Abstract:
Disseminated intravascular coagulation (DIC) is a life-threatening hematologic derangement characterized by dysregulated thrombin generation and excessive fibrinolysis. However, DIC is poorly characterized in the extracorporeal membrane oxygenation (ECMO) population, and the underlying mechanisms are not well understood. Several mechanisms contribute to DIC in ECMO, including consumption of coagulation factors, acquired von Willebrand’s syndrome leading to thrombocytopenia, and hyperfibrinolysis. There are few case reports of DIC in adult ECMO patients. Most are in the context of venoarterial ECMO, which is typically used in the setting of cardiogenic shock and cardiac arrest. These disease states themselves are known to be associated with DIC, liver failure, impaired anticoagulant mechanisms, and increased fibrinolysis. We present an unusual case of a 74-year-old man who developed overt DIC during veno-venous (VV) ECMO. DIC resulted in clinical bleeding and severe hypofibrinogenemia requiring massive cryoprecipitate transfusion of 87 pooled units. When the patient was decannulated from ECMO, his platelet count and fibrinogen concentration improved within 24 hours, suggesting that ECMO was a proximate cause of his DIC.
Keywords: ECMO, coagulation, DIC, coagulopathy.
Disseminated intravascular coagulation (DIC) is typically characterized by the activation of thrombin and fibrin generation in small and midsized vessels, which in turn can lead to either excessive fibrinolysis or consumptive coagulopathy, or both. Venoarterial extracorporeal membrane oxygenation (VA-ECMO) has been known to cause significant hypofibrinogenemia and consumptive coagulopathy (1–4). Although mildly abnormal coagulation parameters may manifest in subtle hemostatic dysfunction known as non-overt DIC, the derangements of the hemostatic system seen in disease states associated with VA-ECMO such as cardiogenic shock and cardiac arrest can cause a decompensated form of DIC known as overt DIC, in which severe bleeding cannot be adequately treated with blood products and hemostatic agents such as fresh frozen plasma, platelets, cryoprecipitate, tranexamic acid, and factor concentrates (3).
On the other hand, VV-ECMO, which is usually used in the context of isolated respiratory failure and acute respiratory distress syndrome, is typically associated with an extreme inflammatory response and a rise in serum fibrinogen (4). The coagulopathic changes seen in VA-ECMO are known to be milder in veno-venous ECMO (VV-ECMO) (5). Here, we describe an unusual case of a patient with acute respiratory failure who developed overt DIC characterized by thrombocytopenia and hypofibrinogenemia during VV ECMO leading to severe bleeding and massive cryoprecipitate transfusion. Written informed consent and health insurance portability and accountability (HIPAA) permission was obtained from the patient’s legally authorized representative to publish the case report.
REPORT
A 74-year-old man with a history of idiopathic pulmonary fibrosis (IPF) and a prior single lung transplant was admitted to the hospital with anemia, fatigue, and a posterior chest wall hematoma in the setting of systemic anticoagulation with apixaban (Eliquis) for chronic atrial flutter. The patient was admitted to the hospital prior to the outbreak of the novel coronavirus (COVID-19) in 2019. His other medical history included obstructive sleep apnea, type 2 diabetes mellitus, and dyslipidemia. On hospital day 10, he underwent a catheter-based left atrial appendage closure. Post-operatively, the patient required long-term hospitalization due to persistent orthostatic hypotension and worsening respiratory status. On hospital day 39, he was admitted to the intensive care unit (ICU) with increased work of breathing and hypercarbia. There was no evidence of allograft rejection based on the donor-specific antibody testing, but a biopsy was not performed because of his tenuous clinical status. Chest roentgenogram revealed an infiltrate in the patient’s native lung. A bronchoalveolar lavage (BAL) was performed and was negative for bacterial growth.
