Abstract
The National Institutes of Health (NIH), and especially its largest and oldest institute, the National Cancer Institute (NCI), have long been among the best - but perhaps not the most well-known supporters - for the life sciences and pharmaceutical industries, providing non-dilutive funding, scientific guidance, education for potential future employees, and importantly, collaboration opportunities including clinical trials and technology licensing partnerships. In this study, various databases were used, including the NCI Technology Transfer Center (TTC) internal agreement database, NIH technology licensing database, Global Data, Pitchbook, and NIH RePORTER, to explore and analyze collaborations and licensing partnerships between NCI TTC and life science and pharmaceutical companies. For early-stage companies receiving funding or taking part in collaborations and licensing, there is a significant relationship between these NIH agreements and successful financial exits. NCI support not only reaches these early-stage firms but also extends to the Top 20 pharmaceutical companies. These two findings highlight the importance of NCI collaborations for both early-stage and established life science and pharmaceutical companies.
Keywords: Collaboration, drug development, National Cancer Institute, technology transfer, drug development, clinical drug
INTRODUCTION
There is growing concern that new drug development and approvals by the Food and Drug Administration (FDA) are at the lowest level since the 2000s. Despite increased spending in research and development and better technologies for the identification and validation of new drug targets, pharmaceutical companies still face serious challenges in developing new drugs (Schofield 2013, Cunningham and Link 2015, Weckowska 2015). Academia, industry, and government all remain important contributors in the drug development ecosystem, and many of the challenges in drug development pathways may be resolved through strong collaborations within the ecosystem of academia, industry, and government.
The National Institutes of Health (NIH) is the one of the largest non-dilutive funding providers for biomedical research globally, with an annual investment of more than $41.7 billion dollars. NIH funding is distributed between Extramural and Intramural research activities. Ninety percent (90%) of NIH’s funding is awarded for extramural research activities that take place in universities, medical schools, and other research institutions (Ferguson and Langer 2021). About ten percent (10%) of NIH’s funding supports intramural projects conducted by scientists in NIH’s own laboratories. NIH has provided financial support to life science companies in the form of grants and contracts; however, NIH’s support for life science companies is not limited to funding. NIH also offers substantive collaboration and technology licensing opportunities to life science companies and academic institutions to advance their research, as well as provide cutting-edge training for their future employees. In this study, the involvement of the NCI and specifically, its Technology Transfer Center (NCI TTC), in the overall success of life science and pharmaceutical companies is explored, highlighting the role of collaborations and licensing partnerships mediated by NCI TTC. We hypothesize that (1) collaborations and licensing partnerships mediated by NCI TTC play an important role in the success of early-stage life science companies, particularly those focused on drug development and (2) NCI TTC also supports the Top 20 global pharmaceutical companies with the highest revenue.
Methods
NIH Institute Study Population
NCI TTC not only advances NCI technology transfer-related programs and partnerships, but also operates as a Service Center for the laboratories and inventions from the following nine NIH institutes and centers:
National Eye Institute (NEI)
National Institute on Minority Health and Health Disparities (NIMHD)
Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD)
NIH Clinical Center (CC)
National Center for Complementary and Integrative Health (NCCIH)
National Institute on Aging (NIA)
National Institute on Drug Abuse (NIDA)
National Library of Medicine (NLM)
NIH Center for Information Technology (CIT)
Thus, this study covers the relationships between early-stage or Top 20 pharmaceutical companies and the NCI plus these nine other NIH institutes and centers listed above.
Databases Used In This Study
To explore collaborations and licensing partnerships between NCI’s intramural programs and life science companies, multiple databases were used, including NCI TTC’s internal agreement database, the NIH technology licensing database, Global Data, Pitchbook, and NIH RePORTER. We searched collaboration agreements including Material Transfer Agreements (MTAs), Clinical Trial Agreements (CTAs), Cooperative Research and Development Agreements (CRADAs) and licensing agreements that were negotiated by NCI TTC (which includes NCI and NIH Institutes served by NCI TTC) and established with early stage life science companies, early-stage drug development companies and the Top 20 pharmaceutical companies.
Pitchbook (https://pitchbook.com): This platform provides access to private and public capital market data for 3.3 million companies, and it delivers data regarding company investors, deals, mergers and acquisitions, funds, advisors and people. Through its daily newsletter and quarterly reports, the company also provides commentary and analysis of current events, trends, and issues relevant to the capital markets.
Global Data (https://globaldata.com): This database provides quantitative and qualitative data including industry forecast reports, sector reports, trend trackers from startups to market leader companies.
NIH RePORTER (https://exporter.nih.gov): This is an electronic tool that provides information regarding NIH-funded research projects, publications and patents resulting from NIH funding.
TIMS: TIMS is NIH’s internal database that is used for recording technology transfer agreements including Material Transfer Agreements (MTAs), Clinical Trial Agreements (CTAs), Confidential Disclosure Agreements (CDAs) and Cooperative Research and Development Agreements (CRADAs). This database records agreements for NCI and the NIH Institutes served by NCI TTC, and this database was used to identify collaboration agreement activities for NCI and the NIH Institutes served by NCI TTC.
TechTracS: TechTracS is the NIH database where licensing agreements are recorded for the entire NIH. TechTracS was used to identify licensing activities for NCI and the NIH Institutes served by NCI TTC.
LinkedIn: LinkedIn is the largest professional network on the internet. It was used to identify former trainees and employees of both NCI and the NIH Institutes served by NCI TTC.
Identification of Early-stage Companies Funded by the NIH and Success Status
Pitchbook Platform: Pitchbook was queried to identify NIH-funded companies. According to Pitchbook data, 1,437 companies received extramural NIH funding between 1992 and 2021. Outcomes of these companies were determined by investigating each company’s exit plan. Out of the 1,437 NIH-funded companies, 206 completed an exit plan. Exit plan completion resulted from either a “Successful Outcome,” which includes being acquired, merged, privately held, publicly held, or in an IPO (Initial Public Offering) registration, or alternatively an “Unsuccessful Outcome,” which includes bankruptcy or an out-of-business status.
NIH RePORTER: To identify which NIH institute provided NIH funding for the 206 early-stage companies identified above, NIH RePORTER was queried. Pitchbook and internet announcements were also used to identify the NIH funding source.
