Abstract
Mechanism-based therapy centred on the molecular understanding of disease-causing pathways in a given patient is still the exception rather than the rule in medicine, even in cardiology. However, recent successful drug developments centred around the second messenger cyclic guanosine-3′-5′-monophosphate (cGMP), which is regulating a number of cardiovascular disease modulating pathways, are about to provide novel targets for such a personalized cardiovascular therapy. Whether cGMP breakdown is inhibited or cGMP synthesis is stimulated via guanylyl cyclases or their upstream regulators in different cardiovascular disease phenotypes, the outcomes seem to be so far uniformly protective. Thus, a network of cGMP-modulating drugs has evolved that act in a mechanism-based, possibly causal manner in a number of cardiac conditions. What remains a challenge is the detection of cGMPopathy endotypes amongst cardiovascular disease phenotypes. Here, we review the growing clinical relevance of cGMP and provide a glimpse into the future on how drugs interfering with this pathway may change how we treat and diagnose cardiovascular diseases altogether.
Keywords: Guanylate cyclase, Natriuretic peptides, Nitric oxide, Cyclic GMP, Biomarkers
1. Background
For decades, the number of approved drugs has been in decline, indicating fundamental problems with respect to the productivity and innovation in basic, translational, and industrial research.1 Potential reasons for this include, amongst others, the underlying concept of disease, which is mainly based on symptoms, an organ and its phenotypic function rather than on molecular pathways. Indeed, causal, mechanistic understanding of disease is still the exception and currently relevant primarily for monogenic diseases.2 Common and complex diseases are primarily treated based on their symptoms, on risk factors or markers; clearly, a low-precision approach evidenced by the high numbers needed to treat and low efficacy of currently available drugs.3–5 Cardiology is no exception to this, and given its many unmet needs, this represents one of the most important knowledge gaps in medicine.3
Therapeutic agents that modulate the second messenger cyclic guanosine-3′ -5′ -monophosphate (cGMP) seem to be one exception to this conceptual roadblock and may lead the way towards a different, mechanism-based approach to a variety of diseases using also the powers of big data, networks and systems medicine.6,7 cGMP modulators have emerged as one of the most promising compounds in recent cardiovascular drug discovery.7 This may be because they do not act only symptomatically but, at least in a subset of suitable patients, target a disease mechanism rather than alleviating symptoms or modulating risk factors.7,8 In contrast, current cardiovascular treatments, such as renin–angiotensin–aldosterone system (RAAS) blockade do not follow a pathomechanistic approach. RAAS-blockers are not chosen as a therapy because in a patient up-regulation of RAAS has been measured, but solely symptomatically because RAAS blockade causes vasodilation. This is not a mechanism-based, causal therapy. The same holds true for other commonly used therapies, such as beta-blockers and calcium channel antagonists.
Cyclic GMP modulating drugs are used in a broad set of cardiovascular symptoms and conditions, such as angina, myocardial infarction, heart failure, pulmonary hypertension (PH), hypertensive crisis, and erectile dysfunction.9–14 Furthermore, preclinical evidence suggests benefit in ischaemic stroke.8 In addition, cGMP-related biomarkers, natriuretic peptides (NPs), are used to monitor heart failure patients.10
In the cardiovascular system, the effects of cGMP are predominantly mediated by cGMP-dependent protein kinases and cGMP-regulated phosphodiesterases (PDEs)15 (Figure 1). Cyclic GMP appears to exert almost exclusively beneficial effects with a single overt limitation, vasodilation which in some patients may lead to hypotension and syncope, in particular in combination therapy.16 Therefore, cGMP increase leverages apparently only additional therapeutic gain, particularly in those cardiovascular conditions associated with a proven, i.e. mechanism-based, deficit in cGMP signalling. Clinically, this is achieved mainly by two approaches, either by (i) activating guanylyl cyclases to increase cGMP synthesis or by (ii) inhibiting relevant PDEs to inhibit cGMP breakdown. Future cGMP-centric strategies will most likely include combinations of different types of cGMP-modulating drugs and be increasingly guided by additional innovative plasma- or cell-based biomarker panels yielding powerful therapeutic and diagnostic (‘theranostic’) couples with cGMP-modulating drugs for cardiovascular precision medicine.
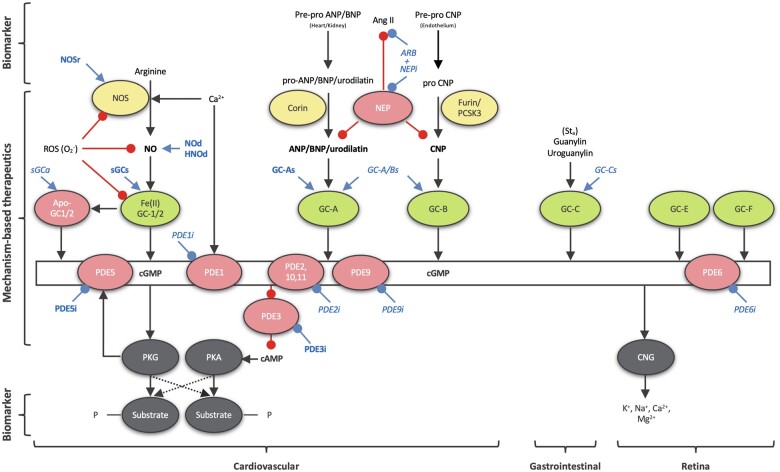
Figure 1.
Classical, curated representation of cGMP signalling. Shown in green are the GCs and in yellow their positive regulators; NO produced by NOS for soluble GC and NPs (ANP, BNP, and CNP) for particulate GC. Negative regulators (cGMP metabolizing PDEs 1, 2, 5, 6, 9, 10, 11, and NPs degrading NEP) and pathophysiological conditions (oxidized/heme-free apo-sGC) are shown in pink. All clinically relevant cGMP-modulating drugs are shown in blue (bold for approved drugs and in italic for drugs under investigation): ARB, Angiotensin II receptor blockers; GC-As, GC-A stimulators; GC-A/Bs, GC-A/B stimulators; NEPi, neprilysin inhibitors; NOd, NO donors; NOSr, NOS recoupling nutraceuticals; sGCa, sGC activators; sGCs, sGC stimulators; PDEi, PDE inhibitors. cGMP effector proteins (PKG and cyclic nucleotide-gated ion channels, CNG) and their substrates are shown in grey. cGMP can also inhibit some isoforms of the PDE enzyme family. In turn, this leads to an altered phosphoprotein profile, a decrease in intracellular calcium levels and sensitivity, and altered cGMP and cAMP levels (proffering crosstalk between cGMP and cAMP networks). GC-C is localized in intestines and GC-E and -F in the retina, thus not relevant to the context of this review. Ang II, angiotensin II; Sta, heat-stable enterotoxin I STa.
2. Cyclic GMP, a mechanism-based approach for cardiology
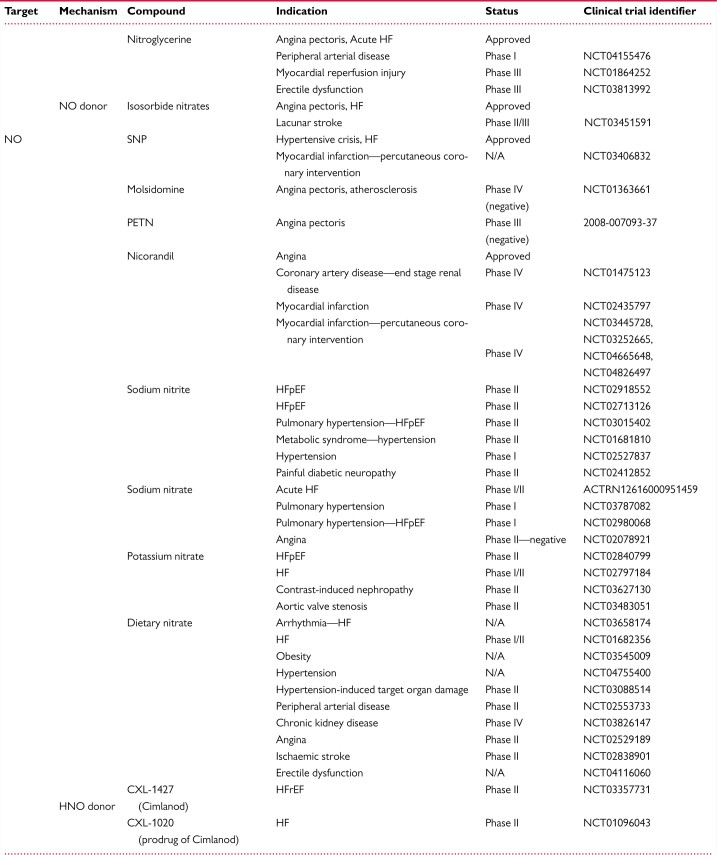
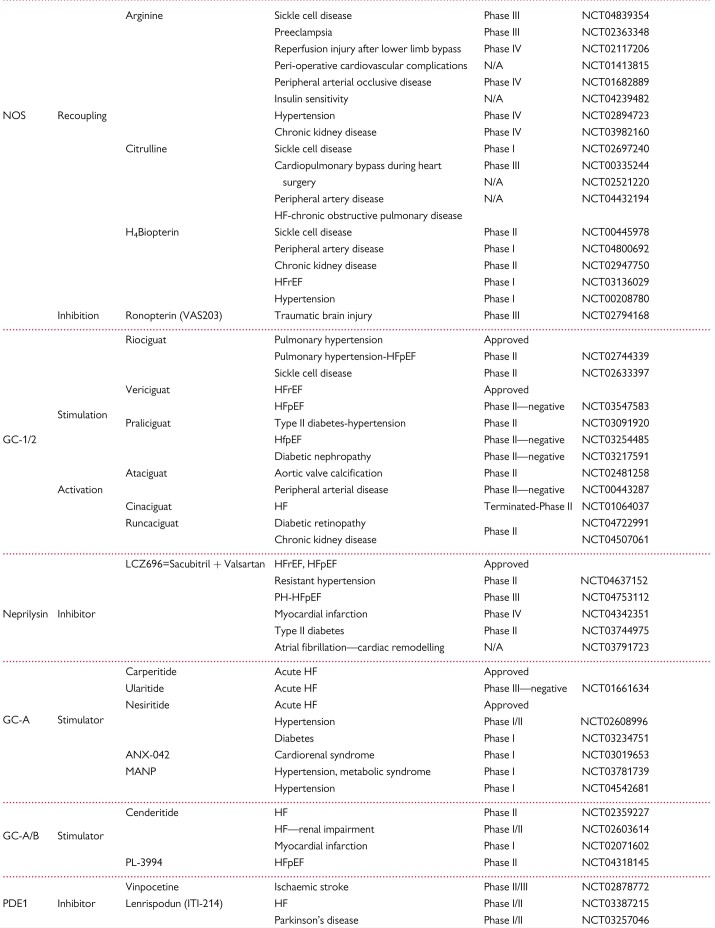
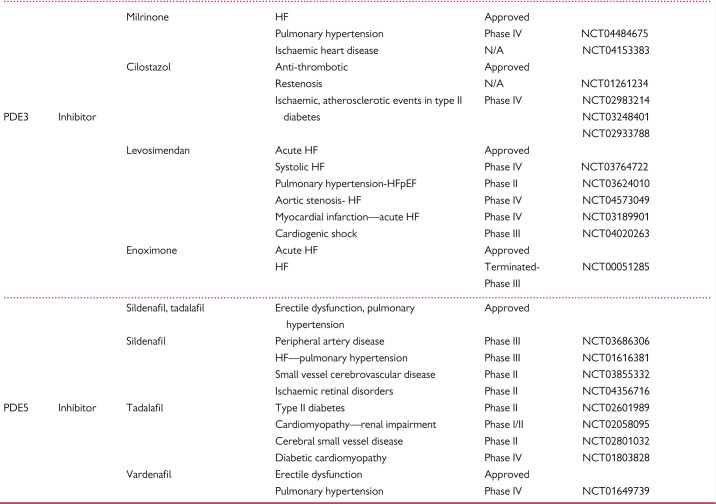
cGMP-modulating drugs are very promising in cardiovascular medicine and provide a broad clinical applicability. Indeed, cGMP-modulating drugs provide protective effects within the heart and vasculature by inhibiting vascular smooth muscle contraction and proliferation, suppressing platelet and leukocyte reactivity, and both anti-fibrotic and anti-hypertrophic actions.15 A second, probably even more promising aspect is the fact that dysfunctional cGMP formation and signalling appear to play direct pathomechanistic roles in cardiovascular disease. Genome-wide association studies have identified single nucleotide polymorphisms in genes encoding several components of this pathway to be correlated with cardiovascular diseases (Table 1).17,18 Thus, any up-regulation of cGMP has the potential to act and cure in a unique mechanism-based manner.8,15Table 2 demonstrates cGMP-related drugs for therapeutic cardiovascular applications, either approved or under clinical investigation.
Table 1.
cGMP-related loci. Identified to be associated with cardiovascular diseases by genome-or exome-wide association studies. NPPA genetic variant rs5068 and GUCY1A3 variant α1-A680T may protect against metabolic syndrome and PH, respectively
| Gene | Chromosome | Association with |
|---|---|---|
| ANP [NPPA] | 1 | AF23, BP17, MetS24, VR178 |
| BNP [NPPB] | 1 | BP17 |
| Furin [PCSK3] | 15 | BP17, MI/CAD179 |
| eNOS [NOS3] | 7 | BP180, CAD181, MetS19 |
| NPR1 [NPR1] | 1 | BP22, VR178 |
| PDE5A [PDE5A] | 4 | CAD18 |
| sGCα1 [GUCY1A3] | 4 | BP17,182, PH183 |
| sGCα1 [GUCY1A3] + CCTη [CCT7] | 4+2 | MI/CAD21 |
| sGCβ1 [GUCY1B3] | 4 | BP17,182 |
AF, atrial fibrillation; BP, blood pressure; CAD, coronary artery disease; CCT7, chaperonin containing TCP1 subunit 7; MetS, metabolic syndrome; MI, myocardial infarction; PH, pulmonary hypertension; VR, ventricular remodelling.
Table 2.
cGMP-modulating drugs. NO donors, NOS targeting compounds, soluble GC (GC-1/2) stimulators and activators, GC-A/B stimulators, NEP inhibitors, and PDE inhibitors, either approved or under clinical investigation for therapeutic cardiovascular applications
|
|
|
2.1 cGMPopathies
cGMPopathies describe a dysregulation of cGMP signalling by reduced cGMP synthesis, increased cGMP breakdown, or defective cGMP downstream signalling.7 This term is a part of an overall new approach to disease namely, not to define diseases based on a symptom in an organ but by a causal mechanism. This mechanism may lead to symptoms in different organs. Coronary artery disease (CAD), myocardial ischaemia (MI), heart failure (HF), hypertension, diabetic nephropathy, and metabolic syndrome belong to cGMPopathies.15,18,19NOS3 polymorphisms have been associated with CAD/MI, hypertension, metabolic syndrome, diabetes mellitus, and diabetic nephropathy;18,19 a shorter half-life variant of NOS3 with reduced event-free survival in HF with systolic dysfunction;20 a PDE5A variant with CAD;18 genetic variants of GUCY1A3 with blood pressure, CAD and MI.18 Mutations in the α1-soluble guanylate cyclase (sGC) subunit and CCT7η encoding genes, leading to decreased sGC activity, have been associated with myocardial infarction risk.21 A deletion mutation in the type A human NP receptor gene was associated with essential hypertension and ventricular hypertrophy.22 In families with atrial fibrillation, a frameshift mutation in the atrial natriuretic peptide (ANP) encoding gene has been identified and possibly involved in the development of the disease.23 Moreover, the ANP (NPPA) genetic variant rs5068 characterized by increased production of ANP in humans has demonstrated a phenotype of lower blood pressure, reduced risk of hypertension, and decreased prevalence of metabolic syndrome.24 The genetic involvement of cGMP signalling in these diseases is further confirmed by pathophysiological data.
