Abstract
Oligodendrocytes are highly specialized glial cells, responsible for producing myelin in the central nervous system (CNS). The multi-stage process of oligodendrocyte development is tightly regulated to ensure proper lineage progression of oligodendrocyte progenitor cells (OPCs) to mature myelin producing oligodendrocytes. This developmental process involves complex interactions between several intrinsic signaling pathways that are modulated by an array of extrinsic factors. Understanding these regulatory processes is of crucial importance, as it may help to identify specific molecular targets both to enhance plasticity in the normal CNS and to promote endogenous recovery following injury or disease. This review describes two major regulators that play important functional roles in distinct phases of oligodendrocyte development: OPC proliferation and differentiation. Specifically, we highlight the roles of the extracellular astrocyte/radial glia-derived protein Endothelin-1 in OPC proliferation and the intracellular Akt/mTOR pathway in OPC differentiation. Lastly, we reflect on how recent advances in neuroscience and scientific technology will enable greater understanding into how intrinsic and extrinsic regulators interact to generate oligodendrocyte diversity.
Keywords: Oligodendrocyte progenitors, Subventricular zone, Proliferation, Differentiation, Endothelin-1, Erk1/2, mTOR
1. Introduction
Myelination is a remarkable and complex developmental process essential for normal nervous system function. In the central nervous system (CNS), myelin is produced by oligodendrocytes and the development of this cell lineage has been intensively investigated for more than 40 years. This well-characterized developmental program can be defined by stages of oligodendrocyte progenitor cell (OPC) specification, proliferation and differentiation, followed by maturation of the myelinating oligodendrocytes. This lineage progression is very complex, i.e., modulated by many extracellular signals and intracellular regulatory pathways. It is clearly essential for normal brain development, but many studies demonstrate that this signaling is also important for recovery and remyelination following brain injury at all ages - for example, in neonatal white matter injury, multiple sclerosis, and other neurological disorders. Thus, characterizing the regulation of oligodendrocyte development will help to identify potential therapeutics to enhance repair in the immature or adult brain.
Extensive studies and review articles have identified the individual and/or synergistic roles of specific growth factors and other extracellular signals that regulate different stages of oligodendrocyte development (see Dubois-Dalcq et al. 2000). These types of signals include morphogens such as sonic hedgehog, which drives initial specification of early precursor cells to the oligodendrocyte lineage (Pringle et al. 1996; Qi et al. 2002), and growth factors, such as platelet derived growth factor (PDGF) or fibroblast growth factor (FGF), which drive proliferation of the resulting OPCs (Collarini et al. 1991). Other molecules actively repress initial differentiation of OPCs into premyelinating oligodendrocytes, for example bone morphogenetic protein (See et al. 2009), while others drive differentiation, e.g., thyroid hormone (Rodríguez-Peña et al. 1999).
While understanding the numerous extracellular signals that drive different phases of the oligodendrocyte developmental program is essential, many investigators have also focused on the intracellular signals that mediate outcomes of the complex network of extracellular signaling (Gaesser et al. 2016). Several intracellular pathways have been identified that impact one or more aspects of oligodendrocyte development, including the MAP kinase pathway, the mTOR pathway, and the Wnt pathway (Gaesser et al. 2016).
Given the wide variety of extracellular signals and intracellular pathways that modulate oligodendrocyte development, it is clear that no simple model of oligodendrocyte development can integrate these signals into specific developmental processes. Furthermore, diverse local extracellular signals undoubtedly drive the recently identified regional heterogeneity of oligodendrocyte gene expression (Marques et al. 2016). Given the extensive literature on this broad topic and the complexity of this developmental program, we choose to focus this review on two specific extracellular/intracellular drivers of oligodendrocyte development that our laboratories have investigated in great detail over the years. We first analyze a specific extracellular signal and its downstream signaling that drives OPC proliferation - Endothelin-1 (ET-1), followed by an intrinsic signal that drives OPC differentiation and myelination – the Akt/mTOR pathway. These pathways provide examples of the intricate phasic signaling mechanisms regulating oligodendrocyte development, how cross-regulation may allow fine-tuning of cellular outcomes, and importantly, how many questions still remain to be answered.
2. Maintaining the proliferative OPC state
During CNS development, radial glial cells (or neural stem cells) in germinal zones give rise to OPCs in a temporal and region-specific manner. OPCs are bipolar, proliferative cells that migrate extensively throughout the CNS – ultimately residing in both gray and white matter regions, where they differentiate into myelinating oligodendrocytes or remain undifferentiated, serving as a pool of progenitors that respond to environmental cues. The density of OPCs is tightly controlled by multiple mechanisms, including programmed cell death (Kessaris et al. 2005), proliferation of existing OPCs (Hughes et al. 2013), and de novo genesis of OPCs from stem cell niches (Menn et al. 2006). Importantly, OPCs continue to be generated from neural stem cells postnatally and throughout adulthood, with the subventricular zone (SVZ) of the lateral ventricles being the main source in the adult brain (Menn et al. 2006). OPCs are essential not only for developmental myelination and remyelination following injury, but also for myelin plasticity and neural circuit modulation, as it has been shown that OPCs respond to neuronal activity with increased proliferation and differentiation (Nagy et al. 2017). Therefore, understanding the regulatory processes maintaining the progenitor state of OPCs is of high importance as it may lead to identifying molecular targets to enhance neural plasticity, and to promote endogenous recovery following injury, disease, or in neurodevelopmental disorders.
2.1. Endothelin-1: An extrinsic regulator of OPC development
Multiple extracellular signaling proteins regulate OPC proliferation and migration. How these different signals integrate and co-regulate each other is an important question that remains largely unknown. Furthermore, identifying how these signals differ across different CNS regions (i.e, brain versus spinal cord, gray versus white matter) will lead to critical insight into OPC heterogeneity. Recently, the Gallo laboratory identified a novel role for the signaling protein ET-1 as an extrinsic regulator of OPC proliferation within the postnatal SVZ (Adams et al. 2020). ET-1 has been extensively characterized for its role in vasoconstriction and, more recently, been shown to modulate nervous system development and regeneration (Gadea et al. 2008; Gadea et al. 2009; Hammond et al. 2014, 2015). ET-1 is a small secreted signaling protein that binds to two G-protein-coupled receptors (GPCRs), Ednra and Ednrb, the latter of which is highly expressed by OPCs (Gadea et al. 2009; Adams et al. 2020). Within the developing postnatal SVZ, ET-1 is primarily expressed by radial glial cells (Adams et al. 2020), mirroring its expression in astrocytes (Gadea et al. 2008; Gadea et al. 2009; Hammond et al. 2014). Interestingly, ablation of Ednrb expression in OPCs reduces the total number of NG2+ OPCs and the percentage of proliferating OPCs within the early postnatal SVZ (Adams et al. 2020). This phenotype is also observed when ET-1 levels are reduced in the postnatal SVZ (Adams et al. 2020). Conversely, exogenous ET-1 treatment increases both the density of OPCs and the percentage of proliferating OPCs within the SVZ of organotypic brain slice cultures (Adams et al. 2020). Therefore, ET-1 binds directly to Ednrb receptors on OPCs within the SVZ to promote their proliferation (Figure 1A).
Figure 1: Mechanisms of ET-1 signaling in OPCs.
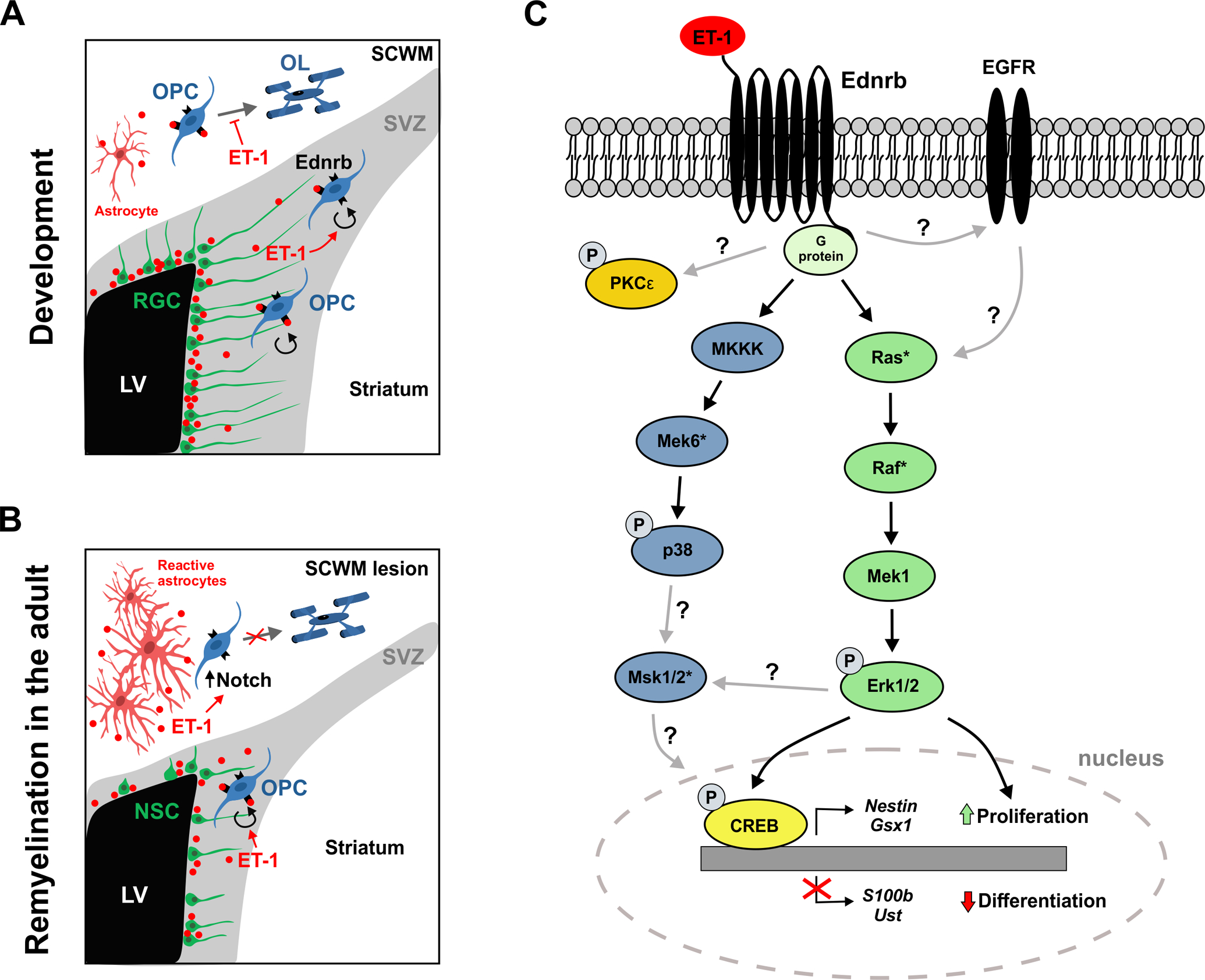
(A) Schematic describing inter-cellular ET-1 signaling in the early postnatal mouse subventricular zone (SVZ) and subcortical white matter (SCWM). Radial glial cells (RGC) in the SVZ secrete ET-1 which binds to Ednrb receptors on OPCs to promote their proliferation. Astrocytes in the SCWM secrete ET-1 which binds to Ednrb receptors on OPCs to block their differentiation. LV = lateral ventricle. (B) Schematic describing inter-cellular ET-1 signaling in the adult mouse following focal demyelination of the SCWM. Neural stem cells (NSCs) in the SVZ upregulate ET-1 which binds to Ednrb receptors on OPCs to promote their proliferation. Reactive astrocytes in the SCWM lesion secrete high levels of ET-1, which indirectly activates Notch signaling in OPCs and blocks their differentiation. LV = lateral ventricle. (C) Proposed intracellular signaling pathways activated by ET-1 signaling in OPCs. ET-1 binding to the Ednrb receptor primarily activates the MAPK/Erk pathway, leading to CREB phosphorylation and transcriptional changes that promote proliferation and progenitor maintenance. Black arrows indicate well-established protein interactions in OPCs, while gray arrows indicate protein interactions that have been described in other cell types, but remain uncharacterized in OPCs. Asterisks denote proteins that still require confirmation of activation upon ET-1 signaling in OPCs.
