Abstract:
Small increases in serum creatinine postoperatively reflect an acute kidney injury (AKI) that likely occurred during cardiopulmonary bypass (CPB). Maintaining adequate oxygen delivery (DO2) during CPB, known as GDP (goal-directed perfusion), improves outcomes. Whether GDP improves outcomes of patients at high risk for acute renal failure (ARF) is unknown. Forty-seven adult patients undergoing cardiac surgery with CPB utilizing GDP with Cleveland Clinic Acute Renal Failure Score of 3 or greater were compared with a matched cohort of patients operated upon using a flow-directed strategy. CPB flow in the GDP cohort was based on a DO2 goal of 260 mL/min/m2. Serum creatinine values were used to determine whether postoperative AKI occurred according to AKIN (Acute Kidney Injury Network) guidelines. We examined the distribution of all variables using proportions for categorical variables and means (standard deviations) for continuous variables and compared treatment groups using t tests for categorical variables and tests for differences in distributions for continuous and count variables. We used inverse probability of treatment weighting to adjust for treatment selection bias. In adjusted models, GDP was not associated with a decrease in AKI (odds ratio [OR]: .97; confidence interval [CI]: .62, 1.52), but was associated with higher odds of ARF (OR: 3.13; CI: 1.26, 7.79), mortality (OR: 3.35; CI: 1.14, 9.89), intensive care unit readmission (OR: 2.59; CI: 1.31, 5.15), need for intraoperative red blood cell transfusion (OR: 2.02; CI: 1.26, 3.25), and postoperative platelet transfusion (OR: 1.78; CI: 1.05, 3.01) when compared with the historic cohort. In patients who are at high risk for postoperative renal failure, GDP was not associated with a decrease in AKI when compared to the historical cohort managed traditionally by determining CPB flows based on body surface area. Surprisingly, the GDP cohort performed significantly worse than the retrospective control group in terms of ARF, mortality, intensive care unit readmission, and RBC and platelet transfusions.
Keywords: CPB, physiology, pathophysiology, kidney, perioperative care.
Acute kidney injury (AKI) after cardiac surgery is associated with poor short- and long-term outcomes and is a signal for adverse outcomes (1–6). Small increases (.3 mg/dL) in serum creatinine (SCr) postoperatively reflect a kidney injury that most likely occurred in the operating room during cardiopulmonary bypass (CPB). This delayed signal provides an opportunity to scrutinize intraoperative processes of care and determine strategies to decrease its incidence. One of the possible sources of the renal injury is poor oxygen delivery during CPB. The renal medulla is a reliable hypoxemic signal for this research purpose and is vulnerable to small shifts of oxygen delivery (DO2) that can result in organ dysfunction and cell death. Small changes in SCr can provide a surrogate marker for hypoxemia and inadequate organ perfusion.
Maintaining DO2 levels above a recommended level during CPB improves physiological and clinical outcomes (7–9). This strategy is described as goal-directed perfusion (GDP) (10). DO2 is measured in real time during CPB, using a software calculation (Sorin Connect® Sorin Group Italia Srl, LivaNova PLC Via Statale 12 Nord, 86–41037 Mirandola (MO) Italy), the variables being flow, oxygenation, and hemoglobin concentration. The resulting value drives strategies (Figure 1) to expeditiously improve DO2. When these strategies to improve DO2 are exhausted, low DO2 values guide the administration of red blood cells (RBC).
Figure 1.
Oxygen delivery strategies.
