Abstract
Octanol–water partitioning experiments in the presence of carboxylate-, phosphate-, and sulfate-containing anionic lipids revealed that Ac-Cav-NH2 (where Cav refers to δ-oxa-arginine) partitions less into octanol than does Ac-Arg-NH2, suggesting that a cell-penetrating peptide based on canavanine would be relatively ineffective.
Graphical Abstract

Introduction
Since the discovery of HIV-TAT peptide and penetratin, arginine-based cell-penetrating peptides (CPPs) have achieved broad use for transporting small molecules, proteins, nucleic acids, and nanoparticles into cells.1 Wender, Rothbard, and coworkers were pioneers in this field, defining the role of peptide length, stereochemistry, and ability to form hydrogen bonds in cell-penetrating ability.2 Subsequent approaches that deployed a variety of molecular architectures have extended the landscape.3
Recently, Schmuck and coworkers investigated the use of guanidiniocarbonyl-pyrroles (GCPs) in cell penetration and found that dimers of this moiety appended onto streptavidin induced uptake whereas dimers of arginine did not.4 The GCP moiety has high affinity for oxoanions such as carboxylates, in part due to its low pKa of ~7.5 Guanidinium groups with lower pKa values are likewise known to form stronger hydrogen bonds with oxoanions.6 These findings inspired us to study a natural guanidinium group with a low pKa value.
Canavanine (Cav), which is δ-oxa-arginine, is a non-proteinogenic amino acid found in the seeds of leguminous plants. Herbivores are discouraged from consuming these seeds because the ribosomal misincorporation of canavanine residues in the place of arginine has deleterious consequences.7 We were intrigued by canavanine because of an attribute that derives from its side-chain oxygen—a low guanidinium pKa. The pKa of the alkylguanidinium group of arginine is 13.8.8 In contrast, the pKa of the alkoxyguanidinium group of canavanine has been reported to be 7.01 and 7.40.8a,9 Arginine is the best of the canonical twenty amino-acid residues at facilitating the translocation of molecules into mammalian cells.2a,10 For two reasons, canavanine could be better still. First, arginine is the most polar proteinogenic amino acid,11 and the translocation of a cationic arginine residue across a nonpolar lipid bilayer is especially endergonic. In contrast, the cationic and neutral forms of canavanine have similar free energies at physiological pH, potentially facilitating membrane transversal. Secondly, stronger acids donate stronger hydrogen bonds.6,12 Accordingly, the salt bridges formed by a cationic canavanine residue with cell-surface anionic groups could be stronger than those formed by a cationic arginine.
Wender, Rothbard, and coworkers demonstrated that octanol–water partitioning can report on desirable attributes of a CPP.2b For example, they observed that fluorophore-labeled Arg8 was transported into the octanol layer upon binding to an amphiphilic lipid, dodecanoate. In contrast, an 8-mer of ornithine (Orn) was less capable of binding to dodecanoate and was retained in the water layer. Because Arg8 enters cells whereas Orn8 does not, an octanol–water partitioning experiment can serve as a proxy for determining cell-penetrating ability.2b
Results and Discussion
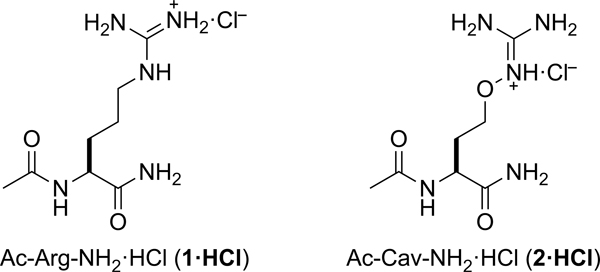
How do the prospects of canavanine as a CPP compare to those of arginine? To answer this question, we sought to measure the partitioning of arginine and canavanine residues in the presence of carboxylate-, phosphate-, and sulfate-containing anionic lipids. To replicate the environment within a peptide, we amidated each amino acid on its N and C termini (Scheme 1). Amidated arginine Ac-Arg-NH2 (1) was obtained from a commercial vendor as an acetic acid salt. Ac-Cav-NH2 (2) was accessed by synthesis (Scheme 2).
Scheme 1.
Structures of Ac-Arg-NH2·HCl (1·HCl) and Ac-Cav-NH2·HCl (2·HCl).
Scheme 2.
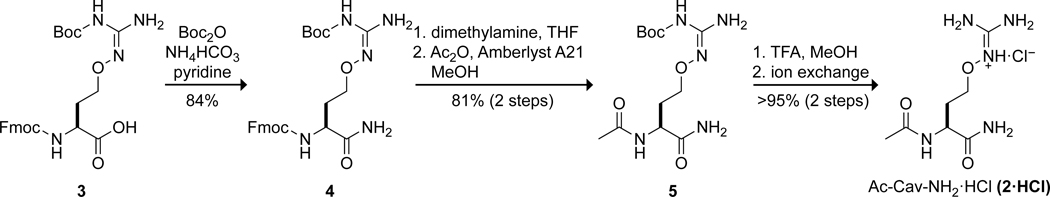
Synthetic route to Ac-Cav-NH2·HCl (2·HCl).