The cause of the patient’s respiratory failure was thought to be exacerbation of his native IPF, and he was treated with intravenous (IV) steroids. His respiratory function was supported with high-flow nasal cannula oxygen and bilevel positive airway pressure (BIPAP). On hospital day 75, his respiratory status worsened, requiring endotracheal intubation, mechanical ventilation, and subsequently VV-ECMO1 to treat severe hypoxemic and hypercarbic respiratory failure. A computed tomography scan of his chest showed pneumonia in his native lung. A repeat BAL grew multiple pathogens, and he was treated with meropenem and micafungin. The patient had completed his steroid course by the time he was cannulated with ECMO. At this point, coagulation monitoring performed on the patient did not demonstrate any disruption of the hemostatic system.
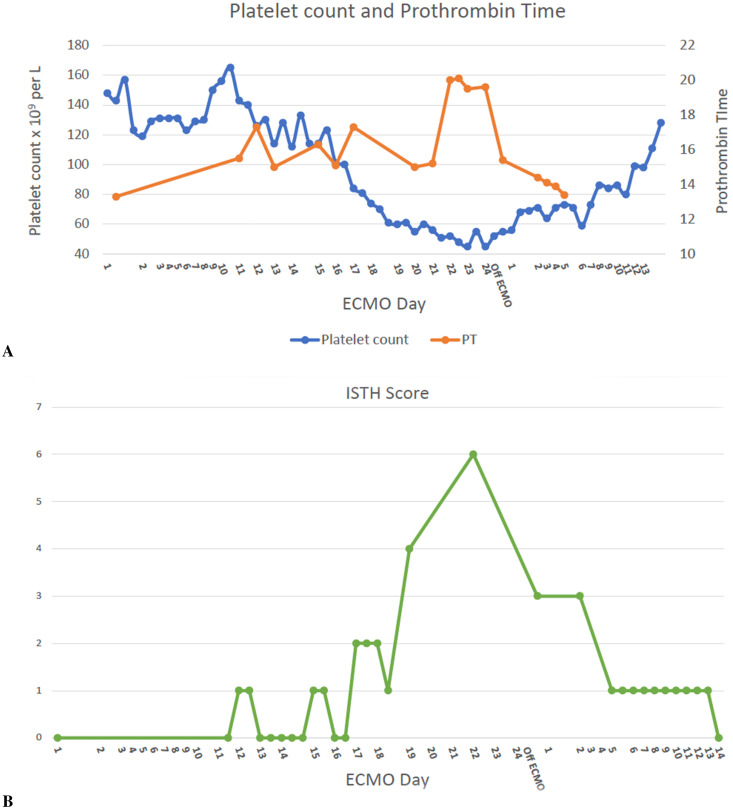
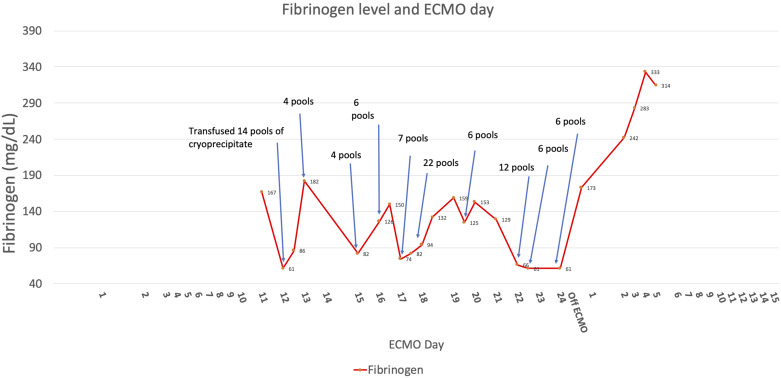
The patient’s initial ECMO course was unremarkable. The ECMO circuit was bonded with heparin. The oxygenator was visually inspected for clots every 12 hours. Transmembrane gradients were measured daily as well and were consistently within the normal range (<50 mmHg). Flows were maintained at >4 liters per minute for a cardiac index of at least 2.2 L/min/m2 (body surface area [BSA] 1.8 m2), and sweep flow was titrated to maintain SpO2 ≥ 92%, pH 7.35–7.45, pCO2 35–45 mmHg, or respiratory rate of <25 breaths/min. He was anticoagulated with continuous unfractionated heparin infusion, with a goal partial thromboplastin time (PTT) of 45–55 seconds, which is the standard anticoagulation goal at the author’s institution for VV-ECMO. There was no difficulty in reaching anticoagulation goals, and heparin infusion rates were not excessively high or low. A complete blood count (CBC) on ECMO day 10 showed a hemoglobin of 10.1 g/dL, platelets 168 × 109 per liter, and WBC 8.9 × 109 per liter. On his 11th ECMO day, he developed spontaneous hematuria and upper gastrointestinal (GI) tract bleeding. Heparin infusion was held during these bleeding episodes. The patient had been on stress ulcer prophylaxis with pantoprazole 40 mg IV once daily since his admission to the ICU. His fibrinogen was <61 mg/dL, platelet count was 126 × 109 per liter, and international normalized ratio (INR) was 1.4. PTT was elevated at 48 seconds. He was transfused 14 pools of cryoprecipitate. An esophagogastroduodenoscopy (EGD) was performed, and two bleeding gastric ulcers were clipped. Despite this intervention, he continued to experience hematuria and bloody nasogastric tube output while on