Using GDP during cardiac surgery is not a novel concept. Ranucci introduced GDP in 2005 and 2007 (10,11). deSomer and Ranucci defined GDP management in 2011 with the goal being oxygen delivery, a departure from traditional CPB management that bases CPB flows on body surface area (BSA) (12). In 2018, Ranucci et al. published the GIFT trial (The Goal dIrected perFusion Trial in Cardiac Surgery) in which patients were randomized to GDP vs. traditional, flow-directed, perfusion. They concluded that patients in the GDP arm were significantly less likely to suffer from AKIN (Acute Kidney Injury Network) stage 1 AKI (7). This study generated debate over the role of GDP in the prevention of AKI in those undergoing cardiac surgery. One criticism of the GIFT trial is the exclusion of patients with severe chronic renal failure (defined as need for dialysis or serum Cr > 3.0 mg/dL) (7). While the previously described studies showed the advantage of using GDP in the general population of cardiac surgery patients undergoing CPB, whether GDP plays a role in preventing AKI in patients at high risk for acute renal failure (ARF) is not known. Our intention herein was to see whether GDP had an effect on this subset of patients who, according to a known prediction model, have a combination of comorbidities putting them at high risk for postoperative ARF.
MATERIALS AND METHODS
Patient Population
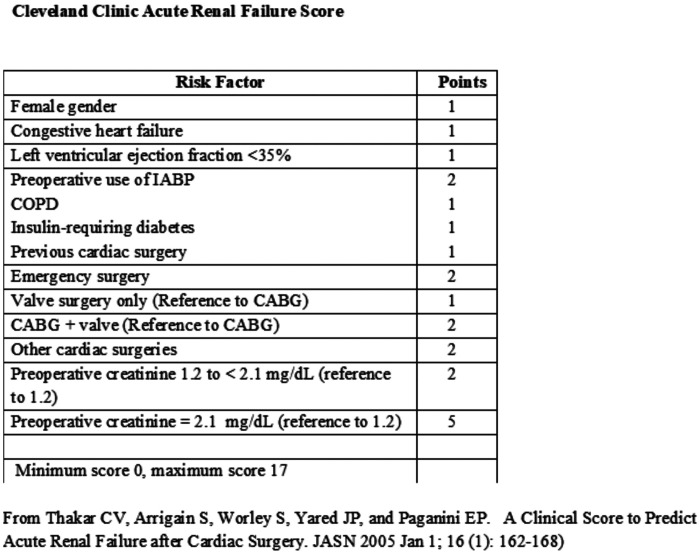
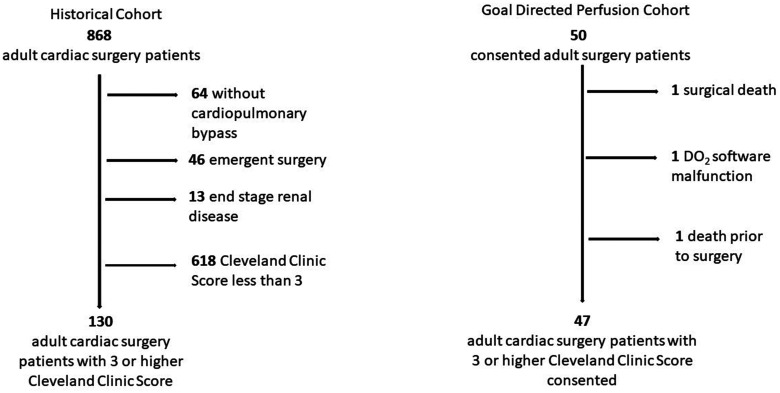
With approval from the Maine Medical Center Institutional Review Board (# 958,918), 50 subjects were consented and enrolled to have cardiac surgery with CPB utilizing the GDP strategy. Included were adults (>18 years) who were to undergo cardiac operations with CPB and had a Cleveland Clinic Acute Renal Failure Score of 3 or greater (6) (Figure 2). The Cleveland Clinic Score predicts ARF requiring dialysis during the postoperative period. Using the scoring system developed by Thaker et al. (Figure 2) (6), we sought to determine whether GDP could reduce AKI in patients who are predicted to be at high risk for postoperative ARF requiring dialysis. We focused on the subset of patients predicted to have an increased risk of developing ARF, while excluding low risk patients and patients with end stage renal disease preoperatively. One patient died in the operating room, one patient was withdrawn due to malfunction of DO2 software and one died preoperatively, leaving 47 patients for the analysis. As our control cohort, we chose a retrospective group as GDP was being used in all contemporaneous cases (Figure 3). The retrospective group had their surgery performed before we had the ability to determine DO2 in real time. The patients in this cohort experienced traditional, flow-directed perfusion, giving us a comparator group that did not have a DO2 driven strategy to one that did, using appropriate software to determine DO2 in real time and respond appropriately.