NIH Technology Transfer Databases: Two NIH databases were used in this study, namely, TIMS and TechTracS. These two NIH internal databases were queried to determine the collaborative agreements and licensing partnerships executed between NCI and the NIH Institutes served by NCI TTC and the 206 early-stage companies previously identified to have been funded by the NIH and who completed an exit plan.
Identification of the Top 20 Pharmaceutical Companies
Global Data Platform: Global Data was used to identify the Top 20 pharmaceutical companies in the world based on revenue.
NIH Technology Transfer Databases: TIMS and TechTracS were queried to determine the NCI TTC-mediated collaboration agreements and licenses, negotiated on behalf of NCI and the NIH Institutes served by NCI TTC, with the Top 20 pharmaceutical companies.
Identification of Companies With NIH-trained Employees
LinkedIn: LinkedIn was used to identify employees of the Top 20 pharmaceutical companies listed on their LinkedIn profiles training and/or work experience from NCI and the NIH Institutes served by NCI TTC. For early-stage companies, there were few examples of current and former employees with any NIH training and/or work experience, so these individuals were exempted from the study.
Results
Exit Plan Outcomes of Early-stage Life Science Companies with NIH-funding
According to Pitchbook data, 1,437 companies received NIH funding between 1992 and 2021, and 206 out of the 1,437 NIH-funded companies completed an exit plan and were included in this analysis. Of these, 54.9% had a “Successful Outcome” defined by the following: “Acquired,” “Merged,” “Privately Held,” “Publicly Held,” or “in IPO (Initial Public Offering) registration” situations. On the other hand, 45.1% had an “Unsuccessful Outcome” defined by: “Bankruptcy” or “Out of Business.” (Figure 1).
Figure 1:
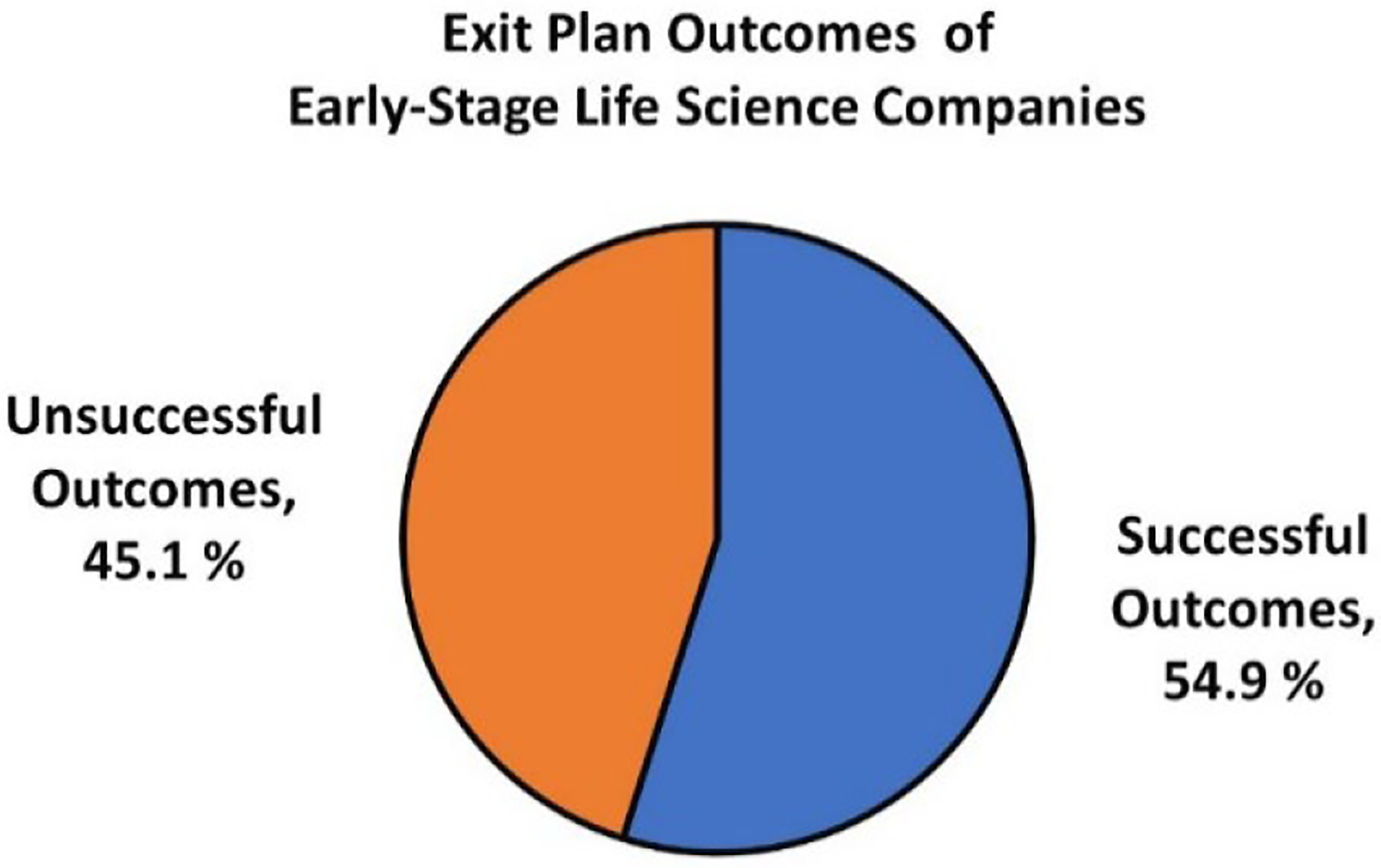
Exit plan outcomes of early-stage life science companies with NIH funding
NIH Funding And Collaboration Correlated With The Successful Outcomes Of Early-Stage Life Science Companies
In this part of the study, we examined the correlation between collaboration and the successful outcomes of early-stage life science companies that perform their activities in various industrial platforms including drug development, diagnostic equipment, therapeutic devices, surgical devices, discovery tools, and biomaterial supply. It was found that 25.7% of early-stage NIH-funded life science companies had collaborations mediated by NCI TTC (n=53) (Figure 2A). For these early-stage companies material transfer agreements (MTAs), were identified as the most common agreements (38.7), followed by licensing, cooperative research and development agreements (CRADAs), and clinical trial agreements (CTAs). ). Licensing agreements were calculated in two categories: (1) All NIH Institutes called as “TOTAL” (33.6%) and (2) NCI and NCI TTC served NIH Institutes (21.8%) (Figure 2B). While 36.3% of early-stage life science companies with “Successful Outcomes,” had established collaborations mediated by NCI TTC, only 12.9% of early-stage life science companies with “Unsuccessful Outcomes”, had established collaborations (Figure 2C). Thus, companies with “Successful Outcomes” were 3 times more likely to have collaborations mediated by NCI TTC than companies with an “Unsuccessful Outcome” (Figure 2D).
