Abstract:
Hematologic complications are a source of morbidity and mortality for patients receiving extracorporeal membrane oxygenation (ECMO) support. There is no consensus strategy for monitoring anticoagulation for children supported with ECMO. This study evaluated a novel measurement of anticoagulation for children on ECMO. This was a single-center observational study of children supported with ECMO from 2015 to 2020. Each patient’s current unfractionated heparin dose was multiplied by the current antithrombin III (AT) level to obtain a novel anticoagulation value, the heparin-antithrombin product (HAP). This value was compared with the heparin dose, AT, and activated clotting time (ACT) to predict anti-Xa value using linear correlation and decision tree methods. Data were obtained from 128 patients supported with ECMO. The HAP value was more highly correlated with anti-Xa level than heparin dose, AT level, and ACT. This correlation was highest in the neonatal population (r = .7). The variable importance metrics from the regression tree and random forest models both identified the HAP value as the most influential predictor variable for anti-Xa value. The HAP value is more highly correlated with the anti-Xa level than heparin dose, AT level, or ACT. Further research is needed to evaluate the effectiveness of the HAP value as a measurement of anticoagulation for children on ECMO.
Keywords: extracorporeal membrane oxygenation, anticoagulation, pediatrics, critical care medicine.
Extracorporeal membrane oxygenation (ECMO) remains a life-saving modality used to stabilize critically ill neonates and children with cardiorespiratory failure that is refractory to conventional measures. Despite advancements made in the field of extracorporeal life support, hematologic complications, including both thrombotic and hemorrhagic events, remain a major source of morbidity and mortality for patients receiving ECMO support (1). The interaction between patient’s bloodstream and the non-endothelial surface of the ECMO circuit leads to a state of systemic inflammation and hypercoagulability, with the subsequent risk of ECMO circuit thrombosis. Systemic anticoagulation is required to overcome the risk of ECMO circuit thrombosis, and historically unfractionated heparin (UFH) has been chosen by the majority of ECMO centers for anticoagulation (2). UFH exerts its anticoagulant effects by potentiating the ability of antithrombin III (AT) to inhibit both thrombin and factor Xa (3).
To date, there is no consensus laboratory strategy for monitoring the anticoagulant effect of UFH for children supported with ECMO, and institutions are advised to develop a method tailored to their unique patient populations (4). Historically, the activated clotting time (ACT) has been the primary laboratory parameter to guide titration of UFH during ECMO (2). However, more recent evidence suggests that the anti-Xa assay has a superior correlation to UFH dose on ECMO over ACT, activated partial thromboplastin time (aPTT), and thromboelastography (TEG) values (5). The anti-Xa assay, as a plasma test, reflects the ability of the heparin-AT complex to inhibit factor X (6). Previous work has evaluated the effect of UFH dose on pediatric ECMO outcomes (7), and studies have also examined the impact of AT within this patient population (8–10). However, the effect of UFH dose and AT activity working synergistically during pediatric ECMO has yet to be explored. Thus, we developed the heparin-antithrombin product (HAP) as a novel anticoagulation value. This study seeks to determine the utility of the HAP value to predict overall anticoagulation during pediatric ECMO compared to other traditional measurements, using the anti-Xa level as a gold standard.
MATERIALS AND METHODS
Approval for this study was obtained from the Institutional Review Board. Data from 128 pediatric patients requiring ECMO support in our institution from the years 2015 to 2020 were retrospectively reviewed and collected from the medical record. ECMO circuit components for patients in our institution include the Stockert S5 roller pump (LivaNova, London, UK), the adult QuadroxID membrane oxygenator (Getinge, Goteborg, Sweden), and Intersept®class VI ¼-inch tubing with Cortiva™BioActive surface coating (Medtronic, Minneapolis, MN). Initiation of ECMO anticoagulation in our institution begins with a 50–100 U/kg bolus dose of UFH at the time of ECMO cannulation followed by an infusion of UFH at 30 U/kg/h for neonates and infants and 20 U/kg/h for children. Thereafter, UFH is titrated via anti-Xa values (Instrumentation Laboratory, Bedford, MA) obtained every 6 hours. Two options exist for the goal anti-Xa range depending on the treating clinician’s concern for patient-specific bleeding complications: 1) a “standard bleeding risk” algorithm, with an anti-Xa range of .7–1.0 IU/mL, or 2) a “high bleeding risk” algorithm, with an anti-Xa range of .3–.7 IU/mL. Measurement of ACT values were also routinely obtained. Lastly, our institution’s ECMO anticoagulation guidelines recommend replacement of AT to keep AT activity >60%. AT replacement dosing in our institution is based on the equation: ((Desired AT activity − current AT activity) × weight in kg)/1.4.