Does ET-1 also play a role in OPC maintenance and/or differentiation? During the first month of postnatal development, OPCs within the subcortical white matter differentiate and mature into oligodendrocytes that myelinate callosal axons (Sturrock 1980). Ablation of the Ednrb receptor from OPCs at early postnatal ages decreases the percentage of NG2+ OPCs and increases the percentage of mature CC1+ oligodendrocytes within the subcortical white matter (Adams et al. 2020). This corresponds with an increase in myelin basic protein (MBP) expression in the corpus callosum (Adams et al. 2020). Therefore, loss of ET-1 signaling promotes OPC differentiation in the developing postnatal subcortical white matter. This confirms previous in vitro findings, where exogenous ET-1 delays the progression of cultured O4+ pre-oligodendrocytes to O1+ oligodendrocytes (Gadea et al. 2009). In addition, blocking endothelin receptor activity with the antagonist Bosentan results in increased oligodendrocyte differentiation in organotypic brain slices (Gadea et al. 2009). Importantly, no differences in cell survival were observed in vivo or in vitro upon loss of ET-1 signaling, indicating that ET-1 does not regulate OPC or oligodendrocyte cell survival (Adams et al. 2020; Gadea et al. 2008) Interestingly, blocking ET-1 signaling did not affect OPC proliferation within the subcortical white matter (Adams et al. 2020) or in primary OPC cultures (Gadea et al. 2009). Therefore, ET-1 appears to specifically promote OPC proliferation within the SVZ and repress differentiation within the subcortical white matter. This is likely due to ET-1 acting in concert with, and potentially amplifying, other extrinsic and intrinsic signaling pathways that regulate OPC function within each brain region. Future studies will need to investigate possible mechanisms underlying this heterogeneity. However, it is important to note that ET-1 promotes the same differential response in each respective brain region following focal demyelination of the subcortical white matter in adult mice (Hammond et al. 2014, 2015; Adams et al. 2020) (Figure 1B). Therefore, ET-1-dependent developmental signaling pathways are reactivated in the injured adult brain.
In addition to proliferation, OPCs are also highly migratory cells. Migration is tightly linked to the progenitor state, as OPCs lose their ability to migrate as they start to differentiate (Bergles and Richardson 2016). Exogenous ET-1 stimulates OPC migration in vitro, while the addition of anti-ET-1 antibodies to organotypic brain slices inhibits progenitor migration (Gadea et al. 2009). Interestingly, this effect on migration requires the presence of PDGF and FGF signaling molecules, as ET-1 alone does not increase OPC migration (Gadea et al. 2009). The role of ET-1 in OPC migration in vivo has yet to be determined and poses significant challenges, as it is difficult to distinguish whether altered cell numbers are due to changes in migration, effects on proliferation, or a combination of both. However, RNAseq analysis of primary SVZ OPCs treated with ET-1 identified multiple differentially expressed genes, a subset of which function in cellular migration and movement (Adams et al. 2020, unpublished data). Therefore, ET-1 likely promotes both OPC proliferation and migration – two critical aspects of OPC function.
2.2. ET-1 downstream signaling pathways
The effect of ET-1 on cellular proliferation is not unique to OPCs as ET-1 has been shown to promote proliferation of smooth muscle cells (Alberts et al. 1994), endothelial cells (Spinella et al. 2009), astrocytes (Gadea et al. 2008), and radial glial cells (Adams et al. 2020). The endothelin proteins are also known regulators of cell migration – the most noteworthy being Endothelin-3’s regulation of enteric neural crest cell migration, as dysregulation of this pathway results in Hirschsprung’s disease (Sanchez-Mejias et al. 2010). Interestingly, ET-1 activates a variety of downstream pathways within each cell population and tissue microenvironment. The receptors Ednra and Ednrb are seven transmembrane domain GPCRs that bind to many types of G-proteins, predominantly signaling through G-protein alpha-q/11 and alpha-i family subunits (Shraga-Levine et al. 2000; Cramer et al. 2001). In OPCs, ET-1 binds to Ednrb, initiating signaling cascades that result in activation of the MAP kinase (MAPK) pathways. ET-1 treatment of cultured OPCs results in increased phosphorylation of Erk and p38 MAPK within 5 minutes (Gadea et al. 2009). This suggests that ET-1 activates multiple MAPK pathways, as Erk and p38 typically function in parallel pathways, although they are capable of cross-regulation (Koga et al. 2019) (Figure 1C). Interestingly, Ednrb activation has also been shown to trans-activate the epidermal growth factor (EGF) receptor in vascular smooth muscle cells (Iwasaki et al. 1998) and Müller cells of the chicken retina (Harun-Or-Rashid et al. 2016). As EGF is a principal regulator of OPC proliferation and migration in the postnatal SVZ (Aguirre et al. 2007), it will be important to confirm whether ET-1 signaling augments the EGF downstream signaling cascade in OPCs to ultimately synergize with EGF in regulating OPC function. Lastly, ET-1 also induces phosphorylation of cyclic AMP response element-binding protein (Creb) in cultured OPCs (Gadea et al. 2009). Creb activation is downstream of multiple signal transduction pathways, including the MAPK/Erk pathway, and promotes a variety of cellular responses, including growth and proliferation (Koga et al. 2019). Together, these results indicate that ET-1 likely activates several signal transduction pathways in OPCs, but primarily activates the MAPK/Erk pathway (Figure 1C). The roles of MAPK signaling in oligodendrocyte development will be further discussed in the next two sections; however, additional characterization of the intracellular signaling cascades induced by ET-1 binding to OPCs is greatly needed, as large gaps in our knowledge of these pathways remain (Figure 1C).
In addition to protein phosphorylation, significant changes in OPC gene expression following ET-1 treatment have also been recently established (Adams et al. 2020). Using cultured SVZ OPCs, seventy-two differentially expressed genes were identified at 24 hours following ET-1 treatment. Identification of upstream regulators using Ingenuity Pathway Analysis software pinpointed several molecular mechanisms as potential upstream activators of these genes, including Erk, Map2k1 (also known as Mek1), and Creb1 (Adams et al. 2020). This indicates that ET-1-induced changes in gene expression are consistent with activation of the MAPK/Erk pathway. Importantly, while the majority of the seventy-two genes have unknown functions in oligodendrocyte development, there are several genes with interesting known roles. For example, the transcription factor Gsx1 and the stem/progenitor gene Nestin are upregulated following ET-1 treatment. The exact role of Gsx1 in OPCs remains to be investigated, but a recent study found that Gsx1/2 mutant embryos display reduced OPC proliferation (Chapman et al 2018), and Nestin expression is higher in OPCs and downregulated at more mature developmental stages (Gallo and Armstrong, 1995). Additionally, ET-1 treatment decreases expression of S100b, which promotes oligodendrocyte differentiation and maturation (Deloulme et al. 2004). This is consistent with ET-1’s role in progenitor state maintenance and preventing differentiation. Therefore, ET-1 signaling likely maintains OPCs through both upregulation of pro-progenitor genes and downregulation of maturation genes. Further understanding of the roles that these differentially expressed genes play in OPC physiology, including cell migration, will be important for obtaining a complete picture of ET-1 signaling in oligodendrocyte development.