ECMO. Urinalysis demonstrated free red blood cells (RBC) and no plasma-free hemoglobin. His platelet count and fibrinogen also continued to become severely low despite continued transfusions of cryoprecipitate, reaching a nadir on ECMO day 23 (Figures 1 and 2). Thromboelastogram showed severely decreased maximum amplitude at 10 mm. At this time, his International Society of Thrombosis and Hemostasis (ISTH) DIC score was 6 (D-Dimer > 20,000 ng/mL fibrinogen equivalent units, prothrombin time (PT) 20 seconds, platelet count 52 × 109 per liter, and fibrinogen 70 mg/dL), which was consistent with overt DIC.
Figure 1.
(A) Shows the platelet count and prothrombin time (PT) during and after extracorporeal membrane oxygenation (ECMO). (B) Shows the International Society of Thrombosis and Hemostasis (ISTH) disseminated intravascular coagulation (DIC) score during and after ECMO.
Figure 2.
Figure shows fibrinogen concentration during and after ECMO and also cryoprecipitate transfusion during ECMO.
Other than exposure to extracorporeal circulatory support, there was no apparent cause for his DIC. His pneumonia had improved, his white blood cell counts normalized, he was afebrile, and all repeat respiratory and blood cultures were negative for growth. The ECMO circuit was visually inspected for thrombus, but none was identified. The circuit and oxygenator had been changed once at the onset of the first GI bleeding episode as a precaution and changed again on ECMO day 22. His lactate dehydrogenase level was unremarkable at 800 units/L, suggesting that there was minimal hemolysis. Hepatic function tests were normal. The patient was hemodynamically stable and did not require vasopressors or inotropes. Given his persistent GI and urinary tract bleeding, his severe hypofibrinogenemia was treated with multiple cryoprecipitate transfusions (87 pooled units over 7 days) to maintain a fibrinogen above 150 mg/dL. During the same time period, he received 12 RBC units and 2 apheresis platelet units. No fresh frozen plasma was given due to normal range PT, PTT, and INR values—the highest PT recorded for the patient was 20 seconds. It is estimated that he lost approximately 4.2 L of blood over his entire ECMO run. On ECMO day 24, ECMO support was discontinued, and the patient was decannulated. One day after decannulation, his ISTH DIC score fell to 3. Platelet and fibrinogen levels increased, and he did not require further allogeneic transfusion. Later in his hospital course, he developed recurrent hypoxemic respiratory failure and life sustaining treatment was withdrawn by his family.
