Abstract
Species interactions are influenced by the trait structure of local multi‐trophic communities. However, it remains unclear whether mutualistic interactions in particular can drive trait patterns at the global scale, where climatic constraints and biogeographic processes gain importance. Here we evaluate global relationships between traits of frugivorous birds and palms (Arecaceae), and how these relationships are affected, directly or indirectly, by assemblage richness, climate and biogeographic history. We leverage a new and expanded gape size dataset for nearly all avian frugivores, and find a positive relationship between gape size and fruit size, that is, trait matching, which is influenced indirectly by palm richness and climate. We also uncover a latitudinal gradient in trait matching strength, which increases towards the tropics and varies among zoogeographic realms. Taken together, our results suggest trophic interactions have consistent influences on trait structure, but that abiotic, biogeographic and richness effects also play important, though sometimes indirect, roles in shaping the functional biogeography of mutualisms.
Keywords: Functional biogeography, seed dispersal, birds, palms, structural equation modeling
We quantified global patterns and drivers of the functional biogeography of mutualistic trait matching using a unique gape size dataset for nearly all avian frugivores, paired with data on palm fruit size. We find bird gape size and palm fruit size are globally coupled, and that this coupling is stronger in the tropics. In addition, climatic constraints, biogeographic history and richness effects all have important, though sometimes indirect, influences on trait matching patterns.
INTRODUCTION
Species interactions are important for structuring local communities and influence ecosystem function (Bertness & Callaway, 1994; Callaway et al., 2002; Tilman, 1982), but whether and how these interactions scale up to create global diversity patterns is currently debated. For example, Schemske et al. (2009) suggested that a gradient in the strength of species interactions is one cause of the latitudinal diversity gradient. This hypothesis has been challenging to test empirically because of a lack of data on species interactions, though some studies have used functional traits as proxies for interactions (Freeman et al., 2022). One way to overcome this challenge is to quantify spatial patterns of trait matching (Dehling et al., 2014), in which traits of interacting taxa are compared to infer the strength of species interactions. However, understanding how species interactions are linked with trait variation at large scales is difficult using trait‐based approaches alone, because interaction‐relevant traits may also be influenced by the abiotic environment (Burns, 2004). Such environmental effects can act either directly on a species trait, or indirectly by altering the traits of the species with which it interacts (Maruyama et al., 2018). Thus, the extent to which trait matching is shaped by species interactions or by direct or indirect effects of climate, and how the strength of trait matching varies across the globe is not well‐understood.
Mutualistic interactions between fruiting plants and frugivores are a useful system to explore the effect of species interactions on macroecological patterns because seed dispersal can influence the structure and diversity of ecosystems at many scales (Bello et al., 2015; Gardner et al., 2019; Levin et al., 2003). Fruit and seed traits in plants and mandibular traits in frugivores are thought to be adaptations, at least in part, for seed dispersal (Janson, 1983, Figure 1, path g). Consequently, the functional relationship between plants and their dispersers is often reflected in a pattern of trait matching at local scales (Dehling et al., 2014). However, it is difficult to determine the underlying causes of trait matching at larger scales because the same traits may also be influenced by the abiotic environment. For example, the avian thermoregulation hypothesis (Tattersall et al., 2017) suggests that bird beak sizes will correlate positively with temperature as an adaptation for thermoregulation, via the heat shedding properties of large beaks (Figure 1, path a). Conversely, the plant productivity hypothesis (Moles et al., 2007) states that more productive environments, such as tropical forests, will favour larger plants with larger seeds able to germinate in low light (Figure 1, path d). Climatic constraints on traits involved in seed dispersal, such as mandible size in frugivores, can therefore act either directly on traits or indirectly by shaping the traits of the resources being consumed (e.g. Boag & Grant, 1981).
FIGURE 1.
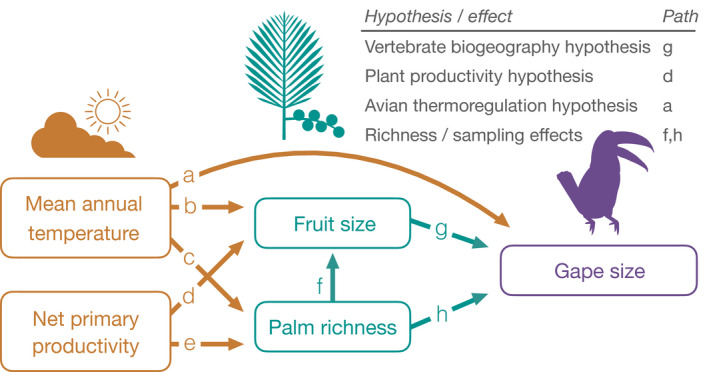
Hypothesised direct and indirect links between climatic variables, palm fruit size, palm richness and bird gape size, shown as a path diagram. Fruit size and palm richness can have direct effects on gape size (green arrows), while climate can have direct effects on fruit size and palm richness, as well as gape size, in addition to indirect effects on gape size through its effect on fruit size and palm richness (orange arrows). All relationships are predicted to be positive
In addition to climatic constraints, global geographic variation in the size, foraging strategy and other functional attributes of vertebrate seed dispersers (Corlett & Primack, 2011) may generate spatial variation in selective pressure on, and ecological sorting of fruiting plant species. This process may in turn lead to trait matching between fruits and frugivores among size‐related or other traits (Kissling, 2017; Mack, 1993; Sinnott‐Armstrong et al., 2021). Testing this idea‐ hereafter the vertebrate biogeography hypothesis‐ requires quantifying traits, and trait matching across many biogeographic realms and a range of latitudes. Furthermore, it may be expected that the largest observed trait values of animal and plant assemblages will be most correlated, as compared to averages, because frugivores cannot swallow fruits much larger than the width of the mandibles. This is particularly important for birds, which tend to swallow fruits whole (Wheelwright, 1985).
Trait matching patterns may also be influenced by the richness of the plant and animal communities being compared (Maruyama et al., 2018, Figure 1, paths f and h). For example, highly diverse assemblages may be more likely to contain species with matching traits or extreme trait values due to sampling effects (Baraloto et al., 2010). In addition, species‐rich assemblages also tend to be found in tropical forest regions, which have higher levels of dietary specialisation among vertebrates (Belmaker et al., 2012) and often have long evolutionary histories (Davis et al., 2005) with ample time for diffuse coevolution, ecological sorting and ecological fitting (sensu Janzen, 1985). This is important because mutualistic interactions such as seed dispersal occur across many regions that vary in richness (Sinnott‐Armstrong et al., 2018), enabling analyses that infer the relative importance of biotic and richness effects on trait patterns.