Endothelial dysfunction characterized by NO dysregulation and inflammation coincides with reactive oxygen species (ROS) formation leading to cardiovascular disease states.25 Notably in heart failure, neurohumoral activation, secretion of inflammatory messengers, and altered shear stress lead to ROS generation that interferes with NO.26 The resulting endothelial dysfunction causes a further imbalance of NO and unphysiological ROS formation that worsens HF.26 Reduced PKG activity and cGMP concentrations, probably resulting from low NO bioavailability, are related to cardiomyocyte stiffness in the HF with preserved ejection fraction (HFpEF) myocardium.27 Higher levels of uncoupled eNOS and PDE9A were also shown in HFpEF myocardium.28,29 Elevated ANP levels have been associated with HF, but blunted responses to ANP infusion in HF patients indicate the possibility of down-regulation of ANP receptors30 or up-regulation of the NP-metabolizing receptor.31 In human failing hearts, guanylate cyclase (GC)-A in cardiomyocytes does not respond to ANP stimulation,32 and PDE1C and PDE5 levels are up-regulated.33,34
Endothelial dysfunction, as a result of dysregulated ROS formation and inflammation, correlates with atherosclerosis.35 Increased activity of NADPH oxidase (as a source of superoxide) was associated with decreased endothelial vasorelaxations and increased atherosclerotic risk factors.36 In CAD patients, the oxidized form of sGC was increased37 and asymmetric-dimethyl-L-arginine (ADMA) levels associated with eNOS uncoupling.38 In hyperlipidaemia, cGMP modulators are unable to induce cardioprotective effects, suggesting a dysfunction downstream of cGMP formation.39 In the same study, PKG activity is down-regulated in hyperlipidaemic rats as assessed by troponin I phosphorylation.39
Nitric oxide is implicated in impaired vasodilation in hypertensive patients.40 Endothelial NO production by eNOS is decreased and systemic NO production by iNOS increased (resulting in hyperproduction of toxic NO levels) in patients with coronary heart disease (CHD) associated with hypertension; these effects are more expressed in CHD with hypertension compared to isolated CHD patients.41 In addition, diminished L-arginine transport has been proposed as a link from dysfunctional NO signalling to essential hypertension.42 In pulmonary arterial hypertension (PAH) patients, arginine levels in airway epithelial cells are inversely associated with pulmonary arterial pressures, while in pulmonary artery endothelial cells NO production is reduced and arginase activity higher.43
Importantly, when moving from organ- and symptom-based to mechanistic disease definitions not all patients with a given clinical disease phenotype is expected to suffer from the same cause. cGMPopathy rather represents one endotype, and there will be others that could lead to a similar phenotype. NOX5-induced uncoupling of eNOS as a causal mechanism of age-related hypertension is a good example of this as it affects approximately only one in four or five patients with hypertension.44 Different endotypes of one phenotype may have different symptoms or comorbidities, which in multiscale modelling is used to identify the mechanism of unclear endotypes.
3. Drugs increasing cGMP generation
3.1 Nitric oxide and its receptors, GC-1 & GC-2
The traditionally defined ‘soluble guanylate cyclase (sGC)’, more recently termed guanylyl cyclases GC-1 and GC-2 (to differentiate them from the membrane-spanning, guanylyl cyclases, GC-A, GC-B, activated by NPs), is a heterodimeric haemoprotein comprised of one of two alpha subunits (α1 or α2) and a beta subunit (β1). An N-terminal pocket binds Fe(II)haem via a proximal histidine and thereby confers sensitivity to NO.45,46 Binding of NO cleaves the proximal histidine-Fe(II) haem bond and induces a structural shift that activates the catalytic site converting GTP into cGMP.45,46 Inappropriate formation of ROS, in particular superoxide, can interfere with NO-cGMP signalling in at least three ways: (i) by chemically scavenging NO; (ii) by uncoupling NO synthase (NOS); or (iii) by oxidizing the haem group within GC-1/2 from Fe(II) to Fe(III) eventually resulting in heme-deficient apo-GC. The latter is not only insensitive to NO but also prone to rapid degradation.47
Therapeutically, three avenues are clinically promising for reinstating or augmenting NO-GC-1/2 signalling: (i) repairing or replacing NO synthesis; (ii) sensitizing GC-1/2 to lower levels of NO by allosteric modulator compounds, so-called sGC stimulators;48 or (iii) re-activating NO-insensitive, haem-free apo-GC by haem-mimetics, so-called sGC activators, which also prevent enzyme degradation.48,49
3.1.1 Repairing or replacing NO synthesis
Recoupling NOS, by dietary supplementation of its redox-sensitive cofactor tetrahydrobiopterin or its substrate L-arginine, is pre-clinically effective. So far, there are, however, no clinical trials with positive outcomes to demonstrate the efficacy of such a nutraceutical approach.50 Therapeutically, NO substitution with so-called NO-donor or nitrovasodilator compounds has the longest history (e.g. in angina, heart failure), but also limitations, such as pharmacokinetic51 and pharmacodynamic52 tolerance, which requires therapy-free intervals to regain nitrate sensitivity. According to ESC/AHA guidelines, the use of sodium nitroprusside, chemically an NO+ donor, is limited to i.v. application in hypertensive emergencies e.g. as first-line treatment in acute cardiogenic pulmonary oedemas, and in acute HF as second-line therapy.10,14,53,54 Short-acting nitrates, such as nitroglycerine and isosorbide dinitrate (ISDN), can be used as first-line therapy for pain relief of an angina attack, whereas long-acting nitrate formations of nitroglycerine, ISDN, or isosorbide mononitrate (ISMN) are used as second-line treatments for angina prophylaxis.9,12,55,56 Nitroglycerine and ISDN are also considered second-option vasodilators in acute HF.10,54 A combination of ISDN and hydralazine can be used as second-line therapy in HF with reduced ejection fraction (HFrEF).10,54 Additionally, molsidomine, an NO-donor upon metabolism, is an antianginal drug; but not yet recommended in routine use.57 Three NO donors, pentaerythritol tetranitrate, nicorandil and nitroxyl (HNO or NO−), seem to be devoid of tolerance, which awaits to be exploited therapeutically.58,59 However, PETN is not recommended for stable angina yet due to not sufficient efficacy evidence.58 Nitroxyl donors, such as BMS-986231 (previously CXL-1427) differ from pure NO donors and showed a favourable safety and haemodynamic profile in acute decompensated HF.60 Nicorandil, a nicotinamide- nitrate ester and K+ channel opener, is suggested as a second-line antianginal drug for patients with chronic coronary syndromes in Europe but not approved in USA.9,10,12,58 In addition to nitrate tolerance, a general concern is that under conditions of elevated ROS levels, NO donors may lead to unwanted reactive nitrogen species and endothelial dysfunction.51,61
Two more targeted and mechanism-based strategies circumvent these shortcomings and risks i.e. sGC stimulators and sGC activators. Despite their very similar sounding names, they have distinct targets, i.e. Fe(II)haem-containing GC-1/2 and apo-GC-1/2, respectively. Importantly, both enhance cGMP synthesis independently of modulating NO levels and are thus devoid of tolerance.48
3.1.2 sGC stimulators
These compounds interact with an allosteric site to sensitize (FeII)haem containing GC-1/2 for NO.46 If tissue levels of NO are low, this will result in a mechanism-based ‘recovery’ of a physiological cGMP response. However, if levels of NO are high, these compounds have limited or no additional effect on cGMP.
Riociguat (BAY 63–2521) was the first registered sGC stimulator approved for use in PH, i.e. chronic thromboembolic pulmonary hypertension and PAH.62 No evidence-based first-line therapy is suggested for PH, but riociguat is one of the initial monotherapies that can be chosen according to ESC/CHEST guidelines.11,63 However, following the early termination of the phase II RISE-IIP trial because of serious adverse events, riociguat is not suggested to patients with PH associated with idiopathic interstitial pneumonia.64 Riociguat was also evaluated in PH associated with left systolic heart failure, and, despite not meeting the primary endpoint of change in mean pulmonary artery pressure (mPAP), it had favourable effects on secondary outcomes.65 The DILATE-1 trial tested riociguat in patients with HFpEF and PH; stroke volume and cardiac index were increased, systolic blood pressure and right ventricular end-diastolic area decreased, but there was no significant change on peak decrease in mPAP.66 At the moment, riociguat is under investigation for its long-term treatment in PH associated with HFpEF (NCT02744339).67
Vericiguat (BAY-1021189) reached the primary outcome in reducing cardiovascular mortality or hospitalization for HF in a Phase 3 clinical trial for HFrEF (VICTORIA) and recently received approval in USA.68,69 It was also further evaluated in a phase IIb HFpEF trial (VITALITY-HFpEF) where it failed to improve the quality of life (physical limitation score of the KCCQ), which was the previously suggested beneficial outcome in phase IIb SOCRATES-PRESERVED.70,71
Another sGC stimulator with promising effects in an animal model of cardiorenal failure, praliciguat (IW-1973), showed favourable trends in metabolic and hemodynamic variables in patients with type 2 diabetes (T2D) and hypertension.72,73 However, it failed to reach the primary endpoints of improved peak rate of oxygen consumption and reduction in albuminuria in Phase 2 trials for HFpEF and diabetic nephropathy, respectively.74,75
A shortcoming of all these trials still is that they stratified patients purely on clinical grounds and did not use biomarkers to identify HFpEF and HFrEF patients with a mechanistic endotype indicating cGMP dysregulation. By failing to do so, potential benefits in some patients may have been diluted through non-responders with different underlying pathomechanisms. Of note, the terms HFpEF and HFrEF are purely descriptive overarching terms, recently complemented by Heart Failure with mid-range or intermediate ejection fraction (HFmrEF or HFiEF).
3.1.3 sGC activators
These molecules specifically bind to the NO-insensitive, haem-free or -oxidized apo-GC-1/2.48 Large molecules, such as cinaciguat (BAY58–2667), but not the smaller ataciguat (HMR1766) binding the oxidized form,76 occupy the empty haem site and prevent its ubiquitination and proteasomal degradation,47 thereby both an activating and stabilizing apo-sGC. However, clinical phase II trials (COMPOSE programme) with cinaciguat in patients with acute heart failure had to be stopped prematurely due to severe hypotension.77 Moreover, the safety of ataciguat (HMR1766) has been evaluated in patients with moderate aortic valve stenosis (NCT02049203) and efficacy in patients with aortic valve calcification (NCT02481258) and peripheral arterial disease (NCT00443287); however, ataciguat’s development was discontinued.78 A novel compound with improved physicochemical and pharmacokinetic characteristics, runcaciguat, is now investigated in chronic kidney disease and diabetic retinopathy.78
3.2 NPs and their GC-coupled receptors
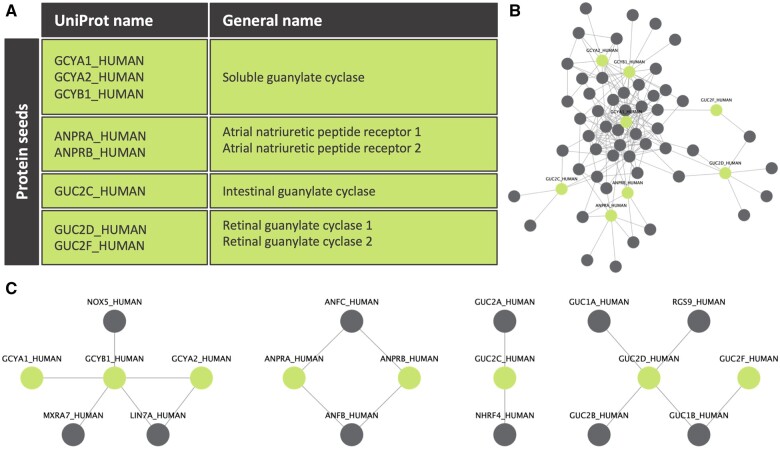
The second cGMP forming family is plasma membrane-spanning GCs, often referred to as particulate GCs due to their subcellular localization in the particulate fraction. They comprise seven members (GC-A to GC-G), of which two, GC-D and GC-G, are pseudogenes and three, GC-C, GC-E, and GC-F, are—as far as we know—not relevant for the cardiovascular system.79,80 This organ- and function-based GCs compartmentalization is further confirmed in silico (Figure 2). Here, we make use of experimentally validated protein–protein interaction (PPI) data from the Integrative Interactive Database (IID).81 Starting from GC-coupled receptors, we look at their direct protein interactions in IID to build the first neighbour PPI network. After pruning the network from highly connected but non-relevant protein interactions, four different subnetworks or signalling modules are extracted: (i) sGC module, (ii) ANP receptors module, (iii) intestinal GC module, and (iv) retinal GCs module. GC-A and GC-B are homodimers containing an N-terminal extracellular ligand-binding domain for NPs.79 They are therefore also termed NP receptors NPR-A and NPR-B, respectively.
Figure 2.
Unbiased, PPI-based GCs signalling modules. (A) Table of clinically relevant GCs. These were used as seed proteins to start building their first neighbour PPI network. (B) First neighbour PPI network of GCs from IID. Seed proteins are shown in green and their first neighbour PPI interactions in grey. A line was drawn between two proteins when they interact with each other according to IID. Only experimentally validated data were used. (C) Four different modules are extracted after curating the network with a 0.1 subnetwork participation degree (SPD) cut-off. The SPD cut-off removes highly connected and non-relevant proteins, thus, confirming in silico the literature function- and organ-based distinction of different GCs.
Humans express four types of NPs, atrial (ANP), brain (BNP), and C-type natriuretic peptide (CNP) and urodilatin.82 ANP, BNP, and urodilatin each activate GC-A; CNP is the sole endogenous GC-B agonist. Via GC-A/B, NPs have a wide range of cardio- and vaso-protective effects, i.e. natriuresis, diuresis, inhibition of vasoconstriction, as well as anti-hypertrophic, anti-fibrotic and anti-proliferative effects and possibly also metabolic actions, such as lipolysis and browning of adipocytes.82 NPs bind to another NP receptor, natriuretic peptide clearance receptor (NPR-C), which has no GC activity and is responsible for NPs clearance from the circulation. However, CNP activation of NPR-C plays a crucial role in cardiac function and vascular homeostasis.83
Elevated NP levels are also disease biomarkers, in particular in heart failure. Paradoxically though, increased expression and release of NPs does not necessarily translate into enhanced activation of the particulate GC-cGMP pathways. Instead, there appears to be a disconnect. In heart failure, proBNP, the precursor of mature BNP, is the predominant circulating form and lacks significant GC-A activating properties compared to BNP.84 More recently, studies have revealed the presence of glycosylation of ANP, resulting in a molecular form with reduced GC-A activation.85 The presence of altered molecular forms of ANP and BNP with reduced cGMP production supports the use of native and designer synthetic NPs to rescue these NP structural abnormalities. Such a hormone replacement strategy is also underscored by the presence of an ANP deficiency in human heart failure due to either reduced production and/or increased peptide degradation.86 Therefore, even when plasma levels of NPs are elevated in heart failure and other conditions, pharmacological GC-A/B stimulation may still be beneficial. Three therapeutic approaches to enhance NP signalling have entered the clinic; natural peptides, such as neseritide or carperitide, designer peptides, such as uralitide, and molecules that inhibit peptide breakdown via neutral endopeptidase (NEP), such as sacubitril.
3.2.1 Recombinant and designer NPs
The clinical utility of GC-A/B-cGMP stimulation was first examined with recombinant ANP, carperitide, in acute heart failure,87,88 but its impact on in-hospital mortality and length of hospitalization was inferior to nitrates.89 LASCAR-AHF now tests the long-term effects of carperitide in acute HF.89 Despite the lack of sufficient evidence, carperitide is used in Japanese practice as second-line treatment in acute HF.90 In the J-WIND trial, recombinant ANP decreased infarct size and improved ejection fraction in patients with myocardial infarction undergoing percutaneous coronary intervention91 but had no effect on in-hospital mortality.92
Another NP, a synthetic form of urodilatin, ularitide, neither affected a clinical composite endpoint nor cardiovascular mortality in patients with acute HF.93 Similarly, the recombinant BNP, nesiritide, despite a small change in dyspnoea, neither improved all-cause death nor re-hospitalization for HF in patients with acute, decompensated HF.94 Even worse, a meta-analysis associated the use of nesiritide to an increase in the short-term risk of death in such patients.95 This perhaps provides a warning that excessive GC-A activation may be detrimental due to significant hypotension that may compromise renal function and lead to sympathetic activation, both unfavourable events in patients with heart failure. Currently, it is considered a second-line intravenous vasodilator for acute HF in Europe and USA.10,54
An alternative strategy has been the development of ‘designer’ NPs, which aim to combine beneficial effects of different endogenous peptides. CD-NP, i.e. cenderitide (CD-NP), is a modified CNP with 15 additional amino acids at the C-terminal tail of DNP (a related peptide identified in the venom of the green mamba, Dendroaspis angusticeps).96 The rationale behind this combination is to promote the vasodilator and anti-fibrotic properties of C-type natriuretic peptide (at least in part via GC-B stimulation), with the natriuretic properties of DNP (which stimulates GC-A but is thought to avoid a dose-limiting hypotension). Both these pharmacodynamics as well as safety, i.e. absence of hypotension, were established in stable HF patients.97 ANX-042, a peptide designed based on an alternative spliced variant of BNP, is currently under investigation as a non-hypotensive drug in cardiorenal syndrome (NCT03019653).98 Beyond heart failure, the designer ANP-analogue (MANP) was engineered as a novel ANP mimetic whose biological properties of natriuresis, blood pressure-lowering, and aldosterone suppression are greater than ANP.99 This analogue retains the 28 amino acids of ANP but possesses a novel 12 amino acid extension to the carboxyl terminus resulting in greater resistance to enzymatic degradation by neprilysin and reduced clearance by the NPR-C.99 MANP has been recently investigated in hypertension and metabolic syndrome, where it showed a safe profile, a borderline significant blood pressure decline and a significant increase of cGMP and non-esterified fatty acids levels.100
3.2.2 NEP inhibitors
NEP (also known as neprilysin) is a membrane-bound metalloproteinase responsible for the breakdown of many vasoactive mediators, including NPs, but also glucagon, bradykinin, oxytocin, substance P, angiotensin II, endothelin, and beta-amyloid.101 Clinically, however, NEP inhibitors (NEPi) have little or no effect on blood pressure despite significantly elevated plasma NP concentrations.102 This paradox was attributed to the fact that NEP metabolizes both vasodilating (e.g. NPs, bradykinin) and vasoconstricting (e.g. angiotensin II and endothelin) peptides, thereby possibly outweighing any hemodynamic benefit.
