Abstract
Anti‐human epidermal growth factor receptor 2 (HER2) therapy is an effective treatment for HER2‐positive gastric and breast malignancies. However, the efficacy of HER2‐targeted therapy in non‐small cell lung cancer (NSCLC) patients with HER2 alterations remains controversial. We searched studies on HER2‐targeted therapy in NSCLC patients that reported objective response rate (ORR), disease control rate (DCR) and progressionfree survival (PFS) published from database inception to 30 May 2021. A total of 32 trials involving 958 patients were included. The ORRs of HER2‐TKIs targeted therapy, humanised monoclonal antibody, trastuzumab‐based treatment and antibody‐drug conjugate (ADC) (T‐DM1) were 22% (95% CI 11–31), 23% (95% CI 20–65), 26% (95% CI 14–39) and 16% (95% CI _6–37), while that of ADC (DS‐8201) was 60% (95% CI 35–85). The DCRs of these groups were 59% (95% CI 49–69), 39% (95% CI _9–88), 63% (95% CI 37–89), 31% (95% CI 4–58) and 87% (95% CI 62–112), respectively. In the subgroup analysis, numerically higher ORRs and DCRs were observed in the poziotinib (38%; 75%) and pyrotinib (35%; 83%) groups. The median PFSs of these groups were 5.51 months, 3.09 months, 4.61 months, 2.65 months and 12.04 months, respectively. HER2‐targeted therapy can be considered an acceptable treatment strategy for NSCLC patients with HER2 alterations. In particular, ADC (DS‐8201), pyrotinib and poziotinib demonstrated promising anti‐tumour activity in HER2‐positive NSCLC.
Keywords: biomarkers, ERBB2, HER2, meta‐analysis, non‐small cell lung cancer, review, targeted therapy
1. INTRODUCTION
Lung cancer is the leading cause of cancer‐related deaths worldwide. Non‐small cell lung cancer (NSCLC) accounts for more than 80% of all lung cancers. 1 NSCLC is one of the most genomically diverse cancers, making it very difficult to treat. For all NSCLC patients who have the adenocarcinoma subtype, testing for alterations in epidermal growth factor receptor (EGFR), mesenchymal epithelial transition factor (MET), b‐raf proto‐oncogene (BRAF), anaplastic lymphoma kinase (ALK), ROS proto‐oncogene 1 (ROS1), ret proto‐oncogene (RET) and neurotrophic tyrosine receptor kinase (NTRK) is recommended by the National Comprehensive Cancer Network (NCCN) and American Society of Clinical Oncology (ASCO) guidelines. 2 , 3
Human epidermal growth factor receptor 2 (HER2) is a member of the ERBB receptor tyrosine kinase family. 4 , 5 HER2 mutations are commonly found in never‐smokers and adenocarcinoma. 6 HER2 alterations, including gene amplification, mutations and overexpression, have also been found in many other cancers and are associated with poor clinical prognoses. 7 , 8 , 9 , 10 The frequency of HER2 alterations varies according to the detection methods used, tumour biology, and heterogeneity. In lung cancer, 2–6% HER2 mutations have been detected by polymerase chain reaction (PCR) or next‐generation sequencing (NGS); 11 , 12 , 13 2–20% HER2 amplification was detected by NGS or fluorescence in situ hybridisation (FISH); 13 , 14 , 15 and 2–38% HER2 protein was detected by immunohistochemistry (IHC), 14 , 15 , 16 , 17 , 18 but IHC 3+ was found only in 2–6% of patients. 14 , 15 , 16 , 17 , 18 , 19 These three HER2 alterations are correlated with the clinical efficacy of HER2‐targeted therapy. 14 , 15 , 19 , 20 , 21 Thus, HER2 alterations are considered as independent biomarkers for HER2‐targeted therapy.
Current HER2‐targeted medicines, including tyrosine kinase inhibitors (TKIs), monoclonal antibodies and antibody‐drug conjugates (ADC), have been developed for breast and gastric adenocarcinoma patients with HER2 amplification and protein overexpression. To allow clarity and consistency in pharmacology, the nomenclature of HER2‐targeted drugs conforms to the IUPHAR/BPS Guide to PHARMACOLOGY. 4 , 5 TKIs, including afatinib, lapatinib, poziotinib, pyrotinib, neratinib, dacomitinib, mobocertinib, tucatinib and tarloxotinib, bind to the intracellular domain of HER2. On the other hand, monoclonal antibodies and ADC, such as pertuzumab, trastuzumab, trastuzumab deruxtecan and ado‐trastuzumab emtansine, bind to the extracellular domain of HER2. There is no association between HER2 overexpression, mutations and amplification, 22 which makes evaluating the efficacy of HER2‐targeted medicines difficult. Nonetheless, evaluating the efficacy of such therapies is important in optimising cancer treatment. This study aimed to investigate the efficacy of targeted therapy in HER2‐positive NSCLC patients.
2. METHODS
2.1. Search strategy
We searched multiple databases, including Web of Science, Embase, Medline and Cochrane, for trials using the terms “{[HER2(Title/Abstract)] OR [ERRB (Title/Abstract)]} AND {[non‐small cell lung cancer (Title/Abstract)] OR [NSCLC (Title/Abstract)]}” with no restrictions in the publication language or period. We searched for reports published from database inception to May 30, 2021. We also retrieved the references of all the included studies to minimise error and bias. Keywords in related conference articles were also used to retrieve the studies. The results were recorded according to the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines. 23 This study was registered with PROSPERO (number CRD42021257794). The following clinical trials were eligible: (1) randomised controlled trials (RCTs), single‐arm and cohort studies; (2) trials conducted in HER2‐positive patients with NSCLC; and (3) trials reporting data on the objective response rate (ORR), disease control rate (DCR) and progression‐free survival (PFS). Patients were excluded if they had received chemotherapy, surgery, radiotherapy anti‐HER2 therapy, or other targeted therapies within four weeks before the first dose of the medication of interest. Patients admitted for other medical problems were excluded. Trials on pregnant women, reviews, editorials, case reports, congress articles and articles unrelated to our theme were omitted. The outcome measures were the ORR, DCR and PFS in patients with NSCLC treated with HER2‐targeted therapy.
2.2. Study selection
Hong‐Xia Wu and Kai‐Quan Zhuo performed the study selection. First, the abstract and titles were examined, and duplicates and unavailable full‐text articles were discarded. Next, qualified trials were selected by screening the full texts. The reasons for exclusion were documented. A third reviewer (Ke Wang) was consulted if no consensus was reached.
2.3. Data extraction
Hong‐Xia Wu and Kai‐Quan Zhuo independently extracted and recorded the demographic characteristics and outcome measures of each study in a standard form advised by Cochrane. 24 The corresponding authors were emailed if any information was missing.
2.4. Quality assessment
The Cochrane Risk of Bias tool was used to assess the quality of the studies. 24 The quality of non‐randomised studies was evaluated using the Newcastle–Ottawa Scale (NOS). 25 Two investigators (Hong‐Xia Wu and Kai‐Quan Zhuo) conducted the quality assessment. Any disagreements were resolved by a third investigator (Ke Wang).
2.5. Quantitative data synthesis
A random‐effects model and the inverse variance heterogeneity method were used to analyse PFS and heterogeneity (I 2). The Mantel–Haenszel method and a random‐effects model were used to analyse the ORR, DCR and I 2. Analyses were performed using STATA (Version 16; Stata Corp., College Station, TX, USA). The outcomes are expressed in forest plots. Dichotomous and continuous variables are shown as odds ratios (OR) and median values, respectively. I 2 > 50% was considered significant for the heterogeneity test. A fixed‐effects model was used in cases of no heterogeneity; otherwise, a random‐effects model was used.
3. RESULTS
3.1. Study selection process
A total of 116 studies were obtained by screening the abstracts. After removing duplicate records, 54 studies remained for subsequent screening. After excluding irrelevant documents, 42 studies were selected by qualification evaluation and full‐text reviews. Finally, 32 studies 9 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 were assessed in this meta‐analysis. The details of the study selection process are presented in Figure 1.
FIGURE 1.
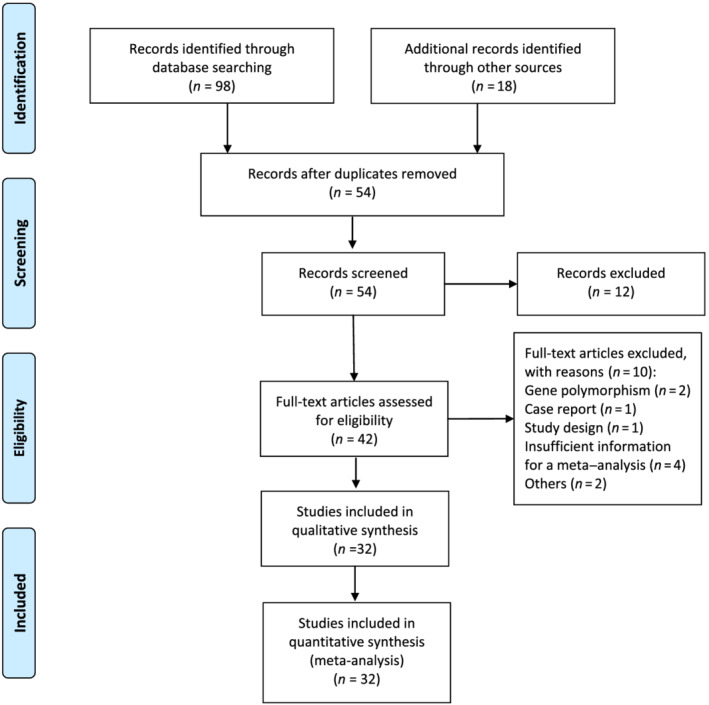
Flowchart diagram of selected searches for inclusion in meta‐analysis
3.2. Characteristics of clinical trials
Thirty‐two studies involving 958 patients were included in this study. Details of the study characteristics and outcomes are presented in Table 1. Among the 32 studies, there were 4 RCTs, 11 cohort studies, 17 single‐arm studies, 1 phase I study and 15 phase II studies. Some trials have a relatively small population, but HER2 genomic alterations have emerged as distinct oncogenic drivers. Thus, few tests fulfilled our meta‐analysis inclusion criteria. The ORR and DCR of single‐arm RCTs were used and combined with data from cohort and single‐arm trials.