To determine if a global signal of morphological matching exits, and to assess variability across zoogeographic realms in the functional biogeography of palm and bird traits, we integrate newly available trait databases for birds and palms within a single multi‐trophic framework (Figure 1). As a test of the plant productivity hypothesis (Moles et al., 2007) and the avian thermoregulation hypothesis (Tattersall et al., 2017), respectively, we predict that fruit sizes will be larger in more productive regions (path d), and that regions with warmer temperatures will have birds with larger beak sizes (path a). Second, as a test of the vertebrate biogeography hypothesis (Kissling, 2017; Mack, 1993), we predict palm fruit size will correlate positively with bird gape size, and that these correlations will be stronger when comparing maximum trait values of assemblages as opposed to median values (path g). Finally, we predict assemblage richness will be positively related to gape and fruit size (paths f, h), and the range of gape and fruit sizes within assemblages, because diverse regions may be more likely to contain species with extreme trait values.
To test our predictions, we compare palm fruit size with the gape sizes and beak volumes of frugivorous birds, which have previously been shown to be related at local and regional scales (Bender et al., 2018; Burns, 2013; Chen & Moles, 2015). To evaluate climatic influences on traits, we use mean annual temperature and net primary productivity (NPP). We focus here on palms because they are ubiquitous and ecologically important elements of many tropical and subtropical regions, and have likely interacted with frugivores for millions of years (Onstein et al., 2017, 2020). Similarly, birds are known to be important consumers of palm fruits (Benzing & Seemann, 1989; Muñoz et al., 2019). We test our hypotheses using structural equation models, as well as regression and residual analysis, and find that both climate and fruit size influence bird gape size, with climate having an indirect effect via its effect on fruit size and palm richness. In addition, we discover a positive relationship between gape size and fruit size globally, and a latitudinal gradient of increasing trait matching strength towards the equator.
MATERIALS AND METHODS
Functional trait data
To test for plant–frugivore trait matching at the global scale, we used bird gape size and beak volume, and palm fruit size. To assemble trait measurements for frugivorous birds, we compiled a dataset of beak measurements taken from wild‐caught and released individuals, as well as specimens accessed in numerous museums and research collections worldwide (Tobias et al., 2022). We defined frugivores as those species with a diet containing 50% or more fruit, and only kept species matching this criterion. We attempted to measure at least two males and two females of each frugivore species, with the final dataset containing measurements from 12,925 individuals and 1129 species. For beak volume and diet traits, we used data from Pigot et al. (2020) and Tobias et al. (2022). To quantify beak volume (n = 1129 spp., units = mm3), we multiplied beak length, measured from the tip of the beak along the culmen to the base of the skull, with beak width and beak depth, both measured at the anterior edge of the nostrils.
To quantify avian frugivore gape size (previously termed ‘gape width’ or ‘beak width’ in some studies), we used data from Pigot et al. (2016), Bender et al. (2018) and Hanz et al. (2019), and also collected previously unpublished measurements on thousands of additional individuals. We defined gape size as the horizontal width of the beak measured between the points at which the upper and lower mandibles meet (units = mm). This trait is important because birds most often swallow fruits whole, and thus gape size should tend to set an upper bound to fruit sizes birds can consume (Burns, 2013; Wheelwright, 1985). Unlike standard beak measurements, such as those in Tobias et al. (2022), the global dataset of gape size is unique to this study (doi.org/10.5061/dryad.tqjq2bw05). We include gape size data in two ways. First, as species averages used in our models, and second, by providing the underlying individual‐level data from all museum specimens and wild‐caught birds along with metadata on source, collection locality and measurer. We found that gape size and beak volume are strongly correlated at the botanical country scale (Figure S1a, r = 0.95), so we chose to include only gape size in our main analyses because of its strong link with maximum ingestible fruit size.
For palm fruit size (units = cm), we used the PalmTraits database (Kissling et al., 2019), which contains vegetative and reproductive traits for nearly all palm species. We first removed three species not thought to be dispersed by animals (Dransfield et al., 2008): the coconut (Cocos nucifera), the coco de mer (Lodoicea maldivica) and the nipa palm (Nypa fruticans). Then, we extracted species mean fruit width (n = 1992 spp.) and length (n = 2049 spp.), which were highly correlated (r = 0.87). Next, we combined fruit width and length measurements to obtain a measure of fruit size (n = 2051 spp.). Fruit width was used when available, and for species with only length data (n = 59) we estimated fruit width via the allometric relationship between length and width for species in the PalmTraits database with both traits, using the equation . Fruit size, rather than seed size, was used because this trait should tend to limit the gape size of birds foraging on fruits, especially because most palms have only one seed (Dransfield et al., 2008).
Species distribution data
To determine how biogeographic history, species richness and climate influence global‐scale trait matching, we compiled geographic distribution data for birds and palms, and aggregated these data via several methods into the same geographic units (Figure S2). Geographic range maps of birds were extracted from the BirdLife International database (Birdlife International & NatureServe, 2015) and palm distribution data from the PalmTraits database (Kissling et al., 2019). As with other plant clades, detailed range maps for many palm species are not yet available, thus species distributions in the PalmTraits database are specified as presences or absences within botanical countries, which are standardized regions defined by the International Taxonomic Databases Working Group (TDWG, Brummitt et al., 2001). To match the scale of palm distribution data, assemblages of bird species were defined by overlapping bird ranges with botanical country polygons, thus creating species lists for each botanical country. We removed the Rufous‐necked hornbill (Aceros nipalensis) from Tibet, as this species is known to be a vagrant in this region. We then calculated the maximum and median trait values for each botanical country using all species occurring within. We excluded from further analysis regions with less than three species, because trait matching can only be quantified in interacting assemblages of several species. Finally, we assigned botanical countries to zoogeographic realms (sensu Holt et al., 2013), placing islands not previously classified this way within the realm most geographically close to the island. Figures S3–S5 show variation across botanical countries in both trait values and richness for birds and palms.