Figure 2.
Cleveland clinic acute renal failure score.
Figure 3.
The consolidated standards of reporting trials flow diagram. Historical Cohort: a total of 868 patients were identified in the institutional database who had undergone cardiac surgery. After exclusion of subjects per specified criteria 130 patients remained for analysis. Goal Directed Perfusion Cohort: a total of 50 patients were consented. One patient died prior to surgery, one died during surgery, and one patient was withdrawn due to DO2 software malfunction leaving 47 patients for analysis.
Data Collection and Definitions
In this analysis, we used the SCr values to determine whether postoperative AKI occurred according to AKIN guidelines (13). Guidelines such as AKIN, RIFLE (Risk, Injury, Failure, Loss of kidney function, and End-stage kidney disease), and KDIGO (Kidney Disease Improving Global Outcomes) are criteria that continue to evolve in defining AKI in patients undergoing cardiac surgery. For Stage 1 AKI, they all have in common a small SCr increase (.3 mg/dL) within the 48-hour postoperative period over baseline (the last recorded SCr) before cardiac surgery. Future guidelines will be even more sensitive to renal injury, measuring biomarkers that detect the presence of the urinary biomarkers tissue inhibitor metalloproteinases 2 (TIMP-2) and insulin-like growth factor–binding protein 7 (IGFBP7) (14,15).
In this analysis, the perfusionists calculated and recorded DO2 values while reacting to them in real time. The Cleveland Clinic scoring system used ARF requiring dialysis in its prediction model was used to screen patients for inclusion. For the outcome of ARF in this analysis, we used the wider definition in the Society of Thoracic Surgeons (STS) database (STS SCA Data Specifications v4.20.2, pages 415–416) as follows: Acute renal failure or worsening renal function at any time postoperatively resulting in one or both of the following: (1) Increase in SCr level ×3.0, or SCr > mg/dL 4.0 with at least a .5 mg/dL rise, or (2) A new requirement for dialysis postoperatively.
Goal Directed Perfusion Management
Studies have demonstrated that DO2 during CPB affects renal outcomes in cardiac surgery (1,16–18). To our knowledge, this study is the first to focus on subjects predicted to be at a high risk for postoperative ARF using real-time DO2 data. Ranucci et al. excluded patients with severe renal failure defined as requiring hemodialysis or having a SCr level of >3.0 mg/dL (10) as did our study that also excluded any other patient with an estimated glomerular filtration rate < 30 mL/min. The GDP strategy was observed by trained research personnel in the operating room for every case with GDP CPB flow based on DO2 goal of 260 mL/min/m2 or greater as described by de Somer et al. (12). The retrospective comparison cohort consisted of 130 patients using a traditional perfusion strategy with flows based on BSA. Both groups were at high risk for ARF with a Cleveland Clinic Score ≥3 (6) (Figure 2). The reason for using a retrospective, non-contemporaneous cohort as the comparator was to find a group of patients managed in the traditional fashion (flow rate based on BSA) before we acquired the Sorin Connect® monitors. This retrospective comparison cohort’s flow was maintained between a cardiac index of 2.0 and 2.6, with an initial target index of 2.4 based on the patient’s BSA during periods of normothermia and mild hypothermia. During the time of this analysis, all of our CPB machines were equipped with Sorin Connect® monitors. Centrifugal Sorin Revolution® pumps were used on all patients and flow was measured using a 3/8-inch Transonic flow probe (Transonic Systems, Inc, Ithaca, NY) placed distal to all shunts in the CPB circuit. Traditional 1:4 del Nido solution or whole blood microplegia were used on these patients and delivered through the Quest MPS2 System (Quest Medical, Inc, Allen, TX). The choice of which type of cardioplegia used was both surgeon and case dependent. Hemoconcentration was only used if needed as an additional oxygen delivery strategy as stated in Figure 1. This was determined either preoperatively by way of our internal Patient Care Plan or during any event while on CPB necessitating the need for removal of volume.