Data obtained included patient age, sex, race, ethnicity, diagnostic category, and ECMO modality. Patient ages were classified into the following categories: neonate (≤28 days of life), infant (29 days to 12 months of age), and child (>12 months). Diagnostic categories included cardiac, pediatric respiratory, and neonatal respiratory. Laboratory and medication dosing values obtained included the following: UFH infusion dosing (U/kg/h), ACT (seconds), anti-Xa level (IU/mL), and AT activity (%). In our institution, all patients on ECMO have a full panel of anticoagulation laboratory tests drawn at 06:00 each morning. Each observation included the ECMO anticoagulation labs drawn at that time and the UFH dose the patient was receiving at 06:00 that day.
The HAP was calculated as the multiplication product of the patient’s UFH dose and AT activity. Single-variable regression models were created using the patient’s anti-Xa level as the dependent variable of interest, and the independent variables of UFH dosing, AT activity, ACT, and the HAP. The Pearson correlation coefficients between each independent variable and the anti-Xa value were obtained. A multivariable linear regression model was also constructed and included heparin dose, AT level, and the HAP value being the interaction of the heparin dose and AT level. These models were applied to the entire patient cohort initially, and subsequently applied to each stratified age cohort and modality.
As the laboratory values obtained were repeated samples, our data does not completely satisfy the assumptions for linear regression. To test for a non-linear relationship between the dependent and independent variables, two additional models were constructed. The first was a smoothing spline that seeks to fit a non-linear function to the relationship between the dependent and independent variables with a defined measure of constraint to prevent overfitting and high variance in the model (11). The second model is a generalized additive model that seeks to accommodate for non-linear variable effects in an additive fashion by calculating a specific function parameter for each individual variable included in the model (12).
Finally, two tree-based regression methods were used to measure both the variable importance for each independent variable (UFH dose, AT activity, and HAP), as well as to measure the prediction value of the model regarding the dependent variable (anti-Xa). These models were built using a randomly selected sample of 75% of the available data. The models were then applied to the remaining 25% of the data as a test dataset. The mean squared error (MSE) values of the tree models were obtained from the test data application. First, a simple regression tree model was created using the 10-fold k cross validation. This model provided a measure of variable importance for each of the independent variables (UFH dose, AT activity, and HAP) (13). Second, a random forest model was built using 500 bootstrapped samples. The random forest model utilizes bootstrap aggregate sampling methods (bagging) to decrease variance in the model (14). This model uses what is known as the Gini index to measure the importance of each independent variable in the estimation of the dependent variable.
All models were constructed using the entire patient cohort. The MSE for each of the models was obtained as a measurement of the overall accuracy of the model in predicting the anti-Xa value based on UFH dosing, AT activity, and HAP value. All figures, data manipulation, data models, and statistical tests were completed using R 4.0.3 (Vienna, Austria). (15).
RESULTS
A total of 132 ECMO runs encompassing 128 patients were included in this study. Table 1 depicts the patient characteristics and demographics of the study cohort. The majority of patients were infants and neonates. The most common diagnostic category was cardiac.
Table 1.
Patient demographics.
| Total patients | 128 |
| Gender | |
| Male | 71 (55%) |
| Female | 57 (45%) |
| Race | |
| American Indian | 1 (1%) |
| Asian | 3 (2%) |
| White | 99 (77%) |
| Black or African American | 25 (20%) |
| Ethnicity | |
| Hispanic or Latino | 9 (7%) |
| Non-Hispanic or Latino | 119 (93%) |
| Total ECMO runs | 132 |
| Age category | |
| Neonate | 53 (40%) |
| Infant | 46 (35%) |
| Child | 33 (25%) |
| Diagnostic category | |
| Cardiac | 76 (58%) |
| Pediatric respiratory | 29 (22%) |
| Neonatal respiratory | 27 (20%) |
| ECMO modality | |
| VA | 111 (84%) |
| VV | 19 (14%) |
| VV convert to VA | 1 (1%) |
| VA convert to VV | 1 (1%) |
ECMO, extracorporeal membrane oxygenation; VA, venoarterial; VV, venovenous.