Climatic data
We obtained climatic data from several sources, including NPP derived from MODIS (Running et al., 2004) averaged over the years 2001–2011, and mean annual temperature from CHELSA (Karger et al., 2017). Though NPP and MAT are somewhat interrelated we retained both variables because each is related to a climatic hypothesis we test, that is, the plant productivity and avian thermoregulation hypotheses, respectively, and because the correlation between them is weak in our dataset (Figure S1). Climatic data were then aggregated in the same way as trait data‐ maximum and median MAT and NPP values were calculated for each botanical country containing bird and palm species.
Structural equation modeling
To determine how direct and indirect effects can create or obscure trait matching, and how these effects vary across zoogeographic realms, we used piecewise structural equation models (piecewise SEMs, Lefcheck, 2016). Piecewise SEMs differ from classic path analyses because each component model is solved separately instead of using a single variance‐covariance matrix, which allows for greater flexibility in the specification of each path relationship. After log‐transforming and scaling each continuous variable (to μ = 0, σ = 1), we used linear mixed effects models to fit SEM paths between plant traits, bird traits and climate, specifying in each case zoogeographic realm as a random effect. We used the maximum trait and climatic values of each botanical county in the main model; however, we also fit the model with median values to compare results using the centers of distributions. We then extracted standardised model coefficients for each direct and indirect path, as well as the marginal R 2‐ the variance explained by fixed factors, and the conditional R 2‐ the variance explained by both fixed and random, here zoogeographic realm, effects.
Trait mapping and regression analysis
To visualise trait matching globally, we co‐plotted the maximum values of gape and fruit size for each botanical country in space and via linear regressions. We also incorporated sampling effects due to species richness by fitting linear regressions of size traits with richness for both birds (log gape size ~log bird richness) and palms (log fruit size ~palm richness). We then used the residuals of these regressions to plot a richness‐corrected relationship between gape and fruit size. Next, we used model II regression to test for significant deviations in these relationships from the line of isometry. Finally, to test whether diverse regions are more likely to contain species with extreme trait values, which may influence our path model via palm richness (Figure 1, paths f–h), we quantified for each botanical country the range of trait values (max–min) for bird gape size and palm fruit size. We then fit linear and 2nd degree polynomial regressions of fruit size range and gape size range with palm richness, and between bird gape size range and palm fruit size range across all botanical countries.
To understand how trait matching varies across latitude and among realms, we extracted the residuals of the linear regression between fruit and gape size, as well as fitted values of gape size. We interpret small residuals for a given botanical country as an indication of strong trait matching. Compared to regions where residuals are large, that is, traits are more mismatched, small residuals suggest fruit size is predictive of, or matched to, gape size in this region. We then examined the relationship between residuals, that is, trait matching, and latitude, compared average residual values among zoogeographic realms and plotted residuals in space for all botanical countries.
RESULTS
Structural equation models
Using a structural equation modeling approach, we found that climate, acting indirectly via effects on palm richness, is an important predictor of bird gape size (Figure 2, see Figure S6 for SEM using median trait values). For example, temperature was significantly and positively linked to both fruit size and richness (Figure 2). However, we did not find support for the avian thermoregulation hypothesis, as temperature was negatively related to gape size (Figure 2), and to beak volume when used instead of gape size in the SEM (not shown, see Figure S1A for pairwise correlations). Counter to the plant productivity hypothesis, NPP was not significantly related to fruit size, though it was linked indirectly via an effect on palm richness (Figure 2). In addition to indirect effects, there was a direct effect of fruit size on gape size (Figure 2), in both models using maximum and median fruit and gape sizes (Figure 2, Figure S6). Our results also indicate large spatial variation in trait matching across zoogeographic realms: conditional R 2 values incorporating realm as a random effect were substantially higher, on average explaining 27% more variance than marginal R 2 values which include only fixed effects. When we added botanical countries with one or two species to the analysis, the link between fruit size and gape size became non‐significant (Figure S7a), which may reflect limited trait‐matching in assemblages containing very few species.
FIGURE 2.
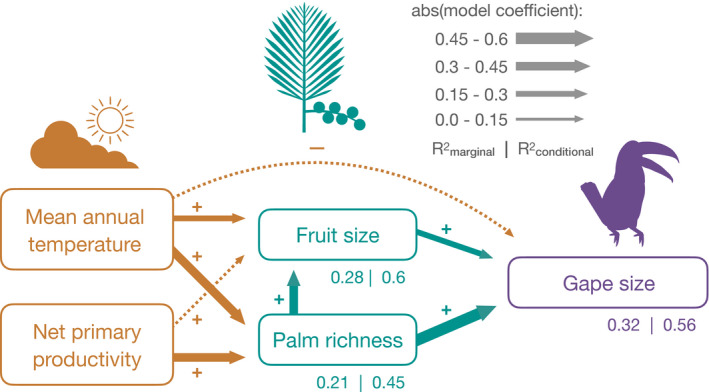
Path diagram showing how climate, fruit size and palm richness affect bird gape size. For all variables the maximum observed value of each botanical country (see Methods) was used, and Wallace realm (Holt et al., 2013) was specified as a random effect in the piecewise SEM sub‐models. Line widths are scaled to the absolute value of model coefficients, solid lines indicate significant and dotted lines non‐significant relationships. See Table S1 for path coefficients and Figure S6 for a path model fit with median values for botanical countries
Trait mapping and regression analysis
Gape and fruit size trait values broadly co‐varied in space and exhibited a high degree of spatial clustering (Figure 3a). For example, gape and fruit sizes were both lowest in the Southeast United States and highest in Southeast Asia, while tropical South America and much of Africa tended to have intermediate values of both traits. In contrast, islands tended to have traits that differed to a larger degree from one another, that is, were less matched. Overall, we found a positive relationship between maximum fruit and gape sizes across botanical countries (Figure 3b, r = 0.57, p < 0.001), and similarly for residuals from regressions of bird and palm traits correcting for assemblage richness (Figure 3c, r = 0.34, p < 0.001). For both relationships (Figure 3b,c), model II regression demonstrated that the observed slope was flatter than the 1:1 line (non‐residual values: slope = 0.62, 95% CI: 0.54–0.71; residual values: slope = 0.42, 95% CI: 0.35–0.49). Thus, best fit lines fell closer to the fruit size axis than the gape size axis in both cases, suggesting that the largest palm fruits were on average somewhat bigger than the widest bird beaks. As predicted, when median trait values of botanical countries were used the relationship between gape and fruit size was somewhat weaker (r = 0.28, p = 0.001, Figure S1b). Results were similar when species poor botanical countries with one to two species were included in the analysis (Figure S7b,c). In addition, we found the range of fruit and gape size traits within botanical countries increased with assemblage richness (Figure 4a,b, R 2 = 0.43 and 0.25, respectively; all p < 0.001) and that the ranges of both traits were themselves positively correlated (Figure 4c, R 2 = 0.31, p < 0.001). Botanical countries with low palm richness had small to large gape size ranges, while areas with high palm richness had only large gape size ranges (Figure 4b).