Figure 2:
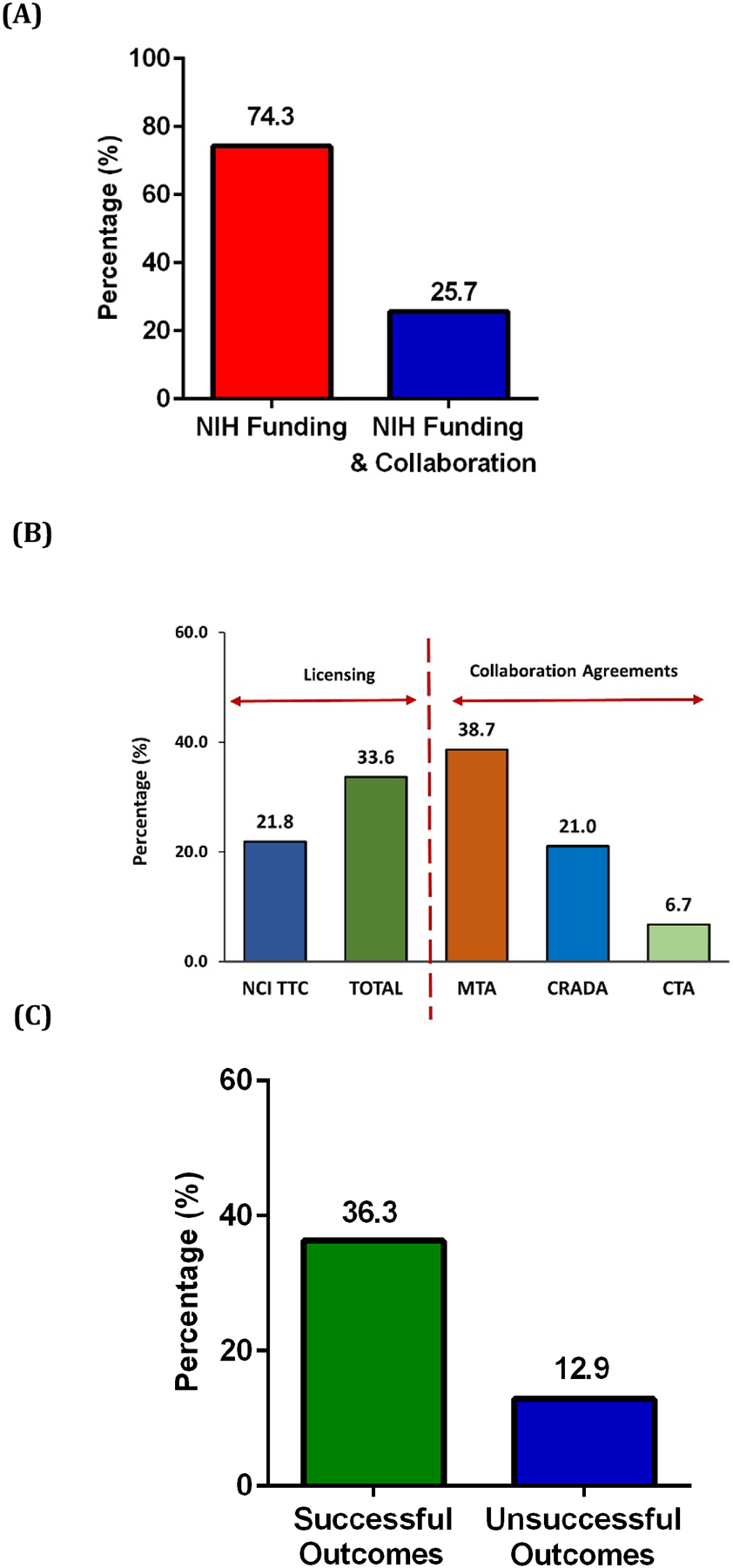
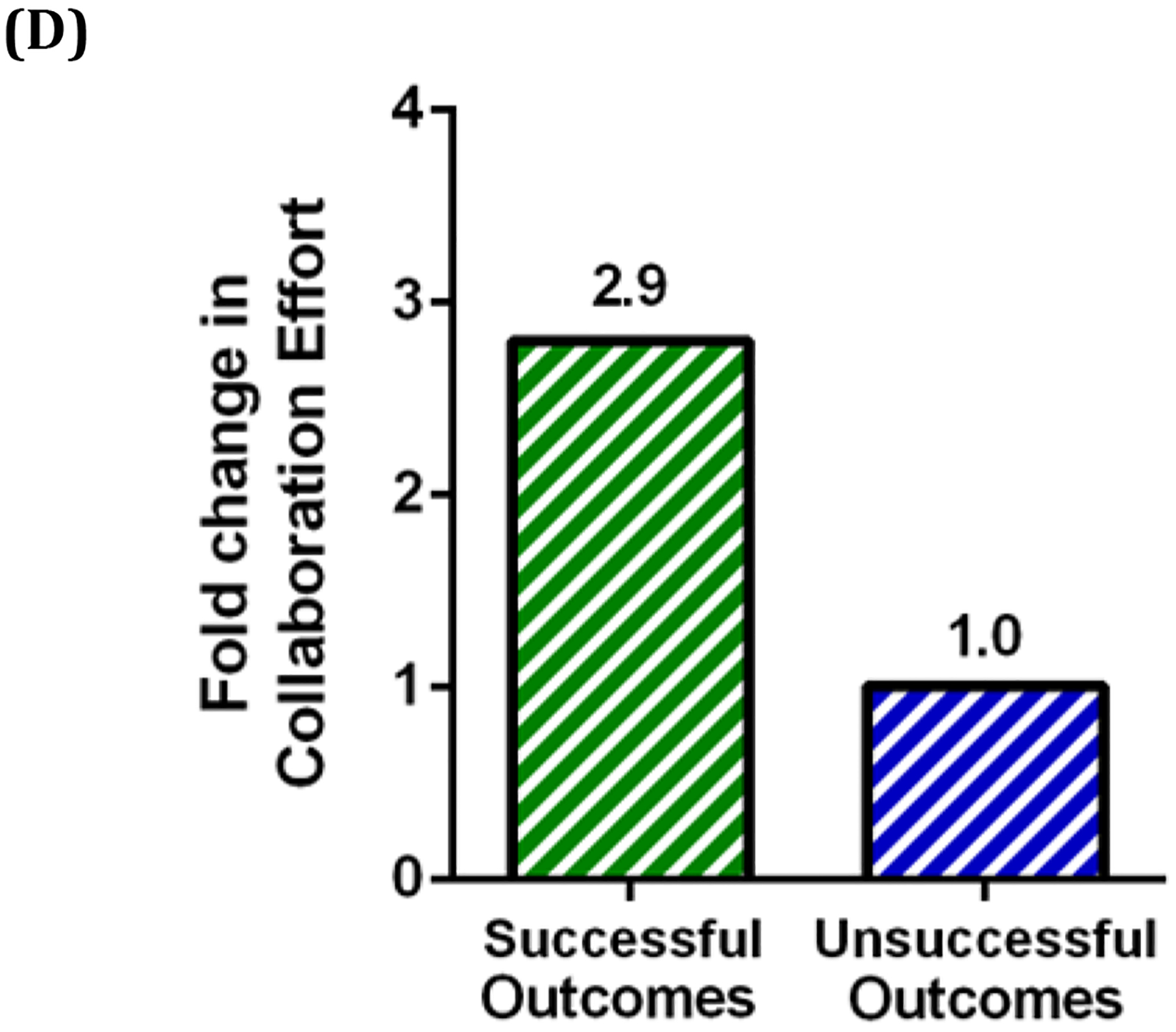
Percentage of collaborations and agreement types for early-stage life science companies. (A) NCI TTC-mediated collaboration among NIH-funded early-stage life science companies. (B) Licenses and collaboration agreements with early-stage life science companies. Licensing agreements were calculated in two categories: (1) All NIH Institutes called as “TOTAL” (33.6%) and (2) NCI and NCI TTC served NIH Institutes (21.8%) (C) Percentage of NCI TTC-mediated collaboration of early-stage life science companies with either a Successful or Unsuccessful Outcome. (D) Fold change in the collaboration effort of early-stage life science companies with either a Successful or Out of Business status.
