Abstract
Background
C-reactive protein (CRP) has shown promise as a triage tool for pulmonary tuberculosis (TB) in adults living with the human immunodeficiency virus. We performed the first assessment of CRP for TB triage in children.
Methods
Symptomatic children less than 15 years old were prospectively enrolled in Kampala, Uganda. We completed a standard TB evaluation and measured CRP using a point-of-care assay. We determined the sensitivity and specificity of CRP to identify pulmonary TB in children using 10 mg/L and 5 mg/L cut-off points and generated a receiver operating characteristic (ROC) curve to determine alternative cut-offs that could approach the target accuracy for a triage test (≥90% sensitivity and ≥70% specificity).
Results
We included 332 children (median age 3 years old, interquartile range [IQR]: 1–6). The median CRP level was low at 3.0 mg/L (IQR: 2.5–26.6) but was higher in children with Confirmed TB than in children with Unlikely TB (9.5 mg/L vs. 2.9 mg/L, P-value = .03). At a 10 mg/L cut-off, CRP sensitivity was 50.0% (95% confidence interval [CI], 37.0–63.0) among Confirmed TB cases and specificity was 63.3% (95% CI, 54.7–71.3) among children with Unlikely TB. Sensitivity increased to 56.5% (95% CI, 43.3–69.0) at the 5 mg/L cut-off, but specificity decreased to 54.0% (95% CI, 45.3–62.4). The area under the ROC curve was 0.59 (95% CI, 0.51–0.67), and the highest sensitivity achieved was 66.1% at a specificity of 46.8%.
Conclusions
CRP levels were low in children with pulmonary TB, and CRP was unable to achieve the accuracy targets for a TB triage test.
Keywords: C-reactive protein, child, tuberculosis, triage
In a prospective cohort of children with respiratory symptoms in Kampala, Uganda, C-reactive protein levels were low regardless of tuberculosis status and did not achieve the target accuracy for a triage test for pulmonary tuberculosis.
INTRODUCTION
Over half of the estimated tuberculosis (TB) cases in children are not reported to public health systems [1]. To reduce this large case detection gap, novel triage tools at the point-of-care are needed to improve screening and referral of children for TB diagnostic testing. Symptom-based screening is non-specific [2, 3], and chest X-ray (CXR) findings are highly variable in childhood TB [4, 5]. A rapid, biomarker-based triage test that can be performed in primary care clinics and as part of contact investigations is needed for children.
C-reactive Protein (CRP) is an acute-phase protein that is increased in adults with TB and correlates with other host gene expression signatures for TB diagnosis [6–9]. Among adults living with human immunodeficiency virus (HIV), multiple studies have found that CRP is elevated in TB and can approach the target product profile for a triage test (≥90% sensitivity and ≥70% specificity) [8, 10, 11]. Consequently, the World Health Organization (WHO) has endorsed CRP as a TB triage tool for adults living with HIV [12].
CRP levels are increased in infectious and inflammatory conditions in children and have been used for screening, severity staging, and treatment monitoring [13]. Children with bacterial pneumonia have elevated CRP, and several studies have found a moderate ability of CRP to distinguish bacterial from viral etiologies of lower respiratory disease [14–17]. Despite its frequent use in pediatric medicine, the role of CRP as a tool for identifying pulmonary TB is unclear. CRP has been shown to be elevated in children with TB compared to healthy controls [18–21], but older studies found only modest increases in CRP in childhood TB [22, 23].
Given the recent WHO recommendation and growing evidence of CRP’s role for TB triage in adults, we examined the performance as a triage test for pulmonary TB in children.
METHODS
Study Design and Participants
We conducted a prospective study among children in Kampala, Uganda between November 2018 and January 2021. We approached consecutive parents or guardians of children who presented for care at Mulago National Referral Hospital, as well as health centers and hospitals affiliated with the Kampala Capital City Authority and the Infectious Diseases Institute. Children (<15 years) were eligible for enrollment if they reported having a cough for at least 1 week and at least two of the following TB risk factors: (1) unexplained weight loss or failure to thrive; (2) unexplained fever for at least 1 week; (3) unexplained lethargy or reduced playfulness for at least 1 week; (4) an abnormal CXR; or (5) contact with an individual diagnosed with pulmonary TB. Children referred to the study with a positive Xpert MTB/RIF or Ultra results were also eligible for enrollment. We excluded children if they were initiated on anti-TB treatment or prophylaxis in the last 12 months, or were receiving antibiotics that had anti-TB activity such as fluoroquinolones. In addition, we excluded children from the analysis if unable to obtain a respiratory specimen and did not have a previous positive Xpert MTB/RIF or Ultra result, or did not undergo CRP testing. We obtained parental consent for all children enrolled in the study, as well as assent for participants 8 years and older. The study was approved by the University of California San Francisco Committee on Human Research, the Mulago Hospital Research and Ethics Committee, and the Uganda National Council of Science and Technology.