FIGURE 3.
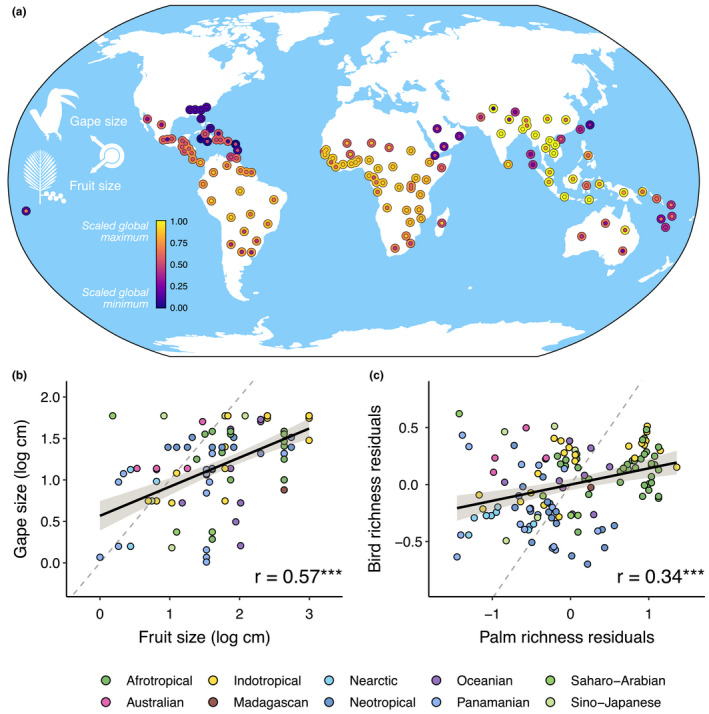
Global associations between palm fruit size and bird gape size. (a) Spatial variation in bird and palm trait matching across botanical countries (see Methods). Outer ring of each point is coloured by gape size while inner points are coloured by fruit size, warmer colours indicate higher values. Values are the maximum for each botanical country, logged and rescaled to 0–1, and plotted at the centroid position. See Figure S2 for names of each botanical country. (b) Fruit and gape size are correlated at the global scale, as are residuals calculated from a regression with log‐transformed gape and fruit size and log transformed bird and palm richness values for each botanical country (c, see Methods). Points are coloured according to Wallace realm (sensu Holt et al., 2013), dashed line is the line of isometry, ***p < 0.001
FIGURE 4.
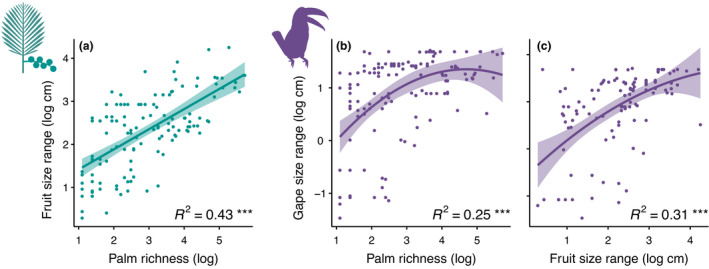
Effect of assemblage richness on trait ranges. (a) The range of fruit sizes within botanical countries (see Methods) increases with palm richness. (b, c) The range of gape sizes within botanical countries also increases with both palm richness and fruit size range. Lines and 95% confidence intervals are from linear (a) and 2nd degree polynomial (b, c) fits. ***p < 0.001
Trait matching, as measured via residuals from the relationship between gape size and fruit size, significantly decreased away from the equator, that is, residuals were larger further away from the equator (Figure 5a). The major tropical forest‐dominated realms‐ Afrotropical, Indotropical and Neotropical‐ all tended to have comparatively strong trait matching, that is, small average absolute residual values (mean = 0.11, Figure 5b). However, these regions differed in whether fruit size or gape size was larger than expected (negative vs. positive residuals respectively). Island and desert regions tended to be more mismatched in their traits (e.g., Madagascar, Oceania, the Arabian Peninsula) than most other realms, often due to larger fruit sizes compared to gape sizes (Figure 5c). We found similar results when botanical countries with one to two species were included in the analysis (Figure S7d,e).
FIGURE 5.
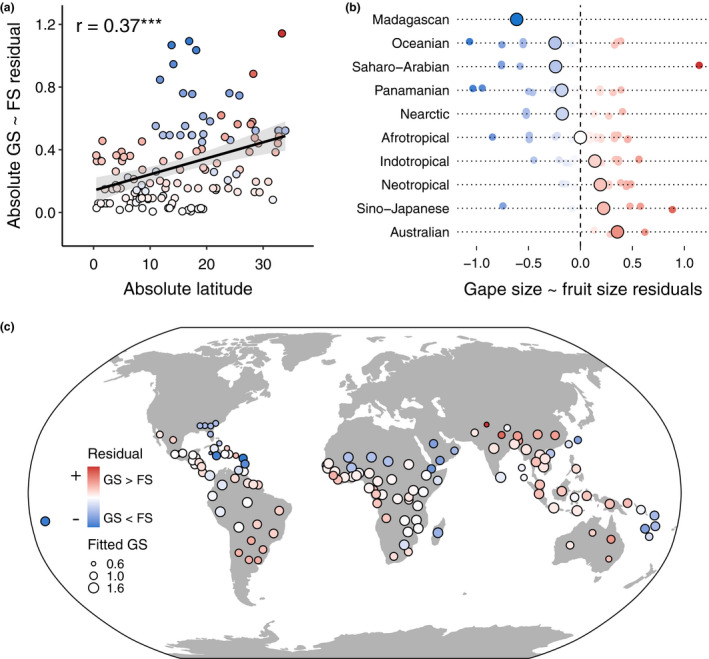
Global matching patterns of gape size (GS) and fruit size (FS). (a) Absolute residual values from a regression between log‐transformed gape and fruit size (see Figure 3b) decrease towards the equator (absolute latitude = 0), suggesting the strength of trait matching is strongest in equatorial regions. More red colours indicate a location has frugivores with larger gape sizes than predicted by fruit size, and more blue colours the opposite. (b) Trait matching strength differs among zoogeographic realms, as quantified via residuals from the gape size ~fruit size regression (Figure 3b). (c) Global spatial variation in the residuals of the gape size ~fruit size regression used to make panels a and b, with points coloured as in panels a and b, and point size scaled to the fitted value of gape size from the same regression (Figure 3b). See Figure S2 for names of each botanical country
DISCUSSION
From our analysis of frugivore and fruit trait matching, we find (1) a positive global association between fruit size and gape size, with significant variation among zoogeographic realms, (2) that indirect effects of climate on trait matching are more important than direct effects, and (3) a latitudinal gradient in the strength of trait matching, increasing towards the tropics. Our study provides evidence that trait matching is likely created not only by traits of interacting partners, but also by indirect climatic influences on species phenotypes. In addition, assemblage richness was an important factor shaping trait patterns, suggesting that sampling effects caused by geographic variation in richness, or unmeasured variation that covaries with richness, can influence large‐scale trait matching patterns. In total, our results suggest that trait matching consistently emerges from seed dispersal interactions over large areas, but that species richness, climatic variation and biogeographic history have important, but often indirect, effects on the functional biogeography of mutualisms.