Statistical Analysis
We examined the distribution of all variables using proportions for categorical variables and means (standard deviations) for continuous variables (Table 1). We compared treatment groups using t tests for categorical variables and tests for differences in distributions (Mann–Whitney U test) for continuous and count variables. We used inverse probability of treatment weighting (IPTW) to adjust for treatment selection bias in this study. The IPTW uses a propensity score, a subject’s probability of treatment selection conditioned on observed baseline covariates. IPTW is used to assess associations of outcomes with treatments when the researcher cannot conduct a controlled (randomized) experiment. Weighting subjects by the IPTW received creates a synthetic sample in which treatment assignment is independent of measured baseline covariates. Propensity scores were calculated using a logistic regression model, with treatment assignment as the dependent variable and all patient characteristics that differed between treatment groups with a p-value of .2 or less as covariates.
Table 1.
Demographic, preoperative, and procedure comparisons.
| Characteristic | Raw data | p Value | Weighted data | p Value | ||
|---|---|---|---|---|---|---|
| Control (n = 130) | GDP (n = 47) | Control (n = 130) | GDP (n = 47) | |||
| Age years, mean (SD) | 68 (11) | 68 (11) | .86 | 68 (13) | 69 (18) | .36 |
| Gender, n (%) female | 46 (35%) | 23 (49%) | .14 | 39% | 38% | .93 |
| Body mass index, mean (SD) | 29 (5.7) | 28 (6.1) | .14 | 29 (7) | 29 (12) | .83 |
| Body surface area, mean (SD) | 2.0 (.26) | 1.9 (.26) | .08 | 2.0 (.30) | 2.0 (.47) | .73 |
| Diabetic, n (%) | 52 (40%) | 19 (40%) | 1.00 | 42% | 45% | .69 |
| Congestive heart failure, n (%) | 86 (66%) | 32 (68%) | .95 | 67% | 69% | .87 |
| Preoperative hematocrit, mean (SD) | 38 (5.3) | 39 (5.7) | .66 | 38 (6.3) | 39 (11.8) | .19 |
| Preoperative hemoglobin, mean (SD) | 13 (1.9) | 13 (2.0) | .79 | 13 (2.3) | 13 (4.1) | .47 |
| Preoperative platelets (thou/uL), mean (SD) | 216 (63) | 228 (130) | .57 | 218 (74) | 221 (245) | .85 |
| Previous cardiac surgery, n (%) | 19 (15%) | 5 (11%) | .66 | 14% | 11% | .66 |
| Cleveland clinic score, mean (SD) | 4.1 (1.1) | 4.0 (1.1) | .62 | 4.1 (1.3) | 4.1 (2.2) | .94 |
| Intra-aortic balloon pump, n (%) | 13 (10%) | 13 (28%) | .007 | 14% | 15% | .82 |
| Surgery urgency level | .02 | .04 | ||||
| Elective, n (%) | 61 (47%) | 12 (26%) | 41% | 30% | ||
| Urgent, n (%) | 69 (53%) | 35 (74%) | 59% | 70% | ||
| Procedure | .003 | .51 | ||||
| Isolated CABG, n (%) | 26 (20%) | 12 (26%) | 22% | 27% | ||
| Isolated valve, n (%) | 10 (8%) | 7 (15%) | 10% | 10% | ||
| CABG + Valve, n (%) | 33 (25%) | 20 (43%) | 29% | 31% | ||
| Other, n (%) | 61 (47%) | 8 (17%) | 40% | 32% | ||
| Cardiopulmonary bypass time, min, mean (SD) | 150 (64) | 141 (57) | .41 | 149 (77) | 151 (108) | .91 |
| Preoperative creatinine, mean (SD) | 1.1 (.42) | 1.1 (.38) | .28 | 1.1 (.48) | 1.1 (.72) | .80 |
CABG, coronary artery bypass grafting; GDP, goal-directed perfusion; SD, standard deviation.
