Abstract
Multisystem inflammatory syndrome in children (MIS-C) after COVID-19 is commonly associated with cardiac involvement. Studies found myocardial dysfunction, as measured by decreased ejection fraction and abnormal strain, to be common early in illness. However, there is limited data on longitudinal cardiac outcomes. We aim to describe the evolution of cardiac findings in pediatric MIS-C from acute illness through at least 2-month follow-up. A retrospective single-center review of 36 patients admitted with MIS-C from April 2020 through September 2021 was performed. Echocardiographic data including cardiac function and global longitudinal strain (GLS) were analyzed at initial presentation, discharge, 2–4-week follow-up, and at least 2-month follow-up. Patients with mild and severe disease, normal and abnormal left ventricular ejection fraction (LVEF), and normal and abnormal GLS at presentation were compared. On presentation, 42% of patients with MIS-C had decreased LVEF < 55%. In patients in whom GLS was obtained (N = 18), 44% were abnormal (GLS < |− 18|%). Of patients with normal LVEF, 22% had abnormal GLS. There were no significant differences in troponin or brain natriuretic peptide between those with normal and abnormal LVEF. In most MIS-C patients with initial LVEF < 55% (90%), LVEF normalized upon discharge. At 2-month follow-up, all patients had normal LVEF with 21% having persistently abnormal GLS. Myocardial systolic dysfunction and abnormal deformation were common findings in MIS-C at presentation. While EF often normalized by 2 months, persistently abnormal GLS was more common, suggesting ongoing subclinical dysfunction. Our study offers an optimistic outlook for recovery in patients with MIS-C and carditis, however ongoing investigation for longitudinal effects is warranted.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00246-022-02972-3.
Keywords: Multisystem inflammatory syndrome in children, Echocardiography, Left ventricular function, Global longitudinal strain, Coronary arteries
Background
The novel severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) identified in 2019 causing coronavirus disease 2019 (COVID-19) became a global pandemic in 2020. As of February 2022, there have been an estimated 424,000,000 confirmed cases with 5,800,000 deaths worldwide [1]. Initially, reports suggested that children experienced a mild form of COVID-19. However, in April 2020, the UK documented cases of children with a similar presentation to Kawasaki Disease (KD) or toxic shock syndrome. Subsequent worldwide experience has described children with fever, severe multisystem inflammation, and shock, leading to a newly recognized condition by the Centers for Disease Control and Prevention (CDC) and World Health Organization (WHO) termed multisystem inflammatory syndrome in children (MIS-C) associated with COVID-19 [2]. Cardiac involvement in MIS-C is common, with echocardiography demonstrating left ventricular (LV) dysfunction, coronary artery (CA) dilation, valvular regurgitation, and pericardial effusion [3]. Several large case series have found 30–40% with decreased LV function and 8–24% with CA abnormalities early in the illness [3–7]. A few studies have also reported abnormal strain in patients with LV dysfunction at time of admission [8–11]; however, there is limited data on longitudinal cardiac outcomes in these patients.
Two-dimensional speckle-tracking echocardiography (STE) allows for assessment of subtle changes in myocardial velocities and deformation [12]. Clinical application of STE in pediatric cardiology continues to evolve, including strain assessment in patients with myocarditis, muscular dystrophy, and after chemotherapy which may detect subclinical LV systolic dysfunction in the presence of a normal ejection fraction (EF) [12]. Patients with acute KD or acute myocarditis with normal LV EF have been shown to have abnormal strain suggesting it may be a more sensitive indicator of myocardial dysfunction [13].
We aim to describe the evolution of cardiac findings in pediatric MIS-C from initial diagnosis through at least 2-month follow-up.
Methods
All children who were admitted with MIS-C to UPMC Children’s Hospital of Pittsburgh from April 2020 through September 2021 were identified for analysis. All met the CDC diagnostic criteria for MIS-C [14], affirmed by real-time multidisciplinary institutional committee review of all possible MIS-C cases for mandated reporting to the state health department. Demographics, hospital outcomes, and laboratory and treatment data were collected from medical records. Based on a national consensus pathway for management of MIS-C, patients were stratified into mild disease with no respiratory support, vasoactive requirement, or cardiac dysfunction, and severe disease with significant respiratory support, vasoactive requirement, or ventricular dysfunction [15]. Echocardiographic data were reviewed in the acute and follow-up period.
Echocardiography
All patients underwent transthoracic, two-dimensional echocardiograms with Doppler at the time of diagnosis by pediatric cardiac sonographers using either Phillips IE33, Epiq 7C (Philips Medical Systems, Andover, MA), or GE VIVID E95 (GE Healthcare, Chicago, IL) ultrasound machines. Follow-up echocardiograms were routinely performed at discharge, 2–4-week follow-up (short-term), and > 2-month (medium-term) follow-up with more or less frequent echocardiograms per clinician judgment. LVEF, LV end-diastolic and systolic volumes and dimensions, fractional shortening (FS), right ventricular function by tricuspid annular plane systolic excursion (TAPSE), coronary artery dimensions, qualitative grades of valvular regurgitation, and pericardial effusion size were abstracted from the clinical echocardiogram reports produced at the time of study acquisition. Z-scores were indexed to body surface area using the Boston z-score database [16]. LVEF was defined as normal ≥ 55%, mildly reduced 41–54%, moderately reduced 30–40%, and severely reduced < 30%. Coronary artery abnormalities were defined and classified according to the American Heart Association guidelines (dilation: Z-score 2 to < 2.5 or z-score < 2 with decrease in z-score ≥ 1 on follow-up echocardiogram, small aneurysm: Z-score ≥ 2.5 to 5, medium aneurysm: Z-score ≥ 5 to < 10, large or giant aneurysm: ≥ 10) [17].
