Abstract
Aims
Elevated concentrations of growth differentiation factor 15 (GDF‐15) in patients with heart failure (HF) have been consistently associated with worse clinical outcomes, but what disease mechanisms high GDF‐15 concentrations represent remains unclear. Here, we aim to identify activated pathophysiological pathways related to elevated GDF‐15 expression in patients with HF.
Methods and results
In 2279 patients with HF, we measured circulating levels of 363 biomarkers. Then, we performed a pathway over‐representation analysis to identify key biological pathways between patients in the highest and lowest GDF‐15 concentration quartiles. Data were validated in an independent cohort of 1705 patients with HF. In both cohorts, the strongest up‐regulated biomarkers in those with high GDF‐15 were fibroblast growth factor 23 (FGF‐23), death receptor 5 (TRAIL‐R2), WNT1‐inducible signalling pathway protein 1 (WISP‐1), tumour necrosis factor receptor superfamily member 11a (TNFRSF11A), leucocyte immunoglobulin‐like receptor subfamily B member 4 (LILRB4), and trefoil factor 3 (TFF3). Pathway over‐representation analysis revealed that high GDF‐15 patients had increased activity of pathways related to inflammatory processes, notably positive regulation of chemokine production; response to interleukin‐6; tumour necrosis factor and death receptor activity; and positive regulation of T‐cell differentiation and inflammatory response. Furthermore, we found pathways involved in regulation of insulin‐like growth factor (IGF) receptor signalling and regulatory pathways of tissue, bones, and branching structures. GDF‐15 quartiles significantly predicted all‐cause mortality and HF hospitalization.
Conclusion
Patients with HF and high plasma concentrations of GDF‐15 are characterized by increased activation of inflammatory pathways and pathways related to IGF‐1 regulation and bone/tissue remodelling.
Keywords: Heart failure, Pathway analysis, Growth differentiation factor 15, Biomarkers
We identified activated pathophysiological pathways related to elevated plasma growth differentiation factor 15 (GDF‐15) levels in patients with heart failure (HF) using pathway over‐representation analysis of 363 circulating biomarkers. Patients with HF with high plasma concentrations of GDF‐15 were characterized by increased activation of inflammatory pathways and pathways related to insulin‐like growth factor (IGF)‐1 regulation and bone/tissue remodelling.
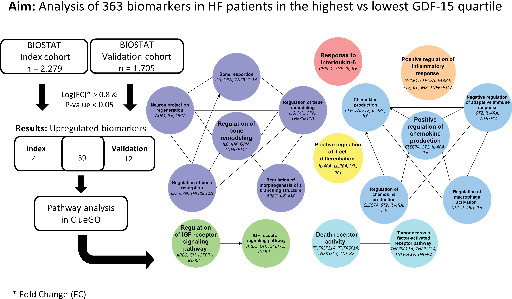
Introduction
The benchmark prognostic biomarker for heart failure (HF) is N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP), a hormone that is secreted by cardiomyocytes after stretching of the ventricle. 1 This measure of mechanical stretching and pressure is valuable in HF. However, NT‐proBNP does not cover the full pathophysiology of HF and other biomarkers might reflect other disease mechanisms. 1 , 2 , 3 , 4
Elevated concentrations of growth differentiation factor 15 (GDF‐15) have been consistently associated with a higher risk of mortality and HF hospitalization in patients with both HF with preserved ejection fraction (HFpEF) and HF with reduced ejection fraction, 2 , 5 , 6 , 7 , 8 , 9 even on top of NT‐pro‐BNP and established clinical predictors. 10 , 11 GDF‐15 is part of the transforming growth factor‐β cytokine family and is a pleiotropic protein involved in various biological processes including inflammation, apoptosis, fibrosis, hypertrophy, and endothelial dysfunction in both the acute and chronic phase. 6 GDF‐15 is non‐cardiac specific, and is lowly expressed by many tissues in physiological states, but is elevated in renal, pulmonary, and cardiac disease among others. 6 GDF‐15 could thus serve as a predictor for various systemic processes in HF. 2 However, due to its complex function, it remains unclear what specific disease mechanisms elevated GDF‐15 concentrations represent in HF. This study utilizes differential expression and pathways analysis of a large number of biomarkers from different disease domains to identify the pathophysiological mechanisms related to elevated GDF‐15 expression in HF.
Methods
Study patients
Overall, 2279 patients with new onset or worsening signs of HF from the multicentre, multinational, prospective, observational index trail of BIOSTAT‐CHF were included between 2010 and 2012. 12 Data were validated in the BIOSTAT‐CHF validation cohort consisting of 1705 hospitalized or outpatients with HF from six centres in Scotland that were enrolled between 2010 and 2014. Inclusion criteria for both cohorts are in the online supplementary material. Patients were grouped into quartiles based on their GDF‐15 levels, after which the highest versus the lowest quartiles were compared.
Biomarkers
Four Olink Proseek Multiplex panels each measuring 92 biomarkers were used to measure circulating biomarker concentrations: cardiovascular (CVD) II, CVD III, immuno‐oncology, and inflammation [https://www.olink.com/products/]. The kits use a proximity extension assay (PEA) technology, which binds oligonucleotide‐labelled antibody probe pairs to the target biomarker. 13 Real‐time polymerase chain reaction was performed for further quantification. For overlapping biomarkers present in multiple panels (B‐type natriuretic peptide and NT‐proBNP; amphiregulin; stem cell factor; and interleukin‐6 [IL‐6]), the mean values of duplicates were calculated leading to a final number of 363 biomarkers. Details of assay reliability for the six most strongly associated with GDF‐15 biomarkers are in the online supplementary Table S1 . 14 Further, sample storage conditions can be found in the online supplementary material. GDF‐15 concentrations were measured using electrochemiluminescence by a Cobas e 411 analyser at baseline using a standard Roche Diagnostics GmbH method in the lab of the Experimental Cardiology department in the UMCG, Groningen. GDF‐15 assays had a level of detection of ≤400 pg/ml and intra‐assay coefficients of variation of <1.5% regardless of GDF‐15 concentration. 15
Statistical analysis
We performed Kaplan–Meier analysis to evaluate differences in survival between patients in GDF‐15 quartiles for two outcomes: all‐cause mortality and HF hospitalization at 2 years. Further, we performed a Cox proportional hazard analysis to investigate the predictive strength of GDF‐15 quartiles on 2‐year all‐cause mortality and hospitalization (with competing risk for mortality) when adjusted for age, sex, estimated glomerular filtration rate (eGFR), diabetes, NT‐proBNP and New York Heart Association (NYHA) class. To establish the strongest associated variables of high GDF‐15 concentrations, we carried out a multivariable logistic regression model in the index cohort. Variables that were significantly different between the highest and lowest GDF‐15 quartile in both cohorts (Tables 1 and 2 ) and had missing data in <20% of patients were included in a multivariable model. Backward selection was performed with a p‐value cut‐off of 0.05 using the fastbw function from the rms package in R. 16 The differential expression analysis was performed using the Limma package in R. 17 In the differential expression analysis, the patients with the highest GDF‐15 quartile were compared to the patients with the lowest GDF‐15 quartile (GDF‐15 high vs. low) in both cohorts. Biomarkers with false discovery rate (FDR, Benjamini–Hochberg) <5% and log fold change value >0.8 were considered to be significantly upregulated. Only biomarkers that were found upregulated in both the index and validation cohorts were used in the pathway analysis. Further, we performed a differential expression analysis for the highest versus the lowest quartiles for each of the six most strongly associated with GDF‐15 biomarkers separately in the index cohort. Then, we created a heatmap, also including GDF‐15 expression results, including all biomarkers that were significant in at least one analysis. As a sensitivity analysis, using full data (without exclusion of the second and third quartiles), we performed linear regression analyses for each biomarker as a dependent variable of log‐transformed GDF‐15 levels. Pathway analysis was performed using a Cystoscope plug‐in, ClueGO, utilizing Gene Ontology Biological Process (08‐05‐2020); more details are in the online supplementary material. 18 P‐values of each pathway term were Bonferroni corrected.
Table 1.
