ABSTRACT
Background
Isolated REM sleep behavior disorder (iRBD) is considered a prodromal stage of parkinsonism. Neurodegenerative changes in the substantia nigra pars compacta (SNc) in parkinsonism can be detected using neuromelanin‐sensitive MRI.
Objective
To investigate SNc neuromelanin changes in iRBD patients using fully automatic segmentation.
Methods
We included 47 iRBD patients, 134 early Parkinson's disease (PD) patients and 55 healthy volunteers (HVs) scanned at 3 Tesla. SNc regions‐of‐interest were delineated automatically using convolutional neural network. SNc volumes, volumes corrected by total intracranial volume, signal‐to‐noise ratio (SNR) and contrast‐to‐noise ratio were computed. One‐way general linear models (GLM) analysis of covariance (ANCOVA) was conducted while adjusting for age and sex.
Results
All SNc measurements differed significantly between the three groups (except SNR in iRBD). Changes in iRBD were intermediate between those in PD and HVs.
Conclusions
Using fully automated SNc segmentation method and neuromelanin‐sensitive imaging, iRBD patients showed neurodegenerative changes in the SNc at a lower level than in PD patients. © 2022 The Authors. Movement Disorders published by Wiley Periodicals LLC on behalf of International Parkinson and Movement Disorder Society
Keywords: substantia nigra, neuromelanin, isolated REM sleep behavior disorder, parkinsonism, convolutional neural networks, deep learning, artificial intelligence
Introduction
Isolated rapid eye movement (REM) sleep behavior disorder (iRBD) is characterized by abnormal behaviors and loss of normal muscle atonia during REM sleep, without daytime neurological disorders. Previous studies have suggested that most iRBD subjects will develop Parkinson's disease (PD) or dementia with Lewy bodies (DLB) with a median conversion time of 7 years.1, 2 At PD onset, 30% to 60% of the dopaminergic neurons in the substantia nigra pars compacta (SNc) are already lost.3, 4 Because most of the iRBD subjects are in a prodromal parkinsonism stage, they present mild SNc impairment as shown using transcranial sonography, 5 and magnetic resonance imaging (MRI) with a loss of the dorsolateral nigral hyperintensity,6, 7 diffusion changes,2, 8 and disruption of basal ganglia connectivity. 9 Reduced striatal dopaminergic function was also reported.5, 10
Neurodegenerative changes in the SNc can be studied using neuromelanin‐sensitive MRI. Neuromelanin is a pigment contained in SNc dopaminergic neurons that acquires paramagnetic properties when associated with metals, therefore, becoming visible using MRI. 11 Concordant studies have reported reduced SNc size and signal intensity in PD using neuromelanin‐sensitive imaging with high diagnostic accuracy.12, 13, 14 Changes predominated in the ventral posterolateral SN. 15 Only two studies have used neuromelanin‐sensitive MRI to study the SNc changes in iRBD in a small number of patients without any comparison with PD patients, 2 or in a larger number, but at a group level. 16 Both studies used manual SNc segmentation, a method that is dependent on the experimenter training.
Here, we investigated neuromelanin signal changes in a large group of patients with iRBD as compared to PD patients and healthy volunteers (HVs) by automatically segmenting the SNc using a convolutional neural network (ConvNet)‐based architecture called the U‐net. 17 For methodological validation, the automated measurements were compared with the manual ones.
Subjects and Methods
Subjects were prospectively recruited from May 2015 to March 2020 as part of the ICEBERG study (ClinicalTrials.gov: NCT02305147). For inclusion, patients were clinically diagnosed by a movement disorder specialist, with an age range between 18 and 75 years, had no/minimal cognitive disturbances, and a disease duration <4 years. Patients with iRBD met the international diagnostic criteria for RBD and had no parkinsonism or cognitive disturbances, nor did they take any drug that could increase muscle tone during REM sleep.18, 19 We confirmed RBD using video polysomnography in all cases, following the International Classification of Sleep Disorders‐3 criteria, by sleep neurologists (I.A. and S.L.S.). The isolated character of RBD was ascertained in absence of the MDS criteria for PD, multiple system atrophy and DLB, by three movement disorders specialists (M.V., G.M., and J.C.C.). HVs had no history of neurological or psychiatric disorders. The local ethics committee approved this study and all subjects provided written informed consent (IRB of Paris VI, RCB 2014‐A00725‐42).