As a result, drug development in this area focused on a combined blockade of NEP and angiotensin-converting enzyme (ACE)—to prevent the accumulation of pro-hypertensive angiotensin II—leading to the so-called vasopeptidase inhibitors.103 However, in heart failure, the vasopeptidase inhibitor, omapatrilat, did not meet its primary endpoint of all-cause death or hospitalization for HF vs. enalapril but was associated with an increased incidence of angioedema (likely because both NEP and ACE are involved in the degradation of bradykinin).104 In hypertensive patients, the effect of omapatrilat on systolic blood pressure change and use of adjunctive antihypertensive therapy exceeded that of an ACE inhibitor alone, but again at the expense of a higher incidence of angioedema.105 Accordingly, omapatrilat did not make it to its clinical use.
Co-crystallizing the NEPi sacubitril with the angiotensin II type 1 receptor blocker, valsartan, in a one-to-one molar ratio as LCZ696, jointly termed an angiotensin receptor-neprilysin inhibitor (ARNI), was more successful than valsartan in reducing diastolic blood pressure in hypertensive patients, with no reports of angioedema.106 The rationale was to avoid the double hit on bradykinin breakdown and angioedema by blocking angiotensin II type 1 receptors rather than inhibiting ACE. In HFrEF patients, sacubitril-valsartan reduced the risk of cardiovascular death and HF hospitalization more effectively than the ACE inhibitor, enalapril (PARADIGM-HF).107 PARAGON-HF compared sacubitril-valsartan vs. valsartan alone in HFpEF, but the primary outcome of total hospitalizations and death from cardiovascular causes did not differ.108 A high heterogeneity within the HFpEF population and the definition of HFpEF itself might be the underlying explanations of the failure of PARAGON-HF.108,109 Indeed, sacubitril-valsartan was beneficial in a subgroup with lower ejection fraction, a patient population more likely to represent early HFrEF rather than HFpEF.108 The protective effect in women remains unclear and warrants further investigation.108 The abovementioned studies have led LCZ696 to get FDA approval for HFrEF110 and also very recently for HFpEF patients with stronger evidence for those with below-normal LVEF.111 LCZ696 is recommended to replace ACE inhibitor as first-line treatment for HFrEF ambulatory symptomatic patients despite optimal therapy with ACE inhibitor, beta-blocker and a mineralocorticoid receptor antagonist according to ESC/AHA guidelines.10,112 In addition, a meta-analysis showed a potent antihypertensive effect of sacubitril-valsartan vs. valsartan alone or olmesartan in elderly hypertensives.113
4. Drugs preventing cGMP breakdown
In addition to enhancing cGMP production, PDE inhibitors can exert, in principle, similar effects by inhibiting cGMP degradation. However, therapeutic exploitation of PDE inhibition has not been as great as one might have anticipated. A total of 11 superfamilies of PDE isoforms are present at different subcellular localizations, thereby targeting different cGMP (or cAMP) enzymatic sources and pools. With respect to cGMP, especially PDE1, 2, 3, 5, and 9 have been implicated in cardiovascular disorders.114
4.1 PDE5
Sildenafil and tadalafil are used in erectile dysfunction, as first-line treatments in Europe and USA,13,115 and in PH; among the initial treatments that can be chosen since there are not available head-to-head comparisons between compounds according to ESC/CHEST guidelines.11,63 Sildenafil also improved peak oxygen uptake in PH due to HFrEF116 and pulmonary pressure and right ventricular function in PH due to HFpEF.117 It showed beneficial effects on glycometabolic control and P-selectin in T2D.118 In HFrEF, sildenafil improves left ventricular (LV) diastolic function and cardiac geometry, while in diabetic cardiomyopathy benefits LV contraction.119,120 In another use-extension trial in HFpEF, sildenafil showed no improvement in exercise capacity or clinical status.121 However, in HFpEF, cGMP concentrations are down-regulated due to low NO bioavailability,27 while sildenafil minimally increases plasma cGMP;121 thus, PDE5 inhibition would not be expected to represent an effective mechanism-based approach whilst the cGMP dysfunction most likely comes from a source different from the targeted one.
4.2 PDE3
The PDE3 inhibitor milrinone is licenced in Europe and USA for acute HF in its intravenous form as second-line treatment,10,54 while oral milrinone was associated with increased all-cause and cardiovascular mortality in severe chronic heart failure.122 Another PDE3 inhibitor, cilostazol, has antithrombotic properties and, as such, has been under investigation for its antiplatelet effects in T2D (NCT02983214, NCT03248401, NCT02933788). In T2D patients with symptomatic lower extremity artery disease, cilostazol reduced the incidence of acute ischaemic stroke/transient ischaemic attack, acute myocardial infarction, and vascular causes-associated death.123 Moreover, in T2D with carotid atherosclerotic plaques, it diminished the carotid plaque progression.124 This benefit can be explained mechanistically by a crosstalk between cGMP and cAMP, where cAMP-specific PDE3 is inhibited by cGMP through direct competition at the catalytic site. Thus, some effects of cGMP, e.g. in platelets, are likely to be mediated at least in part via the cAMP-PKA axis.125 More recently, PDE3 inhibition was explored in HFpEF, focusing on a new extended-release version of milrinone.126 This small pilot study showed a safe profile and improved quality of life in HFpEF patients.126
The inotrope-dilator molecule levosimendan is used in 60 countries outside the USA as second-line treatment for acute HF10,127 and, in addition to its calcium-sensitizing properties, also inhibits PDE3.128 A recent study found that in HFpEF with PH, levosimendan improved the 6-min walk test, although the exercise pulmonary capillary wedge pressure was not significantly reduced.129 Important questions remain regarding the population of patients for which this would be beneficial and how mechanism-based patient stratification can be performed.
4.3 PDE1
The PDE1 inhibitor, vinpocetine, improved clinical outcomes and reduced lesion size in acute ischaemic stroke through inhibition of NF-κB-dependent inflammation.130 This agent, however, is a weak PDE1 inhibitor and also blocks sodium channels and regulates NF-κB signalling.131 The novel and potent PDE1 inhibitor, ITI-214, was recently tested in patients with HFrEF (NCT03387215). This double-blind, placebo-controlled multi-dosage trial revealed that ITI-214 induces systemic arterial vasodilation and increases cardiac output and mean LV power.132
4.4 PDE9A
PDE9A is the most selective cGMP-hydrolysing PDE of the superfamily.29 In 2015, a study performed in mice demonstrated a role in a model of cardiac pressure-overload, with both global genetic deletion and treatment with a selective PDE9 inhibitor reducing hypertrophy and fibrosis while improving cardiac function.29 The study established a close linkage of PDE9A with the regulation of cGMP generated by NP (rather than nitric oxide) signalling.29 In a recent study in mice, the PDE9 inhibitor CRD-733 improved HF characteristics; human trials in HF using CRD-733 are now underway.133
4.5 PDE10A
PDE10A is a dual cAMP/cGMP PDE. In a recent study in mice, PDE10A inhibition with TP-10 improved pathological cardiac remodelling.134 PDE10A inhibition has been clinically tested in schizophrenia and Huntington’s disease, proving that it is a safe target for drug treatment and a potential therapeutic option for diseases related to cardiac remodelling.134
5. Network pharmacology
As indicated above, cGMPopathies can emerge from different dysfunctions within cGMP formation, breakdown or signalling. Network medicine analysis, however, shows that the specific cGMP source matters. PPI networks of validated seed genes suggest that cGMP signalling is segregated into modules. These modules are likely to define the therapeutic (and diagnostic, see below) targets. Thus, NP analogues may not necessarily compensate for a loss of GC-1/2 function, while vice versa, sGC stimulators may not compensate for a loss of GC-A or GC-B-mediated cGMP production; a phenomenon exemplified in experimental heart failure.135
A complex disease mechanism is comprised of a protein network rather than being definable by a single target protein. Within these network modules, the specific source of cGMP matters, as they are not interchangeable. Moreover, another important therapeutic option emerges from that i.e. network pharmacology. A dysfunctional multi-protein network is more likely to be remedied to a more physiological state by several drugs targeting different proteins of the same module. This should occur in a synergistic manner allowing reductions in the dose of each individual drug whilst retaining overall efficacy but likely reducing side effects.136 This mechanism-based network pharmacology approach is different and not to be confused with classical combination therapy, where drugs are combined that have different mechanism of action, none of which is causal for the disease, and effects are maximally additive. A clinical application of this approach is a triple-drug combination in patients with cystic fibrosis; these drugs target a causal mechanistic dysfunction increasing the eligibility for up to 90% of cystic fibrosis patients.137 Since cystic fibrosis has cardiovascular symptoms or phenotypes,138 this is also a good example for both network pharmacology and an organ-agnostic approach to disease. With respect to cGMPopathies, one approach may be to enhance cGMP production and at the same time inhibit cGMP degradation,139 but such combinations have to be chosen in an evidence-based manner, strictly within one disease module (Figure 3). Preclinical models indicate that PDE5 is more involved in regulating GC-1/2 signalling (e.g. in erectile dysfunction), whereas PDE9 and PDE2 are biased towards NP signalling (e.g. in heart failure).29,140 It remains to be seen whether some of the negative clinical studies (e.g. cinaciguat and sildenafil in heart failure) can be explained by the use of a suboptimal cGMP-modulating therapy.77,121 However, such combinations may also be contraindicated in one setting and indicated in another. A possible example of this is sildenafil and NO donors, which are contraindicated as they lead to severe hypotension.141 A similar excessive decrease in systemic blood pressure was observed with riociguat and sildenafil in patients with PH (PATENT PLUS trial).142 Conversely, a small (six patients), pilot clinical trial investigating a combination of the tried-and-tested organic nitrate, ISMN, and the PDE5 inhibitor, sildenafil, appeared to achieve better regulation of the blood pressure in patients afflicted with ‘resistant’ hypertension.143 Another proof-of-concept example came from the phase IIa trial of neprilysin inhibition in PAH patients already stable on PDE5i; this mechanistic combination seems to have an additional benefit in PAH.144
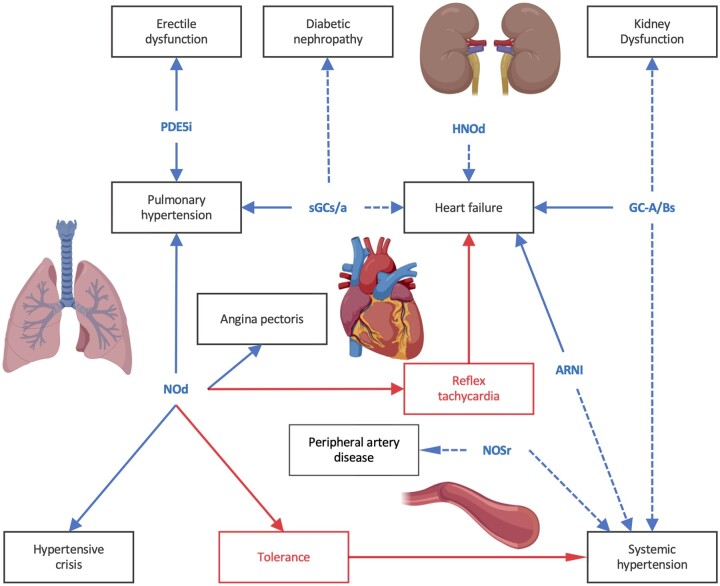
Figure 3.
cGMPopathies and evidence-based therapies. Potential combinatorial cGMP therapies in cardiovascular applications. Blue dashed lines show drugs in clinical trials, whereas solid blue lines approved drugs. Red lines show limitations of usage in these indications. ARNI, angiotensin receptor-neprilysin inhibitor; GC-A/Bs, GC-A/B stimulators; NOd, NO donors; HNOd, nitroxyl donors; NOSr, NOS recoupling drugs; PDEi, PDE inhibitors; sGCs/a, sGC stimulators/activators. Created with BioRender.com.
6. How to diagnose cGMPopathies and stratify patients?
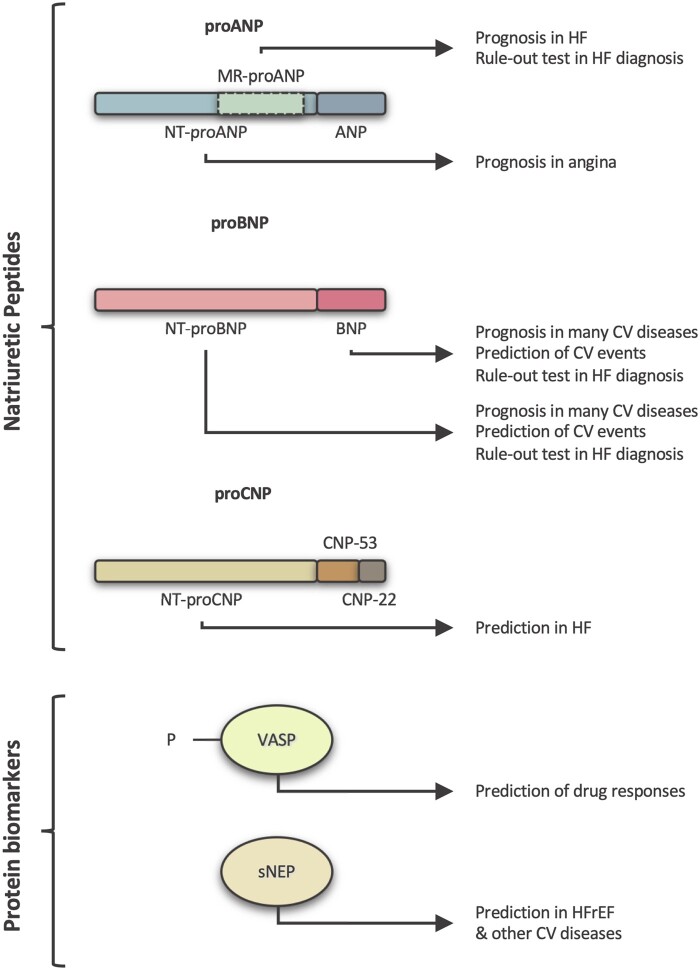
The missing link between cardiovascular phenotypes and cGMP-modulating treatments are mechanism-based biomarkers. Such tools would identify the patients that have a cGMPopathy and also the specific part of the pathway that should be targeted. Therefore, who would benefit from a cGMP therapy and which cGMP-targeting drug or drugs combination to choose remain unknown. Here, we review the current cGMP-related biomarkers and their applications. In principle, three approaches exist to assess endogenous cGMP signalling in patients: (i) cGMP itself, (ii) cGMP-PKG-dependent protein phosphorylation (e.g. of the vasodilator-stimulated phosphoprotein, VASP); or (iii) levels of endogenous GC stimulators (NO- or NP-related). Of clinical relevance, so far, are only circulating NPs and phospho-VASP (Figure 4).
Figure 4.
cGMP diagnostic toolbox. cGMP-related biomarkers currently used in the clinic for cardiovascular diseases. NPs and their precursors, sNEP and P-VASP have several diagnostic, prognostic or predicting applications in cardiovascular disorders. CV, cardiovascular.
