Abstract
Growth hormone (GH) acts in several hypothalamic neuronal populations to modulate metabolism and the autoregulation of GH secretion via negative-feedback loops. However, few studies have investigated whether GH receptor (GHR) expression in specific neuronal populations is required for the homeostatic control of GH secretion and energy homeostasis. In the present study, we investigated the consequences of the specific GHR ablation in GABAergic (VGAT-expressing) or glutamatergic (VGLUT2-expressing) cells. GHR ablation in GABAergic neurons led to increased GH secretion, lean mass, and body growth in male and female mice. VGAT-specific GHR knockout (KO) male mice also showed increased serum insulin-like growth factor-1, hypothalamic Ghrh, and hepatic Igf1 messenger RNA levels. In contrast, normal GH secretion, but reduced lean body mass, was observed in mice carrying GHR ablation in glutamatergic neurons. GHR ablation in GABAergic cells increased weight loss and led to decreased blood glucose levels during food restriction, whereas VGLUT2-specific GHR KO mice showed blunted feeding response to 2-deoxy-D-glucose both in males and females, and increased relative food intake, oxygen consumption, and serum leptin levels in male mice. Of note, VGLUT2-cre female mice, independently of GHR ablation, exhibited a previously unreported phenotype of mild reduction in body weight without further metabolic alterations. The autoregulation of GH secretion via negative-feedback loops requires GHR expression in GABAergic cells. Furthermore, GHR ablation in GABAergic and glutamatergic neuronal populations leads to distinct metabolic alterations. These findings contribute to the understanding of the neuronal populations responsible for mediating the neuroendocrine and metabolic effects of GH.
Keywords: growth hormone, hypothalamus, metabolism, neuroendocrinology, VGAT, VGLUT2
Growth hormone (GH) is the dominant endocrine factor that controls growth velocity and height in children and adolescents (1, 2). GH also regulates several cellular functions, including protein synthesis, cell proliferation and regeneration, as well as numerous metabolic processes. Thus, given the vital physiological functions regulated by GH, GH secretion is tightly regulated. GH is produced and secreted by somatotropic cells of the anterior pituitary gland. The pulsatile pattern of GH secretion is mainly regulated by hypothalamic neuropeptides released into the hypophyseal portal system (3-7). In this regard, GH-releasing hormone (GHRH) stimulates GH secretion (3, 4). In contrast, somatostatin (SST), secreted by hypophysiotropic neurons in the periventricular (PV) and paraventricular (PVH) nuclei (8, 9), inhibits GH secretion. In addition, neuropeptide Y (NPY) signaling is involved in fasting-induced suppression of GH secretion by regulating hypothalamic Sst messenger RNA (mRNA) expression (10).
The regulation of GH secretion relies on negative-feedback loops that control the activity of hypophysiotropic neurons in the hypothalamus (11-16). Both hypophysiotropic SST and GHRH neurons are sensitive to changes in circulating GH levels, which affect their activity, and Sst and Ghrh mRNA levels (11, 16-19). GH receptor (GHR) is highly expressed in the arcuate nucleus of the hypothalamus (ARH), particularly in neurons that coexpress NPY and agouti-related protein (AgRP) (20, 21). Importantly, GH depolarizes ARHAgRP/NPY neurons (22) and induces c-fos gene expression, a marker of neuronal activation, in these cells (14, 16). A recent study showed that GHR ablation in tyrosine hydroxylase (TH)-expressing cells disturbs the autoregulation of GH secretion, suggesting that TH neurons also control GH secretion via short-loop negative feedback (23). Therefore, several neuronal populations, including GHRH-, SST-, AgRP/NPY-, and TH-expressing cells, are possibly involved in the autoregulation of GH secretion via feedback loops. However, although these neuronal populations are identified by their respective peptidergic/catecholaminergic signature, each of these subpopulations is actually composed of heterogeneous groups of neurons that coexpress different genes and classical neurotransmitters, such as glutamate or γ-aminobutyric acid (GABA) (24-26). For example, only half of ARHTH neurons express the vesicular inhibitory amino acid transporter (VGAT), a marker of GABAergic cells (27). Hypothalamic SST neurons express markers of either GABAergic (28) or glutamatergic cells (29, 30). Considering the important communication between these neuronal populations in the hypothalamus for the control of GH secretion (3, 10, 31) and our incomplete knowledge of the neurochemical identity of the subpopulations necessary for the regulation of GH secretion by negative feedback, this study aimed to investigate the physiological consequences of GHR ablation in cells that express either VGAT or the vesicular glutamate transporter 2 (VGLUT2), as markers of GABAergic and glutamatergic neurons, respectively. The effects on body growth, pulsatile GH secretion, circulating insulin-like growth factor 1 (IGF-1) levels, and several metabolic aspects were determined in VGAT- or VGLUT2-specific GHR knockout (KO) male and female mice. Thus, our findings can narrow down, within the known neuronal populations that control GH secretion, which subgroup of cells is actually required to mediate the short-loop negative feedback that controls the somatotropic axis.