Subjects were scanned at 3 Tesla (PRISMA, Siemens, Germany) using a 64‐channel head coil. The MRI protocol included whole brain three‐dimensional (3D) T1‐weighted (T1‐w) imaging (sagittal MP2RAGE with a 1‐mm isotropic resolution) and two‐dimensional (2D) T1‐w neuromelanin‐sensitive imaging (axial turbo spin echo [TSE] with pulse repetition time [TR]/echo time [TE]/flip angle: 890 ms/13 ms/180°, 3 averages, voxel size: 0.4 × 0.4 × 3 mm3).
Image analysis was performed in MATLAB (vR2017b; The MathWorks, Natick, MA, USA) using FSL (FMRIB v5.0; Oxford, UK) and Statistical Parametric Mapping (SPM12; London, UK). Image preprocessing and post‐processing were performed using MRtrix (v3.0.1).
For manual segmentation, SNc and background contours were delineated using FreeSurfer viewer (v5.3.0; MGH, USA) on the neuromelanin‐sensitive images by two independent trained raters blind to the subject's clinical status as described previously (Supplementary Fig. S1). 2
For automatic segmentation, a deep learning pipeline was implemented in Python 3.6.0 (Data S1). From the 236 manually segmented images, a random dataset of 60 images was randomly split into 54 principal training images and six external validation images. We ran the U‐net model to predict the SNc ROI fully automatically on the remaining 176 external subjects that were excluded during the training phase.
We calculated SNc volumes (Vol), corrected volume (Cvol = Vol/total intracranial volume) to normalize for the subject head size, signal‐to‐noise ratio (SNR) and contrast‐to‐noise ratio (CNR) by normalizing the mean signal in SNc relative to the background signal, as described in previous studies (Data S1).2, 20 Statistical analyses were performed using R (R Core Team 2019, v3.6.1) and MATLAB. The between‐group comparisons of demographic and clinical variables were performed using the parametric Student's t test. Sex proportions were assessed using the χ2 test. Between‐group differences in Vol, Cvol, SNR, and CNR were evaluated using one‐way general linear model (GLM) analysis of covariance (ANCOVA) by keeping group (iRBD, PD, and HV) as the only between‐group factor while treating both sex and age as covariates and also by using post hoc t tests. Receiver operating characteristic (ROC) analysis was performed to obtain a diagnostic value. Dice similarity co‐efficient and intraclass correlation coefficient (ICC) was computed to evaluate the overall segmentation performance by comparing the automatic and manual segmentations.
We calculated the Pearson's correlation coefficients between the SNc measurements and both age and clinical scores. An approximate multivariate permutation test was performed to adjust for multiple comparisons. 21
Results
We analyzed 236 participants comprising 47 subjects with iRBD, 134 with PD, and 55 HVs. Patients with iRBD were older than HVs (P < 0.001) and there was no significant difference in age between PD and HVs. There was a larger proportion of males among iRBD and PD patients than in HVs (χ2 = 16.484, P < 0.001). Using both methods, we observed a significant sex effect in Cvol between the three groups and in CNR between iRBD and PD groups. All three groups had significantly larger Cvol in females than males (F value = 23.40, P value<0.001). All SNc measurements (Vol, Cvol, SNR, and CNR) differed significantly between the three groups (Table 1). Furthermore, all SNc measurements were significantly lower in PD than in both iRBD and HVs and in iRBD than in HVs except for SNR that did not differ between iRBD and HVs (Fig. 1, Table 1).
TABLE 1.