Baseline characteristics of the highest versus lowest growth differentiation factor 15 quartile in the index cohort
| Index cohort characteristics | Lowest GDF‐15 quartile (n = 570) | Highest GDF‐15 quartile (n = 569) | p‐value |
|---|---|---|---|
| Female sex | 156 (27.4) | 139 (24.4) | 0.287 |
| Age | 63.7 [54.5–71.8] | 73.9 [65.1–80.2] | <0.001 |
| Race | 0.696 | ||
| Caucasian | 563 (98.8) | 565 (99.3) | |
| Asian | 2 (0.35) | 2 (0.35) | |
| Black | 2 (0.35) | 0 (0.00) | |
| Other | 3 (0.53) | 2 (0.35) | |
| BMI (kg/m2) | 27.5 [24.6–30.8] | 26.9 [23.9–30.4] | 0.054 |
| Weight (kg) | 82.0 [71.0–93.2] | 79.0 [69.0–90.0] | 0.002 |
| Height (cm) | 172 [166–178] | 170 [165–176] | 0.008 |
| NYHA class | <0.001 | ||
| I | 23 (4.11) | 7 (1.27) | |
| II | 291 (52.0) | 125 (22.6) | |
| III | 207 (37.0) | 321 (58.2) | |
| IV | 39 (6.96) | 99 (17.9) | |
| LVEF (%) | 30.0 [25.0–35.0] | 30.0 [21.0–37.0] | 0.867 |
| Pulmonary congestion/oedema with rales/crackles | <0.001 | ||
| No | 326 (59.7) | 196 (35.1) | |
| Single base | 67 (12.3) | 85 (15.2) | |
| Bi‐basilar | 153 (28.0) | 278 (49.7) | |
| Orthopnoea present | 130 (22.8) | 258 (45.7) | <0.001 |
| Peripheral oedema | 455 (79.8) | 492 (86.6) | 0.003 |
| Pulmonary congestion/oedema >1/3 up lung fields | 27 (12.2) | 77 (21.3) | 0.008 |
| Elevated JVP | <0.001 | ||
| No | 311 (78.1) | 186 (46.4) | |
| Yes | 67 (16.8) | 185 (46.1) | |
| Uncertain | 20 (5.03) | 30 (7.48) | |
| Hepatomegaly | 51 (8.95) | 119 (21.1) | <0.001 |
| SBP (mmHg) | 125 [110–140] | 120 [106–130] | <0.001 |
| DBP (mmHg) | 79.0 [70.0–85.0] | 70.0 [62.0–80.0] | <0.001 |
| Heart rate (bpm) | 75.0 [66.0–85.0] | 77.0 [67.5–90.0] | 0.039 |
| Admitted to ICU/CCU | 20 (32.8) | 22 (20.0) | 0.094 |
| Years since first diagnosis | 0.12 [0.01–1.80] | 0.52 [0.01–4.44] | 0.333 |
| Ischaemia | 233 (41.7) | 325 (57.8) | <0.001 |
| Previous HF hospitalizzation(s) in last year | 146 (25.6) | 215 (37.8) | <0.001 |
| Hypertension | 325 (57.0) | 364 (64.0) | 0.019 |
| Atrial fibrillation | 194 (34.0) | 311 (54.7) | <0.001 |
| Myocardial infarction | 175 (30.7) | 252 (44.3) | <0.001 |
| PCI | 99 (17.4) | 136 (23.9) | 0.008 |
| CABG | 62 (10.9) | 143 (25.1) | <0.001 |
| Diabetes mellitus | 105 (18.4) | 262 (46.0) | <0.001 |
| COPD | 63 (11.1) | 105 (18.5) | 0.001 |
| Peripheral arterial disease | 33 (5.79) | 81 (14.2) | <0.001 |
| Stroke | 36 (6.32) | 74 (13.0) | <0.001 |
| Haemoglobin (g/dl) | 13.9 [12.8–14.9] | 12.4 [11.1–13.7] | <0.001 |
| Serum creatinine (µmol/L) | 88.4 [75.0–106] | 126 [99.9–166] | <0.001 |
| Urinary creatinine (mmol/L) | 7.10 [3.48–12.0] | 3.85 [2.20–6.53] | <0.001 |
| Urea (mmol/L) | 8.60 [6.30–13.3] | 15.9 [9.84–25.3] | <0.001 |
| Sodium (mmol/L) | 140 [138–142] | 139 [136–141] | <0.001 |
| Potassium (mmol/L) | 4.30 [4.00–4.60] | 4.20 [3.90–4.60] | 0.069 |
| BNP (pg/ml) | 417 [185–738] | 1101 [678–1989] | <0.001 |
| NT‐proBNP (pg/ml) | 1108 [476–2233] | 5962 [2800–12 855] | <0.001 |
| eGFR (ml/min/1.73 m2) | 75.1 [60.4–90.5] | 46.0 [32.3–60.1] | <0.001 |
| LVEDD (mm) | 62.0 [57.0–68.0] | 60.0 [53.0–66.0] | <0.001 |
| LVESD (mm) | 50.0 [45.0–57.0] | 49.0 [42.0–56.8] | 0.033 |
| Left atrial diameter (mm) | 46.0 [42.0–51.0] | 49.0 [43.5–55.0] | <0.001 |
| GDF‐15 (pg/ml) | 1224 [943–1472] | 7071 [5598–10 510] | <0.001 |
Data are presented as n (%), or median [quartiles].
BMI, body mass index; BNP, B‐type natriuretic peptide; CABG, coronary artery bypass graft; COPD, chronic obstructive pulmonary disease; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; GDF‐15, growth differentiation factor 15; HF, heart failure; ICU, intensive care unit; JVP, jugular venous pressure; LVEDD, left ventricular end‐diastolic diameter; LVEF, left ventricular ejection fraction; LVESD, left ventricular end‐systolic diameter; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; SBP, systolic blood pressure.
Table 2.
Baseline characteristics of the highest versus lowest growth differentiation factor quartile in the validation cohort
| Validation cohort characteristics | Lowest GDF‐15 quartile (n = 427) | Highest GDF‐15 quartile (n = 426) | p‐value |
|---|---|---|---|
| Female sex | 155 (36.3) | 128 (30.0) | 0.062 |
| Age | 68.2 [60.9–75.4] | 78.9 [71.7–84.4] | <0.001 |
| Race | 0.624 | ||
| Caucasian | 425 (99.5) | 423 (99.3) | |
| Black | 1 (0.23) | 0 (0.00) | |
| Asian | 0 (0.00) | 2 (0.47) | |
| Hispanic | 1 (0.23) | 1 (0.23) | |
| BMI (kg/m2) | 28.7 [25.6–33.2] | 27.1 [23.9–32.0] | <0.001 |
| Weight (kg) | 83.5 [71.0–98.0] | 78.0 [65.0–89.0] | <0.001 |
| Height (cm) | 170 [162–178] | 168 [160–175] | 0.003 |
| NYHA class | <0.001 | ||
| I | 11 (2.58) | 2 (0.47) | |
| II | 251 (58.9) | 105 (24.6) | |
| III | 143 (33.6) | 219 (51.4) | |
| IV | 21 (4.93) | 100 (23.5) | |
| LVEF (%) | 39.5 [35.0–48.0] | 40.0 [35.0–50.0] | 0.131 |
| Pulmonary congestion/oedema with rales/crackles | <0.001 | ||
| No | 302 (75.7) | 152 (36.9) | |
| Single base | 16 (4.01) | 33 (8.01) | |
| Bi‐basilar | 81 (20.3) | 227 (55.1) | |
| Peripheral oedema | 363 (85.0) | 400 (94.1) | <0.001 |
| Pulmonary congestion/oedema >1/3 up lung fields | 5 (4.07) | 15 (9.04) | 0.158 |
| Elevated JVP | <0.001 | ||
| No | 288 (80.9) | 226 (59.6) | |
| Yes | 67 (18.8) | 152 (40.1) | |
| Uncertain | 1 (0.28) | 1 (0.26) | |
| Hepatomegaly | 4 (1.08) | 30 (7.52) | <0.001 |
| SBP (mmHg) | 124 [112–140] | 121 [107–137] | 0.044 |
| DBP (mmHg) | 72.0 [64.2–80.0] | 66.0 [59.0–75.0] | <0.001 |
| Heart rate (bpm) | 70.0 [60.0–80.0] | 74.0 [64.0–87.0] | <0.001 |
| Years since first diagnosis | 0.99 [0.13–3.66] | 1.59 [0.05–5.46] | 0.134 |
| Ischemia | 263 (94.9) | 282 (92.2) | 0.232 |
| Previous HF hospitalization(s) in last year | 100 (23.7) | 136 (32.6) | 0.005 |
| Hypertension | 212 (49.6) | 268 (63.1) | <0.001 |
| Atrial fibrillation | 155 (36.7) | 214 (50.6) | <0.001 |
| Myocardial infarction | 193 (45.2) | 213 (50.1) | 0.171 |
| PCI | 97 (22.9) | 69 (16.3) | 0.020 |
| CABG | 66 (15.5) | 83 (19.5) | 0.140 |
| Diabetes mellitus | 80 (18.8) | 208 (49.1) | <0.001 |
| COPD, n (%) | 64 (15.1) | 79 (18.8) | 0.178 |
| Peripheral arterial disease | 64 (15.4) | 100 (24.3) | 0.002 |
| Stroke | 58 (13.7) | 88 (20.8) | 0.009 |
| Haemoglobin (g/dl) | 14.0 [13.1–15.2] | 12.1 [10.9–13.3] | <0.001 |
| Serum creatinine (µmol/L) | 83.0 [69.0–96.0] | 127 [100–168] | <0.001 |
| Urinary creatinine (mmol/L) | 4.10 [2.00–7.90] | 3.80 [2.20–6.50] | 0.286 |
| Urea (mmol/L) | 6.80 [5.50–8.22] | 12.5 [9.00–17.1] | <0.001 |
| Sodium (mmol/L) | 140 [138–141] | 139 [136–141] | 0.001 |
| Potassium (mmol/L) | 4.30 [4.10–4.50] | 4.30 [3.90–4.60] | 0.268 |
| NT‐proBNP (pg/ml) | 548 [229–1357] | 3994 [1459–8712] | <0.001 |
| eGFR (ml/min/1.73 m2) | 70.3 [58.8–84.6] | 38.0 [27.0–52.2] | <0.001 |
| LVEDD (mm) | 56.0 [50.0–62.0] | 54.0 [47.0–60.0] | 0.001 |
| LVESD (mm) | 44.6 (13.1) | 42.6 (12.0) | 0.226 |
| Left atrial diameter (mm) | 44.0 [40.0–49.0] | 46.0 [42.0–50.2] | 0.002 |
| GDF‐15 (pg/ml) | 1343 [1084–1600] | 6955 [5436–9583] | <0.001 |
Data are presented as n (%), median [quartiles], or mean (standard deviation).
