Abstract
Background
Azole resistance complicates treatment of patients with invasive aspergillosis with an increased mortality. Azole resistance in Aspergillus fumigatus is a growing problem and associated with human and environmental azole use. Denmark has a considerable and highly efficient agricultural sector. Following reports on environmental azole resistance in A. fumigatus from Danish patients, the ministry of health requested a prospective national surveillance of azole‐resistant A. fumigatus and particularly that of environmental origin.
Objectives
To present the data from the first 2 years of the surveillance programme.
Methods
Unique isolates regarded as clinically relevant and any A. fumigatus isolated on a preferred weekday (background samples) were included. EUCAST susceptibility testing was performed and azole‐resistant isolates underwent cyp51A gene sequencing.
Results
The azole resistance prevalence was 6.1% (66/1083) at patient level. The TR34/L98H prevalence was 3.6% (39/1083) and included the variants TR34/L98H, TR34 3/L98H and TR34/L98H/S297T/F495I. Resistance caused by other Cyp51A variants accounted for 1.3% (14/1083) and included G54R, P216S, F219L, G54W, M220I, M220K, M220R, G432S, G448S and Y121F alterations. Non‐Cyp51A‐mediated resistance accounted for 1.2% (13/1083). Proportionally, TR34/L98H, other Cyp51A variants and non‐Cyp51A‐mediated resistance accounted for 59.1% (39/66), 21.2% (14/66) and 19.7% (13/66), respectively, of all resistance. Azole resistance was detected in all five regions in Denmark, and TR34/L98H specifically, in four of five regions during the surveillance period.
Conclusion
The azole resistance prevalence does not lead to a change in the initial treatment of aspergillosis at this point, but causes concern and leads to therapeutic challenges in the affected patients.
Keywords: antifungal susceptibility, Aspergillus fumigatus, azole resistance, environmental route, itraconazole, medical route, TR34/L98H, voriconazole
1. INTRODUCTION
Azole resistance in Aspergillus fumigatus due to the specific molecular mechanisms TR34/L98H or TR46/Y121F/T289A has been reported from all seven continents except Antarctica. 1 These mechanisms are found in environmental A. fumigatus isolates and in isolates from azole naïve as well as from exposed patients. Azole resistance can also arise in A. fumigatus in patients receiving long‐term azole treatment. 2 Most resistant isolates harbour mutations in cyp51A, which encodes the azole target 14α‐sterol‐demethylase, essential for ergosterol biosynthesis. 2 However, azole resistance has also been ascribed to efflux pumps and other non‐cyp51A‐mediated resistance mutations. 2 , 3
In Denmark, the first isolation of TR34/L98H and TR46/Y121F/T289A were from clinical samples in 2007 and 2014, respectively. 4 , 5 Subsequently, TR34/L98H and TR46/Y121F/T289A have also been found in environmental samples since 2009 and 2019, respectively. 6 , 7 Moreover, an increase in the prevalence of azole resistance among Danish cystic fibrosis (CF) patients was found over a 10‐year period. 8
Clinical manifestations with Aspergillus vary according to patient group. In the CF population, Aspergillus occurs most often as part of colonisation, allergic bronchopulmonary aspergillosis (ABPA) and bronchitis. 9 ABPA is also a well‐known condition in patients with asthma. 10 Invasive aspergillosis mainly occurs in patients who are immunosuppressed and chronic aspergillosis in patients with impaired lung tissue architecture. Azoles are the drugs of choice in the management of aspergillosis. 10 Voriconazole and isavuconazole are first choice in invasive aspergillosis, 11 , 12 itraconazole (and voriconazole) in chronic aspergillosis 13 and posaconazole as prophylaxis or salvage treatment, but with potential future broadening of its licensed indication due to non‐inferior to voriconazole for primary therapy. 10 , 12 , 14 At this point, azoles are the only antifungal agents against aspergillosis for oral administration. The emergence of azole resistance complicates patient treatment, and invasive aspergillosis with azole resistance is associated with an inferior outcome compared to invasive aspergillosis with a susceptible strain. 15 , 16
An international expert opinion suggested that when the environmental resistance rate exceeds 10% in a region, the initial treatment for invasive aspergillosis should be either liposomal amphotericin B or voriconazole combined with an echinocandin. 17 This recommendation was based on two observations. First, the significantly increased mortality found in patients who received voriconazole initially for invasive aspergillosis due to resistant A. fumigatus 15 , 16 and second, the superior activity of voriconazole for those with susceptible A. fumigatus (~70% survival vs. 55% for amphotericin B and 50% for echinocandins). 17 , 18 This approach requires reliable epidemiological data on the prevalence of azole resistance in A. fumigatus due to the environmental route of acquisition (which may occur even in azole naïve patients) and medical route (which is limited to the azole exposed patient population).
The Danish national surveillance programme on azole resistance was established in 2018 upon request from the ministry of health due to the rising concerns for azole resistance of environmental origin. The objective was to determine the prevalence of azole‐resistant A. fumigatus isolates among A. fumigatus colonised and infected patients in Denmark and determine the underlying resistance mechanism. We present data from the first 2 years of the surveillance.