It is debated whether biotic interactions influence plant and animal traits and assemblage structure in a consistent way at the global scale (Dugger et al., 2019; Sinnott‐Armstrong et al., 2018). Using a new and expanded dataset of gape sizes for nearly all frugivorous birds, we found a positive relationship between bird gape and fruit size globally, suggesting seed dispersal interactions are generally important drivers of trait patterns, and thus of the ecosystem functions these interactions mediate (Bello et al., 2015; Harrison et al., 2013). Nevertheless, historical and environmental factors were also important. Our results add to a growing body of evidence that biotic interactions play an important role in generating broad‐scale patterns of trait distributions (Hargreaves et al., 2019; Maruyama et al., 2018). For example, a positive relationship between mammal body mass and palm fruit size was also recently found (Lim et al., 2020), suggesting that seed dispersal mutualisms are generally important for shaping trait patterns of both plants and frugivores.
Though we show species traits and climatic factors are important drivers of trait matching, the form and strength of this relationship may be influenced by the number and type of interacting species (Šímová et al., 2013), and by unmeasured interactions. For example, in our SEM the effect of palm richness on gape size was significant for assemblages of three or more species. However, this relationship was not significant when including botanical countries with only one or two species (Figure S7). This result suggests that trait matching is less likely to occur if there are too few species with which to match. Also, interactions between birds and fruits are known to be diffuse in seed dispersal networks, and the diffuse nature of these interactions may make 1:1 trait matching unlikely (Guimarães et al., 2017). Indeed, our results indicated maximum sizes of palm fruits tended to be larger than maximum bird gape sizes. One explanation for this result is that palms are also dispersed by mammalian frugivores and omnivores, which tend to have larger body sizes than birds (French & Smith, 2005), especially when including extinct megafauna (Lim et al., 2020).
We predicted that there is a higher chance of sampling extreme trait values in species‐rich assemblages, which may increase the probability of trait matching through evolutionary dynamics (Onstein et al., 2017), ecological fitting (Janzen, 1985) or biotic specialisation (Maruyama et al., 2018). In line with the prediction, we found that as botanical countries became more species rich the range of trait values within them increased, suggesting that sampling effects increase the strength of trait matching. Therefore, trait matching appears more likely to emerge in species rich assemblages, which reinforces our finding that trait matching strength increases towards the equator (Figure 5a), where biodiversity often peaks. One potential mechanism for these patterns is that character displacement and specialisation on specific partners is more likely to occur in species rich areas (Maruyama et al., 2018), which also may have high functional diversity (Dehling et al., 2014). Future studies should therefore account for variability in species richness in large‐scale analyses of trait matching and explore in more depth the underlying mechanisms of how richness shapes trait matching patterns.
Many traits involved in mutualisms are thought to be also influenced by abiotic factors (Burns, 2004; Sales et al., 2021). However, using our SEM framework we found little support for direct climatic constraints on fruit and beak traits, as suggested by the plant productivity (Moles et al., 2007) and avian thermoregulation hypotheses (Tattersall et al., 2017). Thus, biotic effects such as seed dispersal mutualisms (e.g. Galetti et al., 2013) and the sampling effects of assemblage richness may be more important than thermoregulation or ecosystem productivity in determining these global trait patterns. Nonetheless, we only focus here on palms and frugivorous birds, and cannot make inferences about the validity of the hypotheses for other clades and functional groups. However, our results do provide evidence for indirect effects of climate on plant and animal traits, and thus both types of effects should be assessed in future studies of trait biogeography, particularly for traits thought to shape mutualistic interactions.
A latitudinal gradient in the strength of species interactions has long been suggested (Schemske et al., 2009), though tests have been scarce (but see Hargreaves et al., 2019; Freeman et al., 2022). Here, we find that the strength of trait matching in a seed dispersal mutualism increases towards the equator (Figure 5a), which is in line with previous results that found increased morphological matching towards low latitudes in pollination mutualisms (Sonne et al., 2020). Our results may reflect higher levels of trophic specialisation in tropical ecosystems (Belmaker et al., 2012) or the greater evolutionary age of tropical clades, providing more time for diffuse coevolution to generate matching traits among interacting partners (Davis et al., 2005; Schemske et al., 2009). As mentioned above, trait matching may also be more likely to occur in diverse tropical assemblages with many interacting species because some species may possess matching traits by chance, or because trait matching emerges more rapidly due to the high number of interactions.
In contrast to latitudinal patterns, we found that the strength of trait matching in seed dispersal mutualisms was less consistent among zoogeographic regions. For example, Southeast Asia has some of the largest frugivore gape sizes globally, driven largely by hornbills (Bucerotidae). In contrast, mammal body sizes of extant and extinct species in Southeast Asia tend to be small compared to other regions such as Africa (Lim et al., 2020). The degree of matching outside tropical regions also varies idiosyncratically. For example, we found greater mismatching in small islands and other depauperate areas compared to the equatorial tropics. This result could be due to the effects of island isolation, filtering out taxa that cannot disperse to them (Yap et al., 2018), or the environmental filtering of non‐adapted taxa from deserts or other xeric areas, or both.