BMI, body mass index; BNP, B‐type natriuretic peptide; CABG, coronary artery bypass graft; COPD, chronic obstructive pulmonary disease; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; GDF‐15, growth differentiation factor 15; HF, heart failure; ICU, intensive care unit; JVP, jugular venous pressure; LVEDD, left ventricular end‐diastolic diameter; LVEF, left ventricular ejection fraction; LVESD, left ventricular end‐systolic diameter; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; SBP, systolic blood pressure.
Results
Clinical characteristics
Tables 1 and 2 show the baseline characteristics for the index and validation cohorts, respectively. Patients in the highest GDF‐15 quartile had significantly higher serum creatinine and urea, lower eGFR and lower haemoglobin compared to patients in the lowest GDF‐15 quartile. In both the index and validation cohorts, patients with higher GDF‐15 had significantly more often a history of a diabetes mellitus, percutaneous coronary intervention, stroke, atrial fibrillation (AF), myocardial infarction (MI), a higher NYHA class, hepatomegaly, higher NT‐proBNP concentrations and lower weight. Multivariable logistic regression analysis showed that the strongest associated variables for higher GDF‐15 were diabetes, higher NT‐proBNP, lower eGFR, lower haemoglobin and smaller left ventricular end‐diastolic diameter as shown in online supplementary Table S2 . Baseline characteristics for all four quartiles are presented in online supplementary Tables S3 and S4 . Higher GDF‐15 quartiles had significantly higher probability of all‐cause mortality and HF hospitalization at 2 years (Figure 1 ). Further, after adjusting for potential confounders including age, sex, eGFR, diabetes, NT‐proBNP and NYHA class, the association between GDF‐15 quartiles and all‐cause mortality remained significant and was gradually increased per quarter (compared to the first quarter): hazard ratio (HR) of quartile 2 = 1.46 and 2.82, HR of quartile 3 = 1.78 and 3.24, and HR of quartile 4 = 2.26 and 5.26 in the index and validation cohort, respectively. The associations were not significant for 2‐year hospitalization (online supplementary Table S5 ).
Figure 1.
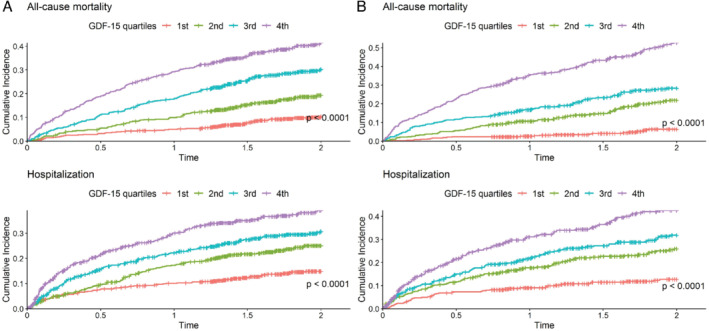
Kaplan–Meier survival curves and p‐value of log‐rank test for all‐cause mortality and hospitalization at 2 years in the index cohort (A) and the validation cohort (B) stratified according to growth differentiation factor 15 (GDF‐15) quartiles.
Differential expression analysis
In both the index and validation cohorts, higher GDF‐15 was associated with upregulation of several other biomarkers (Figure 2 ). A total of 43 and 51 proteins were upregulated in the index and validation cohort, respectively, of which 39 upregulated proteins were replicated in both cohorts (Figure 3 ). In both cohorts, the strongest upregulated biomarkers in patients with high GDF‐15 concentrations were fibroblast growth factor 23 (FGF‐23), death receptor 5 (TRAIL‐R2), WNT1‐inducible signalling pathway protein 1 (WISP‐1), tumour necrosis factor (TNF) receptor superfamily member 11a (TNFRSF11A), leucocyte immunoglobulin‐like receptor subfamily B member 4 (LILRB4) and trefoil factor 3 (TFF3) (Figure 2 and online supplementary Table S6 ). Furthermore, 7 out of 39 biomarkers belonged to the TNF receptor superfamily. Heatmap analysis (online supplementary Figure S1 ) showed GDF‐15 had a distinct expression profile compared to FGF‐23, TRAIL‐R2, WISP‐1, TNFRSF11A, LILRB4 and TFF3. Results from the linear regression analyses (sensitivity analysis) after correction for FDR of 5% are presented in online supplementary Table S7 .
Figure 2.
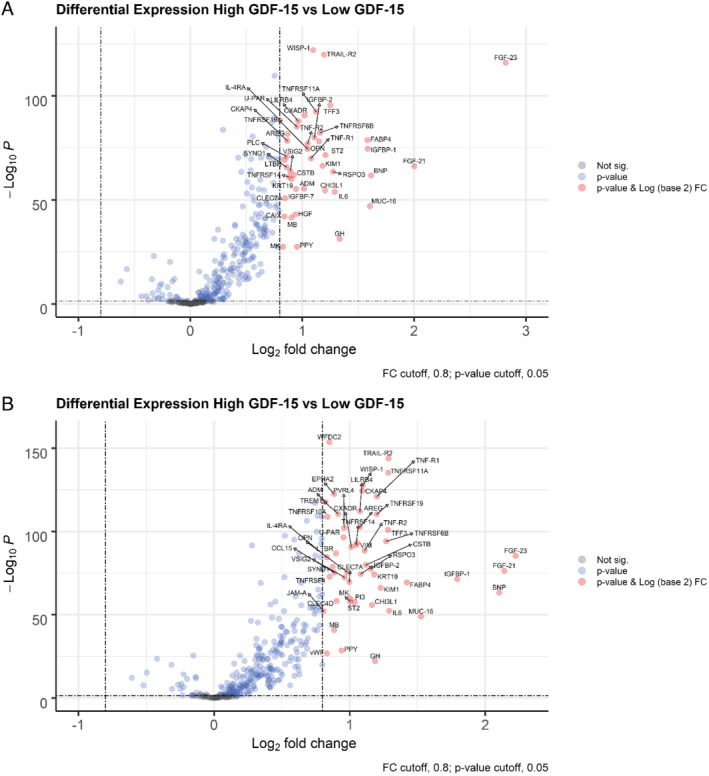
Volcano plots presenting differentially expressed biomarkers in heart failure patients with high growth differentiation factor 15 (GDF‐15) concentrations compared to heart failure patients with low GDF‐15 concentrations — (A) the index cohort, (B) the validation cohort. ADM, adrenomedullin; AREG, amphiregulin; BNP, B‐type natriuretic peptide; CAIX, carbonic anhydrase 9; CCL15, C‐C motif chemokine ligand 15; CHI3L1, chitinase 3 like 1; CKAP4, cytoskeleton associated protein 4; CLEC4D, C‐type lectin domain family 4 member D; CLEC7A, C‐type lectin domain containing 7A; CSTB, cystatin B; CXADR, CXADR Ig‐like cell adhesion molecule; EPHA2, EPH receptor A2; FABP4, fatty acid binding protein 4; FGF‐21, fibroblast growth factor 21; FGF‐23, fibroblast growth factor 23; GH, growth hormone 1; HGF, hepatocyte growth factor; IGFBP‐1, insulin‐like growth factor binding protein 1; IGFBP‐2, insulin‐like growth factor binding protein 2; IGFBP‐7, insulin‐like growth factor binding protein 7; JAM‐A, F11 receptor; KIM1, hepatitis A virus cellular receptor 1; KRT19, keratin 19; IL‐4RA, interleukin‐4 receptor; IL6, interleukin‐6; LILRB4, leucocyte immunoglobulin like receptor B4; LTBR, lymphotoxin beta receptor; MB, myoglobin; MK, midkine; MUC‐16, mucin 16, cell surface associated; PI3, peptidase inhibitor 3; PLC, heparan sulfate proteoglycan 2; PPY, pancreatic polypeptide; PVRL4, nectin cell adhesion molecule 4; RSPO3, R‐spondin 3; OPN, secreted posphoporetin 1; ST2, interleukin 1 receptor like 1; SYND1, syndecan 1; TFF3, trefoil factor 3; TNF‐R1, TNF receptor superfamily member 1A; TNF‐R2, TNF receptor superfamily member 1B; TNFRSF4, TNF receptor superfamily member 4; TNFRSF6B, TNF receptor superfamily member 6b; TNFRSF10A, TNF receptor superfamily member 10a; TNFRSF11A, TNF receptor superfamily member 11a; TNFRSF14, TNF receptor superfamily member 14; TNFRSF19, TNF receptor superfamily member 19; TRAIL‐R2, TNF receptor superfamily member 10b; TREM1, triggering receptor expressed on myeloid cells 1; U‐PAR, urokinase plasminogen activator receptor; VIM, vimentin; VSIG2, V‐set and immunoglobulin domain containing 2; vWF, von Willebrand factor; WFDC2, WAP four‐disulfide core domain 2; WISP‐1, WNT1‐inducible signalling pathway protein 1.
Figure 3.