To synthesize Ac-Cav-NH2, the carboxylate of commercial Fmoc-Cav(Boc)-OH (3) was amidated by exposure to Boc2O and ammonium bicarbonate in pyridine to produce Fmoc-Cav(Boc)-NH2 (4). Notably, using traditional solid-phase methods to produce this amide (e.g., loading onto a Rink amide resin with PyBOP, N-acylation with acetic anhydride, and cleavage with TFA and TIPS) were unsuccessful. Subsequently, the Fmoc protecting group was removed in a THF solution of dimethylamine, which was easier to separate via evaporation than the traditional piperidine. Acetylation with acetic anhydride in the presence of the basic resin Amberlyst A-21 produced Ac-Cav(Boc)-NH2 (5). The Boc group was removed by TFA to produce Ac-Cav-NH2 (2) as its trifluoroacetic acid salt.
A rigorous comparison of the ability of Ac-Arg-NH2 (1) and Ac-Cav-NH2 (2) to bind to oxoanions requires that both residues contain the same counterion. To remove the strongly associated acetate and trifluoroacetate counterions, we exposed both 1·HOAc and 2·TFA to excess HCl(aq) and lyophilization. Under these acidic conditions, we observed significant hydrolysis of the C-terminal amide in both residues. To avoid this decomposition, we synthesized a guanidine resin from a commercial 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) resin, charged the guanidine resin with HCl, and used the resulting guanidinium chloride resin to perform ion-exchange. This procedure was successful in replacing the oxoanions with chloride ions, as evidenced by the disappearance of the HOAc protons and TFA fluoro groups by 1H-NMR and 19F-NMR, respectively.
To compare the abilities of canavanine and arginine to bind to oxoanions, 1·HCl and 2·HCl were mixed with three oxoanion lipids that served as models for the functional groups found in membrane phospholipids and cell-surface glycans. Briefly, 1·HCl and 2·HCl were dissolved in D2O (pD 7.0; pH 7.4) and washed with octanol. An aliquot of the D2O layer was carefully excised, and the 1 and 2 content was analyzed by 1H-NMR spectroscopy using an added standard (Figure S1). This procedure enabled us to quantify the octanol–water partitioning without installing a pendant fluorophore or other label, which could be perturbative.
We observed that all of the 1·HCl and 2·HCl remained in the aqueous layer after partitioning with octanol only. Next, we added sodium dodecanoate (6), bis(2-ethylhexyl) phosphate (7), or dodecylsulfate (8) to the octanol wash with the expectation that these oxoanions could bind to the guanidinium groups and, due to their amphipathic nature, draw 1·HCl and 2·HCl into the octanol layer.2b For each combination of amino acid and anionic lipid, we did indeed observe substantial partitioning of the amino acid into the octanol layer (Figure 1). We had hypothesized that canavanine (2), with its significantly lower pKa value, would partition more than arginine (1) into the octanol layer. Surprisingly, with each lipid, less arginine than canavanine remained in the aqueous layer.
Figure 1.
Graph showing the octanol–water partitioning of 1·HCl and 2·HCl in the presence of anionic lipids 6, 7, or 8 (2.5 equiv2b) at pH 7.4 (unless indicated otherwise). Values were determined by 1H-NMR spectroscopy in duplicate experiments (Figures S2–S4).
Finally, we determined whether the lesser ability of canavanine to partition into octanol was due to its lower level of protonation at pH 7.4. To do so, we measured the octanol–water partitioning of arginine (1·HCl) and canavanine (2·HCl) at pH 3.5 with carboxylate 6. We found that the partitioning of canavanine into octanol did not increase at low pH (Figure 1), suggesting that fully protonated canavanine was a less effective transporter than fully protonated arginine.
Why are anionic lipids relatively ineffective at pulling canavanine into octanol? One reason could be the location of its cationic charge, which resides largely on the bridging Nε–H group rather than the two terminal Nη–H groups (Figure S4). That location could engender steric hindrance in interactions with a carboxylate, phosphate diester, or sulfate monoester. In addition, the two lone pairs on the proximal δ oxygen of canavanine could repel the oxygens of the anionic groups, weakening hydrogen bonding.
Conclusions
We sought to assess the prospects of canavanine (2) in comparison to arginine (1) as an effective CPP. Although oxoanionic lipids draw both canavanine and arginine into the octanol layer during octanol–water partitioning, canavanine partitions significantly less extensively into octanol than does arginine. These data suggest that canavanine-based CPPs would be less capable of binding to cell-surface anions and mediating cell penetration than traditional arginine-based CPPs.
Supplementary Material
Acknowledgments
V.M.T. was supported by the Undergraduate Research Opportunities Program at the Massachusetts Institute of Technology. The authors are grateful to N. S. Abularrage and J. V. Jun for comments on the manuscript. This work was supported by Grant R01 GM044783 (NIH).