6.1 cGMP
cGMP has been used to monitor drug-induced increase as proof of target engagement e.g. with ARNis, designer NPs, and PDE-5 inhibitors.145–147 However, variation in cGMP concentrations between individuals has hindered its use as a biomarker for primary diagnosis.148
6.2 P-VASP
P-VASP was introduced two decades ago as a new biomarker able to monitor the vascular NO/cGMP/PKG signalling.149 In principle, cell-based assays could be suited to detect defective endogenous cGMP signalling, e.g. via lower than normal phosphorylation of VASP or other PKG target proteins. However, both the phosphorylation and dephosphorylation kinetics150 would require extremely reproducible procedures with respect to blood collection, work-up and analysis. So far, this has prevented the establishment of basal P-VASP levels as a biomarker. In contrast, P-VASP assays are clinically established to assess drug responses, e.g. to predict responders and non-responders to antiplatelet drugs to reduce major cardiovascular and cerebral events.151,152 P-VASP responses to sGC activators have been used to detect a higher apo-GC-1/2/Fe(II)GC-1/2 ratio in CAD patients,37 which could be used for mechanism-based therapy in patients with elevated apo-GC-1/2 levels.
6.3 NPs and soluble neprilysin
Each of the NPs has been proposed as a predictive biomarker for cardiovascular diseases or to guide cardiovascular therapy. The best characterized is BNP and its N-terminal fragment post-processing NT-proBNP. The lack of NT-proBNP degradation by NEP and analogous enzymes makes it superior to BNP for monitoring patients. Plasma levels of BNP, NT-proBNP, and mid-regional pro-atrial natriuretic peptide (MR-proANP) have been used to aid the heart failure diagnosis as ‘rule-out’ tests, excluding significant cardiac dysfunction.10 Of note, the 2016 ESC Guidelines on the Management of Acute and Chronic Heart Failure recommend the use of BNP and NT-proBNP for the diagnosis of HF.10 Furthermore, BNP levels are associated with poor prognosis in stroke,153 NT-proBNP levels in hypertrophic cardiomyopathy,154 while both peptides predict cardiovascular events in the general population,155 and poor outcome in heart failure.156,157 However, NPs do have some limitations as diagnostic markers since there are many confounding factors.10 Indeed, age, sex, renal function but also cardiovascular diseases, including volume expansion and possibly increased wall stress, ischaemia, and hypertension, all affect circulating NP concentrations.10,158
N-Terminal pro C-Type Natriuretic Peptide predicts HFpEF outcome159 and is negatively associated with myocardial ageing;160 N-terminal pro ANP has been proposed as a prognostic biomarker in stable angina;161 MR-proANP, in heart failure.162 Finally, high levels of circulating soluble NEP (sNEP) predict outcome in HFrEF,163 but not HFpEF,164 diabetes and other cardiovascular diseases.165 Application of the above correlations for NP-guided therapy is less developed and its value uncertain: HF-related hospitalization may be reduced166 and patients with cardiovascular risk factors but not heart failure may benefit.167
6.4 Nitrogen oxides (NOx)
Many pathological conditions have been associated with altered levels of nitric oxide through measurement of more stable metabolites, nitrite and nitrate (collectively abbreviated NOx) or nitrotyrosine. Rapidly measured plasma nitrite rather than nitrate reflects endothelial nitric oxide synthase activity.168 Nitrotyrosine, either scavenging of nitric oxide through ROS or myeloperoxidase activity, is associated with increased inflammation.169 Lower levels of NOx are associated with a more severe outcome in stroke and with increased mortality in idiopathic PAH,170,171 while higher levels of NOx and nitrotyrosine correlate with increasing severity of chronic HF.169 Finally, higher levels of NOx correlate with cardiovascular mortality.172
6.5 Asymmetric dimethylarginine (ADMA)
Endogenous ADMA and NG-monomethyl-L-arginine (L-NMMA) attenuate L-arginine-dependent NO production by inhibiting and uncoupling NOS.173 Elevated levels of ADMA impair endothelial function and thus promote atherosclerosis.174 ADMA and L-NMMA are possibly strong and independent risk factors for cardiovascular disorders, such as hypertension, CAD, atherosclerosis, PH, atrial fibrillation, stroke, and peripheral artery disease.173,175 However, ADMA-guided interventional studies are missing.
7. Summary and outlook
Several cGMP-modulating drugs have entered the clinical arena with indications across a wide spectrum of cardiovascular disease states. Based on genetic evidence,18,19 correcting dysfunctional cGMP signalling, i.e. cGMPopathies, has the potential to become one of the few mechanism-based, causal interventions in cardiovascular medicine. Whilst all necessary drugs seem to be available, the key challenge will be to identify those patients with the right indications that present not only a suitable phenotype but, importantly, also exhibit cGMP dysfunction, i.e. the mechanotype. Some of the recent failures in HFpEF drug development may have been preventable by mechanism-based patient stratification. PKG phosphoprotein panels in combination with markers, such as ADMA and nitrotyrosine, may be components of such a cGMPopathy diagnostic algorithm. Once this milestone is achieved, diagnostic-enabled cGMP precision therapy will be possible, most likely by network pharmacology i.e. using multiple cGMP-modulating drugs with different targets in a synergistic manner and in doses that are lower than single drug approaches and, consequently, lower side effects. Based on the compartmentalization of cGMP and unique functions, there is a rationale for further drug discovery on both sGC and pGC. Moving from reductionistic approaches of disease development to molecular network modules is vital to understand the underlying mechanism of a disease state and the connection with its comorbidities, which is one of the reasons preclinical research fails to be translated in the clinic.176,177 Clearly, we are in an era of increasing clinical relevance and high precision, mechanism-based and curative applications of cGMP-modulating drugs.
Acknowledgements
We thank Dr Andreas Daiber, Medical University Clinics, Mainz, Germany, for helpful discussions.
Conflict of interest: D.A.K. has received a grant from National Institutes of Health (NIH-NHLBI: R35: HL-135827) and has a PDE9 inhibitors-related patent pending. T.K. has been an employee of Bayer AG until 08/2019, and he is a shareholder of Bayer AG. T.F.L. has received educational grants from Abbot Inc., AstraZeneca, Boehringen Ingelheim, Novartis, Servier and Vifor and a research grant from Amgen Inc. UK and USA. He is a consultant for Amgen Inc., Daichi- Sankyo, Pfizer, Cor2ED and Philipps; DSMB or advisory board member for Amgen Inc. and Sanofi; treasurer of ESC board; and member of the education committee in British CV Society. H.M. is a co-inventor on a hypertension-related patent application. H.H.H.W.S. has received a European Horizon 2020 grant (REPO-TRIAL) and has an sGC stimulators-related patent pending. He is a consultant for TransMIT, the president of the International Network and Systems Medicine Association and co-Editor in Chief of Network and Systems Medicine journal.
Funding
This project has received funding from the European Union s Horizon 2020 research and innovation programme under grant agreement No 777111 (REPO-TRIAL). This reflects only the author's view and the European Commission is not responsible for any use that may be made of the information it contains.
Contributor Information
Alexandra Petraina, Department of Pharmacology and Personalised Medicine, School for Mental Health and Neuroscience, Faculty of Health, Medicine and Life Sciences, Maastricht University, Maastricht University, Universiteitssingel 50, 6229 ER Maastricht, The Netherlands.
Cristian Nogales, Department of Pharmacology and Personalised Medicine, School for Mental Health and Neuroscience, Faculty of Health, Medicine and Life Sciences, Maastricht University, Maastricht University, Universiteitssingel 50, 6229 ER Maastricht, The Netherlands.
Thomas Krahn, Department of Pharmacology and Personalised Medicine, School for Mental Health and Neuroscience, Faculty of Health, Medicine and Life Sciences, Maastricht University, Maastricht University, Universiteitssingel 50, 6229 ER Maastricht, The Netherlands.
Hermann Mucke, H.M. Pharma Consultancy, Enenkelstrasse 28/32, A-1160, Vienna, Austria.
Thomas F Lüscher, Royal Brompton & Harefield Hospitals, Heart Division and National Heart and Lung Institute, Guy Scadding Building, Imperial College, Dovehouse Street London SW3 6LY, United Kingdom; Center for Molecular Cardiology, Schlieren Campus, University of Zurich, Wagistreet 12, CH-8952 Schlieren, Switzerland.
Rodolphe Fischmeister, INSERM UMR-S 1180, Faculty of Pharmacy, Université Paris-Saclay, F-92296 Châtenay-Malabry, France.
David A Kass, Division of Cardiology, Department of Medicine, Ross Research Building, Rm 858, Johns Hopkins Medical Institutions, 720 Rutland Avenue, Baltimore, MD 21205, USA.
John C Burnett, Jr, Department of Cardiovascular Medicine, Mayo Clinic, 200 First St. SW, Rochester, MN 55905, USA.
Adrian J Hobbs, William Harvey Research Institute, Barts and The London School of Medicine and Dentistry, Queen Mary University of London, Charterhouse Square, EC1M 6BQ, London, UK.
Harald H H W Schmidt, Department of Pharmacology and Personalised Medicine, School for Mental Health and Neuroscience, Faculty of Health, Medicine and Life Sciences, Maastricht University, Maastricht University, Universiteitssingel 50, 6229 ER Maastricht, The Netherlands.
References
- 1. Loscalzo J. Personalized cardiovascular medicine and drug development. Circulation 2012;125:638–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Claussnitzer M, Cho JH, Collins R, Cox NJ, Dermitzakis ET, Hurles ME, Kathiresan S, Kenny EE, Lindgren CM, MacArthur DG, North KN, Plon SE, Rehm HL, Risch N, Rotimi CN, Shendure J, Soranzo N, McCarthy MI.. A brief history of human disease genetics. Nature 2020;577:179–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Leopold JA, Loscalzo J.. Emerging role of precision medicine in cardiovascular disease. Circ Res 2018;122:1302–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schmidt HHHW, Baumbach J, Loscalzo J, Agusti A, Silverman EK, Azevedo V.. Expert panel discusses the importance of systems medicine. Syst Med 2018;1:3–8. [Google Scholar]
- 5. Baumbach J, Schmidt HHHW.. The end of medicine as we know it: introduction to the new journal, Systems Medicine. Syst Med 2018;1:1–2. [Google Scholar]
- 6. Garmaroudi FS, Handy DE, Liu Y-Y, Loscalzo J.. Systems pharmacology and rational polypharmacy: nitric oxide−cyclic GMP signaling pathway as an illustrative example and derivation of the general case. PLoS Comput Biol 2016;12:e1004822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Oettrich J, Dao V, Frijhoff J, Kleikers P, Casas A, Hobbs A, Schmidt H.. Clinical relevance of cyclic GMP modulators: a translational success story of network pharmacology. Clin Pharmacol Ther 2016;99:360–362. [DOI] [PubMed] [Google Scholar]
- 8. Langhauser F, Casas AI, Dao V-T-V, Guney E, Menche J, Geuss E, Kleikers PWM, López MG, Barabási A-L, Kleinschnitz C, Schmidt HHHW.. A diseasome cluster-based drug repurposing of soluble guanylate cyclase activators from smooth muscle relaxation to direct neuroprotection. NPJ Syst Biol Appl 2018;4:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rousan TA, Thadani U.. Stable angina medical therapy management guidelines: a critical review of guidelines from the European Society of Cardiology and National Institute for Health and Care Excellence. ECR 2019;14:18–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González-Juanatey JR, Harjola V-P, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, E.S.C. Scientific Document Group . 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129–2200. [DOI] [PubMed] [Google Scholar]
- 11. Galiè N, Humbert M, Vachiery J-L, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M, E.S.C. Scientific Document Group . 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016;37:67–119. [DOI] [PubMed] [Google Scholar]
- 12. Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, Prescott E, Storey RF, Deaton C, Cuisset T, Agewall S, Dickstein K, Edvardsen T, Escaned J, Gersh BJ, Svitil P, Gilard M, Hasdai D, Hatala R, Mahfoud F, Masip J, Muneretto C, Valgimigli M, Achenbach S, Bax JJ, E.S.C. Scientific Document Group . 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes: the Task Force for the diagnosis and management of chronic coronary syndromes of the European Society of Cardiology (ESC). Eur Heart J 2020;41:407–477. [DOI] [PubMed] [Google Scholar]
- 13. Hatzimouratidis K, Amar E, Eardley I, Giuliano F, Hatzichristou D, Montorsi F, Vardi Y, Wespes E, European Association of Urology . Guidelines on male sexual dysfunction: erectile dysfunction and premature ejaculation. Eur Urol 2010;57:804–814. [DOI] [PubMed] [Google Scholar]
- 14. Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, Kahan T, Mahfoud F, Redon J, Ruilope L, Zanchetti A, Kerins M, Kjeldsen SE, Kreutz R, Laurent S, Lip GYH, McManus R, Narkiewicz K, Ruschitzka F, Schmieder RE, Shlyakhto E, Tsioufis C, Aboyans V, Desormais I, Scientific Document Group ESC, ESC Scientific Document Group . 2018 ESC/ESH Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH). Eur Heart J 2018;39:3021–3104. [DOI] [PubMed] [Google Scholar]
- 15. Tsai EJ, Kass DA.. Cyclic GMP signaling in cardiovascular pathophysiology and therapeutics. Pharmacol Ther 2009;122:216–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Golshiri K, Ataei Ataabadi E, Portilla Fernandez EC, Jan Danser AH, Roks AJM.. The importance of the nitric oxide-cGMP pathway in age-related cardiovascular disease: focus on phosphodiesterase-1 and soluble guanylate cyclase. Basic Clin Pharmacol Toxicol 2020;127:67–80. [DOI] [PubMed] [Google Scholar]