TABLE 1.
Characteristics of included studies
| Classification of treatment | First author | Year | Registration number | Study design | Treatment | Population | Gene type | Number of patients | Outcomes | Country | Male (%) | Age, median (range) | Never‐smokers (%) | Molecular diagnostics | Tumour stage |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HER2‐TKIs therapy | Mazières et al. 26 | 2013 | NM | Retrospective cohort study | Afatinib, Lapatinib, Masatinib | NSCLC | HER2 exon‐20 insertion | 4 | ORR, DCR | France, Germany, Switzerland and Spain | 31 | 60.4 (31–86) | 52.3 | Direct sequencing or FISH | I‐IV |
| Mazières et al. 27 | 2015 | NM | Retrospective cohort study | Neratinib, Lapatinib, Afatinib | NSCLC | HER2 exon‐20 insertion | 29 | ORR, DCR, PFS | France, Switzerland, Spain, Italy, Poland, Portugal and the Netherlands | 37.6 | 61 (30–87) | 60.4 | PCR or NGS | I‐IV | |
| De Grève et al. cohort 1 28 | 2015 | EudraCT: 2008–001546‐67 | Prospective cohort study | Afatinib | Lung adenocarcinoma | HER2 mutation | 7 | ORR, DCR | Belgium, Spain, United Kingdom and United States | 0 | 62 (50–79) | 71 | FISH | IIIB/IV | |
| De Grève et al. cohort 2 28 | 2015 | EudraCT: 2008–001546‐67 | Prospective cohort study | Afatinib plus Paclitaxel | Lung adenocarcinoma | HER2 mutation | 3 | ORR, DCR | Belgium, Spain, United Kingdom and United States | 0 | 62 (50–79) | 71 | FISH | IIIB/IV | |
| Kris et al. 29 | 2015 | NCT0114286 | Phase II study | Dacomitinib | HER2‐mutant or amplified tumours | HER2 mutation or amplification | 30 | ORR, DCR | United States, China and Japan | 50 | 58.7 (37–74) | 60 | FISH or sequencing by multiplexed testing | IIIB‐IV | |
| Song et al. 30 | 2016 | NM | Retrospective multicentre single‐arm study | Afatinib | NSCLC | HER2 exon‐20 insertion | 4 | DCR, PFS | China | 33.3 | 60 (39–70) | 81 | NGS | I‐IV | |
| Lai et al. 31 | 2017 | NM | Retrospective study | Afatinib | Lung adenocarcinoma | HER2mutation | 23 | ORR, DCR | Europe, Australia and North America | 59 | 63 (40–84) | 67 | PCR or NGS | IV or recurrent | |
| Gandhi et al. cohort 1 32 | 2017 | NCT01827267 | Randomised controlled trial | Neratinib | Lung cancers | HER2 mutation | 17 | ORR, DCR | NM | 32 | 66 | 60 | NM | IIIB/IV | |
| Gandhi et al. cohort 2 32 | 2017 | NCT01827267 | Randomised controlled trial | Neratinib + Temsirolimus | Lung cancers | HER2 mutation | 43 | ORR, DCR | NM | 32 | 66 | 60 | NM | IIIB/IV | |
| Oh et al. 33 | 2018 | NCT02979821 | Retrospective study | Poziotinib or Afatinib | Lung adenocarcinoma | HER2 mutation | 7 | ORR, DCR | Korea | 0 | 48.5 (55–61) | 100 | NGS | I‐IV | |
| Zhao et al. 34 | 2018 | NM | Retrospective single‐arm study | Afatinib | NSCLC | HER2 mutation | 23 | ORR, PFS | China | NM | NM | NM | NGS | NM | |
| Peters et al. 35 | 2018 | NM | Retrospective single‐arm study | Afatinib | Lung adenocarcinoma | HER2 exon 20 mutation | 16 | ORR,DCR | Switzerland, Israel, Taiwan, Slovenia, Austria and United States | 43 | 55 (39–93) | NM | NM | IV | |
| Liu et al. 36 | 2018 | NM | Retrospective single‐arm study | Afatinib | Lung cancers | HER2 exon 20 insertion | 19 | ORR, DCR | China | 37 | 57 (41–86) | NM | NGS | NM | |
| Robichaux et al. 37 | 2018 | NCT03066206 | Single‐arm phase II trial | Poziotinib | NSCLC | HER2 exon 20 mutation | 11 | ORR, DCR | United States | NM | 57.6 (52–66) | NM | PCR‐based NGS | NM | |
| Hyman et al. 38 | 2018 | NCT01953926 | Single‐arm phase II trial | Neratinib | AdvancedNSCLC | HER2 mutation | 26 | ORR, DCR | United States, Spain and Australia | 34.6 | 62 (46–74) | NM | DNA sequencing | NM | |
| Dziadziuszko et al. 39 | 2019 | NCT02369484 | Single‐arm phase II trial | Afatinib | NSCLC | HER2 exon 20 mutation | 13 | ORR, DCR, PFS | Poland, Netherlands, Greece, Germany, Ireland Switzerland and Spain | 30.8 | 59 (39–82) | 61.5 | NM | IIIB/IV | |
| Robichaux et al. 9 | 2019 | NCT03066206 | Single‐arm phase II trial | Poziotinib | NSCLC | HER2 mutation | 12 | ORR, DCR | United States | 16.7 | 59.5 (56.5–61) | NM | NM | NM | |
| Wang et al. 40 | 2019 | NCT02535507 | Single‐arm phase II study | Pyrotinib | NSCLC | HER2 exon 20 insertion | 15 | ORR, DCR, PFS | China | 53 | 58 (42–78) | 67 | ADx HER2 Mutation Detection Kit, ARMS, NGS, or DNA direct sequencing | NM | |
| Zhou et al. 41 | 2020 | NM | Single‐arm phase II study | Pyrotinib | Lung adenocarcinoma | HER2 mutation | 60 | ORR, DCR | China | 45 | 57 (40–72) | 71.7 | NGS or ADx HER2 Mutation Detection Kit | IIIB/IV | |
| Zhou et al. 42 | 2020 | NM | Retrospective cohort study | Afatinib or Pyrotinib | Advanced lung cancers | HER2 mutation | 25 | ORR, DCR, PFS | China | 45 | 56 (32–76) | 73 | ARMS‐PCR or PCR | IIIB/IV | |
| Liu et al. 43 | 2020 | NCT03805841 | Cohort study | Tarloxotinib | NSCLC | HER2 mutation | 9 | ORR, DCR | United States, China and Canada | NM | NM | NM | NM | NM | |
| Socinski et al. 44 | 2020 | NCT03318939 | Cohort phase II study | Poziotinib | NSCLC | HER2 exon‐20 insertion | 74 | ORR, DCR, PFS | United States, Belgium, Canada, France, Israel, Italy, Netherlands and Spain | 36 | 60 | 66 | NM | NM | |
| Humanised monoclonal antibody | Lara et al. 45 | 2003 | NM | Randomised controlled trial | Trastuzumab | NSCLC | HER2 protein overexpression or HER2 amplification | 4 | ORR, DCR | United States | 61.5 | 66 (42–82) | NM | IHC, ELISA and FISH | NM |
