Abstract
Background/Objective
Patients with moderate‐to‐severe atopic dermatitis (AD) have increased risk of cutaneous and extracutaneous infections. Dupilumab has previously been associated with reduced risk of serious/severe infections and non‐herpetic skin infections in adults with moderate‐to‐severe AD. This analysis assessed infection rates with dupilumab versus placebo in pediatric patients with moderate‐to‐severe and severe AD participating in clinical trials.
Methods
This is a pooled analysis from two 16‐week, randomized, placebo‐controlled, phase 3 clinical trials of dupilumab: monotherapy in adolescents aged 12–17 years with moderate‐to‐severe AD (LIBERTY AD ADOL, NCT03054428) and with concomitant topical corticosteroids in children aged 6–11 years with severe AD (LIBERTY AD PEDS, NCT03345914). Data were pooled according to treatment received: placebo/approved dupilumab doses/other studied dupilumab doses/all dupilumab doses. Exposure‐adjusted rates (patients with ≥1 event per 100 patient‐years [nP/100 PY]) were used to compare treatment groups.
Results
Overall, 612 patients were included: 205 received placebo and 407 received dupilumab (261 received approved dupilumab doses and 146 received other studied dupilumab doses). Overall infection rates were numerically lower with dupilumab versus placebo (nP/100 PY: placebo, 227; approved dupilumab, 173; other dupilumab, 206; all dupilumab, 184). Total skin infections were numerically less frequent in all dupilumab‐treated groups versus placebo (nP/100 PY: placebo, 67; approved dupilumab, 30; other dupilumab, 46; all dupilumab, 36).
Conclusions
These data suggest that dupilumab treatment in children and adolescents with AD does not increase infection risk overall and is associated with lower rates of skin infections compared with placebo.
Keywords: atopic dermatitis, dupilumab, herpetic skin infections, IL‐13, IL‐4, skin infections, systemic infections
1. INTRODUCTION
Patients with atopic dermatitis (AD) are at increased risk of infection, including serious cutaneous and systemic infections. Risk factors include skin barrier defects, immune dysregulation, and skin microbiome alterations. 1 , 2 Traditional systemic medications used for treating AD, as well as new‐generation Janus kinase inhibitors, have immunosupressive properties that may contribute to systemic and skin infections. 3 , 4 , 5 , 6 Dupilumab is selective for the type 2 immune cytokines interleukin (IL)‐4 and IL‐13, which are not thought to have a primary role in host defense mechanisms against bacterial, fungal, or viral infections. 7 , 8 , 9
Dupilumab, a fully human monoclonal antibody directed against the IL‐4 receptor‐alfa, specifically binds to the shared alfa chain subunit of the IL‐4 and IL‐13 receptors, selectively inhibiting signaling of cytokines IL‐4 and IL‐13. 7 , 8 In clinical trials in adolescents with moderate‐to‐severe AD (LIBERTY AD ADOL) and children with severe AD (LIBERTY AD PEDS), dupilumab improved clinical signs and symptoms of AD, as well as quality of life outcomes, versus placebo with acceptable safety. 10 , 11 , 12 Previous analyses also demonstrated that adults with moderate‐to‐severe AD treated with dupilumab are not at increased risk of overall or systemic infections and had lower rates of bacterial and other non‐herpetic skin infections. In adults, rates of herpesvirus infections were slightly higher with dupilumab treatment (mostly due to oral herpes), but clinically important herpesvirus infections (herpes zoster and eczema herpeticum) were fewer with dupilumab treatment versus placebo. 13 , 14 , 15 , 16 Here, we present a comprehensive analysis of infections from two randomized, placebo‐controlled, phase 3 clinical trials of dupilumab in adolescents 11 and children. 10
2. MATERIALS AND METHODS
2.1. Study design
This was a pooled analysis of data from two 16‐week, randomized, placebo‐controlled phase 3 clinical trials of dupilumab as monotherapy in adolescent patients (aged 12–17 years) with moderate‐to‐severe AD (LIBERTY AD ADOL) and dupilumab with concomitant topical corticosteroids (TCS) in children (aged 6–11 years) with severe AD (LIBERTY AD PEDS) for whom topical treatment was inadequate or medically inadvisable. Detailed study designs are provided in the respective publications. 10 , 11 Patients with active infections requiring systemic treatment within 2 weeks of baseline visit were excluded. During the study, if patients had an infection requiring systemic treatment, dupilumab treatment was discontinued.
Data were pooled into groups according to treatment received: placebo, approved dupilumab doses, other dupilumab doses studied, and all dupilumab doses (Table 1). The other dupilumab doses studied are unapproved doses from the clinical trials and are lower or less frequent doses than the approved doses.
TABLE 1.
Dupilumab treatment groups presented
| Pooled approved doses | Pooled other doses studied | All doses (approved + other) a |
|---|---|---|
| LIBERTY AD PEDS (aged 6–11) | ||
| 200 mg q2w (≥30 kg) | 100 mg q2w (<30 kg) | 100/200 mg q2w |
| 300 mg q4w b , c | 300 mg q4w | |
| LIBERTY AD ADOL (aged 12–17) | ||
| 200 mg q2w (<60 kg) | 300 mg q4w | 200/300 mg q2w |
| 300 mg q2w (≥60 kg) | 300 mg q4w | |
Abbreviations: EMA, European Medicines Agency; FDA, Food and Drug Administration; q2w, every 2 weeks; q4w, every 4 weeks.
All doses include both approved doses and other doses studied as per the LIBERTY AD PEDS and LIBERTY AD ADOL study designs.
FDA approved for children (aged 6–11 years) <30 kg, and EMA approved for children (aged 6–11 years) 15–60 kg.
Per EMA recommendation, the dose may be increased to 200 mg every other week based on the doctor's opinion.
2.2. Endpoints
Study endpoints were based on reports of treatment‐emergent adverse events made during the study treatment period and described according to the System Organ Class (SOC) and Preferred Term (PT) of the Medical Dictionary for Regulatory Activities (MedDRA) version 20.1. Endpoints included overall infections, infections leading to treatment discontinuation, severe or serious infections, herpesvirus infections (manually adjudicated, including PTs: herpes simplex, herpesvirus infection, eczema herpeticum, herpes zoster, oral herpes, varicella), non‐herpetic skin infections (manually adjudicated), and helminthic infections. Overall infections reported are from the infections and infestations SOC. The analysis of non‐herpetic skin infections was pre‐specified in the study protocols; other endpoints presented herein were analyzed post hoc.