Clinical Procedures
We administered a demographic and clinical questionnaire and performed a standard TB evaluation, which included a physical exam, HIV testing, tuberculin skin testing (TST), CXR, and microbiological TB testing (for children without a microbiologically confirmed TB result). We obtained up to two respiratory specimens for Xpert MTB/RIF Ultra (Cepheid, Sunnyvale, USA), smear microscopy, and mycobacterial culture. If sputum expectoration was not possible, we performed sputum induction, gastric aspiration, and/or nasopharyngeal aspiration. We measured the concentration of CRP using ichroma II (Boditech, South Korea), a United States Food and Drug Administration-approved point-of-care diagnostic device that has been used in adult TB triage studies [24]. The test’s detection range is 2.5–300 mg/L. Following the manufacturer’s instructions, we used a lancet to prick the participant’s finger and collect approximately 10 μL blood into the sample collector, which was then dispensed into the detection buffer tube. After inverting the sample mixture ten times, we discarded the first two drops on a paper towel and added the next two drops to the sample well on the test cartridge. We inserted the test cartridge into the ichroma II reader and recorded the displayed value in mg/L after 3 min. CRP testing was performed during the initial evaluation before microbiological testing was performed and before TB status was known for most participants.
Decisions regarding initiation of anti-TB treatment were made by TB clinic staff in accordance with Uganda national guidelines [25]. All children were asked to return for a follow-up visit 60 days after enrollment, during which a clinical examination and CXR were performed. All children initiated on anti-TB treatment were asked to return 180 days after treatment initiation to evaluate any clinical response.
TB Testing Procedures
We performed Xpert MTB/RIF Ultra, solid culture (Lowenstein-Jensen [LJ]), and liquid culture (Mycobacterial Growth Indicator Tube [MGIT]) for our reference standard tests. All tests were performed at the Mulago National Referral Hospital TB Laboratory and the Makerere University Mycobacteriology Laboratory in accordance with standard protocols. For Xpert Ultra, we added sample reagent to the respiratory specimen in a 2:1 ratio and added 2 mL of the mixture to the Xpert MTB/RIF Ultra cartridge for testing. For culture, the respiratory specimen was digested and decontaminated with sodium hydroxide and N-acetyl-cysteine, neutralized using a sterile phosphate-buffered solution, centrifuged and resuspended in a sterile phosphate-buffered solution. One LJ slant and one MGIT tube were inoculated in 0.5mL of the resulting decontaminated sample and the remaining sample was used to perform AFB smear microscopy. MGIT tubes were incubated in a BACTEC MGIT 960 instrument (BD, New Jersey, USA) for up to 6 weeks and LJ slants were incubated at 37°C for up to 8 weeks. The presence of Mycobacterium tuberculosis was confirmed with SD Bioline strips (SD MPT64 TB Ag kit, South Korea).
Definitions
We defined a positive TST result as an induration 48–72 h after placement of ≥10 mm in children without HIV and ≥5mm in children with HIV. Underweight was defined as a weight-for-age z-score of < −2 (under 5 years) or a body mass index of <18.5 kg/m2 (5 years and older).
Two clinicians read CXRs and assigned TB classification, with a third clinician as the tiebreaker for discrepancies. CXRs were classified as Normal, Abnormal and Likely TB, or Abnormal and Equivocal using a standardized form [26, 27]. Participants with Confirmed TB either had a positive molecular result or a positive culture result. The remaining participants were classified as Unconfirmed TB or Unlikely TB per the National Institutes of Health consensus definitions [28]. Participants with Unconfirmed TB had respiratory symptoms consistent with TB and other evidence supporting a TB diagnosis, such as a TB contact, CXR consistent with TB, or improvement within 2 months of treatment initiation. Participants with Unlikely TB had respiratory symptoms, but did not have additional evidence of TB disease and had improved TB signs and symptoms at follow-up without TB treatment.
