus is damaged, tympanum mucosa is smooth, stapes is intact, low position of the facial nerve | Second surgery for hearing reconstruction |
| 7 | Left, epitympanum | Tympanic mucosa is smooth, ossicular chain is intact | – |
| 8 | Left, posterior epitympanum | Tympanic mucosa is smooth, ossicular chain is intact | – |
| 9 | Right, anterior epitympanum + posterior epitympanum | Tympanic mucosa is smooth, ossicular chain is intact | – |
| 10 | Left, posterior attic + posterior epitympanum | Intraoperative eardrum fracture, long process of incus is damaged, tympanum mucosa is smooth, stapes is intact, | Myringoplasty, implantation of 1.0-mm PORP |
| 11 | Left, posterior attic + posterior epitympanum | Tympanic mucosa is smooth, ossicular chain is intact | – |
PORP = partial ossicular replacement prosthesis, TORP = total ossicular replacement prosthesismillimeter.
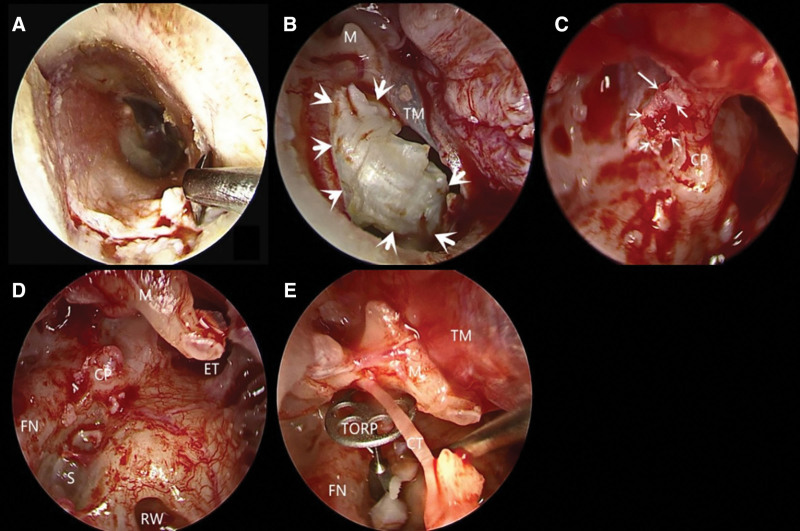
Figure 1.
Case 4 surgical procedure. (A) A rectangular flap was opened under otoendoscopy. (B) Exposure of cholesteatoma cysts. (C) Placement of a 30° otoendoscope to reveal the cholesteatoma epithelium in the medial side of the tensor tympani tendon. (D) Residual cholesteatoma was observed under a 30° otoendoscope. € Total ossicular replacement prosthesis. CP = cochleariformis process, CT = chorda tympani nerve, ET = eustachian tube, FN = facial nerve, M = malleus, RW = round window, S = stapes, TM = tympanic membrane, TORP = total ossicular replacement prosthesis.
3.3. Postoperative situation
The patients ate 4 to 6 hours after surgery and slept well at night. There were no cases of postoperative fever, dizziness, tinnitus, sensorineural deafness, facial paralysis, and other serious complications. In case 1, the chorda tympani nerve was intraoperatively severed without taste aberrations. A second examination was performed at 3 postoperative weeks. The ear canal packing was removed and a checkup otoendoscopy was performed. It revealed that the incision of the external ear canal healed well, the tympanic membrane was intact, and there was no ear discharge or stenosis. At 6 postoperative months, as shown in Table 3, there was no significant change in the bone conductance threshold (S = 0.639, P > .05); moreover, there was a significant decrease in the air conductance threshold and ABG (F = 0.00 and 0.00, respectively, P < .05). One year after surgery, otoendoscopic examination and temporal bone CT revealed good healing of the tympanic membrane without invagination and cholesteatoma recurrence. Figures 2 to 5 show the pre- and postoperative outcomes of representative case 4.
Table 3.