2. METHODS
2.1. Organisation of the national surveillance programme of azole‐resistant A. fumigatus
The surveillance programme was initiated on October 1st 2018 with participation from all 10 Danish clinical microbiological departments. Inclusion criteria were as follows: (a) unique A. fumigatus isolates that were regarded clinically significant and (b) any A. fumigatus isolated on a preferred weekday (regardless of clinical significance) were included when marked ‘Background’. The adherence to the inclusion criteria varied. Six departments followed the instructions with ‘Background’ samples with a potential uncertainty of whether the isolate represented a clinical condition with aspergillosis or a contamination. Two departments sent all isolates, and two departments sent only clinically relevant isolates. The centres are quite in‐homogeneous in patient up‐take and size of uptake area. For example, three are district hospitals (Vejle, Sønderborg and Esbjerg), two hold CF‐centres (AUH and RH) and one has a centre for chronic pulmonary aspergillosis (OUH). Isolates from the same patients were deemed unique if one of the following conditions were met: (1) when sampled more than 30 days apart, (2) if the isolate had a different susceptibility or (3) a different molecular resistance mechanism.
The clinical microbiological departments referred isolates to the reference mycological laboratory at Statens Serum Institut prospectively. Some departments performed species identification of moulds to the species level and only referred A. fumigatus while others referred all Aspergillus isolates or all mould isolates for species identification and susceptibility testing. One department at Aarhus University Hospital (AUH) performed EUCAST susceptibility testing (E. Def 10.1 and E. Def 9.3.1 as described below) of most A. fumigatus isolates locally and referred the MIC data and all resistant isolates for cyp51A sequencing (and confirmatory MIC determination) thus ensuring that all A. fumigatus isolates from AUH were included in the data analysis. Monthly reports on referred isolates were communicated to the participating laboratories to motivate and ensure adherence to the surveillance programme.
2.2. Culturing and species identification
Primary cultures were performed using Sabouraud glucose agar (SSI Diagnostika or bioMérieux) or YGC agar (yeast glucose agar; SSI Diagnostika) with incubation at 35–37°C for 5 days. Species identification included classical techniques including macro‐ and micromorphology and thermotolerance testing supplemented with MALDI‐TOF MS and β‐tubulin sequencing as needed as previously described. 8 Only A. fumigatus sensu stricto isolates were included in the surveillance.
2.3. Susceptibility testing and target gene sequencing
A. fumigatus isolates underwent screening for azole resistance following the EUCAST E. Def 10.1 method using VIPcheck azole agar plates (Mediaproducts BV). 19 Screening positive isolates underwent EUCAST E. Def 9.3.1 susceptibility testing. 20 For consistency, the MIC values from the reference laboratory were used throughout. The applied antifungal concentration ranges for the MIC testing varied slightly during the study period. Susceptibility classification was performed according to the current EUCAST breakpoints v. 10.0. 21 cyp51A sequencing was performed for isolates classified as azole resistant to at least one azole. The promoter and full coding region of the cyp51A gene were sequenced as previously described, 5 with the exception that for Sanger sequencing, 0F was replaced with a new primer 1F (5′‐GTGCGTAGCAAGGGAGAAGGA‐3′) for improved results.
2.4. Data management
The azole resistance prevalence was determined at patient level and compared to the Dutch national surveillance, 2013–2018. 22 Azole resistance was divided into environmentally driven resistance (presence of TR34/L98H or TR46/Y121F/T289A), other cyp51A mutations and non‐cyp51A‐mediated resistance (when resistant, but no cyp51A mutations were identified).
A χ2 was used for comparing azole resistance prevalence at patient level in the Dutch and the Danish population using R studio (R version 4. 1. 1) R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R‐project.org/.
The surveillance was requested by the Danish Ministry of Health and the scientific study approved by the QA & Compliance at Statens Serum Institut (journal number 21/00765).
Preliminary results have previously been presented in part at the Trends in Medical Mycology 2019 and at European Congress on Clinical Microbiology and Infectious Disease (ECCMID) in 2020 and 2021 conferences and briefly summarised as part of the national Danish Integrated Antimicrobial Resistance Monitoring and Research Programme (DANMAP) established by the Danish Ministry of Food, Agriculture and Fisheries and the Danish Ministry of Health in annual reports 2018, 2019 and 2020 reports. 7 , 23 , 24 The results presented here are updated since then.
3. RESULTS
A total of 1820 susceptibility‐tested A. fumigatus isolates from 1083 patients were included in the analysis. The vast majority originated from airways including nose/sinus (1609/1820) and ear samples (182/1820) (Table 1).
TABLE 1.
Number of patients and sample types with Aspergillus fumigatus isolates
| Clinical samples |
Samples marked Background samples |
Total | Proportion of resistant isolates | |
|---|---|---|---|---|
| Male/Female (n) | 527/462 | 48/46 | 575/508 | — |
| Isolates (n) | 1721 | 99 | 1820 | — |
| Sample type (n) | ||||
| Sputum samples/sinus/nose a | 1113 | 52 | 1165 | 8.5% (99/1165) |
| Tracheal aspirate | 155 | 21 | 166 | 3.6% (6/166) |
| BAL/Pleura fluid/Lung/Lung biopsy b | 167 | 11 | 278 | 4.3% (12/278) |
| Other deep samples c | 12 | 12 | 8.3% (1/12) | |
| Ear | 168 | 14 | 182 | 0.5% (1/182) |
| Cornea/Eye swab | 6 | 6 | Not detected | |
| Tissues not specified/scar/puncture site | 10 | 1 | 11 | Not detected |
Includes samples marked as sputum/laryngeal aspirate, sinus, nose/nose vestibule biopsy/nasal aspirate/nose‐throat.
Includes samples marked as BAL/bronchial aspirate/pleura fluid and lung biopsy/lung/pleura.
Cerebrospinal fluid, abscess/drain fluid/drain/abdominal swab, biopsy abdominal/biopsy organ not specified/pericardium/pericardial fluid and bone.