Speckle tracking analysis was performed on echocardiographic images using QLAB Autostrain LV version 13.0 (Phillips Ultrasound, Inc, Bothell, WA) to provide global longitudinal strain (GLS) based on apical two, three, and four chamber views. Strain data were not available for every patient due to limited image quality or technical software limitations.
Cardiac Magnetic Resonance Imaging
A small subset of the MIS-C cohort underwent clinical cardiac magnetic resonance imaging (CMR) on a 1.5 T GE Signa HDxt scanner (GE Healthcare, Waukesha, WI) including functional analysis by standard cine SSFP and evaluation for myocardial fibrosis using late myocardial enhancement imaging. CMR images were reviewed by a single cardiologist (ABC) and feature tracking strain was estimated using CVI42 V5.13 (Circle cardiovascular Inc, Calgary, Canada).
Statistics
Summary data are presented as mean ± standard deviation, median and interquartile range (IQR), or count and percentage, as appropriate. Comparisons of MIS-C patients with mild and severe disease, normal and abnormal LVEF (< 55%) at initial echocardiogram, and normal and abnormal GLS (< |− 18|%) at initial echocardiogram were made using Mann–Whitney tests (continuous measures) or Chi-square or Fisher’s exact tests (categorical measures). Analysis was performed using SAS v9.4 (copyright 2016 SAS Institute Inc., Cary, NC, USA) and for all comparisons a two-sided alpha < 0.05 was considered significant.
Results
Patient Characteristics
Thirty-six patients with MIS-C were identified. Demographic and clinical data of the subjects are represented in Table 1. MIS-C patients had an average age of 9.7 ± 4.4 years, and there was a trend toward increased prevalence among black children. The average length of stay (LOS) was 7.1 ± 5.8 days with 80.6% requiring ICU admission. ECG abnormalities were common, occurring in 52.8% of patients, with the majority having ST-T wave abnormalities, and 5% having first-degree atrioventricular block.
Table 1.
Demographic and clinical data of MIS-C patients, patients normal and abnormal LVEF, and patients with normal and abnormal global longitudinal strain on presentation
| MIS-C (N = 36) | Normal LVEF (N = 15) | Abnormal LVEF (N = 18) | p-value | Normal Strain (N = 10) | Abnormal Strain (N = 8) | p-value | |
|---|---|---|---|---|---|---|---|
| Age (years) | 9.7 ± 4.4 | 7.7 ± 3.5 | 11.4 ± 4.8 | 0.02 | 9.3 ± 3.0 | 8.6 ± 3.8 | 0.66 |
| Female | 15 (41.7%) | 7 (46.7%) | 7 (38.9%) | 0.73 | 3 (30.0%) | 2 (25.0%) | 1.00 |
| BMI (kg/m2) | 22.4 ± 7.6 | 20.1 ± 6.5 | 25.1 ± 8.1 | 0.11 | 18.9 ± 5.9 | 23.3 (8.1%) | 0.28 |
| LOS (days) | 7.1 ± 5.8 | 5.9 ± 4.0 | 8.2 ± 7.0 | 0.44 | 8.6 ± 5.2 | 6.3 ± 2.9 | 0.57 |
| ICU Admission | 29 (80.6%) | 14 (93.3%) | 12 (66.7%) | 0.10 | 8 (80.0%) | 6 (75.0%) | 1.00 |
| Race | 0.49 | 1.00 | |||||
| White | 22/35 (62.9%) | 10 (66.7%) | 9/17 (52.9%) | 6 (60.0%) | 4/7 (57.1%) | ||
| Black | 13/35 (37.1%) | 5 (33.3%) | 8/17 (47.1%) | 4 (40.0%) | 3/7 (42.9%) | ||
| Asian | 0/35 (0.0%) | 0 (0.0%) | 0/17 (0.0%) | 0 (0.0%) | 0/7 (0.0%) | ||
| COVID testing | |||||||
| PCR + | 11/34 (32.4%) | 3/14 (21.4%) | 6/17 (35.3%) | 0.46 | 4 (40.0%) | 2 (25.0%) | 0.64 |
| Serology + | 33/33 (100%) | 14/14 (100%) | 16/16 (100%) | – | 10 (100%) | 7/7 (100%) | – |
| ECG | |||||||
| Normal | 17/36 (47.2%) | 7 (46.7%) | 8 (44.4%) | 1.00 | 4 (40.0%) | 4 (50.0%) | 1.00 |
| ST-T wave abnormality | 17/36 (47.2%) | 8 (53.3%) | 8 (44.4%) | 0.73 | 5 (50.0%) | 4 (50.0%) | 1.00 |
| Labs | |||||||