49% of Control patients and 34% of GDP patients were unable to be scored.
We did not include STS risk scores in the propensity score model because these variables do not exist (no prediction model because of heterogeneity) for any patient who had a surgery that was neither coronary artery bypass grafting (CABG) nor valve nor a combined CABG/valve procedure, therefore, not missing at random and not amenable to imputation. We then used IPTW derived from the propensity score model in adjusted analyses. We used logistic regression models for categorical outcomes, negative binomial models for count models, and quantile regression comparing medians for continuous outcomes, all using the IPTW. Logistic regression models resulted in odds ratios (OR) and 95% confidence intervals (CIs) comparing GDP with control; negative binomial models resulted in rate ratios with 95%CIs; quantile regression resulted indifferences in medians and 95% CIs. Analyses were performed using SPSS version 20 (IBM Corp, Armonk, NY) and SAS EG version 7.1(SAS Institute, Cary, NC). We tested the performance of Poisson, negative binomial, and ordinal logistic models for the number of transfusions. We did not fit zero-inflated Poisson or negative binomial data because we believe that there is no justification for assuming structural zeros in transfusion data (i.e., there is no cardiac surgery patient who is not at risk of transfusion). There was a clear evidence of over dispersion in the Poisson models. Using the log likelihood and Akaike Information Center criteria, the negative binomial models fit the data best and we report rate ratio estimates from these models. When outcomes were binary (i.e., the only two values for the number of transfusions were either 0 or 1), we used logistic regression models to estimate ORs.
RESULTS
We analyzed 47 cardiac surgery patients fitting the inclusion criteria with the intent to compare with a matched cohort of patients operated upon in a previous era. The GDP cohort had a median DO2 of 287 mL/min/m2 with a median time of 8 minutes under the DO2 target of 260 mL/min/m2. Looking at the raw data the two groups were well balanced with regard to age, sex, body mass index (BMI), BSA, diabetes, congestive heart failure (CHF), preoperative hematocrit (Hct), hemoglobin (Hgb), and platelet count, previous cardiac surgeries, Cleveland clinic score, CPB time, and preoperative creatinine. However, significant baseline differences existed with the control group having fewer urgent patients. The control group had 47% elective patients and 53% urgent patients, while the GDP group had 26% elective and 74% urgent (p = .01). While the difference is significant statistically, it is not clinically meaningful as the difference in the urgent vs. nonurgent is to some degree administrative as those designated as urgent are referred while hospitalized and those designated as nonurgent, those met in the practice office. Emergent cases were excluded. The control group had less complex cases in that control had 20.0% isolated CABG, 8% isolated valve, 25% CABG + valve, and 47% “other” while the GDP group had 26% isolated CABG, 15% isolated valve, 43% CABG + valve, and 17% “other” (p = .003) (Table 1). With weighting the procedure undergone between the two groups became no longer statistically different p = .51, the urgency of surgery stayed significant with a p = .04.
In adjusted models, GDP was associated with higher odds of ARF (OR:3.13; CI:1.26,7.79), mortality (OR: 3.35; CI:1.14,9.89), intensive care unit readmission (OR: 2.59; CI: 1.31,5.15), need of intraoperative RBC transfusion (OR:2.02; CI:1.26,3.25), and postoperative platelet transfusion (OR: 1.78; CI: 1.05,3.01) when compared with the historic cohort (Table 2). Intraoperative transfusions in the control group were at the discretion of the team and in the GDP group, driven by DO2. In the GDP group, median Hct was 22 and for the control group the median Hct 19. No significant difference was observed between either groups regarding AKI (OR: .97; CI: .62, 1.52), or need for Extracorporeal Membrane Oxygenator (ECMO) (OR: 3.53; CI: .64, 19.60). In addition, no significant difference was observed in need for intraoperative platelets (OR: 1.27; CI: .78, 2.07), intraoperative fresh frozen plasma (OR: 1.51; CI: .90, 2.54), postoperative of RBC transfusion (OR: 1.50; CI: .97, 2.30), and postoperative fresh frozen plasma (OR 1.04; CI: .58, 1.86) (Table 2).