Itraconazole resistance was found in 5.9% (108/1820) of isolates and voriconazole resistance in 5.6% (102/1820) (Figure 1). Posaconazole resistance was detected in 103 isolates due to MICs of ≥0.5 mg/L, and 85 had MIC 0.25 mg/L (defined as area of technical uncertainty [ATU]) of which 6 were classified as resistant due to an itraconazole MIC >1 mg/L. Isavuconazole resistance with MICs of ≥4 mg/L was detected in 90 isolates, and 235 isolates had MIC 2 mg/L (ATU) of which 13 were classified as resistant due to a voriconazole MIC >1 mg/L. Overall, susceptibility testing identified 119 isolates resistant to at least one azole from 66 patients leading to a resistance prevalence among patients of 6.1% (66/1083, 95% CI 4.8%–7.7%). The proportion of isolates that were azole resistant was 6.5% (119/1820). From lower airways, the proportion of resistant isolates were 4.3% (12/278) compared to 3.6% (6/166) of isolates from tracheal aspirates and 8.5% (99/1165) of isolates in the upper airways (Table 1).
FIGURE 1.
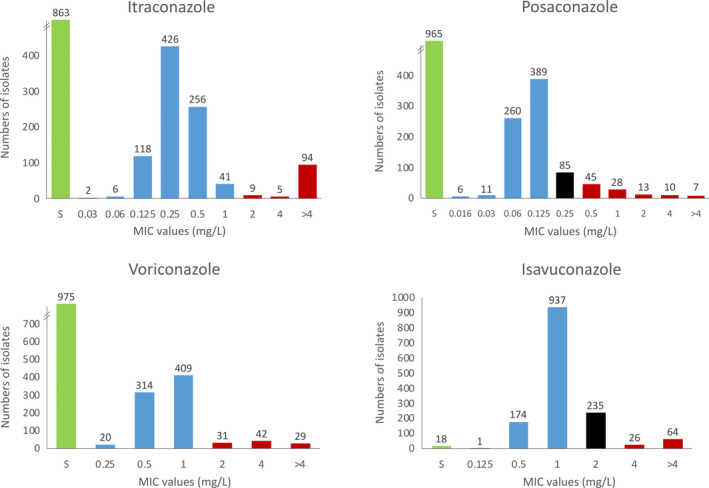
MIC values for the included Aspergillus fumigatus isolates. Susceptible isolates (S) are shown green when susceptible at azole resistance screening. Susceptible isolates with an MIC are shown in blue, resistant isolates in red and isolates in the ATU for which the classification depends on the susceptibility of either itraconazole or voriconazole, respectively, are indicated in black. MIC values above 4 mg/L are shown as >4 mg/L. Isolates with no MICs for posaconazole (n = 1) and isavuconazole (n = 365) are not included in the diagrams
The proportional distribution of resistance mechanisms at patient level are shown in Figure 2. cyp51A sequencing of azole resistant‐A. fumigatus demonstrated environmental resistance (TR34/L98H, TR34 3/L98H or TR34/L98H/297T/F495I) in isolates from 39 patients. This corresponds to an environmental resistance prevalence among the patients of 3.6% (39/1083 patients; 95% CI: 2.6%–4.9%) and accounted for 59.1% (39/66 patients; 95% CI: 47.0%–70.1%) of patients with resistant A. fumigatus isolates (Figure 2). Resistance with a tandem repeat was detected in samples from airways (Sputum [n = 44], BAL [n = 6] and tracheal aspirate [n = 4]) and one ear sample.
FIGURE 2.
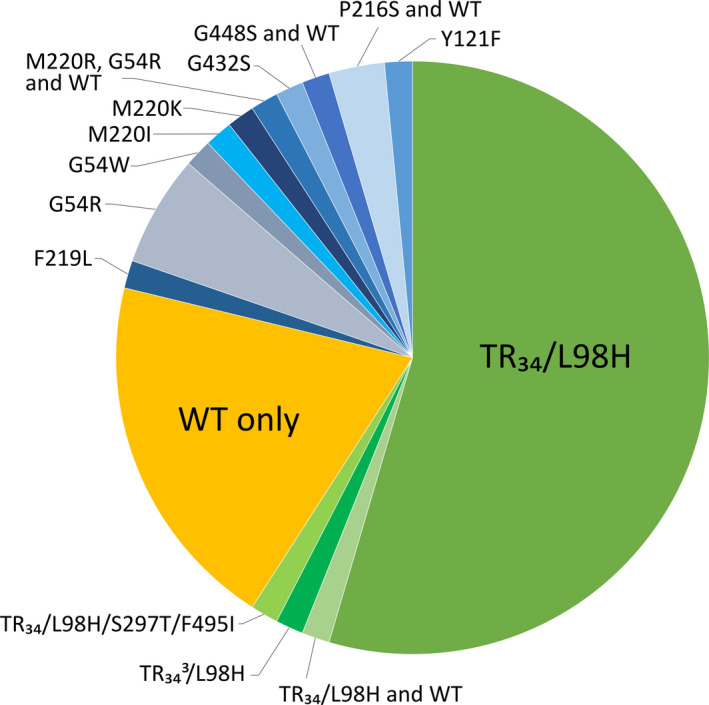
Cyp51A amino acid profiles found in the 66 patients with at least one resistant isolate. Each patient is shown only once. Some patients harbour resistant isolates with a Cyp51A resistance mechanism and resistant isolates with a non‐Cyp51A related mechanism (WT) (for example the two patients with P216S and wild‐type isolates)
Resistance involving other cyp51A mutations accounted for 21.2% (14/66; 95% CI: 13.1%–32.5%). The corresponding alterations were G54R (n = 5), P216S (n = 2), F219L (n = 1), G54W (n = 1), M220I (n = 1), M220K (n = 1), M220R (n = 1), G432S (n = 1), G448S (n = 1) and Y121F (n = 1). One patient had sequential isolates with either M220R or G54R.