Materials and Methods
Mice
VGAT-cre mice (Jackson Laboratory; RRID: IMSR_JAX:028862) and VGLUT2-cre mice (RRID: IMSR_JAX:028863) were crossed with a Cre-dependent green fluorescent protein (GFP) reporter mouse ((RRID: IMSR_JAX:026175) to allow the visualization of GABAergic and glutamatergic cells, respectively, or with mice carrying loxP-flanked Ghr alleles (32) to induce cell-specific GHR ablation. The control group was composed of Ghrflox/flox littermates that were negative for the Cre transgenes, whereas VGAT GHR KO and VGLUT2 GHR KO mice present ablation of GHR in GABAergic and glutamatergic cells, respectively. A group of wild-type, VGATCre/+ and VGLUT2Cre/+ mice without carrying the loxP-flanked Ghr alleles was produced to investigate the physiological consequences of Cre expression, independently of GHR ablation. Mice were weaned and genotyped within 3 to 4 weeks of life. The mutations were confirmed by polymerase chain reaction (PCR) using the DNA that had been previously extracted from the tail tip (REDExtract-N-Amp Tissue PCR Kit, Sigma-Aldrich). Mice were produced and maintained in standard conditions of light (12-h light/dark cycle; lights on at 8 am) with ad libitum access to regular rodent chow (2.99 kcal/g; 9.4% kcal derived from fat; Nuvilab CR-1, Quimtia) and filtered water. The experimental procedures were approved by the ethics committee on the use of animals of the Institute of Biomedical Sciences at the University of São Paulo, and were performed according to the ethical guidelines adopted by the Brazilian College of Animal Experimentation.
Detection of Growth Hormone Responsive Neurons
The detection of GH responsive neurons was performed as previously described (33, 34). Briefly, adult mice received an intraperitoneal injection of porcine pituitary GH (20 µg/g of body weight, from Dr A. F. Parlow, National Institute of Diabetes and Digestive and Kidney Diseases–National Hormone and Pituitary Program [NIDDK-NHPP]) and were perfused approximately 90 minutes later. Mice were deeply anesthetized with isoflurane and perfused transcardially with saline, followed by a 10% buffered formalin solution. Brains were collected and post-fixed in the same fixative for 45 minutes and cryoprotected overnight at 4 °C in 0.1 M phosphate-buffered saline (PBS) containing 20% sucrose. Brains were cut in 30-µm-thick sections using a freezing microtome. Brain slices were rinsed in 0.02 M potassium PBS, pH 7.4 (KPBS), followed by pretreatment in water solution containing 1% hydrogen peroxide and 1% sodium hydroxide for 20 minutes. After rinsing in KPBS, sections were incubated in 0.3% glycine and 0.03% lauryl sulfate for 10 minutes each. Next, slices were blocked in 3% normal donkey serum for 1 hour, followed by incubation in a primary antibody cocktail containing anti-phosphoTyr694-signal transducer and activator of transcription 5 (pSTAT5; 1:1000; Cell Signaling Technology; catalog No. 9351; RRID: AB_2315225) and anti-GFP (1:5,000; Aves Labs Inc; catalog No. GFP-1020; RRID: AB_10000240) for 40 hours. Subsequently, sections were rinsed in KPBS and incubated for 90 minutes in Alexa Fluor-conjugated secondary antibodies (1:500, Jackson ImmunoResearch Laboratories). After rinses in KPBS, sections were mounted onto gelatin-coated slides and covered with Fluoromount G mounting medium (Electron Microscopic Sciences).