Reference Standards
We used three reference standards for our analyses. We first assessed the diagnostic accuracy of CRP among children with Confirmed TB versus children with Unlikely TB. We then included children with Unconfirmed TB and classified them as either having TB (composite reference standard [CRS]) or not having TB (microbiological reference standard [MRS]).
Statistical Analysis
We summarized demographic and clinical characteristics using descriptive statistics. We used two CRP level cut-offs (10 mg/L and 5 mg/L), as recommended by the WHO [12]. For each cut-off, we calculated the sensitivity and specificity of CRP with 95% confidence intervals (CIs) against each reference standard. We then performed receiving operating characteristic (ROC) analysis to identify the optimal cut-off point(s), guided by the WHO target profile for a TB triage test (≥90% sensitivity and ≥70% specificity) [29]. We further analyzed the sensitivity and specificity of CRP using these cut-off points overall and among key clinical subgroups. Statistical significance was defined as a P-value < .05. All analyses were performed using Stata version 16.1 (StataCorp, College Station, TX, USA) and R version 3.6.3 (https://www.R-project.org/).
RESULTS
Sample Characteristics
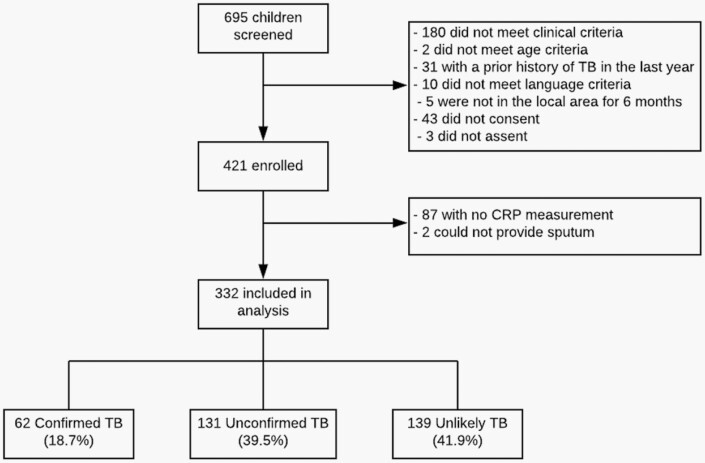
Of the 695 patients screened between November 2018 and January 2021, 421 were eligible and enrolled in the study (Figure 1). We excluded 89 patients from the analysis because a CRP measurement was not available (n = 87), or respiratory specimen was not collected and a previous positive Xpert or Ultra result was not available (n = 2). Of the 332 patients included in the analysis, 62 had Confirmed TB, 131 had Unconfirmed TB, and 139 had Unlikely TB. Sample characteristics are summarized in Table 1. The median age was 3 years (interquartile range [IQR]: 1–6 years), approximately half were male (50.3%, n = 167), and 11.3% were living with HIV (n = 37).
Figure 1.
Participant flowchart.
Table 1.
Participant Characteristics (n = 332)
| Characteristic | n (%) or median (IQR)a |
|---|---|
| Age (years) | 3 (1–6) |
| Age (category) | |
| <5 years | 211 (63.6%) |
| 5–10 years | 101 (30.4%) |
| 11–14 years | 20 (6.0%) |
| HIV-positive | 37/329 (11.3%) |
| CD4 cell count (cells/µL) (n = 27) | 715 (336–1462) |
| Inpatient | 67 (20.2%) |
| Weight loss | 206 (62.1%) |
| Underweight | 179/330 (54.2%) |
| Fever (≥38.3°C) | 19 (5.7%) |
| Abnormal chest X-ray | 132/301 (43.9%) |
| TST positive | 163/310 (52.6%) |
| Xpert or Xpert Ultra positive | 53 (16.0%) |
| Mycobacterial culture positive | 32/329 (9.7%) |
| AFB smear microscopy positive | 18/325 (5.5%) |
| CRP | 3.0 mg/L (2.5–26.6) |
Abbreviations: AFB, acid fast bacilli; CRP, C-reactive protein; HIV, human immunodeficiency virus; IQR, interquartile range.
N=332 unless otherwise indicated.