Table 2.
Effect of GDP on cardiac surgery outcomes (odds ratios compared to control condition, 95% confidence intervals).
| Outcome | Crude GDP effect | IPTW GDP effect* |
|---|---|---|
| Acute kidney injury† | 1.01 (.50, 2.04) | .97 (.62, 1.52) |
| Acute renal failure‡ | 2.33 (.60, 9.06) | 3.13 (1.26, 7.79) |
| Mortality | 5.04 (1.16,21.99) | 3.35 (1.14, 9.89) |
| Intensive care unit readmission | 2.35 (.82, 6.73) | 2.59 (1.31, 5.15) |
| Intraoperative transfusion | ||
| Red blood cells | 1.71 (.81, 3.59) | 2.02 (1.26, 3.25) |
| Platelets | .68 (.29, 1.62) | 1.27 (.78, 2.07) |
| Fresh frozen plasma | .74 (.30, 1.83) | 1.51 (.90, 2.54) |
| Postoperative transfusion | ||
| Red blood cells | 1.39 (.70, 2.73) | 1.50 (.97, 2.30) |
| Platelets | 1.10 (.47, 2.59) | 1.78 (1.05, 3.01) |
| Fresh frozen plasma | 1.49 (.64, 3.46) | 1.04 (.58, 1.86) |
GDP, goal-directed perfusion; IPTW, inverse probability of treatment weighted.
*IPTW, adjusted for urgency of procedure due to imbalance after weighting.
†Acute kidney injury, an increase in serum creatinine by ≥.3 mg/dL within 48 hours of end of surgery.
‡Acute renal failure, (1) increase in serum creatinine level X 3.0, or serum creatinine >4.0 mg/dL with at least a .5 mg/dL rise, or (2) a new requirement for dialysis postoperatively.
DISCUSSION
In this analysis, the use of GDP in patients at high risk for ARF was not associated with a decrease in AKI when compared to the historical cohort managed traditionally with CPB flows based on BSA. In fact, the GDP cohort performed worse than the retrospective control group in terms of ARF, mortality, and intensive care unit readmission. Unlike what has been found in lower risk patients, there does not appear to be any reduction in AKI in patients at high risk of ARF.
Given the context of the current debate in the literature regarding the efficacy of GDP in the prevention of AKI in cardiac surgery patients, we find our results surprising. In 2017, Magruder et al. published an observational pilot study comparing 88 patients undergoing GDP with a threshold DO2 of >300 mL/min/m2 vs. historic controls (9). Similar to the GIFT Trial, Magruder excluded patients from the final analysis who presented with end-stage renal disease on renal replacement therapy. Also, they were careful to exclude patients who experienced a significant perioperative event that might plausibly explain AKI postoperatively (i.e., cardiac arrest, massive transfusion of over 10 units of blood, receiving nephrotoxic medications, periods of hypotension with a mean arterial pressure <60 mmHg for >15 minutes in the intensive care unit postoperatively, or with signs or symptoms of sepsis).
Magruder was able to demonstrate a significant reduction in AKI in those undergoing GDP compared with the historic cohort (9.1% vs. 23.9% p = .008) (9). In the GIFT trial Rannuci et al. in a multicenter randomized control trial analyzed the results of 326 patients, 156 of which underwent GDP with a DO2 threshold of >280 mL/min/m2 and demonstrated that those undergoing GDP were less likely to suffer AKIN stage1 AKI (7,8). Furthermore, the study was terminated early because “the efficacy endpoint at the 50% interim analysis had been met.” (7) There is concern about the statistical validity of the GIFT trial as the stopping boundary at the 50% interim analysis was set at .05 rather than .005 exposing the results to an increased possibility of type I error (2,7,9,17–19). More recently, Magruder et al. analyzed the relationship of DO2 on CPB with regard to STS outcomes. They concluded that maintaining DO2 > 280 mL/min/m2 favorably influences outcomes after cardiac surgery in addition to its known association with AKI, strengthening the argument promoting the use of GDP (20).