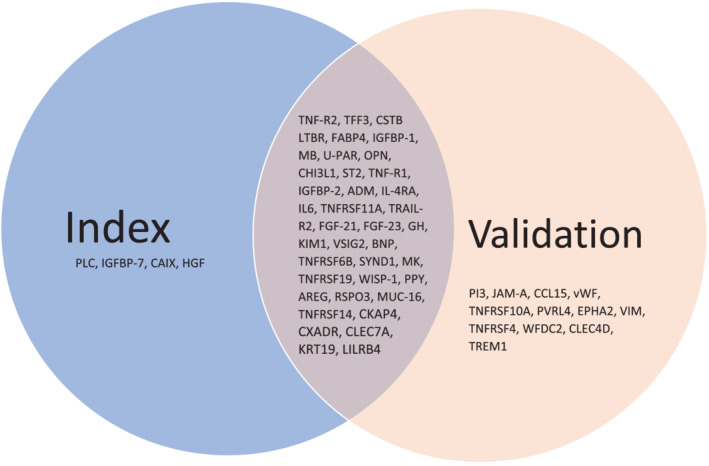
Upregulated biomarkers after differential expression analysis. For abbreviations see Figure 2 .
Pathway analysis
Pathway over‐representation analysis revealed that patients in the highest GDF‐15 quartile had increased activity of pathways related to inflammatory processes, notably regulation of chemokine production, macrophages, and adaptive immune response; response to IL‐6; TNF and death receptor activity; and positive regulation of T‐cell differentiation and inflammatory response (Figure 4 ), as compared to patients in the lowest GDF‐15 quartile. Furthermore, pathways involved in regulation of insulin‐like growth factor (IGF) receptor signalling were found, in addition to regulatory pathways of bones, tissues, neurons and branching structures. Biomarkers related to each pathway are presented in Figure 4A.
Figure 4.
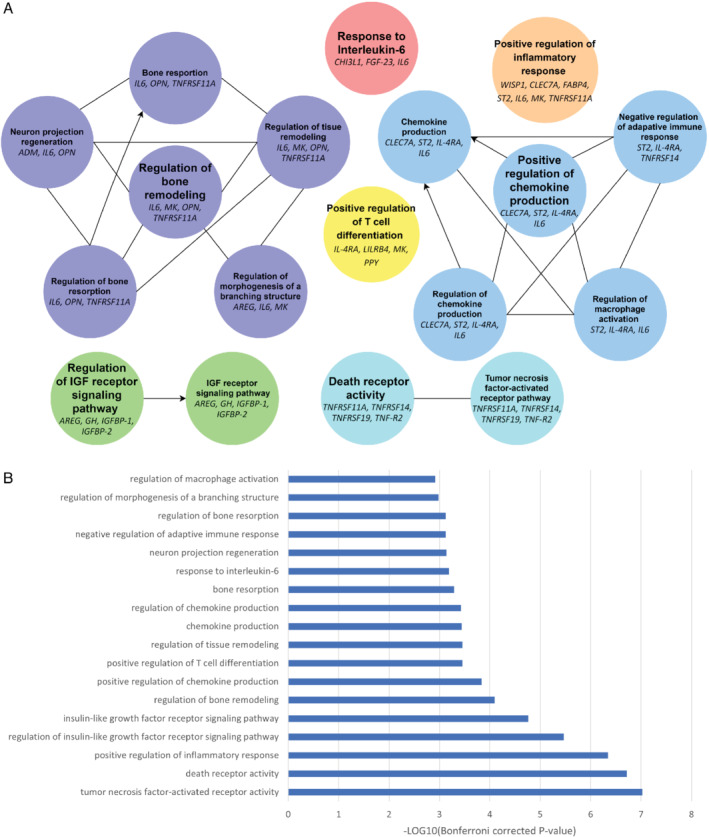
Pathway over‐representation results for heart failure patients with high growth differentiation factor 15 (GDF‐15) concentrations compared to heart failure patients with low GDF‐15 concentrations. (A) Pathway networks, (B) Bonferroni corrected p‐values per pathway term. AREG, amphiregulin; CHI3L1, chitinase 3 like 1; CLEC7A, C‐type lectin domain family 7 member A; FABP4, fatty acid binding protein 4; FGF‐23, fibroblast growth factor 23; GH, growth hormone; IGFBP‐1, insulin like growth factor binding protein 1; IGFBP‐2, insulin like growth factor binding protein 2; IL‐4RA, interleukin‐4 receptor; IL6, interleukin‐6; LILRB4, leucocyte immunoglobulin like receptor B4; MK, midkine; OPN, secreted posphoporetin 1/osteopontin; ST2, interleukin 1 receptor‐like 1; TNF‐R2, TNF receptor superfamily member 1B; TNFRSF11A, TNF receptor superfamily member 11a; TNFRSF14, TNF receptor superfamily member 14; TNFRSF19, TNF receptor superfamily member 19; WISP‐1, WNT1‐inducible signalling pathway protein 1.
Discussion
In two independent cohorts of patients with HF we consistently found that high GDF‐15 concentrations identified patients with increased activity of pathways related to inflammation and those involved in regulation of IGF receptor signalling and bone/tissue remodelling (Graphical Abstract).
Normal physiology of GDF‐15
GDF‐15 is expressed in the kidney, liver, lung, pancreas, gastrointestinal tract and heart, with physiological serums levels between 0.1–1.2 ng/ml. 19 , 20 Increased GDF‐15 levels are linked to older age, excessive exercise, pregnancy, oxidized low‐density lipoprotein (oxLDL) and smoking. 2 , 19 Furthermore, GDF‐15 elevations are seen in many pathological conditions, including metabolic disease, cancer, inflammation, and cardiovascular disease. 2 , 19 , 20 It is believed that GDF‐15 plays a protective, anti‐inflammatory role in the liver, kidney, heart and lungs after injury and inflammation. 2 , 19 , 21 Further, GDF‐15 has a bone regulatory function, as it attenuates osteocyte differentiation in response to hypoxia and in cancer and spondylarthritis. 22 , 23 , 24 , 25 Furthermore, GDF‐15 blocks polymorphonuclear leucocytes recruitment which reduces cardiac rupture after MI and inhibits atherosclerosis progression, 2 , 20 in addition to inhibiting nuclear factor kappa B (NF‐κB). 19 Moreover, GDF‐15 protects against ischaemia–reperfusion injury by inhibiting neutrophil infiltration and apoptosis, decreases thrombus formation by inhibiting platelet integrin activation, and GDF‐15 treatment or cardiac overexpression decreases cardiac hypertrophy. 20 The exact protein–receptor interactions through which GDF‐15 exerts these effects, are still unclear. 19 , 20 GDF‐15 also inhibits appetite, thereby reducing obesity and potentially increasing insulin sensitivity. 20 Moreover, GDF‐15 produced by cardiomyocytes inhibits growth hormone (GH) signalling in the liver by reducing plasma IGF‐1 and IGF binding protein 3 (IGFBP‐3), without affecting GH concentrations, potentially inducing the GH‐tolerance seen in HF. 26
Inflammatory pathways
We found an association between elevated GDF‐15 and biomarkers related to macrophage and adaptive immune response. These findings are in line with earlier studies, where GDF‐15 directly decreased myeloid cell adhesion and transmigration after myocardial infarction, protecting the heart in the chronic phase. 27 Macrophages and monocytes are the major source for TNF in the human body, what may explain the link between GDF‐15 and TNF. As shown in a previous study, TNF has a direct negative inotropic and hypertrophic effect on myocardial cells; uncouples adenylyl cyclase from beta‐adrenergic receptors via its effect on G inhibitory protein; leads to release of cytokines; is directly cytotoxic to endothelial cells and activates metalloproteinases which leads to extracellular matrix remodelling. 28 TNF is expressed in cardiac cells in HF, but not in healthy cells, and is known to be associated with HF severity. 28 Moreover, TNF is increased earlier in the disease process than NT‐proBNP. 29 Furthermore, similar to GDF‐15, TNF is linked to cachexia, anorexia and muscle wasting. 28 , 30 , 31 , 32 , 33 TNF‐α exerts its cachexic effect through activation of death receptors TNF receptor 1 and 2 (TNFR1 and 2), which in turn leads to long‐term overexpression of NF‐κB dependent gene products and results in increased apoptosis, 34 , 35 and through interfering with neuropeptide Y release in the hypothalamus. 34 , 35 The upregulation of TNF‐related proteins could thus partly explain why weight was consistently decreased, and NYHA class increased in upper compared to the lower quartiles of GDF‐15 patients in both cohorts.
Interleukin‐6 is an important pleiotropic cytokine that has pro‐ and anti‐inflammatory effects through its activation of transmembrane receptor subunit glycoprotein 130 (gp130). In the heart, IL‐6 predominantly has its effect through the Janus kinase/signal transducer and activator of transcription 1/3 (JAK/STAT1/3) pathway. 36 Like TNF proteins, IL‐6 has protective effects in the acute phase as a survival pathway, but leads to reduced contractility, diastolic disturbances, disturbed calcium handling and cardiac hypertrophy when chronically elevated or during unbalanced expression. 36 , 37 Further, JAK2/STAT3 activation by IL‐6 increases hepcidin activation, which in turn reduces plasma iron levels through inhibition of ferroportin. 38 In large heterogeneous cohorts, elevated IL‐6 can be found in over half of chronic HF patients, is significantly related to HFpEF, NT‐proBNP, iron deficiency and AF. 39 Furthermore, IL‐6 was found to be a significant predictor of mortality and hospitalization in HF. 37 The upregulation in the IL‐6 pathway in patients with high GDF‐15 levels could be representative of a chronic maladaptation and inflammatory state in patients with elevated concentrations of GDF‐15 and contributes to the observed reduced haemoglobin levels.