Figure 1 displays the single-variable linear regression correlation values between each independent variable and anti-Xa value. For the entire cohort, each of the independent variables has a positive correlation with anti-Xa, with the HAP value having the highest correlation (r = .511). While the HAP value is positively correlated with the anti-Xa value in each of the age categories, the correlation is highest in the neonate category (r = .702). This correlation is again higher than the correlation for both UFH dose and AT activity. The study population was also stratified by ECMO modality into a veno-venous (VV) cohort and a venoarterial (VA) cohort. The VA cohort demonstrated the same pattern with the HAP value being the most strongly correlated with anti-Xa level compared to heparin dose and AT level. The VV cohort had only 19 patients, and results showed poor correlation between anti-Xa level and heparin, AT, and HAP value. Correlation between the ACT level, anti-Xa value, heparin dosing, AT level, and HAP value was poor, with the highest correlation between ACT level and anti-Xa level at .37.
Figure 1.
Bar chart showing correlation values by lab and age category.
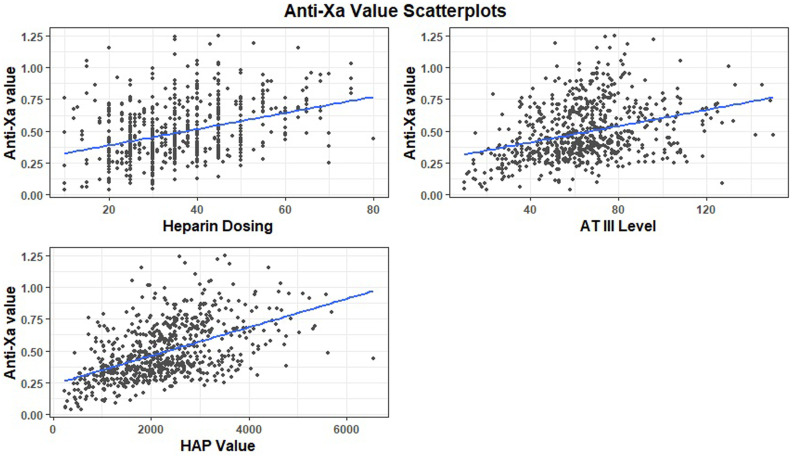
Figure 2 depicts the scatterplots for each independent variable with the anti-Xa level. The single-variable linear regression models showed a positive relationship between each independent variable and the anti-Xa value. The slope was the most positive for the HAP value, as seen in the figure. The multivariable linear regression model did not show a strong predictive value when applied to the entire cohort, with an adjusted R-squared value of .28. This model showed statistical significance of the UFH dose and AT activity, but not the HAP. When the multivariable linear regression model was applied to the neonatal cohort, the adjusted R-squared value increased to .5446, and each of the independent variables was statistically significant with p-values of less than .05, including the HAP.
Figure 2.
Scatterplot of anti-Xa value by heparin dosing with regression line (top left). Scatterplot of anti-Xa value by antithrombin III (AT) level with regression line (top right). Scatterplot of anti-Xa value by heparin-antithrombin product (HAP) value with regression line (bottom).
As the goal of the study was to determine the effectiveness of the HAP value on predicting anti-Xa levels in the general population, we sought to determine whether there was a non-linear relationship between the independent variables (UFH dose, AT activity, and HAP) and the dependent variable (anti-Xa). Both the smoothing spline and generalize additive model (GAM) were created to attempt to model the relationship in a non-linear manner. Upon review of the model shape and MSE values, neither model was definitively superior to the linear regression model, and in some respects were inferior to it. Both models showed high model variability, with very minor improvement in MSE compared to the linear regression model. Thus, we can conclude that it is reasonable to assume a linear relationship between the independent variables and the Anti-Xa level.