- 17. Ehret GB, Munroe PB, Rice KM, Bochud M, Johnson AD, Chasman DI, Smith AV, Tobin MD, Verwoert GC, Hwang S-J, Pihur V, Vollenweider P, O'Reilly PF, Amin N, Bragg-Gresham JL, Teumer A, Glazer NL, Launer L, Zhao JH, Aulchenko Y, Heath S, Sõber S, Parsa A, Luan J, Arora P, Dehghan A, Zhang F, Lucas G, Hicks AA, Jackson AU, Peden JF, Tanaka T, Wild SH, Rudan I, Igl W, Milaneschi Y, Parker AN, Fava C, Chambers JC, Fox ER, Kumari M, Go MJ, van der Harst P, Kao WHL, Sjögren M, Vinay DG, Alexander M, Tabara Y, Shaw-Hawkins S, Whincup PH, Liu Y, Shi G, Kuusisto J, Tayo B, Seielstad M, Sim X, Nguyen K-DH, Lehtimäki T, Matullo G, Wu Y, Gaunt TR, Onland-Moret NC, Cooper MN, Platou CGP, Org E, Hardy R, Dahgam S, Palmen J, Vitart V, Braund PS, Kuznetsova T, Uiterwaal CSPM, Adeyemo A, Palmas W, Campbell H, Ludwig B, Tomaszewski M, Tzoulaki I, Palmer ND, Aspelund T, Garcia M, Chang Y-PC, O'Connell JR, Steinle NI, Grobbee DE, Arking DE, Kardia SL, Morrison AC, Hernandez D, Najjar S, McArdle WL, Hadley D, Brown MJ, Connell JM, Hingorani AD, Day INM, Lawlor DA, Beilby JP, Lawrence RW, Clarke R, Hopewell JC, Ongen H, Dreisbach AW, Li Y, Young JH, Bis JC, Kähönen M, Viikari J, Adair LS, Lee NR, Chen M-H, Olden M, Pattaro C, Bolton JAH, Köttgen A, Bergmann S, Mooser V, Chaturvedi N, Frayling TM, Islam M, Jafar TH, Erdmann J, Kulkarni SR, Bornstein SR, Grässler J, Groop L, Voight BF, Kettunen J, Howard P, Taylor A, Guarrera S, Ricceri F, Emilsson V, Plump A, Barroso I, Khaw K-T, Weder AB, Hunt SC, Sun YV, Bergman RN, Collins FS, Bonnycastle LL, Scott LJ, Stringham HM, Peltonen L, Perola M, Vartiainen E, Brand S-M, Staessen JA, Wang TJ, Burton PR, Soler Artigas M, Dong Y, Snieder H, Wang X, Zhu H, Lohman KK, Rudock ME, Heckbert SR, Smith NL, Wiggins KL, Doumatey A, Shriner D, Veldre G, Viigimaa M, Kinra S, Prabhakaran D, Tripathy V, Langefeld CD, Rosengren A, Thelle DS, Corsi AM, Singleton A, Forrester T, Hilton G, McKenzie CA, Salako T, Iwai N, Kita Y, Ogihara T, Ohkubo T, Okamura T, Ueshima H, Umemura S, Eyheramendy S, Meitinger T, Wichmann H-E, Cho YS, Kim H-L, Lee J-Y, Scott J, Sehmi JS, Zhang W, Hedblad B, Nilsson P, Smith GD, Wong A, Narisu N, Stančáková A, Raffel LJ, Yao J, Kathiresan S, O'Donnell CJ, Schwartz SM, Ikram MA, Longstreth WT, Mosley TH, Seshadri S, Shrine NRG, Wain LV, Morken MA, Swift AJ, Laitinen J, Prokopenko I, Zitting P, Cooper JA, Humphries SE, Danesh J, Rasheed A, Goel A, Hamsten A, Watkins H, Bakker SJL, van Gilst WH, Janipalli CS, Mani KR, Yajnik CS, Hofman A, Mattace-Raso FUS, Oostra BA, Demirkan A, Isaacs A, Rivadeneira F, Lakatta EG, Orru M, Scuteri A, Ala-Korpela M, Kangas AJ, Lyytikäinen L-P, Soininen P, Tukiainen T, Würtz P, Ong RT-H, Dörr M, Kroemer HK, Völker U, Völzke H, Galan P, Hercberg S, Lathrop M, Zelenika D, Deloukas P, Mangino M, Spector TD, Zhai G, Meschia JF, Nalls MA, Sharma P, Terzic J, Kumar MVK, Denniff M, Zukowska-Szczechowska E, Wagenknecht LE, Fowkes FGR, Charchar FJ, Schwarz PEH, Hayward C, Guo X, Rotimi C, Bots ML, Brand E, Samani NJ, Polasek O, Talmud PJ, Nyberg F, Kuh D, Laan M, Hveem K, Palmer LJ, van der Schouw YT, Casas JP, Mohlke KL, Vineis P, Raitakari O, Ganesh SK, Wong TY, Tai ES, Cooper RS, Laakso M, Rao DC, Harris TB, Morris RW, Dominiczak AF, Kivimaki M, Marmot MG, Miki T, Saleheen D, Chandak GR, Coresh J, Navis G, Salomaa V, Han B-G, Zhu X, Kooner JS, Melander O, Ridker PM, Bandinelli S, Gyllensten UB, Wright AF, Wilson JF, Ferrucci L, Farrall M, Tuomilehto J, Pramstaller PP, Elosua R, Soranzo N, Sijbrands EJG, Altshuler D, Loos RJF, Shuldiner AR, Gieger C, Meneton P, Uitterlinden AG, Wareham NJ, Gudnason V, Rotter JI, Rettig R, Uda M, Strachan DP, Witteman JCM, Hartikainen A-L, Beckmann JS, Boerwinkle E, Vasan RS, Boehnke M, Larson MG, Järvelin M-R, Psaty BM, Abecasis GR, Chakravarti A, Elliott P, van Duijn CM, Newton-Cheh C, Levy D, Caulfield MJ, Johnson T, The International Consortium for Blood Pressure Genome-Wide Association Studies, CARDIoGRAM consortium, CKDGen Consortium, KidneyGen Consortium, EchoGen consortium, Charge-HF consortium . Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature 2011;478:103–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dang TA, Schunkert H, Kessler T.. cGMP signaling in cardiovascular diseases: linking genotype and phenotype. J Cardiovasc Pharmacol 2020;75:516–525. [DOI] [PubMed] [Google Scholar]
- 19. Oliveira-Paula GH, Lacchini R, Tanus-Santos JE.. Clinical and pharmacogenetic impact of endothelial nitric oxide synthase polymorphisms on cardiovascular diseases. Nitric Oxide 2017;63:39–51. [DOI] [PubMed] [Google Scholar]
- 20. McNamara DM, Holubkov R, Postava L, Ramani R, Janosko K, Mathier M, MacGowan GA, Murali S, Feldman AM, London B.. Effect of the Asp298 variant of endothelial nitric oxide synthase on survival for patients with congestive heart failure. Circulation 2003;107:1598–1602. [DOI] [PubMed] [Google Scholar]
- 21. Erdmann J, Stark K, Esslinger UB, Rumpf PM, Koesling D, de Wit C, Kaiser FJ, Braunholz D, Medack A, Fischer M, Zimmermann ME, Tennstedt S, Graf E, Eck S, Aherrahrou Z, Nahrstaedt J, Willenborg C, Bruse P, Brænne I, Nöthen MM, Hofmann P, Braund PS, Mergia E, Reinhard W, Burgdorf C, Schreiber S, Balmforth AJ, Hall AS, Bertram L, Steinhagen-Thiessen E, Li S-C, März W, Reilly M, Kathiresan S, McPherson R, Walter U, Ott J, Samani NJ, Strom TM, Meitinger T, Hengstenberg C, Schunkert H, CardioGram . Dysfunctional nitric oxide signalling increases risk of myocardial infarction. Nature 2013;504:432–436. [DOI] [PubMed] [Google Scholar]
- 22. Nakayama T, Soma M, Takahashi Y, Rehemudula D, Kanmatsuse K, Furuya K.. Functional deletion mutation of the 5'-flanking region of type A human natriuretic peptide receptor gene and its association with essential hypertension and left ventricular hypertrophy in the Japanese. Circ Res 2000;86:841–845. [DOI] [PubMed] [Google Scholar]
- 23. Hodgson-Zingman DM, Karst ML, Zingman LV, Heublein DM, Darbar D, Herron KJ, Ballew JD, de Andrade M, Burnett JC, Olson TM.. Atrial natriuretic peptide frameshift mutation in familial atrial fibrillation. N Engl J Med 2008;359:158–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cannone V, Cefalu’ AB, Noto D, Scott CG, Bailey KR, Cavera G, Pagano M, Sapienza M, Averna MR, Burnett JC.. The atrial natriuretic peptide genetic variant rs5068 is associated with a favorable cardiometabolic phenotype in a Mediterranean population. Diabetes Care 2013;36:2850–2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Premer C, Kanelidis AJ, Hare JM, Schulman IH.. Rethinking endothelial dysfunction as a crucial target in fighting heart failure. Mayo Clin Proc Innov Qual Outcomes 2019;3:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Marti CN, Gheorghiade M, Kalogeropoulos AP, Georgiopoulou VV, Quyyumi AA, Butler J.. Endothelial dysfunction, arterial stiffness, and heart failure. J Am Coll Cardiol 2012;60:1455–1469. [DOI] [PubMed] [Google Scholar]
- 27. Heerebeek L, Hamdani N, Falcão-Pires I, Leite-Moreira AF, Begieneman MPV, Bronzwaer JGF, Velden J, Stienen GJM, Laarman GJ, Somsen A, Verheugt FWA, Niessen HWM, Paulus WJ.. Low myocardial protein kinase G activity in heart failure with preserved ejection fraction. Circulation 2012;126:830–839. [DOI] [PubMed] [Google Scholar]
- 28. Franssen C, Chen S, Unger A, Korkmaz HI, Keulenaer GWD, Tschöpe C, Leite-Moreira AF, Musters R, Niessen HWM, Linke WA, Paulus WJ, Hamdani N.. Myocardial microvascular inflammatory endothelial activation in heart failure with preserved ejection fraction. JACC Heart Fail 2016;4:312–324. [DOI] [PubMed] [Google Scholar]
- 29. Lee DI, Zhu G, Sasaki T, Cho G-S, Hamdani N, Holewinski R, Jo S-H, Danner T, Zhang M, Rainer PP, Bedja D, Kirk JA, Ranek MJ, Dostmann WR, Kwon C, Margulies KB, Van Eyk JE, Paulus WJ, Takimoto E, Kass DA.. Phosphodiesterase 9A controls nitric-oxide-independent cGMP and hypertrophic heart disease. Nature 2015;519:472–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tsutamoto T, Kanamori T, Morigami N, Sugimoto Y, Yamaoka O, Kinoshita M.. Possibility of downregulation of atrial natriuretic peptide receptor coupled to guanylate cyclase in peripheral vascular beds of patients with chronic severe heart failure. Circulation 1993;87:70–75. [DOI] [PubMed] [Google Scholar]
- 31. Kuhn M, Voss M, Mitko D, Stypmann J, Schmid C, Kawaguchi N, Grabellus F, Baba HA.. Left ventricular assist device support reverses altered cardiac expression and function of natriuretic peptides and receptors in end-stage heart failure. Cardiovasc Res 2004;64:308–314. [DOI] [PubMed] [Google Scholar]
- 32. Dickey DM, Dries DL, Margulies KB, Potter LR.. Guanylyl cyclase (GC)-A and GC-B activities in ventricles and cardiomyocytes from failed and non-failed human hearts: GC-A is inactive in the failed cardiomyocyte. J Mol Cell Cardiol 2012;52:727–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Knight WE, Chen S, Zhang Y, Oikawa M, Wu M, Zhou Q, Miller CL, Cai Y, Mickelsen DM, Moravec C, Small EM, Abe J, Yan C.. PDE1C deficiency antagonizes pathological cardiac remodeling and dysfunction. Proc Natl Acad Sci USA 2016;113:E7116–E7125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pokreisz P, Vandenwijngaert S, Bito V, Van den Bergh A, Lenaerts I, Busch C, Marsboom G, Gheysens O, Vermeersch P, Biesmans L, Liu X, Gillijns H, Pellens M, Van Lommel A, Buys E, Schoonjans L, Vanhaecke J, Verbeken E, Sipido K, Herijgers P, Bloch KD, Janssens SP.. Ventricular phosphodiesterase-5 expression is increased in patients with advanced heart failure and contributes to adverse ventricular remodeling after myocardial infarction in mice. Circulation 2009;119:408–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Andreadou I, Iliodromitis EK, Lazou A, Görbe A, Giricz Z, Schulz R, Ferdinandy P.. Effect of hypercholesterolaemia on myocardial function, ischaemia-reperfusion injury and cardioprotection by preconditioning, postconditioning and remote conditioning. Br J Pharmacol 2017;174:1555–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Guzik TJ, West NEJ, Black E, McDonald D, Ratnatunga C, Pillai R, Channon KM.. Vascular superoxide production by NAD(P)H oxidase. Circ Res 2000;86:e85–e90. [DOI] [PubMed] [Google Scholar]
- 37. Ahrens I, Habersberger J, Baumlin N, Qian H, Smith BK, Stasch J-P, Bode C, Schmidt HHHW, Peter K.. Measuring oxidative burden and predicting pharmacological response in coronary artery disease patients with a novel direct activator of haem-free/oxidised sGC. Atherosclerosis 2011;218:431–434. [DOI] [PubMed] [Google Scholar]
- 38. Antoniades C, Shirodaria C, Leeson P, Antonopoulos A, Warrick N, Van-Assche T, Cunnington C, Tousoulis D, Pillai R, Ratnatunga C, Stefanadis C, Channon KM.. Association of plasma asymmetrical dimethylarginine (ADMA) with elevated vascular superoxide production and endothelial nitric oxide synthase uncoupling: implications for endothelial function in human atherosclerosis. Eur Heart J 2009;30:1142–1150. [DOI] [PubMed] [Google Scholar]
- 39. Giricz Z, Görbe A, Pipis J, Burley DS, Ferdinandy P, Baxter GF.. Hyperlipidaemia induced by a high-cholesterol diet leads to the deterioration of guanosine-3',5'-cyclic monophosphate/protein kinase G-dependent cardioprotection in rats. Br J Pharmacol 2009;158:1495–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Panza JA, García CE, Kilcoyne CM, Quyyumi AA, Cannon RO.. Impaired endothelium-dependent vasodilation in patients with essential hypertension. Circulation 1995;91:1732–1738. [DOI] [PubMed] [Google Scholar]
- 41. Besedina A. NO-synthase activity in patients with coronary heart disease associated with hypertension of different age groups. J Med Biochem 2016;35:43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Schlaich MP, Parnell MM, Ahlers BA, Finch S, Marshall T, Zhang W-Z, Kaye DM.. Impaired L-arginine transport and endothelial function in hypertensive and genetically predisposed normotensive subjects. Circulation 2004;110:3680–3686. [DOI] [PubMed] [Google Scholar]
- 43. Xu W, Kaneko FT, Zheng S, Comhair SAA, Janocha AJ, Goggans T, Thunnissen FBJM, Farver C, Hazen SL, Jennings C, Dweik RA, Arroliga AC, Erzurum SC.. Increased arginase II and decreased NO synthesis in endothelial cells of patients with pulmonary arterial hypertension. Faseb J 2004;18:1746–1748. [DOI] [PubMed] [Google Scholar]
- 44. Elbatreek MH, Sadegh S, Anastasi E, Guney E, Nogales C, Kacprowski T, Hassan AA, Teubner A, Huang P-H, Hsu C-Y, Schiffers PMH, Janssen GM, Kleikers PWM, Wipat A, Baumbach J, De Mey JGR, Schmidt HHHW.. NOX5-induced uncoupling of endothelial NO synthase is a causal mechanism and theragnostic target of an age-related hypertension endotype. PLoS Biol 2020;18:e3000885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kang Y, Liu R, Wu J-X, Chen L.. Structural insights into the mechanism of human soluble guanylate cyclase. Nature 2019;574:206–210. [DOI] [PubMed] [Google Scholar]
- 46. Horst BG, Yokom AL, Rosenberg DJ, Morris KL, Hammel M, Hurley JH, Marletta MA.. Allosteric activation of the nitric oxide receptor soluble guanylate cyclase mapped by cryo-electron microscopy. Elife 2019;8:e50634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Meurer S, Pioch S, Pabst T, Opitz N, Schmidt PM, Beckhaus T, Wagner K, Matt S, Gegenbauer K, Geschka S, Karas M, Stasch J-P, Schmidt HHHW, Müller-Esterl W.. Nitric oxide-independent vasodilator rescues heme-oxidized soluble guanylate cyclase from proteasomal degradation. Circ Res 2009;105:33–41. [DOI] [PubMed] [Google Scholar]
- 48. Evgenov OV, Pacher P, Schmidt PM, Haskó G, Schmidt HHHW, Stasch J-P.. NO-independent stimulators and activators of soluble guanylate cyclase: discovery and therapeutic potential. Nat Rev Drug Discov 2006;5:755–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Stasch J-P, Schmidt PM, Nedvetsky PI, Nedvetskaya TY, H S AK, Meurer S, Deile M, Taye A, Knorr A, Lapp H, Müller H, Turgay Y, Rothkegel C, Tersteegen A, Kemp-Harper B, Müller-Esterl W, Schmidt HHHW.. Targeting the heme-oxidized nitric oxide receptor for selective vasodilatation of diseased blood vessels. J Clin Invest 2006;116:2552–2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Alkaitis MS, Crabtree MJ.. Recoupling the cardiac nitric oxide synthases: tetrahydrobiopterin synthesis and recycling. Curr Heart Fail Rep 2012;9:200–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Münzel T, Daiber A.. Inorganic nitrite and nitrate in cardiovascular therapy: a better alternative to organic nitrates as nitric oxide donors? Vascul Pharmacol 2018;102:1–10. [DOI] [PubMed] [Google Scholar]
- 52. Dao VT-V, Elbatreek MH, Deile M, Nedvetsky PI, Güldner A, Ibarra-Alvarado C, Gödecke A, Schmidt HHHW.. Non-canonical chemical feedback self-limits nitric oxide-cyclic GMP signaling in health and disease. Sci Rep 2020;10:10012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Unger T, Borghi C, Charchar F, Khan NA, Poulter NR, Prabhakaran D, Ramirez A, Schlaich M, Stergiou GS, Tomaszewski M, Wainford RD, Williams B, Schutte AE.. 2020 International Society of Hypertension Global Hypertension Practice Guidelines. Hypertension 2020;75:1334–1357. [DOI] [PubMed] [Google Scholar]
- 54. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJV, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WHW, Tsai EJ, Wilkoff BL, American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines . 2013 ACCF/AHA guideline for the management of heart failure. Circulation 2013;128:e240–e327. [DOI] [PubMed] [Google Scholar]
- 55. Amsterdam EA, Wenger NK, Brindis RG, Casey DE, Ganiats TG, Holmes DR, Jaffe AS, Jneid H, Kelly RF, Kontos MC, Levine GN, Liebson PR, Mukherjee D, Peterson ED, Sabatine MS, Smalling RW, Zieman SJ, ACC/AHA Task Force Members . 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes. Circulation 2014;130:e344–e426. [DOI] [PubMed] [Google Scholar]
- 56. Fihn SD, Gardin JM, Abrams J, Berra K, Blankenship JC, Dallas AP, Douglas PS, Foody JM, Gerber TC, Hinderliter AL, King SB, Kligfield PD, Krumholz HM, Kwong RYK, Lim MJ, Linderbaum JA, Mack MJ, Munger MA, Prager RL, Sabik JF, Shaw LJ, Sikkema JD, Smith CR, Smith SC, Spertus JA, Williams SV, Anderson JL, American College of Cardiology Foundation/American Heart Association Task Force . 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease. Circulation 2012;126:e354–e471. [DOI] [PubMed] [Google Scholar]
- 57. Husted SE, Ohman EM.. Pharmacological and emerging therapies in the treatment of chronic angina. Lancet 2015;386:691–701. [DOI] [PubMed] [Google Scholar]
- 58. Daiber A, Münzel T.. Organic nitrate therapy, nitrate tolerance, and nitrate-induced endothelial dysfunction: emphasis on redox biology and oxidative stress. Antioxid Redox Signal 2015;23:899–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Andrews KL, Lumsden NG, Farry J, Jefferis A-M, Kemp-Harper BK, Chin-Dusting JPF.. Nitroxyl: a vasodilator of human vessels that is not susceptible to tolerance. Clin Sci 2015;129:179–187. [DOI] [PubMed] [Google Scholar]
- 60. Tita C, Gilbert EM, Van Bakel AB, Grzybowski J, Haas GJ, Jarrah M, Dunlap SH, Gottlieb SS, Klapholz M, Patel PC, Pfister R, Seidler T, Shah KB, Zieliński T, Venuti RP, Cowart D, Foo SY, Vishnevsky A, Mitrovic V.. A Phase 2a dose-escalation study of the safety, tolerability, pharmacokinetics and haemodynamic effects of BMS-986231 in hospitalized patients with heart failure with reduced ejection fraction. Eur J Heart Fail 2017;19:1321–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Dikalov S, Fink B, Skatchkov M, Sommer O, Bassenge E.. Formation of reactive oxygen species in various vascular cells during glyceryltrinitrate metabolism. J Cardiovasc Pharmacol Ther 1998;3:51–61. [DOI] [PubMed] [Google Scholar]
- 62. Conole D, Scott LJ.. Riociguat: first global approval. Drugs 2013;73:1967–1975. [DOI] [PubMed] [Google Scholar]
- 63. Klinger JR, Elliott CG, Levine DJ, Bossone E, Duvall L, Fagan K, Frantsve-Hawley J, Kawut SM, Ryan JJ, Rosenzweig EB, Sederstrom N, Steen VD, Badesch DB.. Therapy for pulmonary arterial hypertension in adults: update of the CHEST Guideline and Expert Panel Report. Chest 2019;155:565–586. [DOI] [PubMed] [Google Scholar]