Randomized control trials are unusual in the context of cardiac surgery (16,17) and the results of the GIFT trial and of the Magruder group suggest that GDP merits further use and investigation. In our high-risk group of patients, we did not observe the reduction in AKI seen in the GIFT trail or in the Magruder study. Based on the results of our study, if they had excluded patients at high risk for ARF in both the GIFT and the Magruder analyses, the advantage of using GDP may have been shown to be more substantial, even though GIFT excluded patients with SCr > 3 mg/dL.
AKI has a multifactorial etiology and may be caused by many events, with CPB and oxygen delivery being potential causal factors (10). There is a wide variation of flow-based DO2 targets among institutions and clinicians worldwide (2,10–12,20,21) The real question now is whether maintaining a certain level of oxygen delivery helps high-risk patients at all. DO2 may not correlate with outcomes in this critically ill patient group as those outcomes may be driven by the presence of comorbidities that may eclipse the beneficial effects of adequate renal oxygen delivery. Total minutes of DO2 below target may not accurately represent the entire story. In addition, the comorbidities driving anemia are not modified by intraoperative transfusions (20), and stored allogeneic RBCs may satisfy the DO2 formula but may not adequately satisfy the oxygen requirements of the renal medulla.
Limitations of this study include its retrospective nature, the dependence on enrolling patients who were referred to cardiac surgery while inpatients and categorized as urgent, and the DO2 threshold of 260 mL/min/m2. Over the course of analyzing and producing this manuscript for publication, the DO2 threshold rose to 272 mL/min/m2 and then to its current level of 300 mL/min/m2. However, it should be noted that there were no emergent patients and that the median DO2 value was 287 mL/min/m2 in this study.
In future analyses, determining the area under the curve (AUC) could be a more accurate way of identifying deficiencies and AKI correlative studies. AUC is the way to measure the function of the absolute value below the target DO2 multiplied by the duration of time below the threshold. The capability to analyze AUC during CPB was not available at the time of this study. Future GDP strategies will include higher DO2 thresholds that are already in place.
Others have shown the effectiveness of GDP in reducing postoperative AKI in low-risk patients (7,9,21). The analysis of this small case series generates the hypothesis that GDP during CPB does not reduce the risk of AKI and may even increase the risk of ARF, mortality, and ICU readmission in patients at high risk for ARF. Other strategies such as oxygen extraction ratios, SVO2 management, lactate production awareness, utilization of cerebral saturations, and cardioplegia type should be further explored to decrease AKI rates in these high-risk patients.
ACKNOWLEDGMENTs
This work was supported in part by the Northern New England Clinical and Translational Research grant U54GM115516 (FLL) as well as NIH grant 1UM1HL177371-01 Subaward GC10197-00-01 (RK).