GDF‐15 was also related to positive chemokine production regulation through pathways including interleukin‐4 receptor (IL4‐RA), interleukin 1 receptor‐like 1 (ST2) and IL‐6, which are all increased in, or a predictor of, HF. 40 Other studies have found that GDF‐15 inhibits chemokine production in HF, possibly highlighting its protective, anti‐inflammatory function. 27 Further, we found an association between positive regulation of T lymphocytes and GDF‐15. Global increases in T lymphocytes are an essential driving factor for both mortality and remodelling in ischaemic HF, with CD4+/CD28null subsets playing a role in the development of AF, and an increase in CD4+/CD57+ being related to cardiovascular events and increased TNF‐α expression. 41 , 42 , 43
Insulin‐like growth factor regulation
Similar to GDF‐15, IGF‐1 has long been linked to diabetes and HF. 44 , 45 IGFBP‐1 and 2 and GH were found to be upregulated in patients with elevated GDF‐15. IGFBP‐2 is a significant predictor for mortality in HF. 46 , 47 This mechanism is likely through IGFBP‐2 inhibition of IGF‐1. IGF‐1 has a cardioprotective effect through a downregulation of the renin–angiotensin system. 47 In addition, IGF‐1 increases contractility, is indicated in physiological cardiac hypertrophy, delays myocyte apoptosis, and is found to be reduced in HF, particularly with comorbid cachexia. 45 , 48 These findings suggest that IGF‐1 might become a novel treatment target of cachectic HF patients. Paradoxically, patients with higher GDF‐15 concentrations also had higher GH concentrations. GH increases IGF production and improves cardiac function. 49 However, in severe HF, especially in cachexic patients, GH resistance occurs, increasing circulating GH concentrations but reducing its cardio‐protective effects. 48 , 49 GDF‐15 seems thus to be related to an unbalanced IGF/GH axis.
Bone, tissue, and branched structure remodelling
Pathway analysis found upregulation in bone and tissue remodelling, which is in line with previous literature, as GDF‐15 has been related to osteoclast differentiation, being attenuated by bone hypoxia. 23 , 50 Key players in these pathways were osteopontin, IL‐6 and TNFRSF11A. Osteopontin inhibits myeloid cell recruitment and metalloproteinase expression, regulates osteoclast differentiation, and has a pleiotropic effect on vascular remodelling. Moreover, osteopontin has strong links to left ventricular dysfunction and remodelling, particularly in HFpEF. 51 , 52 , 53 GDF‐15 has been found to suppress osteopontin secretions in fibrotic liver disease, likely acting as a liver protective protein, in line with our findings, as high GDF‐15 was significantly linked to hepatomegaly. 54 , 55 The bone regulatory effects of GDF‐15 have been linked to bone metastasis in cancer and spondylarthritis, however its exact role in HF remains unclear. 24 , 25 , 50
Strongest GDF‐15 associated biomarkers
WISP‐1 is an extracellular matrix protein expressed by fibroblasts in the heart, lungs, kidneys, spleen, pancreas and brain among others. 56 Further, WISP‐1 is highly expressed in visceral adipose tissue where it may reflect adipose tissue inflammation and insulin resistance, and is elevated in obesity and in reaction to oxLDL. 56 Moreover, it is upregulated in the left ventricle after MI where it might be a therapeutic target for revascularization as it stimulates cardiac‐specific angiogenesis. 57 Although early results are promising, effects were measured 7 days post‐MI. Future studies using knockout mice and/or recombinant WISP‐1 should investigate both the long‐ and short‐term effects of WISP‐1 attenuation and aim identify the receptor for WISP‐1 on endothelial cells. 57
TFF3 belongs to the trefoil factor family, which are expressed in a variety of mucin‐producing cells in the intestines and kidney. 58 It is increased in diabetes, after use of antihypertensive medication and in kidney injury. 58 In the latter it may reflect regenerative function through restitution, apoptosis inhibition and modulation of differentiation/immune function. 58 TFF3 is a Food and Drug Administration‐approved biomarker of drug‐induced kidney injury and is associated with chronic kidney disease. 58 Moreover, TFF3 is upregulated and cardioprotective after MI, 59 and predicts a composite endpoint including HF hospitalization and cardiac death in HF, even after adjustment for established risk predictors. 60 Why TFF3 is highly predictive of HF outcomes is unknown, future studies utilizing TFF3 knockout in HF‐induced mice models could shed light onto the mechanisms of TFF3‐related pathophysiology.
LILRB4 is part of the immunoglobulin superfamily and is expressed on the surface of monocytes, macrophages and dendritic cells. 61 , 62 LILRB4 may be a future therapeutic target as it protects against cardiac hypertrophy and fibrosis by inhibition of NF‐κB. 62 , 63 Moreover, LILRB4 improves plaque stability due to decreasing NF‐κB, IL‐6 and TNF‐α mediated inflammation. 61 , 62 The upregulation of LILRB4 in antigen presenting cells might thus reflect a cardioprotective function. Further, LILRB4 expression is reduced in pressure‐overloaded hearts, 63 thus LILRB4 might improve established cardiac hypertrophy which warrants additional investigation, for example by LILRB4 induction in hypertrophic mice models.
FGF‐23 is a glycoprotein mainly expressed by osteoblasts and is associated with cardiovascular diseases, chronic kidney disease, HF and mortality. 64 FGF‐23 contributes to arterial stiffness/inflammation, left ventricular hypertrophy, renin–angiotensin system stimulation and endothelial dysfunction, although it might stabilize and improve contractility acutely post‐MI. 64 Our finding of elevated FGF‐23 is likely due to both increased skeletal and cardiac expression, because even though not expressed in the healthy heart, cardiac specific FGF‐23 is increased post‐MI, in cardiomyopathy and is augmented by left ventricular hypertrophy. 64 Currently, it is unclear whether FGF‐23 secreted by the myocardium is protective or deleterious. Future studies could investigate optimal systemic and cardiac FGF‐23 levels, explaining the conflicting results so far. 64
TRAIL‐R2 is a cell surface death receptor that is expressed on endothelium, vascular smooth muscle cells (VSMCs) and macrophages, is highly expressed in the heart, and can also be released in a soluble form (sTRAIL). 65 , 66 , 67 It predicts all‐cause mortality after MI 68 and HF development. 67 In most cells TRAIL‐R2 stimulates apoptosis, whereas in cardiomyocytes it promotes hypertrophy while inhibiting fibrosis after injury. 67 , 69 However, more research is needed if TRAIL‐R2 therapy has a future in established HF, as TRAIL‐R2 expression and decoy receptor mediated regulation may be altered in HF. 67
TNFRSF11A, or receptor activator of NF‐kB (RANK), binds to RANK ligand (RANKL) resulting in NF‐kB signalling and osteoprotegerin (OPG) production. 70 The RANK–RANKL–OPG signalling axis is a main regulator of vessel wall calcification/inflammation, bone remodelling, and T‐cell functioning. 70 Although widely expressed in the gastrointestinal tract, adipose tissue and arteries among others, cardiomyocyte RANK expression is increased response to systemic RANKL, but also in VSMCs and endothelial cells in the failing myocardium. 71 Future studies utilizing TNFRSF11A knockout in mice might elucidate TNFRSF11A exact role in HF pathophysiology.
Utility and diagnostics
This study found that patients in the upper GDF‐15 quartiles had significantly lower weight. GDF‐15 exerts its anorexic effect through binding to glial cell‐derived neurotrophic factor (GDNF) family receptor alpha‐like (GFRAL) in the nucleus of the solitary tract and the area postrema, leading to a reduction in appetite. 32 Furthermore, acute induction of the GDF‐15/GFRAL pathway has been shown to combat obesity in mice and non‐human primates, whilst blockade of the pathway helps prevent anorexia and weight loss in cancer patients undergoing chemotherapy. 72 , 73 Moreover, the weight lowering effects of metformin may be mediated through its acute upregulation of GDF‐15. 74 Chronic elevation of GDF‐15 through genetic proxying, however, relates to higher body mass index. 75 This study thus strengthens the argument for additional research on GDF‐15 therapy in HF to promote weight loss.
We found that patients in high GDF‐15 quartiles were more likely to have AF. Previous studies have identified GDF‐15 as a predictor for bleeding risk, but not stroke, in AF patients undergoing anticoagulation therapy. 76 , 77 Therefore, GDF‐15 was included into the ABC (age, biomarkers, clinical history) bleeding score which improved discrimination and utility to pre‐existing models. In line with literature, GDF‐15 quartiles showed to significantly predict both all‐cause mortality and HF hospitalization, highlighting the value of GDF‐15 inclusion in future prognostic multi‐marker models. 2 , 5 , 6 , 7 Furthermore, we found that GDF‐15 is associated with various inflammatory and metabolic pathways, giving insight into why GDF‐15 is such a powerful predictor for patients with HF.