CRP Measurements in Children With and Without Pulmonary TB
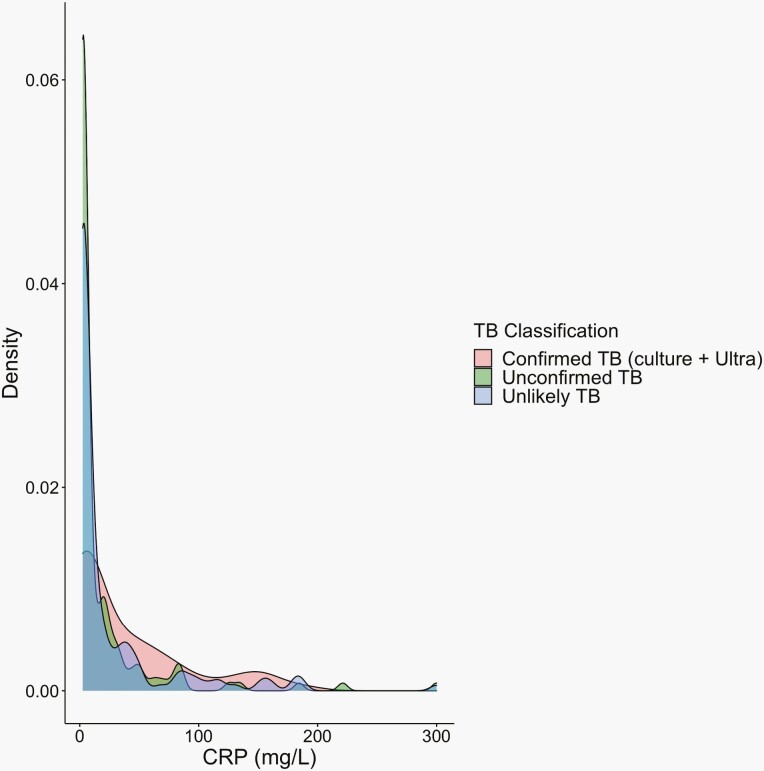
The median CRP level was 3.0 mg/L (IQR: 2.5–26.6) and ranged from 2.5 mg/L (lower limit of detection) to 300 mg/L (upper limit of detection). As shown in Figure 2, the distribution of CRP was low regardless of TB classification. However, there was a right skew among children with Confirmed TB (9.5 mg/L [IQR: 2.5–62.3]) with higher median CRP levels compared to children with Unlikely TB (2.9 mg/L [IQR: 2.5–25.9], P-value = .03) and Unconfirmed TB (2.5 mg/L [IQR: 2.5-18.6], P-value = .003). When stratified by subgroup, median CRP was higher in children who were 5 years and older, underweight, HIV-negative, TST positive, and outpatients (Supplementary Figure 1).
Figure 2.
Distribution of C-reactive protein levels by pediatric TB classification. Density plot of each C-reactive protein (CRP) level plotted by TB status of Confirmed, Unconfirmed, or Unlikely TB.
CRP Performance
Using the 10 mg/L cut-off, the sensitivity of CRP was 50.0% (95% CI: 37.0–63.0) among children with Confirmed TB and specificity was 63.3% (95% CI, 54.7–71.3) among children with Unlikely TB (Table 2). The positive predictive value (PPV) was 37.8% (95% CI, 27.3–49.2) and the negative predictive value (NPV) was 73.9% (95% CI: 65.1–81.6). The sensitivity decreased in the CRS, while specificity was similar for the MRS (Table 2). There was no subgroup that reached the 90% sensitivity threshold, but sensitivity was higher in children who were underweight and living with HIV (Supplementary Table 1).
Table 2.
Diagnostic Accuracy of C-Reactive Protein for Childhood TB
| Cut-off | Sensitivity n/N % (95% CI) |
Specificity n/N % (95% CI) |
|---|---|---|
| Confirmed TB versus Unlikely TB | ||
| 10 mg/L | 31/62 50.0% (37.0–63.0) |
88/139 63.3% (54.7-71.3) |
| 5 mg/L | 35/62 56.5% (43.3–69.0) |
75/139 54.0% (45.3–62.4) |
| CRS | ||
| 10 mg/L | 73/193 37.8% (31.0–45.1) |
88/139 63.3% (54.7–71.3) |
| 5 mg/L | 87/193 45.1% (37.9–52.4) |
75/139 54.0% (45.3–62.4) |
| MRS | ||
| 10 mg/L | 31/62 50.0% (37.0–63.0) |
177/270 65.6% (59.6–71.2) |
| 5 mg/L | 35/62 56.5% (43.3–69.0) |
154/270 57.0% (50.9–63.0) |
Abbreviations: CRS, composite reference standard; MRS, microbiological reference standard; TB, tuberculosis.