DISCUSSION
DIC is a life-threatening hematologic derangement caused by an event such as sepsis, trauma, amniotic fluid embolism, or massive intravascular hemolysis. The incidence of overt DIC in critically ill patients is estimated to be around 1% and mortality is near 50% (6). In a 7-year observational cohort study that included 12,721 ICU admissions and 154 patients with overt DIC, the most common risk factors for DIC were sepsis and acute respiratory failure (7).
The incidence of overt DIC in adult ECMO patients is not described, although DIC has been a known complication of cardiac surgery with cardiopulmonary bypass (CPB) since the 1970s (8). The pathophysiology of DIC is not fully understood, but uncontrolled thrombin generation occurs and intrinsic anticoagulants (antithrombin, protein C, and tissue factor pathway inhibitor) are exhausted (9).
Thrombocytopenia and acquired von Willebrand syndrome are two factors that contribute to bleeding, and exposure of blood to extracorporeal circulation leads to multiple coagulation changes including platelet surface receptor shedding and impaired primary hemostasis (5,10,11).
Fibrinolysis leads to the generation of fibrin degradation products, which inhibit fibrin polymerization.
To our knowledge, our case is the first to suggest that VV-ECMO itself may lead to DIC in a non-surgical patient. Further studies would, of course, be required to solidify this relationship. Thrombin generation occurs in adult ECMO patients on heparin, but it is significantly less than what occurs during cardiac surgery with CPB (11). VV-ECMO patients often demonstrate elevated fibrinogen concentration (4), and it is uncommon for fibrinogen concentration to fall during ECMO unless significant hemorrhage or major surgery occurs (12). The patient was noted to be on IV steroids prior to ECMO cannulation, and, although steroids were discontinued prior to initiation of ECMO, the authors recognize that this may have contributed to low fibrinogen levels during the initial stages of the patient’s ECMO run. Nonetheless, it seemed that ECMO was indeed the inciting factor for the patient’s DIC, as it persisted for 13 consecutive days, and fibrinogen concentration and platelet count immediately improved when he was decannulated. It is noteworthy that his initial bleeding was from a gastric ulcer. Prior animal studies have shown that DIC induces stomach and duodenal bleeding within 80 minutes, perhaps because of tissue hypoxemia from microvascular thrombosis (13). Although the patient did develop pneumonia, DIC did not develop until later in his ECMO course when his pneumonia had improved. Although COVID-19 has been described to cause similar hematologic derangements in patients on VV-ECMO (6), our patient was admitted prior to the outbreak of COVID-19 in the United States, and there was no reported travel to outside countries. Our report has several limitations. First, we cannot rule out the possibility that there was an indolent infection in our patient, which may have contributed to DIC. Pneumonia may have set up the immunosuppressive conditions required for exposure to VV-ECMO to result in development of DIC. Although he was not septic when he developed DIC, immunosuppressed patients frequently have different responses to infection. Second, there were no specific aspects of our patient’s ECMO course that explained his DIC. There was no obvious hemolysis, circuit thrombosis, or other inciting factors. However, we did not conduct an exhaustive deconstruction and inspection of the ECMO circuit and oxygenators for clot, relying instead on point-of-care visual inspection. Furthermore, fibrin degradation products (D-dimer) were not measured before oxygenator changes. The authors acknowledge that D-dimer has been shown to predict clot volume inside membrane oxygenators during ECMO (14). If such clots did exist, this may indicate a different cause of the patient’s coagulopathy.
In summary, we present an unusual case of overt DIC that occurred in a patient with acute respiratory failure on VV-ECMO. The patient experienced severe hypofibrinogenemia and refractory bleeding requiring massive cryoprecipitate transfusion. This case suggests that VV-ECMO alone can induce overt DIC, and the only effective treatment may be to decannulate the patient if feasible.