The customized upper respiratory tract infection (URTI) cluster is a medically adjudicated selection of PTs limited to the upper respiratory tract within the infections and infestations SOC, where treatment‐emergent infections and infestations were reported in ≥5 patients. This cluster includes all PTs that indicate URTIs, regardless of MedDRA coding differences between individual physicians: URTI, streptococcal pharyngitis, viral URTI, rhinitis, nasopharyngitis, and sinusitis.
2.3. Statistical analysis
The analysis included all patients who received ≥1 dose of dupilumab or placebo (safety analysis set). Exposure‐adjusted rates (number of patients with ≥1 event per 100 patient years [nP/100 PY]) were used to compare treatment groups. Confidence intervals (CIs) were calculated using normal approximation. Risk ratio (RR) and p‐values were from a time‐to‐event exponential regression model with treatment, randomization factors, and study identifier as fixed factors.
3. RESULTS
3.1. Patients
This analysis included 612 patients (placebo n = 205; all dupilumab doses n = 407: approved dupilumab doses n = 261, and other dupilumab n = 146). The proportion of patients with medical history of skin infections requiring pharmacological treatment in the year prior to the study was similar among treatment groups (nP/100 PY who had ≥1 infection ranged from 58 to 70) (Figure S1).
3.2. Exposure‐adjusted infection rates
Overall infection rates were numerically lower for all dupilumab doses versus placebo, with a trend toward significance for lower overall infection rates in the approved dupilumab dose groups. Patients in the other studied doses group (receiving lower/unapproved dupilumab doses) had higher infection rates than those on approved doses (Table 2). Approximately 227.1 patients/100 PY in the placebo group had ≥1 infection, compared with 173.3 (RR 0.76, 95% CI [0.57, 1.00], p = .051) in approved dupilumab doses, 205.6 (RR 0.93, 95% CI [0.68, 1.28], p = .651) in other dupilumab doses studied, and 184.4 (RR 0.82, 95% CI [0.64, 1.05], p = .111) in the all dupilumab doses group (Table 2).
TABLE 2.
Exposure‐adjusted numbers of patients with treatment‐emergent infections during the study treatment period
| Patients with ≥1 event, nP (nP/100 PY) |
Comparison with placebo, risk ratio a (95% CI) p‐value b |
||||||
|---|---|---|---|---|---|---|---|
| Placebo | Dupilumab | ||||||
| Pooled placebo groups (n = 205) | Pooled approved doses (n = 261) | Pooled other doses studied (n = 146) | Pooled all dupilumab doses (n = 407) | Pooled approved doses (n = 261) | Pooled other doses studied (n = 146) | Pooled all doses (n = 407) | |
| Overall infections (SOC) | 98 (227.1) | 107 (173.3) | 66 (205.6) | 173 (184.4) |
0.76 (0.57, 1.00) .051 |
0.93 (0.68, 1.28) .651 |
0.82 (0.64, 1.05) .111 |
| Infections leading to treatment discontinuation c | 0 (0) | 1 (1.1) | 0 (0) | 1 (0.7) |
3.093E11 (0.00, NE) 1.000 |
37.58 (0.00, NE) 1.000 |
2.986E11 (0.00, NE) 1.000 |
| Serious or severe infections | 5 (8.1) | 2 (2.5) | 1 (2.2) | 3 (2.4) |
0.27 (0.05, 1.43) .125 |
0.34 (0.04, 3.19) .346 |
0.29 (0.07, 1.22) .092 |
| Total skin infections | 37 (67.0) | 23 (30.3) | 19 (46.4) | 42 (35.9) |
0.45 (0.27, 0.77) .003 |
0.69 (0.39, 1.21) .194 |
0.54 (0.34, 0.83) .001 |
| Non‐herpetic skin infections d | 32 (56.9) | 20 (26.1) | 13 (30.9) | 33 (27.8) |
0.47 (0.27, 0.83) .01 |
0.52 (0.27, 1.00) .049 |
0.49 (0.30, 0.80) .004 |
| Herpes viral infections e | 9 (14.7) | 4 (5.0) | 7 (16.0) | 11 (8.9) |
0.32 (0.10, 1.05) .060 |
1.22 (0.43, 3.44) .710 |
0.60 (0.25, 1.46) .262 |
| Eczema herpeticum f | 1 (1.6) | 1 (1.2) | 0 (0) | 1 (0.8) |
0.72 (0.04, 12.02) .816 |
0.00 (0.00, NE) 1.0 |
0.50 (0.03, 8.06) .628 |
| Herpes zoster g | 0 (0) | 1 (1.2) | 0 (0) | 1 (0.8) |
3.226E11 (0.00, NE) 1.000 |
34.79 (0.00, NE) 1.000 |
3.11E11 (0.00, NE) 1.000 |
| Varicella | 1 (1.6) | 0 (0) | 0 (0) | 0 (0) |
0.00 (0.00, NE) 1.000 |
0.00 (0.00, NE) 1.000 |
0.00 (0.00, NE) 1.000 |
| Oral herpes | 3 (4.8) | 1 (1.2) | 2 (4.5) | 3 (2.4) |
0.28 (0.03, 2.72) .272 |
0.82 (0.13, 5.04) .826 |
0.50 (0.10, 2.47) .393 |
| Herpes virus infection | 2 (3.2) | 0 (0) | 2 (4.5) | 2 (1.6) |
0.00 (0.00, NE) 1.0 |
1.90 (0.18, 19.79) .591 |
0.50 (0.07, 3.53) .485 |
| Herpes simplex | 2 (3.2) | 2 (2.5) | 5 (11.3) | 7 (5.6) |
0.74 (0.10, 5.33) .764 |
3.92 (0.71, 21.74) .118 |
1.77 (0.37, 8.50) .479 |
| Helminthic infections | 1 (1.4) | 1 (1.1) | 0 (0) | 1 (0.7) |
1.03 (0.06, 16.50) .983 |
0.00 (0.00, NE) 1.000 |
0.50 (0.03, 8.01) .625 |
Abbreviations: IGA, Investigator's Global Assessment; MedDRA, Medical Dictionary for Regulatory Activities; NE, not estimable; nP/100 PY, number of patients with ≥1 event per 100 PY; PT, MedDRA Preferred Term; PY, patient‐years; q2w, every 2 weeks; q4w, every 4 weeks; SOC, MedDRA System Organ Class.