Lowering the threshold to 5 mg/L cut-off did not significantly improve performance, with a sensitivity of 56.5% (95% CI, 43.3–69.0) for Confirmed TB and specificity of 54.0% (95% CI: 45.3–62.4) for Unlikely TB (Table 2). The PPV and NPV were similar to the 10 mg/L cut-off, at 35.4% (95% CI: 26.0–45.6) and 73.5% (95% CI: 63.9–81.8), respectively. At this cut-off, none of the evaluated subgroups achieved the target sensitivity or specificity thresholds (Supplementary Table 2).
CRP Performance Using the Optimal Cut-Off Points
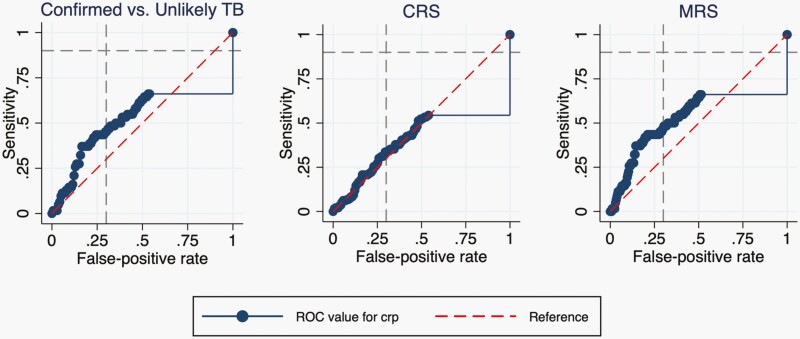
To determine the optimal cut-off point, we created a ROC curve for each standard as shown in Figure 3. The AUC was 0.59 (95% CI, 0.51-0.67) for children with Confirmed versus Unlikely TB and was similar for the MRS and CRS (Table 3). There was no cut-off point that could achieve the target accuracy of at least 90% sensitivity and 70% specificity. When specificity was fixed at 70%, we achieved a sensitivity of 45.2%, 33.7%, and 46.8% for the Confirmed TB versus Unlikely TB, CRS, and MRS reference standards, respectively.
Figure 3.
Receiver operating characteristic (ROC) curves for C-reactive protein to diagnose childhood TB. ROC Curves for three reference standards: Confirmed TB versus Unlikely TB, composite reference standard (CRS), and microbiological reference standard (MRS). The gray-dashed lines indicate the target accuracy for a triage test for TB (≥90% sensitivity and ≥70% specificity), and the red-dashed line is the reference line with an area under the curve (AUC) of 0.5.
Table 3.
Area Under the ROC Curve for C-Reactive Protein Levels to Detect Childhood TB
| AUC (95% CI) | Cut-off (mg/L)a | Sensitivity % (95% CI) | Specificity % (95% CI) | |
|---|---|---|---|---|
| Confirmed versus Unlikely TB | 0.59 (0.51–0.67) | 2.66 | 66.1% (53.0–77.7) | 46.8% (38.3–55.4) |
| CRS | 0.51 (0.45–0.57) | 2.56 | 54.4% (47.1–61.6) | 46.0% (37.6–54.7) |
| MRS | 0.61 (0.53–0.69) | 2.66 | 66.1% (53.0–77.7) | 49.3% (43.1–55.4) |
Abbreviations: AUC, area under the ROC; CRS, composite reference standard; MRS, microbiological reference standard; ROC, receiver operating characteristic curve ;TB, tuberculosis.
Cut-off based on maximizing sensitivity for a TB triage test.
DISCUSSION
While CRP has shown promise for TB triage among adults living with HIV [8], we found that CRP levels had modest performance to discriminate TB disease from other respiratory diseases in children, and did not achieve the goal of 90% sensitivity for a TB triage test for any specified cut-off point or reference standard. Our findings demonstrate that CRP has a limited role in TB triage in children.
CRP levels were notably low in children with Confirmed TB. The median CRP level was only 9.5 mg/L in children with Confirmed TB, as compared to 15–83 mg/L in adult TB patients with and without HIV [11, 30–33]. However, this is consistent with the limited pediatric TB data available, ranging from 0.03 to 4.8 mg/L [19, 22, 23]. The lower CRP level in children with TB may be related to having primary TB as opposed to postprimary disease commonly seen in adults. One study in South Africa compared CRP levels among children with primary TB, adolescents and adults with postprimary TB without lung destruction, and adults with postprimary TB with lung destruction [23]. CRP was similarly low in the children with primary TB (median 6.5 mg/L), and increased with greater parenchymal involvement (median 105 mg/L with lung destruction). An early study in the United States also found that the majority of children with primary TB had negative CRP testing [22]. The few studies that have shown elevated CRP in childhood TB included predominantly older children that are more likely to have postprimary TB [18, 20]. In primary TB, M. tuberculosis spreads to the lymphatics and is less likely to cause significant lung injury and cavitation [5], resulting in a less robust acute phase response. It is also important to recognize that the immune response to TB is different for children than adults [34, 35], which may influence CRP levels. In the study, lower inflammatory responses may have occurred if participants were previously receiving antibiotics with anti-mycobacterial activity, but we excluded children who were on antibiotics that could have an anti-TB effect. While further work is needed to elucidate the exact mechanism, our results support that CRP is low in childhood pulmonary TB.