Footnotes
ECMO circuit consisted of 25 Fr femoral venous drainage and 23 Fr venous return cannula, and Maquet Quadrox-i Adult oxygenator (Getinge, Wayne, NJ) attached to heparin bonded circuit, driven by Maquet ROTAFLOW RF-32 centrifugal pump (Getinge, Wayne, NJ).
REFERENCES
- 1.Mazzeffi MA, Tanaka K, Roberts A, et al. Bleeding, thrombosis, and transfusion with two heparin anticoagulation protocols in venoarterial ECMO patients. J Cardiothorac Vasc Anesth. 2019;33:1216–20. [DOI] [PubMed] [Google Scholar]
- 2.Cheng R, Hachamovitch R, Kittleson M, et al. Complications of extracorporeal membrane oxygenation for treatment of cardiogenic shock and cardiac arrest: A meta-analysis of 1,866 adult patients. Ann Thorac Surg. 2014;97:610–6. [DOI] [PubMed] [Google Scholar]
- 3.Bembea MM. Complications during extracorporeal membrane oxygenation: Why collaboration is key. Pediatr Crit Care Med. 2015;16:193–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cartwright B, Bruce HM, Kershaw G, et al. Hemostasis, coagulation and thrombin in venoarterial and venovenous extracorporeal membrane oxygenation: The HECTIC study. Sci Rep. 2021;11:7975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mazzeffi M, Hasan S, Abuelkasem E, et al. Von Willebrand factor-GP1balpha interactions in venoarterial extracorporeal membrane oxygenation patients. J Cardiothorac Vasc Anesth. 2019;33:2125–32. [DOI] [PubMed] [Google Scholar]
- 6.Hakeem Y, Vasileios Z, Daniel B. Thrombosis and coagulopathy in COVID-19 patients requiring extracorporeal membrane oxygenation. ASAIO J. 2020;66:844–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh B, Hanson AC, Alhurani R, et al. Trends in the incidence and outcomes of disseminated intravascular coagulation in critically ill patients (2004–2010): A population-based study. Chest. 2013;143:1235–42. [DOI] [PubMed] [Google Scholar]
- 8.Boyd AD, Engelman RM, Beaudet RL, et al. Disseminated intravascular coagulation following extracorporeal circulation. J Thorac Cardiovasc Surg. 1972;64:685–93. [PubMed] [Google Scholar]
- 9.Boral BM, Williams DJ, Boral LI. Disseminated intravascular coagulation. Am J Clin Pathol. 2016;146:670–80. [DOI] [PubMed] [Google Scholar]
- 10.Chen Z, Mondal NK, Zheng S, et al. High shear induces platelet dysfunction leading to enhanced thrombotic propensity and diminished hemostatic capacity. Platelets. 2019;30:112–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mazzeffi M, Strauss E, Meyer M, et al. Coagulation factor levels and underlying thrombin generation patterns in adult extracorporeal membrane oxygenation patients. Anesth Analg. 2019;129:659–66. [DOI] [PubMed] [Google Scholar]
- 12.Nair P, Hoechter DJ, Buscher H, et al. Prospective observational study of hemostatic alterations during adult extracorporeal membrane oxygenation (ECMO) using point-of-care thromboelastometry and platelet aggregometry. J Cardiothorac Vasc Anesth. 2015;29:288–96. [DOI] [PubMed] [Google Scholar]
- 13.Linder MM, Hartel W, Lenz J. Acute ischemic gastric mucosal and duodenal lesions in dogs: “Stress ulcer” after experimental disseminated intravascular coagulation. Helv Chir Acta. 1978;45:119–23. [PubMed] [Google Scholar]
- 14.Doyle AJ, Hunt BJ. Current understanding of how extracorporeal membrane oxygenators activate haemostasis and other blood components. Front Med (Lausanne). 2018;5:352. [DOI] [PMC free article] [PubMed] [Google Scholar]