| Herbst et al. 46 | 2007 | NM | Multicentre phase II study | Pertuzumab | Advanced or metastatic NSCLC | HER2 mutation | 43 | ORR, DCR, PFS | United States | 60 | 62 (33–79) | NM | IHC | NM | |
| Mazières et al. 27 | 2015 | NM | Retrospective cohort study | Trastuzumab, T‐DM1 | NSCLC | HER2 exon‐20 insertion | 58 | ORR, DCR, PFS | France, Switzerland, Spain, Italy, Poland, Portugal and the Netherlands | 37.6 | 61 (30–87) | 60.4 | PCR or NGS | I‐IV | |
| Trastuzumab‐based therapy | Lara et al. 45 | 2003 | NM | Randomised controlled trial | Trastuzumab plus Docetaxel | Advanced, recurrent, or metastatic NSCLC | HER2 mutation | 13 | ORR, DCR | United States | 61.5 | 66 (42–82) | NM | IHC | NM |
| Langer et al. 47 | 2004 | ECOG 2598 | Phase II trial | Trastuzumab plus Carboplatin and Paclitaxel | NSCLC | HER2 overexpression | 53 | ORR, DCR, PFS | United States | 50.9 | 59 (52–65) | NM | IHC | IIIB/IV, recurrent | |
| Gatzemeier et al. 48 | 2004 | NM | Randomised phase II study | Gemcitabine–cisplatin plus Trastuzumab | NSCLC | HER2‐positive | 51 | ORR, DCR, PFS | United States | 65.3 | 58 (35–76) | NM | IHC, FISH or ELISA | IB, IIIB/IV | |
| Mazières et al. 26 | 2013 | NM | Retrospective cohort study | Trastuzumab in combination with chemotherapy | NSCLC | HER2 exon‐20 insertion | 15 | ORR, DCR, PFS | France, Germany, Switzerland and Spain | 31 | 60.4 (31–86) | 52.3 | Direct sequencing Or FISH | I‐IV | |
| Hainsworth et al. 49 | 2018 | NCT02091141 | Phase II study | Trastuzumab plus Pertuzumab | NSCLC | HER2 mutation, amplification, or overexpression | 30 | ORR, DCR | United States and United Kingdom | 51 | 62 (23–86) | NM | IHC, FISH, CISH, or NGS | NM | |
| de Langen et al. 50 | 2018 | NCT02226757 | Single‐arm phase II study | Trastuzumab and Paclitaxel | Non‐squamous NSCLC | HER2 overexpression | 24 | ORR, DCR, PFS | The Netherlands | 29 | 68 (40–82) | NM | IHC | IV | |
| Zhou et al. 42 | 2020 | NM | Retrospective cohort study | Trastuzumab‐based therapy | Advanced lung cancers | HER2 mutation | 3 | ORR, DCR | China | 45 | 56 (32–76) | 73 | ARMS‐PCR, and PCR | IIIB/IV | |
| Li et al. 51 | 2021 | NCT01953926 | Phase II study | Neratinib plus Trastuzumab | NSCLC | HER2 mutation | 52 | ORR, DCR | United States, France, Spain and Israel | 34 | 57.5 (62–66) | NM | NM | NM | |
| Antibody drug conjugate, DS‐8201 | Smit et al. 52 | 2020 | NCT03505710 | Multicentre phase II study | Trastuzumab deruxtecan (DS‐8201) | NSCLC | HER2 mutation | 42 | ORR, DCR, PFS | United States, France, Japan, Netherlands and Spain | 35.7 | 60 (34–83) | NM | NM | Unresectable and/or metastatic |
| Tsurutani et al. 53 | 2020 | NCT02564900 | Phase I study | Trastuzumab deruxtecan (DS‐8201) | NSCLC | HER2 overexpression, or mutation | 18 | ORR, DCR, PFS | Japan and United States | 27.8 | 58 (23–83) | NM | IHC or NGS | NM | |
| Antibody drug conjugate, T‐DM1 | Hotta et al. 54 | 2017 | NM | Randomised controlled trial | Trastuzumab emtansine (T‐DM1) | Adenocarcinomas | HER2 overexpression | 15 | ORR, DCR, PFS | Japan | 47 | 67 (45–77) | 67 | IHC and FISH | IV/recurrence |
| Li et al. 55 | 2018 | NCT02675829 | Phase II basket trial | Ado‐trastuzumab emtansine | Lung cancers | HER2 mutation, overexpression or amplification | 18 | ORR, PFS | United States | 28 | 64 (47–74) | 39 | NGS, IHC or FISH | IV/recurrence | |
| Peters et al. 56 | 2019 | NCT02289833 | Prospective multicentre single‐arm study | Trastuzumab Emtansine (T‐DM1) | NSCLC | HER2 overexpression | 49 | ORR, PFS | Switzerland, United States, Poland, Spain, Germany and Italy | 59.2 | 61 (36–80) | 20.4 | IHC | Advanced, recurrent, or metastatic | |
| Total | 32 studies | 958 |
Abbreviations: NM, not mentioned; NSCLC, non‐small cell lung carcinoma; ORR, objective response rate; DCR, disease control rate; PFS, progression‐free survival; ARMS‐PCR: amplification refractory mutation system‐polymerase chain reaction; PCR, polymerase chain reaction; NGS, next generation sequencing; FISH, fluorescent in‐situ hybridisation; CISH, chromogenic in‐situ hybridisation; ICH, immunohistochemistry; ELISA, enzyme‐linked immunosorbent assay.
3.3. Quality assessment and heterogeneity
The assessment of study quality is detailed in Supplementary Figures S1 and S2 and Supplementary Table S1. No studies were excluded because of low quality. Significant statistical heterogeneity was found in the following outcomes: PFS of HER2 TKI‐targeted therapy (I 2 = 93.3%, P = .000) (Figure 6); ORR, DCR, and PFS of the humanised monoclonal antibody group (I 2 = 71.6%, P = .029; I 2 = 78.5%, P = .009; I 2 = 93.1%, P = .000) (Figures 3, 5 and 7); and DCR and PFS of the trastuzumab‐based treatment group (I 2 = 66.1%, P = .012; I 2 = 81.6%, P = .001) (Figures 5 and 7). A sensitivity analysis was used to assess the stability of the results (Supplementary Figures S3, S7, S11 and S15). One study was removed at a time, and the summary median/OR value and overall effect were recalculated. The absence of a statistically significant reversal of the overall effects (I 2) confirmed the stability of the results.
FIGURE 6.
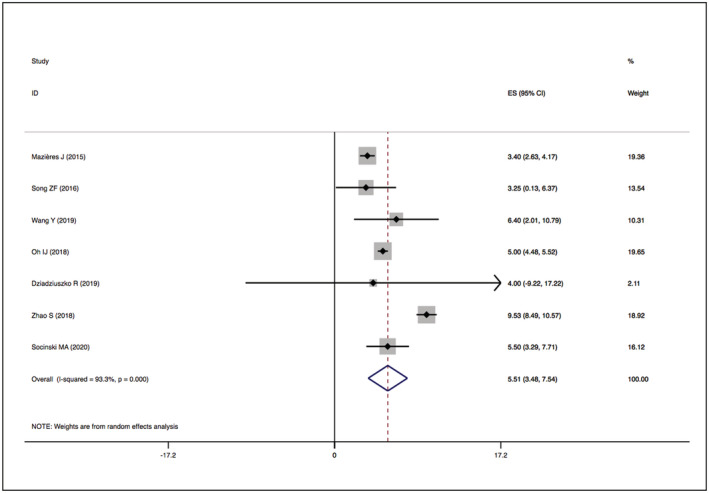
Forest plot of PFS among patients treated with HER2‐TKIs targeted therapy
FIGURE 3.
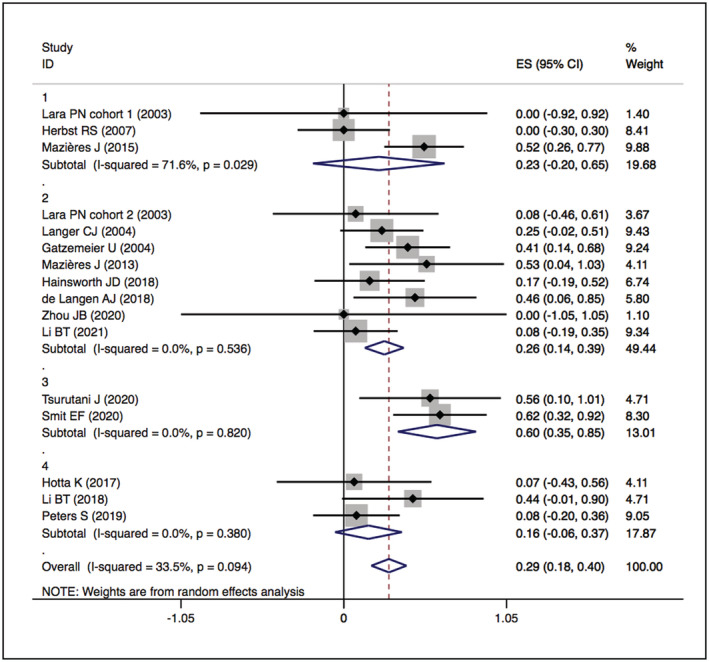
Forest plot of cumulative incidence of ORR among patients treated with HER2‐targeted therapy
FIGURE 5.
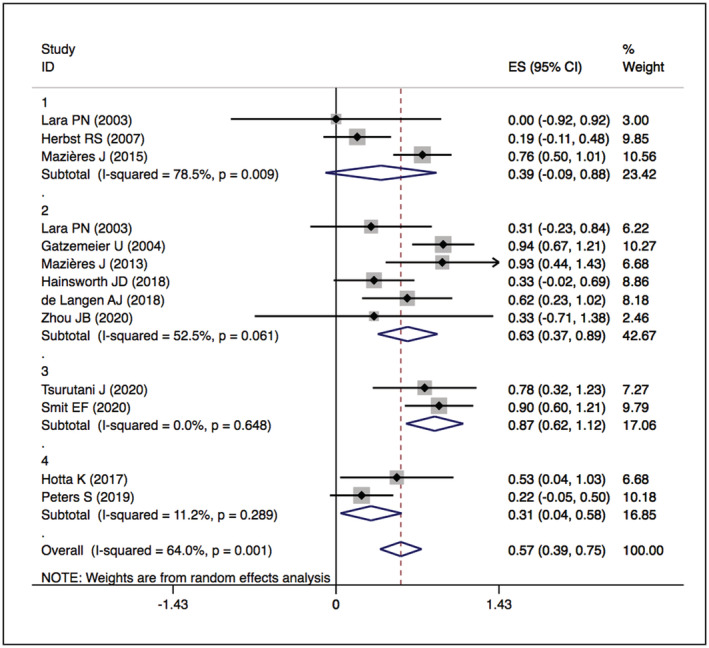
Forest plot of cumulative incidence of DCR among patients treated with HER2‐targeted therapy
FIGURE 7.