Non‐cyp51A‐mediated resistance (wild‐type cyp51A) accounted for 19.7% (13/66; 95% CI: 11.9%–30.8%). Isolates from 12 of these patients were voriconazole resistant with MICs ≥4 mg/L or with MIC 2 mg/L and cross‐resistance to the other azoles. One patient harboured an isolate that was classified as resistant solely due to a voriconazole MIC of 2 mg/L.
All TR34/L98H variants, M220R, G432S, G448S and Y121F were associated with pan azole high‐level resistance (Table 2). In contrast, F219L, G54R, G54W, M220I, M220K and P216S primarily affected itraconazole susceptibility and to some degree posaconazole with limited or no MIC elevations to voriconazole and isavuconazole.
TABLE 2.
MIC values for Aspergillus fumigatus isolates resistant to at least one azole and which underwent cyp51A sequencing
| Resistance mechanism | Isolates (n) | MIC medians and ranges (mg/L) | |||
|---|---|---|---|---|---|
| ITR | POS | VOR | ISA | ||
| Environmental | |||||
| TR34/L98H a | 50 | >16 (2–>16) | 0.5/1 (0.5–>4) | 4 (2–16) | 8 (4–>16) |
| TR34 3/L98H | 4 | >16 | 1 (1–2) | 8 (4–8) | 16 |
| TR34/L98H/S297T/F495I | 1 | >16 | 2 | 4 | >16 |
| Single point mutations | |||||
| Y121F | 2 | >4–>16 | 1–4 | >4–>16 | >8 |
| G448S | 2 | >4 | (0.5–1) | >4 | >8 |
| G432S | 3 | >4 (>4–>16) | 2 (0.5–4) | 4 (2–4) | 8 (4–16) |
| M220R | 4 | >4/>16 (>4–>16) | 1/4 (0.5–>4) | 2/4 (1–4) | 4/8 (4–8) |
| M220K | 9 | >16 (4–>16) | 2 (1–>4) | 2 (1–2) | 2 (1–2) |
| M220I | 1 | >16 | 0.5 | 0.5 | 2 |
| G54R | 6 | >16 (>4–>16) | 4 (2–>4) | 1 (0.25–4) | 2 (0.5–4) |
| G54W | 1 | >16 | >4 | 0.5 | 0.5 |
| F219L | 1 | >4 | 0.5 | 1 | 2 |
| P216S | 3 | 2–>16 | 0.25–0.5 | 1 | 1 |
| Non‐cyp51A mediated b | 27 | 2 (0.5–>16) | 0.25 (0.125–2) | 2 (1–4) | 4 (2–8) |
Resistance mechanisms are shown according to environmental, single point mutations and non‐cyp51A‐mediated. Single point mutations are shown according to decreasing resistance.
Abbreviations: ISA, Isavuconazole; ITR, Itraconazole; POS, Posaconazole; VOR, Voriconazole.
One resistant isolate is not shown in this table since it was found with a F46Y/M172V/E427K, which is not associated with azole resistance, and the same patient had other resistant isolates with TR34/L98H. Four isolates did not undergo cyp51A‐sequencing and are not shown in the table
One isolate with TR34/L98H was mixed with a wild‐type isolate resulting in lower MICs than normally observed for TR34/L98H.
One resistant isolate with N248K was classified as non‐cyp51A‐mediated since this mutation is not associated with azole resistance, and since same patient had another resistant isolate with no detected cyp51A‐mediated resistance.
Among patients with a resistant isolate, both susceptible and resistant isolates were cultured intermittently during the surveillance from 38/66 (58%). Twenty‐five patients had only one resistant isolate, and three patients had several consecutive resistant isolates.
Four unique resistant isolates did not undergo cyp51A‐sequencing. Three isolates from a patient who had several resistant isolates with M220K, and another isolate from a patient who had isolates with P216S.
Isolates marked as ‘Background samples’ included 99 isolates from 94 patients (Table 1). A. fumigatus from three patients (3.2%; 95% CI: 0.9%–9.0%) were azole resistant and all harboured the TR34/L98H resistance mechanism. One patient had consecutive isolates with TR34/L98H‐marked background and not marked as background.
Azole resistance was detected in samples from all five Danish regions (Figure 3). TR34/L98H isolates were detected in four out of five regions and in samples from both the hospital and the primary health care sector, whereas isolates with single point mutations in cyp51A were found in three of five regions.
FIGURE 3.