- 64. Nathan SD, Behr J, Collard HR, Cottin V, Hoeper MM, Martinez FJ, Corte TJ, Keogh AM, Leuchte H, Mogulkoc N, Ulrich S, Wuyts WA, Yao Z, Boateng F, Wells AU.. Riociguat for idiopathic interstitial pneumonia-associated pulmonary hypertension (RISE-IIP): a randomised, placebo-controlled phase 2b study. Lancet Respir Med 2019;7:780–790. [DOI] [PubMed] [Google Scholar]
- 65. Bonderman D, Ghio S, Felix SB, Ghofrani H-A, Michelakis E, Mitrovic V, Oudiz RJ, Boateng F, Scalise A-V, Roessig L, Semigran MJ, on behalf of the Left Ventricular Systolic Dysfunction Associated With Pulmonary Hypertension Riociguat Trial (LEPHT) Study Group . Riociguat for patients with pulmonary hypertension caused by systolic left ventricular dysfunction. Circulation 2013;128:502–511.23775260 [Google Scholar]
- 66. Bonderman D, Pretsch I, Steringer-Mascherbauer R, Jansa P, Rosenkranz S, Tufaro C, Bojic A, Lam CSP, Frey R, Ochan Kilama M, Unger S, Roessig L, Lang IM.. Acute hemodynamic effects of riociguat in patients with pulmonary hypertension associated with diastolic heart failure (DILATE-1): a randomized, double-blind, placebo-controlled, single-dose study. Chest 2014;146:1274–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Mascherbauer J, Grünig E, Halank M, Hohenforst-Schmidt W, Kammerlander AA, Pretsch I, Steringer-Mascherbauer R, Ulrich S, Lang IM, Wargenau M, Frey R, Bonderman D.. Evaluation of the pharmacoDYNAMIC effects of riociguat in subjects with pulmonary hypertension and heart failure with preserved ejection fraction. Wien Klin Wochenschr 2016;128:882–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Armstrong PW, Pieske B, Anstrom KJ, Ezekowitz J, Hernandez AF, Butler J, Lam CSP, Ponikowski P, Voors AA, Jia G, McNulty SE, Patel MJ, Roessig L, Koglin J, O'Connor CM, VICTORIA Study Group . Vericiguat in patients with heart failure and reduced ejection fraction. N Engl J Med 2020;382:1883–1893. [DOI] [PubMed] [Google Scholar]
- 69. Markham A, Duggan S.. Vericiguat: first approval. Drugs 2021;81:721–726. [DOI] [PubMed] [Google Scholar]
- 70. Armstrong PW, Lam CSP, Anstrom KJ, Ezekowitz J, Hernandez AF, O'Connor CM, Pieske B, Ponikowski P, Shah SJ, Solomon SD, Voors AA, She L, Vlajnic V, Carvalho F, Bamber L, Blaustein RO, Roessig L, Butler J, for the VITALITY-HFpEF Study Group . Effect of vericiguat vs placebo on quality of life in patients with heart failure and preserved ejection fraction: the VITALITY-HFpEF randomized clinical trial. JAMA 2020;324:1512–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Pieske B, Maggioni AP, Lam CSP, Pieske-Kraigher E, Filippatos G, Butler J, Ponikowski P, Shah SJ, Solomon SD, Scalise A-V, Mueller K, Roessig L, Gheorghiade M.. Vericiguat in patients with worsening chronic heart failure and preserved ejection fraction: results of the SOluble guanylate Cyclase stimulatoR in heArT failurE patientS with PRESERVED EF (SOCRATES-PRESERVED) study. Eur Heart J 2017;38:1119–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hanrahan JP, Seferovic JP, Wakefield JD, Wilson PJ, Chickering JG, Jung J, Carlson KE, Zimmer DP, Frelinger AL, Michelson AD, Morrow L, Hall M, Currie MG, Milne GT, Profy AT.. An exploratory, randomised, placebo-controlled, 14 day trial of the soluble guanylate cyclase stimulator praliciguat in participants with type 2 diabetes and hypertension. Diabetologia 2020;63:733–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Shea CM, Price GM, Liu G, Sarno R, Buys ES, Currie MG, Masferrer JL.. Soluble guanylate cyclase stimulator praliciguat attenuates inflammation, fibrosis, and end-organ damage in the Dahl model of cardiorenal failure. Am J Physiol Renal Physiol 2020;318:F148–F159. [DOI] [PubMed] [Google Scholar]
- 74. Udelson JE, Lewis GD, Shah SJ, Zile MR, Redfield MM, Burnett J Jr, Parker J, Seferovic JP, Wilson P, Mittleman RS, Profy AT, Konstam MA.. Effect of praliciguat on peak rate of oxygen consumption in patients with heart failure with preserved ejection fraction: the CAPACITY HFpEF randomized clinical trial. JAMA 2020;324:1522–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Hanrahan JP, de Boer IH, Bakris GL, Wilson PJ, Wakefield JD, Seferovic JP, Chickering JG, Chien Y-T, Carlson K, Cressman MD, Currie MG, Milne GT, Profy AT.. Effects of the soluble guanylate cyclase stimulator praliciguat in diabetic kidney disease. Clin J Am Soc Nephrol 2020;16:59–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Schindler U, Strobel H, Schönafinger K, Linz W, Löhn M, Martorana PA, Rütten H, Schindler PW, Busch AE, Sohn M, Töpfer A, Pistorius A, Jannek C, Mülsch A.. Biochemistry and pharmacology of novel anthranilic acid derivatives activating heme-oxidized soluble guanylyl cyclase. Mol Pharmacol 2006;69:1260–1268. [DOI] [PubMed] [Google Scholar]
- 77. Gheorghiade M, Greene SJ, Filippatos G, Erdmann E, Ferrari R, Levy PD, Maggioni A, Nowack C, Mebazaa A, on behalf of the COMPOSE Investigators and Coordinators . Cinaciguat, a soluble guanylate cyclase activator: results from the randomized, controlled, phase IIb COMPOSE programme in acute heart failure syndromes. Eur J Heart Fail 2012;14:1056–1066. [DOI] [PubMed] [Google Scholar]
- 78. Hahn MG, Lampe T, El Sheikh S, Griebenow N, Woltering E, Schlemmer K-H, Dietz L, Gerisch M, Wunder F, Becker-Pelster E-M, Mondritzki T, Tinel H, Knorr A, Kern A, Lang D, Hueser J, Schomber T, Benardeau A, Eitner F, Truebel H, Mittendorf J, Kumar V, van den Akker F, Schaefer M, Geiss V, Sandner P, Stasch J-P.. Discovery of the soluble guanylate cyclase activator runcaciguat (BAY 1101042). J Med Chem 2021;64:5323–5344. [DOI] [PubMed] [Google Scholar]
- 79. Potter LR. Guanylyl cyclase structure, function and regulation. Cell Signal 2011;23:1921–1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Kuhn M. Molecular physiology of membrane guanylyl cyclase receptors. Physiol Rev 2016;96:751–804. [DOI] [PubMed] [Google Scholar]
- 81. Kotlyar M, Pastrello C, Malik Z, Jurisica I.. IID 2018 update: context-specific physical protein–protein interactions in human, model organisms and domesticated species. Nucleic Acids Res 2019;47:D581–D589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Buglioni A, Burnett JCJr.. New pharmacological strategies to increase cGMP. Annu Rev Med 2016;67:229–243. [DOI] [PubMed] [Google Scholar]
- 83. Preedy MEJ, Baliga RS, Hobbs AJ.. Multiplicity of nitric oxide and natriuretic peptide signaling in heart failure. J Cardiovasc Pharmacol 2020;75:370–384. [DOI] [PubMed] [Google Scholar]
- 84. Huntley BK, Sandberg SM, Heublein DM, Sangaralingham SJ, Burnett JC, Ichiki T.. Pro-B-type natriuretic peptide-1-108 processing and degradation in human heart failure. Circ Heart Fail 2015;8:89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Hansen LH, Madsen TD, Goth CK, Clausen H, Chen Y, Dzhoyashvili N, Iyer SR, Sangaralingham SJ, Burnett JC, Rehfeld JF, Vakhrushev SY, Schjoldager KT, Goetze JP.. Discovery of O-glycans on atrial natriuretic peptide (ANP) that affect both its proteolytic degradation and potency at its cognate receptor. J Biol Chem 2019;294:12567–12578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Reginauld SH, Cannone V, Iyer S, Scott C, Bailey K, Schaefer J, Chen Y, Sangaralingham SJ, Burnett JC.. Differential regulation of ANP and BNP in acute decompensated heart failure: deficiency of ANP. JACC Heart Fail 2019;7:891–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Nomura F, Kurobe N, Mori Y, Hikita A, Kawai M, Suwa M, Okutani Y.. Multicenter prospective investigation on efficacy and safety of carperitide as a first-line drug for acute heart failure syndrome with preserved blood pressure: COMPASS: carperitide effects observed through monitoring dyspnea in acute decompensated heart failure study. Circ J 2008;72:1777–1786. [DOI] [PubMed] [Google Scholar]
- 88. Hata N, Seino Y, Tsutamoto T, Hiramitsu S, Kaneko N, Yoshikawa T, Yokoyama H, Tanaka K, Mizuno K, Nejima J, Kinoshita M.. Effects of carperitide on the long-term prognosis of patients with acute decompensated chronic heart failure: the PROTECT multicenter randomized controlled study. Circ J 2008;72:1787–1793. [DOI] [PubMed] [Google Scholar]
- 89. Nagai T, Iwakami N, Nakai M, Nishimura K, Sumita Y, Mizuno A, Tsutsui H, Ogawa H, Anzai T, JROAD-DPC investigators . Effect of intravenous carperitide versus nitrates as first-line vasodilators on in-hospital outcomes in hospitalized patients with acute heart failure: insight from a nationwide claim-based database. Int J Cardiol 2019;280:104–109. [DOI] [PubMed] [Google Scholar]
- 90. Tsutsui H, Isobe M, Ito H, Ito H, Okumura K, Ono M, Kitakaze M, Kinugawa K, Kihara Y, Goto Y, Komuro I, Saiki Y, Saito Y, Sakata Y, Sato N, Sawa Y, Shiose A, Shimizu W, Shimokawa H, Seino Y, Node K, Higo T, Hirayama A, Makaya M, Masuyama T, Murohara T, Momomura S-I, Yano M, Yamazaki K, Yamamoto K, Yoshikawa T, Yoshimura M, Akiyama M, Anzai T, Ishihara S, Inomata T, Imamura T, Iwasaki Y-K, Ohtani T, Onishi K, Kasai T, Kato M, Kawai M, Kinugasa Y, Kinugawa S, Kuratani T, Kobayashi S, Sakata Y, Tanaka A, Toda K, Noda T, Nochioka K, Hatano M, Hidaka T, Fujino T, Makita S, Yamaguchi O, Ikeda U, Kimura T, Kohsaka S, Kosuge M, Yamagishi M, Yamashina A, on behalf of the Japanese Circulation Society and the Japanese Heart Failure Society Joint Working Group . JCS 2017/JHFS 2017 guideline on diagnosis and treatment of acute and chronic heart failure― digest version ―. Circ J 2019;83:2084–2184. [DOI] [PubMed] [Google Scholar]
- 91. Kitakaze M, Asakura M, Kim J, Shintani Y, Asanuma H, Hamasaki T, Seguchi O, Myoishi M, Minamino T, Ohara T, Nagai Y, Nanto S, Watanabe K, Fukuzawa S, Hirayama A, Nakamura N, Kimura K, Fujii K, Ishihara M, Saito Y, Tomoike H, Kitamura S, J-WIND investigators . Human atrial natriuretic peptide and nicorandil as adjuncts to reperfusion treatment for acute myocardial infarction (J-WIND): two randomised trials. Lancet 2007;370:1483–1493. [DOI] [PubMed] [Google Scholar]
- 92. Isogai T, Matsui H, Tanaka H, Fushimi K, Yasunaga H.. Atrial natriuretic peptide therapy and in-hospital mortality in acute myocardial infarction patients undergoing percutaneous coronary intervention. Int J Cardiol 2016;222:163–170. [DOI] [PubMed] [Google Scholar]
- 93. Packer M, O'Connor C, McMurray JJV, Wittes J, Abraham WT, Anker SD, Dickstein K, Filippatos G, Holcomb R, Krum H, Maggioni AP, Mebazaa A, Peacock WF, Petrie MC, Ponikowski P, Ruschitzka F, van Veldhuisen DJ, Kowarski LS, Schactman M, Holzmeister J, TRUE-AHF Investigators . Effect of ularitide on cardiovascular mortality in acute heart failure. N Engl J Med 2017;376:1956–1964. [DOI] [PubMed] [Google Scholar]
- 94. O'Connor CM, Starling RC, Hernandez AF, Armstrong PW, Dickstein K, Hasselblad V, Heizer GM, Komajda M, Massie BM, McMurray JJV, Nieminen MS, Reist CJ, Rouleau JL, Swedberg K, Adams KF, Anker SD, Atar D, Battler A, Botero R, Bohidar NR, Butler J, Clausell N, Corbalán R, Costanzo MR, Dahlstrom U, Deckelbaum LI, Diaz R, Dunlap ME, Ezekowitz JA, Feldman D, Felker GM, Fonarow GC, Gennevois D, Gottlieb SS, Hill JA, Hollander JE, Howlett JG, Hudson MP, Kociol RD, Krum H, Laucevicius A, Levy WC, Méndez GF, Metra M, Mittal S, Oh B-H, Pereira NL, Ponikowski P, Tang WHW, Wilson WH, Tanomsup S, Teerlink JR, Triposkiadis F, Troughton RW, Voors AA, Whellan DJ, Zannad F, Califf RM.. Effect of nesiritide in patients with acute decompensated heart failure. N Engl J Med 2011;365:32–43. [DOI] [PubMed] [Google Scholar]
- 95. Sackner-Bernstein JD, Kowalski M, Fox M, Aaronson K.. Short-term risk of death after treatment with nesiritide for decompensated heart failure: a pooled analysis of randomized controlled trials. JAMA 2005;293:1900–1905. [DOI] [PubMed] [Google Scholar]
- 96. Dickey DM, Burnett JC Jr, Potter LR.. Novel bifunctional natriuretic peptides as potential therapeutics. J Biol Chem 2008;283:35003–35009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Kawakami R, Lee CYW, Scott C, Bailey KR, Schirger JA, Chen HH, Benike SL, Cannone V, Martin FL, Sangaralingham SJ, Ichiki T, Burnett JC.. A human study to evaluate safety, tolerability, and cyclic GMP activating properties of cenderitide in subjects with stable chronic heart failure. Clin Pharmacol Ther 2018;104:546–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Pan S, Chen HH, Dickey DM, Boerrigter G, Lee C, Kleppe LS, Hall JL, Lerman A, Redfield MM, Potter LR, Burnett JC, Simari RD.. Biodesign of a renal-protective peptide based on alternative splicing of B-type natriuretic peptide. Proc Natl Acad Sci USA 2009;106:11282–11287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. McKie PM, Cataliotti A, Huntley BK, Martin FL, Olson TM, Burnett JC.. A human atrial natriuretic peptide gene mutation reveals a novel peptide with enhanced blood pressure-lowering, renal-enhancing, and aldosterone-suppressing actions. J Am Coll Cardiol 2009;54:1024–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. McKie PM, Iyer SR, Scott C, Bailey K, Johnson BK, Benike SL, Chen H, Miller WL, Volpi R, Cabassi A, Burnett JC, Cannone V.. Abstract 15205: MANP: a novel ANP analog for hypertension associated with obesity and metabolic syndrome. Circulation 2020;142:A15205–A15205. [Google Scholar]
- 101. Bayes-Genis A, Barallat J, Richards AM.. A test in context: neprilysin: function, inhibition, and biomarker. J Am Coll Cardiol 2016;68:639–653. [DOI] [PubMed] [Google Scholar]
- 102. Ferro CJ, Spratt JC, Haynes WG, Webb DJ.. Inhibition of neutral endopeptidase causes vasoconstriction of human resistance vessels in vivo. Circulation 1998;97:2323–2330. [DOI] [PubMed] [Google Scholar]
- 103. Corti R, Burnett JC, Rouleau JL, Ruschitzka F, Lüscher TF.. Vasopeptidase inhibitors: a new therapeutic concept in cardiovascular disease? Circulation 2001;104:1856–1862. [DOI] [PubMed] [Google Scholar]
- 104. Packer M, Califf RM, Konstam MA, Krum H, McMurray JJ, Rouleau J-L, Swedberg K, for the OVERTURE Study Group . Comparison of omapatrilat and enalapril in patients with chronic heart failure. Circulation 2002;106:920–926. [DOI] [PubMed] [Google Scholar]
- 105. Kostis JB, Packer M, Black HR, Schmieder R, Henry D, Levy E.. Omapatrilat and enalapril in patients with hypertension: the Omapatrilat Cardiovascular Treatment vs. Enalapril (OCTAVE) trial. Am J Hypertens 2004;17:103–111. [DOI] [PubMed] [Google Scholar]
- 106. Ruilope LM, Dukat A, Böhm M, Lacourcière Y, Gong J, Lefkowitz MP.. Blood-pressure reduction with LCZ696, a novel dual-acting inhibitor of the angiotensin II receptor and neprilysin: a randomised, double-blind, placebo-controlled, active comparator study. Lancet 2010;375:1255–1266. [DOI] [PubMed] [Google Scholar]
- 107. McMurray JJV, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR, for the PARADIGM-HF Investigators and Committees . Angiotensin–neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014;371:993–1004. [DOI] [PubMed] [Google Scholar]
- 108. Solomon SD, McMurray JJV, Anand IS, Ge J, Lam CSP, Maggioni AP, Martinez F, Packer M, Pfeffer MA, Pieske B, Redfield MM, Rouleau JL, van Veldhuisen DJ, Zannad F, Zile MR, Desai AS, Claggett B, Jhund PS, Boytsov SA, Comin-Colet J, Cleland J, Düngen H-D, Goncalvesova E, Katova T, Kerr Saraiva JF, Lelonek M, Merkely B, Senni M, Shah SJ, Zhou J, Rizkala AR, Gong J, Shi VC, Lefkowitz MP, for the PARAGON-HF Investigators and Committees . Angiotensin–neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med 2019;381:1609–1620. [DOI] [PubMed] [Google Scholar]
- 109. Sanders-van Wijk S, Barandiarán Aizpurua A, Brunner-La Rocca H-P, Henkens MTHM, Weerts J, Knackstedt C, Uszko-Lencer N, Heymans S, van Empel V.. The HFA-PEFF and H2FPEF scores largely disagree in classifying patients with suspected heart failure with preserved ejection fraction. Eur J Heart Fail 2021;23:838–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Sauer AJ, Cole R, Jensen BC, Pal J, Sharma N, Yehya A, Vader J.. Practical guidance on the use of sacubitril/valsartan for heart failure. Heart Fail Rev 2019;24:167–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. ENTRESTO [prescribing information] . East Hanover, NJ: Novartis Pharmaceuticals Corp; 2021.