Risk ratio and p‐values are from time‐to‐event exponential regression model with treatment, randomization factors, and study identifier as fixed factors.
Stratification factors are baseline disease severity (IGA = 3 vs. IGA = 4) and baseline weight group (<60 kg vs. ≥60 kg) for LIBERTY AD ADOL, and region (North America versus Europe) and baseline weight group (<30 kg vs. ≥30 kg) for LIBERTY AD PEDS.
One patient on dupilumab discontinued treatment (LIBERTY AD PEDS study) due to bacterial conjunctivitis infection.
Manually adjudicated.
Manually adjudicated, including the PTs herpes simplex, herpesvirus infection, eczema herpeticum, herpes zoster, oral herpes, and varicella.
Eczema herpeticum: one was a moderate case (LIBERTY AD PEDS, dupilumab 200 mg q2w group) lasting 10 days that resolved with oral acyclovir treatment; the other was a moderate case (LIBERTY AD ADOL, placebo group) lasting 19 days that resolved with valacyclovir treatment.
This was a mild case of herpes zoster that resolved after treatment with acyclovir in the LIBERTY AD PEDS study dupilumab 300 mg q4w treatment group.
Only one study patient (dupilumab approved doses group) discontinued treatment due to an infection (bacterial conjunctivitis; Table 2). Severe and serious infections were uncommon in all treatment groups, with fewer serious or severe infections in the dupilumab groups versus placebo. In the placebo group, 8.1 patients/100 PY had ≥1 serious or severe infection; 2.5 patients/100 PY in the approved dupilumab doses group (RR 0.27, 95% CI [0.05, 1.43], p = .125); 2.2 patients/100 PY in the other dupilumab doses group (RR 0.34, 95% CI [0.04, 3.19], p = .346); and 2.4 patients/100 PY in the all dupilumab doses group (RR 0.29, 95% CI [0.07, 1.22], p = .092; Table 2).
Total skin infections (non‐herpetic skin infections and herpesvirus infections) were significantly less frequent in all dupilumab‐treated groups compared with placebo (RR 0.54, 95% CI [0.34, 0.83], p = .001; Table 2). A total of 67.0 patients/100 PY in the placebo group had ≥1 skin infection versus 30.3 in approved dupilumab doses (p = .003), 46.4 in other dupilumab doses (p = .194), and 35.9 in all dupilumab doses. Non‐herpetic skin infections were significantly less frequent in the dupilumab groups than in the placebo group, affecting 56.9 patients/100 PY in the placebo group compared with 26.1 in approved dupilumab doses (RR 0.47, 95% CI [0.27, 0.83], p = .01), 30.9 in other dupilumab doses studied (RR 0.52, 95% CI [0.27, 1.00], p = .049), and 27.8 in all dupilumab doses (RR 0.49, 95% CI [0.30, 0.80], p = .004; Table 2 and Figure 1A). Percentages of patients with skin infections (excluding herpetic infections) were numerically lower in dupilumab‐treated patients compared with placebo and were similar in both studies (Figure 1B). Time to onset of non‐herpetic skin infections and herpesvirus infections is shown in Figure [Link], [Link].
FIGURE 1.
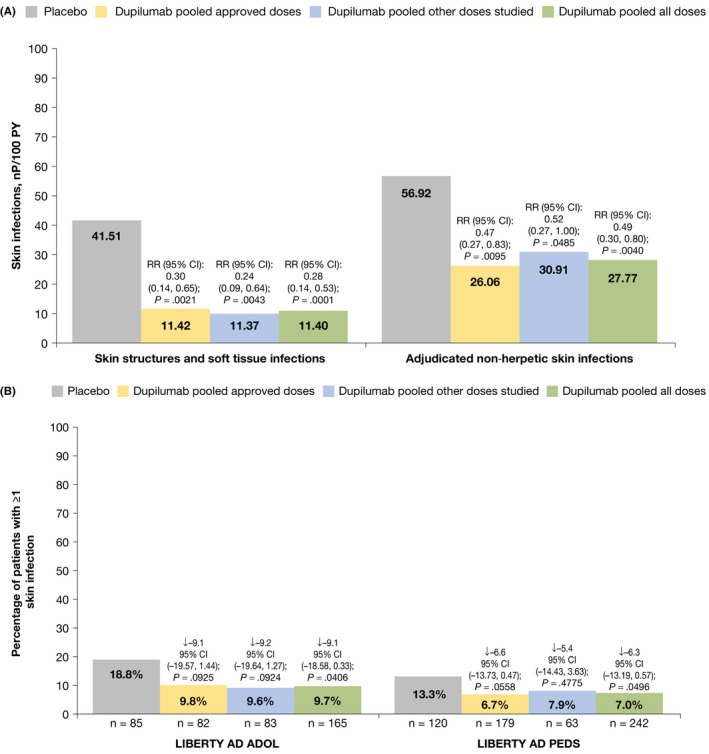
Exposure‐adjusted numbers of patients with treatment‐emergent skin infections (non‐herpetic) during the study treatment period. (A) Skin infections by HLT and adjudicated skin infections. (B) Proportion of patients having at least 1 skin infection treatment‐emergent adverse event (excluding herpetic infections) through week 16, by study. HLT selected from records of adjudicated skin infections excluding herpetic infections. ↓ = Difference versus placebo CI calculated using normal approximation. p‐values were derived by Cochran‐Mantel‐Haenszel (CMH) test stratified by baseline disease severity (IGA = 3 vs. IGA = 4) and baseline weight group (<60 kg vs. ≥60 kg) for study LIBERTY AD ADOL; by region (North America vs. Europe) and baseline weight group (<30 kg vs. ≥30 kg) for study LIBERTY AD PEDS. CI, confidence interval; HLT, MedDRA high‐level term; IGA, Investigator's Global Assessment; MedDRA, Medical Dictionary for Regulatory Activities; nP, number of patients with ≥1 event; PY, patient‐years; RR, risk ratio
Rates of herpesvirus infections were also numerically lower for the all dupilumab doses (8.9 patients/100 PY, p = .262) and the approved dupilumab doses (5.0 patients/100 PY, p = .060) groups versus placebo (14.7 patients/100 PY) but were more frequent in the other dupilumab group (16.0 patients/100 PY, p = .710). Serious herpetic infections were infrequent: one case of eczema herpeticum in the placebo group and one in the dupilumab group, one case of herpes zoster in the dupilumab group, and one case of varicella in the placebo group (Table 2). Time to onset of non‐herpetic and herpesvirus skin infections was similar between treatment groups (Figure [Link], [Link]). One case of coxsackievirus infection (hand, foot, and mouth disease) was reported in the LIBERTY AD PEDS placebo group. Helminthic infections were infrequent and comparable for all dupilumab doses versus placebo (one dupilumab‐treated and one placebo‐treated patient). One case, in the placebo group, was Enterobius vermicularis and one, in the dupilumab group, was ascariasis. These infections did not lead to study treatment discontinuation (Table 2).