Some important factors may affect our results due to the complex nature and global scale of the datasets used. First, the spatial resolution of palm distribution data required aggregating bird ranges and climatic data to the same scale, that of botanical countries. Therefore, the spatial units in our analysis were larger than traditionally defined communities of interacting species, here representing regional assemblages. Second, it should be noted that trait matching within a region does not imply co‐occurring species are necessarily interacting partners (Blanchet et al., 2020). In our analysis, we could not include interaction data due to a lack of sufficient coverage over large areas (Poisot et al., 2021). Third, while birds are ecologically important dispersers of palms, several other groups also feed on palms, including mammals, lizards and insects (Benzing & Seemann, 1989). Conversely, the diets of frugivorous birds include other fruit species in addition to palms, and may also include insects and small mammals (Corlett & Primack, 2011). Despite these potential constraints, we find predictable structure in trait patterns both in terms of trait matching across trophic levels and a consistent latitudinal gradient in the strength of this relationship.
CONCLUSIONS
In summary, we find evidence for gape and fruit size trait matching between birds and palms at the global scale, suggesting that the seed dispersal mutualism influences and constrains the size of both plant and frugivore traits. We also find that climate can influence bird traits via links with plant richness, though not directly as has been hypothesised (Moles et al., 2007; Tattersall et al., 2017). These results contribute to our understanding of how biotic interactions influence diversity at large scales through both direct and indirect effects, with large‐scale analyses across trophic levels now increasingly possible thanks to open, global trait data (Kissling et al., 2019; Kattge et al., 2020; Tobias et al., 2022). This study and future research on other clades could be used to predict how biotic interactions mediate global change impacts on biodiversity (Schleuning et al., 2020), and to what extent these interactions are themselves vulnerable to human impacts from biotic (e.g., introduced species, habitat loss) and abiotic factors such as climate change (Tobias et al., 2020). Finally, the analyses presented here should be expanded to include all clades involved in seed dispersal mutualisms, supported by the continued collection of large plant and animal trait databases, to gain a more holistic understanding of the role trophic interactions play in generating ecosystem function and resilience.
AUTHORSHIP
Conceptualisation: IRM, CHG, MS, JAT, WDK, SAF; data collection: JAT, MS, WDK, SAF; data analysis and visualisation: IRM; writing (original draft): IRM; writing (reviewing and editing): IRM, CHG, JAT, MS, WDK, NEZ, SAF, LP.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/ele.13890.
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
We are grateful to Adam Devenish and Julie Marin for help with data organisation, and also to all measurers of wild‐caught birds, bird specimens and the respective collection holders who have been key to assembling the global bird trait dataset (Tobias et al. 2022) and the gape size dataset provided with this paper (doi.org/10.5061/dryad.tqjq2bw05). We thank members of the WDK, CHG, MS and SAF research groups for constructive feedback on the work, and Jeremy Kerr and two anonymous reviewers for helpful comments. Bird trait data collection was supported by the UK Natural Environment Research Council (Nos. NE/I028068/1 and NE/P004512/1 to JAT) and by the German Research Foundation (Nos. SCHL 1934/3‐1 and SCHL 1934/2‐3 to MS). IM, CG were supported by the European Research Council (ERC) under the European Union Horizon 2020 research and innovation program (No. 787638 granted to CG) and the Swiss National Science Foundation (No. 173342 granted to CG). WDK acknowledges funding from the Netherlands Organisation for Scientific Research (No. 824.15.007) and University of Amsterdam (via a starting grant and Faculty Research Cluster ‘Global Ecology’). SAF was funded by the German Research Foundation (FR 3246/2‐2) and the Leibniz Association (Leibniz Competition P52/2017). This publication is a contribution of the Swiss WSL Institute Biodiversity Center. Open Access Funding provided by Lib4RI Library for the Research Institutes within the ETH Domain Eawag Empa PSI and WSL.
McFadden, I.R. , Fritz, S.A. , Zimmermann, N.E. , Pellissier, L. , Kissling, W.D. , Tobias, J.A. , et al. (2022) Global plant‐frugivore trait matching is shaped by climate and biogeographic history. Ecology Letters, 25, 686–696. 10.1111/ele.13890
DATA AVAILABILITY STATEMENT
Bird beak and gape size trait data at the species and individual level, with associated metadata, is available via Dryad (doi.org/10.5061/dryad.tqjq2bw05). Aggregated data on bird and palm traits as well as climate at the level of botanical countries, with code to reproduce the results, is accessible via Zenodo repository (doi.org/10.5281/zenodo.5552240). Palm trait data at the species level are from PalmTraits (doi.org/10.5061/dryad.ts45225) and global climatic data are from CHELSA (chelsa‐climate.org).
REFERENCES
- Baraloto, C. , Timothy Paine, C.E. , Patino, S. , Bonal, D. , Hérault, B. & Chave, J. (2010) Functional trait variation and sampling strategies in species‐rich plant communities. Functional Ecology, 24, 208–216. [Google Scholar]
- Bello, C. , Galetti, M. , Pizo, M.A. , Magnago, L.F.S. , Rocha, M.F. , Lima, R.A.F. et al. (2015) Defaunation affects carbon storage in tropical forests. Science Advances, 1, e1501105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmaker, J. , Sekercioglu, C.H. & Jetz, W. (2012) Global patterns of specialization and coexistence in bird assemblages. Journal of Biogeography, 39, 193–203. [Google Scholar]
- Bender, I.M.A. , Kissling, W.D. , Blendinger, P.G. , Böhning‐Gaese, K. , Hensen, I. , Kühn, I. et al. (2018) Morphological trait matching shapes plant–frugivore networks across the Andes. Ecography, 41, 1910–1919. [Google Scholar]
- Benzing, D.H. & Seemann, J. (1989) A review of animal‐mediated seed dispersal of palms. Selbyana, 2, 133–148. [Google Scholar]
- Bertness, M.D. & Callaway, R. (1994) Positive interactions in communities. Trends in Ecology & Evolution, 9, 191–193. [DOI] [PubMed] [Google Scholar]
- Birdlife International and NatureServe (2015) Bird species distribution maps of the world. Cambridge, UK: BirdLife International. [Google Scholar]
- Blanchet, F.G. , Cazelles, K. & Gravel, D. (2020) Co‐occurrence is not evidence of ecological interactions. Ecology Letters, 23, 1050–1063. [DOI] [PubMed] [Google Scholar]
- Boag, P.T. & Grant, P.R. (1981) Intense natural selection in a population of Darwin’s finches (geospizinae) in the Galápagos. Science, 214, 82–85. [DOI] [PubMed] [Google Scholar]
- Brummitt, R.K. (2001). World Geographical Scheme for Recording Plant Distributions Edition 2. Hunt Institute for Botanical Documentation, Pittsburgh; 2001. Group. International working group on taxonomic databases for plant sciences (TDWG), Pittsburgh.