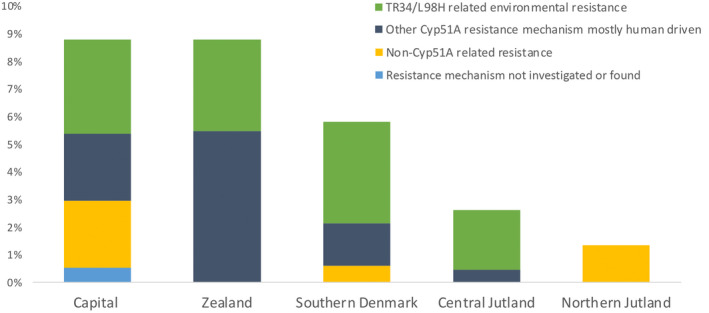
Proportion of resistant Aspergillus fumigatus isolates and associated underlying resistance mechanism across the five Danish Regions. Each Region represented is the Region of the health care facility from which the isolate was referred. As some health care services are centralised this will not in all cases represent the patients' place of residence or the place where the resistant fungus was acquired. Total numbers of isolates were for Capital n = 910, Zealand n = 91, Southern Denmark n = 326, Central Jutland n = 419 and Northern Jutland n = 74. The resistance mechanism remained uncharacterised in five isolates from five patients whom were known to harbour other cyp51A mutant isolates (blue bar). These included four resistant isolates that did not undergo cyp51A sequencing of which three isolates derived from a patient who had other isolates with M220K, and one isolate from a patient who had other isolates with P216S, and one isolate with F46Y/M172/E427K from a patient who also had isolates with TR34/L98H
Comparing surveillances at national level, azole resistance prevalence was lower in Denmark than in the Netherlands (66/1083 [6.1%] vs. 508/4496 [11.3%]) (p < .0001). 22
4. DISCUSSION
An azole resistance prevalence of 6.1% including a TR34/L98H‐related environmental resistance of 3.6% was documented at patient level during the first 2 years of the Danish nationwide surveillance programme. Whereas the first figure represents the current burden of azole resistance, the second provides information on what the chances are for facing azole resistance among azole‐naïve patients. The resistance prevalence was higher in samples from the upper airways than in tracheal aspirates and lower airway specimens. We speculate, that this may reflect that out‐patients with chronic lung disease including CF and aspergillosis are often provided with sputum containers for regular submission of sputum by mail and thus that the resistance frequencies across the different sample types are not directly comparable. Overall the resistance prevalence remains well below 10%, and azole therapy therefore remains the first choice for the initial treatment for aspergillosis in our country.
Several observations suggest that azole resistance in A. fumigatus and resistance due to TR34/L98H specifically is increasing in Denmark. In 2007, 1.9% A. fumigatus isolates were azole resistant and none due to TR34/L98H mechanisms in a 3 month multicentre survey. 25 From 2010 to 2014, the azole resistance prevalence at patient level was 2.1% among referred (and thus selected) isolates with approximately half involving TR34/L98H mechanisms. 26 Moreover, in the Danish CF population, specifically, an azole resistance prevalence of 4.5% including 1.5% due to TR34/L98H was observed in 2007 and 2009 compared to 7.3% including 3.7% TR34/L98H in 2018. 5 , 8 Although the studies are not directly comparable, we speculate that azole resistance is rising both overall and among CF patients and is driven by both medical and environmental azole use. Of note, we did not observe any isolates harbouring TR46/Y121F/T289A during the 2‐year surveillance although this resistance genotype has been found once in DK in 2014. 4
Three single point amino acid alterations (G54A, G54R and G432S) have been associated with azole resistance in both azole‐treated patients and the environment. 27 , 28 , 29 , 30 Five patients in this surveillance programme harboured isolates with a G54R and one patient an isolate with a G432S alteration. Unfortunately, we did not have access to clinical information or prior medication data to enable a discussion of the origin of these resistance mechanisms.
The number of patients with azole‐resistant A. fumigatus was unevenly distributed across the five regions in Denmark. The reason for a higher occurrence in the capital region is likely that this is the largest region based on population size, and that one of the two CF centres is based in the capital region. TR34/L98H was not detected in northern Jutland during the study period, but was detected in a clinical isolate shortly before the surveillance programme was initiated. 24 We therefore argue that TR34/L98H is found all over the country and pose a risk for any patient in Denmark susceptible to Aspergillus infections.
In comparison to other surveillance studies, our azole resistance prevalence was higher than the 3.2% found in 2009 to 2011 in a multicentre study with 19 countries, 31 but lower than the 11% in the more recent Dutch nationwide surveillance in 2017 and 2018. 22 Larger studies and surveillances on azole resistance in A. fumigatus are summarised in Table 3. 22 , 25 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 These studies show that the azole resistance prevalence in the present surveillance is in line with other European studies from 2011 to 2018 and the Netherlands from 2007 to 2009.
TABLE 3.
Studies and surveillances with azole resistance in Aspergillus fumigatus
| Country and study period | Study type/Setting | Azole resistance prevalence |
TR34/L98H and/or TR46/Y121F proportion of resistance |
|---|---|---|---|
| Europe | |||
|
Denmark 2007 (Mortensen et al.) 25 |
Nationwide surveillance 3‐month |
1.9% isolate level (2/107) |
Not detected |
|
The Netherlands 2013–2018 (Lestrade et al.) 22 |
Nationwide surveillance |
11.3% patient level (508/4496) |
556/640 of resistant isolates a |
|
The Netherlands 2007–2009 (Van der linden et al.) 32 |
Multicentre study |
5.3% patient level (63/1192) 4.6% isolate level (82/1792) |
4.1% isolate level (74/1792) |
|
Belgium 2011–2012 (Vermeulen et al.) 33 |
Multicentre study |
5.5% patient level (9/164) |
4.3% patient level (7/164) |
|
Spain 2019 (Escribano et al.) 34 |
Multicentre study |
4.7% patient level (34/715) |
2.8% patient level (20/715) |
|
Italy 2016–2018 (Prigitano et al.) 35 |
Multicentre study |
6.6% isolate level (19/286) |
4.2% isolate level (12/286) |
| North America | |||
|
USA 2015–2017 (Berkow et al.) 36 |
Multicentre study/surveillance |
1.5% isolate level (20/1356) |
0.4% isolate level (5/1356) |
| Asia | |||
|
Japan 2017–2018 (Tsuchido et al.) 39 |
Multicentre surveillance |
12.7% isolate level (7/55) |
3.6% isolate level (2/55) |
|
Taiwan 2011–2018 (Wu et al.) 37 |
Multicentre study |
4% patient level (12/297) 5.1% isolate level (19/375) |
3.4% patient level (10/297) 3.5% isolate level (13/375) |
|
China 2010–2015 (Chen et al.) 38 |
Multicentre surveillance |
2.5% isolate level (8/317) |
2.5% isolate level (8/317) |
Studies shown involve those who are either nationwide surveillances or multicentre studies in one country and not limited to a certain patient group or a referral hospital. Studies included are those that report azole resistance in A. fumigatus specifically. Azole resistance in A. fumigatus prevalence is shown in numbers of patients unless other specified.