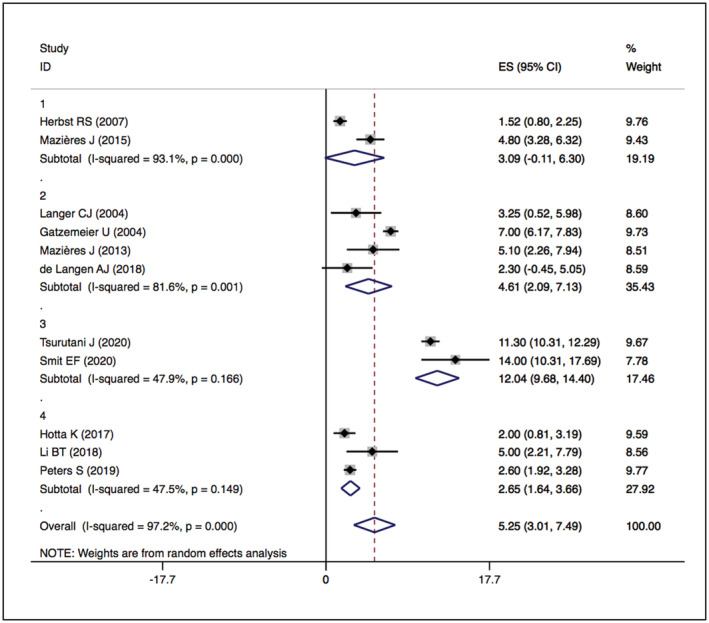
Forest plot of PFS among patients treated with HER2‐targeted therapy
3.4. Outcome measures
3.4.1. ORR
The overall ORRs of HER2‐TKIs targeted therapy, humanised monoclonal antibody, trastuzumab‐based treatment and ADC (T‐DM1) were 22% (95% CI 11–31), 23% (95% CI 20–65), 26% (95% CI 14–39) and 16% (95% CI −6–37), while that of ADC (DS‐8201) was 60% (95% CI 35–85). When restricted to the subgroup, numerically higher ORRs were observed in the pyrotinib (ORR 35%, 95% CI 12–57) and poziotinib (ORR 38%, 95% CI 19–57) groups. The details are presented in Figures 2 and 3.
FIGURE 2.
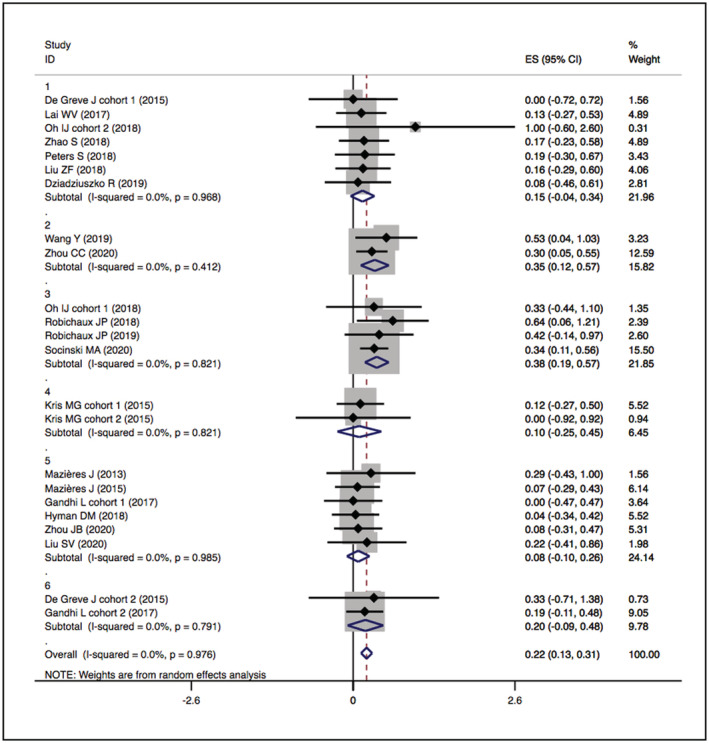
Forest plot of cumulative incidence of ORR among patients treated with HER2‐TKIs targeted therapy
3.4.2. DCR
The overall DCRs of HER2‐TKIs targeted therapy, humanised monoclonal antibody, trastuzumab‐based treatment and ADC (T‐DM1) were 59% (95% CI 49–69), 39% (95% CI −9–88), 63% (95% CI 37–89) and 31% (95% CI 4–58), respectively, while that of ADC (DS‐8201) was 87% (95% CI 62–112). When restricted to the subgroup, numerically higher DCRs were observed in the pyrotinib (83%, 95% CI 60–105) and poziotinib (75%, 95% CI 56–94) groups. The details are presented in Figures 4 and 5.
FIGURE 4.
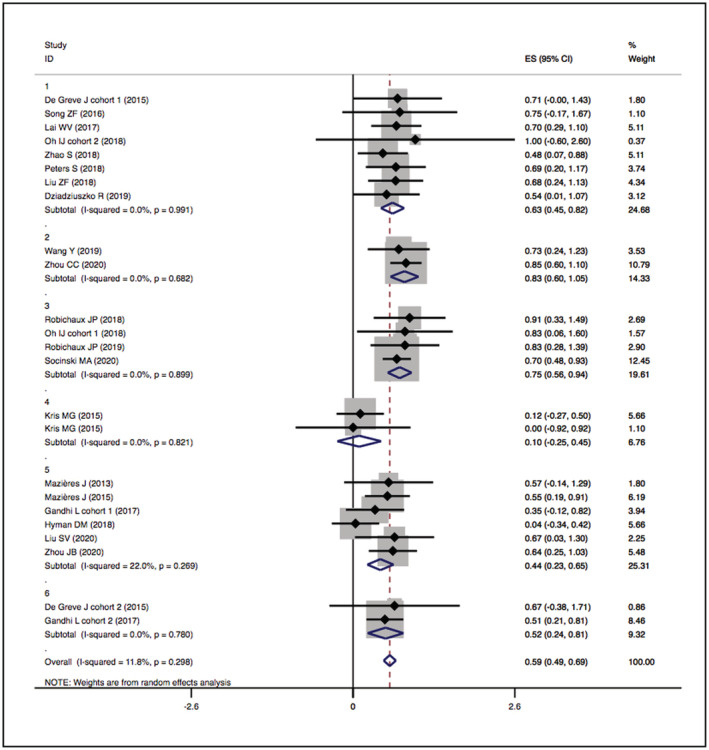
Forest plot of cumulative incidence of DCR among patients treated with HER2‐TKIs targeted therapy
3.4.3. PFS
The overall median PFSs of HER2‐TKIs therapy, humanised monoclonal antibody, trastuzumab‐based treatment, ADC (DS‐8201) and ADC (T‐DM1) were 5.51 months (95% CI 3.48–7.54), 3.09 months (95% CI −0.11–6.30), 4.61 months (95% CI 2.09–7.13), 12.04 months (95% CI 9.68–14.40) and 2.65 months (95% CI 1.64–3.66), respectively. The details are presented in Figures 6 and 7.
3.4.4. Publication bias
Publication bias was evaluated using funnel plots, Egger's test and Begg's test (Supplementary Figures S4–S6, S8–S10, S12–S14, S16–S18). The trim‐and‐fill method was used if publication bias was suspected. No publication bias was found in the studies.
4. DISCUSSION
In our meta‐analysis, numerically higher response rates were observed in the poziotinib (ORR 37%; DCR 71%) and pyrotinib (ORR 35%; DCR 83%) groups in the subgroup analysis of HER2‐TKIs therapy. Poziotinib is a new oral, irreversible inhibitor of EGFR/HER4/HER2. 57 The molecular structure of poziotinib is smaller and more flexible than that of second‐ and third‐generation inhibitors. 37 Robichaux et al., 9 Oh et al., 33 Robichaux et al. 37 and Socinski et al. 44 discussed the efficacy of poziotinib in NSCLC patients with HER2 mutations. Cohort C2 of the phase II study ZENITH20 conducted by Socinski et al. 44 evaluated the efficacy of poziotinib in NSCLC patients with HER2 alterations. This cohort was comprised of 90 patients pre‐treated with HER2 exon 20 insertions. The ORR, DCR and median PFS were 35.1% (74 evaluable patients), 70% and 5.5 months, respectively. In cohort 1 of the study performed by Oh et al., 33 ORR and DCR were 33% and 83%, respectively, in HER2 mutant lung adenocarcinoma. The survey conducted by Robichaux et al. 37 showed an ORR of 64% and DCR of 91% for poziotinib in NSCLC patients with HER2 mutation. The recommended dose of poziotinib is 16 mg daily, but 55% of the patients received a dosage reduction due to skin rash and diarrhoea. In a subsequent study, Robichaux et al. 9 reported an ORR of 42% and a DCR of 83% in NSCLC patients with HER2 exon 20 mutation. The survey conducted by Oh et al. 33 included patients with tumour stages I–IV, and all patients were female and non‐smokers. However, the other three studies did not detail the status of smoking and the patients' tumour stage.