- Burns, K.C. (2004) Scale and macroecological patterns in seed dispersal mutualisms. Global Ecology and Biogeography, 13, 289–293. [Google Scholar]
- Burns, K.C. (2013) What causes size coupling in fruit‐frugivore interaction webs? Ecology, 94, 295–300. [DOI] [PubMed] [Google Scholar]
- Callaway, R.M. , Brooker, R.W. , Choler, P. , Kikvidze, Z. , Lortie, C.J. , Michalet, R. et al. (2002) Positive interactions among alpine plants increase with stress. Nature, 417, 844–848. [DOI] [PubMed] [Google Scholar]
- Chen, S.C. & Moles, A.T. (2015) A mammoth mouthful? A test of the idea that larger animals ingest larger seeds. Global Ecology and Biogeography, 24, 1269–1280. [Google Scholar]
- Corlett, R.T. & Primack, R.B. (2011) Tropical rain forests: an ecological and biogeographical comparison, 2nd edition. Oxford, UK: John Wiley & Sons. [Google Scholar]
- Davis, C.C. , Webb, C.O. , Wurdack, K.J. , Jaramillo, C.A. & Donoghue, M.J. (2005) Explosive radiation of Malpighiales supports a mid‐cretaceous origin of modern tropical rain forests. American Naturalist, 165, E36–E65. [DOI] [PubMed] [Google Scholar]
- Dehling, D.M. , Töpfer, T. , Schaefer, H.M. , Jordano, P. , Böhning‐Gaese, K. & Schleuning, M. (2014) Functional relationships beyond species richness patterns: trait matching in plant‐bird mutualisms across scales. Global Ecology and Biogeography, 23, 1085–1093. [Google Scholar]
- Dransfield, J. , Uhl, N.W. , Asmussen, C.B. , Baker, W. , Harley, M.M. & Lewis, C.E. (2008) Genera Palmarum: the evolution and classification of palms. London, UK: Kew Publishing. [Google Scholar]
- Dugger, P.J. , Blendinger, P.G. , Böhning‐Gaese, K. , Chama, L. , Correia, M. , Dehling, D.M. et al. (2019) Seed‐dispersal networks are more specialized in the Neotropics than in the Afrotropics. Global Ecology and Biogeography, 28, 248–261. [Google Scholar]
- Freeman, B.G. , Weeks, T. , Schluter, D. & Tobias, J.A. (2022). The latitudinal gradient in rates of evolution for bird beaks, a species interaction trait. Ecology Letters, 25, 635–646. [DOI] [PubMed] [Google Scholar]
- French, A.R. & Smith, T.B. (2005) Importance of body size in determining dominance hierarchies among diverse tropical frugivores. Biotropica Biological Conservation, 37, 96–101. [Google Scholar]
- Galetti, M. , Guevara, R. , Cortes, M.C. , Fadini, R. , Von Matter, S. , Leite, A.B. et al. (2013) Functional extinction of birds drives rapid evolutionary changes in seed size. Science, 340, 1086–1090. [DOI] [PubMed] [Google Scholar]
- Gardner, C.J. , Bicknell, J.E. , Baldwin‐Cantello, W. , Struebig, M.J. & Davies, Z.G. (2019) Quantifying the impacts of defaunation on natural forest regeneration in a global meta‐analysis. Nature Communications, 10, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimarães, P.R. , Pires, M.M. , Jordano, P. , Bascompte, J. & Thompson, J.N. (2017) Indirect effects drive coevolution in mutualistic networks. Nature, 550, 511–514. [DOI] [PubMed] [Google Scholar]
- Hargreaves, A.L. , Suárez, E. , Mehltreter, K. , Myers‐Smith, I. , Vanderplank, S.E. , Slinn, H.L. et al. (2019) Seed predation increases from the Arctic to the Equator and from high to low elevations. Science Advances, 5, eaau4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison, R.D. , Tan, S. , Plotkin, J.B. , Slik, F. , Detto, M. , Brenes, T. et al. (2013) Consequences of defaunation for a tropical tree community. Ecology Letters, 16, 687–694. [DOI] [PubMed] [Google Scholar]
- Holt, B.G. , Lessard, J.‐P. , Borregaard, M.K. , Fritz, S.A. , Araújo, M.B. , Dimitrov, D. et al. (2013) An update of Wallace’s zoogeographic regions of the world. Science, 339, 74–78. [DOI] [PubMed] [Google Scholar]
- Janson, C.H. (1983) Adaptation of fruit morphology to dispersal agents in a neotropical forest. Science, 219, 187–188. [DOI] [PubMed] [Google Scholar]
- Janzen, D.H. (1985) On ecological fitting. Oikos, 45, 308. [Google Scholar]
- Karger, D.N. , Conrad, O. , Böhner, J. , Kawohl, T. , Kreft, H. , Soria‐Auza, R.W. et al. (2017) Climatologies at high resolution for the earth’s land surface areas. Scientific Data, 4, 170122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kattge, J. , Bönisch, G. , Díaz, S. , Lavorel, S. , Prentice, I.C. , Leadley, P. et al. (2020) TRY plant trait database – enhanced coverage and open access. Global Change Biology, 26, 119–188. [DOI] [PubMed] [Google Scholar]
- Kissling, W.D. (2017) Has frugivory influenced the macroecology and diversification of a tropical keystone plant family? Research Ideas and Outcomes, 3, e14944. [Google Scholar]
- Kissling, W.D. , Balslev, H. , Baker, W.J. , Dransfield, J. , Göldel, B. , Lim, J.Y. et al. (2019) PalmTraits 1.0, a species‐level functional trait database of palms worldwide. Scientific Data, 6, 178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefcheck, J.S. (2016) piecewiseSEM: piecewise structural equation modelling in r for ecology, evolution, and systematics. Methods in Ecology and Evolution, 7, 573–579. [Google Scholar]
- Levin, S.A. , Muller‐Landau, H.C. , Nathan, R. & Chave, J. (2003) The ecology and evolution of seed dispersal: a theoretical perspective. Annual Review of Ecology Evolution and Systematics, 34, 575–604. [Google Scholar]