Clinical implications
To our knowledge, this is the first study linking GDF‐15 with WISP‐1, TFF3, LILRB4, TRAIL‐R2 and TNFRSF11A in HF. Besides improving prognosis, GDF‐15 could be a valuable marker that indicates repair processes after myocardial and renal damage and can potentially guide therapy based on several repair mechanisms found in this study. Studies applying the Mendelian randomization analysis have found a causal effect of genetically predicted TNF levels on coronary artery disease and ischemic stroke. 78 Further, there was a causal relation established between IL‐6 signalling and coronary artery disease and AF. 79 Thus, we cannot exclude the possibility that there are causal links between biomarkers tested in our study and HF, and this could be addressed in the future with the Mendelian randomization approach. Moreover, further studies could help understand whether WISP‐1, TFF3, LILRB4, FGF‐23 and TRAIL‐R2 carry a clinical potential, and whether GDF‐15 could predict treatment response for these agents. Furthermore, the link between GDF‐15 and the IGF‐1/GH signalling axis could be further investigated, as GDF‐15 might be a therapeutic target to alleviate GH resistance in HF.
Strengths and limitations
The main strength in the design of our study is the validation of our findings in an independent cohort. Another strength is the high number of analysed biomarkers (n = 363), which cover a broad range of proteins such as cytokines, enzymes, apoptotic and growth factors. Further, biomarkers were analysed using PEA, which shows good performance in plasma samples due to its high sensitivity, specificity, scalability, and low sample consumption, 13 , 80 and is shown not to be affected by freeze–thaw cycles. 81 We acknowledge limitations of our study. Firstly, since the analysed biomarkers come from the following panels: cardiovascular II and III, oncological, and immunological, any upregulated pathway could be potentially biased into one of these four categories. Secondly, since gene ontology annotation data are continuously manually updated by curators, and more extensively studied proteins might be more comprehensively annotated, updates and choice of GO data source might slightly affect results of the pathway analysis. In addition, although circulating plasma protein levels are influenced by factors such as metabolism and clearance besides tissue expression, an increasing amount of evidence suggests that plasma proteins reliably detect myocardial tissue changes. 82 However, because tissue sample analytes were not measured, verification that the 363 circulating biomarkers truly reflect tissue‐based events was limited. Furthermore, although the enrichment/depletion analysis (two‐sided hypergeometric test) in ClueGO showed that the relation between the identified pathways and the upregulated biomarkers was highly significant (Figure 4B), whether the number of biomarkers was sufficient to comprehensively populate the pathways is still uncertain. Lastly, although our findings reflected previous literature, enrolled patients were largely of Caucasian descent (99%), limiting generalizability. Further studies in more diverse populations will have to be performed to replicate our findings.
Conclusion
Patients with HF and high plasma concentrations of GDF‐15 are characterized by increased activation of inflammatory pathways and pathways related to IGF‐1 regulation and bone/tissue remodelling.
Funding
This work was supported by an educational grant from Roche Diagnostics. The BIOSTAT‐CHF project was funded by a grant from the European Commission (FP7‐242209‐BIOSTAT‐CHF; EudraCT 2010–020808–29). Dr. De Boer is supported by a grant from the European Research Council (ERC CoG 818715, SECRETE‐HF).
Conflict of interest: The UMCG, which employs several of the authors, has received research grants and/or fees from AstraZeneca, Abbott, Boehringer Ingelheim, Cardior Pharmaceuticals Gmbh, Ionis Pharmaceuticals, Inc., Novo Nordisk, and Roche. J.T. has received speaker and or consultancy fees from Roche diagnostics, Us2.ai and holds a patent (U.S. Patent No. 10,702,247) unrelated to the present work. R.A.d.B. received speaker fees from Abbott, AstraZeneca, Bayer, Novartis, and Roche. C.C.L. declares receiving consultancy fees and/or research grants from Amgen, Applied Therapeutics, AstraZeneca, Boehringer Ingelheim, MSD, Novartis, and Novo Nordisk. S.D.A. reports receiving fees from Abbott, Bayer, Boehringer Ingelheim, Cardiac Dimension, Cordio, Impulse Dynamics, Novartis, Occlutech, Servier, and Vifor Pharma, and grant support from Abbott and Vifor Pharma. M.M. has received personal fees from Actelion, Amgen, AstraZeneca, Abbott Vascular, Servier, Edwards Therapeutics, Livanova, Vifor pharma, WindTree Therapeutics, as member of Trials' Committees or for speeches at sponsored meetings in the last three years. All other authors have nothing to disclose.
Supporting information
Table S1. Assay coefficients of variation for the most strongly associated biomarkers with GDF‐15.
Table S2. Multivariable logistic model investigating association with the highest GDF‐15 quartile in the index cohort.
Table S3. Baseline characteristics of all GDF‐15 quartiles in the index cohort.
Table S4. Baseline characteristics of all GDF‐15 quartiles in the validation cohort.
Table S5. Hazard ratio and P‐value for GDF‐15 quartiles in Cox regression predicting 2‐year all‐cause mortality and hospitalization in the index and validation cohort, adjusted for established risk factors.
Table S6. Differential expression results in the index and validation cohort.
Table S7. Associations between circulating biomarkers and GDF‐15 levels.
Figure S1. Heatmap for expression profiles of GDF‐15, TFF3, FGF‐23, LILRB4, WISP‐1, TRAIL‐R2, TNFRSF11A in the index cohort.
References
- 1. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18:891–975. [DOI] [PubMed] [Google Scholar]
- 2. Wesseling M, de Poel JHC, de Jager SCA. Growth differentiation factor 15 in adverse cardiac remodelling: from biomarker to causal player. ESC Heart Fail. 2020;7:1488–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Van Linthout S, Tschöpe C. Inflammation – cause or consequence of heart failure or both? Curr Heart Fail Rep. 2017;14:251–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shirazi LF, Bissett J, Romeo F, Mehta JL. Role of inflammation in heart failure. Curr Atheroscler Rep. 2017;19:27. [DOI] [PubMed] [Google Scholar]
- 5. Sharma A, Stevens SR, Lucas J, Fiuzat M, Adams KF, Whellan DJ, et al. Utility of growth differentiation factor‐15, a marker of oxidative stress and inflammation, in chronic heart failure: insights from the HF‐ACTION study. JACC Heart Fail. 2017;5:724–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. George M, Jena A, Srivatsan V, Muthukumar R, Dhandapani V. GDF 15 – a novel biomarker in the offing for heart failure. Curr Cardiol Rev. 2016;12:37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cotter G, Voors AA, Prescott MF, Felker GM, Filippatos G, Greenberg BH, et al. Growth differentiation factor 15 (GDF‐15) in patients admitted for acute heart failure: results from the RELAX‐AHF study. Eur J Heart Fail. 2015;17:1133–43. [DOI] [PubMed] [Google Scholar]
- 8. Chan MMY, Santhanakrishnan R, Chong JPC, Chen Z, Tai BC, Liew OW, et al. Growth differentiation factor 15 in heart failure with preserved vs. reduced ejection fraction. Eur J Heart Fail. 2016;18:81–8. [DOI] [PubMed] [Google Scholar]
- 9. Wollert KC, Kempf T, Wallentin L. Growth differentiation factor 15 as a biomarker in cardiovascular disease. Clin Chem. 2017;63:140–51. [DOI] [PubMed] [Google Scholar]
- 10. Richter B, Koller L, Hohensinner PJ, Zorn G, Brekalo M, Berger R, et al. A multi‐biomarker risk score improves prediction of long‐term mortality in patients with advanced heart failure. Int J Cardiol. 2013;168:1251–7. [DOI] [PubMed] [Google Scholar]
- 11. Lok DJ, Klip IT, Lok SI, Bruggink‐André de la Porte PW, Badings E, van Wijngaarden J, et al. Incremental prognostic power of novel biomarkers (growth‐differentiation factor‐15, high‐sensitivity C‐reactive protein, galectin‐3, and high‐sensitivity troponin‐T) in patients with advanced chronic heart failure. Am J Cardiol. 2013;112:831–7. [DOI] [PubMed] [Google Scholar]
- 12. Voors AA, Anker SD, Cleland JG, Dickstein K, Filippatos G, van der Harst P, et al. A systems BIOlogy Study to TAilored Treatment in Chronic Heart Failure: rationale, design, and baseline characteristics of BIOSTAT‐CHF. Eur J Heart Fail. 2016;18:716–26. [DOI] [PubMed] [Google Scholar]
