Abstract
IMPORTANCE
In the US, approximately 12.7% of reproductive age women seek treatment for infertility each year. This review summarizes current evidence regarding diagnosis and treatment of infertility.
OBSERVATIONS
Infertility is defined as the failure to achieve pregnancy after 12 months of regular unprotected sexual intercourse. Approximately 85% of infertile couples have an identifiable cause. The most common causes of infertility are ovulatory dysfunction, male factor infertility, and tubal disease. The remaining 15% of infertile couples have “unexplained infertility.” Lifestyle and environmental factors, such as smoking and obesity, can adversely affect fertility. Ovulatory disorders account for approximately 25% of infertility diagnoses; 70% of women with anovulation have polycystic ovary syndrome. Infertility can also be a marker of an underlying chronic disease associated with infertility. Clomiphene citrate, aromatase inhibitors such as letrozole, and gonadotropins are used to induce ovulation or for ovarian stimulation during in vitro fertilization (IVF) cycles. Adverse effects of gonadotropins include multiple pregnancy (up to 36% of cycles, depending on specific therapy) and ovarian hyperstimulation syndrome (1%–5% of cycles), consisting of ascites, electrolyte imbalance, and hypercoagulability. For individuals presenting with anovulation, ovulation induction with timed intercourse is often the appropriate initial treatment choice. For couples with unexplained infertility, endometriosis, or mild male factor infertility, an initial 3 to 4 cycles of ovarian stimulation may be pursued; IVF should be considered if these approaches do not result in pregnancy. Because female fecundity declines with age, this factor should guide decision-making. Immediate IVF may be considered as a first-line treatment strategy in women older than 38 to 40 years. IVF is also indicated in cases of severe male factor infertility or untreated bilateral tubal factor.
CONCLUSIONS AND RELEVANCE
Approximately 1 in 8 women aged 15 to 49 years receive infertility services. Although success rates vary by age and diagnosis, accurate diagnosis and effective therapy along with shared decision-making can facilitate achievement of fertility goals in many couples treated for infertility.
Infertility, defined as the failure to achieve pregnancy after 12 months of regular unprotected sexual intercourse, affects 8.8% of US women aged 15 to 49 years1 and is often associated with significant physical and emotional stress. This review summarizes current evidence regarding the pathophysiology, diagnosis, and management of infertility for heterosexual couples.2
Methods
We searched the PubMed and Cochrane databases for English-language studies of the epidemiology, diagnosis, and management of infertility published from January 2015 to November 2020, including randomized clinical trials (RCTs), meta-analyses, systematic reviews, and observational studies. Based on these criteria, 71 articles were identified, including 5 clinical trials, 31 systematic reviews, 29 meta-analyses, and 6 practice guidelines. We manually searched references for additional relevant publications. RCTs, meta-analyses, and systematic reviews applicable to a general medical readership were prioritized for inclusion.
Approach to the Patient With Infertility
Heterosexual women desiring pregnancy who have not conceived after 12 months of unprotected intercourse or donor insemination should be offered an infertility evaluation. Earlier evaluation is recommended for women older than 35 years who have not conceived for 6 months, and more immediate evaluation is warranted for women older than 40 years.3 Fertility evaluation is also recommended for women with oligomenorrhea or amenorrhea, known or suspected uterine, tubal, or peritoneal disease (including stage III or IV endometriosis), and male partners with known or suspected male factor infertility (Figure).4 Infertility is caused by identifiable abnormalities in normal physiology or underlying disease in 85% of infertile couples. The most common causes of infertility are ovulatory dysfunction, male factor infertility, and tubal disease. The remaining 15% of infertile couples have “unexplained infertility.”5
Figure.
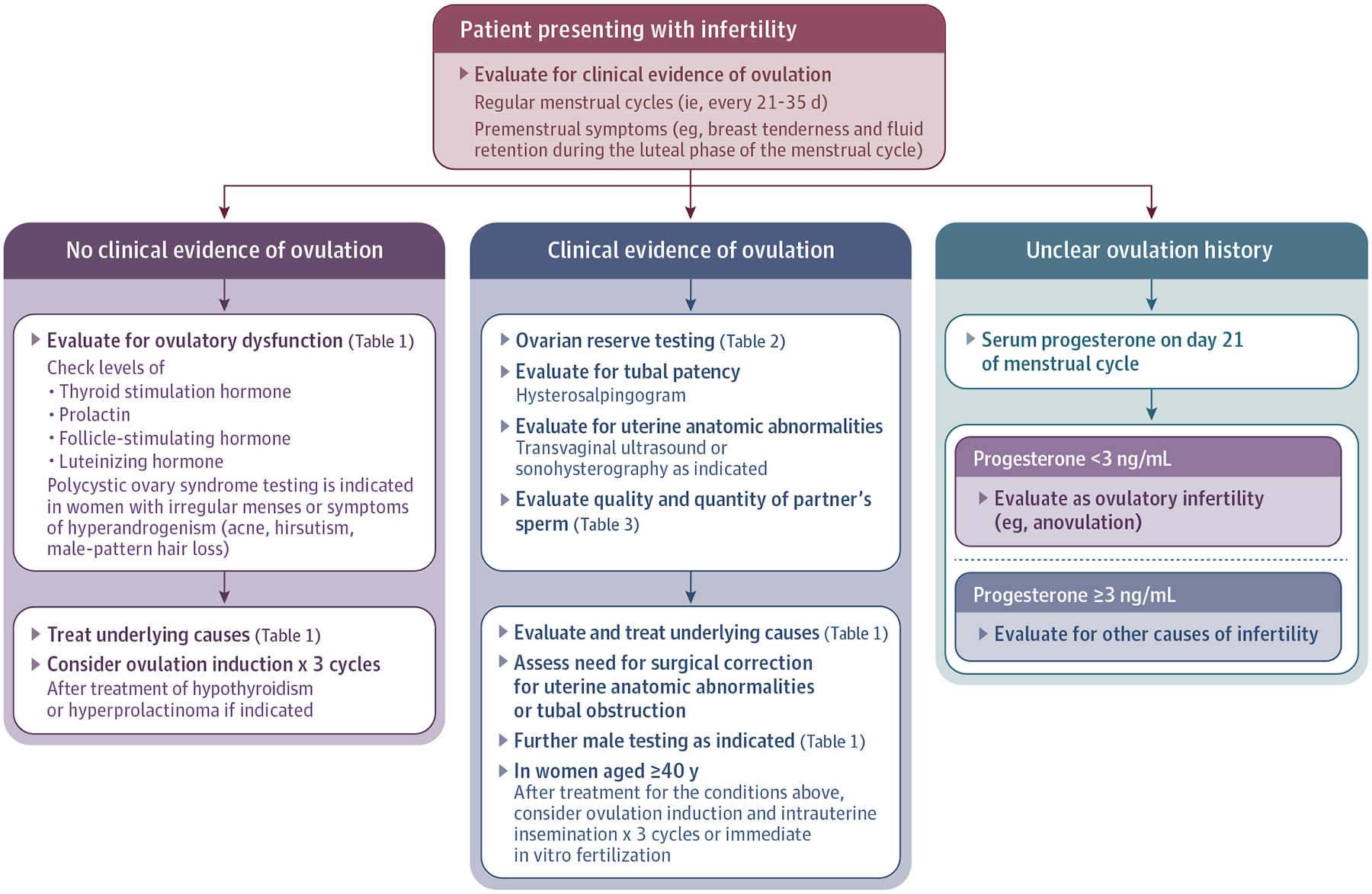
Suggested Evaluation for Patients Presenting With Infertility
Women who have not achieved pregnancy after 12 months of unprotected intercourse or donor insemination should be offered an infertility evaluation. Earlier evaluation is recommended for women older than 35 years who have failed to conceive for 6 months; for women older than 40 years, immediate evaluation is warranted.3 Evaluation is also recommended for women with oligomenorrhea or amenorrhea, known or suspected uterine, tubal disease, or peritoneal disease (including stage III or IV endometriosis) and known or suspected male infertility.4
Major Categories of Infertility
Ovulatory Dysfunction and Anovulation
A history of regular, cyclic menstrual cycles with premenstrual symptoms (eg, breast tenderness, fluid retention) is adequate to establish ovulation. According to the World Health Organization, ovulatory disorders (Table 1)6–10 account for approximately 25% of infertility diagnoses.11 Anovulation should be suspected when menstrual cycles occur irregularly, in cycles shorter than 21 or longer than 35 days (although for most women, cycle length is >25 days), or if the patient reports abnormal uterine bleeding or amenorrhea.9 Ovulation typically occurs 14 days before onset of menstruation. When the menstrual history is unclear or inadequate, ovulation may be documented with a postovulatory serum progesterone level obtained in the expected midluteal phase, approximately 1 week before the expected menses. The most common cause of anovulation is polycystic ovary syndrome (PCOS),12 which affects 70% of women with anovulation. Obesity itself is associated with anovulation apart from PCOS; women with a body mass index (BMI, calculated as weight in kilograms divided by height in meters squared) greater than 27 have an increased risk of anovulatory infertility compared with women with a normal-range BMI (relative risk, 3.1 [95% CI, 2.2–4.4]; absolute rates were not given in the American Society for Reproductive Medicine guideline).13 Other causes include thyroid disease (2%–3%), pituitary disease (eg, prolactinoma, 13%), elevated androgens from adrenal hyperplasia or adrenal tumor (2%), idiopathic chronic anovulation (7%–8%), and functional hypothalamic amenorrhea (eg, due to underweight, eating disorders, and excessive exercise). Patients with eating disorders have anovulatory infertility more often than women without an eating disorder (16.2% vs 5.6%; n = 271).14
Table 1.