Common infections (MedDRA PTs) that were numerically more frequent in the all dupilumab doses group than in the placebo group included nasopharyngitis, conjunctivitis, streptococcal pharyngitis, and molluscum contagiosum (MC) (Table 3). It should be noted that the MedDRA PT conjunctivitis represents conjunctivitis of unspecified or undetermined etiology and defaults to the infections and infestations MedDRA SOC, although the conjunctivitis is often not infectious. For streptococcal pharyngitis, the proportion of cases was lower in the dupilumab versus placebo groups in LIBERTY AD PEDS, with three cases (1.2%) in the dupilumab‐treated group and three (2.5%) in the placebo group. This proportion was reversed in LIBERTY AD ADOL, with seven cases (4.2%) in the dupilumab‐treated group and zero in the placebo group (for details on the individual cases, see Table S1). Infections more frequent in the placebo group than in the all dupilumab groups included URTI (cluster and PT), impetigo, and folliculitis (Table 3).
TABLE 3.
Treatment‐emergent infections (by PT) by incidence rate: number of patients per 100 PY (includes any PT reported in ≥5 patients) a
| Patients with infection, nP (nP/100 PY) | Comparison with placebo, p‐value | ||||||
|---|---|---|---|---|---|---|---|
| Placebo | Dupilumab b | ||||||
| Pooled placebo groups (n = 205) | Pooled approved doses (n = 261) | Pooled other doses studied (n = 146) | Pooled all doses (n = 407) | Pooled approved doses (n = 261) | Pooled other doses studied (n = 146) | Pooled all doses (n = 407) | |
| Upper respiratory tract infection cluster c | 50 (93.0) | 57 (80.3) | 36 (93.9) | 93 (85.1) | 0.483 | 0.997 | 0.625 |
| Upper respiratory tract infection | 27 (47.1) | 28 (36.8) | 11 (25.8) | 39 (32.8) | 0.440 | 0.071 | 0.153 |
| Nasopharyngitis | 12 (19.8) | 20 (25.8) | 15 (35.3) | 35 (29.2) | 0.587 | 0.087 | 0.252 |
| Pharyngitis streptococcal | 3 (4.8) | 5 (6.3) | 4 (9.0) | 9 (7.3) | 0.698 | 0.443 | 0.535 |
| Viral upper respiratory tract infection | 7 (11.2) | 4 (5.0) | 4 (9.0) | 8 (6.4) | 0.257 | 0.545 | 0.277 |
| Rhinitis | 3 (4.8) | 3 (3.7) | 1 (2.2) | 4 (3.2) | 0.852 | 0.431 | 0.595 |
| Sinusitis | 1 (1.6) | 1 (1.2) | 3 (6.7) | 4 (3.2) | 0.910 | 0.278 | 0.540 |
| Other infections | |||||||
| Conjunctivitis | 4 (6.5) | 10 (12.6) | 9 (20.6) | 19 (15.4) | 0.311 | 0.043 | 0.115 |
| Molluscum contagiosum d | 1 (1.6) | 4 (5.0) | 4 (9.1) | 8 (6.4) | 0.462 | 0.043 | 0.191 |
| Conjunctivitis bacterial | 1 (1.6) | 2 (2.5) | 5 (11.3) | 7 (5.6) | 0.908 | 0.031 | 0.246 |
| Gastroenteritis viral | 2 (3.2) | 6 (7.5) | 1 (2.2) | 7 (5.6) | 0.259 | 0.698 | 0.484 |
| Herpes simplex | 2 (3.2) | 2 (2.5) | 5 (11.3) | 7 (5.6) | 0.764 | 0.118 | 0.479 |
| Impetigo | 9 (14.8) | 4 (5.0) | 3 (6.8) | 7 (5.6) | 0.115 | 0.145 | 0.056 |
| Bronchitis | 0 (0) | 5 (6.2) | 0 (0) | 5 (4.0) | 1.000 | 1.000 | 1.000 |
| Folliculitis | 5 (8.1) | 3 (3.7) | 2 (4.5) | 5 (4.0) | 0.260 | 0.588 | 0.267 |
| Furuncle | 4 (6.5) | 3 (3.7) | 2 (4.5) | 5 (4.0) | 0.463 | 0.697 | 0.471 |
| Gastroenteritis | 1 (1.6) | 2 (2.5) | 2 (4.5) | 4 (3.2) | 0.666 | 0.477 | 0.537 |
| Influenza | 8 (13.0) | 4 (5.0) | 0 (0) | 4 (3.2) | 0.186 | 1.000 | 0.022 |
| Otitis media | 3 (4.8) | 3 (3.7) | 1 (2.2) | 4 (3.2) | 0.882 | 0.383 | 0.574 |
| Urinary tract infection | 2 (3.2) | 3 (3.7) | 1 (2.2) | 4 (3.2) | 0.892 | 0.796 | 0.992 |
| Oral herpes | 3 (4.8) | 1 (1.2) | 2 (4.5) | 3 (2.4) | 0.272 | 0.826 | 0.393 |
| Dermatitis infected | 6 (9.7) | 1 (1.2) | 0 (0) | 1 (0.8) | 0.058 | 1.000 | 0.020 |
| Ear infection | 5 (8.1) | 0 (0) | 0 (0) | 0 (0) | 1.000 | 1.000 | 1.000 |
Abbreviations: AE, adverse event; MedDRA, Medical Dictionary for Regulatory Activities; nP, number of patients with ≥1 event; nP/100 PY, number of patients with ≥1 event per 100 PY; PT, MedDRA preferred term; PY, patient‐years; q2w, every 2 weeks; SOC, MedDRA System Organ Class.