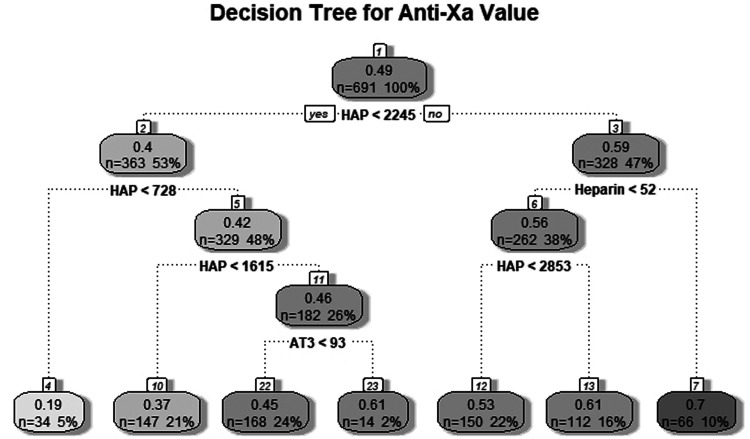
Tree-based models are a class of data models useful for both causal inference as well as prediction. These models do not rely on the same assumptions required for linear regression models. As our data are repeated laboratory samples from the same cohort of patients, it does not meet the assumption of independence. For this reason, two tree-based models were created to further evaluate the utility of the HAP value. Figure 3 illustrates the decision tree for a simple regression tree model. Each branch point indicates an independent variable value split point, and the end of each branch indicates a terminal node predicting the anti-Xa value. Each of the independent variables (UFH dose, AT activity, and HAP value) was included as a significant predictor in the model.
Figure 3.
Regression tree diagram for predicting anti-Xa value.
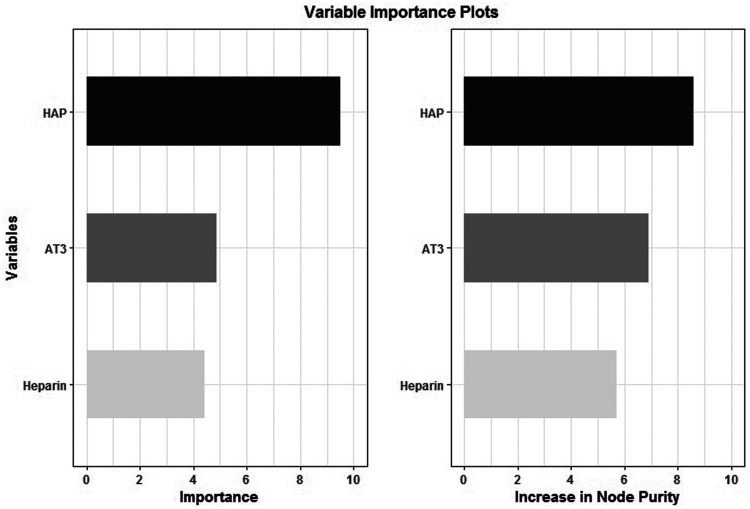
Figure 4 illustrates the variable importance value assigned to each independent variable. While each predictor was given a high importance value, the HAP value was ranked highest at 9.52 in the regression tree model. A random forest model was also created with anti-Xa value as the dependent variable and UFH dose, AT activity, and HAP values as the independent variables. This model utilizes a measure of model impurity called the Gini index to measure the importance of specific predictor variables in the model. A perfect random forest model would have no impurity in the decision nodes, which would equate to a Gini index value of 0. A completely imperfect random forest model with maximum impurity in the decision nodes would equate to a Gini index value of 1. The influence of each variable to decrease impurity in the model and minimize the Gini index in the random forest model created for our study is shown in Figure 4. Each independent variable is given a reasonably high influence on decreasing node impurity, with the HAP value again receiving the highest at 8.6. Both tree models conclude that the HAP value is the most influential independent variable in predicting the anti-Xa value.
Figure 4.
Variable importance values for the simple regression tree (left), and amount of increase in node purity for each variable in the random forest model (right).
The MSE of each model was measured to determine which was most accurate in predicting a patient’s anti-Xa level based on the morning values of heparin infusion dose, AT level, and HAP value. The linear regression model had the highest MSE at .0348, whereas the random forest model had the lowest MSE at .0333.
DISCUSSION
In this retrospective cohort study of 128 pediatric patients encompassing 132 ECMO runs, we introduced the HAP as a novel measurement in the field of ECMO anticoagulation. The HAP value consistently proved to have a stronger influence in predicting patients’ anti-Xa value when compared to the dose of UFH or AT activity individually.