2.3. The MAPK pathway: An intrinsic regulator of OPC proliferation
In addition to ET-1, other extracellular signaling molecules activate MAPK pathways in OPCs. These include FGF and PDGF, which both promote OPC proliferation via activation of Erk1/2, p38 MAPK, and pp70 S6 kinase (Baron et al. 2000), and PDGF can regulate OPC migration via activation of Erk (Frost et al. 2008; Singh et al. 2019). In addition, BDNF activates MAPK signaling via TrkB receptor activation, resulting in one study in enhanced proliferation of basal forebrain OPCs in vitro (Veer et al. 2009), although a different study reported that knockdown of TrkB in cultured OPCs resulted in increased OPC proliferation (Wong et al. 2013). Therefore, further investigation into BDNF’s impact on OPC proliferation is needed. Other known regulators of OPC proliferation and differentiation, such insulin growth factor 1 (IGF-1) (Cui and Almazan 2007) and neurotrophin-3 (NT-3) (Xiao et al. 2009) also activate MAPK signaling. Therefore, MAPK pathways integrate multiple extracellular signals in OPCs, likely resulting in signal amplification, as well as more robust and prolonged intracellular and transcriptional changes. Importantly, the strength and duration of MAPK activation can be key determinants of the subsequent biological outcome (Ramos 2008); therefore, future studies should take into account the exact level and timing of MAPK activation or inhibition when interpreting results.
Because of the convergence of multiple extracellular signals onto MAPK signaling transduction pathways, researchers have also examined the role of specific kinases in OPC proliferation, producing conflicting results. The majority of studies on MAPK/Erk signaling in OPCs have been in vitro assays using pharmacological inhibitors or activators of different kinases in cultured OPCs. Multiple reports show that blocking Erk1/2 activation in cultured OPCs reduces proliferation, thereby blocking the mitogenic effect of growth factors mentioned above (Baron et al. 2000; Frederick et al. 2007). However, there are also several studies that failed to observe changes in OPC proliferation in vitro following modulation of MAPK signaling, including Erk2 ablation (Fyffe-Maricich et al. 2011) and expression of constitutively activated-Mek1 (Xiao et al. 2012). These inconsistencies are likely due to different experimental designs, including starting cell populations and culture conditions, as well as potential compensatory effects of other MAPK proteins. Therefore, in vivo studies of MAPK signaling in OPCs provide a potentially more relevant and complete, albeit more complex, analysis. Sustained activation of Erk1/2 by expression of constitutively active Mek1 in Olig1+ cells results in transient hyperproliferation and overproduction of OPCs in the mouse spinal cord (Ishii et al. 2013). Conversely, loss of Erk1/2 reduces OPC proliferation but only when ablation of Erk1/2 occurred in Olig1+ cells, not NG2+ or CNP+ cells (Ishii et al. 2013; Ishii et al. 2012). The authors hypothesize that Erk1/2 signaling plays a significant role in proliferation at the earlier Olig1+ stage, but is dispensable once cells reach the NG2+ stage. Another study found that Erk1/2 deletion in Olig2+ cells also reduces OPC proliferation in the embryonic spinal cord (Newbern et al. 2011). Therefore, MAPK/Erk signaling does appear to regulate OPC proliferation in vivo. However, additional studies using tamoxifen inducible Cre mouse strains are needed to assess the role of Erk1/2 in OPC development postnatally, especially in the brain. Furthermore, defining the role of specific MAPK proteins in specific stages of oligodendrocyte development remains a high priority.
2.4. Other functions of ET-1 and MAPK in oligodendrocyte development
A puzzling question that remains in developmental biology is how does the same signal result in differential cellular outcomes, such as proliferation and differentiation? In addition to ET-1’s role in OPC maintenance as described above, ET-1 can also regulate CNS myelination, suggesting that ET-1 regulates multiple stages of oligodendrocyte development. Ablation of Ednrb expression from embryonic OPCs decreases the number of myelin sheaths per oligodendrocyte in the prefrontal cortex of juvenile mice (Swire et al. 2020). Interestingly, in contrast to other studies, there was no difference in the total number of Olig2+ cells or mature CC1+ oligodendrocytes following Ednrb ablation. However, this may be due to the long experimental timeline, which may allow other extrinsic signaling mechanisms, such as FGF or EGF, to compensate for the loss of Ednrb (Baron et al 2000; Aguirre et al. 2007). This may also reflect regional differences in the functional role of ET-1 signaling in oligodendrocytes. A comparable myelin sheath phenotype was also seen in a global Ednrb knockout zebrafish model, along with an increase in oligodendrocyte numbers (Swire et al. 2020), which is likely due to Ednrb ablation in radial glial cells (Adams et al. 2020). Ednrb activation in mouse oligodendrocyte cultures induced phosphorylation of serine-729 of PKC epsilon, an isoform of the Protein Kinase C (PKC) family (Swire et al. 2020), which suggests that ET-1 may activate distinct intracellular signaling pathways, depending on the developmental stage. As PKC epsilon can activate Erk1/2 in other cell types (Schönwasser et al. 1998), it is also possible that MAPK/Erk signaling is involved in both early and late roles of ET-1 signaling. The question of whether ET-1 activates PKC signaling in OPCs remains unanswered.
The role of MAPK signaling in OPC differentiation and maturation has also been widely investigated. Several reports identified Erk1/2 signaling as a regulatory pathway of OPC differentiation to oligodendrocytes (Fyffe-Maricich et al. 2011; Baron et al. 2000; Dai et al. 2004; Suo et al. 2018), but separate studies found no changes in OPC differentiation following Erk1/2 manipulation in vitro and in vivo (Ishii et al. 2012; Ishii et al. 2013; Xiao et al. 2012). These conflicting reports are difficult to reconcile, but again likely stem from different experimental approaches and conditions. As genetic manipulation of Erk1/2 in oligodendrocyte lineage cells in vivo has failed to reveal any effects on differentiation, it strongly indicates that MAPK/Erk signaling does not play an important role in this process. However, there is consistent evidence of MAPK signaling regulating myelination. Activation of Erk1/2 signaling in oligodendrocytes in vivo results in dramatic increases in myelin sheath thickness (Ishii et al. 2013), whereas Erk1/2 knockout mice display thinner myelin sheaths (Ishii et al. 2012). Furthermore, Erk1/2 activation in myelinating co-cultures increases myelin protein expression and the number of myelinated axonal segments (Xiao et al. 2012). There are several possible explanations for how MAPK/Erk signaling promotes myelination, which have been nicely reviewed elsewhere (Gonsalvez et al. 2016). Interestingly, a recent study found that Mek/Erk1/2 cooperates with the Akt/mTOR pathway, at the level of mTOR, to promote efficient growth of the myelin sheath (Ishii et al. 2019). This suggests that interactions between intracellular signaling pathways regulating OPC proliferation and differentiation may allow a level of fine-tuning that subsequently results in diverse cellular outcomes.
3. Regulation of oligodendrocyte differentiation
Once sufficient OPCs have been specified, they migrate to appropriate sites where they proliferate and begin to differentiate in response to local cues (Levison et al. 1993; Davies et al. 2001; Tsai et al. 2016). The process of differentiation requires that oligodendrocytes increase in size, through increased transcription and translation of membrane proteins, primarily myelin proteins (Barbarese et al. 1995), and the upregulation of membrane lipid synthesis (Morell et al. 1984). Differentiating oligodendrocytes extend membrane protrusions, transport lipids and proteins to the leading edge of that membrane, wrap the membrane around a target axon, and then finally compact the membrane (Snaidero et al. 2017). Generating the characteristic oligodendrocyte morphology additionally requires the dynamic activity of cytoskeleton components, such as actin and tubulin, as well as proteins that regulate the cytoskeleton (Gillespie et al. 1989; Nawaz et al. 2015; Zuchero et al. 2015). These varied developmental activities are initiated by extrinsic signaling factors but are regulated and driven by intrinsic signaling pathways.
3.1. Extracellular signaling driving oligodendrocyte differentiation
There is evidence that OPCs have an intrinsic capability of differentiating. For example, purified OPCs incubated with nanofibers in the presence of basal media without excess growth or differentiation factors differentiate and wrap the nanofibers (Mei et al. 2014; Ong et al. 2018). Nevertheless, the classic differentiation factor enhancing oligodendrocyte differentiation is thyroid hormone (Bhat et al. 1979), and thyroid deficiencies have clear impact on developmental myelination. Neuregulin-1 is another extrinsic factor that enhances oligodendrocyte differentiation (Canoll et al. 1994; Vartanian et al. 1999; Taveggia et al. 2008). Neuregulin-1 has an impact on spinal cord oligodendrocyte differentiation but not myelination (Vartanian et al. 1999; Taveggia et al. 2008). In contrast, loss of neuregulin-1 signaling in the forebrain has minimal impact on oligodendrocyte differentiation, but does impact myelination (Brinkmann et al. 2008; Taveggia et al. 2008; Makinodan et al. 2012). The extracellular factor laminin-2 (Lm2) is also an extrinsic factor that regulates oligodendrocyte differentiation (Buttery et al. 1999). Lm2 signals through receptors α6β1 integrin and dystroglycan to activate the tyrosine-protein kinase, Fyn. Upon binding Lm2, the Lm2 receptors form a complex with receptor-like protein tyrosine phosphatase alpha (PTPα) to signal for differentiation (Ly et al. 2018). Another extracellular signal, pleiotrophin (PTN) promotes oligodendrocyte differentiation through inhibition of protein tyrosine phosphatase receptor type Z (PTPRZ) (Kuboyama et al. 2012; Kuboyama et al. 2015). Lastly, FGF signaling through the FGF Receptor 2 (FGFR2) functions in the later stages of myelination by increasing myelin thickness (Furusho et al. 2017). Overall, it is clear that many different extracellular factors promote oligodendrocyte differentiation. We can then ask what are the intracellular signals that are being activated by these extracellular factors to drive cellular activities necessary for differentiation.