Categories of Infertility, Potential Testing, and Treatment Optionsa
| Category | Categories and examples of infertility etiology | Diagnostic testing | Treatment |
|---|---|---|---|
| Ovulatory dysfunctionb | Thyroid dysfunction; hyperprolactinemia; PCOS, hypothalamic amenorrhea | History and physical examination; TSH, prolactin If PCOS is suspected: free and total testosterone; DHEAS; 17-OHP; transvaginal ultrasound FSH/LH/estradiol |
Abnormal TSH or prolactin: correction of the specific defect can stimulate ovulation PCOS: ovulation induction (unless other infertility factors are present); for obese women, weight loss of 15% of body weight can prompt ovulation to resume6 Hypogonadotropic hypogonadism may be treated with pulsatile GnRH or gonadotropin therapy; hypergonadotropic hypogonadism may necessitate donor oocytes |
| Tubal occlusionc | Related to sexually transmitted infections; endometriosis; peritubal adhesions; hydrosalpinx | Hysterosalpingogram (sensitivity: 65%; specificity: 83%) Laparoscopy with chromotubation (ie, instillation of a fluid through the tubes and visualization of it spilling from the tube) |
Surgical repair (eg, hysteroscopy with tubal cannulation for proximal tubal obstruction, tubal reanastomosis, or fimbrioplasty for distal obstruction) or IVF |
| Endometriosisd | Risk factors include early menarche, short menstrual cycles, heavy menstrual periods, nulliparity, and family history of endometriosis | Transvaginal ultrasound | Diagnostic laparoscopy has minimal therapeutic benefit in women with mild endometriosis and typically is not warranted to rule out endometriosis in asymptomatic women with infertility Ovulation induction (see Table 4) vs IVF if other infertility factors are present |
| Diminished ovarian reservee | Age-related oocyte loss Chemotherapy/radiation Fragile X premutationf |
Ovarian reserve testing (eg, AMH, FSH/estradiol, AFC; see Table 2) | Variable, depending on age/history and ovarian reserve testing |
| Uterine factorsg | Endometrial polyps/fibroids, uterine synechiae | TVUS Sonohysterogram 3-D ultrasound/MRI |
Hysteroscopic removal, as appropriate |
| Male factorsh | Obstructive causes (eg, cystic fibrosis mutation, retrograde ejaculation); nonobstructive causes (eg, testicular failure) | Semen analysis (repeat if abnormal) If sperm count <10 million/mL or symptoms/examination findings suggest endocrinopathy,7 FSH and total testosterone, prolactin, karyotype/Y chromosome microdeletion testingi Physical examination: ultrasound if findings suggest varicocele; cystic fibrosis testing if findings suggest absent vas deferens Routine use of other sperm testing (such as antisperm antibodies, sperm DNA fragmentation, or sperm chromosome aneuploidy) is controversial and generally not recommended7 |
Treatment based on results of initial evaluation Diagnostic testing and consultation with a reproductive specialist if indicated based on results of the initial evaluation; see Penzias et al8 for comprehensive evaluation and managementj Surgical repair, surgical sperm retrieval, and/or IUI or IVF with ICSI, as indicated |
Abbreviations: AFC, antral follicle count; AMH, anti-Müllerian hormone; ART, assisted reproductive technology; DHEAS, dehydroepiandrosterone sulfate; FSH, follicle-stimulating hormone; GnRH, gonadotropin-releasing hormone; ICSI, intracytoplasmic sperm injection; IUI, intrauterine insemination; IVF, in vitro fertilization; 17-OHP, 17-hydroxyprogesterone; PCOS, polycystic ovary syndrome; LH, luteinizing hormone; MRI, magnetic resonance imaging; TSH, thyroid-stimulating hormone; TVUS, transvaginal ultrasound.
Infertility is attributable to an identifiable cause as outlined in the table in 85% of women and couples; however, 15% of couples have unexplained infertility5 after diagnostic workup has been completed.
Testing indicated if menstrual cycles are irregular or patient reports abnormal uterine bleeding or amenorrhea; also indicated if serum progesterone levels are suggestive of anovulation (see the Ovulatory Dysfunction and Anovulation section).
Testing may be deferred in anovulatory infertile women but should be performed if the patient does not conceive after 3 to 6 cycles of successful ovulation induction.9
Testing indicated if patient reports cyclic or chronic pelvic pain, dysmenorrhea, or dyspareunia or if examination demonstrates adnexal mass and/or uterosacral ligament nodularity, thickening, or tenderness.10
Testing indicated for women at increased risk of diminished ovarian reserve (age >35 years, family history of early menopause, prior ovarian surgery or absent ovary, history of chemotherapy or pelvic radiation).
A premutation is defined as 55 to 200 unmethylated CGG repeats in the 5′ UTR of the X-linked FMR1 gene.
Testing may be deferred in anovulatory infertile women but should be performed if patient does not conceive after 3 to 6 cycles of successful ovulation induction.9
Testing indicated for all male partners of couples presenting for infertility evaluation or in men presenting with risk factors for male factor infertility (eg, history of chemotherapy or pelvic radiation, prior testicular surgery).7
In cases of nonobstructive azoospermia, a 2019 systematic review and meta-analysis of 117 studies found surgical sperm retrieval rates of 47%, with live birth rates of 24% per ART cycle using retrieved sperm.
Testing for a mutation in the azoospermic factor (AZF) region, found on the long arm of the Y chromosome, can help guide decision-making regarding sperm retrieval. Complete deletions in the AZFa region result in azoospermia; men with deletions in the AZFb and AZFb-c regions frequently also have azoospermia (19 of 21 patients or 90.5% in a prospective study of infertile men). A Y chromosome defect in a man is transmissible to his male offspring, and genetic counseling should be considered before undertaking ART.
Tubal Infertility
Tubal infertility, defined as either blocked fallopian tubes or inability of the tubes to pick up an oocyte from the ovary due to pelvic adhesions, accounts for between 11%11 and 67%15 of infertility diagnoses, depending on the population studied. Tubal infertility should be suspected in women with a history of sexually transmitted infection (the most common cause of tubal disease16), cervical dysplasia, abdominal surgery, or previous intraabdominal infection (eg, ruptured appendix). The severity of tubal abnormalities helps determine the most effective treatment. Hysterosalpingography (HSG), a procedure in which radiopaque dye (either oil or water soluble) is injected through the uterine cervix into the uterine cavity and followed through the fallopian tubes with fluoroscopy, has a sensitivity and specificity of 65% and 83%, respectively,17 and is a first-line diagnostic tool for tubal infertility. A recent systematic review and meta-analyses of 6 RCTs determined that the use of oil-soluble contrast media (OSCM) was associated with significantly higher rates of pregnancy (defined as a positive fetal heartbeat on ultrasonographic examination after 12 weeks’ gestation) after HSG compared with water-soluble contrast media (WSCM) (rates were 32.1% for oil contrast vs 23.6% for water contrast).18 In one study of 1119 women randomly assigned to HSG with OSCM or WSCM, 379 ongoing pregnancies were observed in the OSCM group vs 326 ongoing pregnancies in the WSCM group (odds ratio, 1.47 [95% CI, 1.12–1.93]).19 The underlying mechanisms by which oil contrast might enhance fertility are unclear. Sonohysterography (SHG), in which spillage of contrast from the fallopian tubes is introduced into the uterine cavity and assessed with ultrasound, can be used to assess tubal patency with a sensitivity and specificity of 76% and 67%, respectively.20 Laparoscopy with chromopertubation, in which indigo carmine is inserted transcervically into the uterus and evaluated directly for tubal spillage with laparoscopic visualization, is considered the gold standard for evaluating tubal disease.
Compared with infertile women with bilateral tubal patency, those with unilateral proximal tubal blockage have similar pregnancy rates after ovarian stimulation with intrauterine insemination.21 However, when bilateral tubal obstruction exists, surgery to restore tubal patency or ovarian stimulation with in vitro fertilization (IVF) can be considered. To our knowledge, there are no high-quality RCTs comparing surgery vs IVF for tubal infertility. The selection of tubal surgery or IVF (which bypasses tubal blockage) should be based on the female partner’s age, infertility duration, and presence of other diagnoses (such as male factor infertility), prior pregnancy success and number of desired pregnancies, extent of tubal disease, and financial resources. For example, a woman who is younger than 35 years, has no other infertility factors, and desires more than 1 child may opt for tubal surgery, especially if she does not have the financial resources to support IVF. Laparoscopic tubal ligation (interruption of the tubes) or salpingectomy (removal of the fallopian tubes) should be considered prior to IVF in women with hydrosalpinges (fluid-filled fallopian tubes, typically due to a long-standing untreated infection involving the fallopian tubes). This approach increases the clinical pregnancy rate (396 clinical pregnancies in the surgical treatment group per 1000 patients vs 123 clinical pregnancies per 1000 patients in the nonsurgical group; risk ratio, 3.21 [95% CI, 1.72–5.99]; 2 RCTs).22
Endometriosis
Endometriosis is the presence of endometrial tissue outside the uterine cavity and affects 25% to 40% of women with infertility.23 Anatomic distortion, such as the presence of adhesions blocking the fallopian tubes or impairing tubal patency, or ovarian masses (eg, endometriomas) occurring between the tube and site of ovulation can impair tubal patency, oocyte quality, and retrieval of oocytes by tubal fimbria.24 Data are conflicting regarding whether endometriosis can affect endometrial receptivity.25 Although laparoscopic surgery for endometriosis improves spontaneous pregnancy rates,26 it is not recommended as part of a routine fertility evaluation in women without endometriosis symptoms.24
Diminished Ovarian Reserve
In a study of fecundity in women undergoing artificial insemination with frozen donor semen, cumulative success rates over 12 cycles were 74.1% for the group aged 26 to 30 years, 61.5% for the group aged 31 to 35 years, and 53.6% for the group older than 35 years.27 This decline in fecundity with older age occurs in part due to the progressive loss of follicles and oocytes (the “ovarian reserve”) and the deterioration of gamete quality with age. Other risk factors for diminished ovarian reserve include a history of ovarian surgery, chemotherapy, radiation therapy with exposure to the ovaries, a family history of premature menopause, or a fragile X (FMR1) pre-mutation, defined as between 55 and 200 CGG repeats in the fragile X gene. Ovarian reserve can be assessed with serum markers such as anti-Müllerian hormone or ultrasound (Table 2).8,28–36 Anti-Müllerian hormone, which is expressed by small growing ovarian follicles and declines with age, reflects the size of the follicular pool and correlates with the number of oocytes retrieved after ovarian hyperstimulation.37 As the follicle pool diminishes in size, inhibition of pituitary follicle-stimulating hormone (FSH) secretion by estrogen is lost and early follicular phase serum FSH rises. As ovarian reserve diminishes further, early follicular phase estradiol rises and inhibits FSH elevation.