Pre- and postoperative pure-tone audiometry.
| No. | Pre-AC (dB HL) | Pre-BC (dB HL) | Pre-ABG (dB HL) | Post-AC (dB HL) | Post-BC (dB HL) | Post-ABG (dB HL) |
|---|---|---|---|---|---|---|
| 1 | 37.5 | 7.5 | 30 | 20 | 6.25 | 13.75 |
| 2 | 38.25 | 6.25 | 32 | 10 | 5 | 5 |
| 3 | 25 | 5 | 20 | 11.25 | 6.25 | 5 |
| 4 | 61.25 | 6.25 | 55 | 10 | 10 | 0 |
| 5 | 45 | 5 | 40 | 18.75 | 5 | 13.75 |
| 6 | 25 | 5 | 20 | 35 | 5 | 30 |
| 7 | 37.5 | 7.5 | 30 | 15 | 6.25 | 8.75 |
| 8 | 30 | 5 | 25 | 12.5 | 5 | 7.5 |
| 9 | 41.25 | 6.25 | 35 | 16.25 | 6.25 | 10 |
| 10 | 25 | 5 | 20 | 17.5 | 5 | 12.5 |
| 11 | 31.25 | 6.25 | 25 | 10 | 5 | 5 |
| Average | 36.19 ± 10.75 | 5.90 ± 0.98 | 30.27 ± 10.41 | 17.84 ± 14.67 | 5.45 ± 0.63 | 12.39 ± 14.76 |
Post-ABG = postoperative air–bone gap, Post-AC = postoperative air conductance, Post-BC = postoperative bone conductance, Pre-ABG = preoperative air–bone gap, Pre-AC = preoperative air conductance, Pre-BC = preoperative bone conductance.
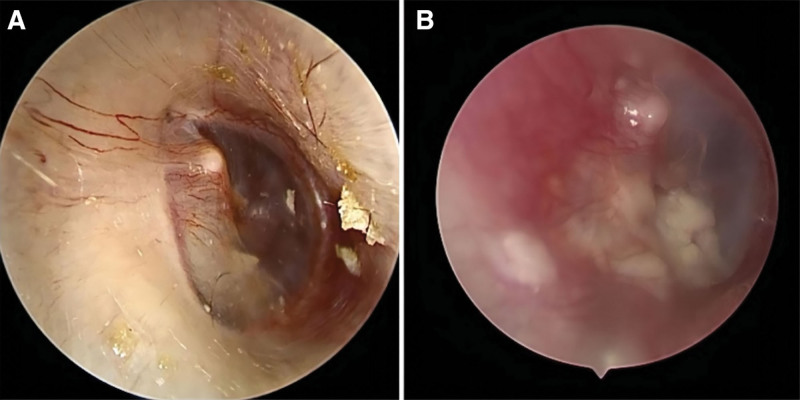
Figure 2.
Otoendoscopic examination in case 4. (A) A white patchy shadow behind the intact tympanic membrane was observed before surgery. (B) The tympanic membrane was intact and the cartilage was visible inside after surgery.
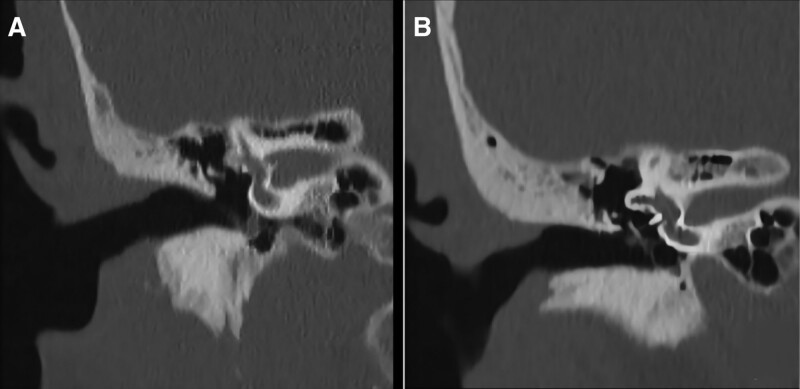
Figure 5.
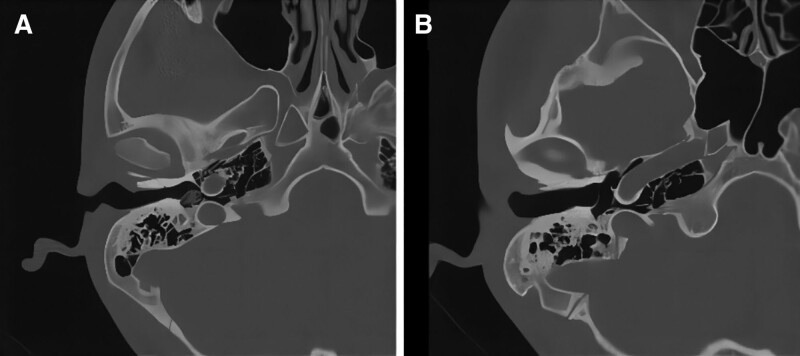
CT images in the coronal view in case 4. (A) Intact scute, hypodensity shadow of the inferior tympanum, and incomplete ossicular chain before surgery. (B) The scute was intact, the hypodensity shadow of the inferior tympanum disappeared, and artificial ossicles were present in the vestibular window. CT = computerized tomography.