- 112. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBride PE, Peterson PN, Stevenson LW, Westlake C.. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2017;136:e137–e161. [DOI] [PubMed] [Google Scholar]
- 113. De Vecchis R, Ariano C, Soreca S.. Antihypertensive effect of sacubitril/valsartan: a meta-analysis. Minerva Cardioangiol 2019;67:214–222. [DOI] [PubMed] [Google Scholar]
- 114. Bobin P, Belacel-Ouari M, Bedioune I, Zhang L, Leroy J, Leblais V, Fischmeister R, Vandecasteele G.. Cyclic nucleotide phosphodiesterases in heart and vessels: a therapeutic perspective. Arch Cardiovasc Dis 2016;109:431–443. [DOI] [PubMed] [Google Scholar]
- 115. Burnett AL, Nehra A, Breau RH, Culkin DJ, Faraday MM, Hakim LS, Heidelbaugh J, Khera M, McVary KT, Miner MM, Nelson CJ, Sadeghi-Nejad H, Seftel AD, Shindel AW.. Erectile dysfunction: AUA guideline. J Urol 2018;200:633–641. [DOI] [PubMed] [Google Scholar]
- 116. Lewis GD, Shah R, Shahzad K, Camuso JM, Pappagianopoulos PP, Hung J, Tawakol A, Gerszten RE, Systrom DM, Bloch KD, Semigran MJ.. Sildenafil improves exercise capacity and quality of life in patients with systolic heart failure and secondary pulmonary hypertension. Circulation 2007;116:1555–1562. [DOI] [PubMed] [Google Scholar]
- 117. Guazzi M, Vicenzi M, Arena R, Guazzi MD.. Pulmonary hypertension in heart failure with preserved ejection fraction. Circulation 2011;124:164–174. [DOI] [PubMed] [Google Scholar]
- 118. Mandosi E, Giannetta E, Filardi T, Lococo M, Bertolini C, Fallarino M, Gianfrilli D, Venneri MA, Lenti L, Lenzi A, Morano S.. Endothelial dysfunction markers as a therapeutic target for Sildenafil treatment and effects on metabolic control in type 2 diabetes. Expert Opin Ther Targets 2015;19:1617–1622. [DOI] [PubMed] [Google Scholar]
- 119. Guazzi M, Vicenzi M, Arena R, Guazzi MD.. PDE5 inhibition with sildenafil improves left ventricular diastolic function, cardiac geometry, and clinical status in patients with stable systolic heart failure. Circ Heart Fail 2011;4:8–17. [DOI] [PubMed] [Google Scholar]
- 120. Giannetta E, Isidori AM, Galea N, Carbone I, Mandosi E, Vizza CD, Naro F, Morano S, Fedele F, Lenzi A.. Chronic inhibition of cGMP phosphodiesterase 5A improves diabetic cardiomyopathy. Circulation 2012;125:2323–2333. [DOI] [PubMed] [Google Scholar]
- 121. Redfield MM, Chen HH, Borlaug BA, Semigran MJ, Lee KL, Lewis G, LeWinter MM, Rouleau JL, Bull DA, Mann DL, Deswal A, Stevenson LW, Givertz MM, Ofili EO, O'Connor CM, Felker GM, Goldsmith SR, Bart BA, McNulty SE, Ibarra JC, Lin G, Oh JK, Patel MR, Kim RJ, Tracy RP, Velazquez EJ, Anstrom KJ, Hernandez AF, Mascette AM, Braunwald E, for the RELAX Trial . Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA 2013;309:1268–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Packer M, Carver JR, Rodeheffer RJ, Ivanhoe RJ, DiBianco R, Zeldis SM, Hendrix GH, Bommer WJ, Elkayam U, Kukin ML, Mallis GI, Sollano JA, Shannon J, Tandon PK, DeMets DL.. Effect of oral milrinone on mortality in severe chronic heart failure. N Engl J Med 1991;325:1468–1475. [DOI] [PubMed] [Google Scholar]
- 123. Kalantzi K, Tentolouris N, Melidonis AJ, Papadaki S, Peroulis M, Amantos KA, Andreopoulos G, Bellos GI, Boutel D, Bristianou M, Chrisis D, Dimitsikoglou NA, Doupis J, Georgopoulou C, Gkintikas SA, Iraklianou S, Kanellas Κ, Kotsa K, Koufakis T, Kouroglou M, Koutsovasilis AG, Lanaras L, Liouri E, Lixouriotis C, Lykoudi A, Mandalaki E, Papageorgiou E, Papanas N, Rigas S, Stamatelatou MI, Triantafyllidis I, Trikkalinou A, Tsouka AN, Zacharopoulou O, Zoupas C, Tsolakis I, Tselepis AD.. Efficacy and safety of adjunctive cilostazol to clopidogrel-treated diabetic patients with symptomatic lower extremity artery disease in the prevention of ischemic vascular events. J Am Heart Assoc 2021;10:e018184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Lee D-H, Chun EJ, Moon JH, Yun HM, Lim S.. Effect of cilostazol on carotid plaque volume measured by three-dimensional ultrasonography in patients with type 2 diabetes: the FANCY study. Diabetes Obes Metab 2020;22:2257–2266. [DOI] [PubMed] [Google Scholar]
- 125. Maurice DH, Haslam RJ.. Molecular basis of the synergistic inhibition of platelet function by nitrovasodilators and activators of adenylate cyclase: inhibition of cyclic AMP breakdown by cyclic GMP. Mol Pharmacol 1990;37:671–681. [PubMed] [Google Scholar]
- 126. Nanayakkara S, Byrne M, Mak V, Carter K, Dean E, Kaye DM.. Extended-release oral milrinone for the treatment of heart failure with preserved ejection fraction. J Am Heart Assoc 2020;9:e015026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Papp Z, Agostoni P, Alvarez J, Bettex D, Bouchez S, Brito D, Černý V, Comin-Colet J, Crespo-Leiro MG, Delgado JF, Édes I, Eremenko AA, Farmakis D, Fedele F, Fonseca C, Fruhwald S, Girardis M, Guarracino F, Harjola V-P, Heringlake M, Herpain A, Heunks LM, Husebye T, Ivancan V, Karason K, Kaul S, Kivikko M, Kubica J, Masip J, Matskeplishvili S, Mebazaa A, Nieminen MS, Oliva F, Papp J-G, Parissis J, Parkhomenko A, Põder P, Pölzl G, Reinecke A, Ricksten S-E, Riha H, Rudiger A, Sarapohja T, Schwinger RH, Toller W, Tritapepe L, Tschöpe C, Wikström G, von Lewinski D, Vrtovec B, Pollesello P.. Levosimendan efficacy and safety: 20 years of SIMDAX in clinical use. Card Fail Rev 2020;6:e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Ørstavik Ø, Ata SH, Riise J, Dahl CP, Andersen GØ, Levy FO, Skomedal T, Osnes J-B, Qvigstad E.. Inhibition of phosphodiesterase-3 by levosimendan is sufficient to account for its inotropic effect in failing human heart. Br J Pharmacol 2014;171:5169–5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Burkhoff D, Borlaug BA, Shah SJ, Zolty R, Tedford RJ, Thenappan T, Zamanian RT, Mazurek JA, Rich JD, Simon MA, Chung ES, Raza F, Majure DT, Lewis GD, Preston IR, Rich S.. Levosimendan improves hemodynamics and exercise tolerance in PH-HFpEF: results of the randomized placebo-controlled HELP trial. JACC Heart Fail 2021;9:360–370. [DOI] [PubMed] [Google Scholar]
- 130. Zhang F, Yan C, Wei C, Yao Y, Ma X, Gong Z, Liu S, Zang D, Chen J, Shi F-D, Hao J.. Vinpocetine inhibits NF-κB-dependent inflammation in acute ischemic stroke patients. Transl Stroke Res 2018;9:174–184. [DOI] [PubMed] [Google Scholar]
- 131. Wennogle LP, Hoxie H, Peng Y, Hendrick JP, Phosphodiesterase 1: a unique drug target for degenerative diseases and cognitive dysfunction. In Zhang H-T, Xu Y, O'Donnell JM (eds). Phosphodiesterases: CNS Functions and Diseases. Advances in Neurobiology Springer, Cham, 2017. p349–384. [DOI] [PubMed] [Google Scholar]
- 132. Gilotra NA, Devore A, Hays A, Hahn V, Agunbiade TA, Chen R, Davis R, Satlin A, Povsic T, Kass D.. Cardiac and hemodynamic effects of acute phosphodiesterase-1 inhibition in human heart failure. J Card Fail 2020;26:S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Richards DA, Aronovitz MJ, Liu P, Martin GL, Tam K, Pande S, Karas RH, Bloomfield DM, Mendelsohn ME, Blanton RM.. CRD-733, a novel PDE9 (phosphodiesterase 9) inhibitor, reverses pressure overload-induced heart failure. Circ Heart Fail 2021;14:e007300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Chen S, Zhang Y, Lighthouse JK, Mickelsen DM, Wu J, Yao P, Small EM, Yan C.. A novel role of cyclic nucleotide phosphodiesterase 10A in pathological cardiac remodeling and dysfunction. Circulation 2020;141:217–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. McKie PM, Cataliotti A, Ichiki T, Sangaralingham SJ, Chen HH, Burnett JC.. M-atrial natriuretic peptide and nitroglycerin in a canine model of experimental acute hypertensive heart failure: differential actions of 2 cGMP activating therapeutics. JAHA 2014;3:e000206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Casas AI, Hassan AA, Larsen SJ, Gomez-Rangel V, Elbatreek M, Kleikers PWM, Guney E, Egea J, López MG, Baumbach J, Schmidt HHHW.. From single drug targets to synergistic network pharmacology in ischemic stroke. Proc Natl Acad Sci USA 2019;116:7129–7136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Ridley K, Condren M.. Elexacaftor-tezacaftor-ivacaftor: the first triple-combination cystic fibrosis transmembrane conductance regulator modulating therapy. J Pediatr Pharmacol Ther 2020;25:192–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Labombarda F, Saloux E, Brouard J, Bergot E, Milliez P.. Heart involvement in cystic fibrosis: a specific cystic fibrosis-related myocardial changes? Respir Med 2016;118:31–38. [DOI] [PubMed] [Google Scholar]
- 139. Lukowski R, Krieg T, Rybalkin SD, Beavo J, Hofmann F.. Turning on cGMP-dependent pathways to treat cardiac dysfunctions: boom, bust, and beyond. Trends Pharmacol Sci 2014;35:404–413. [DOI] [PubMed] [Google Scholar]
- 140. Castro LRV, Verde I, Cooper DMF, Fischmeister R.. Cyclic guanosine monophosphate compartmentation in rat cardiac myocytes. Circulation 2006;113:2221–2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Webb DJ, Muirhead GJ, Wulff M, Sutton JA, Levi R, Dinsmore WW.. Sildenafil citrate potentiates the hypotensive effects of nitric oxide donor drugs in male patients with stable angina. J Am Coll Cardiol 2000;36:25–31. [DOI] [PubMed] [Google Scholar]
- 142. Galiè N, Müller K, Scalise A-V, Grünig E.. PATENT PLUS: a blinded, randomised and extension study of riociguat plus sildenafil in pulmonary arterial hypertension. Eur Respir J 2015;45:1314–1322. [DOI] [PubMed] [Google Scholar]
- 143. Oliver JJ, Hughes VEC, Dear JW, Webb DJ.. Clinical potential of combined organic nitrate and phosphodiesterase type 5 inhibitor in treatment-resistant hypertension. Hypertension 2010;56:62–67. [DOI] [PubMed] [Google Scholar]
- 144. Hobbs AJ, Moyes AJ, Baliga RS, Ghedia D, Ochiel R, Sylvestre Y, Doré CJ, Chowdhury K, Maclagan K, Quartly HL, Sofat R, Smit A, Schreiber BE, Coghlan GJ, MacAllister RJ.. Neprilysin inhibition for pulmonary arterial hypertension: a randomized, double-blind, placebo-controlled, proof-of-concept trial. Br J Pharmacol 2019;176:1251–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Morrow DA, Velazquez EJ, DeVore AD, Prescott MF, Duffy CI, Gurmu Y, McCague K, Rocha R, Braunwald E.. Cardiovascular biomarkers in patients with acute decompensated heart failure randomized to sacubitril-valsartan or enalapril in the PIONEER-HF trial. Eur Heart J 2019;40:3345–3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Lee CYW, Chen HH, Lisy O, Swan S, Cannon C, Lieu HD, Burnett JC.. Pharmacodynamics of a novel designer natriuretic peptide, CD-NP, in a first-in-human clinical trial in healthy subjects. J Clin Pharmacol 2009;49:668–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Mirone V, d'Emmanuele di Villa Bianca R, Mitidieri E, Imbimbo C, Fusco F, Verze P, Vitale DF, Sorrentino R, Cirino G.. Platelet cyclic guanosine monophosphate as a biomarker of phosphodiesterase type 5 inhibitor efficacy in the treatment of erectile dysfunction: a randomized placebo-controlled study. Eur Urol 2009;56:1067–1073. [DOI] [PubMed] [Google Scholar]
- 148. Ulvestad L, Sager G.. Cyclic GMP as a biomarker for cardiovascular disease and cancer. Tidsskr nor Laegeforen 2005;125:27–28. [PubMed] [Google Scholar]
- 149. Oelze M, Mollnau H, Hoffmann N, Warnholtz A, Bodenschatz M, Smolenski A, Walter U, Skatchkov M, Meinertz T, Münzel T.. Vasodilator-stimulated phosphoprotein serine 239 phosphorylation as a sensitive monitor of defective nitric oxide/cGMP signaling and endothelial dysfunction. Circ Res 2000;87:999–1005. [DOI] [PubMed] [Google Scholar]
- 150. Geiger J, Brandmann T, Hubertus K, Tjahjadi B, Schinzel R, Walter U.. A protein phosphorylation-based assay for screening and monitoring of drugs modulating cyclic nucleotide pathways. Anal Biochem 2010;407:261–269. [DOI] [PubMed] [Google Scholar]