NIH funding and its distribution to the previously identified 206 early-stage life science companies was calculated. Of those with NIH funding, 64% were able to achieve “Successful Outcomes” (Figure 3A). NIH funding distribution for companies with “Successful Outcomes” and “Unsuccessful Outcomes” with or without NCI TTC-mediated collaboration is summarized below and depicted in Figure 3B:
Figure 3:
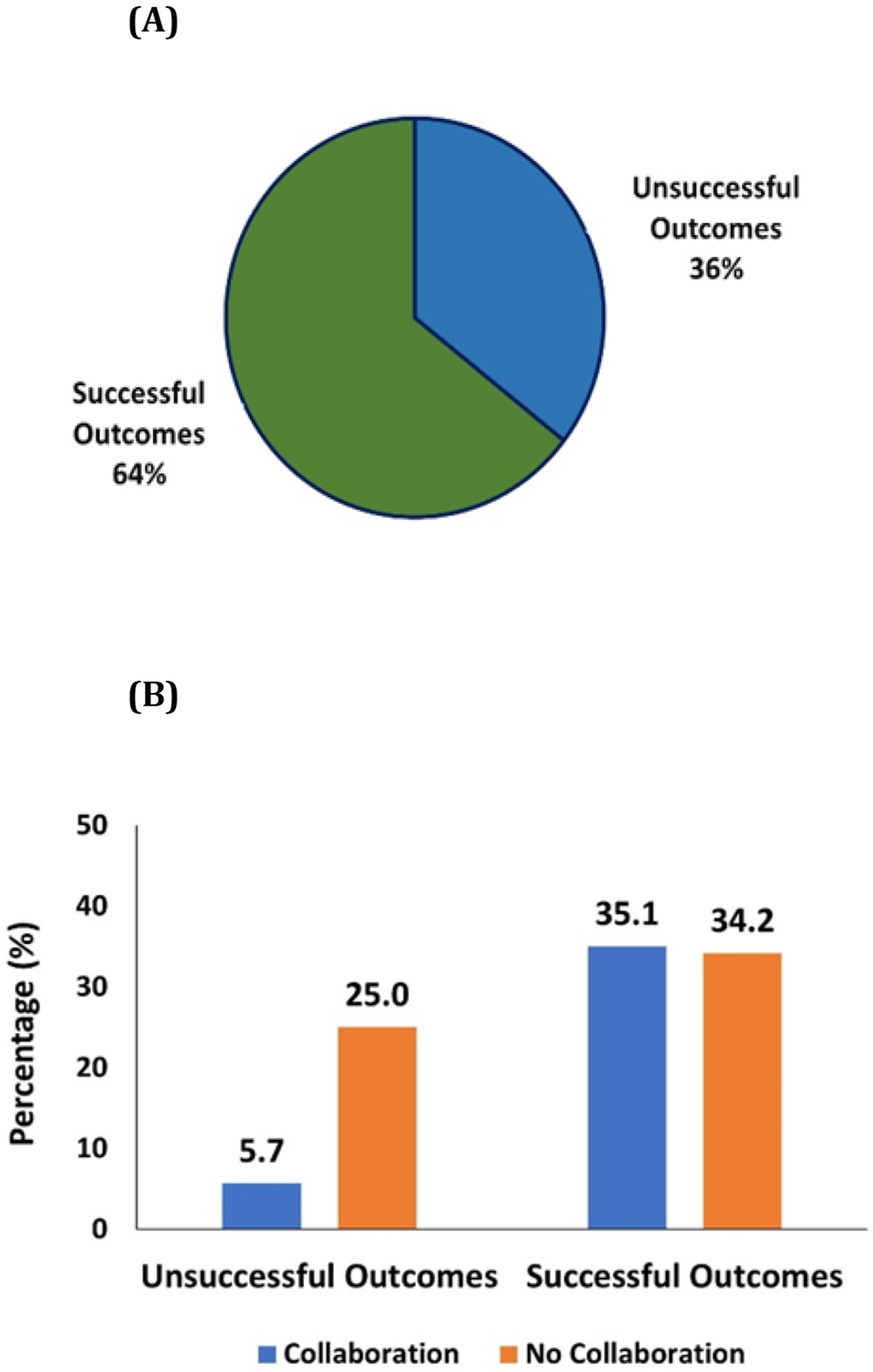
Funding distribution for early-stage life science companies with or without collaboration and the collaboration effect on their “Successful Outcome”. (A) NIH funding distribution between companies with “Successful Outcome” and “Unsuccessful Outcomes”. (B) NIH funding distribution for companies with “Successful Outcomes” and “Unsuccessful Outcomes” with or without collaborations mediated by NCI TTC.