| Troponin I, initial (ng/mL) | 0.24 ± 0.44 | 0.18 ± 0.21 | 0.32 ± 0.59 | 0.67 | 0.34 ± 0.69 | 0.08 ± 0.09 | 0.74 |
| Troponin I, peak (ng/mL) | 0.54 ± 1.10 | 0.24 ± 0.24 | 0.88 ± 1.51 | 0.56 | 1.17 ± 1.93 | 0.10 ± 0.13 | 0.25 |
| BNP, initial (pg/mL) | 470.1 ± 628.0 | 529.1 ± 368.7 | 494.1 ± 825.4 | 0.16 | 362.5 ± 463.1 | 462.3 ± 398.6 | 0.46 |
| BNP, peak (pg/mL) | 943.0 ± 884.9 | 964.7 ± 539.1 | 869.6 ± 981.0 | 0.14 | 1023.5 ± 1012.5 | 867.5 ± 497.0 | 1.00 |
| WBC (× 109 cells/L) | 9.8 ± 4.2 | 10.7 ± 3.9 | 9.4 ± 4.6 | 0.26 | 9.1 ± 3.4 | 13.3 ± 3.4 | 0.03 |
| Hgb (g/dL) | 11.7 ± 1.3 | 11.7 ± 1.6 | 11.6 ± 1.2 | 0.86 | 12.4 ± 1.9 | 11.2 ± 0.8 | 0.26 |
| Platelets (× 109 cells/L) | 182.4 ± 97.3 | 157.5 ± 53.8 | 185.4 ± 89.4 | 0.46 | 187.7 ± 87.1 | 169.3 ± 57.3 | 0.97 |
| ANC (× 109 cells/L) | 8.4 ± 3.8 | 9.1 ± 3.8 | 8.0 ± 4.2 | 0.29 | 7.7 ± 3.2 | 11.7 ± 3.4 | 0.03 |
| ALC (× 109 cells/L) | 0.87 ± 0.68 | 0.95 ± 0.85 | 0.70 ± 0.43 | 0.43 | 0.68 ± 0.30 | 0.87 ± 0.60 | 0.79 |
| ESR (mm/hr) | 42.7 ± 24.4 | 39.1 ± 25.5 | 47.1 ± 25.0 | 0.35 | 39.0 ± 22.9 | 45.6 ± 30.7 | 0.85 |
| CRP (mg/dL) | 15.5 ± 8.4 | 14.7 ± 8.2 | 17.6 ± 8.4 | 0.16 | 12.2 ± 6.6 | 20.8 ± 10.4 | 0.09 |
| Procalcitonin (ng/mL) | 11.4 ± 14.0 | 13.1 ± 12.6 | 11.5 ± 16.2 | 0.27 | 17.0 ± 18.6 | 14.1 ± 16.7 | 0.90 |
| D-dimer (µg/mL) | 4.3 ± 3.7 | 4.8 ± 4.5 | 3.7 ± 2.4 | 0.36 | 5.8 ± 5.5 | 4.3 ± 1.4 | 0.83 |
| Fibrinogen (mg/dL) | 466.3 ± 152.2 | 435.6 ± 201.0 | 494.8 ± 123.2 | 0.23 | 461.3 ± 71.4 | 489.8 ± 279.0 | 1.00 |
| Ferritin (ng/mL) | 695.1 ± 771.5 | 870.6 ± 996.3 | 600.2 ± 566.2 | 0.37 | 791.8 ± 822.2 | 1057.4 ± 1258.8 | 0.46 |
| Creatinine (mg/dL) | 0.71 ± 0.39 | 0.63 ± 0.32 | 0.81 ± 0.46 | 0.23 | 0.64 ± 0.32 | 0.92 ± 0.63 | 0.63 |
| AST (unit/L) | 54.1 ± 48.3 | 57.7 ± 43.8 | 54.2 ± 55.5 | 0.67 | 60.6 ± 70.0 | 64.3 ± 60.4 | 1.00 |
| ALT (unit/L) | 54.6 ± 49.1 | 48.3 ± 34.3 | 62.3 ± 61.3 | 0.69 | 55.0 ± 73.2 | 61.5 ± 39.3 | 0.16 |
| Treatment | |||||||
| Anticoagulation | 11 (30.6%) | 3 (20.0%) | 8 (44.4%) | 0.27 | 3 (30.0%) | 2 (25.0%) | 1.00 |
| ASA | 34 (94.4%) | 15 (100%) | 16 (88.9%) | 0.49 | 9 (90.0%) | 8 (100%) | 1.00 |
| IVIg | 36 (100%) | 15 (100%) | 18 (100%) | – | 10 (100%) | 8 (100%) | – |
| Steroids | 36 (100%) | 15 (100%) | 18 (100%) | – | 10 (100%) | 8 (100%) | – |
| Infliximab | 3 (8.3%) | 0 (0.0%) | 2 (11.1%) | 0.49 | 2 (20.0%) | 0 (0.0%) | 0.48 |
| Anakinra | 10 (27.8%) | 3 (20.0%) | 5 (27.8%) | 0.7 | 6 (60.0%) | 2 (25.0%) | 0.19 |
Values are a number (%) or mean ± standard deviation. p-value <0.05 are bolded
ALC absolute lymphocyte count; ALT alanine aminotransferase; ANC absolute neutrophil count; ASA aspirin; AST aspartate aminotransferase; BMI body mass index; BNP brain natriuretic peptide; CRP C-reactive protein; ECG electrocardiogram; ESR erythrocyte sedimentation rate; GLS global longitudinal strain; Hgb hemoglobin; ICU intensive care unit; IVIg intravenous immunoglobulin; LOS length of stay; LVEF left ventricular ejection fraction; PCR polymerase chain reaction; WBC white blood cell count
On admission, 16 patients (44%) in the MIS-C cohort had an elevated troponin-I (≥ 0.1 ng/mL) and 22 (61%) had an elevated brain natriuretic peptide (BNP ≥ 100 pg/mL). All MIS-C patients had elevated acute phase reactants and 31 (86%) had lymphopenia.
All MIS-C patients were treated with intravenous immunoglobulin and systemic steroids, with 10 (28%) receiving additional treatment with anakinra and 3 (8%) with infliximab. Most patients (94%) received aspirin therapy.