Double Immunofluorescence
Brain sections were rinsed in KPBS, followed by incubation in 3% normal serum for 1 hour. Next, sections were incubated overnight in an antibody cocktail containing anti-GFP antibody (1:5000) with either anti-SST (1:1000; Peninsula Laboratories International; catalog No. T4103; RRID: AB_518614), anti-TH (1:1000; Abcam; catalog No. ab112; RRID: AB_297840), or anti-AgRP (1:4000, Phoenix Pharmaceuticals Inc; catalog No. H-003-53; RRID: AB_2313908) antibodies. Subsequently, sections were incubated for 90 minutes in Alexa Fluor–conjugated secondary antibodies (1:500; Jackson ImmunoResearch Laboratories) and mounted onto gelatin-coated slides.
Image Analysis
A Zeiss Axiocam 512 color camera adapted to an Axioimager A1 microscope (Zeiss) was used to obtain the photomicrographs. The photomicrographs obtained using a 20× objective were analyzed using ImageJ software (http://rsb.info.nih.gov/ij/). Only neurons within the respective subregions displaying a well stained soma in sharp focus were considered for analysis, plotted electronically, and classified as either single- or double-labeled. The colocalizations in each animal were determined in 2 or 3 levels of the PV (bregma between –0.38 and –0.65), PVH (bregma between –0.55 and –0.88), ARH (bregma between –1.25 and –1.55), ventromedial nucleus of the hypothalamus (VMH; bregma between –1.25 and –1.55), dorsomedial nucleus (DMH; bregma between –1.65 and –1.75), posterodorsal medial nucleus of the amygdala (MEApd; bregma between –1.75 and –1.95), and ventral premammillary nucleus (PMv; bregma between –2.15 and –2.25). The Allen Brain Atlas (https://mouse.brain-map.org/static/atlas) was used as an anatomical reference. The values obtained at different rostrocaudal levels were averaged, representing the data for each animal. The colocalizations were determined in 3 to 5 animals.
RNAScope
For the in situ hybridization, we used the RNAscope Multiplex Fluorescent Assay v2 from Advanced Cell Diagnostics (ACD) combined with Tyramide Signal Amplification technology (TSA) and dyes from Akoya Biosciences. Fresh-frozen brains collected from wild-type male and female mice (n = 2 per sex) were sliced on a cryostat at 16 µm and mounted directly onto Superfrost Plus slides (Fisher). The tissue was fixed in 10% neutral buffered formalin for 15 minutes and then dehydrated in ethanol. Endogenous peroxidase was blocked with H2O2 for 10 minutes, washed in DEPC-treated water, and then tissue was gently digested for 30 minutes at room temperature using Protease IV from the ACD kit. The tissue was incubated for 2 hours at 40 °C using probes targeting Mm-Slc32a1 (VGAT), Mm-Slc17a6 (VGLUT2), and Mm-Sst, Mm-Ghrh, and Mm-Slc32a1 or Mm-Ghrh and Mm-Slc17a6, and ACD’s 3-plex RNAscope Positive Control Probes for mouse tissue. After hybridization, probes were labeled with Akoya Opal fluorophore reagents. Sections were counterstained with 4′6-diamidino-2-phenylindole (DAPI) and cover-slipped with ProLong Gold antifade mounting medium.
Evaluation of Body Growth
To analyze somatic growth, the body weight of male and female mice was determined weekly from weaning up to age 5 months. Body fat mass and lean mass were determined every 2 weeks by time-domain nuclear magnetic resonance using the LF50 body composition mice analyzer (Bruker). Eight-week-old mice were anesthetized with isoflurane and the naso-anal length was determined.