Pyrotinib is an oral, irreversible pan‐HER TKI inhibitor of HER2. 40 , 58 Pyrotinib was first applied in HER2‐positive breast cancer patients and obtained encouraging results. 59 An in vitro cell proliferation assay, 40 in which plasma concentrations of afatinib and pyrotinib were obtained from NSCLC patients with HER2 exon 20 mutations in two previous studies, 59 , 60 showed that the inhibition of cell growth was more robust in the pyrotinib group. A phase II study of pre‐treated NSCLC patients with HER2 exon 20 mutation showed a 53.3% partial response rate, 20.0% stable disease rate and a median PFS of 6.4 months. 40 Gao et al. 61 published a multicentre phase II study focusing on pre‐treated NSCLC patients with HER2 insertion (n = 60), which showed an ORR of 31.7% and a median PFS of 6.8 months. In the same study, patients with a history of other HER2‐targeted treatments or brain metastases were excluded. 61 In our analysis, Wang et al. 40 and Zhou et al. 41 detailed the efficacy of pyrotinib in NSCLC patients with HER2 mutations. Wang et al. 40 conducted a phase II study of pyrotinib in NSCLC patients with HER2 exon 20 insertion (n = 15). They reported an ORR of 53%, a DCR of 73% and a median PFS of 6.4 months (95% CI 1.60–11.20). However, Wang et al. did not detail the tumour stage nor the proportion of enrolled patients with each stage. In addition, pyrotinib showed a significantly more promising anti‐tumour effect than afatinib in vitro (P = .0038) and a significantly stronger growth inhibition of organoids than afatinib (P = .0471) and T‐DM1 (P = .0138) in the patient‐derived xenograft (PDX) model. On the other hand, Zhou et al. 41 included 60 patients with HER2‐mutant lung adenocarcinoma of the tumour stage IIIB or IV, who had a history of platinum‐based chemotherapy. In their study, pyrotinib showed promising anti‐tumour activity (ORR of 30%; DCR of 85%; median PFS of 6.9 months) and acceptable safety. In these two studies by Wang et al. and Zhou et al., all enrolled patients were diagnosed with lung adenocarcinoma, and never‐smokers accounted for 67% and 71.17%, respectively. These results are consistent with those of previous studies. Male patients accounted for 53% and 45% of the participants, respectively, suggesting that molecular testing should not be restricted to females. Other concomitant mutations in the study by Zhou et al. may affect the efficacy of HER2‐targeted therapy. 41 It is important to note that these two studies lacked a control arm and had relatively small sample sizes. Nonetheless, pyrotinib showed numerically greater anti‐tumour potential than chemotherapy or other licensed HER2‐targeted drugs (afatinib and T‐DM1) in patients with HER2 mutations. The multicentre phase II trial, which tested afatinib in 13 NSCLC patients with HER2 mutations pre‐treated with platinum‐based therapy, with a median PFS of 15.9 weeks and overall survival (OS) of 56 weeks, did not show the expected efficacy. 39
Trastuzumab is a monoclonal humanised antibody that binds to the HER2 receptor. 62 Trastuzumab is the standard therapy for HER2‐positive gastric and breast cancers and is also being examined for other HER2‐positive cancers. 63 Three studies were included in the humanised monoclonal antibody group in our analysis. The overall ORR, DCR and median PFS of humanised monoclonal antibody were 23%, 39% and 3.09 months, respectively. Lara et al. 45 found no response to trastuzumab; Herbst et al. 46 reported a DCR of 19% and a median PFS of 1.92 months; and Mazières et al. 27 suggested an ORR of 52%, DCR of 76% and a median PFS of 4.8 months. In the study by Mazières et al., 57 patients were treated with trastuzumab, but one patient had T‐DM1. 27 Eight studies were included in the trastuzumab‐based therapy group. A phase II trial evaluated 53 NSCLC patients with HER2 alterations. In patients with IHC 3+, the chemotherapy plus trastuzumab therapy group had a longer OS than the chemotherapy group. 47 In a randomised phase II study including patients with HER2 alterations, 51 patients were treated with trastuzumab plus gemcitabine–cisplatin, and 50 patients were treated with gemcitabine–cisplatin alone. There was no significant difference between the two groups: ORR 36% vs 41% and a median PFS of 6.1 months vs 7 months. Although ORR (83%) and median PFS (8.5 months) seemed comparatively good in the HER2 IHC 3+ trastuzumab‐based group, the subgroup (n = 6) did not provide convincing information. 48 A phase II study including 13 patients with IHC 2+ or 3+ HER2 alterations after platinum‐based chemotherapy demonstrated that one patient had a partial response in the docetaxel alone group (n = 9), but none of the patients responded to the trastuzumab arm. The overall outcomes followed by combination therapy showed a partial response rate of 8% and a stable disease rate of 23%. Estimated event‐free survival and OS were 4.3 months and 5.7 months, respectively. 45 Overall, the results suggested that the efficacy of trastuzumab‐based therapy was similar to or better than that of chemotherapy alone. In contrast, trastuzumab showed no response in a phase II study of pre‐treated NSCLC patients with HER2 alterations. 64
Ado‐trastuzumab emtansine, also known as T‐DM1, is a HER2‐targeted antibody‐toxin conjugate that is made up of trastuzumab and the potent cytotoxic agent DM1. 65 T‐DM1 has a 3:4 chemotherapy drug‐to‐antibody ratio. Ado‐trastuzumab emtansine is advocated as a treatment for NSCLC patients with HER2 mutations according to the NCCN guidelines. 3 In a phase II study, 55% of patients with HER2 exon 20 mutations responded to T‐DM1 therapy. 55 However, another phase II study (n = 15) showed limited activity. 54
Trastuzumab deruxtecan, also known as T‐DXd or DS‐8201, is an ADC consisting of a direct HER2‐targeting antibody, trastuzumab, and a cytotoxic topoisomerase inhibitor, exatecan derivative. 66 , 67 , 68 Its mechanism of action differs from that of other ADCs. 69 Trastuzumab deruxtecan has an 8:4 chemotherapy drug‐to‐antibody ratio, which improves its efficacy. 67 , 68 Furthermore, trastuzumab deruxtecan has been reported to exert anti‐tumour immune effects. 70 Altogether, trastuzumab deruxtecan is designed to improve the characteristics of prior ADCs. In May 2020, trastuzumab deruxtecan was approved by the US Food and Drug Administration (FDA) as a breakthrough therapy for NSCLC patients with HER2 mutations after platinum‐based treatment failure. 71 The World Conference on Lung Cancer (WCLC) 2020 reported the activity of trastuzumab deruxtecan in patients with HER2 overexpression. 72 A cohort of a phase I study enrolling NSCLC patients with HER2 overexpression or mutations had no encouraging results. 73 However, interstitial lung disease has been reported in patients treated with DS‐8201. 74 In a phase I trial, the ORR of HER2‐positive NSCLC patients was 72.7% (n = 11), whereas the median PFS was 11.3 months. The phase II trial DESTINY‐Lung01, 52 which included 90 NSCLC patients with HER2 mutations, reported an ORR of 61.9%, a DCR of 90.5% and an estimated median PFS of 14 months.
There are no published randomised controlled trials on poziotinib, pyrotinib or trastuzumab deruxtecan in patients with HER2‐positive NSCLC. Therefore, we could not directly compare the efficacy of poziotinib, pyrotinib or trastuzumab deruxtecan with chemotherapy. However, through indirect comparison with other targeted therapies, we can deduce that the trastuzumab deruxtecan, poziotinib and pyrotinib treatment is superior to chemotherapy.
The definition of “HER2‐positive NSCLC” is insufficient to describe the differences in the complexity of HER2 alterations. Indeed, the failure of many clinical studies focusing on HER2‐targeted therapy may be due to these complex alterations. 39 , 54 , 64 A multicentre phase II study is currently recruiting advanced NSCLC patients with HER2 overexpression (cohort 1) or mutation (cohort 2). 75 Distinguishing between HER2 mutation and overexpression, as was done in these two cohorts, may help provide a better understanding of the influence of such alterations.
This study had several advantages. First, it is a comprehensive analysis of the efficacy of targeted therapy in HER2‐positive NSCLC patients. Additionally, the studies included were of high quality. However, the limitations of this study must also be considered. First, most of the included studies were single‐arm or cohort trials. Because HER2‐positive cancer results from a relatively rare genetic alteration, related clinical research is fewer. Nonetheless, nearly half of the investigations consist of clinical research on new drugs. As a result, the studies included in the analysis are considered to be of relatively high quality. Second, the tumour stage and treatment lines were different in the patients included in the studies. A proportion of patients may have other concurrent gene mutations. Third, there may be differences in the exact treatment used in each study. Therefore, we considered drug classification as the grouping principle for analysis. Finally, different detection methods for HER2 alterations may influence the results. These methods are all recommended by the current guidelines, but the sensitivity and specificity of the different detection methods vary. With the enlargement of sample size and the advancement of research, subgroup analysis according to the different detection methods may be performed in the future. Nevertheless, this conclusion has a certain value and significance; but further research is warranted.
5. CONCLUSION
HER2‐targeted therapy can be considered an acceptable treatment strategy for NSCLC patients with HER2 alterations. In particular, ADC (DS‐8201), pyrotinib and poziotinib have demonstrated promising anti‐tumour activity in HER2‐positive NSCLC. However, further trials are required to explore new therapeutic strategies.