3.2. Akt/mTOR signaling: a major pathway enhancing oligodendrocyte differentiation
Many of these extracellular signals that drive oligodendrocyte differentiation can activate the Akt/mTOR pathway (Figure 2A). mTOR is a serine/threonine kinase in the Akt/mTOR pathway, and in general, chemically inhibiting the mTOR pathway dramatically inhibits oligodendrocyte differentiation (Narayanan et al. 2009; Tyler et al. 2009; Lebrun-Julien et al. 2014; Sachs et al. 2014). The initial focus on the mTOR pathway came from early studies on neuregulin-1 signaling in oligodendrocytes (Flores et al. 2008; Narayanan et al. 2009; Goebbels et al. 2012). These and other studies demonstrate that mTOR is a major regulator and driver of oligodendrocyte differentiation and myelination induced by extracellular signals (Bercury et al. 2014; LeBrun-Julien et al. 2014; Wahl et al. 2014; Zou et al. 2014). For example, the activated Lm2 receptors described above that form a complex with PTPα activate Fyn and Akt, inducing oligodendrocyte differentiation (Ly et al. 2018). Additionally, the PTN-mediated inhibition of PTPRZ signaling promotes oligodendrocyte differentiation through actin filament associate protein 1-like 2 (AFAP1L2), which is an adaptor protein in the PI3K/Akt pathway. In a mouse model expressing a catalytically inactive mutant of PTPRZ, the reduced PTN-PTPRZ pro-differentiation signal is partly rescued by the phosphorylation of AFAP1L2 to activate Akt/mTOR (Tanga et al. 2019). Interestingly, FGF signaling through FGFR2, which is typically associated with Erk1/2 signaling, increases myelin thickness, which is typically associated with mTOR signaling (Furusho et al. 2017). Thus, the FGF/FGFR2 signal increases Erk1/2 activation, which acts with Akt signaling and converges on mTOR to increase myelin thickness. Importantly, Akt and Erk1/2 converge on mTOR signaling in varying degrees depending on the stage of differentiation. For example, while Akt signaling to mTOR is required early, Erk1/2 signaling functions through mTOR during later myelination stages (Ishii et al. 2019).
Figure 2: Extracellular signals activate the intracellular signaling pathway mTOR.
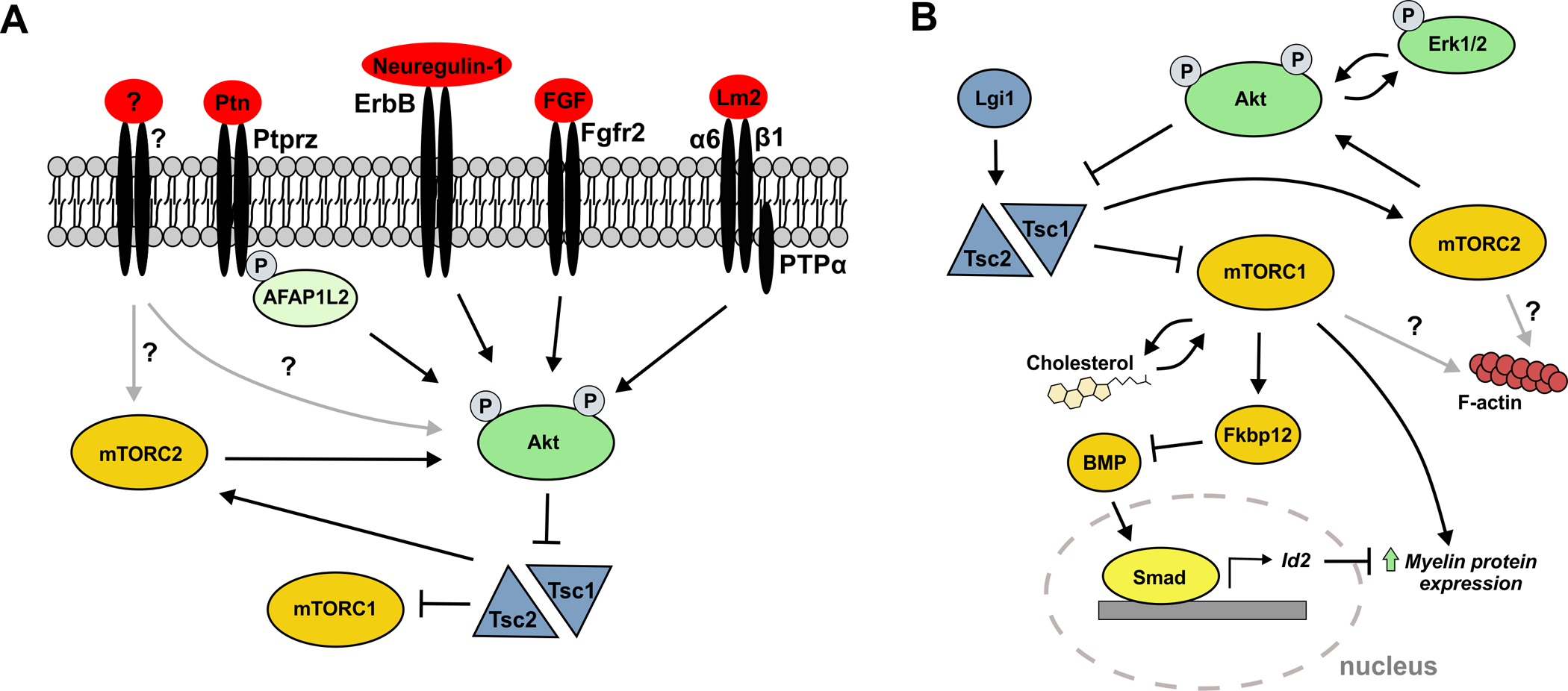
(A) Model of extracellular signals that function as cues for OPC differentiation through the activation of Akt in the Akt/mTOR pathway. Neuregulin is a canonical extracellular factor that triggers the activation of Akt/mTOR in oligodendrocytes to drive differentiation, and several other pro-differentiation extracellular factors have recently been found to also activate Akt. Akt is activated through phosphorylation at threonine 308 by the upstream PI3K pathway, and is further phosphorylated at serine 473 by mTORC2. Activated Akt regulates mTORC1 by inhibiting the TSC1/TSC2 complex which inhibits mTORC1 activation by a guanine nucleotide exchange factor. (B) While activation of Akt can be achieved by extracellular signaling, intracellular regulation functions to internally control Akt/mTOR activity. Cholesterol, an important component of the oligodendrocyte membrane, and mTOR are dependent on each other and mTOR may signal through cholesterol-rich signaling domains for differentiation. Akt and Erk1/2 signaling converge on mTORC1 to drive myelination. Activation and regulation of mTOR activity ultimately impacts downstream targets that function in the cellular activities necessary for differentiation, such as downregulation of BMP signaling and actin polymerization. Bars indicate inhibitory activity; arrows indicate activation or positive interaction. Gray arrows indicate possible protein interactions that remain uncharacterized in oligodendrocytes.
The Akt/mTOR pathway drives numerous cellular changes that oligodendrocytes undergo as they mature, such as altered transcription and translation driving cell growth, lipid biosynthesis, and cytoskeleton regulation. Expression of myelin specific proteins, such as proteolipid protein (PLP) and MBP, is dependent on mTOR signaling (Bercury et al. 2014; Dai et al. 2014; Lebrun-Julien et al. 2014; Musah et al. 2020). mTOR regulates overall lipogenesis in oligodendrocytes, which is necessary for membrane growth (Lebrun-Julien et al. 2014). Cholesterol is a major lipid component of the oligodendrocyte membrane and it is also necessary for myelin protein expression (Saher et al. 2005). One recent study found that mTOR signaling was dependent on cholesterol, potentially through cholesterol-rich signaling domains, while cholesterol-mediated protein expression was in turn dependent on mTOR signaling (Mathews et al. 2016). Thus, mTOR and cholesterol appear to participate in an interactive signaling system in oligodendrocytes to drive myelination, although it is likely quite complex (Figure 2B).
In conclusion, numerous extracellular signals converge on mTOR signaling to drive oligodendrocyte differentiation and myelination. How it is regulated and how it alters oligodendrocyte gene expression and myelination itself are major areas of investigation.
3.3. Regulators of Akt/mTOR
Akt/mTOR signaling is extremely complex and, as in other cells, it is regulated by numerous positive and negative signals in oligodendrocytes (Figure 2B). The TSC1/TSC2 complex is an important negative mTOR regulator, and Tsc1 knockouts result in constitutive activation of mTOR signaling. As expected, conditional knock-out of Tsc1 in oligodendrocytes hyperactivates mTOR signaling (LeBrun-Julien et al. 2014). Unexpectedly, however, the LeBrun-Julien et al., (2014) study and other studies on Tsc1 loss in oligodendrocytes demonstrate that hyperactive mTOR signaling driven by loss of Tsc1 results in hypomyelination, with reduced myelin protein and RNA expression, and eventual oligodendrocyte apoptosis. These studies conclude that the TSC1/mTOR interaction normally maintains oligodendrocyte homoeostasis (LeBrun-Julien et al. 2014), possibly through an ER stress mechanism (Jiang et al. 2016). Regulation of the TSC1/mTOR pathway is itself complex, and leucine-rich glioma inactivation 1 (Lgi1) functions to regulate TSC1. Lgi1 loss results in reduced TSC1 activity and hyperactivation of mTOR signaling, and as with the TSC1 conditional knockouts, its loss also results in hypomyelination (Xie et al. 2018). This series of studies clearly establish that while mTOR signaling is crucial for myelination, it must be downregulated appropriately at specific developmental stages, as continued mTOR activation in oligodendrocytes results in defects in oligodendrocyte differentiation and myelination.
In contrast to the signaling pathways regulating mTOR activation and inactivation, a very different mechanism to downregulate mTOR activity is through degradation of the protein itself. FBXW7, a subunit of a ubiquitin ligase that targets proteins for degradation, is important for decreasing mTOR protein abundance in oligodendrocytes at appropriate times (Mao et al 2008; Kearns et al 2015). In contrast to the hypomyelination resulting from the mTOR hyperactivation after loss of TSC1, the excess mTOR protein levels and mTOR hyperactivity that result from reduced mTOR protein degradation lead to hypermyelination. Thus, FBXW7 in oligodendrocytes normally controls mTOR activity by controlling the level of the protein and FBXW7 mutations result in hypermyelination through decreased mTOR turnover and the resulting increased mTOR activity (Kearns et al. 2015).