Demographic, clinical characteristics and SNc measurements
| Demographics | HVs | iRBD | PD | t test a (P value) | ||
|---|---|---|---|---|---|---|
| HVs vs. PD | HVs vs. iRBD | iRBD vs. PD | ||||
| No. of subjects | 55 | 47 | 134 | |||
| Age, y | 61.4 ± 8.8 | 67.7 ± 5.0 b , c | 61.6 ± 9.4 | 0.90 | <0.001 | <0.001 |
| Male/Female | 27/28 | 41/6 b , c | 84/50 | 0.84 | <0.001 | 0.001 |
| Clinical characteristics | ||||||
| MMSE | 29.4 ± 0.8 | 29.0 ± 1.0 d | 29.0 ± 1.2 e | 0.01 | 0.01 | 0.93 |
| MDS‐UPDRS I | 5.1 ± 3.4 | 8.9 ± 4.9 b | 9.4 ± 3.9 f | <0.001 | <0.001 | 0.53 |
| MDS‐UPDRS II | 1.2 ± 1.8 | 2.3 ± 2.2 b , c | 8.1 ± 3.8 e | <0.001 | 0.00 | <0.001 |
| MDS‐UPDRS III off | 5.7 ± 5.4 | 11.7 ± 6.5 b , c | 29.5 ± 7.9 f | <0.001 | <0.001 | <0.001 |
| MDS‐UPDRS IV | NA | NA | 0.1 ± 0.7 | NA | NA | NA |
| Hoehn and Yahr score | 0.1 ± 0.5 | 0.7 ± 0.9 b , c | 2.0 ± 0.3 f | <0.001 | <0.001 | <0.001 |
| GLM‐ANCOVA: group factor | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SNc imaging measurements | HVs vs. PD vs. iRBD | HVs vs. PD | HVs vs. iRBD | iRBD vs. PD | |||||||
| Automated | F value | P value | F value | P value | F value | P value | F value | P value | |||
| Volume (mm3) | 297.1 ± 55.1 | 240.3 ± 47.5 b , g | 218.8 ± 51.0 f | 44.32 | <0.001 | 85.39 | <0.001 | 29.85 | <0.001 | 6.18 | 0.01 |
| Corrected volume (Cvol) | 0.21 ± 0.04 | 0.16 ± 0.04 b , g | 0.15 ± 0.04 f | 51.00 | <0.001 | 99.29 | <0.001 | 31.47 | <0.001 | 8.16 | 0.00 |
| SNR | 110.1 ± 1.5 | 109.7 ± 1.9 c | 108.4 ± 1.5 f | 25.70 | <0.001 | 47.27 | <0.001 | 1.79 | 0.18 | 20.40 | <0.001 |
| CNR | 1.58 ± 0.27 | 1.45 ± 0.34 d , g | 1.30 ± 0.30 f | 18.34 | <0.001 | 37.74 | <0.001 | 4.80 | 0.03 | 8.28 | 0.00 |
| Manual | |||||||||||
| Volume (mm3) | 261.8 ± 41.3 | 229.5 ± 26.3 b | 217.9 ± 56.3 f | 16.06 | <0.001 | 27.50 | <0.001 | 21.41 | <0.001 | 1.82 | 0.17 |
| Corrected volume (Cvol) | 0.18 ± 0.03 | 0.16 ± 0.02 b | 0.14 ± 0.04 f | 21.74 | <0.001 | 38.31 | <0.001 | 20.61 | <0.001 | 3.37 | 0.07 |
| SNR | 111.1 ± 1.5 | 110.7 ± 1.7 c | 109.4 ± 1.6 f | 25.75 | <0.001 | 43.39 | <0.001 | 1.68 | 0.20 | 21.38 | <0.001 |
| CNR | 1.74 ± 0.28 | 1.60 ± 0.33 d , g | 1.46 ± 0.34 f | 16.05 | <0.001 | 30.94 | <0.001 | 5.20 | 0.02 | 6.76 | 0.01 |
Demographical and clinical characteristics were compared using t‐tests. Significant differences are considered at P value <0.05.Trends are indicated in italics. Data represented as mean ± standard deviation.
Sex was compared using χ2.
For P value <0.001.
For P value <0.001.
Indicates significant difference between HVs and iRBD with P value <0.01.
Indicates significant difference between HVs and PD with P value <0.01.