Table 2.
Tests to Assess Ovarian Reservea
| Test | Description | Timing | Thresholds suggesting increased probability of low ovarian reserve | Costb |
|---|---|---|---|---|
| AMH28–30 | Glycoprotein produced by granulosa cells of growing follicles High predictive value for ovarian response to stimulation May be decreased in patients with obesity31,32 Interpretation of AMH is laboratory assay-dependent |
Low intercycle variability; can be performed throughout cycle | <1.66 ng/mL33 | $76-$9534 |
| FSH and estradiol | FSH is secreted by pituitary; early follicular levels reflect unsuppressed hypothalamic-pituitary-ovarian axis function Significant intra- and intercycle variability; thus, a highly abnormal FSH (eg, >15 mIU/mL) is specific for DOR but less sensitive than AMH and does not detect more subtle declines in ovarian reserve35 Estradiol is useful in interpreting FSH concentrations; prospective studies suggest that day 3 estradiol levels >80pg/mL result in higher cycle cancellation rates and lower pregnancy rates |
Early follicular phase (if patient has regular menstrual cycles) or random (if suspected anovulation) | FSH >10–15 mIU/mL Estradiol >60–80 pg/mL | $95-$12534 |
| Antral follicle count34 | Sum of follicles measuring 2–10 mm in both ovaries observed by ultrasound Good intercycle reliability Limited ability to evaluate overweight or obese women Should be performed in experienced centers |
Can be performed throughout cycle | <4 Follicles 2–10 mm in both ovaries | $300-$50034 |
Abbreviations: AMH, anti-Müllerian hormone; DOR, diminished ovarian reserve; FSH, follicle-stimulating hormone.
In general, there is not consensus on the threshold values suggestive of reduced fertility potential; listed thresholds are suggestive of low ovarian reserve and/or ovarian response to stimulation.
Cost data from Tal and Seifer.34
Uterine and Cervical Factors
Uterine cavity abnormalities are associated with adverse pregnancy outcomes such as miscarriage and preterm birth (outcomes that are not limited to infertile women).28,38 Factors that distort the uterine cavity include endometrial polyps, leiomyomas, intrauterine synechiae, and congenital uterine malformations such as septate uterus. SHG detects polyps or leiomyomas with a sensitivity and specificity of 91% and 84%, respectively,29 making SHG superior to HSG and transvaginal ultrasound for evaluating the uterine cavity. If a congenital malformation (eg, bicornuate uterus) is suspected, further evaluation with pelvic magnetic resonance imaging or 3-dimensional ultrasound is warranted. To our knowledge, there is no high-quality evidence to support the routine use of hysteroscopy as a diagnostic test for infertility etiology in the general population of infertile women with a normal ultrasound or HSG.
Surgery to correct uterine cavity defects is commonly performed to improve reproductive outcomes. A 2018 Cochrane review based on 2 RCTs including 309 women compared operative hysteroscopy vs control for suspected uterine cavity abnormalities including uterine fibroids, endometrial polyps, intrauterine adhesions, and uterine septum. It concluded that removing endometrial polyps may improve pregnancy rates, but that more research is needed to measure the effectiveness of surgery on other structural uterine abnormalities.30 A 2015 Cochrane review of 2 randomized trials found very low–quality evidence to support hysteroscopic removal of submucous fibroids for infertile women (39% clinical pregnancy rate after surgery vs 21% without surgery among 94 women; odds ratio, 2.44 [95% CI, 0.97–6.17]; P = .06).30 Although limited, based on these data, surgery is frequently considered for infertile women with cavity-distorting defects, especially if other symptoms (eg, abnormal uterine bleeding) are present.
Cervical factor infertility is defined as an anatomical abnormality, postsurgical scarring, or decreased cervical mucous that interferes with the natural progression of sperm into the uterus. Congenital cervical anomalies are rare (1/80 000)39; cervical stenosis may occur as a result of surgery (eg, loop electrosurgical excision procedure or cervical cone biopsy for cervical neoplasia), but studies regarding the effect of cervical surgery on fertility are limited by small sample size, short follow-up, and insufficient detail regarding the extent of surgery.40 The use of the postcoital test, historically performed after intercourse to assess the viability of sperm in mucus, is not recommended.
Male Factor
Disorders of male physiology, such as low testosterone concentrations or low sperm count, occur in 35% of infertile couples.41 A couple may also have multiple factors contributing to infertility; therefore, an evaluation for male factor infertility should be performed concurrently to the female evaluation. In addition to a reproductive history, semen analysis should be performed to determine semen volume and sperm production (Table 3).42,43 When the ejaculate does not contain sperm (azoospermia), the presence of sperm in a urine specimen confirms retrograde ejaculation. Obstructive azoospermia is defined as the absence of sperm in the ejaculate due to an obstruction of sperm transport. In men with obstructive azoospermia, the finding of congenital bilateral absence of the vas deferens should prompt evaluation for a mutation in the cystic fibrosis transmembrane conductance regulator, the protein absent in patients with cystic fibrosis. The most common cause of nonobstructive azoospermia is primary testicular failure,44 a diagnosis that requires serum total testosterone and FSH levels and subsequent testing based on initial results (Table 1). Treatment for azoospermia includes surgical sperm retrieval from the testes to obtain sperm for immediate use in assisted reproductive technology (ART) cycles or cryopreservation for later use.
Table 3.
World Health Organization Lower Limits of Normal Semen Parametersa
| Parameter | Normal values |
|---|---|
| pH | 7.2–7.8 |
| Volume | 1.5 cc |
| Total count | 39 million |
| Concentration | ≥15 Milllion sperm/mL(<15 million sperm/mL indicates oligozoospermia) |
| Motility | ≥40% Forward progression (<40% forward progression indicates asthenozoospermia) |
| Morphology | ≥4% Normalforms (by Kruger criteria42) (<4% normally formed sperm indicates teratospermia) |
| White blood cell count | <1 Million/μL |
Total motile sperm count (TMC), or the number of moving sperm in the entire ejaculate, can be calculated by multiplying the volume by the concentration (million sperm/mL) by the motility (% moving). TMCs less than 20 million are significantly associated with a lower probability of fathering a child.43 Values are based on samples from men who had fathered a pregnancy in the previous year and taken after 2 to 7 days of abstinence. Values represent the fifth percentile. A diagnosis of an “abnormal” semen analysis should only be made after a repeat analysis is performed, at least 1 month later.
Treatment for Infertility
Commonly used infertility treatments include ovulation induction, which refers to the use of pharmacologic treatments to induce ovulation, and ovarian stimulation, which is performed with the goal of inducing multiple mature ovarian follicles. Either timed intercourse or intrauterine insemination (IUI) may be used to achieve fertilization at the time of ovulation. Alternatively, mature oocytes may be retrieved directly from the ovary for fertilization using an ultrasound-guided needle (IVF).
Treatment Options
Two oral medications are used for ovulation induction. Clomiphene citrate is a selective estrogen receptor modifier that blocks the negative feedback effect of circulating estradiol and causes an increased hypothalamic gonadotropin-releasing hormone (GnRH) pulse frequency and subsequent pituitary FSH and luteinizing hormone (LH) production,45 promoting ovarian follicular growth. Letrozole blocks aromatase, reducing serum concentrations of estradiol and stimulating pituitary gonadotropins. Both clomiphene citrate and aromatase inhibitors have a multiple pregnancy rate of less than 10%, the majority of which are twin gestations.46 In women with PCOS undergoing ovulation induction, letrozole is the first-line therapy based on the Pregnancy in Polycystic Ovary II Trial, which demonstrated that letrozole results in higher live birth rates compared with clomiphene (103 of 374 [27.5% live birth rate] vs 72 of 376 [19.1% live birth rate]).46 A 2018 Cochrane review of 13 RCTs involving 2954 women comparing clomiphene vs letrozole reached a similar conclusion (314 pregnancies per 1000 women treated with letrozole vs 214 pregnancies with clomiphene; odds ratio, 1.68 [95% CI, 1.42–1.99]), without differences in ovarian hyperstimulation rates, miscarriage rates, or multiple pregnancy rates.47
These oral agents are less useful in women with hypogonadotropic hypogonadism, who may exhibit limited or no endogenous pituitary gonadotropin response. In these patients, the use of pulsatile GnRH administration restores physiological stimulation of endogenous FSH and LH with the goal of inducing follicular maturation and ovulation. The frequency of pulses is adjusted to mimic physiologic variation in GnRH pulse variability. Treatment with pulsatile GnRH results in pregnancy rates of 93% to 100% after up to 6 months48 and is well tolerated with no reported cases of severe ovarian hyperstimulation syndrome. As an alternative, exogenous gonadotropins can be used to directly stimulate ovarian follicles. In women with hypogonadotropic hypogonadism, intrinsic ovulatory dysfunction necessitates use of an exogenous ovulatory trigger (Table 4).8,45,46,48–55
Table 4.
