ABSTRACT
Measles is a vaccine-preventable viral disease whose vaccination coverage remains low in Zambia, where the target group for vaccination is children aged 9 to 18 months. In addition to inadequate measles vaccination coverage among children, few studies address potential resultant immunity gaps among adults. We analyzed data from a simulated HIV vaccine efficacy trial (SiVET) conducted from 2015–2017 among adult Zambian women of childbearing age to determine measles antibody seroprevalence before and after vaccination with the measles, mumps and rubella (MMR) vaccine. We used MMR vaccine as a substitute for an experimental HIV vaccine as part of a simulation exercise to prepare for an HIV vaccine efficacy trial. We found that 75% of women had measles antibodies prior to receiving MMR, which increased to 98% after vaccination. In contrast, mumps and rubella antibody prevalence was high before (93% and 97%, respectively) and after (99% and 100%, respectively) vaccination. The low baseline measles seropositivity suggests an immunity gap among women of childbearing age. We recommend that measles vaccination programs target women of childbearing age, who can pass antibodies on to neonates. Moreover, administering the MMR vaccine to clinical trial candidates could prevent measles, mumps or rubella-related adverse events during actual trials.
KEYWORDS: Simulated vaccine efficacy trials, measles, mumps, rubella, Zambia
Introduction
Measles is a highly contagious viral disease that poses a public health burden despite being vaccine preventable.1 The burden of measles disproportionately impacts low resource settings where health systems struggle to achieve adequate immunization coverage.2,3 In Zambia, measles vaccination is performed routinely for infants at 9 months of age, with a booster dose administered at 18 months.4 Despite routine vaccination, national immunization coverage remains below the 95% level required to achieve herd immunity; recent estimates place current measles vaccination coverage rate at 93% for the first dose and 66% for the second dose.5 Zambia only started routine inoculation of children aged 9 months to 14 years with combined measles and rubella vaccination in 2016.6
Most epidemiological studies in Zambia have characterized measles and rubella immunity among children.7,8 But few studies document the immunization status of adults who may have immunity gaps due to waning immunity from childhood vaccination or being unvaccinated. During the last major measles outbreak in Zambia in 2010–2011, females of reproductive age (17%) and unvaccinated neonates under 9-months-old (13%) accounted for around a third of cases.9 It is thus vital that women of childbearing age are vaccinated and can therefore transmit maternal antibodies that grant neonates some immunity before vaccination at 9 months. However, evidence of measles immunity status among women of childbearing age—a high-risk group for measles in Zambia—remains scant. We assessed measles, mumps and rubella immunity before and after vaccination with MMR among a cohort of women of reproductive age.
Methods
Study design
This was a simulated vaccine efficacy trial (SiVET) nested within a large prospective HIV-incidence cohort of women at high-risk of acquiring HIV. The goal of the SiVET was to enhance preparedness for future HIV vaccine trials. The study was conducted between October 2015 and May 2017 at the two Centers for Family Health Research in Zambia, located in Lusaka and Ndola. SiVET participants spent a maximum of 12 months in the study from enrollment to completion, with vaccination visits occurring at Month 0 and Month 3.
Ethics
Ethical approval for the SiVET was granted by the University of Zambia Biomedical Research Ethics Committee (Protocol number: 011-03-15, Lusaka, Zambia) and the Emory University Institutional Review Board (Protocol number: IRB00080202, Atlanta, GA, USA). The trial was registered with ClinicalTrials.gov with identifier NCT02589678.
Study Participants
Eligibility criteria for the SiVET mirrored those of a hypothetical HIV vaccine trial, hence we included participants who were: women aged 18 to 40, either a self-identified female sex worker or single mother—both of whom are at high-risk of HIV,10–12 not pregnant or intending to be pregnant for the study duration, willing to use a long-acting contraceptive method (intrauterine device, hormonal implant or injectable), living in Lusaka or Ndola, willing to participate for the 12-month trial duration, willing to be tested for HIV, and willing and able to provide informed consent.