At each level of patient summarization, a patient is counted once if the patient reported ≥1 event. TEAEs included in the analysis were those that occurred during the study treatment period. PTs fall under the Infections and infestations SOC.
In LIBERTY AD PEDS, 1 patient with baseline weight <30 kg who was mis‐randomized to 200 mg dupilumab q2w was summarized in baseline weight <30 kg 100 mg dupilumab q2w group. One patient with baseline weight ≥30 kg who was randomized to placebo, but received 100 mg dupilumab inadvertently was summarized in baseline weight ≥30 kg 200 mg dupilumab q2w group.
Includes PT: upper respiratory tract infection, pharyngitis streptococcal, viral upper respiratory tract infection, rhinitis, nasopharyngitis, and sinusitis.
Treatment‐emergent AEs (PT) of molluscum contagiosum (MC) occurred in one patient in the placebo group and seven patients in the dupilumab treatment groups in LIBERTY AD PEDS, and in one patient in LIBERTY AD ADOL, dupilumab 300 mg q4w group. All treatment‐emergent AEs of molluscum contagiosum were non‐serious, mild to moderate in severity, and non‐recurrent (ie, observed only once for each patient), with all patients reported as recovered or recovering.
3.3. Anti‐infective medications
Systemic anti‐infective medication use was numerically lower in all dupilumab‐treated groups versus the placebo group when assessed by nP/100 PY (Figure 2A). When assessed by number of events per 100 PY, systemic anti‐infective medication use was significantly lower in the approved dupilumab (p = .030 and all dupilumab dose groups (p = .018) and numerically lower in the other dupilumab dose group versus placebo (Figure 2B). The most frequently administered systemic anti‐infective medications included the antibiotics cefalexin, trimethoprim/sulfamethoxazole, amoxicillin, and azithromycin, and the antiviral acyclovir.
FIGURE 2.
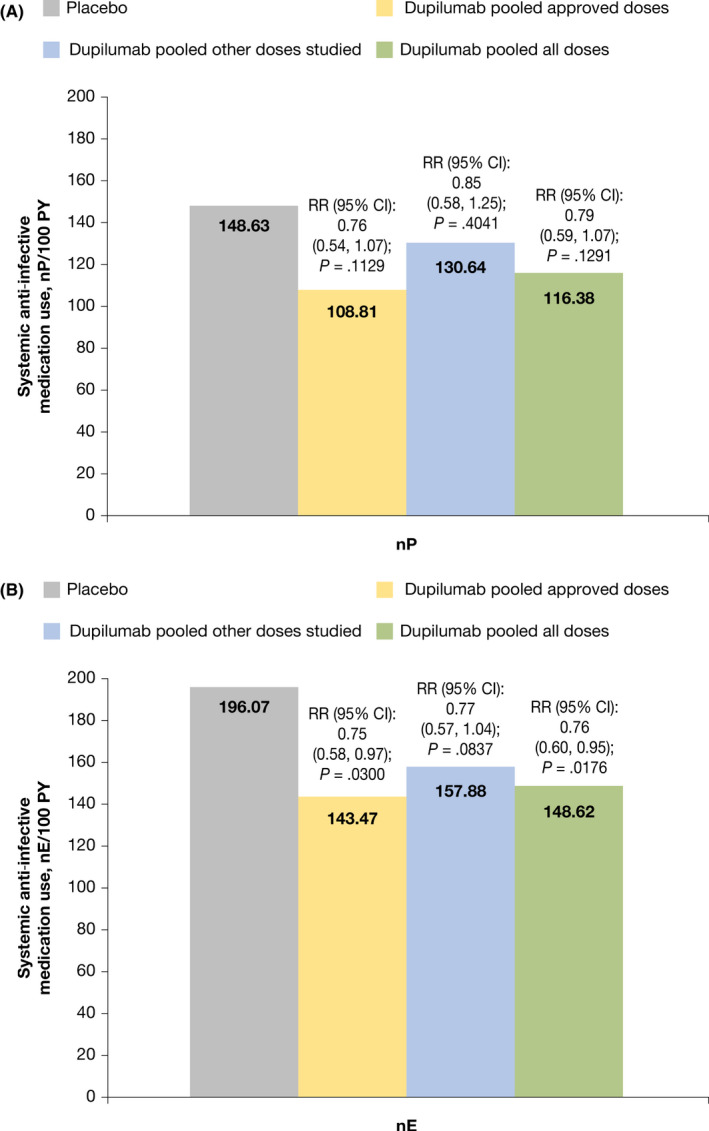
Systemic anti‐infective medication use. (A) Proportion of patients with ≥1 use of systemic anti‐infective medication per 100 PY. (B) Systemic anti‐infective medication use by number of events per 100 PY. CI calculated using normal approximation. RR and p‐values are from time‐to‐event exponential regression model with treatment, randomization factors and study identifier as fixed factors. Stratification factors are baseline disease severity (IGA = 3 vs. IGA = 4) and baseline weight group (<60 kg vs. ≥60 kg) for LIBERTY AD ADOL; stratification factors are region (North America vs. Europe) and baseline weight group (<30 kg vs. ≥30 kg) for LIBERTY AD PEDS. CI, confidence interval; nE, number of events; nP, number of patients with ≥1 event; PY, patient‐years; RR, risk ratio
4. DISCUSSION
In this pooled analysis of phase 3 clinical data from children with severe AD and adolescents with moderate‐to‐severe AD, rates of overall infections were numerically lower in patients treated with dupilumab compared with placebo, approaching statistical significance in the approved dupilumab dose group (p = .051). Rates of infections leading to treatment discontinuation and incidence of severe and serious infections were low across all dose groups. Furthermore, non‐herpetic skin infections were statistically less frequent in dupilumab‐treated patients than in patients receiving placebo, and rates of herpesvirus infections were numerically lower in dupilumab‐treated patients than those receiving placebo. Serious herpesvirus infections (eczema herpeticum, herpes zoster, and varicella) and helminthic infections were rare, and there were no differences between the placebo and dupilumab treatment groups. Rates of infections in the URTI cluster were similar rates between dupilumab and placebo‐treated patients, though slightly higher in placebo‐treated patients. For PTs within this cluster, URTI, viral upper respiratory tract infection, and rhinitis were higher in placebo‐treated patients, and nasopharyngitis, pharyngitis streptococcal, and sinusitis were higher in dupilumab‐treated patients. It should be noted that cases of streptococcal pharyngitis were lower in dupilumab‐treated patients in LIBERTY AD PEDS but not in LIBERTY AD ADOL, and overall cases were similar to those observed in the adult population (data on file). Relevant to the pediatric population, ear infections (ear infection and otitis media PTs) were lower with dupilumab versus placebo; however, cases of conjunctivitis and MC were more common with dupilumab versus placebo. Staphylococcal skin infections such as impetigo/folliculitis and furuncle were more frequent in the placebo group versus dupilumab. The PT dermatitis infected refers to AD lesions that show clinical evidence of infection (most likely staphylococcal), which in turn can lead to flares; these were also lower in the dupilumab groups versus placebo. Anti‐infective medication use was also lower in dupilumab‐treated patients versus placebo.