Medications to Treat Infertilitya
| Example | Dose | Adverse effects (rate) | Efficacy |
|---|---|---|---|
| Selective estrogen receptor modifiers, used for ovulation induction | |||
| Clomiphene citrate | 50 mg/daily orally × 5 d (starting between cycle days 2 and 5 after an induced or spontaneous bleed); may increase up to 150 mg/daily if ovulation does not occurb; identification of an ovulatory LH surge for timing of intercourse or IUI can be done using urinary LH kits or office estradiol/ultrasound | Hot flashes (33%), headache (34%), fatigue (14%), dizziness (7%), irritability (21%); thin endometrium (15%−50%49); visual disturbances (2%); multiple pregnancy (up to 12.5%)45 | 24%−31% Cumulative LBR over 3 cycles when combined with IUI for unexplained infertility8; 19.1% LBR over 5 cycles in women with PCOS46 |
| Aromatase inhibitors, used for ovulation induction (off-label indication) | |||
| Letrozole | 2.5 mg/daily orally × 5d (starting between cycle days 2 and 5 after an induced or spontaneous bleed); may increase up to 7.5 mg/daily if ovulation does not occur; monitoring for ovulation as with clomiphene citrate | Hot flashes (20.3%), headache (41%), fatigue (21.7%), dizziness (12.3%), irritability (18%); multiple pregnancy (up to 14.3%)50 In contrast to clomiphene, letrozole does not appear to adversely affect endometrial thickness and cervical mucus50 |
27.5% Cumulative LBR over 5 cycles in women with PCOS46 |
| Gonadotropins, used for ovarian stimulation in combination with timed intercourse/IUI or IVF | |||
| Follicle-stimulating hormone: urinary FSH (Bravelle) recombinant follitropin beta (Follistim); recombinant follitropin alpha (Gonal-F) | For ovarian hyperstimulation with timed intercourse or IUI: typical starting dose is 37.5–75 IU/d; for IVF, typical starting dose is 150–200 IU/d | Injection site reaction (10%), abdominal bloating/discomfort (27%−34%); ovarian hyperstimulation syndrome (1%−5% of cycles)51; multiple pregnancy (up to 36%)52 | 32%−33% Cumulative LBR over 4 cycles when used in combination with IUI for ovarian stimulation; depending on age, LBR may be >65% per cycle when used for IVF with autologous oocytes8 |
| LH: recombinant luteotropin alpha (Luveris) | |||
| Human menopausal gonadotropin: Menopur, Repronex | |||
| Human chorionic gonadotropin (hCG), used as an ovulatory trigger in ovulation induction and ovarian hyperstimulation cycles | |||
| Recombinant hCG (Ovidrel) | 250-μg Recombinant hCG | Injection site swelling, pain, erythema (10%−20%) | Based on OI or OS regimen used |
| Urinary hCG (Pregnyl, Novarel) | 5000–10 000 Units of urinary hCG | ||
| GnRH agonists, used for pituitary downregulation and as an ovulatory trigger during ovarian hyperstimulation cycles | |||
| Leuprolide acetate (Lupron) | Injection: 0.5–1 mg subcutaneous daily | Short-term menopausal symptoms (eg, hot flashes, mood swings, vaginal dryness, headache) (70%−80%) | Based on OI or OS regimen used |
| Nafarelin acetate (Synarel) | Nasalspray: 400 μg twice daily | ||
| GnRH antagonists, used for pituitary downregulation during ovarian hyperstimulation cycles | |||
| Ganirelix; Cetrorelix acetate (Cetrotide) | Injection: 0.25 mg daily | Similar to GnRH agonists | Based on OI or OS regimen used |
| Pulsatile GnRH therapy, used for ovulation induction in patients with hypothalamic amenorrhea | |||
| GnRH therapy | 75 ng/kg (Discontinued after ovulation) | Injection site adverse effects; multiple gestation (4%−8%53); ovarian hyperstimulation (<1%54) | Pregnancy rates 70%−100% after up to 6 mo48 |
| Dopamine agonists, for treatment of hyperprolactinemic anovulation (titrated to normalize serum prolactin levels) c | |||
| Bromocriptine (Parlodel) | Starting at 1.25mg orally daily | Dizziness (25%), headache (25%−30%), nausea/vomiting (30%−50%) | 52%−72% Resumption of ovulatory cycles55 |
| Cabergoline (Dostinex) | Starting at 0.25 mg orally twice weekly; may be given vaginally to minimize adverse effects | ||
| Progesterone | |||
| Crinone 8% vaginal gel | Gel: 90 mg intravaginal daily | Abdominal discomfort (15%), headache (13%), vaginal discharge (7%), nausea (22%) | |
| Endometrin, 100-mg vaginal tablet | Tablet: 100 mg intravaginal 2–3 times daily | ||
| Intramuscular progesterone in oil | Injection: 50 mg daily | ||
Abbreviations: GnRH, gonadotropin-releasing hormone; IUI, intrauterine insemination; IVF, in vitro fertilization; LBR, live birth rate; LH, luteinizing hormone; OI, ovulation induction; OS, ovarian stimulation; PCOS, polycystic ovary syndrome.
The medications may be used alone or in combination in a fertility treatment cycle.
Higher doses (200–250 mg daily have been used in women refractory to these doses, but these doses exceed US Food and Drug Administration recommendation.
Not available in the US.
Ovarian stimulation can be performed with clomiphene citrate, aromatase inhibitors, gonadotropins, or a combination of these medications using doses similar to those used for ovulation induction. Ovarian stimulation is combined with intrauterine insemination to treat unexplained infertility; live birth rates depend on the diagnosis, sperm viability, and ovarian response. A 2019 Cochrane review concluded that gonadotropins resulted in a higher live birth rate than continued clomiphene citrate after 6 ovulatory cycles for women with PCOS56; however, multiple pregnancy rates as high as 36% have been reported with the use of gonadotropins.52 Ovarian hyperstimulation syndrome (1%–5% of cycles), which can include ascites, electrolyte imbalance, and hypercoagulability, is another serious complication of gonadotropin use. Thus, gonadotropin therapy should be administrated under the supervision of a reproductive endocrinologist.
IUI is accomplished by placing sperm into the uterus 24 to 36 hours after an endogenous LH surge or an exogenous ovulation trigger. IUI is typically first-line therapeutic strategy for mild male sub-fertility, although there is no formally recognized definition for mild male factor infertility. A 2016 Cochrane review of 10 RCTs involving 757 patients found no evidence of a difference between IUI and timed intercourse for male infertility.57 However, evidence was low quality. In patients with unexplained infertility, IUI should be administered in combination with ovulation stimulation because IUI alone does not increase pregnancy rates in this population.58
A typical IVF cycle includes gonadotropin stimulation, followed by aspiration of multiple ovarian follicles. Oocytes can be fertilized in vitro either by mixing with spermatozoa (IVF) or with intracytoplasmic sperm injection (ICSI) if severe male factor infertility exists and sperm can be obtained surgically or from the ejaculate. Embryos are cultured under optimal conditions, then transferred into the uterus under ultrasound guidance.
Preimplantation genetic testing may be used to identify embryos with a single gene disorder or to screen for euploid embryos, defined as an embryo with the correct number of chromosomes, for uterine transfer. Cultured embryos, obtained via IVF, are biopsied to select the most appropriate embryos for transfer. Although several RCTs support this practice,59,60 considerable controversy exists regarding the efficacy of preimplantation genetic testing as a universal screening test for all patients undergoing IVF.61 For example, some embryos develop with multiple cell lines containing both euploid and aneuploid cells within the same embryo (eg, mosaic embryos). These embryos have been shown to result in chromosomally normal pregnancies,62,63 although the likelihood of implantation is significantly less, and the pregnancy loss rate higher, than for nonmosaic euploid embryos.63 Proposed mechanisms by which mosaic embryos may produce chromosomally normal pregnancies include initial misdiagnosis due to biopsy technique or inaccurate analysis, discordance between trophectoderm and inner cell mass of the embryo, or self-correction of chromosomal abnormalities in growing embryos.
Third-party Reproduction
Donor oocytes or sperm may be considered when either partner has severe defects in gamete quality or quantity or a severe genetic condition. These options may also be considered for females without male partners or males without female partners. A gestational carrier may be indicated when a medical condition or absence of a uterus prevents a woman from carrying a pregnancy. Single males or gay male couples may also use a gestational carrier. Uterine transplant, in which a uterus is surgically removed from one individual and placed in another individual, is an experimental procedure for women with uterine factor infertility (eg, congenital or surgical absence of the uterus or presence of a nonfunctioning uterus). The first clinical trial of a living donor uterine transplant resulted in a 32-week delivery in 2014 in Sweden,64 followed by the first live birth from a deceased donor at 36 weeks in 2017 in Brazil.65 Two deceased donor uterine transplant live births have been reported in the US.
Choice of Treatment for Infertility
For individuals presenting with anovulation, ovulation induction with timed intercourse is often the appropriate initial treatment choice. For couples with unexplained infertility, the American Society for Reproductive Medicine recommends an initial 3 to 4 cycles of ovarian stimulation with intrauterine insemination,8 an approach that can also be used for women with endometriosis or partners with mild male factor infertility. IVF should be considered if these approaches do not result in pregnancy. The Fast Track and Standard Treatment Trial, which randomized women with unexplained infertility to either 3 cycles of clomiphene-IUI followed by 3 cycles of gonadotropins-IUI prior to IVF or 3 cycles of clomiphene-IUI followed by 6 cycles of IVF, determined that the time to pregnancy was significantly faster (8 vs 11 months), with a cost savings of $2624 per couple, in the clomiphene to IVF group.66 Thus, gonadotropin-IUI cycles are not recommended for unexplained infertility.8
Age is another consideration that should guide decision-making (Box). Success rates for fertility treatment decline with age: the per-cycle pregnancy rates for clomiphene and intrauterine insemination are 8.2% in women aged 35 to 37 years, 6.5% in women aged 38 to 40 years, 3.6% in women aged 41 to 42 years, and 0.8% in women older than 42 years.67 For IVF cycles, registry data from the Centers for Disease Control and Prevention also reflect decreasing success with advancing age (from a live birth rate of 48.5% per embryo transfer for women <35 years to 11.0% for women >43 years in 2017).68 One RCT evaluating treatment strategies for unexplained infertility in women 38 to 42 years old found higher live birth rates in couples undergoing immediate IVF (31.4% over 2 treatment cycles) as compared with those undergoing ovarian stimulation–IUI with clomiphene (15.7%) or gonadotropins (13.5%).69 Thus, immediate IVF may be considered as a first-line treatment strategy in women older than 38 to 40 years, although women with severely diminished ovarian reserve may be candidates for oocyte donation. Immediate IVF is also indicated in cases of severe male factor infertility, untreated bilateral tubal factor infertility, or in situations where preimplantation genetic testing will be used.