Footnotes
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].a) For reviews, see: Schwarze SR, Hruska KA, Dowdy SF, Trends Cell Biol, 2000, 10, 290–295. [DOI] [PubMed] [Google Scholar]; b) Fuchs SM, Raines RT, Cell. Mol. Life Sci, 2006, 63, 1819–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Heitz F, Morris MC, Divita G, Br. J. Pharmacol, 2009, 157, 195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Copolovici DM, Langel K, Eriste E, Langel Ü, ACS Nano, 2014, 8, 1972–1994. [DOI] [PubMed] [Google Scholar]; e) Dupont E, Prochiantz A, Joliot A, Methods Mol. Biol., 2015, 1324, 29–37. [DOI] [PubMed] [Google Scholar]; f) Takeuchi T, Futaki S, Chem. Pharm. Bull. (Tokyo), 2016, 64, 1431–1437. [DOI] [PubMed] [Google Scholar]; g) Zhu P, Jin L, Curr Protein Pept. Sci, 2018, 19, 211–220. [DOI] [PubMed] [Google Scholar]; h) Derakhshankhah H, Jafari S, Biomed. Pharmacother, 2018, 108, 1090–1096. [DOI] [PubMed] [Google Scholar]; i) Xie J, Bi Y, Zhang H, Dong S, Teng L, Lee RJ, Yang Z, Front. Pharmacol, 2020, 11, 697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].a) Wender PA, Mitchell DJ, Pattabiraman K, Pelkey ET, Steinman L, Rothbard JB, Proc. Natl. Acad. Sci. USA, 2000, 97, 13003–13008. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Rothbard JB, Jessop TC, Lewis RS, Murray BA, Wender PA, J. Am. Chem. Soc, 2004, 126, 9506–9507. [DOI] [PubMed] [Google Scholar]
- [3].a) For examples, see: Umezawa N, Gelman MA, Haigis MC, Raines RT, Gellman SH, J. Am. Chem. Soc, 2002, 124, 368–369. [DOI] [PubMed] [Google Scholar]; b) Okuyama M, Laman HH, Kingsbury SR, Visintin C, Leo E, Eward KL, Stoeber K, Boshoff C, Williams GH, Selwood DL, Nat. Methods, 2007, 4, 153–159. [DOI] [PubMed] [Google Scholar]; c) Nischan N, Herce HD, Natale F, Bohlke N, Budisa N, Cardoso MC, Hackenberger CP, Angew. Chem., Int. Ed, 2015, 54, 1950–1953. [DOI] [PubMed] [Google Scholar]; d) Nagel YA, Raschle PS, Wennemers H, Angew. Chem., Int. Ed, 2017, 56, 122–126. [DOI] [PubMed] [Google Scholar]; e) Kalafatovic D, Giralt E, Molecules, 2017, 22, 1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Li M, Mosel S, Knauer SK, Schmuck C, Org. Biomol. Chem, 2018, 16, 2312–2317. [DOI] [PubMed] [Google Scholar]
- [5].Schmuck C, Chem.—Eur. J., 2000, 6, 709–718. [DOI] [PubMed] [Google Scholar]
- [6].Rether C, Schmuck C, Eur. J. Org. Chem., 2011, 2011, 1459–1466. [Google Scholar]
- [7].a) Pines M, Rosenthal GA, Applebaum SW, Proc. Natl. Acad. Sci. USA, 1981, 78, 5480–5483. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Rosenthal GA, Lambert J, Hoffmann D, J. Biol. Chem, 1989, 264, 9768–9771. [PubMed] [Google Scholar]; c) Rosenthal GA, Reichhart J-M, Hoffmann JA, J. Biol. Chem, 1989, 264, 13693–13696. [PubMed] [Google Scholar]; d) Rosenthal GA, Dahlman DL, J. Biol. Chem, 1991, 266, 15684–15687. [PubMed] [Google Scholar]
- [8].a) Boyar A, Marsh RE, J. Am. Chem. Soc, 1982, 104, 1995–1998. [Google Scholar]; b) Fitch CA, Platzer G, Okon M, Garcia-Moreno BE, McIntosh LP, Protein Sci, 2015, 24, 752–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Tomiyama T, J. Biol. Chem, 1935, 111, 45–49. [Google Scholar]
- [10].Mitchell DJ, Kim DT, Steinman L, Fathman CG, Rothbard JB, J. Pept. Res, 2000, 56, 318–325. [DOI] [PubMed] [Google Scholar]
- [11].Radzicka A, Wolfenden R, Biochemistry, 1988, 27, 1664–1670. [DOI] [PubMed] [Google Scholar]
- [12].a) Gordy W, Stanford SC, J. Chem. Phys, 1940, 8, 170–177. [Google Scholar]; b) Hine J, Physical Organic Chemistry. McGraw–Hill: New York, 1956; pp 35–36. [Google Scholar]; c) Shan S, Loh S, Herschlag D, Science, 1996, 272, 97–101. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.