4. Discussion
An increasing number of CMEC pediatric cases has been recently reported due to the widespread application of outpatient otoendoscopy and improved disease awareness among clinicians. However, because the eustachian tube in children is “short, flat, and straight,” some children are diagnosed with CMEC only after the occurrence of tympanic membrane perforation and ear discharge. Other children who do not meet Levenson’s criteria might be diagnosed after visiting the hospital for secretory otitis media.[5–7] Given the difficulty in early detection of unilateral HL among young children, they are often sent to the specialist after failing hearing screening tests. In our study, 3 children failed hearing screening tests and were diagnosed with CMEC. Gilberto et al[8] recommended routine hearing screening for children entering kindergarten and school. We recommend that children who fail hearing screening tests should undergo otoendoscopic examination and temporal bone CT scan. All 11 children who underwent outpatient otoendoscopy showed white localized mass shadows on the inner side of the intact tympanic membrane. Therefore, early CMEC detection can be facilitated through the use of otoendoscopy for routine examination of children with HL or failed hearing screening tests. Additionally, for children with secretory otitis media, in case long-term treatment does not improve symptoms, temporal bone CT should be considered to exclude CMEC.
The pathogenesis of CMEC remains unclear; however, several theories have been proposed, including epidermoid theory, tympanic annulus development disorder theory, metagenesis theory, and ectoderm implantation theory. Among them, the epidermoid theory is widely accepted. Although the accumulation of keratoid epithelium is benign, it can progressively grow and destroy normal surrounding tissue.[8] CC in the posterior quadrant of mesotympanum is often detected late in routine ear examinations because of the opacity of the eardrum. Due to the adjacent ossicular chain and facial nerve, CC in the posterior quadrant is more invasive and can cause serious intracranial and external complications. In our study, 4 children showed stage III CMEC occurring in the posterior quadrant. Therefore, early CMEC detection and treatment are crucial for avoiding the occurrence of serious complications
Currently, surgical treatment remains the only treatment for CMEC.[9] Its main objectives are to completely remove lesions, prevent recurrence, and preserve or rebuild hearing. For patients with CMEC, the classical surgical methods include modified radical mastoidectomy and tympanoplasty based on the lesion scope. Moreover, enlarged tympanum exploration has been used for localized stage I and stage II lesions.[10] Children with CMEC present good mastoid pneumatization and limited lesions. Compared with tympanoplasty, modified radical mastoidectomy is more traumatic and requires children to be regularly cleaned in the clinic, which results in poor cooperation among children. Moreover, there is limited microscopic exposure of the attic tympanum, facial recess, and sinus tympani, which results in incomplete lesion removal and recurrence. We chose the surgical treatment of CMEC under otoendoscopy based on previous reports that CMEC within stage III can be treated using the ear canal approach under microscopy. This approach can avoid damage to the mastoid bone and air chamber with mild lesions.[11] Stage III CMEC can be treated using the ear canal approach when the lesion is localized without invasion of the mastoid process. All children with stage III underwent surgery under otoendoscopic guidance. Additionally, applying the angle scope facilitated the preservation of the bone wall of the external auditory canal and the lateral wall of the upper drum; moreover, it significantly decreased external auditory canal exudation and the dressing time.
The recurrence rate of CMEC in children is 10.5% to 45%.[12,13] Most studies on CMEC are small scale[8,12,14–17]; therefore, it remains unclear whether otoendoscopy can reduce the CMEC recurrence rate. Advances in otoendoscopy have yielded otoendoscopes with a diameter of 2.7 and 1.9 mm, which can pass through the narrow ear canal to increase the surgical field of vision, improve the surgical field clarity, and observe tissue from multiple angles at close range.[18] Otoendoscopy allows clear vision of the superior tympanum, facial recess, tympanum sinus, eustachian tube, and other concealed anatomical sites. Otoendoscopic ear surgery involves a small incision, which makes it easier to accept even for children scheduled for a second surgery. Another difficulty experienced by children with CMEC is stenosis of the ear canal. In case preoperative CT reveals that the anteroposterior diameter of the middle part of the external auditory canal (midpoint of ear canal opening and umbilical point of eardrum) ear bone wall exceeds 4.5 mm, an otoendoscope with a diameter of >2.7 mm is suitable for operation.[19] The anteroposterior diameter of the isthmus of the external auditory canal in Chinese children is 3.27 ± 0.75 and 4.01 ± 0.75 mm for children aged 0 to 5 and 6 to 15 years, respectively. If the external auditory canal bulge affects the surgical field, curettes or electric drills can be used to expand the external auditory canal to increase the surgical field of vision. Given the thin skin of children’s ear canal, it is important to protect the ear canal flap to avoid postoperative scar stenosis.