As reported in previous publications of dupilumab clinical trials, dupilumab treatment was associated with increased incidence of conjunctivitis. 17 , 18 However, the diagnoses of conjunctivitis were made by dermatologists (not ophthalmologists) without microbiological evaluation, and it is therefore possible that cases of non‐infectious conjunctivitis such as allergic conjunctivitis were labeled as conjunctivitis (which falls under the MedDRA SOC infections and infestations and the PT conjunctivitis). Many, if not most, events of conjunctivitis are not of infectious etiology, and their inclusion in the infections and infestations SOC of MedDRA may have artificially inflated infection rates in dupilumab‐treated patients.
The lower incidence of non‐herpetic skin infections in dupilumab‐treated versus placebo‐treated patients was similar to the findings in the adult dupilumab‐treated population. 13 This decrease in skin infections is likely the result of improved skin barrier function, as dupilumab inhibits the type 2 cytokines that contribute to the dysregulation of protein and lipid structural and functional components of the epidermis, 19 and it also blocks antimicrobial peptide synthesis, thereby decreasing Staphylococcus aureus colonization and increasing skin microbiome diversity. 20 , 21 Finally, dupilumab reduces itch, which reduces scratch‐related mechanical damage. Overall, these data suggest improvements in skin signs of AD may directly lead to protection from infection, although correlation analysis of skin improvement with infection risk was not feasible due to low incidence numbers and sample size.
While there were more cases of MC in the dupilumab‐treated group than in the placebo group, they were only significantly higher in the pooled unapproved doses versus placebo while not significantly higher in pooled approved doses or all doses versus placebo. The rate of 6.4% in dupilumab‐treated patients is within the prevalence of MC reported in the literature in pediatric patients with AD. 22 MC is thought to be prevalent in AD patients due to the disruption of the skin barrier. 23 , 24 While the mechanism is unclear, there are limited case studies reporting transient dissemination followed by clearance of existing MC with dupilumab treatment, 25 as well as enhanced clearance. 24 The clearance is significant, given the challenges presented with treating MC in AD patients, as traditional immunosuppressive AD treatments may exacerbate MC. 26 Defense mechanisms for MC are typically type 1‐mediated, and a selective type 2 inhibitor such as dupilumab is not expected to interfere with these defense mechanisms. 24
By contrast, type 2 immunity is a key component of anti‐helminthic host defense. 9 In this analysis, incidence of helminthic infections was very low and was similar in dupilumab and placebo groups. This finding should be interpreted within the context of a lower prevalence of parasitic infections in the regions in which these studies were conducted (North America and Europe). One of the helminth infection cases involved Enterobius vermicularis, or pinworm. Pinworm is one of the most common human parasitic helminths, and children are the most susceptible age group. 27 The other case of helminth infection was Ascaris, and it was diagnosed based on positive serum IgG for Ascaris. The patient did not have any signs or symptoms of active infection at the time of the event. Further research on how dupilumab may affect parasitic infection susceptibility is needed, especially in geographic areas where prevalence of these types of infections is higher.
An advantage of this pooled analysis is the sample size (approximately 600 patients), which increases the ability to identify potential safety signals. A potential limitation is that, despite the pooled data, the study population was limited to the safety analysis sets from two randomized controlled trials, which may not be reflective of real‐world patient populations. In addition, despite the sample size, some of the infection events were rare, limiting precise estimation of incident rates. In addition, the analysis did not account for the use of TCS as rescue medication, which could potentially reduce incidence of skin infections by reducing inflammation. 28 Finally, many of these diagnoses were clinical, without any microbiological investigation.
In summary, overall infection rates were numerically lower and skin infection (including non‐herpetic skin infections) rates were significantly lower compared with placebo in both children aged 6–11 years and adolescent patients with AD. Herpesvirus infections were also lower in the approved dupilumab doses and all dupilumab doses compared with placebo but were slightly higher in the other doses studied compared with placebo. Systemic anti‐infective medication use was numerically, however, not significantly lower in dupilumab‐treated patients. These findings provide further support for the safety profile of dupilumab in pediatric patients and reflect those of the individual study publications. 10 , 11 Furthermore, these findings are consistent with published results in adults. 13 The results of this analysis support the safety of dupilumab for treatment of adolescents with moderate‐to‐severe AD and children with severe AD.