34.2% of total NIH funding went to companies with “Successful Outcomes” and without NCI TTC collaboration.
35.1% of NIH funding went to companies with “Successful Outcomes” and with collaborations mediated by NCI TTC.
25.0% of NIH funding went to companies with “Unsuccessful Outcomes” and without collaborations mediated by NCI TTC.
Thus, only 5.7% of NIH funding went to companies with “Unsuccessful Outcomes” and with collaborations mediated by NCI TTC.
NCI TTC-mediated Collaborations With Early-stage Drug Development Companies
Of the 206 NIH-funded early-stage life science companies previously identified, 31.7% (n=65) focus on drug development either as a primary focus or one of their foci as determined by searching the Pitchbook database. These early-stage drug development companies were also examined to identify the involvement of NIH collaboration on their success. NCI and the nine client institutes supported by NCI TTC were found to be the second leading funding provider for these companies. 59% of early-stage drug development companies exhibited “Successful Outcomes” (Figure 4), and 40.0% of early-stage drug development companies established collaborations mediated by NCI TTC (Figure 5A). Licensing agreements and MTAs were found to be the most common agreements between NCI TTC and early-stage drug development companies (Figure 5B).
Figure 4:
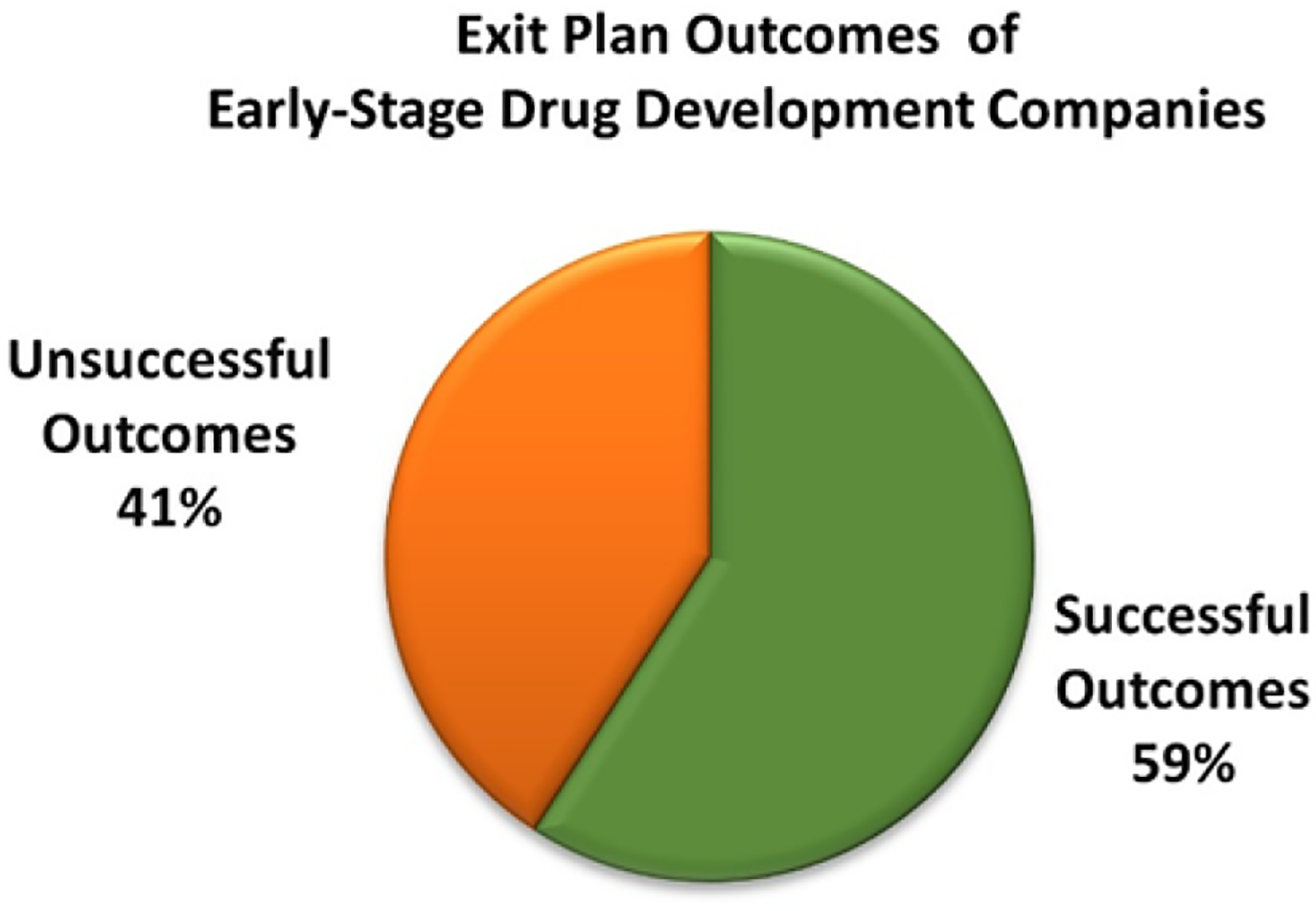
NIH funding distribution to early-stage drug development companies
Figure 5:
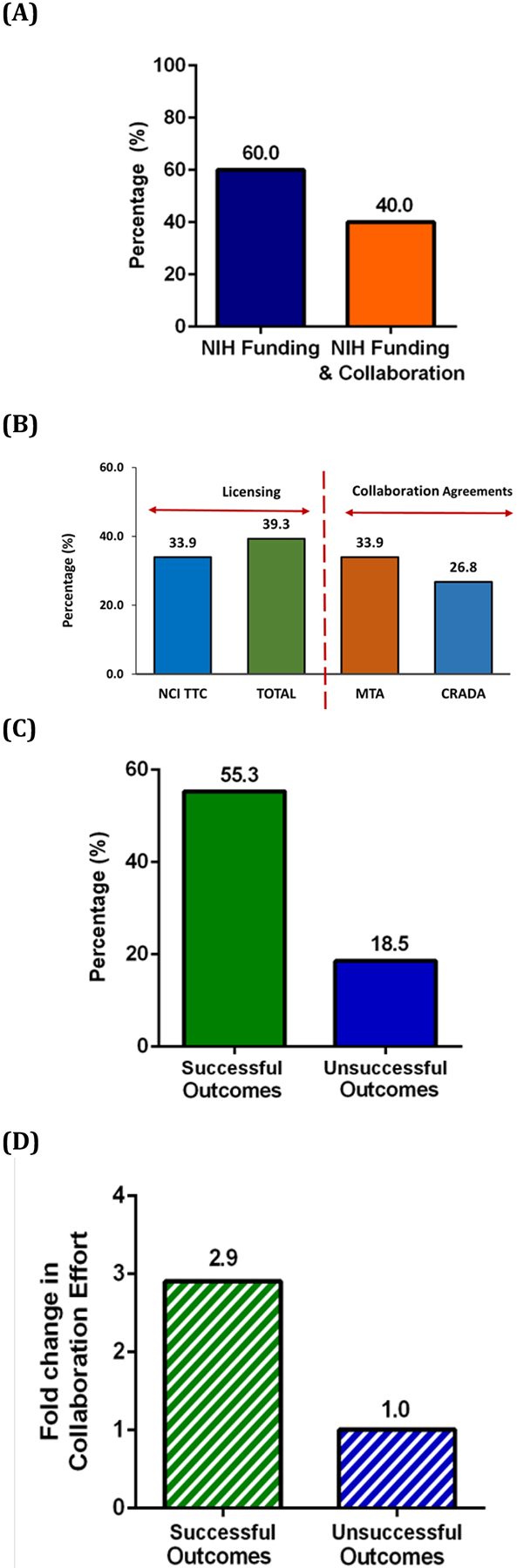
Percentage of collaborations and agreement types for early-stage drug development companies. (A) NCI TTC-mediated collaboration among NIH-funded early-stage drug development companies. (B) Licenses and collaboration agreements with early-stage drug development companies. (C) Percentage of NCI TTC-mediated collaboration of early-stage drug development companies with either a Successful or Unsuccessful Outcome. (D) Fold change in the collaboration effort of early-stage drug development companies with either a Successful or Out of Business status.
Interestingly, while 55.3% of successful early-stage drug development companies had collaborations mediated by NCI TTC, only 18.5% of companies with “Unsuccessful Outcomes” tended to have collaborations mediated by NCI TTC (Figure 5C). This means that companies with “Successful Outcomes” had approximately 2.9 times greater likelihood to have collaborations mediated by NCI TTC than companies with “Unsuccessful Outcomes”. These data suggest that collaborations and licensing partnerships mediated by NCI TTC may contribute to the success of early-stage drug development companies (Figure 5D).