Of the 36 MIS-C patients, 15 (42%) demonstrated decreased LVEF, with all having mildly reduced function (49.7 ± 3.9%). MIS-C patients with abnormal LVEF on presentation were older than those with normal LVEF (11.4 ± 4.8 years vs. 7.7 ± 3.5 years; p = 0.02). There were no significant differences in hospital LOS or ICU admission between the MIS-C patients with normal and abnormal LVEF. We also did not find a significant difference between initial or peak troponin levels or initial or peak BNP levels between the groups. Among those in whom strain analysis was also completed (N = 18), 8 (44%) demonstrated abnormal global longitudinal strain (GLS < |− 18|%). There were no observed differences in demographics, hospital LOS, ICU admission, or troponin or BNP values among those with normal versus abnormal GLS. Those with abnormal strain had a higher WBC count (9.1 ± 3.4 × 109cells/L vs 13.3 ± 3.4 × 109cells/L; p = 0.03) and ANC (7.7 ± 3.2 × 109cells/L vs 11.7 ± 3.4 × 109cells/L; p = 0.03) than those with normal strain.
Twenty-seven of the 36 MIS-C patients had evidence of severe disease. Clinical data of patients based on severity of disease are shown in Supplemental Table 1. Patients with severe disease had higher incidence of ICU admission (96.3% vs. 33.3%; p = 0.0003) and need for vasoactive support. None of the patients with mild disease had abnormal LVEF, while 55.6% of those with severe disease had decreased LVEF (p = 0.0046). GLS was not significantly different between the two groups.
Echocardiographic Trends in MIS-C
2D Echo and Color Doppler Parameters
Echocardiographic data in the MIS-C cohort are shown in Table 2. On initial presentation, all patients had echocardiograms available to review. At discharge (median 3.5 days from admission, IQR 3-8.25), 24 (67%) patients had repeat echocardiography. A total of 32 (89%) patients had short-term follow-up (median 24.5 days, IQR 19.75-38) and 17 (47%) had medium-term follow-up (median 135 days, IQR 57-191). LVEF improved by discharge in most patients (90%) and normalized by medium-term follow-up in all patients (Fig. 1). A small percentage of patients had more than mild valvular regurgitation initially (tricuspid more frequent than mitral), with all returning to normal by short-term follow-up. All patients with pericardial effusion on initial echocardiogram (19%) showed resolution by medium-term follow-up.
Table 2.
Serial echocardiographic data of MIS-C patients
| Initial | Discharge | Short-term | Medium-term | |
|---|---|---|---|---|
| Patients in group | 36 | 24 | 32 | 17 |
| Days from Admission to echocardiography (median (IQR)) | 1 (0–1) | 3.5 (3–8.25) | 24.5 (19.75–38) | 135 (57–191) |
| LVEF % | 56.5 ± 7.5 | 62.3 ± 5.0 | 60.4 ± 4.4 | 60.4 ± 3.5 |
| Mildly reduced | 15/36 (42%) | 1/24 (4%) | 3/29 (10%) | 0/17 (0%) |
| Moderately reduced | 0/33 (0%) | 0/24 (0%) | 0/29 (0%) | 0/17 (0%) |
| Severely reduced | 0/33 (0%) | 0/24 (0%) | 0/29 (0%) | 0/17 (0%) |
| LVEDV (mL) | 94.2 ± 46.1 | 96.8 ± 33.3 | 89.3 ± 41.6 | 91.7 ± 35.8 |
| LVEDV (z-score) | − 0.02 ± 2.36 | 0.06 ± 1.30 | − 0.68 ± 1.28 | − 0.53 ± 1.35 |
| Fractional shortening % | 34.4 ± 5.4 | 36.9 ± 4.5 | 36.4 ± 4.4 | 38.0 ± 4.7 |
| FS (z-score) | − 0.55 ± 1.87 | 0.28 ± 1.48 | 0.17 ± 1.27 | 0.65 ± 1.33 |
| TAPSE (z-score) | − 0.24 ± 3.29 | 1.00 ± 2.58 | − 0.42 ± 2.29 | − 0.17 ± 2.14 |
| Mitral valve regurgitation (> mild) | 1/35 (2%) | 0/24 (0%) | 0/32 (0%) | 0/17 (0%) |
| Tricuspid valve regurgitation (> mild) | 3 (8%) | 3/24 (13%) | 0/32 (0%) | 0/17 (0%) |
| Coronary arteries | ||||
| RCA (z-score) | 0.68 ± 2.43 | 0.37 ± 2.27 | 0.09 ± 2.41 | 0.04 ± 3.70 |
| LMCA (z-score) | 0.13 ± 1.12 | −0.22 ± 0.99 | − 0.51 ± 0.87 | − 0.33 ± 0.65 |
| LAD (z-score) | 0.54 ± 1.67 | 0.28 ± 2.14 | − 0.05 ± 2.73 | 0.06 ± 3.65 |
| Pericardial effusion (≥ trace) | 7 (19%) | 8 (33%) | 4/31 (13%) | 0 (0%) |
| GLS % | − 17.7 ± 4.3 (N = 18) | − 19.0 ± 4.2 (N = 13) | − 18.8 ± 2.3 (N = 23) | − 21.0 ± 3.0 (N = 14) |
| Abnormal GLS ( <|-18|%) | 8/18 (44%) | 5/13 (39%) | 9/23 (39%) | 3/14 (21%) |
Values are a number (%) or mean ± standard deviation
FS fractional shortening; GLS global longitudinal strain; IQR interquartile range; LAD left anterior descending; LMCA left main coronary artery; LOS length of stay; LVEDV left ventricular end-diastolic volume; LVEF left ventricular ejection fraction; RCA right coronary artery; TAPSE tricuspid annular plane systolic excursion
Fig. 1.