- 151. Hu C, Zhang X, Liu Y, Gao Y, Zhao X, Zhou H, Luo Y, Liu Y, Wang X.. Vasodilator-stimulated phosphoprotein-guided Clopidogrel maintenance therapy reduces cardiovascular events in atrial fibrillation patients requiring anticoagulation therapy and scheduled for percutaneous coronary intervention: a prospective cohort study. BMC Cardiovasc Disord 2018;18:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Freynhofer MK, Brozovic I, Bruno V, Farhan S, Vogel B, Jakl G, Willheim M, Hübl W, Wojta J, Huber K.. Multiple electrode aggregometry and vasodilator stimulated phosphoprotein-phosphorylation assay in clinical routine for prediction of postprocedural major adverse cardiovascular events. Thromb Haemost 2011;106:230–239. [DOI] [PubMed] [Google Scholar]
- 153. Rost NS, Biffi A, Cloonan L, Chorba J, Kelly P, Greer D, Ellinor P, Furie KL.. Brain natriuretic peptide predicts functional outcome in ischemic stroke. Stroke 2012;43:441–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Coats CJ, Gallagher MJ, Foley M, O'Mahony C, Critoph C, Gimeno J, Dawnay A, McKenna WJ, Elliott PM.. Relation between serum N-terminal pro-brain natriuretic peptide and prognosis in patients with hypertrophic cardiomyopathy. Eur Heart J 2013;34:2529–2537. [DOI] [PubMed] [Google Scholar]
- 155. Kara K, Lehmann N, Neumann T, Kälsch H, Möhlenkamp S, Dykun I, Broecker-Preuss M, Pundt N, Moebus S, Jöckel K-H, Erbel R, Mahabadi AA.. NT-proBNP is superior to BNP for predicting first cardiovascular events in the general population: the Heinz Nixdorf Recall Study. Int J Cardiol 2015;183:155–161. [DOI] [PubMed] [Google Scholar]
- 156. Veldhuisen van DJ, Linssen GCM, Jaarsma T, van Gilst WH, Hoes AW, Tijssen JGP, Paulus WJ, Voors AA, Hillege HL.. B-type natriuretic peptide and prognosis in heart failure patients with preserved and reduced ejection fraction. J Am Coll Cardiol 2013;61:1498–1506. [DOI] [PubMed] [Google Scholar]
- 157. Salah K, Stienen S, Pinto YM, Eurlings LW, Metra M, Bayes-Genis A, Verdiani V, Tijssen JGP, Kok WE.. Prognosis and NT-proBNP in heart failure patients with preserved versus reduced ejection fraction. Heart 2019;105:1182–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Iwanaga Y, Nishi I, Furuichi S, Noguchi T, Sase K, Kihara Y, Goto Y, Nonogi H.. B-type natriuretic peptide strongly reflects diastolic wall stress in patients with chronic heart failure: comparison between systolic and diastolic heart failure. J Am Coll Cardiol 2006;47:742–748. [DOI] [PubMed] [Google Scholar]
- 159. Lok DJ, Klip IT, Voors AA, Lok SI, Bruggink-André de la Porte PW, Hillege HL, Jaarsma T, van Veldhuisen DJ, van der Meer P.. Prognostic value of N-terminal pro C-type natriuretic peptide in heart failure patients with preserved and reduced ejection fraction. Eur J Heart Fail 2014;16:958–966. [DOI] [PubMed] [Google Scholar]
- 160. Keng BMH, Gao F, Tan RS, Ewe SH, Teo LLY, Xie BQ, Goh GBB, Koh W-P, Koh AS.. N-Terminal pro C-Type Natriuretic Peptide (NTproCNP) and myocardial function in ageing. PLoS One 2018;13:e0209517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Barbato E, Bartunek J, Marchitti S, Mangiacapra F, Stanzione R, Delrue L, Cotugno M, Di Castro S, De Bruyne B, Wijns W, Volpe M, Rubattu S.. NT-proANP circulating level is a prognostic marker in stable ischemic heart disease. Int J Cardiol 2012;155:311–312. [DOI] [PubMed] [Google Scholar]
- 162. Arrigo M, Truong QA, Szymonifka J, Rivas-Lasarte M, Tolppanen H, Sadoune M, Gayat E, Cohen-Solal A, Ruschitzka F, Januzzi JL Jr, Singh JP, Mebazaa A.. Mid-regional pro-atrial natriuretic peptide to predict clinical course in heart failure patients undergoing cardiac resynchronization therapy. EP Europace 2017;19:1848–1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Bayés-Genís A, Barallat J, Galán A, de Antonio M, Domingo M, Zamora E, Urrutia A, Lupón J.. Soluble neprilysin is predictive of cardiovascular death and heart failure hospitalization in heart failure patients. J Am Coll Cardiol 2015;65:657–665. [DOI] [PubMed] [Google Scholar]
- 164. Lyle MA, Iyer SR, Redfield MM, Reddy YNV, Felker GM, Cappola TP, Hernandez AF, Scott CG, Burnett JC, Pereira NL.. Circulating neprilysin in patients with heart failure and preserved ejection fraction. JACC Heart Fail 2020;8:70–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Ramanathan K, Padmanabhan G.. Soluble neprilysin: a versatile biomarker for heart failure, cardiovascular diseases and diabetic complications-a systematic review. Indian Heart J 2020;72:14–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Banerjee S, McCormack S.. Natriuretic Peptide Testing for Monitoring of Heart Failure Therapy: A Review of Clinical Effectiveness, Clinical Utility, Cost-Effectiveness, and Guidelines. Canadian Agency for Drugs and Technologies in Health, Ottawa, 2019. [PubMed] [Google Scholar]
- 167. Sweeney C, Ryan F, Ledwidge M, Ryan C, McDonald K, Watson C, Pharithi RB, Gallagher J.. Natriuretic peptide‐guided treatment for the prevention of cardiovascular events in patients without heart failure. Cochrane Database Syst Rev 2019;10:CD013015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Lauer T, Preik M, Rassaf T, Strauer BE, Deussen A, Feelisch M, Kelm M.. Plasma nitrite rather than nitrate reflects regional endothelial nitric oxide synthase activity but lacks intrinsic vasodilator action. PNAS 2001;98:12814–12819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Eleuteri E, Di Stefano A, Ricciardolo FLM, Magno F, Gnemmi I, Colombo M, Anzalone R, Cappello F, La Rocca G, Genta FT, Zummo G, Giannuzzi P.. Increased nitrotyrosine plasma levels in relation to systemic markers of inflammation and myeloperoxidase in chronic heart failure. Int J Cardiol 2009;135:386–390. [DOI] [PubMed] [Google Scholar]
- 170. Rashid PA, Whitehurst A, Lawson N, Bath PMW.. Plasma nitric oxide (nitrate/nitrite) levels in acute stroke and their relationship with severity and outcome. J Stroke Cerebrovasc Dis 2003;12:82–87. [DOI] [PubMed] [Google Scholar]
- 171. Zhang R, Wang X-J, Zhang H-D, Sun X-Q, Zhao Q-H, Wang L, He J, Jiang X, Liu J-M, Jing Z-C.. Profiling nitric oxide metabolites in patients with idiopathic pulmonary arterial hypertension. Eur Respir J 2016;48:1386–1395. [DOI] [PubMed] [Google Scholar]
- 172. Gumanova NG, Deev AD, Zhang W, Kots AY, Shalnova SA.. Serum nitrite and nitrate levels, NOx, can predict cardiovascular mortality in the elderly in a 3-year follow-up study. Biofactors 2017;43:82–89. [DOI] [PubMed] [Google Scholar]
- 173. Liu X, Xu X, Shang R, Chen Y.. Asymmetric dimethylarginine (ADMA) as an important risk factor for the increased cardiovascular diseases and heart failure in chronic kidney disease. Nitric Oxide 2018;78:113–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Sibal L, Agarwal SC, Home PD, Boger RH.. The role of asymmetric dimethylarginine (ADMA) in endothelial dysfunction and cardiovascular disease. Curr Cardiol Rev 2010;6:82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Gać P, Poręba M, Jurdziak M, Trzmielewska E, Gocławska K, Derkacz A, Mazur G, Szuba A, Poręba R.. Cardiovascular risk factors and the concentration of asymmetric dimethylarginine. Adv Clin Exp Med 2020;29:63–70. [DOI] [PubMed] [Google Scholar]
- 176. Parini P, Altucci L, Balligand J-L, Baumbach J, Ferdinandy P, Filetti S, Maron BA, Petrillo E, Silverman EK, Barabasi A-L, Loscalzo J, International Network Medicine Consortium . The network medicine imperative and the need for an international network medicine consortium. Am J Med 2020;133:e451–e454. [DOI] [PubMed] [Google Scholar]
- 177. Ferdinandy P, Hausenloy DJ, Heusch G, Baxter GF, Schulz R.. Interaction of risk factors, comorbidities, and comedications with ischemia/reperfusion injury and cardioprotection by preconditioning, postconditioning, and remote conditioning. Pharmacol Rev 2014;66:1142–1174. [DOI] [PubMed] [Google Scholar]
- 178. Rubattu S, Bigatti G, Evangelista A, Lanzani C, Stanzione R, Zagato L, Manunta P, Marchitti S, Venturelli V, Bianchi G, Volpe M, Stella P.. Association of atrial natriuretic peptide and type A natriuretic peptide receptor gene polymorphisms with left ventricular mass in human essential hypertension. J Am Coll Cardiol 2006;48:499–505. [DOI] [PubMed] [Google Scholar]
- 179. Yang X, Yang W, McVey DG, Zhao G, Hu J, Poston RN, Ren M, Willeit K, Coassin S, Willeit J, Webb TR, Samani NJ, Mayr M, Kiechl S, Ye S.. FURIN expression in vascular endothelial cells is modulated by a coronary artery disease-associated genetic variant and influences monocyte transendothelial migration. J Am Heart Assoc 2020;9:e014333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Salvi E, Kutalik Z, Glorioso N, Benaglio P, Frau F, Kuznetsova T, Arima H, Hoggart C, Tichet J, Nikitin YP, Conti C, Seidlerova J, Tikhonoff V, Stolarz-Skrzypek K, Johnson T, Devos N, Zagato L, Guarrera S, Zaninello R, Calabria A, Stancanelli B, Troffa C, Thijs L, Rizzi F, Simonova G, Lupoli S, Argiolas G, Braga D, D'Alessio MC, Ortu MF, Ricceri F, Mercurio M, Descombes P, Marconi M, Chalmers J, Harrap S, Filipovsky J, Bochud M, Iacoviello L, Ellis J, Stanton AV, Laan M, Padmanabhan S, Dominiczak AF, Samani NJ, Melander O, Jeunemaitre X, Manunta P, Shabo A, Vineis P, Cappuccio FP, Caulfield MJ, Matullo G, Rivolta C, Munroe PB, Barlassina C, Staessen JA, Beckmann JS, Cusi D.. Genomewide association study using a high-density single nucleotide polymorphism array and case-control design identifies a novel essential hypertension susceptibility locus in the promoter region of endothelial NO synthase. Hypertension 2012;59:248–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Nikpay M, Goel A, Won H-H, Hall LM, Willenborg C, Kanoni S, Saleheen D, Kyriakou T, Nelson CP, Hopewell JC, Webb TR, Zeng L, Dehghan A, Alver M, Armasu SM, Auro K, Bjonnes A, Chasman DI, Chen S, Ford I, Franceschini N, Gieger C, Grace C, Gustafsson S, Huang J, Hwang S-J, Kim YK, Kleber ME, Lau KW, Lu X, Lu Y, Lyytikäinen L-P, Mihailov E, Morrison AC, Pervjakova N, Qu L, Rose LM, Salfati E, Saxena R, Scholz M, Smith AV, Tikkanen E, Uitterlinden A, Yang X, Zhang W, Zhao W, de Andrade M, de Vries PS, van Zuydam NR, Anand SS, Bertram L, Beutner F, Dedoussis G, Frossard P, Gauguier D, Goodall AH, Gottesman O, Haber M, Han B-G, Huang J, Jalilzadeh S, Kessler T, König IR, Lannfelt L, Lieb W, Lind L, Lindgren CM, Lokki M-L, Magnusson PK, Mallick NH, Mehra N, Meitinger T, Memon F-U-R, Morris AP, Nieminen MS, Pedersen NL, Peters A, Rallidis LS, Rasheed A, Samuel M, Shah SH, Sinisalo J, Stirrups KE, Trompet S, Wang L, Zaman KS, Ardissino D, Boerwinkle E, Borecki IB, Bottinger EP, Buring JE, Chambers JC, Collins R, Cupples LA, Danesh J, Demuth I, Elosua R, Epstein SE, Esko T, Feitosa MF, Franco OH, Franzosi MG, Granger CB, Gu D, Gudnason V, Hall AS, Hamsten A, Harris TB, Hazen SL, Hengstenberg C, Hofman A, Ingelsson E, Iribarren C, Jukema JW, Karhunen PJ, Kim B-J, Kooner JS, Kullo IJ, Lehtimäki T, Loos RJF, Melander O, Metspalu A, März W, Palmer CN, Perola M, Quertermous T, Rader DJ, Ridker PM, Ripatti S, Roberts R, Salomaa V, Sanghera DK, Schwartz SM, Seedorf U, Stewart AF, Stott DJ, Thiery J, Zalloua PA, O'Donnell CJ, Reilly MP, Assimes TL, Thompson JR, Erdmann J, Clarke R, Watkins H, Kathiresan S, McPherson R, Deloukas P, Schunkert H, Samani NJ, Farrall M, the CARDIoGRAMplusC4D Consortium . A comprehensive 1000 Genomes–based genome-wide association meta-analysis of coronary artery disease. Nat Genet 2015;47:1121–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Chen Y, Zhu L, Fang Z, Jin Y, Shen C, Yao Y, Zhou C.. Soluble guanylate cyclase contribute genetic susceptibility to essential hypertension in the Han Chinese population. Ann Transl Med 2019;7:620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Wilkins MR, Aldashev AA, Wharton J, Rhodes CJ, Vandrovcova J, Kasperaviciute D, Bhosle SG, Mueller M, Geschka S, Rison S, Kojonazarov B, Morrell NW, Neidhardt I, Surmeli NB, Surmeli NB, Aitman TJ, Stasch J-P, Behrends S, Marletta MA.. α1-A680T variant in GUCY1A3 as a candidate conferring protection from pulmonary hypertension among Kyrgyz highlanders. Circ Cardiovasc Genet 2014;7:920–929. [DOI] [PubMed] [Google Scholar]