Participants were excluded from the study if they: tested positive for HIV, were pregnant/intending to be pregnant, were unable to provide informed consent or locator information, were uninterested in participating, had a chronic illness, had severe allergies, were recently vaccinated, received a recent investigational blood product, had ever had a severe local or systemic reaction to a vaccine.
Study procedures
Screening
Participants from the larger HIV-incidence cohort were screened for eligibility for the SiVET (Figure 1). Knowledge of vaccines was assessed during a vaccine education session, which explained what vaccines are and how they work and introduced the concepts of randomization and blinding to ensure participants understood the essence of a vaccine trial. To assess their understanding, participants were tested pre-and post-vaccine education sessions; participants scoring ≥80% in the post-vaccine education session test and meeting all other eligibility criteria were invited to enroll and signed informed consent to participate in the SiVET. Consent was obtained in English or whichever local language participants were most comfortable in (Bemba or Nyanja).
Figure 1.
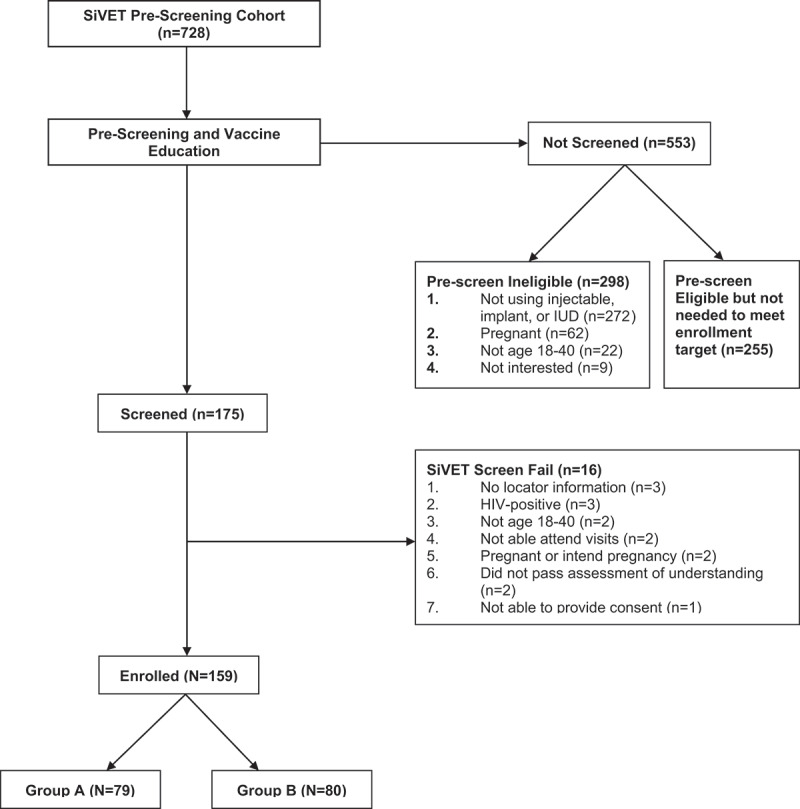
Simulated vaccine efficacy trial participant flowchart (N = 159).
Vaccination
At the enrollment visit (Visit 1), women were randomized into one of two arms: Group A or Group B. At Visit 1, Group A received intramuscular injections of the measles, mumps and rubella (MMR) vaccine (TRIMOVAX), while Group B received intramuscular injections of the tetanus toxoid, diphtheria toxoid, acellular pertussis and poliomyelitis (Tdap-IPV) vaccine (Adacel Quadra). At Month 3, Group A received the Tdap-IPV vaccine and Group B received the MMR vaccine. Clinicians and study participants were blinded about group assignments. HIV risk reduction counseling and testing were provided at every vaccination visit. The vaccine administration was designed to mimic the conditions of an HIV vaccine trial, during which one group receives the investigational product and the other group receives a control. In the case of SiVET, we eventually offered both MMR and Tdap-IPV to all participants to avoid withholding the benefits of both vaccines from either Group A or B. During the 12-month study period, additional follow-up visits that included clinical safety assessments and various lab tests (e.g., pregnancy test, sexually transmitted infection screening, urinalysis, serology) were also performed in line with study procedures.