Numbers of patients with either TR34/L98H or TR46/Y121F were not specified, and total number of isolates was not specified.
This study is associated with both strengths and limitations. The primary strength is that it is nationwide and thus population based. Results in studies limited to specific disease or centre will depend strongly on the case mix and use of azole therapy, which would favour selection of azole‐resistant A. fumigatus. Limitations include a risk of ascertainment bias. We cannot exclude that centres managing many Aspergillus patients are more prone to prioritise referral of isolates – a so‐called cluster sampling. Furthermore, the COVID‐19 pandemic emerged during the surveillance, and we cannot be certain that routine sampling was performed as under regular circumstances. Indeed fewer BALs were performed, and out‐patients with lung diseases were more often encouraged to send sputum samples by mail, than to visit the clinic in person.
Our classification of isolates as background samples is also associated with limitations. Not all laboratories adhered strictly to the inclusion criteria and not all laboratories referred background samples. Clinical data and information on prior antifungal treatment were not collected and therefore we cannot verify that the samples marked ‘Background’ actually represented a clinically insignificant background samples or that all such samples were indeed marked as Background samples. However, the fact that no isolates with medically driven point mutations were found among background samples suggests that Background samples at least are dominated by isolates from patients without prior azole therapy for clinically documented infection and thus representative for the background level of environmental resistance.
In conclusion, azole resistance is a significant problem for patients with clinical disease and in need of azole treatment. Few or no oral alternative drug options combined with long duration of treatment is a clinical challenge and results in a worsened prognosis. Initial treatment of invasive aspergillosis can remain unchanged in Denmark – but optimal treatment strategies do depend on the likelihood of azole resistance – highlighting the importance of continued surveillance, rapid susceptibility testing and a one‐health approach to azole use.
CONFLICT OF INTEREST
MR: Has received research ‐and travel grants from Gilead. RKH: Has over the past 5 years received travel grants and speaker honoraria from Gilead. JBG: Has over the past 5 years received travel grants and speaker honoraria from Gilead. LK: No conflicts of interest. FSR: No conflicts of interest. SS: No conflicts of interest. NA: No conflicts of interest. JB: No conflicts of interest. BLR: No conflicts of interest. EM: No conflicts of interest. KA: Has received travel grant and speaker honoraria from Gilead.MP: No conflicts of interest. ED: No conflicts of interest. SLA: No conflicts of interest. MCA: has outside the current work, over the past 5 years, received research grants/contract work (paid to the SSI) from Amplyx, Basilea, Cidara, F2G, Gilead, Novabiotics and Scynexis, and speaker honoraria (personal fee) from Astellas, Chiesi, Gilead, MSD, and SEGES. She is the current chairman of the EUCAST‐AFST.
AUTHOR CONTRIBUTIONS
Malene Risum: Data curation (lead); Formal analysis (equal); Investigation (equal); Project administration (equal); Writing – original draft (lead). Rasmus Krøger Hare: Data curation (equal); Investigation (equal); Methodology (lead); Writing – review & editing (equal). Jan Berg Gertsen: Data curation (equal); Investigation (equal); Methodology (equal); Project administration (equal); Writing – review & editing (supporting). Lise Kristensen: Investigation (equal); Methodology (equal); Writing – review & editing (supporting). Flemming Schønning Rosenvinge: Investigation (supporting); Methodology (supporting); Writing – review & editing (supporting). Sofia Sulim: Investigation (supporting); Methodology (supporting); Writing – review & editing (supporting). Nissrine Abou‐Chakra: Investigation (supporting); Methodology (equal); Writing – review & editing (supporting). Jette Bangsborg: Investigation (supporting); Methodology (supporting); Writing – review & editing (supporting). Bent Røder: Investigation (supporting); Methodology (supporting); Writing – review & editing (supporting). Ea Sofie Marmolin: Investigation (supporting); Methodology (supporting); Writing – review & editing (supporting). Karen Astavad : Investigation (equal); Methodology (supporting); Writing – review & editing (equal). Michael Pedersen: Investigation (supporting); Methodology (supporting); Writing – review & editing (supporting). Esad Dzajic: Investigation (supporting); Methodology (supporting); Writing – review & editing (supporting). Steen Lomborg Andersen: Investigation (supporting); Methodology (supporting); Writing – review & editing (supporting). Maiken Cavling Arendrup: Conceptualization (lead); Data curation (equal); Formal analysis (equal); Investigation (equal); Methodology (lead); Project administration (lead); Writing – original draft (lead).
ACKNOWLEDGEMENTS
The authors acknowledge the laboratory staff at the mycology unit at Statens Serum Institut.