Although overall levels were low, median CRP was higher among children with Confirmed TB compared to Unlikely TB, suggesting possible utility at a lower cut-off value. However, the performance was modest and could not achieve the target accuracy for a TB triage test at either the 10 mg/L or 5 mg/L threshold. In the ROC analysis, the optimal cut-off was 2.56–2.66 mg/L; this was slightly higher than the lower limit of detection of the assay (2.5 mg/L) and still could not achieve sufficient sensitivity or specificity. CRP had higher accuracy among children who were underweight, outpatients, and older, but still did not meet target accuracy thresholds in these sub-groups. Based on these findings, CRP should not be used alone to guide further assessment and management of pulmonary TB in children.
Our evaluation of CRP was conducted in a large prospective sample of symptomatic children from community and tertiary settings with well-defined TB status. However, there were limitations. We examined CRP as a triage tool, and further evaluation is needed to examine if CRP can be applied in a screening context, such as for child contacts of adults with TB. Our assessment was focused on pulmonary TB, and are unable to comment on the role of CRP for extrapulmonary disease. The study occurred in urban Kampala and may not be generalizable to rural areas, though the low level of CRP in Confirmed childhood TB would also suggest limited utility in that setting. Lastly, we had few adolescents in our cohort, and so additional data is needed to assess the role of CRP in this group as they are more likely to have adult-type disease.
In conclusion, CRP levels were low in children with pulmonary TB, and are unlikely to be useful alone as a TB triage test. These findings have important implications for the translation of host-response biomarkers for children that were initially identified in adults living with HIV. Given the different pathogenesis and immune response of TB in young children [34], our data suggest that it is important to consider host biomarkers that are unique to primary and childhood TB.
Supplementary Data
Supplementary materials are available at Journal of the Pediatric Infectious Diseases Society online (http://jpids.oxfordjournals.org).
Notes
Acknowledgments. We thank the patients, families and staff at Mulago National Referral Hospital, Kampala Capital City Authority Clinics, and Infectious Diseases Institute (IDI) clinics.
Financial support. This work was supported by grants from the National Heart, Lung, and Blood Institute (grant number R01HL139717 to A.C. and K23HL153581 to D.J.) and the National Institute of Child Health and Human Development (grant number K12HD000850 to D.J.).
Potential conflicts of interest. All authors: No reported conflicts.
Contributor Information
Devan Jaganath, Division of Pediatric Infectious Diseases, University of California, San Francisco, San Francisco, California, USA; Division of Pulmonary and Critical Care Medicine, University of California, San Francisco, San Francisco, California, USA; Center for Tuberculosis, University of California, San Francisco, San Francisco, California, USA.
Tania F Reza, Division of Pulmonary and Critical Care Medicine, University of California, San Francisco, San Francisco, California, USA; Center for Tuberculosis, University of California, San Francisco, San Francisco, California, USA.
Peter Wambi, Mulago National Referral Hospital, Kampala, Uganda.
Jascent Nakafeero, Mulago National Referral Hospital, Kampala, Uganda.
Emma Kiconco, Mulago National Referral Hospital, Kampala, Uganda.
Gertrude Nanyonga, Mulago National Referral Hospital, Kampala, Uganda.
Ernest A Oumo, Mulago National Referral Hospital, Kampala, Uganda.
Moses C Nsereko, Mulago National Referral Hospital, Kampala, Uganda.
Moorine P Sekadde, National TB and Leprosy Program, Ministry of Health, Kampala, Uganda.
Mary G Nabukenya-Mudiope, Infectious Diseases Institute, Kampala, Uganda.
Midori Kato-Maeda, Division of Pulmonary and Critical Care Medicine, University of California, San Francisco, San Francisco, California, USA; Center for Tuberculosis, University of California, San Francisco, San Francisco, California, USA.