Box and whisker plots of global longitudinal strain (GLS) (above) and left ventricular ejection fraction (LVEF) (below) on serial assessment in MIS-C. LVEF and GLS at medium-term is significantly improved from admission (LVEF p = 0.03; GLS p = 0.01)
Global Longitudinal Strain
GLS had gradual improvement through follow-up (Fig. 1). Though mean GLS improved over time, 21% of patients had persistently abnormal GLS at medium-term follow-up.
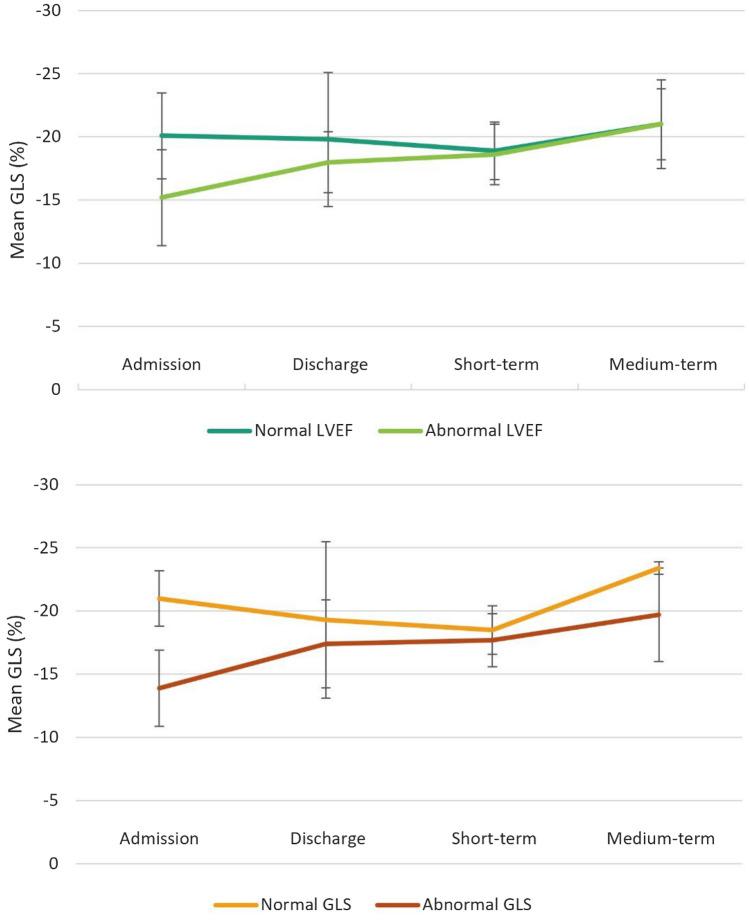
On subgroup analysis of patients with normal versus abnormal LVEF at presentation, GLS was lower in the abnormal LVEF group compared to normal LVEF on initial echocardiogram (− 15.2 ± 3.8% vs. − 20.1 ± 3.4%; p = 0.01). At medium-term follow-up, average GLS was similar in both groups (Fig. 2). Among the patients with normal LVEF initially, 22% had abnormal GLS. Of patients in the normal LVEF group who had medium-term follow-up, 2/6 had abnormal strain.
Fig. 2.
Comparison of mean global longitudinal strain (GLS) values over time for patients with normal and abnormal LVEF (above) and patients with normal and abnormal GLS (below). Error bars represent standard deviation
Subgroup analysis of patients with normal and abnormal strain on presentation revealed a lower initial LVEF in patients with abnormal strain (52.6 ± 4.4% vs. 60.2 ± 9.9%; p = 0.046). While GLS improved in both groups at follow-up, average GLS remained lower in the abnormal GLS group at medium-term follow-up (Fig. 2).
Severity of Disease
Subgroup analysis of left ventricular function in patients with mild and severe disease are displayed in Supplemental Table 2. While average LVEF was normal in both groups on admission, LVEF was significantly lower in those with severe disease (55.5 ± 7.7% vs 61.8 ± 4.6%; p = 0.026). Ejection fraction improved upon discharge with no significant difference between groups. Though LVEF remained normal at follow-up, LVEF was significantly lower in those with severe disease at both short-term and medium-term follow-up (Fig. 3).
Fig. 3.
Comparison of mean global longitudinal strain (GLS) over time (below) and mean LVEF over for time (above) for patients with mild and severe disease. Error bars represent standard deviation
There was no significant difference in GLS on presentation in those with mild or severe disease. While GLS improved in both groups at follow-up, average GLS remained lower in those with severe disease (Fig. 3).
Coronary Arteries
Coronary artery (CA) abnormalities occurred in 5 (14%) patients with MIS-C. Three of these patients with CA abnormalities had aneurysm (1 large, 2 small), while the rest had dilation. On discharge, more patients had evidence of CA dilation with only 2 patients having aneurysms (1 medium, 1 small). At medium-term follow-up, only 1 patient had evidence of persistent aneurysm.
Cardiac Magnetic Resonance Imaging Findings
A small subset of MIS-C patients had available CMR data (5/36). Of this cohort, 1 patient underwent CMR during short-term follow-up with the remainder during medium-term follow-up (median 88 days). MRI data for individual patients are shown in Table 3. The average LVEF was 58.3% and average GLS was − 15.9%. Among those who underwent CMR at medium-term follow-up, average RVEF was normal at 49.1%. No patients had evidence of myocardial fibrosis by late gadolinium enhancement.
Table 3.