Risum M, Hare RK, Gertsen JB, et al. Azole resistance in Aspergillus fumigatus. The first 2‐year's Data from the Danish National Surveillance Study, 2018–2020. Mycoses. 2022;65:419–428. doi: 10.1111/myc.13426
DATA AVAILABILITY STATEMENT
Data are only available for research upon reasonable request to Statens Serum Institut and within the framework of the Danish data protection legislation.
REFERENCES
- 1. Burks C, Darby A, Londoño LG, Momany M, Brewer MT. Azole‐resistant Aspergillus fumigatus in the environment: identifying key reservoirs and hotspots of antifungal resistance. PLoS Pathog. 2021;17:e1009711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rivero‐Menendez O, Alastruey‐Izquierdo A, Mellado E, Cuenca‐Estrella M. Triazole resistance in Aspergillus spp.: a worldwide problem? J Fungi. 2016;2(3):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Camps SMT, Dutilh BE, Arendrup MC, et al. Discovery of a hapE mutation that causes azole resistance in Aspergillus fumigatus through whole genome sequencing and sexual crossing. PLoS One. 2012;7:e50034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Astvad KMT, Jensen RH, Hassan TM, et al. First detection of TR 46 /Y121F/T289A and TR 34 /L98H alterations in Aspergillus fumigatus isolates from azole‐naive patients in Denmark despite negative findings in the environment. Antimicrob Agents Chemother. 2014;58:5096‐5101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mortensen KL, Jensen RH, Johansen HK, et al. Aspergillus species and other molds in respiratory samples from patients with cystic fibrosis: a laboratory‐based study with focus on Aspergillus fumigatus azole resistance. J Clin Microbiol. 2011;49:2243‐2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mortensen KL, Mellado E, Lass‐Florl C, Rodriguez‐Tudela JL, Johansen HK, Arendrup MC. Environmental study of azole‐resistant Aspergillus fumigatus and other Aspergilli in Austria, Denmark, and Spain. Antimicrob Agents Chemother. 2010;54:4545‐4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Risum M, Hare RK, Abou‐Chakra N, Gertsen JB, Kristensen L, Arendrup MC. Azole resistance in Aspergillus spp. Preliminary six months data from the newly established surveillance in Denmark. DANMAP 2018. 2019; (Textbox 5.4 part 2):77‐78.
- 8. Risum M, Hare RK, Gertsen JB, et al. Azole‐resistant Aspergillus fumigatus among Danish cystic fibrosis patients: increasing prevalence and dominance of TR34/L98H. Front Microbiol. 2020;11:1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Felton IC, Simmonds NJ. Aspergillus and cystic fibrosis: old disease – new classifications. Curr Opin Pulm Med. 2014;20:632‐638. [DOI] [PubMed] [Google Scholar]
- 10. Patterson TF, Thompson GR, Denning DW, et al. Practice guidelines for the diagnosis and management of Aspergillosis: 2016 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;63:e1‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Maertens JA, Raad II, Marr KA, et al. Isavuconazole versus voriconazole for primary treatment of invasive mould disease caused by Aspergillus and other filamentous fungi (SECURE): a phase 3, randomised‐controlled, non‐inferiority trial. Lancet. 2016;387:760‐769. [DOI] [PubMed] [Google Scholar]
- 12. Ullmann AJ, Aguado JM, Arikan‐Akdagli S, et al. Diagnosis and management of Aspergillus diseases: executive summary of the 2017 ESCMID‐ECMM‐ERS guideline. Clin Microbiol Infect. 2018;24(Suppl 1):e1‐e38. [DOI] [PubMed] [Google Scholar]
- 13. Denning DW, Cadranel J, Beigelman‐Aubry C, et al. Chronic pulmonary aspergillosis: rationale and clinical guidelines for diagnosis and management. Eur Respir J. 2016;47:45‐68. [DOI] [PubMed] [Google Scholar]
- 14. Maertens JA, Rahav G, Lee D, et al. Posaconazole versus voriconazole for primary treatment of invasive aspergillosis : a phase 3, randomised, controlled, non‐inferiority trial. Lancet. 2021;397(10273):499‐509. [DOI] [PubMed] [Google Scholar]
- 15. Lestrade PP, Bentvelsen RG, Schauwvlieghe AFAD, et al. Voriconazole resistance and mortality in invasive aspergillosis: a multicenter retrospective cohort study. Clin Infect Dis. 2019;68:1463‐1471. [DOI] [PubMed] [Google Scholar]
- 16. Resendiz‐Sharpe A, Mercier T, Lestrade PPA, et al. Prevalence of voriconazole‐resistant invasive aspergillosis and its impact on mortality in haematology patients. J Antimicrob Chemother. 2019;74:2759‐2766. [DOI] [PubMed] [Google Scholar]
- 17. Verweij PE, Ananda‐Rajah M, Andes D, et al. International expert opinion on the management of infection caused by azole‐resistant Aspergillus fumigatus . Drug Resist Updat. 2015;22:30‐40. [DOI] [PubMed] [Google Scholar]
- 18. Herbrecht R, Patterson TF, Slavin MA, et al. Application of the 2008 definitions for invasive fungal diseases to the trial comparing voriconazole versus amphotericin B for therapy of invasive aspergillosis: a Collaborative Study of the Mycoses Study Group (MSG 05) and the European Organization for R. Clin Infect Dis. 2015;60(5):713‐720. [DOI] [PubMed] [Google Scholar]
- 19. Guinea J, Verweij PE, Meletiadis J, et al. How to: EUCAST recommendations on the screening procedure E.Def 10.1 for the detection of azole resistance in Aspergillus fumigatus isolates using four‐well azole‐containing agar plates. Clin Microbiol Infect. 2019;25:681‐687. [DOI] [PubMed] [Google Scholar]
- 20. Arendrup MC, Meletiadis J, Mouton JW, et al. EUCAST technical note on isavuconazole breakpoints for Aspergillus, itraconazole breakpoints for Candida and updates for the antifungal susceptibility testing method documents. Clin Microbiol Infect. 2016;22(571):e1‐e4. [DOI] [PubMed] [Google Scholar]
- 21. Arendrup MC, Friberg N, Mares M, et al. How to: interpret MICs of antifungal compounds according to the revised clinical breakpoints v. 10.0 European committee on antimicrobial susceptibility testing (EUCAST). Clin Microbiol Infect. 2020;26:1464‐1472. [DOI] [PubMed] [Google Scholar]
- 22. Lestrade PPA, Buil JB, Van Der Beek MT, et al. Paradoxal trends in azole‐resistant Aspergillus fumigatus in a National Multicenter Surveillance Program, the Netherlands, 2013–2018. Emerg Infect Dis. 2020;26:1447‐1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Risum M, Hare RK, Arendrup MC. Azole resistance in Aspergillus fumigatus – data from the first 2 years of nationwide surveillance. DANMAP 2020. 2021;(Textbox 8.5):150‐151.