5.1. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, and are permanently archived in the Concise Guide to PHARMACOLOGY 2021/22. 76
COMPETING INTERESTS
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
OPEN RESEARCH BADGES
This article has earned Open Data, Open Materials and Preregistered Research Design badges. Data, materials and the preregistered design and analysis plan are available at https://www.crd.york.ac.uk/PROSPERO/#recordDetails.
Supporting information
Table S1 Quality assessment of the Newcastle–Ottawa Scale (NOS)
Figure S1 Risk of bias summary
Figure S2 Risk of bias graph
Figure S3 Sensitivity analysis of PFS among patients treated with HER2‐TKIs targeted therapy
Figure S4 Funnel graph of PFS among patients treated with HER2‐TKIs targeted therapy
Figure S5 Begg's test of PFS among patients treated with HER2‐TKIs targeted therapy
Figure S6 Egger's test of PFS among patients treated with HER2‐TKIs targeted therapy
Figure S7 Sensitivity analysis of ORR among patients treated with HER2‐targeted therapy
Figure S8 Funnel graph of ORR among patients treated with HER2‐targeted therapy
Figure S9 Begg's test of ORR among patients treated with HER2‐targeted therapy
Figure S10 Egger's test of ORR among patients treated with HER2‐targeted therapy
Figure S11 Sensitivity analysis of DCR among patients treated with HER2‐targeted therapy
Figure S12 Funnel graph of DCR among patients treated with HER2‐targeted therapy
Figure S13 Begg's test of DCR among patients treated with HER2‐targeted therapy
Figure S14 Egger's test of DCR among patients treated with HER2‐targeted therapy
Figure S15 Sensitivity analysis of PFS among patients treated with HER2‐targeted therapy
Figure S16 Funnel graph of PFS among patients treated with HER2‐targeted therapy
Figure S17 Begg's test of PFS among patients treated with HER2‐targeted therapy
Figure S18 Egger's test of PFS among patients treated with HER2‐targeted therapy
ACKNOWLEDGEMENTS
We thank all the people who participated in the primary trials and the teams conducted did them. This work was supported by grants from the National Natural Science Foundation of China (Grants 82070019 and 81870034).
Wu H‐X, Zhuo K‐Q, Wang K. Efficacy of targeted therapy in patients with HER2‐positive non‐small cell lung cancer: A systematic review and meta‐analysis. Br J Clin Pharmacol. 2022;88(5):2019-2034. doi: 10.1111/bcp.15155
Funding information National Natural Science Foundation of China, Grant/Award Numbers: 82070019, 81870034
REFERENCES
- 1. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394‐424. 10.3322/caac.21492 Erratum in: CA Cancer J Clin. 2020;70(4):313. [DOI] [PubMed] [Google Scholar]
- 2. Kalemkerian GP, Narula N, Kennedy EB, et al. Molecular testing guideline for the selection of patients with lung cancer for treatment with targeted tyrosine kinase inhibitors: American Society of Clinical Oncology endorsement of the College of American Pathologists/International Association for the Study of Lung Cancer/Association for Molecular Pathology Clinical Practice guideline update. J Clin Oncol. 2018;36(9):911‐919. 10.1200/JCO.2017.76.7293 [DOI] [PubMed] [Google Scholar]
- 3. Ettinger DS, Wood DE, Aisner DL, et al. NCCN guidelines insights: non‐small cell lung cancer, version 2.2021. J Natl Compr Canc Netw. 2021;19(3):254‐266. 10.6004/jnccn.2021.0013 [DOI] [PubMed] [Google Scholar]
- 4. Alexander SP, Kelly E, Marrion N, et al. The Concise Guide to PHARMACOLOGY 2019/20: Introduction and other protein targets. Br J Pharmacol. 2019;176:S1‐S20. 10.1111/bph.14747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Alexander SP, Kelly E, Marrion NV, et al. The Concise Guide to PHARMACOLOGY 2019/20: Catalytic receptors. Br J Pharmacol. 2019;176:S247‐S296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pillai RN, Behera M, Berry LD, et al. HER2 mutations in lung adenocarcinomas: a report from the Lung Cancer Mutation Consortium. Cancer. 2017;123(21):4099‐4105. 10.1002/cncr.30869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nakamura H, Kawasaki N, Taguchi M, Kabasawa K. Association of HER‐2 overexpression with prognosis in nonsmall cell lung carcinoma: a meta analysis. Cancer. 2005;103(9):1865‐1873. 10.1002/cncr.20957 [DOI] [PubMed] [Google Scholar]
- 8. Chmielecki J, Ross JS, Wang K, et al. Oncogenic alterations in ERBB2/HER2 represent potential therapeutic targets across tumors from diverse anatomic sites of origin. Oncologist. 2015;20(1):7‐12. 10.1634/theoncologist.2014-0234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Robichaux JP, Elamin YY, Vijayan RSK, et al. Pan‐cancer landscape and analysis of ERBB2 mutations identifies poziotinib as a clinically active inhibitor and enhancer of T‐DM1 activity. Cancer Cell. 2020;37(3):420. 10.1016/j.ccell.2020.03.003 Erratum for: Cancer Cell. 2019;36(4):444–457.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. The Clinical Knowledgebase . Erbb2 gene level evidence; 2020. https://ckb.jax.org
- 11. Arcila ME, Chaft JE, Nafa K, et al. Prevalence, clinicopathologic associations, and molecular spectrum of ERBB2 (HER2) tyrosine kinase mutations in lung adenocarcinomas. Clin Cancer Res. 2012;18(18):4910‐4918. 10.1158/1078-0432.CCR-12-0912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sassen A, Rochon J, Wild P, et al. Cytogenetic analysis of HER1/EGFR, HER2, HER3 and HER4 in 278 breast cancer patients. Breast Cancer Res. 2008;10(1):R2. 10.1186/bcr1843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li BT, Ross DS, Aisner DL, et al. HER2 amplification and HER2 mutation are distinct molecular targets in lung cancers. J Thorac Oncol. 2016;11(3):414‐419. 10.1016/j.jtho.2015.10.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kim EK, Kim KA, Lee CY, Shim HS. The frequency and clinical impact of HER2 alterations in lung adenocarcinoma. PLoS ONE. 2017;12(2):e0171280. 10.1371/journal.pone.0171280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Garrido‐Castro AC, Felip E. HER2 driven non‐small cell lung cancer (NSCLC): potential therapeutic approaches. Transl Lung Cancer Res. 2013;2(2):122‐127. 10.3978/j.issn.2218-6751.2013.02.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hirsch FR, Franklin WA, Veve R, Varella‐Garcia M, Bunn PA Jr. HER2/neu expression in malignant lung tumors. Semin Oncol. 2002;29(1 Suppl 4):51‐58. 10.1053/sonc.2002.31523 [DOI] [PubMed] [Google Scholar]
- 17. Heinmöller P, Gross C, Beyser K, et al. HER2 status in non‐small cell lung cancer: results from patient screening for enrollment to a phase II study of herceptin. Clin Cancer Res. 2003;9(14):5238‐5243. 10.1016/S0959-8049(01)80666-3 [DOI] [PubMed] [Google Scholar]
- 18. Zhao J, Xia Y. Targeting HER2 alterations in non‐small‐cell lung cancer: a comprehensive review. JCO Precis Oncol. 2020;(4):411‐425. 10.1200/PO.19.00333 [DOI] [PubMed] [Google Scholar]
- 19. Zinner RG, Glisson BS, Fossella FV, et al. Trastuzumab in combination with cisplatin and gemcitabine in patients with Her2‐overexpressing, untreated, advanced non‐small cell lung cancer: report of a phase II trial and findings regarding optimal identification of patients with Her2‐overexpressing disease. Lung Cancer. 2004;44(1):99‐110. 10.1016/j.lungcan.2003.09.026 [DOI] [PubMed] [Google Scholar]
- 20. Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2‐positive advanced gastric or gastro‐oesophageal junction cancer (ToGA): a phase 3, open‐label, randomised controlled trial. Lancet. 2010;376(9742):687‐697. 10.1016/S0140-6736(10)61121-X Erratum in: Lancet. 2010;376(9749):1302 [DOI] [PubMed] [Google Scholar]
- 21. Wang X, Hu W, Xie L. Response to anti‐HER2‐based treatment in a patient with bladder adenocarcinoma harboring HER2 amplification and S310F mutation discovered by next‐generation sequencing: a case report. Onco Targets Ther. 2020;13:4249‐4255. 10.2147/OTT.S247515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yoshizawa A, Sumiyoshi S, Sonobe M, et al. HER2 status in lung adenocarcinoma: a comparison of immunohistochemistry, fluorescence in situ hybridization (FISH), dual‐ISH, and gene mutations. Lung Cancer. 2014;85(3):373‐378. 10.1016/j.lungcan.2014.06.007 [DOI] [PubMed] [Google Scholar]