Lab procedures
At baseline before vaccine administration and again at one month after vaccination, we collected blood samples from participants to test the presence of measles, mumps and rubella antibodies. We used two qualitative assays, VIDAS Measles IgG and VIDAS Mumps IgG, and a quantitative VIDAS Rubella (RUB) IgG II assay from bioMérieux, France to test participants’ immune responses. The principle of these assays combines a two-step enzyme immunoassay sandwich method with a final enzyme-linked fluorescence assay (ELFA). The miniVidas instrument calculated each sample’s relative fluorescence value (RFV) by subtracting the background reading from the final result generated. The “test value,” used for the result interpretation of the measles and mumps assays, was created by forming the ratio of RFV of the participant’s sample to a standard. The rubella assay reported final results as concentrations in titers (IU/ml). Titers were calculated automatically using calibration curves that are stored by the miniVidas instrument.
For measles, interpretation of test values was as follows: <.50 (negative), ≥.50 to <.70 (equivocal) and ≥.70 (positive). For mumps, interpretation of tests values were <.35 (negative); ≥.35 to .50 (equivocal) and ≥.50 (positive). A rubella assay result of ≥ 15 IU/ml was considered positive; 10 ≤ Titer <15 IU/mL was equivocal and <10 IU/ml was negative. It is worth pointing out that a positive IgG antibody result is not always protective against viral infection but rather strongly indicative of protection.
Data collection
Women in the larger HIV incidence cohort were administered a questionnaire, which collected sociodemographic information such as age, number of live births, education level and place of residence. During the trial, data were collected using data collection forms, entered into REDCap13 and combined with sociodemographic information stored in a Microsoft Access database for the HIV incidence cohort of high-risk women. All survey data and laboratory samples used alphanumeric codes, no patient identifiers were recorded. All electronic data were thus anonymized.
Study outcome
The main outcome of this analysis was to compare seropositivity for measles, mumps and rubella IgG antibodies (separately) from reproductive-age women before and after administration of MMR vaccine.
Statistical analysis
We used frequencies and percentages to summarize participant sociodemographic characteristics and pre-vaccination knowledge. Pearson chi-squared tests with an alpha significance level of p < .05 were used to compare differences in measles, mumps and rubella IgG antibody seroprevalence before and after vaccination. Statistical analysis was performed using SAS 9.4 (Cary, NC).
Results
Sociodemographic characteristics
Figure 1 shows the flow of participants from screening to enrollment. Overall, the SiVET enrolled 159 women, 79 of whom were randomized to Group A and 80 of whom were randomized to Group B. The median age of study participants was 23 years (IQR: 21–28). Most participants had either primary (47%, n = 74) or secondary school (45%, n = 72) level education (Table 1). The majority of participants were single mothers (63%, n = 100), while the rest were female sex workers (37%, n = 59). Enrolled participants had high pre-vaccination knowledge of measles (97%, n = 154) and mumps (93%, n = 148), but low pre-vaccination knowledge of rubella (16%, n = 26) (Table 1).
Table 1.
Sociodemographics of Zambian women enrolled in SiVET.