Box. Common Questions About Infertility.
What Can a Patient Do to Maximize Likelihood of Pregnancy?
There are several steps an individual can take to enhance his or her natural fertility, including maintaining a healthy weight (body mass index, 19–24) and abstaining from cigarette smoking. While data are limited regarding the effects on fertility, reducing alcohol consumption to <2 drinks per day and increasing intake of supplemental folic acid, fruits and vegetables with low pesticide residue, whole grains, seafood, dairy, and soy may be considered. Couples can maximize chances of conceiving by having sexual intercourse regularly during the most fertile part of the menstrual cycle (the 3-day interval ending on the day of ovulation). Timing ovulation with methods, such as monitoring of cervical mucus or use of ovulation predictor kits (available without a prescription), can help determine this timing.
Does Female Age Affect Fertility?
Frequently, infertility can be directly attributed to ovarian aging. With age, a drastic decline in the quantity of follicles and oocytes (the “ovarian reserve”) occurs. Chromosomal segregation errors during meiotic divisions are increasingly common with age and lead to the production of oocytes with an incorrect number of chromosomes, causing deterioration of gamete quality and increasing the risk of birth defects and miscarriage. Moreover, with advancing age, women are also more likely to develop conditions such as uterine fibroids or endometriosis, which can impair fertility. This age-related decline in fertility occurs more rapidly after age 37 years. While less well-studied, data suggest that semen quality also declines with age.
When Should a Patient Be Evaluated for Infertility?
Women <35 years old should be evaluated after 1 year of attempting conception. Women >35 years should be evaluated after 6 months, and women ≥40 years should consider an immediate evaluation. However, women who may have difficulty getting pregnant, such as those with painful periods or endometriosis, irregular menstrual cycles, a history of pelvic inflammatory disease, or a partner with a low sperm count, should be evaluated sooner.
Do Insurance Plans Cover Infertility Treatment?
The degree of services covered depends on the state an individual resides in, as well as the insurance coverage available. Nineteen states have passed laws that require insurance companies to include fertility treatment in their plans. However, these laws vary greatly in their scope of what is and is not required to be covered. For more information about the specific laws for each of those states, an individual can contact his or her insurance carrier or state Insurance Commissioner’s office.
Specific Considerations in Patient Counseling
Role of Lifestyle Factors
Systematic reviews and meta-analyses have shown that female obesity (BMI >30) is negatively associated with live birth rates following IVF.70 For obese women, delaying conception to achieve weight loss should be strongly considered. In a secondary analysis of 2 RCTs of overweight/obese women with PCOS and infertility, delayed treatment with clomiphene after lifestyle modification and weight loss resulted in improved ovulation and live birth rates when compared with immediate treatment.71 However, 2 recent RCTs found that for 962 infertile, obese women (BMI, 30–35) undergoing IVF, randomization to weight reduction with a low calorie diet (880 kcal/d for 12 weeks; mean weight reduction, 9.44 kg) did not improve live birth rates in the index cycle72 or over a 2-year follow-up period.73 Whether weight loss can reverse the deleterious effect of obesity on oocyte quality is also not clear.74 Metformin does not improve live birth rates in women with PCOS and is not recommended for ovulation induction.75
Among the general population, observational studies have suggested an association between specific diets and increased fecundity. An observational prospective study of dietary patterns among 357 women undergoing ART assigned participant scores based on self-reported intake of supplemental folic acid, vitamins, fruits and vegetables with low pesticide residue, whole grains, seafood, dairy, and soy. The study reported a linear increase in odds of live birth with adherence to this dietary pattern (eg, for each 4-point increase in adherence scores, a 53% higher odd of live birth was observed [95% CI, 26%–85%, P < .001; range of profertility diet scores, 9–36).76 This study did not provide absolute rates. A 2017 systematic review of observational studies concluded that male intake of high amounts of alcohol, caffeine, and red meat was negatively associated with pregnancy in the study participants’ partners.77 Tobacco use, alcohol consumption of more than 2 drinks per day, and recreational drugs should also be discouraged for couples trying to conceive,78 although there is no evidence from RCTs that preconception lifestyle counseling improved the chance of a live birth in subfertile individuals.
Fertility Drugs and Birth Defects
A 2012 meta-analysis of 46 studies comparing risk of birth defects among 124 468 children conceived via IVF or ICSI found a significantly increased risk after ART (relative risk, 1.37 [95% CI, 1.26–1.48]; no absolute rates were provided), but ICSI did not increase the risk compared with IVF.79 In contrast, an Australian registry study of 327 420 births found no association between assisted conception and risk of birth defects after adjusting for parental factors, with the exception of cycles with ICSI (165 births with defects from IVF cycles as compared with 139 births with defects from ICSI cycles; odds ratio, 0.68 [95% CI, 0.53–0.87]), although residual confounding related to differences in male infertility factors could not be excluded.80 Letrozole was not included in this study; however, retrospective cohort studies comparing letrozole vs clomiphene81–84 reported no difference in rates of congenital anomalies. More recently, a meta-analysis of 35 cohort studies involving 135 695 multiple births found that multiple pregnancies from ART were associated with a small but significantly higher risk of congenital malformation (risk ratio, 1.11 [95% CI, 1.02–1.22]; no absolute rates were provided in this study) as compared with spontaneously conceived pregnancies.85
Infertility and Cancer Risk
Infertility is a risk factor for breast, endometrial, and ovarian cancers; however, it is not clear that fertility treatment itself increases these risks.86 Factors such as nulliparity, late age at first birth, late age at menopause, and anovulation are characteristics of the infertile population but are also associated with increased risk for ovarian, endometrial, or breast cancer. A 2017 Cochrane review of 19 614 participants across 4 studies found an increased risk of endometrial cancer in subfertile women treated with clomiphene citrate compared with controls (risk ratio, 1.87 [95% CI, 1.00–3.48]) but could not conclude whether this association was related to underlying conditions, such as PCOS, or exposure to clomiphene itself.87 A 2019 Cochrane review that evaluated risks of ovarian cancer in women treated with ovarian-stimulating drugs for infertility (37 studies, 4 684 724 women) concluded that available studies were of low methodological quality, with short follow-up and lack of adjustment for confounders, and that available evidence did not suggest a clinically significant adverse association.86
Multiple systematic reviews and meta-analyses encompassing fertility treatment regimens such as ovulation induction and IVF, including 2 with more than 30 years of follow-up, concluded that hormonal infertility treatments were not associated with increased breast cancer risk. Thus, while infertile women may be at an increased risk of invasive ovarian, endometrial, and breast cancer, there is not definitive evidence that fertility medications increase this risk.88 Similar to female infertility, male infertility is associated with higher overall cancer risk. An analysis of US claims data from 76 083 infertile men found an increased risk of all cancers (600 cases observed, 333.41 cases expected; hazard ratio, 1.80 [95% CI, 1.66–1.95]) in the years after infertility evaluation.89
Infertility as a Risk Factor for Overall Health Conditions
Some causes of infertility are associated with adverse health outcomes. For example, PCOS is associated with obesity, metabolic syndrome, diabetes, dyslipidemia, and insulin resistance. The association of anovulation with endometrial hyperplasia and cancer is well established.90 Primary ovarian insufficiency is associated with an increased risk of osteoporosis, cardiovascular disease, and endocrine disorders including hypothyroidism and adrenal insufficiency.91 A 2019 cross-sectional analysis of National Health and Nutrition Examination Survey data, which examined the association between self-reported infertility and metabolic syndrome and cardiovascular events, found that infertile women had higher odds of metabolic syndrome (1.79 [95% CI, 1.04–3.08]) and of a prior cardiovascular event.92 Further, a 2020 analysis of a multicenter cancer-screening RCT including 75 784 women aged 55 to 74 years found that women with a history of infertility had a 10% increased risk of death during the study period.93 Associations between fertility and overall health exist in men as well. A 2009 case-control study of 344 infertile men compared with 293 age-comparable fertile men found that infertile men scored more poorly on a predictive index for 10-year mortality compared with controls (mean Charlson Comorbidity Index score, 0.33 vs 0.14; P < .001 [95% CI, 0.08–0.29]).94 Meanwhile, a large Danish prospective cohort study of men who had undergone fertility treatment found an increased diabetes risk among men with male factor infertility (129 cases among 12 857 men) as compared with men undergoing fertility treatment for other cases (61 cases among 12 376 controls; hazard ratio, 1.45 [95% CI, 1.06–1.97]).95
The Potential of Reproductive Medicine
Emerging reproductive technologies have the potential to change evaluation and treatment of infertility. Advances in DNA sequencing have generated large data sets that may prove useful in developing personalized treatments. The development of artificial gametes generated in vitro has resulted in live births in animal models, although no study has reported the birth of human offspring from artificial gametes. The use of gene therapies to improve oocyte quality, such as autologous mitochondrial transfer into oocytes, has been explored in prospective cohort studies,96,97 although prognosis was not improved in a randomized pilot study of women who had previously unsuccessful IVF.98 Lack of rigorous testing, as well as ethical concerns including informed consent from future biological children, long-term safety, and questions about natural limits to the reproductive lifespan and the demands of later-life child-rearing, remains a major barrier to widespread use and should be at the forefront of discussions surrounding these innovations. Caution is warranted in implementing fertility treatments that have not been rigorously tested, have insufficient evidence to suggest efficacy, or for which evidence of benefit is extrapolated from unrelated populations.
Limitations
This review has some limitations. First, the search was restricted to English-language publications, including published systematic reviews, meta-analyses, and clinical practice guidelines where available. The search may have excluded relevant non–English-language publications. Second, this review provides an overview of infertility for the general medical clinician and does not represent an exhaustive review of all aspects of infertility diagnosis and treatment. Third, some aspects of the review refer to guidelines, which can be based on expert opinion. Fourth, high-quality data are lacking for some covered topics. Fifth, the review is limited to management of infertility in heterosexual couples.