Alfred Andama, Department of Medicine, Makerere University College of Health Sciences, Kampala, Uganda.
Christina Yoon, Division of Pulmonary and Critical Care Medicine, University of California, San Francisco, San Francisco, California, USA; Center for Tuberculosis, University of California, San Francisco, San Francisco, California, USA.
Swomitra Mohanty, Department of Chemical Engineering, University of Utah, Salt Lake City, Utah, USA; Department of Materials Science Engineering, University of Utah, Salt Lake City, Utah, USA.
Eric Wobudeya, Mulago National Referral Hospital, Kampala, Uganda.
Adithya Cattamanchi, Division of Pulmonary and Critical Care Medicine, University of California, San Francisco, San Francisco, California, USA; Center for Tuberculosis, University of California, San Francisco, San Francisco, California, USA; Center for Vulnerable Populations, Department of Medicine, University of California, San Francisco, San F, rancisco, California, USA.
References
- 1. World Health Organization. Roadmap Towards Ending TB in Children and Adolescents. Geneva: World Health Organization; 2018. Accessed January 18, 2020. https://www.who.int/tb/publications/2018/tb-childhoodroadmap/en/. Last. [Google Scholar]
- 2. Marais BJ, Obihara CC, Gie RP, et al. The prevalence of symptoms associated with pulmonary tuberculosis in randomly selected children from a high burden community. Arch Dis Child 2005; 90:1166–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Marais BJ, Gie RP, Schaaf HS, et al. The natural history of childhood intra-thoracic tuberculosis: a critical review of literature from the pre-chemotherapy era. Int J Tuberc Lung Dis 2004; 8:392–402. [PubMed] [Google Scholar]
- 4. Marais BJ, Gie RP, Schaaf HS, et al. A proposed radiological classification of childhood intra-thoracic tuberculosis. Pediatr Radiol 2004; 34:886–94. [DOI] [PubMed] [Google Scholar]
- 5. Starke JR, Donald PR.. Handbook of Child and Adolescent Tuberculosis. New York, NY: Oxford University Press; 2016. [Google Scholar]
- 6. Sproston NR, Ashworth JJ.. Role of C-reactive protein at sites of inflammation and infection. Front Immunol 2018; 9:754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rajan JV, Semitala FC, Mehta T, et al. A novel, 5-transcript, whole-blood gene-expression signature for tuberculosis screening among people living with human immunodeficiency virus. Clin Infect Dis 2019; 69:77–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yoon C, Chaisson LH, Patel SM, et al. Diagnostic accuracy of C-reactive protein for active pulmonary tuberculosis: a meta-analysis. Int J Tuberc Lung Dis 2017; 21:1013–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Södersten E, Ongarello S, Mantsoki A, et al. Diagnostic accuracy study of a novel blood-based assay for identification of tuberculosis in people living with HIV. J Clin Microbiol 2021; 59:e01643–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chaisson LH, Semitala FC, Asege L, et al. Point-of-care C-reactive protein and risk of early mortality among adults initiating antiretroviral therapy. AIDS 2019; 33:895–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yoon C, Semitala FC, Atuhumuza E, et al. Point-of-care C-reactive protein-based tuberculosis screening for people living with HIV: a diagnostic accuracy study. Lancet Infect Dis 2017; 17:1285–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. World Health Organization. WHO Consolidated Guidelines on Tuberculosis. Module 2: Screening—Systematic Screening for Tuberculosis Disease. Geneva: World Health Organization; 2021. [PubMed] [Google Scholar]
- 13. Jaye DL, Waites KB.. Clinical applications of C-reactive protein in pediatrics. Pediatr Infect Dis J 1997; 16:735–46; quiz 746. [DOI] [PubMed] [Google Scholar]
- 14. Flood RG, Badik J, Aronoff SC.. The utility of serum C-Reactive protein in differentiating bacterial from nonbacterial pneumonia in children: a meta-analysis of 1230 children. Pediatr Infect Dis J 2008; 27:95–9. [DOI] [PubMed] [Google Scholar]
- 15. Higdon MM, Le T, O’Brien KL, et al. ; PERCH Study Group. Association of C-reactive protein with bacterial and respiratory syncytial virus-associated pneumonia among children aged <5 years in the PERCH study. Clin Infect Dis 2017; 64:378–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Koster MJ, Broekhuizen BD, Minnaard MC, et al. Diagnostic properties of C-reactive protein for detecting pneumonia in children. Respir Med 2013; 107:1087–93. [DOI] [PubMed] [Google Scholar]