Individual cardiac MRI data in MIS-C patients on follow-up
| Patient No | Days from admission to MRI | LVEF (%) | LVEDVi (mL/m2) | LVEDV (z-score) |
LVESVi (mL/m2) | LVESV (z-score) |
RVEF (%) | RVEDVi (mL/m2) | RVEDV (z-score) |
RVESVi (mL/m2) | RVESV (z-score) |
LGE present | GLS (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 21 | 57.3 | 57.0 | − 4.5 | 24.0 | − 2.2 | 59.0 | 57 | − 5.1 | 23 | − 3.9 | No | − 15.6 |
| 2 | 57 | 55.5 | 75.0 | − 1.5 | 34.0 | 0.1 | 49.0 | 80 | − 1.7 | 41 | − 0.2 | No | − 12.8 |
| 3 | 110 | 62.6 | 65.5 | − 4.8 | 24.0 | − 3.3 | 51.7 | 69 | − 4.8 | 33 | − 2.9 | No | − 19.4 |
| 4 | 106 | 57.0 | 94.7 | 1.0 | 40.8 | 1.5 | 53.0 | 106 | 1.0 | 50 | 1.1 | No | − 18.8 |
| 5 | 88 | 59.2 | 78.0 | − 2.7 | 32.0 | − 1.3 | 55.6 | 85 | − 2.7 | 38 | − 1.9 | No | − 13.1 |
| Mean ± SD (Median) | 76.4 ± 37.4 (88) | 58.3 ± 2.7 (57.3) | 74.0 ± 14.2 (75) | − 2.50 ± 2.38 (− 2.70) | 31.0 ± 7.1 (32) | − 1.0 ± 1.9 (− 1.3) | 53.7 ± 3.8 (53) | 79.4 ± 18.4 (80) | − 2.66 ± 2.49 (2.70) | 37.0 ± 10.0 (38) |
− 1.56 ± 2.02 (− 1.90) |
0/5 (0.0%) | − 15.9 ± 3.1 (− 15.6) |
GLS global longitudinal strain; i, indexed; LGE late gadolinium enhancement; LVEDV left ventricular end-diastolic volume; LVEF left ventricular ejection fraction; LVESV left ventricular end-systolic volume; RVEDV right ventricular end-diastolic volume; RVEF right ventricular ejection fraction; RVESV right ventricular end-systolic volume; SD, standard deviation
Discussion
We observed that MIS-C patients had a high prevalence of myocardial dysfunction on presentation, as measured by lower LVEF and GLS. While decreased LVEF was seen in 41% of patients with MIS-C on admission, the ventricular function normalized in the majority (90%) by discharge at a median of 3.5 days and in all patients by medium-term follow-up at a median of 135 days. GLS was abnormal in 44% of patients on admission, however, took longer to normalize, with 2 patients having persistently abnormal GLS at medium-term follow-up. As MIS-C remains a novel disease, there is a paucity of data on long-term cardiac outcomes and risk for future cardiac disease. Our study provides medium-term follow-up data regarding cardiac function in MIS-C.
Cardiac Function in MIS-C
The most common cardiac finding in MIS-C that we observed was decreased myocardial function. While myocardial strain has been shown to be a more sensitive marker for ventricular dysfunction even in patients with normal LVEF [12], we observed similar proportions of abnormal LVEF and GLS on initial presentation in our cohort. While patients with abnormal LVEF had a lower strain on admission, we also observed a few individuals with normal LVEF with abnormal strain. In a previous study by Kobayashi et al., similar findings were demonstrated with patients using conventional echocardiographic assessment and myocardial deformation analysis [8]. Additionally, we confirmed that patients with evidence of severe disease were more likely to have abnormal LVEF on admission, though there was no significant difference in GLS. We also observed that while LVEF normalizes by medium-term follow-up, GLS more often remains abnormal.
The recovery in systolic function seen in our patients is supported by recent studies. Initial single-center studies demonstrated that most patients had improvement in LV function upon short-term follow-up (discharge and up to 1 month) [8–10, 18–20]. Following this, a large multicenter study by Feldstein et al. found that 34% of patients had initially reduced LVEF with 91% normalizing within 30 days [21]. Some of these studies which assessed myocardial deformation, demonstrated that many patients had abnormal strain at presentation with a proportion of patients having abnormal strain at discharge and 3–10-week follow-up [10, 11, 22]. Though these studies demonstrated improvement in LV function, they were primarily in the short-term period without much longitudinal data. More recently, a single-center study by Matsubara et al. found rapid improvement in LV function within the first week using both LVEF and strain, with continued gradual improvement through 3-month follow-up [23]. This study was one of first studies to assess cardiac function longitudinally using both LVEF and strain. Our study confirms these findings, demonstrating that most patients have recovery of LVEF by discharge. We also demonstrate that patients with both mild and severe disease have improvement of LVEF and GLS throughout follow-up. It is interesting to note that there was no difference in LVEF at discharge based on disease severity; however, LVEF was significantly lower in those with severe disease at longer-term follow-up. This, as well as our clinical experience suggests a trend toward normal LVEF immediately following steroid therapy; however, this was not within the scope of our study and further investigation is warranted. We also demonstrate a minority of patients with persistently abnormal strain at medium-term follow-up even with normal LVEF. This suggests that even in patients with normal systolic function as measured by conventional echocardiographic parameters, subclinical dysfunction may persist months from initial presentation. As GLS may be a more sensitive marker for dysfunction, further follow-up is likely needed particularly in patients with abnormal GLS. We suggest that these patients be followed until strain normalizes by echocardiography. In addition, in this group, CMR may be a useful tool to evaluate cardiac dysfunction and myocardial characterization in the longer term.