In this study, all 11 children were treated using transcanal endoscopic surgery. In 1 case, a cartilage connection was implanted due to the low position of the facial nerve and the lack of postoperative hearing recovery. Accordingly, a second reconstructive surgery was considered. In another case, tympanic membrane perforation occurred during surgery separation. The eardrum was simultaneously repaired with the perichondrium, with good healing being observed at 3 postoperative weeks. Follow-up examination confirmed significantly improved hearing in all cases. Seven children with stage I and stage II were young and promptly diagnosed. Their external auditory canal and shield plate were not intraoperatively expanded. The ABG was postoperatively reduced to within 10 dB HL, with the dry ear being recovered within approximately 2 months. The remaining 4 children with stage III were aged >7 years, which could be attributed to the lack of early birth examination and hearing screening. Among them, 1 child planned to undergo a second surgery for hearing reconstruction, while the remaining showed significantly improved hearing. To clarify the lesion, there were different degrees of external auditory canal enlargement. All children recovered dry ears within 3 postoperative months, with no cases of auditory ossicle discharge, displacement, and recurrence.
5. Conclusions
Transcanal endoscopic treatment for CMEC in children is feasible, minimally invasive, and functional; additionally, it should be popularized for CMEC treatment in children. Given the small sample size and short follow-up duration, further studies are warranted.
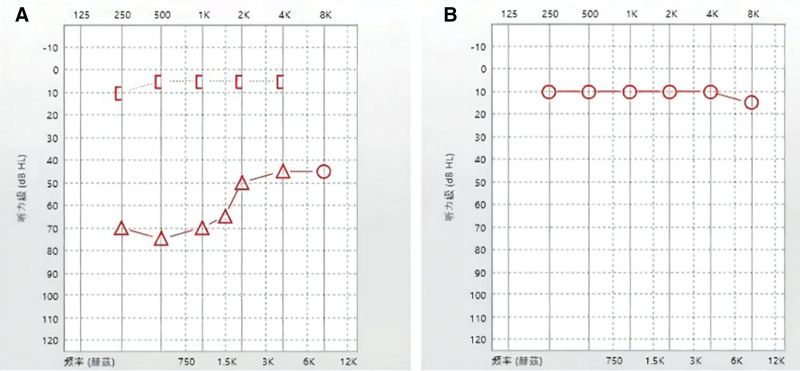
Figure 3.
Pure-tone audiometry in case 4. (A) Severe conductive hearing loss before surgery. (B) Hearing recovery after surgery.
Figure 4.
CT images from the horizontal view in case 4. (A) A hypodensity shadow was observed in the inferior tympanum horizontally before surgery. (B) The tympanic hypodensity shadow disappeared and the posterior wall of the external auditory canal was intact after surgery. CT = computerized tomography.
Author contributions
Nan Zeng, Meng Liang and Shang Yan contributed equally to this work and should be considered co-first authors. Because this research is designed and carried out under the guidance of Qiong Yang and Shuo Li, they are the co-correspondence authors. Lue Zhang Was responsible for the collection and collation of cases.
Abbreviations:
- ABG =
- air–bone gap
- CC =
- congenital cholesteatoma
- CMEC =
- congenital middle ear cholesteatoma
- CT =
- computerized tomography
NZ, ML, and SY contributed equally to this work.
QY and SL contributed equally to this work.
The authors have no funding and conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
How to cite this article: Zeng N, Liang M, Yan S, Zhang L, Li S, Yang Q. Transcanal endoscopic treatment for congenital middle ear cholesteatoma in children. Medicine 2022;101:29(e29631).
Contributor Information
Nan Zeng, Email: 471561785@qq.com.
Meng Liang, Email: liangmeng666@126.com.
Shang Yan, Email: 286280026@qq.com.
Lue Zhang, Email: 545304423@qq.com.