| Group A (N=79) |
Group B (N=80) |
Total (N=159) |
||||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Antibody status | ||||||
| Measles positive | 58 | 73 | 60 | 75 | 118 | 75 |
| Mumps positive | 74 | 94 | 73 | 91 | 147 | 93 |
| Rubella positive | 74 | 94 | 77 | 96 | 151 | 95 |
| Place of residence | ||||||
| Lusaka | 36 | 46 | 43 | 54 | 79 | 49 |
| Ndola | 43 | 54 | 37 | 46 | 80 | 51 |
| Age (years) | ||||||
| 18 -20 | 16 | 20 | 15 | 19 | 31 | 19 |
| 21-23 | 20 | 25 | 32 | 40 | 52 | 33 |
| 24-28 | 20 | 25 | 17 | 21 | 37 | 23 |
| 29-39 | 23 | 30 | 16 | 20 | 39 | 25 |
| Number of live births | ||||||
| 0 | 5 | 6 | 7 | 9 | 12 | 8 |
| 1-2 | 55 | 70 | 54 | 67 | 109 | 69 |
| 3-4 | 15 | 19 | 16 | 20 | 31 | 19 |
| 5-6 | 4 | 5 | 3 | 4 | 7 | 4 |
| Marital statusa | ||||||
| Never married | 56 | 72 | 55 | 69 | 111 | 70 |
| Divorced/separated | 20 | 25 | 24 | 30 | 44 | 28 |
| Widowed | 2 | 3 | 1 | 1 | 3 | 2 |
| Education level | ||||||
| Primary | 38 | 48 | 36 | 45 | 74 | 47 |
| Secondary | 31 | 39 | 41 | 51 | 72 | 45 |
| College | 3 | 4 | 0 | 0 | 3 | 2 |
| None of the above | 7 | 9 | 3 | 4 | 10 | 6 |
| High-risk group | ||||||
| Female sex worker | 31 | 39 | 28 | 35 | 59 | 37 |
| Single mother | 48 | 61 | 52 | 65 | 100 | 63 |
| Measles pre-vaccination knowledge from VESb | ||||||
| Yes | 77 | 99 | 77 | 97 | 154 | 97 |
| No | 1 | 1 | 2 | 3 | 3 | 2 |
| Mumps pre-vaccination knowledge from VESb | ||||||
| Yes | 74 | 96 | 74 | 94 | 148 | 93 |
| No | 3 | 4 | 5 | 6 | 8 | 5 |
| Rubella pre-vaccination knowledge from VESc | ||||||
| Yes | 15 | 19 | 11 | 14 | 26 | 16 |
| No | 63 | 81 | 67 | 86 | 130 | 82 |
Measles, mumps and rubella immunity
Table 2 shows serological results for measles, mumps and rubella immunity, pre- and post-vaccination. Overall, participants’ seropositivity for measles, mumps and rubella antibodies increased after they received the MMR vaccine. We observed that 75% of women tested positive for measles IgG antibody pre-vaccination, which improved to 98% post-vaccination (p < .05). We further noted a 5% increase in pre- and post-vaccination IgG antibody positivity for mumps (94% to 99%, p < .05). Rubella IgG antibody titers increased from 97% pre-vaccination to 100% post-vaccination, although this change was not statistically significant (p > .05).
Table 2.
Antibody status results pre- and post-vaccination.
| Pre-Vaccination (N= 159) |
Post-Vaccination (N=154) |
||||
|---|---|---|---|---|---|
| n | % | n | % | p-value | |
| Measlesa | |||||
| Negative | 30 | 19 | 2 | 1 | |
| Equivocal | 9 | 6 | 2 | 1 | |
| Positive | 118 | 75 | 150 | 98 | <.05 |
| Mumps | |||||
| Negative | 9 | 6 | 2 | 1 | |
| Equivocal | 0 | 0 | 0 | 0 | |
| Positive | 150 | 94 | 152 | 99 | <.05 |
| Rubella | |||||
| Negative | 4 | 3 | 0 | 0 | |
| Equivocal | 0 | 0 | 0 | 0 | |
| Positive | 155 | 97 | 154 | 100 | .123 |
Two participants were missing pre-vaccination samples for measles.