For P value <0.001.
Indicates significant difference between iRBD and PD with P value <0.0.
Abbreviations: SNc, substantia nigra pars compacta; HVs, healthy volunteers; iRBD, isolated REM sleep behavior disorder; PD, Parkinson's disease; MMSE, Mini‐Mental State Examination; SNR, signal‐to‐noise ratio; CNR, contrast‐to‐noise ratio.
FIG 1.
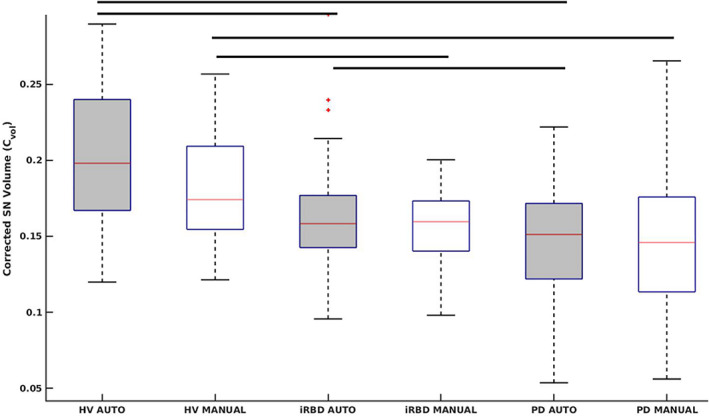
Box plot of corrected substantia nigra pars compacta (SNc) volume (Cvol) between isolated REM sleep behavior disorder (iRBD), Parkinson's disease (PD), and healthy volunteers (HVs) using both automatic and manual segmentation methods. [Color figure can be viewed at wileyonlinelibrary.com]
The ROC analyses provided areas under the curve of 0.78 for Vol, 0.79 for Cvol, 0.56 for SNR, and 0.63 for CNR between iRBD and HVs and 0.83 for Vol, 0.85 for Cvol, 0.79 for SNR, and 0.77 for CNR between PD and HVs (Supplementary Table S1).
The same between‐group differences were obtained using the manual method. The ROC analysis results were also similar (Supplementary Table S1).
Correlation study for automated method showed the following results. Age did not correlate with any SNc measurements in any of the three groups. In the iRBD group, there were significant positive correlations between Vol and Cvol and Movement Disorder Society‐Unified Parkinson's Disease Rating Scale (MDS‐UPDRS) I and II scores and a trend for a positive correlation between Cvol and MDS‐UPDRS III off. In PD, there were significantly negative correlations between Cvol and MDS‐UPDRS III off score and a trend for Vol (Supplementary Table S2). Correlations for manual measurements are presented in Supplementary Table S2.
There was a high overall reproducibility between all automatic and manual segmentations (n = 236, Dice, 0.80; ICC for SNR, 0.75; ICC for CNR, 0.82). Similarly, high between automatic and manual segmentations used in training (Dice, 0.80), and also between the measurements performed by the two manual raters (Dice inter‐observer, 0.82, Dice intra‐observer, 0.85).
Discussion
Using a robust fully automated ConvNet‐based SNc segmentation method; this study confirmed that iRBD presented reduced neuromelanin content and SNc volume in a large number of subjects. The automated method demonstrated significant differences between both iRBD and PD and between iRBD and HVs separately for all SNc measurements. Volumes and signal changes were at an intermediate level between values in HVs and PD patients. All female groups had larger Cvol similar to previous HVs 22 and PD 20 studies.