Conclusions
Approximately 1 in 8 women aged 15 to 49 years receive infertility services. Although success rates vary by age and diagnosis, accurate diagnosis and effective therapy along with shared decision-making can facilitate achievement of fertility goals in many couples treated for infertility.
Conflict of Interest Disclosures:
Dr Carson reported being a member of the board of directors of Agile Therapeutics. Dr Kallen reported receiving grant support from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the Global Consortium for Reproductive Longevity and Equality, loan repayment support through the National Institutes of Health, fees as a medical reviewer for Healthline; serving on the advisory board for Healthline Parenthood; and having a patent to H19 as a biomarker for ovarian reserve status pending.
REFERENCES
- 1.Centers for Disease Control and Prevention. Key statistics from the National Survey of Family Growth—I listing. Accessed April 28, 2020. https://www.cdc.gov/nchs/nsfg/key_statistics/i_2015-2017.htm#infertility
- 2.Ethics T, Medicine R; Ethics Committee of American Society for Reproductive Medicine. Access to fertility treatment by gays, lesbians, and unmarried persons: a committee opinion. Fertil Steril. 2013;100(6):1524–1527. doi: 10.1016/j.fertnstert.2013.08.042 [DOI] [PubMed] [Google Scholar]
- 3.American College of Obstetricians and Gynecologists Committee on Gynecologic Practice and Practice Committee. Female age-related fertility decline: committee opinion No. 589. Fertil Steril. 2014;101(3):633–634. doi: 10.1016/j.fertnstert.2013.12.032 [DOI] [PubMed] [Google Scholar]
- 4.Infertility workup for the women’s health specialist: ACOG committee opinion number 781. Obstet Gynecol. 2019;133(6):1294–1295. doi: 10.1097/AOG.0000000000003272 [DOI] [PubMed] [Google Scholar]
- 5.Gelbaya TA, Potdar N, Jeve YB, Nardo LG. Definition and epidemiology of unexplained infertility. Obstet Gynecol Surv. 2014;69(2):109–115. doi: 10.1097/OGX.0000000000000043 [DOI] [PubMed] [Google Scholar]
- 6.Clark AM, Ledger W, Galletly C, et al. Weight loss results in significant improvement in pregnancy and ovulation rates in anovulatory obese women. Hum Reprod. 1995;10(10):2705–2712. doi: 10.1093/oxfordjournals.humrep.a135772 [DOI] [PubMed] [Google Scholar]
- 7.Pfeifer S, Butts S, Dumesic D, et al. ; Practice Committee of the American Society for Reproductive Medicine. Diagnostic evaluation of the infertile male: a committee opinion. Fertil Steril. 2015;103(3):e18–e25. doi: 10.1016/j.fertnstert.2014.12.103 [DOI] [PubMed] [Google Scholar]
- 8.Penzias A, Bendikson K, Falcone T, et al. ; Practice Committee of the American Society for Reproductive Medicine. Evidence-based treatments for couples with unexplained infertility: a guideline. Fertil Steril. 2020;113(2):305–322. doi: 10.1016/j.fertnstert.2019.10.014 [DOI] [PubMed] [Google Scholar]
- 9.Practice Committee of the American Society for Reproductive Medicine. Diagnostic evaluation of the infertile female: a committee opinion. Fertil Steril. 2015;103(6):e44–e50. doi: 10.1016/j.fertnstert.2015.03.019 [DOI] [PubMed] [Google Scholar]
- 10.Practice Committee of the American Society for Reproductive Medicine. Endometriosis and infertility: a committee opinion. Fertil Steril. 2012; 98(3):591–598. doi: 10.1016/j.fertnstert.2012.05.031 [DOI] [PubMed] [Google Scholar]
- 11.Recent advances in medically assisted conception: report of a WHO scientific group. World Health Organ Tech Rep Ser. 1992;820(820):1–111. [PubMed] [Google Scholar]
- 12.Sirmans SM, Pate KA. Epidemiology, diagnosis, and management of polycystic ovary syndrome. Clin Epidemiol. 2013;6:1–13. doi: 10.2147/CLEP.S37559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Practice Committee of the American Society for Reproductive Medicine. Obesity and reproduction: a committee opinion. Fertil Steril. 2015;104(5): 1116–1126. doi: 10.1016/j.fertnstert.2015.08.018 [DOI] [PubMed] [Google Scholar]
- 14.Boutari C, Pappas PD, Mintziori G, et al. The effect of underweight on female and male reproduction. Metabolism. 2020;107:154229. doi: 10.1016/j.metabol.2020.154229 [DOI] [PubMed] [Google Scholar]
- 15.Audu BM, Massa AA, Bukar M, El-Nafaty AU, Sa’ad ST. Prevalence of utero-tubal infertility. J Obstet Gynaecol. 2009;29(4):326–328. doi: 10.1080/01443610902803625 [DOI] [PubMed] [Google Scholar]
- 16.Practice Committee of the American Society for Reproductive Medicine. Role of tubal surgery in the era of assisted reproductive technology: a committee opinion. Fertil Steril. 2015;103(6):e37–e43. doi: 10.1016/j.fertnstert.2015.03.032 [DOI] [PubMed] [Google Scholar]
- 17.Broeze KA, Opmeer BC, Van Geloven N, et al. Are patient characteristics associated with the accuracy of hysterosalpingography in diagnosing tubal pathology? an individual patient data meta-analysis. Hum Reprod Update. 2011;17(3):293–300. doi: 10.1093/humupd/dmq056 [DOI] [PubMed] [Google Scholar]
- 18.Fang F, Bai Y, Zhang Y, Faramand A. Oil-based versus water-based contrast for hysterosalpingography in infertile women: a systematic review and meta-analysis of randomized controlled trials. Fertil Steril. 2018;110(1):153–160.e3. doi: 10.1016/j.fertnstert.2018.03.021 [DOI] [PubMed] [Google Scholar]
- 19.Dreyer K, van Rijswijk J, Mijatovic V, et al. Oil-based or water-based contrast for hysterosalpingography in infertile women. N Engl J Med. 2017;376(21):2043–2052. doi: 10.1056/NEJMoa1612337 [DOI] [PubMed] [Google Scholar]
- 20.Maheux-Lacroix S, Boutin A, Moore L, et al. Hysterosalpingosonography for diagnosing tubal occlusion in subfertile women: a systematic review with meta-analysis. Hum Reprod. 2014;29(5):953–963. doi: 10.1093/humrep/deu024 [DOI] [PubMed] [Google Scholar]
- 21.Tan J, Tannus S, Taskin O, Kan A, Albert AY, Bedaiwy MA. The effect of unilateral tubal block diagnosed by hysterosalpingogram on clinical pregnancy rate in intrauterine insemination cycles: systematic review and meta-analysis. BJOG. 2019; 126(2):227–235. doi: 10.1111/1471-0528.15457 [DOI] [PubMed] [Google Scholar]
- 22.Melo P, Georgiou EX, Johnson N, et al. Surgical treatment for tubal disease in women due to undergo in vitro fertilisation. Cochrane Database Syst Rev. 2020;10(10):CD002125. doi: 10.1002/14651858.CD002125.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ozkan S, Murk W, Arici A. Endometriosis and infertility: epidemiology and evidence-based treatments. Ann N Y Acad Sci. 2008;1127:92–100. doi: 10.1196/annals.1434.007 [DOI] [PubMed] [Google Scholar]
- 24.Practice Committee of the American Society for Reproductive Medicine. Endometriosis and infertility: a committee opinion. Fertil Steril. 2012; 98(3):591–598. doi: 10.1016/j.fertnstert.2012.05.031 [DOI] [PubMed] [Google Scholar]
- 25.Lessey BA, Young SL. What exactly is endometrial receptivity? Fertil Steril. 2019;111(4): 611–617. doi: 10.1016/j.fertnstert.2019.02.009 [DOI] [PubMed] [Google Scholar]
- 26.Brown J, Farquhar C. Endometriosis: an overview of Cochrane Reviews. Cochrane Database Syst Rev. 2014;2014(3):CD009590. doi: 10.1002/14651858.CD009590.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwartz D, Mayaux MJ; Federation CECOS. Female fecundity as a function of age: results of artificial insemination in 2193 nulliparous women with azoospermic husbands. N Engl J Med. 1982; 306(7):404–406. doi: 10.1056/NEJM198202183060706 [DOI] [PubMed] [Google Scholar]
- 28.Venetis CA, Papadopoulos SP, Campo R, Gordts S, Tarlatzis BC, Grimbizis GF. Clinical implications of congenital uterine anomalies: a meta-analysis of comparative studies. Reprod Biomed Online. 2014; 29(6):665–683. doi: 10.1016/j.rbmo.2014.09.006 [DOI] [PubMed] [Google Scholar]
- 29.Bittencourt CA, Dos Santos Simões R, Bernardo WM, et al. Accuracy of saline contrast sonohysterography in detection of endometrial polyps and submucosal leiomyomas in women of reproductive age with abnormal uterine bleeding: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2017;50(1):32–39. doi: 10.1002/uog.17352 [DOI] [PubMed] [Google Scholar]
- 30.Bosteels J, van Wessel S, Weyers S, et al. Hysteroscopy for treating subfertility associated with suspected major uterine cavity abnormalities. Cochrane Database Syst Rev. 2018;12:CD009461. doi: 10.1002/14651858.CD009461.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bernardi LA, Carnethon MR, de Chavez PJ, et al. Relationship between obesity and anti-Müllerian hormone in reproductive-aged African American women. Obesity (Silver Spring). 2017;25(1):229–235. doi: 10.1002/oby.21681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moy V, Jindal S, Lieman H, Buyuk E. Obesity adversely affects serum anti-müllerian hormone (AMH) levels in Caucasian women. J Assist Reprod Genet. 2015;32(9):1305–1311. doi: 10.1007/s10815-015-0538-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.La Marca A, Sighinolfi G, Radi D, et al. Anti-Mullerian hormone (AMH) as a predictive marker in assisted reproductive technology (ART). Hum Reprod Update. 2010;16(2):113–130. doi: 10.1093/humupd/dmp036 [DOI] [PubMed] [Google Scholar]