Discussion
We found low baseline measles immunity among women enrolled in SiVET. The 75% baseline seropositivity for measles IgG we observed highlights a potential measles immunity gap among sexually active women of childbearing age. It is unclear from our study whether the low baseline seroprevalence of measles IgG antibody was due to poor vaccination coverage or waning immunity after childhood vaccination, as we did not ask women to provide proof of childhood vaccinations. Measles vaccination coverage in Zambia was below 70% in the 1990s (most of our participants were born in or before the 1990s), which could have created an immunity gap in adulthood.9 In Italy, Anichini et al showed that measles antibody titers among vaccinated adults waned significantly 11 years after vaccination.14 In the context of this literature, our findings recommend measles vaccination for Zambian women of childbearing age during supplemental immunization activities, which have historically targeted children.15,16 Such a targeted strategy would have clear public health benefit by mitigating the severe disease associated with adult measles, and the high risk of poor maternal and fetal outcomes associated with measles during pregnancy.17
We also noted high baseline mumps and rubella immunity without previous mumps and rubella vaccination (both were not routinely administered in our participants’ lifetimes), which indicates prior exposure to wild type viruses. Further, post-vaccination measles immunity rose to be comparable to mumps and rubella. These findings support using every opportunity to identify and vaccinate clinical trial candidates at risk of licensed vaccine-preventable diseases. Moreover, urban areas such as Lusaka and Ndola have been the hardest hit by previous measles outbreaks due to the presence of ‘clusters’ of non-immunized individuals within close proximity to each other.18 Hence, trials recruiting adult women from urban areas must be particularly vigilant about offering the MMR vaccine to participants.
We acknowledge certain study limitations. Firstly, our study had a small sample size that only included women from urban areas of Zambia, which prevents our findings from being generalizable to rural areas. Evidence suggests lower measles vaccination coverage in rural regions of Zambia compared to urban regions,3 and exposure to pathogens likely varies across regions and countries. Secondly, our study was not sufficiently powered to measure the factors associated with negative measles serology at baseline. Doing so may shed light on the specific sociodemographic profile of women who require targeted adult vaccination campaigns.
Despite these limitations, our findings have potential implications for women of childbearing age from both a public health perspective and for those being considered for clinical trials. For these women, we recommend serological screening for immunization against vaccine-preventable diseases as standard of care. Such a strategy would carry the dual benefit of preventing participants from acquiring measles, which can be very dangerous and might confuse interpretation of the trial results. Beyond potential clinical trial participants, serological surveys could identify adult Zambian women of reproductive age in need of booster measles vaccinations, which could protect the health of mothers and neonates.
Acknowledgement
We would like to thank all the women who were screened, enrolled and/or completed this trial for their time and effort. We also extend our thanks to the study staff, who diligently followed study procedures and ensured the trial ran successfully. We also acknowledge Research Electronic Data Capture (REDCap) electronic data capture tools hosted at Emory University with IT support from Research and Woodruff Health IT Division grant support (UL1 TR000424).
Funding Statement
This study was supported by the International AIDS Vaccine Initiative (IAVI) with the generous support of the American people through the United States Agency for International Development (USAID, https://www.usaid.gov/). A full list of IAVI donors can be found at https://www.iavi.org/; National Institutes of Health (https://www.nih.gov/) grants (R01 MH66767, R01 HD40125, and R01 MH95503; R01 AI051231); the AIDS International Training and Research Program Fogarty International Center (D43 TW001042); and the Emory Center for AIDS Research (P30 AI050409). The contents of this manuscript are the responsibility of the authors and do not necessarily reflect the views of USAID or the US Government.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 1.Durrheim DN, Crowcroft NS, Strebel PM.. Measles - the epidemiology of elimination. Vaccine. 2014;32(51):6880–5. doi: 10.1016/j.vaccine.2014.10.061. [DOI] [PubMed] [Google Scholar]