Neuromelanin‐based SNc volume in iRBD decreased by 19.1% and SNR by 4.0% as compared to HVs. Changes were milder than in previous study possibly because of the differences in scanners, examiners, and subject characteristics (examinations performed by different neurologists using different clinical scales: UPDRS vs. MDS‐UPDRS). 2 Changes were also milder than in the PD group, in which Vol decreased by 26.3% and SNR by 16.7%, in line with previous studies in de novo PD patients.23, 24, 25, 26
SN changes observed using the automatic method were comparable to the established ground truth that was the manual method. Our model needed a small training dataset. Furthermore, the untrained testing dataset of 176 subjects was more than three times the size of the training dataset. This demonstrated the robustness of our model that it has the potential to segment untrained external neuromelanin data from other scanners as well. Recently, various artificial intelligence (AI) models based on deep learning segmentation techniques have made tremendous progress in the field of quantitative neuroimaging analysis.17, 27, 28, 29 Despite this breakthrough, the potential has been limited because the medical datasets were relatively small and training any AI model is a difficult task with a relatively small size of datasets.27, 28, 29 Here, the Dice coefficient between the automatic and manual method was high, similar to previous studies. 28 We also obtained similar SNc volume decrease between HVs and PD of 26.4% in line with a previous study using U‐net model. 29
There was no correlation between SNc neuromelanin measures in the HVs or iRBD group and age using either of the segmentation methods, in line with previous studies demonstrating a plateau of neuromelanin in midlife from the 5th to 6th decades.22, 30 In the iRBD group, MDS‐UPDRS I and II scores correlated positively with Vol and Cvol and a trend was seen between Cvol and MDS‐UPDRS III off scores. These correlations were unexpected given the negative correlations found in PD and hence, should be interpreted with caution because of the limited number of patients. These different correlations may be explained by a hypothesis derived from animal model of PD. 30 According to this hypothesis, neuromelanin would first accumulate in SN neurons (explaining the positive correlation). Beyond a certain threshold, this accumulation would compromise neuronal function and trigger PD‐type pathology (explaining the negative correlation).
In PD, SNc volume demonstrated a trend for Vol and significantly negative correlation with MDS‐UPDRS III scores in line with previous studies.31, 32, 33, 34, 35, 36 Many of the correlations were found using both methods (eg, MDS‐UPDRS I with Cvol in iRBD, trend for MDS‐UPDRS on with Vol in PD), but not all (eg, SN measurements and age in PD in the manual method as in our previous study, 20 but not in the automated method). Therefore, although overall results were broadly similar between the two methods, there were some differences that could be because of the image resampling procedure or the greater SNc segmentation provided by the automatic method.
The study had several limitations and can be improved in several ways. First, it needs to be further validated using external cohorts scanned with different scanners, which could help us understand the scanner effect using this model. Although such automatic methods have the potential advantages of saving time and being more reproducible, yet manual segmentation allows careful image quality control and removal of areas containing artifacts (eg, caused by blood flow or head motion). As a result, experienced raters can deliver highly reproducible segmentations.37, 38 Second, the implementation of 3D acquisitions may possibly increase the accuracy of the results 39 by enabling isotropic voxel acquisitions, reducing partial voluming and eliminating or reducing artifacts (eg, caused by cross‐talk between slices in 2D sequences). Moreover, more clinical assessments are warranted to understand the clinical significance of SNc neuromelanin loss in iRBD.
Patients with iRBD showed a decrease in SNc volume and signal intensity, and our results confirmed that neuromelanin MRI signal is an early marker of SNc neurodegeneration in parkinsonism. Nonetheless, comparative MRI, histological and molecular studies are needed to better understand the basis of neuromelanin‐based MRI signal changes in iRBD.
In summary, our proposed fully automated ConvNet segmentation method showed comparable performance with the manual method, was faster and user‐independent. Therefore, it could enable reproducible and fast segmentation of the SNc in large patient cohorts, for instance, in clinical trials. Several questions still remain unanswered, in particular whether neuromelanin imaging could serve as a predictor of conversion in these patients or to estimate the time before the appearance of clinical motor signs and the evolution of neuromelanin changes in relation to the striatal dopaminergic function.