References
- [1].Levenson MJ, Michaels L, Parisier SC. Congenital cholesteatomas of the middle ear in children: origin and management. Otolaryngol Clin North Am. 1989;22:941–54. [PubMed] [Google Scholar]
- [2].Lin V, Daniel S, James A, et al. Bilateral cholesteatomas: the hospital for sick children experience. J Otolaryngol. 2004;33:145–50. [DOI] [PubMed] [Google Scholar]
- [3].Gülşen S, Arici M. Endoscopic transcanal versus conventional microscopic tympanoplasty in treatment of anterior tympanic membrane perforations. Eur Arch Otorhinolaryngol. 2019;276:3327–33. [DOI] [PubMed] [Google Scholar]
- [4].Potsic WP, Korman SB, Samadi DS, et al. Congenital cholesteatoma: 20 years’ experience at the children’s hospital of Philadelphia. Otolaryngol Head Neck Surg. 2002;126:409–14. [DOI] [PubMed] [Google Scholar]
- [5].Kazahaya K, Potsic WP. Congenital cholesteatoma. Curr Opin Otolaryngol Head Neck Surg. 2004;12:398–403. [DOI] [PubMed] [Google Scholar]
- [6].Liu L, Gong S, Ma X, et al. Two cases of congenital middle ear cholesteatoma with secretory otitis media as the main manifestation in children. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2016;51:383–5. [DOI] [PubMed] [Google Scholar]
- [7].Potsic WP, Samadi DS, Marsh RR, et al. A staging system for congenital cholesteatoma. Arch Otolaryngol Head Neck Surg. 2002;128:1009–12. [DOI] [PubMed] [Google Scholar]
- [8].Gilberto N, Custodio S, Colaco T, et al. Middle ear congenital cholesteatoma: systematic review, meta-analysis and insights on its pathogenesis. Eur Arch Otorhinolaryngol. 2020;277:987–98. [DOI] [PubMed] [Google Scholar]
- [9].Hao J, Chen M, Liu B, et al. The Significance of Staging in the Treatment of Congenital Cholesteatoma in Children. Ear Nose Throat J. 2018;32:1097–101. [DOI] [PubMed] [Google Scholar]
- [10].Song IS, Han WG, Lim KH, et al. Clinical characteristics and treatment outcomes of congenital cholesteatoma. J Int Adv Otol. 2019;15:386–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kim H, Yoo SY, Choung YH, et al. Is transcanal tympanoplasty an appropriate surgical treatment for congenital middle ear cholesteatoma with ossicular involvement? Int J Pediatr Otorhinolaryngol. 2019;116:102–6. [DOI] [PubMed] [Google Scholar]
- [12].Cho HS, Kim HG, Jung DJ, et al. Clinical aspects and surgical outcomes of congenital cholesteatoma in 93 children: increasing trends of congenital cholesteatoma from 1997 through 2012. J Audiol Otol. 2016;20:168–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hao JS, Chen M, Liu B, et al. Clinical treatment of congenital middle ear cholesteatoma in children. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2018;32:1097–101. [DOI] [PubMed] [Google Scholar]
- [14].Benhammou A, Nguyen DQ, El Makhloufi K, et al. Long term results of congenital middle ear cholesteatoma in children. Ann Otolaryngol Chir Cervicofac. 2005;122:113–9. [DOI] [PubMed] [Google Scholar]
- [15].Bukurov B, Babic B, Dimitrijevic M, et al. Congenital cholesteatoma of the middle ear—uncommon clinical presentation. Vojnosanit Pregl. 2014;71:503–5. [PubMed] [Google Scholar]
- [16].Choi HG, Park KH, Park SN, et al. Clinical experience of 71 cases of congenital middle ear cholesteatoma. Acta Otolaryngol. 2010;130:62–7. [DOI] [PubMed] [Google Scholar]
- [17].Curran JF, Coleman H, Tikka T, et al. Comparison of outcomes of endoscopic ear surgery with microsurgery for cholesteatoma: a prospective study of 91 cases with three-year follow-up. Clin Otolaryngol. 2021;47:197–202. [DOI] [PubMed] [Google Scholar]
- [18].Yaniv D, Tzelnick S, Ulanovski D, et al. Effect of endoscope assistance in tympanomastoidectomy for lowering the rate of residual cholesteatoma: results from 91 paediatric patients. Clin Otolaryngol. 2019;44:1105–8. [DOI] [PubMed] [Google Scholar]
- [19].McCabe R, Lee DJ, Fina M. The endoscopic management of congenital cholesteatoma. Otolaryngol Clin North Am. 2021;54:111–23. [DOI] [PubMed] [Google Scholar]