- 2.WHO . Measles. Measles: World Health Organization; 2019. [Google Scholar]
- 3.Brownwright TK, Dodson ZM, van Panhuis WG. Spatial clustering of measles vaccination coverage among children in sub-saharan africa. BMC Public Health. 2017;17(1):957. doi: 10.1186/s12889-017-4961-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hayford K, Mutembo S, Carcelen A, Matakala HK, Munachoonga P, Winter A, Wanyiri JW, Searle K, Mwansa FD, Mwiche A, et al. Measles and rubella serosurvey identifies rubella immunity gap in young adults of childbearing age in Zambia: the added value of nesting a serological survey within a post-campaign coverage evaluation survey. Vaccine. 2019;37(17):2387–93. doi: 10.1016/j.vaccine.2019.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Katemba B, Gianetti B, Groeneveld C, Mwansa D, Musakanya K, Sikapande B, Simwinga J, Hamoonga R, Mazaba M. A retrospective analysis of measles trends and vaccination coverage in Zambia from 2016 to 2018. Health Press Zambia Bull. 2019;3:1–2. [Google Scholar]
- 6.Silitongo M, Mazaba M, Mulenga D, Chirambo-Kalolekesha M, Njunju E, Daka V, Tinago W, Rudatsikira E, Syapiila P, Banda C, et al. Cluster survey evaluation of reasons of vaccination failure in measles-rubella vaccination campaign in Zambia, 2016. Health Press Zambia Bull. 2019;3(1):21–26. [Google Scholar]
- 7.Carcelen AC, Mutembo S, Matakala KH, Chilumba I, Mulundu G, Monze M, Mwansa FD, Moss WJ, Hayford K. Impact of a measles and rubella vaccination campaign on seroprevalence in southern province, zambia. Am J Trop Med Hyg. 2021;104(6):2229–32. doi: 10.4269/ajtmh.20-1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mazaba ML, Siziya S, Monze M, Cohen D. Epidemiology of acute rubella infection in Zambia during the pre-vaccination period (2005-2016) as a baseline for monitoring rubella epidemiology in the post-rubella vaccine introduction era. BMC Infect Dis. 2020;20(1):101. doi: 10.1186/s12879-020-4806-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mpabalwani M, Matapo B, Katepa-Bwalya M, Mukonka V, Mutambo H, Babaniyi O. The 2010 - 2011 measles outbreak in Zambia: challenges and lessons learnt for future action. East Afr J Public Health. 2013;10(1). [Google Scholar]
- 10.Chanda MM, Ortblad KF, Mwale M, Chongo S, Kanchele C, Kamungoma N, Barresi LG, Harling G, Barnighausen T, Oldenburg CE. Contraceptive use and unplanned pregnancy among female sex workers in zambia. Contraception. 2017;96(3):196–202. doi: 10.1016/j.contraception.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beckham SW, Shembilu CR, Winch PJ, Beyrer C, Kerrigan DL. ‘If you have children, you have responsibilities’: motherhood, sex work and HIV in southern Tanzania. Cult Health Sex. 2015;17(2):165–79. doi: 10.1080/13691058.2014.961034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kilembe W, Inambao M, Sharkey T, Wall KM, Parker R, Himukumbwa C, Tichacek A, Malama K, Visoiu AM, Price M, et al. Single mothers and female sex workers in Zambia have similar risk profiles. AIDS Res Hum Retroviruses. 2019;35(9):814–25. doi: 10.1089/aid.2019.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (redcap)-a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anichini G, Gandolfo C, Fabrizi S, Miceli GB, Terrosi C, Gori Savellini G, Prathyumnan S, Orsi D, Battista G, Cusi MG. Seroprevalence to measles virus after vaccination or natural infection in an adult population, in italy. Vaccines (Basel). 2020;8(1). doi: 10.3390/vaccines8010066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.CDC . Progress in measles control–Zambia, 1999-2004. MMWR Morb Mortal Wkly Rep. 2005;54(23):581–84. [PubMed] [Google Scholar]
- 16.Portnoy A, Jit M, Helleringer S, Verguet S. Impact of measles supplementary immunization activities on reaching children missed by routine programs. Vaccine. 2018;36(1):170–78. doi: 10.1016/j.vaccine.2017.10.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Congera P, Maraolo AE, Parente S, Schiano Moriello N, Bianco V, Tosone G. Measles in pregnant women: a systematic review of clinical outcomes and a meta-analysis of antibodies seroprevalence. J Infect. 2020;80(2):152–60. doi: 10.1016/j.jinf.2019.12.012. [DOI] [PubMed] [Google Scholar]
- 18.Pinchoff J, Chipeta J, Banda GC, Miti S, Shields T, Curriero F, Moss WJ. Spatial clustering of measles cases during endemic (1998-2002) and epidemic (2010) periods in Lusaka, zambia. BMC Infect Dis. 2015;15(121). doi: 10.1186/s12879-015-0842-y. [DOI] [PMC free article] [PubMed] [Google Scholar]