NCI TTC-mediated Collaborations And Licensing Partnerships With The Top 20 Pharmaceutical Companies
NCI TTC mediates collaborations and licensing partnerships with companies of all sizes, in many different stages of development and geographical locations, and with various research and development interests in the life sciences. Large pharmaceutical companies, with substantial capital to perform clinical trials, are key players in bringing cutting-edge technologies to market. Thus, they have a heightened need to be able to access expert medical and research knowledge, experience, and new technologies, making collaborations and licensing partnerships crucial for large pharmaceutical companies as well as small and early-stage companies.
To explore the hypothesis that NCI TTC supports the Top 20 pharmaceutical companies, the pharmaceutical companies with the highest revenue were identified through a Global Data query conducted in May 2021. Then, collaborative and licensing agreement history was collected for the Top 20 pharmaceutical companies using NIH Technology Transfer Databases. The Top 20 pharmaceutical companies have a significant impact on public health with 8,000 marketed drugs (Figure 6A). NCI TTC was discovered to have mediated collaborations and licensing partnerships with all Top 20 pharmaceutical companies in various ways, including Licensing Agreements, Material Transfer Agreements (MTA), Clinical Trial Agreements (CTA), and Cooperative Research and Development Agreements (CRADA) (Figure 6B). Most strikingly, NCI TTC has mediated at least two different collaborative and licensing agreements with all Top 20 pharmaceutical companies. The MTA was the most common agreement executed by NCI TTC with large pharmaceutical companies, followed by Licensing Agreements (Figure 6C-6D). In this study, we did not examine whether NCI or one of the NIH Institutes served by NCI TTC was the provider or recipient. But if a large pharmaceutical company received a material via an MTA executed by NCI TTC, this material could be a very useful tool to advance the company’s technology, e.g., the material could be used to overcome obstacles in the company’s research project. On the other hand, if NCI receives a material from a pharmaceutical company, and develops an invention with the company’s reagent, the large pharmaceutical company would be informed of the invention and would have an opportunity to license it. Eventually, a pharmaceutical company in each situation (recipient or provider) would benefit from an MTA executed by NCI TTC.