Author Roles
(1) Research project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript: A. Writing of the first draft, B. Review and Critique
RG: 1A; 1B; 1C; 2A; 2B; 3A
NP, EB, RV, LYC, GM,SLS, JCC, MV, IA: 2C; 3B
SL: 1A; 2C; 3A; 3B
Financial Disclosures
Rahul Gaurav and Stéphane Lehéricy received grant funding from Biogen Inc. USA Emma Biondetti received grant funding from France Parkinson and Biogen Inc. USA Romain Valabrègue, Nadya Pyatigorskaya, Graziella Mangone, Lydia Yahia‐Cherif, Smaranda Leu‐Semenescu and Marie Vidailhet have nothing to report. Jean‐Christophe Corvol has served in advisory boards for Air Liquide, Biogen Inc., Denali, Ever Pharma, Idorsia, Prevail Therapeutic, Theranexus, UCB; has received grants from Sanofi and the Michael J Fox Foundation. Isabelle Arnulf received honoraria from Idorsia Pharma, unrelated to this study.
Supporting information
Data S1 Automatic and manual segmentation procedure
Figure S1 SNc regions of interest demonstrating an iRBD patient, a PD patient and a healthy volunteer using both automatic and manual segmentation
Table S1 Diagnostic performance of SNc measurements
Table S2 Correlations of SNc measurements with age along with clinical scores
Acknowledgments
The authors would like to thank Energipole (M. Mallart), M. Villain and Société Française de Médecine Esthétique (M. Legrand) for unrestricted support for Research on Parkinson's disease. We would also like to thank all of the participants involved in the study, who have helped to make this research possible.
Relevant conflicts of interest/financial disclosures: R.G. and S.L. received grant funding from Biogen. E.B. received grant funding from France Parkinson and Biogen. R.V., N.P., G.M., L.Y.C., S.L.S., and M.V. have nothing to report. J.C.C. has served in advisory boards for Air Liquide, Biogen., Denali, Ever Pharma, Idorsia, Prevail Therapeutic, Theranexus, and UCB; has received grants from Sanofi and The Michael J. Fox Foundation. I.A. received honoraria from Idorsia Pharma unrelated to this study.
Funding agencies: This study was funded by grants from the Investissements d'Avenir, IAIHU‐06 (Paris Institute of Neurosciences, IHU), ANR‐11‐INBS‐0006, ERA PerMed EU‐wide project DIGIPD (01KU2110), Fondation d'Entreprise EDF, Biogen, Fondation Thérèse and René Planiol, Fondation Saint Michel, unrestricted support for research on Parkinson's disease from Energipole (M. Mallart), M. Villain, and Société Française de Médecine Esthétique (M. Legrand). This work was supported by grants from DHOS‐Inserm, France Parkinson, Ecole des NeuroSciences de Paris (ENP), and Fondation pour la Recherche Médicale (FRM).
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. Iranzo A, Fernández‐Arcos A, Tolosa E, et al. Neurodegenerative disorder risk in idiopathic REM sleep behavior disorder: study in 174 patients. PLoS One 2014;9(2):e89741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pyatigorskaya N, Gaurav R, Arnaldi D, et al. Magnetic resonance imaging biomarkers to assess substantia Nigra damage in idiopathic rapid eye movement sleep behavior disorder. Sleep 2017;40(11):1–8. [DOI] [PubMed] [Google Scholar]
- 3. Poewe W, Seppi K, Tanner CM, et al. Parkinson disease. Nat Rev Dis Prim 2017;3:1–21. [DOI] [PubMed] [Google Scholar]