- 17. Marcus N, Mor M, Amir L, et al. Validity of the quick-read C-reactive protein test in the prediction of bacterial pneumonia in the pediatric emergency department. Eur J Emerg Med 2008; 15:158–61. [DOI] [PubMed] [Google Scholar]
- 18. Herlina M, Nataprawira HM, Garna H.. Association of serum C-reactive protein and leptin levels with wasting in childhood tuberculosis. Singapore Med J 2011; 52:446–50. [PubMed] [Google Scholar]
- 19. Albuquerque VVS, Kumar NP, Fukutani KF, et al. Plasma levels of C-reactive protein, matrix metalloproteinase-7 and lipopolysaccharide-binding protein distinguish active pulmonary or extrapulmonary tuberculosis from uninfected controls in children. Cytokine 2019; 123:154773. [DOI] [PubMed] [Google Scholar]
- 20. Kashyap B, Gupta N, Dewan P, et al. High sensitivity C-reactive protein: an adjunct diagnosis in ruling out pediatric tuberculosis. Indian J Clin Biochem 2020; 35:211–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bajaj G, Rattan A, Ahmad P.. Prognostic value of ‘C’ reactive protein in tuberculosis. Indian Pediatr 1989; 26:1010–3. [PubMed] [Google Scholar]
- 22. Zitrin CM. The C-reactive protein in childhood tuberculosis. Am Rev Respir Dis 1960; 81:266–70. [DOI] [PubMed] [Google Scholar]
- 23. de Beer FC, Nel AE, Gie RP, et al. Serum amyloid A protein and C-reactive protein levels in pulmonary tuberculosis: relationship to amyloidosis. Thorax 1984; 39:196–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yoon C, Semitala FC, Asege L, et al. Yield and efficiency of novel intensified tuberculosis case-finding algorithms for people living with HIV. Am J Respir Crit Care Med 2019; 199:643–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Uganda Ministry of Health. Consolidated Guidelines for Prevention and Treatment of HIV in Uganda. Kampala, Uganda: Ministry of Health;2018. [Google Scholar]
- 26. Den Boon S, Bateman ED, Enarson DA, et al. Development and evaluation of a new chest radiograph reading and recording system for epidemiological surveys of tuberculosis and lung disease. Int J Tuberc Lung Dis 2005; 9:1088–96. [PubMed] [Google Scholar]
- 27. Graham SM, Ahmed T, Amanullah F, et al. Evaluation of tuberculosis diagnostics in children: 1. Proposed clinical case definitions for classification of intrathoracic tuberculosis disease. Consensus from an expert panel. J Infect Dis 2012; 205:S199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Graham SM, Cuevas LE, Jean-Philippe P, et al. Clinical case definitions for classification of intrathoracic tuberculosis in children: an update. Clin Infect Dis 2015; 61:S179–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. World Health Organization. High-Priority Target Product Profiles for New Tuberculosis Diagnostics: Report of a Consensus Meeting. Accessed December 18, 2018. http://apps.who.int/iris/bitstream/handle/10665/135617/WHO_HTM_TB_2014.18_eng.pdf?sequence=12014.
- 30. Wilson D, Badri M, Maartens G.. Performance of serum C-reactive protein as a screening test for smear-negative tuberculosis in an ambulatory high HIV prevalence population. PLoS One 2011; 6:e15248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shapiro AE, Hong T, Govere S, et al. C-reactive protein as a screening test for HIV-associated pulmonary tuberculosis prior to antiretroviral therapy in South Africa. AIDS 2018; 32:1811–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Skogmar S, Schön T, Balcha TT, et al. Plasma levels of neopterin and C-reactive protein (CRP) in tuberculosis (TB) with and without HIV coinfection in relation to CD4 cell count. PLoS One 2015; 10:e0144292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Samuels THA, Wyss R, Ongarello S, Moore DAJ, Schumacher SG, Denkinger CM.. Evaluation of the diagnostic performance of laboratory-based c-reactive protein as a triage test for active pulmonary tuberculosis. PLoS One 2021; 16:e0254002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Basu Roy R, Whittaker E, Seddon JA, Kampmann B.. Tuberculosis susceptibility and protection in children. Lancet Infect Dis 2019; 19:e96–e108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bergamini BM, Losi M, Vaienti F, et al. Performance of commercial blood tests for the diagnosis of latent tuberculosis infection in children and adolescents. Pediatrics 2009; 123:e419–24. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.