- 34.Tal R, Seifer DB. Ovarian reserve testing: a user’s guide. Am J Obstet Gynecol. 2017;217(2): 129–140. doi: 10.1016/j.ajog.2017.02.027 [DOI] [PubMed] [Google Scholar]
- 35.Esposito MA, Coutifaris C, Barnhart KT. A moderately elevated day 3 FSH concentration has limited predictive value, especially in younger women. Hum Reprod. 2002;17(1):118–123. doi: 10.1093/humrep/17.1.118 [DOI] [PubMed] [Google Scholar]
- 36.ACOG committee opinion No. 773: the use of antimüllerian hormone in women not seeking fertility care. Obstet Gynecol. 2019;133(4):e274–e278. doi: 10.1097/AOG.0000000000003162 [DOI] [PubMed] [Google Scholar]
- 37.Seifer DB, MacLaughlin DT, Christian BP, Feng B, Shelden RM. Early follicular serum müllerian-inhibiting substance levels are associated with ovarian response during assisted reproductive technology cycles. Fertil Steril. 2002;77(3):468–471. doi: 10.1016/S0015-0282(01)03201-0 [DOI] [PubMed] [Google Scholar]
- 38.Chan YY, Jayaprakasan K, Tan A, Thornton JG, Coomarasamy A, Raine-Fenning NJ. Reproductive outcomes in women with congenital uterine anomalies: a systematic review. Ultrasound Obstet Gynecol. 2011;38(4):371–382. doi: 10.1002/uog.10056 [DOI] [PubMed] [Google Scholar]
- 39.Fujimoto VY, Miller JH, Klein NA, Soules MR. Congenital cervical atresia: report of seven cases and review of the literature. Am J Obstet Gynecol. 1997;177(6):1419–1425. doi: 10.1016/S0002-9378(97)70085-1 [DOI] [PubMed] [Google Scholar]
- 40.Martyn F, McAuliffe FM, Wingfield M. The role of the cervix in fertility: is it time for a reappraisal? Hum Reprod. 2014;29(10):2092–2098. doi: 10.1093/humrep/deu195 [DOI] [PubMed] [Google Scholar]
- 41.Odisho AY, Nangia AK, Katz PP, Smith JF. Temporal and geospatial trends in male factor infertility with assisted reproductive technology in the United States from 1999–2010. Fertil Steril. 2014;102(2):469–475. doi: 10.1016/j.fertnstert.2014.05.006 [DOI] [PubMed] [Google Scholar]
- 42.Kruger TF, Acosta AA, Simmons KF, Swanson RJ, Matta JF, Oehninger S. Predictive value of abnormal sperm morphology in in vitro fertilization. Fertil Steril. 1988;49(1):112–117. doi: 10.1016/S0015-0282(16)59660-5 [DOI] [PubMed] [Google Scholar]
- 43.van der Steeg JW, Steures P, Eijkemans MJC, et al. ; Collaborative Effort for Clinical Evaluation in Reproductive Medicine Study Group. Role of semen analysis in subfertile couples. Fertil Steril. 2011;95 (3):1013–1019. doi: 10.1016/j.fertnstert.2010.02.024 [DOI] [PubMed] [Google Scholar]
- 44.Practice T, Medicine R; Practice Committee of the American Society for Reproductive Medicine. Management of nonobstructive azoospermia: a committee opinion. Fertil Steril. 2018;110(7):1239–1245. doi: 10.1016/j.fertnstert.2018.09.012 [DOI] [PubMed] [Google Scholar]
- 45.Adashi EY. Clomiphene citrate: mechanism(s) and site(s) of action: a hypothesis revisited. Fertil Steril. 1984;42(3):331–344. doi: 10.1016/S0015-0282(16)48069-6 [DOI] [PubMed] [Google Scholar]
- 46.Legro RS, Brzyski RG, Diamond MP, et al. ; NICHD Reproductive Medicine Network. Letrozole versus clomiphene for infertility in the polycystic ovary syndrome. N Engl J Med. 2014;371(2):119–129. doi: 10.1056/NEJMoa1313517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Franik S, Eltrop SM, Kremer JA, Kiesel L, Farquhar C. Aromatase inhibitors (letrozole) for subfertile women with polycystic ovary syndrome. Cochrane Database Syst Rev. 2018;5(5):CD010287. doi: 10.1002/14651858.CD010287.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Christou F, Pitteloud N, Gomez F. The induction of ovulation by pulsatile administration of GnRH: an appropriate method in hypothalamic amenorrhea. Gynecol Endocrinol. 2017;33(8):598–601. doi: 10.1080/09513590.2017.1296948 [DOI] [PubMed] [Google Scholar]
- 49.Nakamura Y, Ono M, Yoshida Y, Sugino N, Ueda K, Kato H. Effects of clomiphene citrate on the endometrial thickness and echogenic pattern of the endometrium. Fertil Steril. 1997;67(2):256–260. doi: 10.1016/S0015-0282(97)81907-3 [DOI] [PubMed] [Google Scholar]
- 50.Mitwally MFM, Casper RF. Use of an aromatase inhibitor for induction of ovulation in patients with an inadequate response to clomiphene citrate. Fertil Steril. 2001;75(2):305–309. doi: 10.1016/S0015-0282(00)01705-2 [DOI] [PubMed] [Google Scholar]
- 51.Practice Committee of the American Society for Reproductive Medicine. Prevention and treatment of moderate and severe ovarian hyperstimulation syndrome: a guideline. 2016;106(7):1634–1647. doi: 10.1016/j.fertnstert.2016.08.048 [DOI] [PubMed] [Google Scholar]
- 52.Practice Committees of the American Society for Reproductive Medicine and Society for Reproductive Endocrinology and Infertility. Use of exogenous gonadotropins for ovulation induction in anovulatory women: a committee opinion. Fertil Steril. 2020;113(1):66–70. doi: 10.1016/j.fertnstert.2019.09.020 [DOI] [PubMed] [Google Scholar]
- 53.Homburg R, Eshel A, Armar NA, et al. One hundred pregnancies after treatment with pulsatile luteinising hormone releasing hormone to induce ovulation. BMJ. 1989;298(6676):809–812. doi: 10.1136/bmj.298.6676.809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hurley DM, Brian R, Outch K, et al. Induction of ovulation and fertility in amenorrheic women by pulsatile low-dose gonadotropin-releasing hormone. N Engl J Med. 1984;310(17):1069–1074. doi: 10.1056/NEJM198404263101702 [DOI] [PubMed] [Google Scholar]
- 55.Webster J, Piscitelli G, Polli A, Ferrari CI, Ismail I, Scanlon MF; Cabergoline Comparative Study Group. A comparison of cabergoline and bromocriptine in the treatment of hyperprolactinemic amenorrhea. N Engl J Med. 1994;331(14):904–909. doi: 10.1056/NEJM199410063311403 [DOI] [PubMed] [Google Scholar]
- 56.Weiss NS, Kostova E, Nahuis M, Mol BWJ, van der Veen F, van Wely M. Gonadotrophins for ovulation induction in women with polycystic ovary syndrome. Cochrane Database Syst Rev. 2019;1(1): CD010290. doi: 10.1002/14651858.CD010290.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cissen M, Bensdorp A, Cohlen BJ, Repping S, de Bruin JP, van Wely M. Assisted reproductive technologies for male subfertility. Cochrane Database Syst Rev. 2016;2(2):CD000360. doi: 10.1002/14651858.CD000360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bhattacharya S, Harrild K, Mollison J, et al. Clomifene citrate or unstimulated intrauterine insemination compared with expectant management for unexplained infertility: pragmatic randomised controlled trial. BMJ. 2008;337:a716. doi: 10.1136/bmj.a716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Scott RT Jr, Upham KM, Forman EJ, et al. Blastocyst biopsy with comprehensive chromosome screening and fresh embryo transfer significantly increases in vitro fertilization implantation and delivery rates: a randomized controlled trial. Fertil Steril. 2013;100(3):697–703. doi: 10.1016/j.fertnstert.2013.04.035 [DOI] [PubMed] [Google Scholar]
- 60.Rubio C, Bellver J, Rodrigo L, et al. In vitro fertilization with preimplantation genetic diagnosis for aneuploidies in advanced maternal age: a randomized, controlled study. Fertil Steril. 2017; 107(5):1122–1129. doi: 10.1016/j.fertnstert.2017.03.011 [DOI] [PubMed] [Google Scholar]
- 61.Penzias A, Bendikson K, Butts S, et al. ; Practice Committees of the American Society for Reproductive Medicine; Society for Assisted Reproductive Technology. The use of preimplantation genetic testing for aneuploidy (PGT-A): a committee opinion. Fertil Steril. 2018;109 (3):429–436. doi: 10.1016/j.fertnstert.2018.01.002 [DOI] [PubMed] [Google Scholar]
- 62.Victor AR, Tyndall JC, Brake AJ, et al. One hundred mosaic embryos transferred prospectively in a single clinic: exploring when and why they result in healthy pregnancies. Fertil Steril. 2019;111 (2):280–293. doi: 10.1016/j.fertnstert.2018.10.019 [DOI] [PubMed] [Google Scholar]
- 63.Fragouli E, Alfarawati S, Spath K, et al. Analysis of implantation and ongoing pregnancy rates following the transfer of mosaic diploid-aneuploid blastocysts. Hum Genet. 2017;136(7):805–819. doi: 10.1007/s00439-017-1797-4 [DOI] [PubMed] [Google Scholar]
- 64.Brännström M, Johannesson L, Bokström H, et al. Livebirth after uterus transplantation. Lancet. 2015;385(9968):607–616. doi: 10.1016/S0140-6736(14)61728-1 [DOI] [PubMed] [Google Scholar]
- 65.Ejzenberg D, Andraus W, Baratelli Carelli Mendes LR, et al. Livebirth after uterus transplantation from a deceased donor in a recipient with uterine infertility. Lancet. 2019;392 (10165):2697–2704. doi: 10.1016/S0140-6736(18)31766-5 [DOI] [PubMed] [Google Scholar]