- 4. Kish SJ, Shannak K, Hornykiewicz O. Uneven pattern of dopamine loss in the striatum of patients with idiopathic Parkinson's disease. N Engl J Med 1988;318(14):876–880. [DOI] [PubMed] [Google Scholar]
- 5. Iranzo A, Lomeña F, Stockner H, et al. Decreased striatal dopamine transporter uptake and substantia nigra hyperechogenicity as risk markers of synucleinopathy in patients with idiopathic rapid‐eye‐movement sleep behaviour disorder: a prospective study [internet]. Lancet Neurol 2010;9(11):1070–1077. 10.1016/S1474-4422(10)70216-7 [DOI] [PubMed] [Google Scholar]
- 6. Barber TR, Griffanti L, Bradley KM, et al. Nigrosome 1 imaging in REM sleep behavior disorder and its association with dopaminergic decline. Ann Clin Transl Neurol 2020;7(1):26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. De Marzi R, Seppi K, Högl B, et al. Loss of dorsolateral nigral hyperintensity on 3.0 tesla susceptibility‐weighted imaging in idiopathic rapid eye movement sleep behavior disorder [internet]. Ann. Neurol 2016;79(6):1026–1030. [DOI] [PubMed] [Google Scholar]
- 8. Scherfler C, Frauscher B, Schocke M, et al. White and gray matter abnormalities in idiopathic rapid eye movement sleep behavior disorder: a diffusion‐tensor imaging and voxel‐based morphometry study. Ann Neurol 2011;69(2):400–407. [DOI] [PubMed] [Google Scholar]
- 9. Rolinski M, Griffanti L, Piccini P, et al. Basal ganglia dysfunction in idiopathic REM sleep behaviour disorder parallels that in early Parkinson's disease. Brain 2016;139(8):2224–2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Knudsen K, Fedorova TD, Hansen AK, et al. In‐vivo staging of pathology in REM sleep behaviour disorder: a multimodality imaging case‐control study [internet]. Lancet Neurol 2018;17(7):618–628. [DOI] [PubMed] [Google Scholar]
- 11. Sulzer D, Cassidy C, Horga G, et al. Neuromelanin detection by magnetic resonance imaging (MRI) and its promise as a biomarker for Parkinson's disease [internet]. NPJ Park Dis 2018;4(1):11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sasaki M, Shibata E, Tohyama K, et al. Neuromelanin magnetic resonance imaging of locus ceruleus and substantia nigra in Parkinson's disease. Neuroreport 2006;17(11):1215–1218. [DOI] [PubMed] [Google Scholar]
- 13. Schwarz ST, Rittman T, Gontu V, et al. T1‐weighted MRI shows stage‐dependent substantia nigra signal loss in Parkinson's disease. Mov Disord 2011;26(9):1633–1638. [DOI] [PubMed] [Google Scholar]
- 14. Castellanos G, Fernández‐Seara MA, Lorenzo‐Betancor O, et al. Automated Neuromelanin imaging as a diagnostic biomarker for Parkinson's disease [internet]. Mov. Disord 2015;30(7):945–952. [DOI] [PubMed] [Google Scholar]
- 15. Cassidy CM, Zucca FA, Girgis RR, et al. Neuromelanin‐sensitive MRI as a noninvasive proxy measure of dopamine function in the human brain [internet]. Proc Natl Acad Sci USA 2019;116(11):5108–5117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Biondetti E, Gaurav R, Yahia‐Cherif L, et al. Spatiotemporal changes in substantia nigra neuromelanin content in Parkinson's disease. Brain 2020;143(9):2757–2770. [DOI] [PubMed] [Google Scholar]
- 17. Ronneberger O, Fischer P, Brox T. U‐net: convolutional networks for biomedical image segmentation. Lect Notes Comput Sci 2015;9351:234–241. 10.1007/978-3-319-24574-4_28 [DOI] [Google Scholar]
- 18. American Academy of Sleep Medicine . The International Classification of Sleep Disorders:(ICSD‐3). American Academy of Sleep Medicine; 2014.