- Lim, J.Y. , Svenning, J.C. , Göldel, B. , Faurby, S. & Kissling, W.D. (2020) Frugivore‐fruit size relationships between palms and mammals reveal past and future defaunation impacts. Nature Communications, 11, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack, A.L. (1993) The sizes of vertebrate‐dispersed fruits: a neotropical‐paleotropical comparison. American Naturalist, 142, 840–856. [Google Scholar]
- Maruyama, P.K. , Sonne, J. , Vizentin‐Bugoni, J. , Martín González, A.M. , Zanata, T.B. , Abrahamczyk, S. et al. (2018) Functional diversity mediates macroecological variation in plant–hummingbird interaction networks. Global Ecology and Biogeography, 27, 1186–1199. [Google Scholar]
- Moles, A.T. , Ackerly, D.D. , Tweddle, J.C. , Dickie, J.B. , Smith, R. , Leishman, M.R. et al. (2007) Global patterns in seed size. Global Ecology and Biogeography, 16, 109–116. [Google Scholar]
- Muñoz, G. , Trøjelsgaard, K. & Kissling, W.D. (2019) A synthesis of animal‐mediated seed dispersal of palms reveals distinct biogeographical differences in species interactions. Journal of Biogeography, 46, 466–484. [Google Scholar]
- Onstein, R.E. , Baker, W.J. , Couvreur, T.L.P. , Faurby, S. , Svenning, J.C. & Kissling, W.D. (2017) Frugivory‐related traits promote speciation of tropical palms. Nature Ecology & Evolution, 1, 1903–1911. [DOI] [PubMed] [Google Scholar]
- Onstein, R.E. , Vink, D.N. , Veen, J. , Barratt, C.D. , Flantua, S.G.A. , Wich, S.A. et al. (2020) Palm fruit colours are linked to the broad‐scale distribution and diversification of primate colour vision systems. Proceedings of the Royal Society B‐Biological Sciences, 287, 20192731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poisot, T. , Bergeron, G. , Cazelles, K. , Dallas, T. , Gravel, D. , MacDonald, A. et al. (2021) Global knowledge gaps in species interaction networks data. Journal of Biogeography, 48, 1–17. [Google Scholar]
- Running, S.W. , Nemani, R.R. , Heinsch, F.A. , Zhao, M. , Reeves, M. & Hashimoto, H. (2004) A continuous satellite‐derived measure of global terrestrial primary production. BioScience, 54, 547–560. [Google Scholar]
- Sales, L.P. , Kissling, W.D. , Galetti, M. , Naimi, B. , Pires, M. (2021) Climate change reshapes the eco‐evolutionary dynamics of a Neotropical seed dispersal system. Global Ecology and Biogeography, 30, 1129–1138. [Google Scholar]
- Schemske, D.W. , Mittelbach, G.G. , Cornell, H.V. , Sobel, J.M. & Roy, K. (2009) Is there a latitudinal gradient in the importance of biotic interactions? Annual Review of Ecology Evolution and Systematics, 40, 245–269. [Google Scholar]
- Schleuning, M. , Neuschulz, E.L. , Albrecht, J. , Bender, I.M.A. , Bowler, D.E. , Dehling, D.M. et al. (2020) Trait‐based assessments of climate‐change impacts on interacting species. Trends in Ecology & Evolution, 35, 319–328. [DOI] [PubMed] [Google Scholar]
- Šímová, I. , Li, Y.M. & Storch, D. (2013) Relationship between species richness and productivity in plants: the role of sampling effect, heterogeneity and species pool. Journal of Ecology, 101, 161–170. [Google Scholar]
- Sinnott‐Armstrong, M.A. , Donoghue, M.J. & Jetz, W.J. (2021) Dispersers and environment drive global variation in fruit colour syndromes. Ecology Letters, 24, 1387–1399. [DOI] [PubMed] [Google Scholar]
- Sinnott‐Armstrong, M.A. , Downie, A.E. , Federman, S. , Valido, A. , Jordano, P. & Donoghue, M.J. (2018) Global geographic patterns in the colours and sizes of animal‐dispersed fruits. Global Ecology and Biogeography, 27, 1339–1351. [Google Scholar]
- Sonne, J. , Vizentin‐Bugoni, J. , Maruyama, P.K. , Araujo, A.C. , Chávez‐González, E. , Coelho, A.G. et al. (2020) Ecological mechanisms explaining interactions within plant‐hummingbird networks: Morphological matching increases towards lower latitudes. Proceedings of the Royal Society B‐Biological Sciences, 287, 20192873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tattersall, G.J. , Arnaout, B. & Symonds, M.R.E. (2017) The evolution of the avian bill as a thermoregulatory organ. Biological Reviews, 92, 1630–1656. [DOI] [PubMed] [Google Scholar]
- Tilman, D. (1982) Resource competition and community structure, Volume 17, Princeton, NJ: Princeton University Press. [PubMed] [Google Scholar]
- Tobias, J.A. , Ottenburghs, J. & Pigot, A.L. (2020) Avian diversity: speciation, macroevolution, and ecological function. Annual Review of Ecology Evolution and Systematics, 51, 533–560. [Google Scholar]
- Tobias, J.A. , Sheard, C. , Pigot, A.L. , Devenish, A.J. , Yang, J. , Johnson, O. et al. (2022). AVONET: morphological, ecological and geographical data for all birds. Ecology Letters, 25, 581–597. [DOI] [PubMed] [Google Scholar]
- Wheelwright, N.T. (1985) Fruit size, gape width, and the diets of fruit‐eating birds. Ecology, 66, 808–818. [Google Scholar]
- Yap, J.‐Y. , Rossetto, M. , Costion, C. , Crayn, D. , Kooyman, R.M. , Richardson, J. et al. (2018) Filters of floristic exchange: how traits and climate shape the rain forest invasion of Sahul from Sunda. Journal of Biogeography, 45, 838–847. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
Bird beak and gape size trait data at the species and individual level, with associated metadata, is available via Dryad (doi.org/10.5061/dryad.tqjq2bw05). Aggregated data on bird and palm traits as well as climate at the level of botanical countries, with code to reproduce the results, is accessible via Zenodo repository (doi.org/10.5281/zenodo.5552240). Palm trait data at the species level are from PalmTraits (doi.org/10.5061/dryad.ts45225) and global climatic data are from CHELSA (chelsa‐climate.org).