Clearly how and when mTOR activity is reduced is a major regulator of myelination, but the specific modulation of this process is still being investigated. Recent data suggest that the impact of mTOR signaling is dependent on the developmental stage of the oligodendrocyte lineage. Thus, during remyelination, deletion of TSC1 from adult OPCs and subsequent mTOR hyperactivation results in accelerated remyelination, while deletion of TSC1 in more mature oligodendrocytes slows remyelination (McLane et al. 2017). These studies highlight the essential nature of mTOR signaling in oligodendrocyte differentiation, but also establish that mTOR activity impacts specific stages of oligodendrocyte differentiation in dramatically different ways.
3.4. Intracellular impact of Akt/mTOR signaling.
The activation, inhibition, or modification of the Akt/mTOR pathway alters many downstream functional targets driving oligodendrocyte differentiation (Figure 2B). An important initial impact of mTOR signaling is to reduce activity of the molecular pathways that maintain OPCs in a proliferative, non-differentiating state. One pathway that prevents OPC differentiation is bone morphogenetic protein (BMP) (Feigenson et al. 2011; Grinspan et al. 2015; Ornelas et al. 2020), and the BMP pathway is an important target of mTOR signaling in oligodendrocytes. BMP inhibits OPC differentiation by promoting the expression of the transcription factor DNA binding 2 (Id2), which in turn inhibits the transcription of genes encoding myelin proteins. mTOR inhibits BMP signaling during early OPC differentiation by positively regulating the expression of FKBP12, a suppressor of BMP receptor activity (Ornelas et al. 2020). Additionally, it regulates the Smad protein complex that binds to the Id2 promoter region (Ornelas et al. 2020). Thus, mTOR signaling fine tunes the transition from OPC to differentiating oligodendrocyte, not only by regulating aspects of myelin gene expression, but also by reducing signaling of oligodendrocyte differentiation inhibitors.
Actin polymerization and depolymerization are necessary for oligodendrocyte morphology changes during differentiation and myelination. These morphology changes include process extension and wrapping, which require actin polymerization, and membrane compaction, which requires actin depolymerization (Nawaz et al. 2015; Zuchero et al. 2015). Phosphorylated cofilin enhances actin polymerization and dephosphorylated cofilin depolymerizes actin filaments. mTOR signaling positively regulates the phosphorylation of cofilin during early differentiation, allowing for the actin polymerization necessary to drive process extension and axon wrapping (Musah et al. 2020). mTOR signaling also positively regulates the expression of the actin regulating protein profilin2 and a component of the branched actin polymerizing complex, Arpc3. Thus, the loss of mTOR signaling results in reduced polymerized actin in early differentiating oligodendrocytes, and this loss of polymerized actin corresponds with an overall decrease in complex cell morphology (Musah et al. 2020).
mTOR functions in two distinct complexes: mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2), each of which has different substrates and functions. While far more is known about mTORC1 signaling, which regulates protein translation, transcription, and lipid biogenesis, mTORC2 has been studied to a lesser degree. This complex is known to impact cytoskeleton organization (Oh et al. 2011), and likely regulates aspects of the cytoskeletal changes that drive oligodendrocyte differentiation. Loss of mTORC2 signaling in oligodendrocytes by conditional knock out of an essential binding partner, RICTOR, delays myelination in the corpus callosum. However, the early myelin deficit is resolved in adult animals, indicating later compensation for the loss of mTORC2 signaling (Grier et al. 2017).
While many studies have examined how mTOR functions in driving differentiation in oligodendrocytes, many downstream targets are still unknown. For example, mTOR clearly functions in actin regulation but it is unknown whether this is exclusively through mTORC1 signaling or mTORC2 signaling, which as noted above is known to impact the cytoskeleton, or a combination of both. Additionally, we still do not have a clear understanding of how mTORC1 vs mTORC2 are contributing to other aspects of myelination. There appear to be some differences in regional requirements for each complex (Bercury et al. 2014), but it is unknown why that is. Clearly, further investigation of the separate complexes is necessary to fully understand how mTOR is functioning in myelination.
4. Conclusions and Future Perspectives
Understanding the molecular mechanisms underlying the physiological role of specific developmental signals is fundamentally important to define the biology of oligodendrocyte development and the complex cellular interactions that regulate lineage transitions from OPCs to mature, myelinating oligodendrocytes. In this review article, we focused on two specific aspects of OPC development – proliferation and differentiation, in particular on an astrocyte-derived signal that promotes proliferation of OPCs - ET-1 - and a signaling pathway - the mTOR pathway - that play a major role in OPC maturation and myelination. In our view, these provide important examples of how extrinsic and intrinsic developmental signals are identified and functionally characterized, and how their cellular origin and molecular/biochemical properties are determined. These examples also highlight the need for continued investigation of extrinsic and intrinsic signaling pathways at specific developmental stages using inducible, conditional mouse genetics that allow manipulation of these signals at the progenitor and/or mature state. This will help to define what the direct, rather than indirect effects of these pathways are at each stage of oligodendrocyte development.
Since the first characterization of the ET-1 and mTOR roles in oligodendrocyte development, a number of new and advanced experimental tools have become available to researchers working on oligodendrocyte and myelin biology. These tools are particularly valuable to identify new extrinsic and intrinsic signals, to determine their mechanisms of action, and to investigate the heterogeneity of response to cellular factors that might occur in distinct subpopulations of OPCs and at different developmental stages. The opportunity to characterize oligodendrocytes not only based on their antigenic, morphological and physiological properties, but also on molecular profiling by single-cell RNAseq analysis has generated new approaches to identify potential subpopulations of these cells not only across different CNS regions, but also within the same area of the brain (Marques et al. 2016; Marques et al. 2018). These findings also open new opportunities to define new intrinsic and extrinsic regulators of oligodendrocyte development, based on differentially expressed genes and distinct molecular signatures. Furthermore, the analysis and characterization of gene networks that can be specifically activated in selected subpopulations of OPCs or oligodendrocytes will open new avenues to our understanding of how different signals act in concert and in an integrated fashion to promote the generation of myelinating cells. Finally, complementing single-cell RNAseq with single-cell Western blotting (Kang et al. 2016) or single-cell proteomics (Marx 2019) will allow detection of changes in protein phosphorylation/modification and better define the heterogeneity of intracellular signaling cascades within oligodendrocytes.
Another crucial question for future studies is whether experimental results obtained in rodents, which take full advantage of mouse genetics to define mechanistic role of specific developmental signals, are also relevant to human oligodendrocytes and to myelin disorders. The opportunity to generate human iPSCs and brain organoids that can be efficiently differentiated to oligodendrocytes now allows investigation of cellular mechanisms of normal development and disease in human cells (Madhavan et al. 2018). The role of intrinsic molecular pathways and extrinsic cellular signals can be teased out at the cellular level, and links to human disease and specific cellular abnormalities can be established. For example, genetic disorders that result in mutations and dysfunction in specific signaling pathways can be analyzed to determine cell-autonomous or non-autonomous effects. The impact on entire gene networks and gene families can be rapidly established through RNAseq molecular profiling and bioinformatics analyses of human iPSCs and their progenies. A combination of large scale high-throughput gene expression profiling studies and the use of brain tissue organoids are gradually emerging as cutting-edge experimental approaches and tools to identify new signaling pathways potentially involved in oligodendrocyte development and myelin disorders (Marton et al. 2019). These results can be integrated with more extensive analysis in postmortem human tissue, in which expression of new genes identified in iPSC-derived oligodendrocytes can be further analyzed for cellular specificity and regulation in both gray and white matter regions.
As described above, it is now well established that both ET-1 and mTOR signaling play important roles in development and in remyelination after demyelination. However, it is still largely unknown how these regulators of oligodendrocyte development and regeneration are integrated in the extensive network of signals that modulate myelination and repair. The new experimental approaches now available will facilitate answering this question at the single cell level and will help determine whether alterations in these pathways and in gene networks downstream of ET-1 receptor and mTOR activation are involved in myelin disorders.
Acknowledgements
These studies were supported by NIH R01NS090383 and R01NS117434 (K.L.A. and V.G.), 5F32NS098647 (K.L.A.) and NS082203 (W.B.M. and K.D.).