Several studies over the past decade have sought to determine a correlation between the ACT and anti-Xa values during pediatric ECMO, but the relationship between ACT and anti-Xa has remained poor (16–18). Similarly, the correlation between UFH dose and ACT values has been demonstrated in multiple pediatric ECMO studies to be a weak association (7,19,20). Conversely, in 2019, Padhya et al. conducted a systematic review and meta-analysis of 19 studies containing 759 pediatric patients supported with ECMO (5). Among ACT, aPTT, anti-Xa, and TEG-R values, anti-Xa was the only laboratory test shown to have a strong correlation with UFH dosing, with a correlation coefficient of .61. A closer look at the laboratory assays themselves can help to elucidate these findings. The ACT measures the time to fibrin formation after the addition of a clot-activating catalyst such as kaolin or celite. However, as a whole blood sample, many factors influence the ACT value beyond the effect of UFH, including patient temperature, platelet number and function, clotting factors, and hemodilution (21). Conversely, the anti-Xa assay, as a plasma test, reflects the ability of the heparin-AT complex to inhibit factor X (6). Given this specific focus on heparin-AT activity, it is logical for the anti-Xa assay to have better association with heparin dose.
At the conclusion of their systematic review, Padhya et al. concluded that the anti-Xa assay appears to be the most appropriate test for monitoring UFH when used for ECMO anticoagulation. If the practice of pediatric ECMO continues to shift broadly toward an anti-Xa-based strategy for the management of anticoagulation, then it is essential for clinicians to recognize both components that contribute to the anti-Xa value, i.e., UFH and its necessary substrate, AT. This was the rationale for our creation of the HAP. And indeed, in our study, the HAP proved to have a superior correlation to anti-Xa values than did either UFH dose or AT activity, thus supporting the concept that it is the heparin-AT complex that is driving ECMO anticoagulation, more so than either component in isolation. Of note, some laboratories will add exogenous AT to the patient’s plasma sample (22). If this is the case, the anti-Xa value becomes more of a sole reflection of the effect of only heparin in the patient. Omitting exogenous AT to the assay allows the anti-Xa value to reflect the effect of heparin working in conjunction with the patient’s own endogenous AT activity. This latter scenario is clinically preferable, as clinicians have the decision of both titrating heparin dose as well as supplementing AT when its activity is low. Lastly, as a chromogenic assay, the anti-Xa laboratory test may provide falsely low anti-Xa values in the presence of substances that interfere with the analyzer. Free hemoglobin as a result of hemolysis as well as elevated unconjugated bilirubin are two such substances; close monitoring of these values is therefore warranted (23).
In our study, the correlation between HAP and anti-Xa values was strongest in the neonatal ECMO subgroup. Biologically the neonate has unique hematologic considerations, including reduced levels of several coagulation proteins compared to older children and adults (24); but at the same time, neonatal patients also have lower levels of endogenous anticoagulant factors such as protein C and protein S (25). Collectively, these hematologic differences lead to unique markers of coagulation and fibrinolysis when neonates are supported with ECMO (26). From a clinical standpoint, neonatal patients demonstrate several characteristics pertinent to ECMO anticoagulation, including a larger volume of distribution of UFH (27) and lower levels of AT activity (9). These two considerations may make the neonatal ECMO patient more sensitive to both titrations of UFH infusions and supplementation with AT, thus explaining the higher correlation of the HAP value to anti-Xa in our neonatal subgroup.
Several statistical models were created in our study to examine the relationship between the HAP value and the anti-Xa level. These models are useful both for causal inference in determining which specific factors are most influential in predicting a patient’s anti-Xa level, as well as for the overall prediction of anti-Xa level. Causal inference is how researchers can statistically determine which factors have a significant influence on the outcome variable of interest (28). This study sought to determine which of three factors was the most influential in determining a patient’s anti-Xa level. Linear regression is one of the most powerful tools for demonstrating causal inference. Although the smoothing spline and generalized additive models had superior MSE values compared to the multivariable linear regression model, they are both less useful for causal inference and are prone to specific deficiencies. The smoothing spline followed the middle of the data well but lost accuracy at the data tail. The generalized additive model followed the overall shape of the data well with a low MSE approaching that of the random forest model; however, it expressed high variability in the middle of the data, which makes it less useful in predictions for future data sets. Thus, even though the linear regression model had a higher MSE, it is a more useful tool for causal inference, as it is less prone to these issues and has only a slightly higher MSE. Using this method, the HAP value was more highly correlated with a patient’s anti-Xa value than either of the other predictor variables in the total population. This was also seen in the younger patient categories, with particularly high correlation in the neonatal population. The tree-based methods, which do not rely on the same statistical assumptions as the linear regression model does, also determined the importance of the HAP value being higher in predicting the anti-Xa level than either of the other predictor variables. This is of particular interest for ECMO clinicians, as the use of this novel value could be used to predict bleeding risk and/or clotting risk for ECMO patients in the future. While the multivariable linear regression model did not show statistical significance of the HAP value in the entire cohort, this may have several explanations. It is possible that the HAP value is more influential in the neonatal age group, thus explaining the lack of significance in the entire cohort. It is also possible that a larger cohort with more data observations is needed to show the statistical significance of the HAP value in a general population of pediatric patients. The high correlation of the HAP with the anti-Xa value in all age groups, as well as the variable importance metrics of the tree-based models, all give merit to the HAP value’s worth in measuring ECMO anticoagulation.