- 13. Assarsson E, Lundberg M, Holmquist G, Björkesten J, Thorsen SB, Ekman D, et al. Homogenous 96‐plex PEA immunoassay exhibiting high sensitivity, specificity, and excellent scalability. PLoS One. 2014;9:e95192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Document download center – Olink. [Internet]. [cited 2021 Nov 20]. Available from: https://www.olink.com/resources‐support/document‐download‐center/
- 15. Roche Diagnostics International Ltd . Illuminating the hidden severity of cardiovascular disease with GDF‐15. [Internet]. 2017. [cited 2021 Nov 20]. Available from: https://chrono.bg/useruploads/GDF‐15.brochure.pdf
- 16. Regression Modeling Strategies : With Applications to Linear Models, Logistic… ‐ Frank E. Harrell, Jr. ‐ Google Books [Internet] [cited 2021 Jun 29]. Available from: https://books.google.it/books?id=94RgCgAAQBAJ&printsec=frontcover#v=onepage&q&f=false
- 17. Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, et al. Limma powers differential expression analyses for RNA‐sequencing and microarray studies. Nucleic Acids Res. 2015;43:e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bindea G, Mlecnik B, Hackl H, Charoentong P, Tosolini M, Kirilovsky A, et al. ClueGO: a Cytoscape plug‐in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics. 2009;25:1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Assadi A, Zahabi A, Hart RA. GDF15, an update of the physiological and pathological roles it plays: a review. Pflügers Arch. 2020;472:1535–46. [DOI] [PubMed] [Google Scholar]
- 20. Wang D, Day EA, Townsend LK, Djordjevic D, Jørgensen SB, Steinberg GR. GDF15: emerging biology and therapeutic applications for obesity and cardiometabolic disease. Nat Rev Endocrinol. 2021;17:592–607. [DOI] [PubMed] [Google Scholar]
- 21. Abulizi P, Loganathan N, Zhao D, Mele T, Zhang Y, Zwiep T, et al. Growth differentiation factor‐15 deficiency augments inflammatory response and exacerbates septic heart and renal injury induced by lipopolysaccharide. Sci Rep. 2017;7:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hinoi E. Regulation of osteoclastogenesis by osteocytes through growth differentiation factor‐15. Yakugaku Zasshi. 2014;134:1259–63. [DOI] [PubMed] [Google Scholar]
- 23. Hinoi E, Ochi H, Takarada T, Nakatani E, Iezaki T, Nakajima H, et al. Positive regulation of osteoclastic differentiation by growth differentiation factor 15 upregulated in osteocytic cells under hypoxia. J Bone Miner Res. 2012;27:938–49. [DOI] [PubMed] [Google Scholar]
- 24. Duan L, Pang HL, Chen WJ, Shen WW, Cao PP, Wang SM, et al. The role of GDF15 in bone metastasis of lung adenocarcinoma cells. Oncol Rep. 2019;41:2379–88. [DOI] [PubMed] [Google Scholar]
- 25. Wang W, Yang X, Dai J, Lu Y, Zhang J, Keller ET. Prostate cancer promotes a vicious cycle of bone metastasis progression through inducing osteocytes to secrete GDF15 that stimulates prostate cancer growth and invasion. Oncogene. 2019;38:4540–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang T, Liu J, McDonald C, Lupino K, Zhai X, Wilkins BJ, et al. GDF 15 is a heart‐derived hormone that regulates body growth. EMBO Mol Med. 2017;9:1150–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kempf T, Zarbock A, Widera C, Butz S, Stadtmann A, Rossaint J, et al. GDF‐15 is an inhibitor of leukocyte integrin activation required for survival after myocardial infarction in mice. Nat Med. 2011;17:581–8. [DOI] [PubMed] [Google Scholar]
- 28. Feldman AM, Combes A, Wagner D, Kadakomi T, Kubota T, You Li Y, et al. The role of tumor necrosis factor in the pathophysiology of heart failure. J Am Coll Cardiol. 2000;35:537–44. [DOI] [PubMed] [Google Scholar]
- 29. Torre‐Amione G, Kapadia S, Benedict C, Oral H, Young JB, Mann DL. Proinflammatory cytokine levels in patients with depressed left ventricular ejection fraction: a report from the Studies of Left Ventricular Dysfunction (SOLVD). J Am Coll Cardiol. 1996;27:1201–6. [DOI] [PubMed] [Google Scholar]
- 30. Hariyanto TI, Kurniawan A. Appetite problem in cancer patients: pathophysiology, diagnosis, and treatment. Cancer Treat Res Commun. 2021;27:100336. [DOI] [PubMed] [Google Scholar]
- 31. Lerner L, Hayes TG, Tao N, Krieger B, Feng B, Wu Z, et al. Plasma growth differentiation factor 15 is associated with weight loss and mortality in cancer patients. J Cachexia Sarcopenia Muscle. 2015;6:317–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ahmed DS, Isnard S, Lin J, Routy B, Routy JP. GDF15/GFRAL pathway as a metabolic signature for cachexia in patients with cancer. J Cancer. 2021;12:1125–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Molfino A, Amabile MI, Imbimbo G, Rizzo V, Pediconi F, Catalano C, et al. Association between growth differentiation factor‐15 (GDF‐15) serum levels, anorexia and low muscle mass among cancer patients. Cancers (Basel). 2021;13:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rahman A, Jafry S, Jeejeebhoy K, Nagpal AD, Pisani B, Agarwala R. Malnutrition and cachexia in heart failure. J Parenter Enteral Nutr. 2016;40:475–86. [DOI] [PubMed] [Google Scholar]
- 35. Wajant H, Siegmund D. TNFR1 and TNFR2 in the control of the life and death balance of macrophages. Front Cell Dev Biol. 2019;7:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Askevold ET, Gullestad L, Dahl CP, Yndestad A, Ueland T, Aukrust P. Interleukin‐6 signaling, soluble glycoprotein 130, and inflammation in heart failure. Curr Heart Fail Rep. 2014;11:146–55. [DOI] [PubMed] [Google Scholar]
- 37. Piek A, Du W, de Boer RA, Silljé HHW. Novel heart failure biomarkers: why do we fail to exploit their potential? Crit Rev Clin Lab Sci. 2018;55:246–63. [DOI] [PubMed] [Google Scholar]
- 38. Vela D. Balance of cardiac and systemic hepcidin and its role in heart physiology and pathology. Lab Invest. 2017;98:315–26. [DOI] [PubMed] [Google Scholar]
- 39. Markousis‐Mavrogenis G, Tromp J, Ouwerkerk W, Devalaraja M, Anker SD, Cleland JG, et al. The clinical significance of interleukin‐6 in heart failure: results from the BIOSTAT‐CHF study. Eur J Heart Fail. 2019;21:965–73. [DOI] [PubMed] [Google Scholar]
- 40. Alvarez P, Briasoulis A. Immune modulation in heart failure: the promise of novel biologics. Curr Treat Options Cardiovasc Med. 2018;20:26. [DOI] [PubMed] [Google Scholar]
- 41. Bansal SS, Ismahil MA, Goel M, Patel B, Hamid T, Rokosh G, et al. Activated T‐lymphocytes are essential drivers of pathological remodeling in ischemic heart failure. Circ Heart Fail. 2017;10:e003688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sulzgruber P, Kolle L, Winter MP, Richter B, Blum S, Korpak M, et al. The impact of CD4+CD28null T‐lymphocytes on atrial fibrillation and mortality in patients with chronic heart failure. Thromb Haemost. 2017;117:349–56. [DOI] [PubMed] [Google Scholar]
- 43. Youn JC, Jung MK, Yu HT, Kwon JS, Kwak JE, Park SH, et al. Increased frequency of CD4+CD57+ senescent T cells in patients with newly diagnosed acute heart failure: exploring new pathogenic mechanisms with clinical relevance. Sci Rep. 2019;9:12887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kim MS, Lee DY. Insulin‐like growth factor (IGF)‐I and IGF binding proteins axis in diabetes mellitus. Ann Pediatr Endocrinol Metab. 2015;20:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Roof SR, Boslett J, Russell D, del Rio C, Alecusan J, Zweier JL, et al. Insulin‐like growth factor 1 prevents diastolic and systolic dysfunction associated with cardiomyopathy and preserves adrenergic sensitivity. Acta Physiol. 2016;216:421–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ravassa S, Beaumont J, Cediel G, Lupón J, López B, Querejeta R, et al. Cardiorenal interaction and heart failure outcomes. A role for insulin‐like growth factor binding protein 2? Rev Esp Cardiol (Engl Ed). 2020;73:835–43. [DOI] [PubMed] [Google Scholar]
- 47. Barutaut M, Fournier P, Peacock WF, Evaristi MF, Caubère C, Turkieh A, et al. Insulin‐like growth factor binding protein 2 predicts mortality risk in heart failure. Int J Cardiol. 2020;300:245–51. [DOI] [PubMed] [Google Scholar]
- 48. Piccioli L, Arcopinto M, Salzano A, D'Assante R, Schiavo A, Stagnaro FM, et al. The impairment of the growth hormone/insulin‐like growth factor 1 (IGF‐1) axis in heart failure: a possible target for future therapy. Monaldi Arch Chest Dis. 2018;88:20–5. [DOI] [PubMed] [Google Scholar]
- 49. Anker SD, Volterrani M, Pflaum CD, Strasburger CJ, Osterziel KJ, Doehner W, et al. Acquired growth hormone resistance in patients with chronic heart failure: implications for therapy with growth hormone. J Am Coll Cardiol. 2001;38:443–52. [DOI] [PubMed] [Google Scholar]
- 50. Song Y, Cui Y, Zhang X, Lin H, Zhang G, Zeng H, et al. Increased serum levels of MIC1/GDF15 correlated with bone erosion in spondyloarthritis: a pilot study. Medicine (Baltimore). 2018;97:e13733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Icer MA, Gezmen‐Karadag M. The multiple functions and mechanisms of osteopontin. Clin Biochem. 2018;59:17–24. [DOI] [PubMed] [Google Scholar]