Abbreviations:
- afap1l2
actin filament associate protein 1-like 2
- bmp
bone morphogenetic protein
- cns
central nervous system
- creb
cyclic AMP response element-binding protein
- egf
epidermal growth factor
- et-1
endothelin-1
- fgf
fibroblast growth factor
- fgfr2
fibroblast growth factor receptor 2
- gpcrs
g-protein coupled receptors
- id2
dna binding protein inhibitor id-2
- ifg-1
insulin growth factor 1
- lgi1
leucine-rich glioma inactivation 1
- lm2
laminin-2
- opc
oligodendrocyte progenitor cell
- mapk
mitogen-activated protein kinase
- mbp
myelin basic protein
- mtorc1
mtor complex 1
- mtorc2
mtor complex 2
- nt-3
neurotrophin-3
- pdgf
platelet derived growth factor
- pkc
protein kinase c
- plp
proteolipid protein
- ptn
pleiotrophin
- ptp-alpha
protein tyrosine phosphatase alpha
- ptprz
protein tyrosine phosphatase receptor type Z
- svz
subventricular zone
References
- Adams KL, Riparini G, Banerjee P, Breur M, Bugiani M, Gallo V. Endothelin-1 signaling maintains glial progenitor proliferation in the postnatal subventricular zone. Nat. Commun 2020; 11: 2138. doi: 10.1038/s41467-020-16028-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguirre A, Dupree JL, Mangin JM, Gallo V. A functional role for EGFR signaling in myelination and remyelination. Nat. Neurosci 2007; 10: 990–1002. doi: 10.1038/nn1938. [DOI] [PubMed] [Google Scholar]
- Alberts GF, Peifley KA, Johns A, Kleha JF, Winkles JA. Constitutive endothelin-1 overexpression promotes smooth muscle cell proliferation via an external autocrine loop. J. Biol. Chem 1994; 269: 10112–10118. [PubMed] [Google Scholar]
- Barbarese E, Koppel DE, Deutscher MP, Smith CL, Ainger K, Morgan F, Carson JH. Protein translation components are colocalized in granules in oligodendrocytes. J. Cell Sci 1995; 108: 2781–2790. [DOI] [PubMed] [Google Scholar]
- Baron W, Metz B, Bansal R, Hoekstra D, de Vries H. PDGF and FGF-2 signaling in oligodendrocyte progenitor cells: regulation of proliferation and differentiation by multiple intracellular signaling pathways. Mol. Cell. Neurosci 2000; 3: 314–329. doi: 10.1006/mcne.1999.0827 [DOI] [PubMed] [Google Scholar]
- Bercury KK, Dai J, Sachs HH, Ahrendsen JT, Wood TL, Macklin WB. Conditional Ablation of Raptor or Rictor Has Differential Impact on Oligodendrocyte Differentiation and CNS Myelination. J. Neurosci 2014; 34: 4466–4480. 10.1523/JNEUROSCI.4314-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergles DW and Richarson WD. Oligodendrocyte development and plasticity. Cold Spring Harb. Perspect. Biol 2016; 8: a020453. doi: 10.1101/cshperspect.a020453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat NR, Sarlieve LL, Rao GS, Pieringer RA. Investigations on myelination in vitro. Regulation by thyroid hormone in cultures of dissociated brain cells from embryonic mice. J Biol Chem 1979; 254: 9342–9344. [PubMed] [Google Scholar]
- Brinkmann BG, Agarwal A, Sereda MW, Garratt AN, Muller T, Wende H, Stassart RM, Nawaz S, Humml C, Velanac V, Radyushkin K, Goebbels S, Fischer TM, Franklin RJ, Lai C, Ehrenreich H, Birchmeier C, Schwab MH, Nave KA. Neuregulin-1/ErbB signaling serves distinct functions in myelination of the peripheral and central nervous system. Neuron 2008; 59: 581–595. doi: 10.1016/j.neuron.2008.06.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttery PC, ffrench-Constant C. Laminin-2/integrin interactions enhance myelin membrane formation by oligodendrocytes. Mol Cell Neurosci 1999; 14: 199–212. doi: 10.1006/mcne.1999.0781 [DOI] [PubMed] [Google Scholar]
- Chapman H, Riesenberg A, Ehrman LA, Kohli V, Nardini D, Nakafuku M, Campbell K, Waclaw RR. Gsx transcription factors control neuronal versus glial specification in ventricular zone progenitors of the mouse lateral ganglionic eminence. Dev. Biol 2018; 442: 115–126. doi: 10.1016/j.ydbio.2018.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collarini EJ, Pringle N, Mudhar H, Stevens G, Kuhn R, Monuki ES, Lemke G, Richardson WD. Growth factors and transcription factors in oligodendrocyte development. J. Cell Sci. Suppl 1991; 15: 117–123. doi: 10.1242/jcs.1991.supplement_15.16 [DOI] [PubMed] [Google Scholar]
- Cramer H, Schmenger K, Heinrich K, Horstmeye A, Boning H, Breit A, Piiper A, Lundstrom K, Muller-Esterl W, Schroeder C. Coupling of endothelin receptors to the ERK/MAP Kinase pathway. Roles of palmitoylation and G(alpha)q. Eur. J. Biochem 2001; 268: 5449–5459. doi: 10.1046/j.0014-2956.2001.02486.x [DOI] [PubMed] [Google Scholar]
- Cui QL and Almazan G. IGF-I-induced oligodendrocyte progenitor proliferation requires PI3K/Akt, MEK/ERK, and Src-like tyrosine kinases. J. Neurochem 2007; 100: 1480e1493. doi: 10.1111/j.1471-4159.2006.04329.x [DOI] [PubMed] [Google Scholar]
- Dai J, Bercury KK, Macklin WB. Interaction of mTOR and Erk1/2 signaling to regulate oligodendrocyte differentiation. Glia 2014; 62: 2096–2109. 10.1002/glia.22729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies JE, Miller RH. Local Sonic Hedgehog Signaling Regulates Oligodendrocyte Precursor Appearance in Multiple Ventricular Zone Domains in the Chick Metencephalon. Dev. Biol 2001; 233: 513–525. doi: 10.1006/dbio.2001.0224. [DOI] [PubMed] [Google Scholar]
- Deloulme JC, Raponi E, Gentil BJ, Bertacchi N, Marks A, Labourdette G, Baudier J. Nuclear expression of S100B in oligodendrocyte progenitor cells correlates with differentiation toward the oligodendroglial lineage and modulates oligodendrocytes maturation. Mol. Cell Neurosci 2004; 27: 453–465. doi: 10.1016/j.mcn.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Dubois-Dalcq M, Murray K. Why are growth factors important in oligodendrocyte physiology?. Pathol. Biol. (Paris) 2000; 48: 80–86. [PubMed] [Google Scholar]
- Feigenson K, Reid M, See J, Crenshaw III EB, Grinspan JB. Canonical Wnt signalling requires the BMP pathway to inhibit oligodendrocyte maturation. ASN Neuro 2011;3(3):e00061. Published 2011 Jun 16. doi: 10.1042/AN20110004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores AI, Narayanan SP, Morse EN, Shick HE, Yin X, Kidd G, Avila RL, Kirschner DA, Macklin WB. Constitutively active Akt induces enhanced myelination in the CNS. J. Neurosci 2008; 28: 7174–7183. 10.1523/JNEUROSCI.0150-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederick TJ, Min J, Altieri SC, Mitchell NE, Wood TL. Synergistic induction of cyclin D1 in oligodendrocyte progenitor cells by IGF-I and FGF-2 requires differential stimulation of multiple signaling pathways. Glia 2007; 55: 1011e1022. doi: 10.1002/glia.20520 [DOI] [PubMed] [Google Scholar]
- Frost EE, Zhou ZC, Krasnesky K, Armstrong RC. Initiation of oligodendrocyte progenitor cell migration by a PDGF-A activated extracellular regulated kinase (ERK) signaling pathway. Neurochem. Res 2009; 34: 169–181. doi: 10.1007/s11064-008-9748-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furusho M, Ishii A, Bansal R. Signaling by FGF receptor 2, not FGF receptor 1, regulates myelin thickness through activation of ERK1/2–MAPK, which promotes mTORC1 activity in an Akt-independent manner. J. Neurosci 2017; 37: 2931–2946. 10.1523/JNEUROSCI.3316-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyffe-Maricich S, Karlo JC, Landreth GE, Miller RH. The ERK2 mitogen-activated protein kinase regulates the timing of oligodendrocyte differentiation. J. Neurosci 2011; 31: 843–850. doi: 10.1523/JNEUROSCI.3239-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadea A, Aguirre A, Haydar TF, Gallo V. Endothelin-1 regulates oligodendrocyte development. J. Neurosci 2009; 29: 10047–10062. doi: 10.1523/JNEUROSCI.0822-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadea A, Schinelli S, Gallo V. Endothelin-1 regulates astrocyte proliferation and reactive gliosis via a JNK/c-Jun signaling pathway. J. Neurosci 2008; 28: 2394–2408. doi: 10.1523/JNEUROSCI.5652-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo V, Armstrong RC. Developmental and growth factor-induced regulation of nestin in oligodendrocyte lineage cells. J. Neurosci 1995; 15: 394–406. doi: 10.1523/JNEUROSCI.15-01-00394.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaesser JM, Fyffe-Maricich SL. Intracellular signaling pathway regulation of myelination and remyelination in the CNS. Exp. Neurol 2016; 283(Pt B): 501–511. doi: 10.1016/j.expneurol.2016.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinspan JB. Bone Morphogenetic Proteins: Inhibitors of Myelination in Development and Disease. Vitam Horm 2015; 99:195–222. doi: 10.1016/bs.vh.2015.05.005. [DOI] [PubMed] [Google Scholar]
- Gillespie CS, Wilson R, Davidson A, Brophy PJ. Characterization of a cytoskeletal matrix associated with myelin from rat brain. Biochem J 1989; 260: 689–696. doi: 10.1042/bj2600689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebbels S, Wieser GL, Pieper A, Spitzer S, Weege B, Yan K, Edgar JM, Yagensky O, Wichert SP, Agarwal A, Karram K, Renier N, Tessier-Lavigne M, Rossner MJ, Karadottir RT, Nave KA. A neuronal PI(3,4,5)P3-dependent program of oligodendrocyte precursor recruitment and myelination. Nat. Neurosci 2017; 20: 10–15. doi: 10.1038/nn.4425. [DOI] [PubMed] [Google Scholar]
- Gonsalvez D, Ferner AH, Peckham H, Murray SS, Xiao J. The roles of extracellular related-kinases 1 and 2 signaling in CNS myelination. Neuropharmacology 2016; 110: 586–593. doi: 10.1016/j.neuropharm.2015.04.024 [DOI] [PubMed] [Google Scholar]