CONFLICTS OF INTEREST
Paller has served as an investigator for AbbVie, AnaptysBio, Eli Lilly, Incyte, Janssen, Lenus Pharma, LEO Pharma, Novartis, Regeneron Pharmaceuticals, Inc., and UCB; and a consultant for AbbVie, Abeona Therapeutics, Almirall, Asana Biosciences, Boehringer Ingelheim, BridgeBio Pharma, Dermavant, Dermira, Eli Lilly, Exicure, Forté, Galderma, Incyte, InMed Pharmaceuticals, Janssen, LEO Pharma, LifeMax, Novartis, Pfizer, RAPT Therapeutics, Regeneron Pharmaceuticals, Inc., Sanofi Genzyme, Sol‐Gel, and UCB. Beck has received honoraria as a consultant from AbbVie, Allakos, Arena Pharma, AstraZeneca, Connect Biopharma, Eli Lilly, LEO Pharma, Novan, Novartis, Pfizer, Regeneron Pharmaceuticals, Inc., Sanofi, UCB, and Vimalan; has received grants for clinical trials from AbbVie, LEO Pharma, Pfizer, and Regeneron Pharmaceuticals, Inc.; and holds stock in Pfizer and Medtronic. Blauvelt has served as a scientific advisor and/or clinical study investigator for AbbVie, Abcentra, Aligos, Almirall, Amgen, Arcutis, Arena, Aslan, Athenex, Boehringer Ingelheim, Bristol Myers Squibb, Dermavant, Eli Lilly, Evommune, Forte, Galderma, Incyte, Janssen, Landos, LEO Pharma, Novartis, Pfizer, Rapt, Regeneron Pharmaceuticals, Inc., Sanofi Genzyme, Sun Pharma, and UCB. Siegfried has served as a scientific advisor and/or clinical study investigator for Eli Lilly, Janssen, Novartis, Novan, Regeneron Pharmaceuticals, Inc., Sanofi Genzyme, UCB, and Verrica; as a paid speaker for Regeneron Pharmaceuticals, Inc.; and as a DSMB member for GSK, LEO Pharma, Novan, Pfizer, and UCB. Cork has served as a consultant and/or advisory board member and/or received research grants from Almirall, Amgen, Astellas, Bayer, GSK, Hyphens Pharma, Johnson & Johnson, LEO Pharma, Novartis, Perrigo L’Oreal, Pfizer, Sanofi, Stiefel, Regeneron Pharmaceuticals, Inc., and Unilever. Wollenberg has served as an investigator for Eli Lilly, Galderma, LEO Pharma, Novartis, Pfizer, Regeneron Pharmaceuticals, Inc., Sanofi Genzyme, and UCB; as a consultant for AbbVie, Almirall, Anacor, Arena, Eli Lilly, Galapagos, Galderma, LEO Pharma, Novartis, Pfizer, Regeneron Pharmaceuticals, Inc., and Sanofi Genzyme; and has received research grants from LEO Pharma and Pierre Fabre. Chen, Khokhar, Bansal, and Cyr are employees and shareholders of Regeneron Pharmaceuticals, Inc. Vakil and Zhang are employees and may hold stock and/or stock options in Sanofi Genzyme.
Supporting information
Fig S1
Fig S2a
Fig S2b
Supplementary Material
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to thank Brad Shumel, MD, of Regeneron Pharmaceuticals, Inc., for his considerable contributions to the development of this manuscript. Research sponsored by Sanofi and Regeneron Pharmaceuticals, Inc. ClinicalTrials.gov Identifiers: NCT03054428, NCT03345914. Medical writing/editorial assistance provided by Lola MacRae, PhD, of Excerpta Medica, funded by Sanofi Genzyme and Regeneron Pharmaceuticals, Inc. according to the Good Publication Practice guideline .
Paller AS, Beck LA, Blauvelt A, et al. Infections in children and adolescents treated with dupilumab in pediatric clinical trials for atopic dermatitis—A pooled analysis of trial data. Pediatr Dermatol. 2022;39:187–196. doi: 10.1111/pde.14909
DATA AVAILABILITY STATEMENT
Qualified researchers may request access to study documents (including the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan) that support the methods and findings reported in this manuscript. Individual anonymized participant data will be considered for sharing once the product and indication has been approved by major health authorities (e.g., FDA, EMA, PMDA, etc), if there is legal authority to share the data and there is not a reasonable likelihood of participant re‐identification. Submit requests to https://vivli.org/.