REFERENCES
- 1.Karkouti K, Wijeysundera DN, Yau TM, et al. Acute kidney injury after cardiac surgery: Focus on modifiable risk factors. Circulation. 2009;119:495–502. [DOI] [PubMed] [Google Scholar]
- 2.Santarpino G, Di Molfetta P, Melone M. Conventional or oxygen delivery-guided perfusion: Which comes first, the chicken or the egg? J Thorac Cardiovasc Surg. 2019;157:300. [DOI] [PubMed] [Google Scholar]
- 3.Mao H, Katz N, Ariyanon W, et al. Cardiac surgery-associated acute kidney injury. Cardiorenal Med. 2013;3:178–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kramer RS, Herron CR, Groom RC, et al. Acute kidney injury subsequent to cardiac surgery. J Extra Corpor Technol. 2015;47:16–28. [PMC free article] [PubMed] [Google Scholar]
- 5.Brown J, Kramer RS, Coca S, et al. Duration of acute kidney injury impacts long-term survival after cardiac surgery. Ann Thorac Surg. 2010;90:1142–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thakar CV, Arrigain S, Worley S, et al. A clinical score to predict acute renal failure after cardiac surgery. J Am Soc Nephrol. 2005;16:162–8. [DOI] [PubMed] [Google Scholar]
- 7.Ranucci M, Johnson I, Willcox T, et al. Goal-directed perfusion to reduce acute kidney injury: A randomized trial. J Thorac Cardiovasc Surg. 2018;156:1918–1927.e2. [DOI] [PubMed] [Google Scholar]
- 8.Srey R, Rance G, Shapeton AD, et al. A quick reference tool for goal-directed perfusion in cardiac surgery. J Extra Corpor Technol. 2019;51:172–4. [PMC free article] [PubMed] [Google Scholar]
- 9.Magruder JT, Crawford TC, Harness HL, et al. A pilot goal-directed perfusion initiative is associated with less acute kidney injury after cardiac surgery. J Thorac Cardiovasc Surg. 2017;153:118–125.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ranucci M, Romitti F, Isgrò G, et al. Oxygen delivery during cardiopulmonary bypass and acute renal failure after coronary operations. Ann Thorac Surg. 2005;80:2213–20. [DOI] [PubMed] [Google Scholar]
- 11.Ranucci M. Perioperative renal failure: Hypoperfusion during cardiopulmonary bypass? Semin Cardiothorac Vasc Anesth. 2007;11:265–8. [DOI] [PubMed] [Google Scholar]
- 12.de Somer F, Mulholland JW, Bryan MR, Aloisio T, Van Nooten G, Ranucci M. O2 delivery and CO2 production during cardiopulmonary bypass as determinants of acute kidney injury: Time for a goal-directed perfusion management? Crit Care. 2011;15:R192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: Report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lopes JA, Jorge S. The RIFLE and AKIN classifications for acute kidney injury: A critical and comprehensive review. Clin Kidney J. 2013;6:8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.El-Khoury JM, Hoenig MP, Jones GRD, et al. AACC guidance document on laboratory investigation of acute kidney injury. J Appl Lab Med. 2021;6:1316–37. [DOI] [PubMed] [Google Scholar]
- 16.Ranucci M. On the premature termination of the goal-directed perfusion trial. J Thorac Cardiovasc Surg. 2019;157:e277–9. [DOI] [PubMed] [Google Scholar]
- 17.Ferraris VA. Perfusion-induced acute kidney injury: Critiques that do not roll off the tongues of thoracic surgeons. J Thorac Cardiovasc Surg. 2019;157:e279–80. [DOI] [PubMed] [Google Scholar]
- 18.Ferraris VA. Perfusion-induced acute kidney injury: A litany of uncertainty and frustration. J Thorac Cardiovasc Surg. 2019;157:e279–80. [DOI] [PubMed] [Google Scholar]
- 19.Schulte PJ. Questionable interim analyses in the Goal-Directed Perfusion Trial study of goal-directed perfusion. J Thorac Cardiovasc Surg. 2019;157:e277. [DOI] [PubMed] [Google Scholar]
- 20.Magruder JT, Weiss SJ, DeAngelis KG, et al. Correlating oxygen delivery on cardiopulmonary bypass with Society of Thoracic Surgeons outcomes following cardiac surgery. J Thorac Cardiovasc Surg. 2020. doi: 10.1016/j.jtcvs.2020.12.008. [DOI] [PubMed] [Google Scholar]
- 21.Newland R, Baker R, Woodman R, et al. Predictive capacity of oxygen delivery during cardiopulmonary bypass on acute kidney injury. Ann Thorac Surg. 2019;108:1807–14. [DOI] [PubMed] [Google Scholar]