Figure 6:
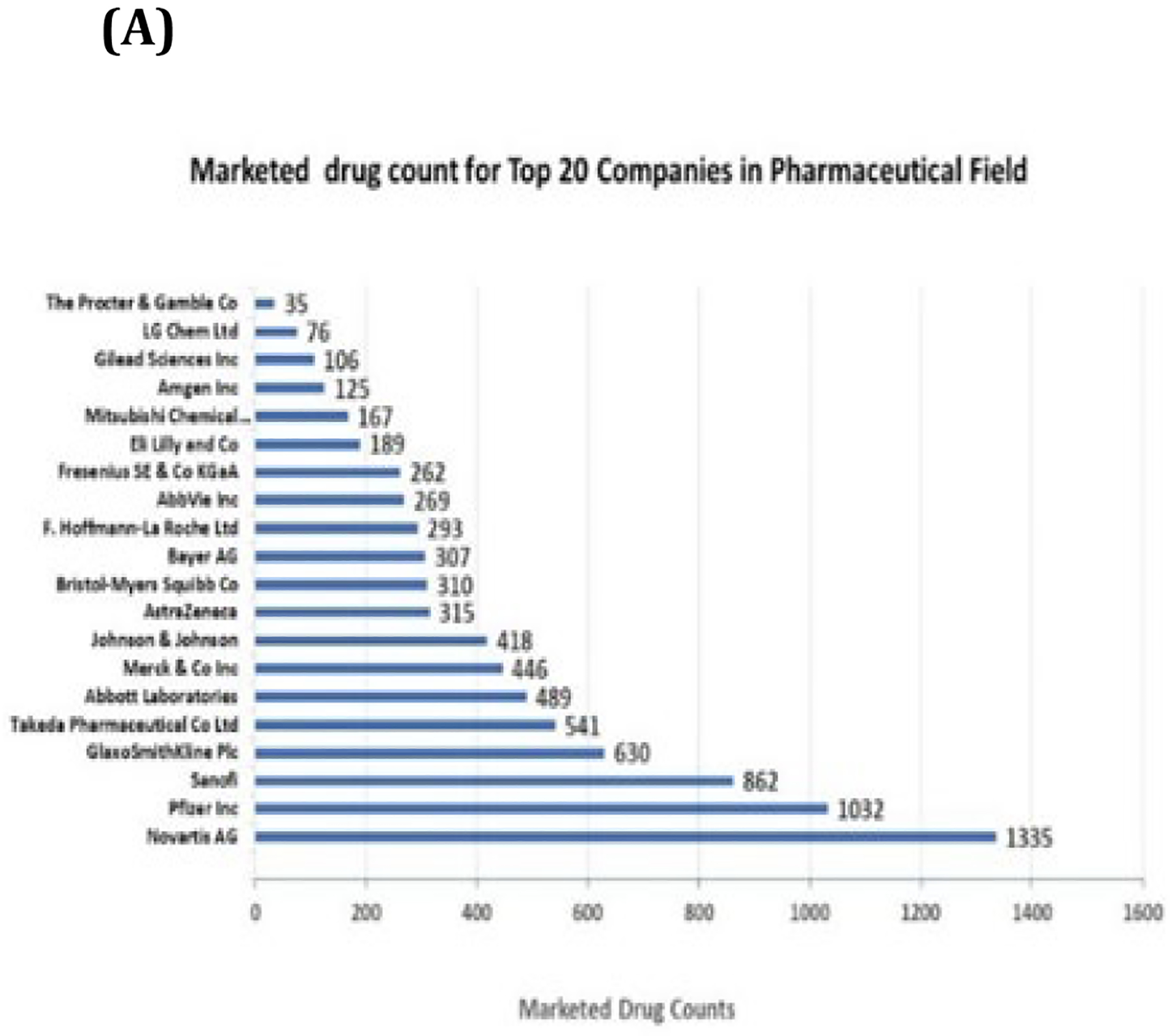
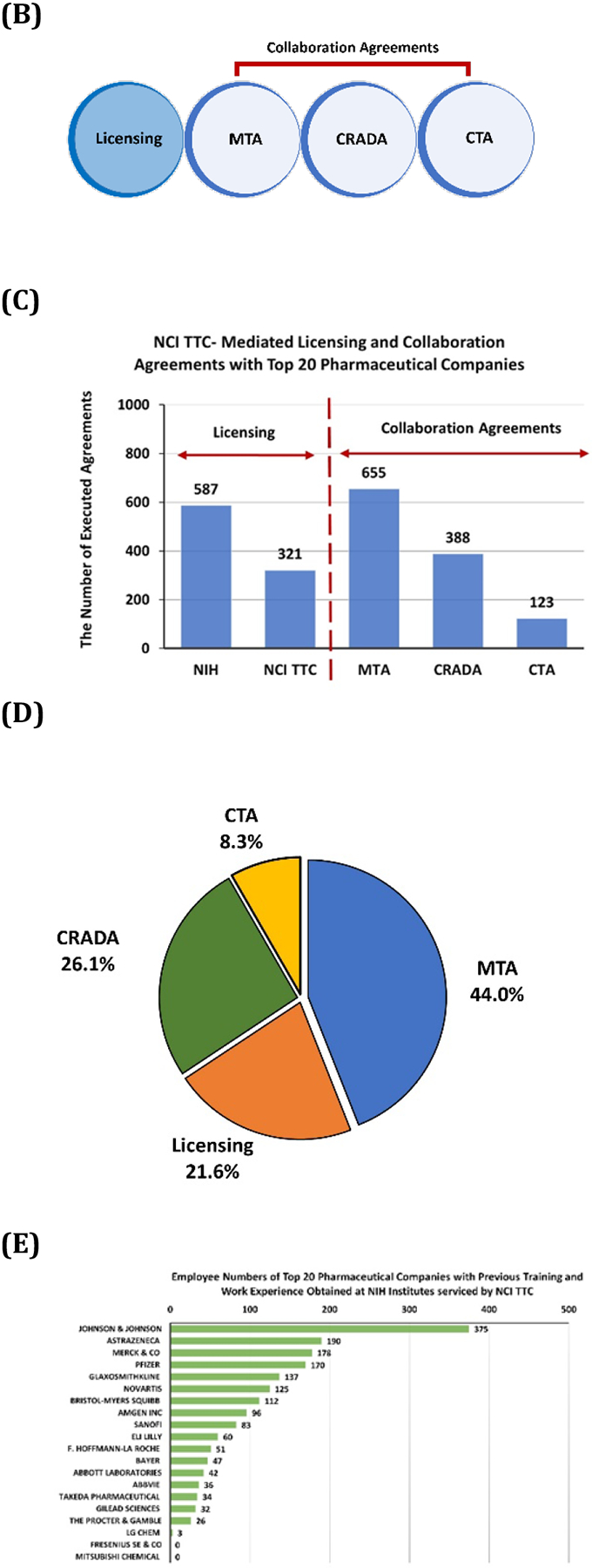
NCI TTC provides collaboration and licensing support to all Top 20 Pharmaceutical Companies. (A) Marketed drug count of Top 20 Pharmaceutical Companies. (B) The types of agreements that NCI TTC executed with the Top 20 Pharmaceutical Companies. (C) Percentage distribution of various NCI TTC-mediated licensing and collaboration agreements. (D) The percentage of licensing and collaboration agreements mediated by NCI TTC with the Top 20 pharmaceutical companies. (E) The number of employees of the Top 20 pharmaceutical companies with prior training and work experience obtained at NCI and the NIH Institutes served by NCI TTC.