Myocardial Inflammation and Injury
Most patients with MIS-C had elevated troponin and BNP values on admission and all patients had evidence of systemic inflammation. When comparing those with normal and abnormal LVEF on admission, it is interesting to note that there was no significant difference in initial or peak troponin or BNP values among the two groups; however, we acknowledge that this is in the setting of a small sample size. Previous studies have suggested differences in inflammatory markers and white blood cell counts (including absolute neutrophil (ANC) and lymphocyte counts (ALC)) between patients with normal and abnormal LVEF; however, our study found no significant difference between these groups [5, 10, 19, 21]. When comparing those with normal and abnormal GLS on admission, there was also no significant difference in initial or peak troponin or BNP values. We did find that those with abnormal strain had a higher WBC count and ANC than those with normal strain, with no difference in ALC. As previous studies have demonstrated that patients with severe clinical disease have higher levels of inflammatory markers, WBC and ANC counts, this may suggest that patients with abnormal GLS have a more severe form of MIS-C that is associated with longer-term ventricular dysfunction [24]. Additionally, there was no difference in ICU admission or length of stay in any groups. These findings suggest that though myocardial injury is evident with elevated cardiac biomarkers, the initial or peak values likely do not predict myocardial systolic dysfunction or cardiac outcomes and thus may not need to be frequently obtained.
Coronary Artery Trends
Coronary artery abnormalities such as dilation or aneurysm are rare in MIS-C. We found that though initial dilation was rare, some patients met criteria for dilation at discharge and short-term follow-up though they had normal CA z-scores. Even at medium-term follow-up, there was some persistence in CA dilation. However, coronary artery aneurysms were very rare with all but 1 aneurysm resolving upon medium-term follow-up. These results are consistent with previous studies which demonstrated that most coronary artery abnormalities normalized within 30 days [8, 9, 18, 21]. No coronary artery thrombosis or stenosis was seen in these patients.
Cardiac Magnetic Resonance Imaging
A small subset of our patients underwent follow-up cardiac MRI, most after 2 months from initial illness, and were found to have normal LVEF with decreased GLS and no evidence of myocardial fibrosis. This is consistent with few previous studies that have studied CMR in children after MIS-C. In a study from the UK assessing myocardial deformation, strain values were significantly lower for patients with MIS-C than controls even though conventional CMR parameters were in the normal range [25]. A single-center study by Barris et al. also found that patients had normal LVEF with no myocardial fibrosis at 9 months from illness [26]. There are few published studies that have assessed cardiac function by CMR after MIS-C; however, to our knowledge, only one prior study evaluated strain by CMR [25]. Though we only present a case series of patients with CMR, our data raises the question of subclinical myocardial dysfunction months after illness, though in a pattern that differs from viral myocarditis.
Limitations
This is a single-center study and thus has multiple limitations such as small sample size, retrospective nature, and limited follow-up. As this was a retrospective study, echocardiograms were obtained for clinical reasons and thus subject to the limited acoustic windows in systemically ill children. Timing of follow-up echocardiograms after discharge was not standardized due to the novelty of the disease and varying disease severity. In addition, some patients were lost to follow-up. This also led to variability in time points of follow-up with our medium-term group having a large range, including some patients evaluated up to 4 months from illness. Given the wide interquartile range for the medium-term follow-up group, it is possible that patients at the later end of the follow-up range had more time for recovery of ventricular function. Our data were also limited by availability of reliable strain data making the sample size for this group smaller. In addition, the number of patients in each subgroup analysis were small, limiting the statistical power of these findings. Aside from echocardiographic data, other limitations include time points for admission labs and ECG. These data were obtained from the patient chart on admission regardless of the number of days into their illness, thus our selected time points may encapsulate a wider range of days than anticipated. Finally, a very small subset of patients underwent CMR; however, this limited data can be useful to understand need for long-term follow-up after MIS-C.
Conclusions
Myocardial systolic dysfunction and abnormal GLS were common findings in MIS-C at presentation. While ejection fraction often normalized by 2 months, persistently abnormal strain was more common. This suggests that a proportion of patients may have continued subclinical dysfunction and may benefit from further follow-up with multimodal imaging. Overall, our study offers an optimistic outlook for recovery in patients with MIS-C and carditis; however, ongoing investigation for longitudinal effects is warranted.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This work was partially supported by the National Institutes of Health (NIH) Clinical and Translational Science Award (CTSA) program, grant UL1 TR001857 through the Clinical and Translational Science Institute at the University of Pittsburgh.
Author Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Nikkan Das, Rachel Hill and Adam Christopher. The first draft of the manuscript was written by Nikkan Das and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization, “WHO Coronavirus (COVID-19) Dashboard,” Feb. 22, 2022. https://covid19.who.int/ (Accessed Feb. 21, 2022).
- 2.Riphagen S, Gomez X, Gonzalez-Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395(10237):1607–1608. doi: 10.1016/S0140-6736(20)31094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.M. B. F. Son and K. Friedman, “COVID-19: Multisystem inflammatory syndrome in children (MIS-C) clinical features, evaluation, and diagnosis,” UpToDate, Apr. 02, 2021. https://www.uptodate.com/contents/covid-19-multisystem-inflammatory-syndrome-in-children-mis-c-clinical-features-evaluation-and-diagnosis?search=misc&source=search_result&selectedTitle=2~89&usage_type=default&display_rank=2 (Accessed Feb. 21, 2022).