- 24. Risum M, Hare RK, Arendrup MC. Azole resistance in Aspergillus fumigatus – Nationwide surveillance data from the first 18 months. DANMAP 2019. 2020;(Textbox 8.3):139‐141.
- 25. Mortensen KL, Johansen HK, Fuursted K, et al. A prospective survey of Aspergillus spp. in respiratory tract samples: prevalence, clinical impact and antifungal susceptibility. Eur J Clin Microbiol Infect Dis. 2011;30:1355‐1363. [DOI] [PubMed] [Google Scholar]
- 26. Jensen RH, Hagen F, Astvad KMT, Tyron A, Meis JF, Arendrup MC. Azole‐resistant Aspergillus fumigatus in Denmark: a laboratory‐based study on resistance mechanisms and genotypes. Clin Microbiol Infect. 2016;22(6):570.e1‐570.e9. [DOI] [PubMed] [Google Scholar]
- 27. Riat A, Plojoux J, Gindro K, Schrenzel J, Sanglard D. Azole resistance of environmental and clinical Aspergillus fumigatus isolates from Switzerland. Antimicrob Agents Chemother. 2018;62:e02088‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Buil JB, Hare RK, Zwaan BJ, Arendrup MC, Melchers WJG, Verweij PE. The fading boundaries between patient and environmental routes of triazole resistance selection in Aspergillus fumigatus . PLoS Pathog. 2019;15:e1007858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jørgensen KM, Helleberg M, Hare RK, Jørgensen LN, Arendrup MC. Dissection of the activity of agricultural fungicides against clinical aspergillus isolates with and without environmentally and medically induced azole resistance. J Fungi. 2021;7:205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sabino R, Gonçalves P, Melo AM, et al. Trends on aspergillus epidemiology—perspectives from a national reference laboratory surveillance program. J Fungi. 2021;7:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. van der Linden JWM, Arendrup MC, Warris A, et al. Prospective multicenter international surveillance of azole resistance in Aspergillus fumigatus . Emerg Infect Dis. 2015;21:1041‐1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. van der Linden JWM, Snelders E, Kampinga GA, et al. Clinical implications of azole resistance in Aspergillus fumigatus, The Netherlands, 2007–2009. Emerg Infect Dis. 2011;17:1846‐1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vermeulen E, Maertens J, De Bel A, et al. Nationwide surveillance of azole resistance in Aspergillus diseases. Antimicrob Agents Chemother. 2015;59:4569‐4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Escribano P, Rodríguez‐Sánchez B, Díaz‐García J, et al. Azole resistance survey on clinical Aspergillus fumigatus isolates in Spain. Clin Microbiol Infect. 2021;27:1170.e1‐1170.e7. [DOI] [PubMed] [Google Scholar]
- 35. Prigitano A, Esposto MC, Grancini A, et al. Azole resistance in Aspergillus isolates by different types of patients and correlation with environment – An Italian prospective multicentre study (ARiA study). Mycoses. 2021;64:528‐536. [DOI] [PubMed] [Google Scholar]
- 36. Berkow EL, Nunnally NS, Bandea A, Kuykendall R, Beer K, Lockhart R. Detection of TR34/L98H CYP51A mutation through passive surveillance for azole‐resistant Aspergillus fumigatus in the United States from 2015 to 2017. Antimicrob Agents Chemother. 2018;62:e02240‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wu C, Liu W, Lai C, et al. Multicenter study of azole‐resistant Aspergillus fumigatus clinical isolates, Taiwan. Emerg Infect Dis. 2020;26:804‐806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chen Y, Lu Z, Zhao J, et al. Epidemiology and molecular characterizations of azole resistance in clinical and environmental Aspergillus fumigatus isolates from China. Antimicrob Agents Chemother. 2016;60:5878‐5884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tsuchido Y, Tanaka M, Nakano S, Yamamoto M, Matsumura Y, Nagao M. Prospective multicenter surveillance of clinically isolated Aspergillus species revealed azole‐resistant Aspergillus fumigatus isolates with TR34/L98H mutation in the Kyoto and Shiga regions of Japan. Med Mycol. 2019;57(8):997‐1003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are only available for research upon reasonable request to Statens Serum Institut and within the framework of the Danish data protection legislation.