This study has several limitations. Most observations in the data are repeated samples from the same patient cohort. There are likely specific patient factors that bias the test results for these patients that are not accounted for in our study. Conversely, all patients were managed under an institutional protocol, thus minimizing the variation in provider practice. Despite the relatively high correlation between the HAP value and a patient’s anti-Xa level seen in the overall study population compared to the other predictor variables, this relationship was significantly higher in the neonatal population than in infants and children. It is possible that this relationship is not as accurate in older patients and therefore not generalizable to all pediatric patients. The VV-ECMO subgroup also demonstrated lower correlation between all independent variables and anti-Xa level. This is likely because the correlation, even in the neonatal population, was only .7. Thus, a larger sample size is likely needed to identify the significant relationship between the variables, and the VV-ECMO subgroup in this study was not large enough to accomplish this. This study is a retrospective observational study from a single center, and is thus prone to institutional bias, and can only be used to recognize associations. To validate the relationships observed in this study as well as the statistical models’ validity, these methods would need to be applied to a larger data set of ECMO laboratory values from other pediatric centers.
CONCLUSION
To our knowledge, this is the first study evaluating the concept of the HAP as a marker for the activity of the heparin-AT complex in the setting of pediatric ECMO. The HAP value shows a superior correlation to anti-Xa values on ECMO when compared to UFH dose and AT activity alone. Thus, the HAP may serve as a valuable marker for titration of anticoagulation during pediatric ECMO. Further work is needed to validate the HAP across larger data sets, as well as to explore the utility of HAP in predicting hemorrhagic and thrombotic ECMO complications.
REFERENCES
- 1.Dalton HJ, Garcia-Filion P, Holubkov R, et al. Association of bleeding and thrombosis with outcome in extracorporeal life support. Pediatr Crit Care Med. 2015;16:167–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bembea MM, Annich G, Rycus P, et al. Variability in anticoagulation management of patients on extracorporeal membrane oxygenation: An international survey. Pediatr Crit Care Med. 2013;14:e77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hirsh J, Anand SS, Halperin JL, et al. Mechanism of action and pharmacology of unfractionated heparin. Arterioscler Thromb Vasc Biol. 2001;21:1094–6. [DOI] [PubMed] [Google Scholar]
- 4.Extracorporeal Life Support Organization . ELSO Anticoagulation Guidelines. 2014. [cited 2021 June 28]. Available at: https://www.elso.org/Portals/0/Files/elsoanticoagulationguideline8-2014-table-contents.pdf. Accessed June 12, 2021.