REFERENCES
- 1. Totte JE, van der Feltz WT, Hennekam M, van Belkum A, van Zuuren EJ, Pasmans SG. Prevalence and odds of Staphylococcus aureus carriage in atopic dermatitis: a systematic review and meta‐analysis. Br J Dermatol. 2016;175(4):687‐695. [DOI] [PubMed] [Google Scholar]
- 2. Narla S, Silverberg JI. Association between atopic dermatitis and serious cutaneous, multiorgan and systemic infections in US adults. Ann Allergy Asthma Immunol. 2018;120(1):66‐72.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Eichenfield LF, Ahluwalia J, Waldman A, Borok J, Udkoff J, Boguniewicz M. Current guidelines for the evaluation and management of atopic dermatitis: a comparison of the Joint Task Force Practice Parameter and American Academy of Dermatology guidelines. J Allergy Clin Immunol. 2017;139(4S):S49‐S57. [DOI] [PubMed] [Google Scholar]
- 4. Wollenberg A, Barbarot S, Bieber T, et al. Consensus‐based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part II. J Eur Acad Dermatol Venereol. 2018;32(6):850‐878. [DOI] [PubMed] [Google Scholar]
- 5. Rinvoq (upadacitinib) Prescribing Information. AbbVie Inc.; 2019. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/211675s000lbl.pdf. Accessed June 30, 2021 [Google Scholar]
- 6. Olumiant (baricitinib) Prescribing information. Lilly USA, LLC; 2018. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/207924s000lbl.pdf. Accessed June 30, 2021. [Google Scholar]
- 7. Gandhi NA, Pirozzi G, Graham NMH. Commonality of the IL‐4/IL‐13 pathway in atopic diseases. Expert Rev Clin Immunol. 2017;13(5):425‐437. [DOI] [PubMed] [Google Scholar]
- 8. Le Floc’h A, Allinne J, Nagashima K, et al. Dual blockade of IL‐4 and IL‐13 with dupilumab, an IL‐4Rα antibody, is required to broadly inhibit type 2 inflammation. Allergy. 2020;75(5):1188‐1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Annunziato F, Romagnani C, Romagnani S. The 3 major types of innate and adaptive cell‐mediated effector immunity. J Allergy Clin Immunol. 2015;135(3):626‐635. [DOI] [PubMed] [Google Scholar]
- 10. Paller AS, Siegfried EC, Thaçi D, et al. Efficacy and safety of dupilumab with concomitant topical corticosteroids in children 6 to 11 years old with severe atopic dermatitis: a randomized, double‐blinded, placebo‐controlled phase 3 trial. J Am Acad Dermatol. 2020;83(5):1282‐1293. [DOI] [PubMed] [Google Scholar]
- 11. Simpson EL, Paller AS, Siegfried EC, et al. Efficacy and safety of dupilumab in adolescents with uncontrolled moderate to severe atopic dermatitis: a phase 3 randomized clinical trial. JAMA Dermatol. 2020;156(1):44‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cork MJ, Thaçi D, Eichenfield LF, et al. Dupilumab in adolescents with uncontrolled moderate‐to‐severe atopic dermatitis: results from a phase IIa open‐label trial and subsequent phase III open‐label extension. Br J Dermatol. 2020;182(1):85‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Eichenfield LF, Bieber T, Beck LA, et al. Infections in dupilumab clinical trials in atopic dermatitis: a comprehensive pooled analysis. Am J Clin Dermatol. 2019;20(3):443‐456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Blauvelt A, de Bruin‐Weller M, Gooderham M, et al. Long‐term management of moderate‐to‐severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1‐year, randomised, double‐blinded, placebo‐controlled, phase 3 trial. Lancet. 2017;389(10086):2287‐2303. [DOI] [PubMed] [Google Scholar]
- 15. Simpson EL, Bieber T, Guttman‐Yassky E, et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375(24):2335‐2348. [DOI] [PubMed] [Google Scholar]
- 16. de Bruin‐Weller M, Thaçi D, Smith CH, et al. Dupilumab with concomitant topical corticosteroid treatment in adults with atopic dermatitis with an inadequate response or intolerance to ciclosporin A or when this treatment is medically inadvisable: a placebo‐controlled, randomized phase III clinical trial (LIBERTY AD CAFE). Br J Dermatol. 2018;178(5):1083‐1101. [DOI] [PubMed] [Google Scholar]
- 17. Akinlade B, Guttman‐Yassky E, Bruin‐Weller M, et al. Conjunctivitis in dupilumab clinical trials. Br J Dermatol. 2019;181(3):459‐473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bansal A, Simpson EL, Paller AS, et al. Conjunctivitis in dupilumab clinical trials for adolescents with atopic dermatitis or asthma. Am J Clin Dermatol. 2021;22(1):101‐115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Beck LA, Cork MJ, Amagai M, et al. Type 2 Inflammation contributes to skin barrier dysfunction in atopic dermatitis. JID Innovations, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Callewaert C, Nakatsuji T, Knight R, et al. IL‐4Rα blockade by dupilumab decreases Staphylococcus aureus colonization and increases microbial diversity in atopic dermatitis. J Invest Dermatol. 2020;140(1):191‐202.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Guttman‐Yassky E, Bissonnette R, Ungar B, et al. Dupilumab progressively improves systemic and cutaneous abnormalities in patients with atopic dermatitis. J Allergy Clin Immunol. 2019;143(1):155‐172. [DOI] [PubMed] [Google Scholar]
- 22. Olsen JR, Gallacher J, Piguet V, Francis NA. Epidemiology of molluscum contagiosum in children: a systematic review. Fam Pract. 2014;31(2):130‐136. [DOI] [PubMed] [Google Scholar]
- 23. Berger EM, Orlow SJ, Patel RR, Schaffer JV. Experience with molluscum contagiosum and associated inflammatory reactions in a pediatric dermatology practice: the bump that rashes. Arch Dermatol. 2012;148(11):1257‐1264. [DOI] [PubMed] [Google Scholar]
- 24. Di Lernia V, Casanova DM, Simonetti V. Dupilumab may facilitate the clearance of molluscum contagiosum in atopic dermatitis patients (Dupilumab kann die Abheilung von Molluscum contagiosum bei Patienten mit atopischer Dermatitis erleichtern [Article in German]). J Dtsch Dermatol Ges. 2020;18(9):1016‐1018. [DOI] [PubMed] [Google Scholar]
- 25. Ferrucci S, Tavecchio S, Veraldi S, Berti E, Benzecry V. Transitory dissemination of molluscum contagiosum in a patient treated with dupilumab for atopic dermatitis. Acta Derm Venereol. 2020;100(14):adv00207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fotiadou C, Lazaridou E, Lekkas D, Kourouklidou A, Demetrios I. Disseminated, eruptive molluscum contagiosum lesions in a psoriasis patient under treatment with methotrexate and cyclosporine. Eur J Dermatol. 2012;22(1):147‐148. [DOI] [PubMed] [Google Scholar]
- 27. Wendt S, Trawinski H, Schubert S, Rodloff AC, Mössner J, Lübbert C. The diagnosis and treatment of pinworm infection. Dtsch Arztebl Int. 2019;116(13):213‐219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gonzalez ME, Schaffer JV, Orlow SJ, et al. Cutaneous microbiome effects of fluticasone propionate cream and adjunctive bleach baths in childhood atopic dermatitis. J Am Acad Dermatol. 2016;75(3):481‐493.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Fig S2a
Fig S2b
Supplementary Material
Supplementary Material
Data Availability Statement
Qualified researchers may request access to study documents (including the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan) that support the methods and findings reported in this manuscript. Individual anonymized participant data will be considered for sharing once the product and indication has been approved by major health authorities (e.g., FDA, EMA, PMDA, etc), if there is legal authority to share the data and there is not a reasonable likelihood of participant re‐identification. Submit requests to https://vivli.org/.