- Grier MD, West KL, Kelm ND, Fu C, Does MD, Parker B, McBrier E, Lagrange AH, Ess KC, Carson RP. Loss of mTORC2 signaling in oligodendrocyte precursor cells delays myelination. PLoS One 2017; 12: 1–18. 10.1371/journal.pone.0188417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond TR, Gadea A, Dupree J, Kerninon C, Nait-Oumesmar B, Aguirre A, Gallo V. Astrocyte-derived endothelin-1 inhibits remyelination through notch activation. Neuron 2014; 81: 588–602. doi: 10.1016/j.neuron.2013.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond TR, McEllin B, Morton PD, Raymond M, Dupree J, Gallo V. Endothelin-B receptor activation in astrocytes regulates the rate of oligodendrocyte regeneration during remyelination. Cell Rep 2015; 13: 2090–2097. doi: 10.1016/j.celrep.2015.11.002. [DOI] [PubMed] [Google Scholar]
- Huges EG, Kang SH, Fukaya M, Bergles DE. Oligodendrocyte progenitors balance growth with self-repulsion to achieve homeostasis in the adult brain. Nat. Neurosci 2013; 16: 668–676. doi: 10.1038/nn.3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang CC, Yamauchi KA, Vlassakis J, Sinkala E, Duncombe TA, Herr AE. Single cell-resolution western blotting. Nat. Protoc 2016; 11: 1508–1530. doi: 10.1038/nprot.2016.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessaris N, Fogarty M, Iannarelli P, Grist M, Wegner M, Richardson WD. Competing waves of oligodendrocytes in the forebrain and postnatal elimination of an embryonic lineage. Nat. Neurosci 2005; 9: 173–179. doi: 10.1038/nn1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga Y, Tsurumaki H, Aoki-Saito H, Sato M, Yatomi M, Takehara K, Hisada T. Roles of cyclic AMP response element binding activation in the ERK1/2 and p38 MAPK signaling pathway in central nervous system, cardiovascular system, osteoclast differentiation and mucin and cytokine production. Int. J. Mol. Sci 2019; 20: 1346. doi: 10.3390/ijms20061346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii A, Fyffe-Maricich SL, Furusho M, Miller RH, Bansal R. ERK1/ERK2 MAPK signaling is required to increase myelin thickness independent of oligodendrocyte differentiation and initiation of myelination. J. Neurosci 2012; 32: 8855–8864. doi: 10.1523/JNEUROSCI.0137-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii A, Furusho M, Bansal R. Sustained activation of ERK1/2 MAPK in oligodendrocytes and schwann cells enhances myelin growth and stimulates oligodendrocyte progenitor expansion. J. Neurosci 2013; 33: 175–186. doi: 10.1523/JNEUROSCI.4403-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii A, Furusho M, Macklin W, Bansal R. Independent and cooperative roles of the Mek/ERK1/2-MAPK and PI3K/Akt/mTOR pathways during developmental myelination and in adulthood. Glia 2019; 67: 1277–1295. doi: 10.1002/glia.23602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki H, Eguchi S, Marumo F, Hirata Y. Endothelin-1 stimulates DNA synthesis of vascular smooth-muscle cells through transactivtion of epidermal growth factor receptor. J. Cardiovasc. Pharmacol 1998; 31: S182–S184. doi: 10.1097/00005344-199800001-00052 [DOI] [PubMed] [Google Scholar]
- Jiang M, Liu L, He X, Wang H, Lin W, Wang H, Yoon SO, Wood TL, Lu QR. Regulation of PERK-eIF2α signalling by tuberous sclerosis complex-1 controls homoeostasis and survival of myelinating oligodendrocytes. Nat Commun 2016; 7: 12185. doi: 10.1038/ncomms12185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearns CA, Ravanelli AM, Cooper K, Appel B. Fbxw7 Limits Myelination by Inhibiting mTOR Signaling. J. Neurosci 2015; 35: 14861–14871. doi: 10.1523/JNEUROSCI.4968-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuboyama K, Fujikawa A, Masumura M, Suzuki R, Matsumoto M, Noda M. Protein tyrosine phosphatase receptor type z negatively regulates oligodendrocyte differentiation and myelination. PLoS One 2012; 7: e48797. doi: 10.1371/journal.pone.0048797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuboyama K, Fujikawa A, Suzuki R, Noda M. Inactivation of protein tyrosine phosphatase receptor type Z by pleiotrophin promotes remyelination through activation of differentiation of oligodendrocyte precursor cells. J. Neurosci 2015; 35: 12162–12171. doi: 10.1523/JNEUROSCI.2127-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebrun-Julien F, Bachmann L, Norrmén C, Trötzmüller M, Köfeler H, Rüegg MA, Hall MN, Suter U. Balanced mTORC1 Activity in Oligodendrocytes Is Required for Accurate CNS Myelination. J. Neurosci 2014; 34: 8432–8448. doi: 10.1523/JNEUROSCI.1105-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levison SW, Chuang C, Abramson BJ, Goldman JE. The migrational patterns and developmental fates of glial precursors in the rat subventricular zone are temporally regulated. Development 1993. Nov;119(3):611–22. [DOI] [PubMed] [Google Scholar]
- Ly PTT, Stewart C, Pallen CJ. PTPα is required for laminin-2-induced Fyn-Akt signaling to drive oligodendrocyte differentiation. J. Cell Sci 2018; 131: jcs212076. doi: 10.1242/jcs.212076. [DOI] [PubMed] [Google Scholar]
- Madhavan M, Nevin ZS, Shick HE, Garrison E, Clarkson-Paredes C, Karl M, Clayton BLL, Factor DC, Allan KC, Barbar L, Jain T, Douvaras P, Fossati V, Miller RH, Tesar PJ. Induction of myelinating oligodendrocytes in human cortical spheroids. Nat. Methods 2018; 15: 700–706. doi: 10.1038/s41592-018-0081-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makinodan M, Rosen KM, Ito S, Corfas G. A critical period for social experience-dependent oligodendrocyte maturation and myelination. Science 2012. Sep 14;337(6100):1357–60. doi: 10.1126/science.1220845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao JH, Kim IJ, Wu D, Climent J, Kang HC, DelRosario R, Balmain A. FBXW7 targets mTOR for degradation and cooperates with PTEN in tumor suppression. Science 2008;321(5895):1499–1502. doi: 10.1126/science.1162981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks V A dream of single-cell proteomics. Nat. Methods 2019; 16: 809–812. doi: 10.1038/s41592-019-0540-6. [DOI] [PubMed] [Google Scholar]
- Marton RM, Miura Y, Sloan SA, Li Q, Revah O, Levy RJ, Huguenard JR, Pasca SP. Differentiation and maturation of oligodendrocytes in human three-dimensional neural cultures. Nat. Neurosci 2019; 22: 484–491. doi: 10.1038/s41593-018-0316-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques S, van Bruggen D, Vanichkina DP, Floriddia EM, Munguba H, Varemo L, Giacomello S, Falcao AM, Meijer M, Bjorklund AK, Hherling-Leffler J, Taft RJ, Castelo-Branco G. Transcriptional convergence of oligodendrocyte lineage progenitors during development. Dev. Cell 2018; 46: 504–517. doi: 10.1016/j.devcel.2018.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques S, Zeisel A, Codeluppi S, van Bruggen D, Falcao AM, Xiao L, Li H, Haring M, Hochgerner H, Romanov RA, Gyllborg D, Manchado AM, Manno GL, Lonnerberg P, Floriddia EM, Rezayee F, Ernfors P, Arenas E, Hjerling-Leffler J, Harkany T, Richardson WD, Linnarsson S, Castelo-Branco G. Oligodendrocyte heterogeneity in the mouse juvenile and adult central nervous system. Science 2016; 352: 1326–1329. doi: 10.1126/science.aaf6463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews ES, Appel B. Cholesterol biosynthesis supports myelin gene expression and axon ensheathment through modulation of P13K/Akt/mTor signaling. J. Neurosci 2016; 36: 7628–7639. doi: 10.1523/JNEUROSCI.0726-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLane LE, Bourne JN, Evangelou AV, Khandker L, Macklin WB, Wood TL. (2017). Loss of tuberous sclerosis complex1 in adult oligodendrocyte progenitor cells enhances axon remyelination and increases myelin thickness after a focal demyelination. J. Neurosci 2017; 37: 7534–7546. doi: 10.1523/JNEUROSCI.3454-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei F, Fancy SPJ, Shen YAA, Niu J, Zhao C, Presley B, Miao E, Lee S, Mayoral SR, Redmond SA, Etxeberria A, Xiao L, Franklin RJM, Green A, Hauser SL, Chan JR. Micropillar arrays as a high-throughput screening platform for therapeutics in multiple sclerosis. Nat. Med 2014; 20: 954–960. doi: 10.1038/nm.3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menn B, Garcia-Verdugo JM, Yaschine C, Gonzalez-Perez O, Rowitch D, Alvarez-Buylla A. Origin of oligodendrocytes in the subventricular zone of the adult brain. J. Neurosci 2006; 26: 7907–7918. doi: 10.1523/JNEUROSCI.1299-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morell P, Toews AD. In vivo metabolism of oligodendroglial lipids. In: Norton WT (eds) Oligodendroglia. Advances in Neurochemistry, vol 5. Springer, Boston, MA. 1984. [Google Scholar]