- 4.Palabiyik F, Akcay N, Sevketoglu E, Hatipoglu N, Sari EE, Inci E. Imaging of multisystem inflammatory disease in children (MIS-C) associated with COVID-19. Acad Radiol. 2021;28(9):1200–1208. doi: 10.1016/j.acra.2021.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramcharan T, et al. Paediatric inflammatory multisystem syndrome: temporally associated with SARS-CoV-2 (PIMS-TS): cardiac features, management and short-term outcomes at a UK tertiary paediatric hospital. Pediatr Cardiol. 2020;41(7):1391–1401. doi: 10.1007/s00246-020-02391-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yasuhara J, Watanabe K, Takagi H, Sumitomo N, Kuno T. COVID-19 and multisystem inflammatory syndrome in children: a systematic review and meta-analysis. Pediatr Pulmonol. 2021;56(5):837–848. doi: 10.1002/ppul.25245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sirico D, et al. Early echocardiographic and cardiac MRI findings in multisystem inflammatory syndrome in children. J Clin Med. 2021;10:15. doi: 10.3390/jcm10153360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kobayashi R, et al. Detailed assessment of left ventricular function in multisystem inflammatory syndrome in children, using strain analysis. CJC Open. 2021;3(7):880–887. doi: 10.1016/j.cjco.2021.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsubara D, et al. Echocardiographic findings in pediatric multisystem inflammatory syndrome Associated With COVID-19 in the United States. J Am Coll Cardiol. 2020;76(17):1947–1961. doi: 10.1016/j.jacc.2020.08.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanil Y, et al. Echocardiographic indicators associated with adverse clinical course and cardiac sequelae in multisystem inflammatory syndrome in children with coronavirus disease 2019. J Am Soc Echocardiogr. 2021;34(8):862–876. doi: 10.1016/j.echo.2021.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He M, et al. Longitudinal assessment of global and regional left ventricular strain in patients with multisystem inflammatory syndrome in children (MIS-C) Pediatr Cardiol. 2022 doi: 10.1007/s00246-021-02796-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kostakou PM, et al. Subclinical left ventricular dysfunction and correlation with regional strain analysis in myocarditis with normal ejection fraction. A new diagnostic criterion. Int J Cardiol. 2018;259:116–121. doi: 10.1016/j.ijcard.2018.01.058. [DOI] [PubMed] [Google Scholar]
- 13.Mccandless RT, Minich LL, Wilkinson SE, McFadden ML, Tani LY, Menon SC. Myocardial strain and strain rate in Kawasaki disease. Eur Heart J Cardiovasc Imaging. 2013;14(11):1061–1068. doi: 10.1093/ehjci/jet041. [DOI] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention, “Multisystem Inflammatory Syndrome (MIS),” May 20, 2021. https://www.cdc.gov/mis/mis-c/hcp/index.html (Accessed Jan. 12, 2021).
- 15.Harwood R, et al. A national consensus management pathway for paediatric inflammatory multisystem syndrome temporally associated with COVID-19 (PIMS-TS): results of a national Delphi process. Lancet Child Adolesc Health. 2021;5(2):133–141. doi: 10.1016/S2352-4642(20)30304-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boston Children’s Hospital, “Boston Children’s Hospital Heart Center Z-Score Calculator,” 2022. https://zscore.chboston.org/ (Accessed Sep. 07, 2021).
- 17.McCrindle BW, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation. 2017;135(17):e927–e999. doi: 10.1161/CIR.0000000000000484. [DOI] [PubMed] [Google Scholar]
- 18.Jhaveri S, et al. Longitudinal echocardiographic assessment of coronary arteries and left ventricular function following multisystem inflammatory syndrome in children. J Pediatr. 2021;228:290–293.e1. doi: 10.1016/j.jpeds.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kavurt AV, et al. Echocardiographic findings and correlation with laboratory values in multisystem inflammatory syndrome in children (MIS-C) associated with COVID-19. Pediatr Cardiol. 2021 doi: 10.1007/s00246-021-02738-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kelly MS, Valle CW, Fernandes ND, Cummings BM, Lahoud-Rahme M, Chiu JS. Multisystem inflammatory syndrome in children: cardiac biomarker profiles and echocardiographic findings in the acute and recovery phases. J Am Soc Echocardiogr. 2020;33(10):1288–1290. doi: 10.1016/j.echo.2020.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feldstein LR, et al. Characteristics and outcomes of US Children and adolescents with multisystem inflammatory syndrome in children (MIS-C) compared with severe acute COVID-19. JAMA J Am Med Assoc. 2021;325(11):1074–1087. doi: 10.1001/jama.2021.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patnaik S, et al. Short-term outcomes in children recovered from multisystem inflammatory syndrome associated with SARS-CoV-2 infection. Rheumatol Int. 2021;41(11):1957–1962. doi: 10.1007/s00296-021-04932-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsubara D, et al. Longitudinal assessment of cardiac outcomes of multisystem inflammatory syndrome in children associated With COVID-19 infections. J Am Heart Assoc. 2022;11:3. doi: 10.1161/jaha.121.023251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao Y, Yin L, Patel J, Tang L, Huang Y. The inflammatory markers of multisystem inflammatory syndrome in children (MIS-C) and adolescents associated with COVID-19: a meta-analysis. J Med Virol. 2021;93(7):4358–4369. doi: 10.1002/jmv.26951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krupickova S, et al. Myocardial deformation assessed by CMR in children after multisystem inflammatory syndrome (MIS-C) Int J Cardiol. 2022;346:105–106. doi: 10.1016/j.ijcard.2021.11.036. [DOI] [PubMed] [Google Scholar]
- 26.Barris DM, et al. Midterm outcomes and cardiac magnetic resonance imaging following multisystem inflammatory syndrome in children. J Pediatr. 2022;241:237–241.e1. doi: 10.1016/j.jpeds.2021.10.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.