- 5.Padhya DR, Prutsky GJ, Nemergut ME, et al. Routine laboratory measures of heparin anticoagulation for children on extracorporeal membrane oxygenation: Systematic review and meta-analysis. Thromb Res. 2019;179:132–9. [DOI] [PubMed] [Google Scholar]
- 6.Newall F. Anti-factor Xa (anti-Xa) assay. Methods Mol Biol. 2013;992:265–72. [DOI] [PubMed] [Google Scholar]
- 7.Baird CW, Zurakowski D, Robinson B, et al. Anticoagulation and pediatric extracorporeal membrane oxygenation: Impact of activated clotting time and heparin dose on survival. Ann Thorac Surg. 2007;83:912–9, discussion 9–20. [DOI] [PubMed] [Google Scholar]
- 8.Perry R, Stein J, Young G, et al. Antithrombin III administration in neonates with congenital diaphragmatic hernia during the first three days of extracorporeal membrane oxygenation. J Pediatr Surg. 2013;48:1837–42. [DOI] [PubMed] [Google Scholar]
- 9.Niebler RA, Christensen M, Berens R, et al. Antithrombin replacement during extracorporeal membrane oxygenation. Artif Organs. 2011;35:1024–8. [DOI] [PubMed] [Google Scholar]
- 10.Wong TE, Nguyen T, Shah SS, et al. Antithrombin concentrate use in pediatric extracorporeal membrane oxygenation: A multicenter cohort study. Pediatr Crit Care Med. 2016;17:1170–8. [DOI] [PubMed] [Google Scholar]
- 11.Cleophas TJ. Clinical trials: Spline modeling is wonderful for nonlinear effects. Am J Ther. 2016;23:e844–9. [DOI] [PubMed] [Google Scholar]
- 12.McLean MW, Hooker G, Staicu AM, et al. Functional feneralized additive models. J Comput Graph Stat. 2014;23:249–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Richardson B, Fuller-Tyszkiewicz M, O’Donnell R, et al. Regression tree analysis of ecological momentary assessment data. Health Psychol Rev. 2017;11:235–41. [DOI] [PubMed] [Google Scholar]
- 14.Rigatti SJ. Random Forest. J Insur Med. 2017;47:31–9. [DOI] [PubMed] [Google Scholar]
- 15.R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria, R Foundation for Statistical Computing. 2022. Available at: https://www.R-project.org.
- 16.Nankervis CA, Preston TJ, Dysart KC, et al. Assessing heparin dosing in neonates on venoarterial extracorporeal membrane oxygenation. ASAIO J. 2007;53:111–4. [DOI] [PubMed] [Google Scholar]
- 17.Bembea MM, Schwartz JM, Shah N, et al. Anticoagulation monitoring during pediatric extracorporeal membrane oxygenation. ASAIO J. 2013;59:63–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deshpande SJ, Vitali S, Thiagarajan R, et al. Coagulations studies do not correlate with each other or with hematologic complications during pediatric extracorporeal membrane oxygenation. Pediatr Crit Care Med. 2021;22:542–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liveris A, Bello RA, Friedmann P, et al. Anti-factor Xa assay is a superior correlate of heparin dose than activated partial thromboplastin time or activated clotting time in pediatric extracorporeal membrane oxygenation. Pediat Crit Care Med. 2014;15:e72–9. [DOI] [PubMed] [Google Scholar]
- 20.McMichael ABV, Hornik CP, Hupp SR, et al. Correlation among antifactor Xa, activated partial thromboplastin time, and heparin dose and association with pediatric extracorporeal membrane oxygenation complications. ASAIO J. 2020;66:307–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saini A, Spinella PC. Management of anticoagulation and hemostasis for pediatric extracorporeal membrane oxygenation. Clin Lab Med. 2014;34:655–73. [DOI] [PubMed] [Google Scholar]
- 22.Winter WE, Flax SD, Harris NS. Coagulation testing in the core laboratory. Lab Med. 2017;48:295–313. [DOI] [PubMed] [Google Scholar]
- 23.Khan J, Chandler WL. Interference in the anti-Xa heparin activity assay due to hemolysis and icterus during pediatric extracorporeal life support. Artif Organs. 2019;43:880–7. [DOI] [PubMed] [Google Scholar]
- 24.Monagle P, Barnes C, Ignjatovic V, et al. Developmental haemostasis. Impact for clinical haemostasis laboratories. Thromb Haemost. 2006;95:362–72. [DOI] [PubMed] [Google Scholar]
- 25.Favaloro EJ, Lippi G. Translational aspects of developmental hemostasis: Infants and children are not miniature adults and even adults may be different. Ann Transl Med. 2017;5:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hundalani SG, Nguyen KT, Soundar E, et al. Age-based difference in activation markers of coagulation and fibrinolysis in extracorporeal membrane oxygenation. Pediatr Crit Care Med. 2014;15:e198–205. [DOI] [PubMed] [Google Scholar]
- 27.Vieira A, Berry L, Ofosu F, et al. Heparin sensitivity and resistance in the neonate: An explanation. Thromb Res. 1991;63:85–98. [DOI] [PubMed] [Google Scholar]
- 28.Stovitz SD, Shrier I. Causal inference for clinicians. BMJ Evid Based Med. 2019;24:109–12. [DOI] [PubMed] [Google Scholar]