NCI-supported Training For Future Pharmaceutical Company Employees
In addition to supporting large pharmaceutical companies with collaborations and licensing partnerships, NIH also provides excellent training for prospective employees of these companies. Current employees of the Top 20 pharmaceutical companies were examined for their educational and work experience history with NCI and the NIH Institutes served by NCI TTC. Interestingly, it was found that over 1,700 former fellows or employees who received training at NCI and the NIH Institutes served by NCI TTC are current employees of the Top 20 pharmaceutical companies (Figure 6E). Thus, NCI and the NIH Institutes served by NCI TTC also contribute toward educating and preparing its trainees to join the Top 20 pharmaceutical companies as a possible career step.
Discussion
It is well-known that collaboration is important in the life sciences. Joint efforts between the public and private sectors may generate promising new therapies and solutions to complex public health challenges. For example, the NIH, Food and Drug Administration (FDA), the Foundation for NIH (FNIH), and the private sector work together in the Partnership for Accelerating Cancer Therapies (PACT) to advance cancer prevention, diagnosis, and treatment by bringing successful cancer immunotherapies to more patients (Baker, Hoos, et al. 2018). This study highlights the value and importance of collaboration and licensing with the NCI and other NIH Institutes supported by NCI TTC.
It is the first study to detail the substantial, but not well-recognized, relationship between collaborating and licensing with the NCI and other NCI TTC client institutes and successful outcomes in early stage life science companies. The primary discovery in these analyses was that early-stage life science companies with “Successful Outcomes” had a nearly 3 times greater likelihood of having collaborative interactions with the NIH, mediated by NCI TTC. This result was maintained when focusing on early-stage companies involved in drug development. Only 12.9% of early-stage life science companies with “Unsuccessful Outcomes” were involved in any collaboration mediated by NCI TTC, and these companies only received 5.7% of NIH funding. Further, the data highlight an important relationship between collaborative and licensing partnerships mediated by NCI TTC and the success of early-stage life science and early-stage drug development companies.
The major benefit of utilizing collaborations with NIH is that it can accelerate drug development in both small and large companies. On average, across the industry, drug development can take 10–15 years (Uygur, et al. 2017), and more than $1.5 billion in investments to take a drug from the discovery stage to FDA approval (Reddy and Chao 2020). To remain competitive and accelerate efforts to develop drugs for unmet medical needs, collaborations and licensing are essential for life science companies to survive the so-called “valley of death” and bring their technologies to market. Thus, the sharing of valuable knowledge and expertise in collaborative and licensing partnerships with NCI and others can help cultivate the rapid development of new therapies and technologies.
Collaborative and licensing relationships between NCI TTC and both the early-stage and Top 20 pharmaceutical companies typically consist of Licensing Agreements, MTAs, CRADAs, and CTAs. For both types of companies. For both types of companies, MTAs and licensing agreements were found to be the most common agreement types. One of the most expensive aspects of the drug development process is clinical trials, and later-phase trials are almost exclusively funded by large pharmaceutical companies. However, NCI is likely the world’s largest holder of Investigational New Drug Applications (INDs) with about 200 active at any given time and thus offers the opportunity for companies to collaborate in advancing their technologies and projects to the clinical stage. Thus, it is not surprising that the NCI TTC was found to mediate many more CTAs for early-stage companies than for large pharmaceutical companies.
It’s also worthwhile to note that NCI TTC provided support to each of the Top 20 pharmaceutical companies in a variety of ways, including collaborative agreements (MTAs, CRADAS, CTAs) and Licensing Agreements. NIH further contributed to the training of some of the employees of the Top 20 pharmaceutical companies. This human capital input is quite significant, with nearly 2,000 current employees of the Top 20 pharmaceutical companies being either former training fellows or former employees of NCI and the NIH Institutes serviced by NCI TTC. If this is expanded to include all NIH Institutes, nearly 6,000 current employees of the Top 20 pharmaceutical companies are either former fellows, interns, or former employees of NIH. As a result, NIH both directly and indirectly impacts drug development in early-stage and large pharmaceutical companies, through funding agreements, collaboration, and licensing agreements as well as training contributions, creating tremendous value and impact for global public health.
Conclusion
In summary, for early-stage life science and drug development companies, collaborative relationships and licensing partnerships mediated by NCI TTC are significantly correlated with “Successful Outcomes”. NIH also plays an important role in providing support to the Top 20 pharmaceutical companies, including educating some of their future employees.
Contributor Information
Berna Uygur, Technology Transfer Center, National Cancer Institute, Rockville, MD.
Steven Ferguson, Office of Technology Transfer, National Institutes of Health, Bethesda, MD.
Michael Pollack, Technology Transfer Center, National Cancer Institute, Rockville, MD.
References
- Baker RG, et al. (2018). “The Partnership for Accelerating Cancer Therapies (PACT).” Cancer journal (Sudbury, Mass.) 24(3): 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham JA and Link AN (2015). “Fostering University-industry R&D Collaborations in European Union Countries.” International Entrepreneurship and Management Journal 11(4): 849–860. [Google Scholar]
- Ferguson SM and Langer LJ (2021). “The US National Institutes Of Health–Founding A National Biomedical “Innovation Ecosystem”.” Journal of Commercial Biotechnology 26(1): 72–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy SSK and Chao S (2020). “Academic Collaborations with Industry: Lessons For the Future.” Journal of Investigative Medicine 68(8): 1305–130. [DOI] [PubMed] [Google Scholar]
- Schofield T (2013). “Critical Success Factors For Knowledge Transfer Collaborations Between University And Industry.” Journal of Research Administration 44(2): 38–56. [Google Scholar]
- Takebe T, et al. (2018). “The Current Status Of Drug Discovery And Development As Originated In United States Academia: The Influence Of Industrial And Academic Collaboration On Drug Discovery And Development.” Clinical and Translational Science 11(6): 597–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uygur B, et al. (2017). “A Guide to Time Lag and Time Lag Shortening Strategies in Oncology-Based Drug Development.” Journal of Commercial Biotechnology 23(2), 75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weckowska DM (2015). “Learning in university technology transfer offices: Transactions-focused And Relations-focused Approaches To Commercialization Of Academic Research.” Technovation 41: 62–74. [Google Scholar]