- Musah AS, Brown TL, Jeffries MA, Shang Q, Hashimoto H, Evangelou AV, Kowalski A, Batish M, Macklin WB, Wood TL. Mechanistic target of rapamycin regulates the oligodendrocyte cytoskeleton during myelination. J. Neurosci 2020; 40: 2993–3007. doi: 10.1523/JNEUROSCI.1434-18.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy B, Hovhannisyan A, Barzan R, Chen TJ, Kukley M. Different patterns of neuronal activity trigger distinct responses of oligodendrocyte precursor cells in the corpus callosum. PLoS Biology 2017; 15: e2001993. doi: 10.1371/journal.pbio.2001993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan SP, Flores AI, Wang F, Macklin WB. Akt signals through the mammalian target of rapamycin pathway to regulate CNS myelination. J. Neurosci 2009; 29: 6860–6870. doi: 10.1523/JNEUROSCI.0232-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawaz S, Sanchez P, Schmitt S, Snaidero N, Mitkovski M, Velte C, Bruckner BR, Alexopoulos I, Czopka T, Jung SY, Rhee JS, Janshoff A, Witke W, Schaap IAT, Lyons DA, Simons M. Actin filament turnover drives leading edge growth during myelin sheath formation in the central nervous system. Dev. Cell 2015; 34: 139–151. doi: 10.1016/j.devcel.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbern JM, Li X, Shoemaker SE, Zhou J, Zhong J, Wu Y, Bonder D, Hollenback S, Coppola G, Geschwind DH, Landreth GE, Snider WD. Specific functions for ERK/MAPK signaling during PNS development. Neuron 2011; 69: 91–105. doi: 10.1016/j.neuron.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh WJ, Jacinto E. mTOR complex 2 signaling and functions. Cell Cycle 2011. Jul 15;10(14):2305–16. doi: 10.4161/cc.10.14.16586. Epub 2011 Jul 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong W, Lin J, Bechler ME, Wang K, Wang M, ffrench-Constant C, Chew SY. Microfiber drug/gene delivery platform for study of myelination. Acta. Biomater 2018; 75: 152–160. doi: 10.1016/j.actbio.2018.06.011 [DOI] [PubMed] [Google Scholar]
- Ornelas IM, Khandker L, Wahl SE, Hashimoto H, Macklin WB, Wood TL. (2020). The mechanistic target of rapamycin pathway downregulates bone morphogenetic protein signaling to promote oligodendrocyte differentiation. Glia 2020; 68: 1274–1290. doi: 10.1002/glia.23776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringle NP, Yu WP, Guthrie S, Roelink H, Lumsden A, Peterson AC, Richarson WD. Determination of neuroepithelial cell fate: induction of the oligodendrocyte lineage by ventral midline cells and sonic hedgehog. Dev. Biol 1996; 177: 30–42. doi: 10.1006/dbio.1996.0142. [DOI] [PubMed] [Google Scholar]
- Qi Y, Stapp D, Qiu M. Origin and molecular specification of oligodendrocytes in the telencephalon. Trends Neurosci 2002; 25: 223–225. doi: 10.1016/s0166-2236(02)02145-8. [DOI] [PubMed] [Google Scholar]
- Ramos JW. The regulation of extracellular signal-regulated kinase (ERK) in mammalian cells. Int. J. Biochem. Cell Biol 2008; 40: 2707–2719. doi: 10.1016/j.biocel.2008.04.009 [DOI] [PubMed] [Google Scholar]
- Rodríguez-Peña A Oligodendrocyte development and thyroid hormone. J. Neurobiol 1999; 40: 497–512. doi: . [DOI] [PubMed] [Google Scholar]
- Sanchez-Mejias A, Fernandez RM, Lopez-Alonso M, Antinolo G, Borrego S. New roles of EDNRB and EDN3 in the pathogenesis of Hirschsprung disease. Genet. Med 2010; 12: 39–43. doi: 10.1097/GIM.0b013e3181c371b0. [DOI] [PubMed] [Google Scholar]
- Sachs HH, Bercury KK, Popescu DC, Narayanan SP, Macklin WB. A new model of cuprizone-mediated demyelination/remyelination. ASN Neuro 2014; 6: 1759091414551955. doi: 10.1177/1759091414551955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saher G, Brügger B, Lappe-Siefke C, Mobius W, Tozawa RI, Wehr MC, Wieland F, Ishibashi S, Nave KA. High cholesterol level is essential for myelin membrane growth. Nat. Neurosci 2005; 8: 468–475. doi: 10.1038/nn1426. [DOI] [PubMed] [Google Scholar]
- See JM, Grinspan JB. Sending mixed signals: bone morphogenetic protein in myelination and demyelination. J. Neuropathol. Exp. Neurol 2009; 68: 595–604. doi: 10.1097/NEN.0b013e3181a66ad9 [DOI] [PubMed] [Google Scholar]
- Schönwasser DC, Marais RM, Marshall CJ, Parker PJ. Activation of the mitogen-activated protein kinase/extracellular signal-regulated kinase pathway by conventional, novel, and atypical protein kinase c isotypes. Mol. Cell. Biol 1998; 18: 790–798. doi: 10.1128/mcb.18.2.790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shraga-Levine Z and Sokolovsky M. Functional coupling of G proteins to Endothelin receptors is ligand and receptor subtype specific. Cell Mol. Neurobiol 2000; 20: 305–317. doi: 10.1023/a:1007010125316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh J, Sharma K, Frost EE, Pillai PP. Role of PDGF-A-activated ERK signaling mediated FAK-Paxillin interaction in oligodendrocyte progenitor cell migration. J. Mol. Neurosci 2019; 67: 564–573. 10.1007/s12031-019-1260-1 [DOI] [PubMed] [Google Scholar]
- Snaidero N, Simons M. The Logistics of Myelin Biogenesis in the Central Nervous System. Glia 2017; 65:1021–1031. doi: 10.1002/glia.23116 [DOI] [PubMed] [Google Scholar]
- Spinella F, Garrafa E, Di Castro V, Rosano L, Nicotra MR, Caruso A, Natali PG, Bagnato A. Endothelin-1 stimulates lymphatic endothelial cells and lymphatic vessels to grow and invade. Cancer Res 2009; 69: 2669–2676. doi: 10.1158/0008-5472.CAN-08-1879. [DOI] [PubMed] [Google Scholar]
- Sturrock RR. Myelination of the mouse corpus callosum. Neuropathol Appl Neurobiol 1980; 6: 415–420. doi: 10.1111/j.1365-2990.1980.tb00219.x. [DOI] [PubMed] [Google Scholar]
- Suo N, Guo Y, He B, Gu H, Xie X. Inhibition of MAPK/ERK pathway promotes oligodendrocytes generation and recovery of demyelinating diseases. Glia 2019; 67: 1320–1332. doi: 10.1002/glia.23606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swire M, Kotelevtsev Y, Webb DJ, Lyons DA, ffrench-Constant C. Endothelin signaling mediates experience-dependent myelination in the CNS. eLife 2019; 8: e49493. doi: 10.7554/eLife.49493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanga N, Kuboyama K, Kishimoto A, Kiyonari H, Shiraishi A, Suzuki R, Watanabe T, Fujikawa A, Noda M. The PTN-PTPRZ signal activates the AFAP1L2-dependent PI3K-AKT pathway for oligodendrocyte differentiation: Targeted inactivation of PTPRZ activity in mice. Glia 2019; 67: 967–984. doi: 10.1002/glia.23583. [DOI] [PubMed] [Google Scholar]
- Taveggia C, Thaker P, Petrylak A, Caporaso GL, Toews A, Falls DL, Einheber S, Salzer JL. Type III neuregulin-1 promotes oligodendrocyte myelination. Glia 2008; 56: 284–293. doi: 10.1002/glia.20612 [DOI] [PubMed] [Google Scholar]
- Tsai HH, Niu J, Munji R, Davalos D, Chang J, Zhang H, Tien AC, Kuo CJ, Chan JR, Daneman R, Fancy SPJ. Oligodendrocyte precursors migrate along vasculature in the developing nervous system. Science 2016; 351: 379–384. doi: 10.1126/science.aad3839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler WA, Gangoli N, Gokina P, Kim HA, Covey M, Levison SW, Wood TL. Activation of the mammalian target of rapamycin (mTOR) is essential for oligodendrocyte differentiation. J. Neurosci 2009; 29: 6367–6378. doi: 10.1523/JNEUROSCI.0234-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vartanian T, Fischbach G, Miller R. Failure of spinal cord oligodendrocyte development in mice lacking neuregulin. Proc. Natl. Acad. Sci. U.S.A 1999; 96: 731–735. doi: 10.1073/pnas.96.2.731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veer AV, Du Y, Fischer TZ, Boetig DR, Wood MR, Dreyfus CF. Brain-derived neurotrophic factor effects on oligodendrocyte progenitors of the basal forebrain are mediated through TrkB and the MAP kinase pathway. J. Neurosci 2009; 87: 69–78. doi: 10.1002/jnr.21841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl SE, McLane LE, Bercury KK, Macklin WB, Wood TL. Mammalian Target of Rapamycin Promotes Oligodendrocyte Differentiation, Initiation and Extent of CNS Myelination. J. Neurosci 2014; 34: 4453–4465. doi: 10.1523/JNEUROSCI.4311-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong AW, Xiao J, Kemper D, Kilpatrick TJ, Murray SS. Oligodendroglial expression of TrkB independently regulates myelination and progenitor cell proliferation. J. Neurosci 2013; 33: 4947–4957. doi: 10.1523/JNEUROSCI.3990-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao J, Kilpatrick TJ, Murray SS. The role of neurotrophins in the regulation of myelin development. Neurosignals 2009; 17: 265–276. doi: 10.1159/000231893. [DOI] [PubMed] [Google Scholar]
- Xiao J, Ferner AH, Wong AW, Denham M, Kilpatrick TJ, Murray SS. Extracellular signal-regulated kinase 1/2 signaling promotes oligodendrocyte myelination in vitro. J. Neurochem 2012; 122: 1167–1180. doi: 10.1111/j.1471-4159.2012.07871.x [DOI] [PubMed] [Google Scholar]
- Xie YJ, Zhou L, Wang Y, Jiang NW, Cao S, Shao CY, Wang XT, Li XY, Shen Y, Zhou L. Leucine-rich glioma inactivated 1 promotes oligodendrocyte differentiation and myelination via TSC-mTOR signaling. Front. Mol. Neurosci 2018; 11: 231. doi: 10.3389/fnmol.2018.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Y, Jiang W, Wang J, Li Z, Zhang J, Bu J, Zou J, Zhou L, Yu S, Cui Y, Yang W, Luo L, Lu QR, Liu Y, Chen M, Worley PF, Xiao B. Oligodendrocyte precursor cell-intrinsic effect of Rheb1 controls differentiation and mediates mTORC1-dependent myelination in brain. J. Neurosci 2014; 34: 15764–15778. doi: 10.1523/JNEUROSCI.2267-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuchero JB, Meng-Meng F, Sloan SA, Ibrahim A, Olson A, Zaremba A, Dugas JC, Wienbar S, Caprariello AV, Kantor C, Leonoudakis D, Lariosa-Willingham K, Kronenberg G, Gertz K, Soderling SH, Miller RH, Barres BA. CNS myelin wrapping Is driven by actin disassembly. Dev. Cell 2015; 34: 152–167. doi: 10.1016/j.devcel.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]