- 52. Yousefi K, Irion CI, Takeuchi LM, Ding W, Lambert G, Eisenberg T, et al. Osteopontin promotes left ventricular diastolic dysfunction through a mitochondrial pathway. J Am Coll Cardiol. 2019;73:2705–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tromp J, Khan MAF, Klip IT, Meyer S, de Boer RA, Jaarsma T, et al. Biomarker profiles in heart failure patients with preserved and reduced ejection fraction. J Am Heart Assoc. 2017;6:e003989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Koo BK, Um SH, Seo DS, Joo SK, Bae JM, Park JH, et al. Growth differentiation factor 15 predicts advanced fibrosis in biopsy‐proven non‐alcoholic fatty liver disease. Liver Int. 2018;38:695–705. [DOI] [PubMed] [Google Scholar]
- 55. Kim KH, Kim SH, Han DH, Jo YS, Lee YH, Lee MS. Growth differentiation factor 15 ameliorates nonalcoholic steatohepatitis and related metabolic disorders in mice. Sci Rep. 2018;8:6789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Murahovschi V, Pivovarova O, Ilkavets I, Dmitrieva RM, Döcke S, Keyhani‐Nejad F, et al. WISP1 is a novel adipokine linked to inflammation in obesity. Diabetes. 2015;64:856–66. [DOI] [PubMed] [Google Scholar]
- 57. Wright LH, Herr DJ, Brown SS, Kasiganesan H, Menick DR. Angiokine Wisp‐1 is increased in myocardial infarction and regulates cardiac endothelial signaling. JCI Insight. 2018;3:e95824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Astor BC, Köttgen A, Hwang S‐J, Bhavsar N, Fox CS, Coresh J. Trefoil factor 3 predicts incident chronic kidney disease: a case‐control study nested within the Atherosclerosis Risk in Communities (ARIC) study. Am J Nephrol. 2011;34:291–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Liu SQ, Tefft BJ, Zhang D, Roberts D, Schuster DJ, Wu A. Cardioprotective mechanisms activated in response to myocardial ischemia. Mol Cell Biomech. 2011;8:319–38. [PubMed] [Google Scholar]
- 60. Brankovic M, Martijn Akkerhuis K, Mouthaan H, Constantinescu A, Caliskan K, van Ramshorst J, et al. Utility of temporal profiles of new cardio‐renal and pulmonary candidate biomarkers in chronic heart failure. Int J Cardiol. 2019;276:157–65. [DOI] [PubMed] [Google Scholar]
- 61. Jiang Z, Qin JJ, Zhang Y, Cheng WL, Ji YX, Gong FH, et al. LILRB4 deficiency aggravates the development of atherosclerosis and plaque instability by increasing the macrophage inflammatory response via NF‐κB signaling. Clin Sci. 2017;131:2275–88. [DOI] [PubMed] [Google Scholar]
- 62. Zhou H, Li N, Yuan Y, Jin YG, Wu Q, Yan L, et al. Leukocyte immunoglobulin‐like receptor B4 protects against cardiac hypertrophy via SHP‐2‐dependent inhibition of the NF‐κB pathway. J Mol Med. 2020;98:691–705. [DOI] [PubMed] [Google Scholar]
- 63. Li Q, Wei G, Tao T. Leukocyte immunoglobulin‐like receptor B4 (LILRB4) negatively mediates the pathological cardiac hypertrophy by suppressing fibrosis, inflammation and apoptosis via the activation of NF‐κB signaling. Biochem Biophys Res Commun. 2019;509:16–23. [DOI] [PubMed] [Google Scholar]
- 64. Stöhr R, Schuh A, Heine GH, Brandenburg V. FGF23 in cardiovascular disease: innocent bystander or active mediator? Front Endocrinol (Lausanne). 2018;9:351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Gonçalves I, Singh P, Tengryd C, Cavalera M, Mattisson IY, Nitulescu M, et al. sTRAIL‐R2 (soluble TNF [tumor necrosis factor]‐related apoptosis‐inducing ligand receptor 2) a marker of plaque cell apoptosis and cardiovascular events. Stroke. 2019;50:1989–96. [DOI] [PubMed] [Google Scholar]
- 66. Kakareko K, Rydzewska‐Rosołowska A, Zbroch E, Hryszko T. Trail and cardiovascular disease – a risk factor or risk marker: a systematic review. J Clin Med. 2021;10:1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Tanner MA, Thomas TP, Grisanti LA. Death receptor 5 contributes to cardiomyocyte hypertrophy through epidermal growth factor receptor transactivation. J Mol Cell Cardiol. 2019;136:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Skau E, Henriksen E, Wagner P, Hedberg P, Siegbahn A, Leppert J. GDF‐15 and TRAIL‐R2 are powerful predictors of long‐term mortality in patients with acute myocardial infarction. Eur J Prev Cardiol. 2017;24:1576–83. [DOI] [PubMed] [Google Scholar]
- 69. Tanner MA, Grisanti LA. A dual role for death receptor 5 in regulating cardiac fibroblast function. Front Cardiovasc Med. 2021;8:699102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Dutka M, Bobiński R, Wojakowski W, Francuz T, Pająk C, Zimmer K. Osteoprotegerin and RANKL‐RANK‐OPG‐TRAIL signalling axis in heart failure and other cardiovascular diseases. Heart Fail Rev. 2021. 10.1007/s10741-021-10153-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ueland T, Yndestad A, Øie E, Florholmen G, Halvorsen B, Frøland SS, et al. Dysregulated osteoprotegerin/RANK ligand/RANK axis in clinical and experimental heart failure. Circulation. 2005;111:2461–8. [DOI] [PubMed] [Google Scholar]
- 72. Breen DM, Kim H, Bennett D, Calle RA, Collins S, Esquejo RM, et al. GDF‐15 neutralization alleviates platinum‐based chemotherapy‐induced emesis, anorexia, and weight loss in mice and nonhuman primates. Cell Metab. 2020;32:938–50. [DOI] [PubMed] [Google Scholar]
- 73. Mullican SE, Lin‐Schmidt X, Chin CN, Chavez JA, Furman JL, Armstrong AA, et al. GFRAL is the receptor for GDF15 and the ligand promotes weight loss in mice and nonhuman primates. Nat Med. 2017;23:1150–7. [DOI] [PubMed] [Google Scholar]
- 74. Coll AP, Chen M, Taskar P, Rimmington D, Patel S, Tadross JA, et al. GDF15 mediates the effects of metformin on body weight and energy balance. Nature. 2020;578:444–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Karhunen V, Larsson SC, Gill D. Genetically proxied growth‐differentiation factor 15 levels and body mass index. Br J Clin Pharmacol. 2021;87:4036–9. [DOI] [PubMed] [Google Scholar]
- 76. Hijazi Z, Oldgren J, Lindbäck J, Alexander JH, Connolly SJ, Eikelboom JW, et al.; ARISTOTLE and RE‐LY Investigators. The novel biomarker‐based ABC (age, biomarkers, clinical history)‐bleeding risk score for patients with atrial fibrillation: a derivation and validation study. Lancet. 2016;387:2302–11. [DOI] [PubMed] [Google Scholar]
- 77. Hijazi Z, Oldgren J, Andersson U, Connolly SJ, Eikelboom JW, Ezekowitz MD, et al. Growth‐differentiation factor 15 and risk of major bleeding in atrial fibrillation: insights from the Randomized Evaluation of Long‐Term Anticoagulation Therapy (RE‐LY) trial. Am Heart J. 2017;190:94–103. [DOI] [PubMed] [Google Scholar]
- 78. Yuan S, Carter P, Bruzelius M, Vithayathil M, Kar S, Mason AM, et al. Effects of tumour necrosis factor on cardiovascular disease and cancer: a two‐sample Mendelian randomization study. EBioMedicine. 2020;59:102956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Rosa M, Chignon A, Li Z, Boulanger MC, Arsenault BJ, Bossé Y, et al. A Mendelian randomization study of IL6 signaling in cardiovascular diseases, immune‐related disorders and longevity. NPJ Genom Med. 2019;4:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Lundberg M, Eriksson A, Tran B, Assarsson E, Fredriksson S. Homogeneous antibody‐based proximity extension assays provide sensitive and specific detection of low‐abundant proteins in human blood. Nucleic Acids Res. 2011;39:e102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Shen Q, Björkesten J, Galli J, Ekman D, Broberg J, Nordberg N, et al. Strong impact on plasma protein profiles by precentrifugation delay but not by repeated freeze‐thaw cycles, as analyzed using multiplex proximity extension assays. Clin Chem Lab Med. 2018;56:582–94. [DOI] [PubMed] [Google Scholar]
- 82. Shimada YJ, Raita Y, Liang LW, Maurer MS, Hasegawa K, Fifer MA, et al. Comprehensive proteomics profiling reveals circulating biomarkers of hypertrophic cardiomyopathy. Circ Heart Fail. 2021;14:e007849. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Assay coefficients of variation for the most strongly associated biomarkers with GDF‐15.
Table S2. Multivariable logistic model investigating association with the highest GDF‐15 quartile in the index cohort.
Table S3. Baseline characteristics of all GDF‐15 quartiles in the index cohort.
Table S4. Baseline characteristics of all GDF‐15 quartiles in the validation cohort.
Table S5. Hazard ratio and P‐value for GDF‐15 quartiles in Cox regression predicting 2‐year all‐cause mortality and hospitalization in the index and validation cohort, adjusted for established risk factors.
Table S6. Differential expression results in the index and validation cohort.
Table S7. Associations between circulating biomarkers and GDF‐15 levels.
Figure S1. Heatmap for expression profiles of GDF‐15, TFF3, FGF‐23, LILRB4, WISP‐1, TRAIL‐R2, TNFRSF11A in the index cohort.