- 23. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. 10.14306/renhyd.18.3.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.3.0. Oxford: The Cochrane Collaboration; 2014. Accessed 30 May 2021. http://www.cochrane-handbook.org. Updated March 2014. [Google Scholar]
- 25. Cota GF, de Sousa MR, Fereguetti TO, et al. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses. Accessed May 30, 2021. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 26. Mazières J, Peters S, Lepage B, et al. Lung cancer that harbors an HER2 mutation: epidemiologic characteristics and therapeutic perspectives. J Clin Oncol. 2013;31(16):1997‐2003. 10.1200/JCO.2012.45.6095 [DOI] [PubMed] [Google Scholar]
- 27. Mazières J, Barlesi F, Filleron T, et al. Lung cancer patients with HER2 mutations treated with chemotherapy and HER2‐targeted drugs: results from the European EUHER2 cohort. Ann Oncol. 2016;27(2):281‐286. 10.1093/annonc/mdv573 [DOI] [PubMed] [Google Scholar]
- 28. De Grève J, Moran T, Graas MP, et al. Phase II study of afatinib, an irreversible ErbB family blocker, in demographically and genotypically defined lung adenocarcinoma. Lung Cancer. 2015;88(1):63‐69. 10.1016/j.lungcan.2015.01.013 [DOI] [PubMed] [Google Scholar]
- 29. Kris MG, Camidge DR, Giaccone G, et al. Targeting HER2 aberrations as actionable drivers in lung cancers: phase II trial of the pan‐HER tyrosine kinase inhibitor dacomitinib in patients with HER2‐mutant or amplified tumors. Ann Oncol. 2015;26(7):1421‐1427. 10.1093/annonc/mdv186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Song Z, Yu X, Shi Z, Zhao J, Zhang Y. HER2 mutations in Chinese patients with non‐small cell lung cancer. Oncotarget. 2016;7(47):78152‐78158. 10.18632/oncotarget.11313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lai WV, Lebas L, Barnes TA, et al. Afatinib in patients with metastatic or recurrent HER2‐mutant lung cancers: a retrospective international multicentre study. Eur J Cancer. 2019;109:28‐35. 10.1016/j.ejca.2018.11.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gandhi L, Besse B, Mazières J, et al. Neratinib ± temsirolimus in HER2‐mutant lung cancers: an international, randomized phase II study. J Thorac Oncol. 2016;12(1):S358‐S359. 10.1016/j.jtho.2016.11.398 [DOI] [Google Scholar]
- 33. Oh IJ, Hur JY, Park CK, et al. Clinical activity of pan‐HER inhibitors against HER2‐mutant lung adenocarcinoma. Clin Lung Cancer. 2018;19(5):e775‐e781. 10.1016/j.cllc.2018.05.018 [DOI] [PubMed] [Google Scholar]
- 34. Zhao S, Fang W, Yang Y, Zhang L. Three specific HER2 mutations predict favorable outcomes in advanced lung cancer patients treated with afatinib. J Thorac Oncol. 2018;13(10):S506‐S507. 10.1016/j.jtho.2018.08.667 [DOI] [Google Scholar]
- 35. Peters S, Curioni‐Fontecedro A, Nechushtan H, et al. Activity of afatinib in heavily pretreated patients with ERBB2 mutation‐positive advanced NSCLC: findings from a global named patient use program. J Thorac Oncol. 2018;13(12):1897‐1905. 10.1016/j.jtho.2018.07.093 [DOI] [PubMed] [Google Scholar]
- 36. Liu Z, Wu L, Cao J, et al. Clinical characterization of ERBB2 exon 20 insertions and heterogeneity of outcomes responding to afatinib in Chinese lung cancer patients. Onco Targets Ther. 2018;11:7323‐7331. 10.2147/OTT.S173391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Robichaux JP, Elamin YY, Tan Z, et al. Mechanisms and clinical activity of an EGFR and HER2 exon 20‐selective kinase inhibitor in non‐small cell lung cancer. Nat Med. 2018;24(5):638‐646. 10.1038/s41591-018-0007-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hyman DM, Piha‐Paul SA, Won H, et al. HER kinase inhibition in patients with HER2‐ and HER3‐mutant cancers. Nature. 2018;554(7691):189‐194. 10.1038/nature25475 Erratum in: Nature 2019;566(7745):E11–E12 [DOI] [PubMed] [Google Scholar]
- 39. Dziadziuszko R, Smit EF, Dafni U, et al. Afatinib in NSCLC with HER2 mutations: results of the prospective, open‐label phase II NICHE trial of European Thoracic Oncology Platform (ETOP). J Thorac Oncol. 2019;14(6):1086‐1094. 10.1016/j.jtho.2019.02.017 [DOI] [PubMed] [Google Scholar]
- 40. Wang Y, Jiang T, Qin Z, et al. HER2 exon 20 insertions in non‐small‐cell lung cancer are sensitive to the irreversible pan‐HER receptor tyrosine kinase inhibitor pyrotinib. Ann Oncol. 2019;30(3):447‐455. 10.1093/annonc/mdy542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhou C, Li X, Wang Q, et al. Pyrotinib in HER2‐mutant advanced lung adenocarcinoma after platinum‐based chemotherapy: a multicenter, open‐label, single‐arm, phase II study. J Clin Oncol. 2020;38(24):2753‐2761. 10.1200/JCO.20.00297 [DOI] [PubMed] [Google Scholar]
- 42. Zhou J, Ding N, Xu X, et al. Clinical outcomes of patients with HER2‐mutant advanced lung cancer: chemotherapies versus HER2‐directed therapies. Ther Adv Med Oncol. 2020;12:1758835920936090. 10.1177/1758835920936090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Liu SV, Villaruz LC, Lee VHF, et al. First analysis of RAIN‐701: study of tarloxotinib in patients with non‐small cell lung cancer (NSCLC) EGFR Exon 20 insertion, HER2‐activating mutations & other solid tumours with NRG1/ERBB gene fusions. Ann Oncol. 2020;31:S1189. 10.1016/j.annonc.2020.08.2294 [DOI] [Google Scholar]
- 44. Socinski M, Cornelissen R, Garassino M, et al. LBA60 ZENITH20, a multinational, multi‐cohort phase II study of poziotinib in NSCLC patients with EGFR or HER2 exon 20 insertion mutations. Ann Oncol. 2020;31:S1188. 10.1016/j.annonc.2020.08.2293 [DOI] [Google Scholar]
- 45. Lara PN Jr, Laptalo L, Longmate J, et al. Trastuzumab plus docetaxel in HER2/neu‐positive non‐small‐cell lung cancer: a California Cancer Consortium screening and phase II trial. Clin Lung Cancer. 2004;5(4):231‐236. 10.3816/clc.2004.n.004 [DOI] [PubMed] [Google Scholar]
- 46. Herbst RS, Davies AM, Natale RB, et al. Efficacy and safety of single‐agent pertuzumab, a human epidermal receptor dimerization inhibitor, in patients with non small cell lung cancer. Clin Cancer Res. 2007;13(20):6175‐6181. 10.1158/1078-0432.CCR-07-0460 [DOI] [PubMed] [Google Scholar]
- 47. Langer CJ, Stephenson P, Thor A, Vangel M, Johnson DH. Trastuzumab in the treatment of advanced non‐small‐cell lung cancer: is there a role? Focus on Eastern Cooperative Oncology Group Study 2598. J Clin Oncol. 2004;22(7):1180‐1187. 10.1200/JCO.2004.04.105 [DOI] [PubMed] [Google Scholar]
- 48. Gatzemeier U, Groth G, Butts C, et al. Randomized phase II trial of gemcitabine‐cisplatin with or without trastuzumab in HER2‐positive non‐small‐cell lung cancer. Ann Oncol. 2004;15(1):19‐27. 10.1093/annonc/mdh031 [DOI] [PubMed] [Google Scholar]
- 49. Hainsworth JD, Meric‐Bernstam F, Swanton C, et al. Targeted therapy for advanced solid tumors on the basis of molecular profiles: results from MyPathway, an open‐label, phase IIa multiple basket study. J Clin Oncol. 2018;36(6):536‐542. 10.1200/JCO.2017.75.3780 Erratum in: J Clin Oncol. 2019;37(4):360 [DOI] [PubMed] [Google Scholar]
- 50. de Langen AJ, Jebbink M, Hashemi SMS, et al. Trastuzumab and paclitaxel in patients with EGFR mutated NSCLC that express HER2 after progression on EGFR TKI treatment. Br J Cancer. 2018;119(5):558‐564. 10.1038/s41416-018-0194-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Li B, Gandhi L, Besse B, et al. Neratinib‐based combination therapy in HER2‐mutant lung adenocarcinomas: findings from two international phase 2 studies. J Thorac Oncol. 2021;16(3):S234. 10.1016/j.jtho.2021.01.158 [DOI] [Google Scholar]
- 52. Smit EF, Nakagawa K, Nagasaka M, et al. Trastuzumab deruxtecan (T‐DXd; DS‐8201) in patients with HER2‐mutated metastatic non‐small cell lung cancer (NSCLC): interim results of DESTINY‐Lung01. J Clin Oncol. 2020;38(15):9504. 10.1200/JCO.2020.38.15_suppl.9504 [DOI] [Google Scholar]