- 66.Reindollar RH, Regan MM, Neumann PJ, et al. A randomized clinical trial to evaluate optimal treatment for unexplained infertility: the Fast Track and Standard Treatment (FASTT) trial. Fertil Steril. 2010;94(3):888–899. doi: 10.1016/j.fertnstert.2009.04.022 [DOI] [PubMed] [Google Scholar]
- 67.Dovey S, Sneeringer RM, Penzias AS. Clomiphene citrate and intrauterine insemination: analysis of more than 4100 cycles. Fertil Steril. 2008;90(6):2281–2286. doi: 10.1016/j.fertnstert.2007.10.057 [DOI] [PubMed] [Google Scholar]
- 68.Centers for Disease Control and Prevention. 2017 Assisted Reproductive Technology Fertility Clinic Success Rates Report. Centers for Disease Control and Prevention; 2017. [Google Scholar]
- 69.Goldman MB, Thornton KL, Ryley D, et al. A randomized clinical trial to determine optimal infertility treatment in older couples: the Forty and Over Treatment Trial (FORT-T). Fertil Steril. 2014;101(6):1574–81.e1, 2. doi: 10.1016/j.fertnstert.2014.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sermondade N, Huberlant S, Bourhis-Lefebvre V, et al. Female obesity is negatively associated with live birth rate following IVF: a systematic review and meta-analysis. Hum Reprod Update. 2019;25(4): 439–451. doi: 10.1093/humupd/dmz011 [DOI] [PubMed] [Google Scholar]
- 71.Legro RS, Dodson WC, Kunselman AR, et al. Benefit of delayed fertility therapy with preconception weight loss over immediate therapy in obese women with PCOS. J Clin Endocrinol Metab. 2016;101(7):2658–2666. doi: 10.1210/jc.2016-1659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Einarsson S, Bergh C, Friberg B, et al. Weight reduction intervention for obese infertile women prior to IVF: a randomized controlled trial. Hum Reprod. 2017;32(8):1621–1630. doi: 10.1093/humrep/dex235 [DOI] [PubMed] [Google Scholar]
- 73.Kluge L, Bergh C, Einarsson S, Pinborg A, Mikkelsen Englund A-L, Thurin-Kjellberg A. Cumulative live birth rates after weight reduction in obese women scheduled for IVF: follow-up of a randomized controlled trial. Hum Reprod Open. 2019;2019(4):hoz030. doi: 10.1093/hropen/hoz030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Reynolds KA, Boudoures AL, Chi MM, Wang Q, Moley KH. Adverse effects of obesity and/or high-fat diet on oocyte quality and metabolism are not reversible with resumption of regular diet in mice. Reprod Fertil Dev. 2015;27(4):716–724. doi: 10.1071/RD14251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Legro RS, Barnhart HX, Schlaff WD, et al. ; Cooperative Multicenter Reproductive Medicine Network. Clomiphene, metformin, or both for infertility in the polycystic ovary syndrome. N Engl J Med. 2007;356(6):551–566. doi: 10.1056/NEJMoa063971 [DOI] [PubMed] [Google Scholar]
- 76.Gaskins AJ, Nassan FL, Chiu YH, et al. ; EARTH Study Team. Dietary patterns and outcomes of assisted reproduction. Am J Obstet Gynecol. 2019; 220(6):567.e1–567.e18. doi: 10.1016/j.ajog.2019.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Salas-Huetos A, Bulló M, Salas-Salvadó J. Dietary patterns, foods and nutrients in male fertility parameters and fecundability: a systematic review of observational studies. Hum Reprod Update. 2017;23(4):371–389. doi: 10.1093/humupd/dmx006 [DOI] [PubMed] [Google Scholar]
- 78.Pfeifer S, Butts S, Fossum G, et al. ; Practice Committee of the American Society for Reproductive Medicine; Society for Reproductive Endocrinology and Infertility. Optimizing natural fertility: a committee opinion. Fertil Steril. 2017;107(1):52–58. doi: 10.1016/j.fertnstert.2016.09.029 [DOI] [PubMed] [Google Scholar]
- 79.Wen J, Jiang J, Ding C, et al. Birth defects in children conceived by in vitro fertilization and intracytoplasmic sperm injection: a meta-analysis. Fertil Steril. 2012;97(6):1331–1337. doi: 10.1016/j.fertnstert.2012.02.053 [DOI] [PubMed] [Google Scholar]
- 80.Davies MJ, Moore VM, Willson KJ, et al. Reproductive technologies and the risk of birth defects. N Engl J Med. 2012;366(19):1803–1813. doi: 10.1056/NEJMoa1008095 [DOI] [PubMed] [Google Scholar]
- 81.Sharma S, Ghosh S, Singh S, et al. Congenital malformations among babies born following letrozole or clomiphene for infertility treatment. PLoS One. 2014;9(10):e108219. doi: 10.1371/journal.pone.0108219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tulandi T, Martin J, Al-Fadhli R, et al. Congenital malformations among 911 newborns conceived after infertility treatment with letrozole or clomiphene citrate. Fertil Steril. 2006;85(6):1761–1765. doi: 10.1016/j.fertnstert.2006.03.014 [DOI] [PubMed] [Google Scholar]
- 83.Forman R, Gill S, Moretti M, Tulandi T, Koren G, Casper R. Fetal safety of letrozole and clomiphene citrate for ovulation induction. J Obstet Gynaecol Can. 2007;29(8):668–671. doi: 10.1016/S1701-2163(16)32551-8 [DOI] [PubMed] [Google Scholar]
- 84.Akbari Sene A, Ghorbani S, Ashrafi M. Comparison of the pregnancy outcomes and the incidence of fetal congenital abnormalities in infertile women treated with letrozole and clomiphene citrate. J Obstet Gynaecol Res. 2018;44(6):1036–1041. doi: 10.1111/jog.13644 [DOI] [PubMed] [Google Scholar]
- 85.Qin J, Wang H, Sheng X, Liang D, Tan H, Xia J. Pregnancy-related complications and adverse pregnancy outcomes in multiple pregnancies resulting from assisted reproductive technology: a meta-analysis of cohort studies. Fertil Steril. 2015;103(6):1492–508.e1, 7. doi: 10.1016/j.fertnstert.2015.03.018 [DOI] [PubMed] [Google Scholar]
- 86.Rizzuto I, Behrens RF, Smith LA. Risk of ovarian cancer in women treated with ovarian stimulating drugs for infertility. Cochrane Database Syst Rev. 2019;6:CD008215. doi: 10.1002/14651858.CD008215.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Skalkidou A, Sergentanis TN, Gialamas SP, et al. Risk of endometrial cancer in women treated with ovary-stimulating drugs for subfertility. Cochrane Database Syst Rev. 2017;3(3):CD010931. doi: 10.1002/14651858.CD010931.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pfeifer S, Butts S, Dumesic D, et al. ; Practice Committee of the American Society for Reproductive Medicine. Fertility drugs and cancer: a guideline. Fertil Steril. 2016;106(7):1617–1626. doi: 10.1016/j.fertnstert.2016.08.035 [DOI] [PubMed] [Google Scholar]
- 89.Eisenberg ML, Li S, Brooks JD, Cullen MR, Baker LC. Increased risk of cancer in infertile men: analysis of US claims data. J Urol. 2015;193(5):1596–1601. doi: 10.1016/j.juro.2014.11.080 [DOI] [PubMed] [Google Scholar]
- 90.Legro RS, Arslanian SA, Ehrmann DA, et al. ; Endocrine Society. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2013;98(12):4565–4592. doi: 10.1210/jc.2013-2350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.ACOG committee opinion number 605: primary ovarian insufficiency in adolescents and young women. Obstetrics. 2002;99(4):679–680. doi: 10.1016/S0029-7844(02)01986-5 [DOI] [PubMed] [Google Scholar]
- 92.Gleason JL, Shenassa ED, Thoma ME. Self-reported infertility, metabolic dysfunction, and cardiovascular events: a cross-sectional analysis among US women. Fertil Steril. 2019;111(1):138–146. doi: 10.1016/j.fertnstert.2018.10.009 [DOI] [PubMed] [Google Scholar]
- 93.Stentz NC, Koelper N, Barnhart KT, Sammel MD, Senapati S. Infertility and mortality. Am J Obstet Gynecol. 2020;222(3):251.e1–251.e10. doi: 10.1016/j.ajog.2019.09.007 [DOI] [PubMed] [Google Scholar]
- 94.Salonia A, Matloob R, Gallina A, et al. Are infertile men less healthy than fertile men? results of a prospective case-control survey. Eur Urol. 2009;56(6):1025–1031. doi: 10.1016/j.eururo.2009.03.001 [DOI] [PubMed] [Google Scholar]
- 95.Glazer CH, Bonde JP, Giwercman A, et al. Risk of diabetes according to male factor infertility: a register-based cohort study. Hum Reprod. 2017;32(7):1474–1481. doi: 10.1093/humrep/dex097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fakih MH, El Shmoury M, Szeptycki J, et al. The AUGMENT treatment: physician reported outcomes of the initial global patient experience. J Fertil. 2015;03(03). doi: 10.4172/2375-4508.1000154 [DOI] [Google Scholar]
- 97.Oktay K, Baltaci V, Sonmezer M, et al. Oogonial precursor cell-derived autologous mitochondria injection to improve outcomes in women with multiple IVF failures due to low oocyte quality: a clinical translation. Reprod Sci. 2015;22(12):1612–1617. doi: 10.1177/1933719115612137 [DOI] [PubMed] [Google Scholar]
- 98.Labarta E, de Los Santos MJ, Herraiz S, et al. Autologous mitochondrial transfer as a complementary technique to intracytoplasmic sperm injection to improve embryo quality in patients undergoing in vitro fertilization: a randomized pilot study. Fertil Steril. 2019;111(1): 86–96. doi: 10.1016/j.fertnstert.2018.09.023 [DOI] [PubMed] [Google Scholar]