- 19. Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico‐pathological study of 100 cases. J Neurol Neurosurg Psychiatry 1992;55(3):181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gaurav R, Yahia‐Cherif L, Pyatigorskaya N, et al. Longitudinal changes in Neuromelanin MRI signal in Parkinson's disease. Mov Disord 2021;2:9–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp 2002;15(1):1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xing Y, Sapuan A, Dineen RA, Auer DP. Life span pigmentation changes of the substantia nigra detected by neuromelanin‐sensitive MRI. Mov Disord 2018;33(11):1792–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Reimão S, Pita Lobo P, Neutel D, et al. Substantia nigra neuromelanin‐MR imaging differentiates essential tremor from Parkinson's disease [internet]. Mov. Disord 2015;30(7):953–959. [DOI] [PubMed] [Google Scholar]
- 24. Reimão S, Ferreira S, Nunes RG, et al. Magnetic resonance correlation of iron content with neuromelanin in the substantia nigra of early‐stage Parkinson's disease. Eur J Neurol 2016;23(2):368–374. [DOI] [PubMed] [Google Scholar]
- 25. Wang J, Huang Z, Li Y, et al. Neuromelanin‐sensitive MRI of the substantia nigra: an imaging biomarker to differentiate essential tremor from tremor‐dominant Parkinson's disease. Parkinson's Relat Disord 2019;58:3–8. [DOI] [PubMed] [Google Scholar]
- 26. Wang J, Li Y, Huang Z, et al. Neuromelanin‐sensitive magnetic resonance imaging features of the substantia nigra and locus coeruleus in de novo Parkinson's disease and its phenotypes. Eur J Neurol 2018;25(7):949–955. [DOI] [PubMed] [Google Scholar]
- 27. Shinde S, Prasad S, Saboo Y, et al. Predictive markers for Parkinson's disease using deep neural nets on neuromelanin sensitive MRI [internet]. NeuroImage Clin 2019;22:101748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Le Berre A, Kamagata K, Otsuka Y, et al. Convolutional neural network‐based segmentation can help in assessing the substantia nigra in neuromelanin MRI. Neuroradiology 2019;61(12):1387–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Krupička R, Mareček S, Malá C, et al. Automatic substantia nigra segmentation in neuromelanin‐sensitive MRI by deep neural network in patients with prodromal and manifest synucleinopathy. Physiol Res 2019;68:S453–S458. [DOI] [PubMed] [Google Scholar]
- 30. Vila M. Neuromelanin, aging, and neuronal vulnerability in Parkinson's disease. Mov Disord 2019;34(10):1440–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Prasad S, Stezin A, Lenka A, et al. Three‐dimensional neuromelanin‐sensitive magnetic resonance imaging of the substantia nigra in Parkinson's disease. Eur J Neurol 2018;25(4):680–686. [DOI] [PubMed] [Google Scholar]
- 32. Isaias IU, Trujillo P, Summers P, et al. Neuromelanin imaging and dopaminergic loss in parkinson's disease. Front Aging Neurosci 2016;8:196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schwarz ST, Xing Y, Tomar P, et al. In vivo assessment of brainstem depigmentation in Parkinson disease: potential as a severity marker for multicenter studies. Radiology 2017;283(3):789–798. [DOI] [PubMed] [Google Scholar]
- 34. Kawaguchi H, Shimada H, Kodaka F, et al. Principal component analysis of multimodal neuromelanin MRI and dopamine transporter PET data provides a specific metric for the nigral dopaminergic neuronal density. PLoS One 2016;11(3):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Okuzumi A, Hatano T, Kamagata K, et al. Neuromelanin or DaT‐SPECT: which is the better marker for discriminating advanced Parkinson's disease? Eur J Neurol 2019;26(11):1408–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Taniguchi D, Hatano T, Kamagata K, et al. Neuromelanin imaging and midbrain volumetry in progressive supranuclear palsy and Parkinson's disease. Mov Disord 2018;33(9):1488–1492. [DOI] [PubMed] [Google Scholar]
- 37. Ohtsuka C, Sasaki M, Konno K, et al. Changes in substantia nigra and locus coeruleus in patients with early‐stage Parkinson's disease using neuromelanin‐sensitive MR imaging [internet]. Neurosci. Lett 2013;541:93–98. [DOI] [PubMed] [Google Scholar]
- 38. Pyatigorskaya N, Magnin B, Mongin M, et al. Comparative study of MRI biomarkers in the substantia nigra to discriminate idiopathic Parkinson disease. Am J Neuroradiol 2018;39(8):1460–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Oshima S, Fushimi Y, Okada T, et al. Neuromelanin‐sensitive magnetic resonance imaging using DANTE pulse. Mov Disord 2021;36(4):874–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1 Automatic and manual segmentation procedure
Figure S1 SNc regions of interest demonstrating an iRBD patient, a PD patient and a healthy volunteer using both automatic and manual segmentation
Table S1 Diagnostic performance of SNc measurements
Table S2 Correlations of SNc measurements with age along with clinical scores
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