- 53. Tsurutani J, Iwata H, Krop I, et al. Targeting HER2 with trastuzumab deruxtecan: a dose‐expansion, phase I study in multiple advanced solid tumors. Cancer Discov. 2020;10(5):688‐701. 10.1158/2159-8290.CD-19-1014 Erratum in: Cancer Discov. 2020;10(7):1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hotta K, Aoe K, Kozuki T, et al. A phase II study of trastuzumab emtansine in HER2‐positive non‐small cell lung cancer. J Thorac Oncol. 2018;13(2):273‐279. 10.1016/j.jtho.2017.10.032 [DOI] [PubMed] [Google Scholar]
- 55. Li BT, Shen R, Buonocore D, et al. Ado‐trastuzumab emtansine for patients with HER2‐mutant lung cancers: results from a phase II basket trial. J Clin Oncol. 2018;36(24):2532‐2537. 10.1200/JCO.2018.77.9777 Erratum in: J Clin Oncol. 2019;37(4):362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Peters S, Stahel R, Bubendorf L, et al. Trastuzumab emtansine (T‐DM1) in patients with previously treated HER2‐overexpressing metastatic non‐small cell lung cancer: efficacy, safety, and biomarkers. Clin Cancer Res. 2019;25(1):64‐72. 10.1158/1078-0432.CCR-18-1590 [DOI] [PubMed] [Google Scholar]
- 57. Cha MY, Lee KO, Kim M, et al. Antitumor activity of HM781‐36B, a highly effective pan‐HER inhibitor in erlotinib‐resistant NSCLC and other EGFR‐dependent cancer models. Int J Cancer. 2012;130(10):2445‐2454. 10.1002/ijc.26276 [DOI] [PubMed] [Google Scholar]
- 58. Li X, Yang C, Wan H, et al. Discovery and development of pyrotinib: a novel irreversible EGFR/HER2 dual tyrosine kinase inhibitor with favorable safety profiles for the treatment of breast cancer. Eur J Pharm Sci. 2017;110:51‐61. 10.1016/j.ejps.2017.01.021 [DOI] [PubMed] [Google Scholar]
- 59. Ma F, Li Q, Chen S, et al. Phase I study and biomarker analysis of pyrotinib, a novel irreversible pan‐ErbB receptor tyrosine kinase inhibitor, in patients with human epidermal growth factor receptor 2‐positive metastatic breast cancer. J Clin Oncol. 2017;35(27):3105‐3112. 10.1200/JCO.2016.69.6179 [DOI] [PubMed] [Google Scholar]
- 60. Yap TA, Vidal L, Adam J, et al. Phase I trial of the irreversible EGFR and HER2 kinase inhibitor BIBW 2992 in patients with advanced solid tumors. J Clin Oncol. 2010;28(25):3965‐3972. 10.1200/JCO.2009.26.7278 [DOI] [PubMed] [Google Scholar]
- 61. Gao G, Li X, Wang Q, et al. Single‐arm, phase II study of pyrotinib in advanced non‐small cell lung cancer (NSCLC) patients with HER2 exon 20 mutation. J Clin Oncol. 2019;37(15_suppl):9089. 10.1200/JCO.2019.37.15_suppl.9089 [DOI] [Google Scholar]
- 62. Klapper LN, Waterman H, Sela M, Yarden Y. Tumor‐inhibitory antibodies to HER‐2/ErbB‐2 may act by recruiting c‐Cbl and enhancing ubiquitination of HER‐2. Cancer Res. 2000;60(13):3384‐3388. [PubMed] [Google Scholar]
- 63. Oh DY, Bang YJ. HER2‐targeted therapies—a role beyond breast cancer. Nat Rev Clin Oncol. 2020;17(1):33‐48. 10.1038/s41571-019-0268-3 [DOI] [PubMed] [Google Scholar]
- 64. Kinoshita I, Goda T, Watanabe K, et al. 1491PA phase II study of trastuzumab monotherapy in pretreated patients with non‐small cell lung cancers (NSCLCs) harboring HER2 alterations: HOT1303‐B trial. Ann Oncol. 2018;29(suppl_8):viii540. 10.1093/annonc/mdy292.112 [DOI] [Google Scholar]
- 65. Phillips GDL, Li G, Dugger DL, et al. Targeting HER2‐positive breast cancer with trastuzumab‐DM1, an antibody‐cytotoxic drug conjugate. Cancer Res. 2008;68(22):9280‐9290. 10.1158/0008-5472.CAN-08-1776 [DOI] [PubMed] [Google Scholar]
- 66. Doi T, Shitara K, Naito Y, et al. Safety, pharmacokinetics, and antitumour activity of trastuzumab deruxtecan (DS‐8201), a HER2‐targeting antibody‐drug conjugate, in patients with advanced breast and gastric or gastro‐oesophageal tumours: a phase 1 dose‐escalation study. Lancet Oncol. 2017;18(11):1512‐1522. 10.1016/S1470-2045(17)30604-6 [DOI] [PubMed] [Google Scholar]
- 67. Ogitani Y, Aida T, Hagihara K, et al. DS‐8201a, a novel HER2‐targeting ADC with a novel DNA topoisomerase I inhibitor, demonstrates a promising antitumor efficacy with differentiation from TDM1. Clin Cancer Res. 2016;22(20):5097‐5108. 10.1158/1078-0432.CCR-15-2822 [DOI] [PubMed] [Google Scholar]
- 68. Ogitani Y, Hagihara K, Oitate M, Naito H, Agatsuma T. Bystander killing effect of DS‐8201a, a novel anti‐human epidermal growth factor receptor 2 antibody‐drug conjugate, in tumors with human epidermal growth factor receptor 2 heterogeneity. Cancer Sci. 2016;107(7):1039‐1046. 10.1111/cas.12966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Pommier Y. Topoisomerase I inhibitors: camptothecins and beyond. Nat Rev Cancer. 2006;6(10):789‐802. 10.1038/nrc1977 [DOI] [PubMed] [Google Scholar]
- 70. Iwata TN, Ishii C, Ishida S, Ogitani Y, Wada T, Agatsuma T. A HER2‐targeting antibody‐drug conjugate, trastuzumab deruxtecan (DS‐8201a), enhances antitumor immunity in a mouse model. Mol Cancer Ther. 2018;17(7):1494‐1503. 10.1158/1535-7163.MCT-17-0749 [DOI] [PubMed] [Google Scholar]
- 71. The ASCO Post Staff . FDA Pipeline: Two breakthrough therapy designations for fam‐trastuzumab deruxtecan‐nxki, and more. The ASCO Post. May 18, 2020. Accessed May 30, 2021. https://ascopost.com/news/may-2020/two-breakthrough-therapy-designations-for-fam-trastuzumab-deruxtecan-nxki-and-more/#:~:text=Fam%2Dtrastuzumab%20deruxtecan%2Dnxki%20(,or%20more%20prior%20regimens%2C%20including [Google Scholar]
- 72. Nakagawa K, Nagasaka M, Felip E, et al. OA04.05 Trastuzumab deruxtecan in HER2‐overexpressing metastatic non‐small cell lung cancer: interim results of DESTINY‐Lung01. J Thorac Oncol. 2021;16(3):S109‐S110. 10.1016/j.jtho.2021.01.285 [DOI] [Google Scholar]
- 73. Tsurutani J, Park H, Doi T, et al. OA02.07 updated results of phase 1 study of DS‐8201a in HER2‐expressing or ‐mutated advanced non‐small‐cell lung cancer. J Thorac Oncol. 2018;13(10):S324. 10.1016/j.jtho.2018.08.244 [DOI] [Google Scholar]
- 74. Powell CA, Camidge DR, Gemma A, et al. Abstract P6‐17‐06: characterization, monitoring and management of interstitial lung disease in patients with metastatic breast cancer: analysis of data available from multiple studies of DS‐8201a, a HER2‐targeted antibody drug conjugate with a topoisomerase I inhibitor payload. Am Assoc Cancer Res. 2019;79(Suppl 4). 10.1158/1538-7445.SABCS18-P6-17-06 [DOI] [Google Scholar]
- 75. Planchard D, Li BT, Murakami H, et al. A phase II study of trastuzumab deruxtecan (DS‐8201a) in HER2‐overexpressing or ‐mutated advanced non‐small cell lung cancer. Ann Oncol. 2019;30(Suppl_2):ii66‐ii67. 10.1093/annonc/mdz063.081 [DOI] [Google Scholar]
- 76. Alexander SPH, Fabbro D, Kelly E, et al. The Concise Guide to PHARMACOLOGY 2021/22: Enzymes. Br J Pharmacol. 2021;178(S1):S313‐S411. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Quality assessment of the Newcastle–Ottawa Scale (NOS)
Figure S1 Risk of bias summary
Figure S2 Risk of bias graph
Figure S3 Sensitivity analysis of PFS among patients treated with HER2‐TKIs targeted therapy
Figure S4 Funnel graph of PFS among patients treated with HER2‐TKIs targeted therapy
Figure S5 Begg's test of PFS among patients treated with HER2‐TKIs targeted therapy
Figure S6 Egger's test of PFS among patients treated with HER2‐TKIs targeted therapy
Figure S7 Sensitivity analysis of ORR among patients treated with HER2‐targeted therapy
Figure S8 Funnel graph of ORR among patients treated with HER2‐targeted therapy
Figure S9 Begg's test of ORR among patients treated with HER2‐targeted therapy
Figure S10 Egger's test of ORR among patients treated with HER2‐targeted therapy
Figure S11 Sensitivity analysis of DCR among patients treated with HER2‐targeted therapy
Figure S12 Funnel graph of DCR among patients treated with HER2‐targeted therapy
Figure S13 Begg's test of DCR among patients treated with HER2‐targeted therapy
Figure S14 Egger's test of DCR among patients treated with HER2‐targeted therapy
Figure S15 Sensitivity analysis of PFS among patients treated with HER2‐targeted therapy
Figure S16 Funnel graph of PFS among patients treated with HER2‐targeted therapy
Figure S17 Begg's test of PFS among patients treated with HER2‐targeted therapy
Figure S18 Egger's test of PFS among patients treated with HER2‐targeted therapy