Metabolic Measurements
After the longitudinal evaluation of body composition and weight, mice were single-housed for acclimation. Then, food intake was measured for approximately 4 consecutive days. O2 consumption (VO2), CO2 production, ambulatory activity (by infrared sensors), and respiratory quotient (CO2 production/O2 consumption) were determined using the Oxymax/Comprehensive Lab Animal Monitoring System (Columbus Instruments) for approximately 5 days. The data from the first 2 days of analysis were discarded because we considered the acclimatization period. The results used for each animal were the average of the analyzed days. Glucoprivic hyperphagia was evaluated by recording the cumulative food intake 4 hours after PBS or 0.5 g/kg of 2-deoxy-D-glucose (2DG; Sigma) intraperitoneal injections. Changes in body weight and glycemia were determined in mice exposed for 5 days to 60% food restriction. In this protocol, mice received 40% of their basal food intake 3 hours before lights off for 5 consecutive days.
Evaluation of Pulsatile Growth Hormone Secretion
Another group of 4-week-old male and female mice was daily handled for 30 days to acclimate to the procedure of tail-tip blood sampling and minimize the interference of stress. Then, the blood collection started at the beginning of the light cycle (~8 am) and 36 sequential tail-tip blood samples of 5 μL were collected from each mouse at 10-minute intervals (23, 35). Immediately before the first sample collection, a small portion of the tail tip (1 mm) was cut with a surgical blade to allow the collection of small drops of blood. During the whole period of the experiment, mice were allowed to move freely in their home cage with ad libitum access to food and water. For each blood collection, mice were placed inside a cardboard tube and quickly held by the base of the tail. Using a 10-μL pipette, a 5-μL sample of whole blood was collected and transferred to 105 μL of PBS with 0.05% Tween-20 (PBS-T). After each blood collection, fingertip pressure was gently applied to the tail tip to stop bleeding. Samples were immediately placed on dry ice and stored at –80 °C.
Hormone Assessment
To determine GH levels in the blood, a sensitive sandwich enzyme-linked immunosorbent assay (ELISA) for GH was adapted from a protocol published by Steyn et al (35). A 96-well high-binding plate (9018, Corning) was coated with 50 µL of monkey anti-rat GH antibody (NIDDK-NHPP; rGH-IC-1, catalog No. AFP411S; RRID: AB_2665564) diluted in PBS at 1:50 000 overnight at 4 °C. After decanting the coating antibody, wells were incubated with 200 µL of blocking buffer (5% skim milk powder in PBS-T) for 2 hours at room temperature. The standard curve consisted of a 2-fold serial dilution of mouse recombinant GH (mGH reference preparation; NIDDK-NHPP; catalog No. AFP-10783B) in 0.2% bovine serum albumin PBS-T. The wells were incubated with 50 µL of samples at a 1:22 dilution for 24 hours at room temperature. The plate was washed in PBS-T and the wells were incubated with 50 µL of rabbit anti-rat GH antibody (NIDDK-NHPP; catalog No. AFP5672099; RRID: AB_2721132) diluted in blocking buffer at 1:100 000 for 24 hours at 4 °C. After washing in PBS-T, wells were incubated with 50 μL of horseradish peroxidase–conjugated goat anti-rabbit immunoglobulin G antibody (A9169-2ml, Sigma-Aldrich) diluted in 50% PBS, and 50% blocking buffer at 1:30 000 for 90 minutes at room temperature. After a final wash in PBS-T, wells were incubated with 100 μL of 2 mg/mL o-phenylenediamine dihydrochloride (OPD, P1526, Sigma-Aldrich) in citrate-phosphate buffer (pH 5.0) containing 0.02% hydrogen peroxide for 45 minutes at room temperature. The reaction was stopped with 50 μL of 3 M HCl. The absorbance was determined at 490 nm with a microplate reader (Epoch, Biotek) and a wavelength of 650 nm was used for background correction. The standard curve ranged from 0.03 to 7.5 ng/mL and the concentration of GH was calculated by interpolating the optical density of the samples against a nonlinear regression of the standard curve. The lower limit of detection was 0.04 ng/mL, and the intra-assay and interassay coefficients of variation were 2.6% and 9.7%, respectively. GH pulses were detected using default parameters of the DynPeak pulse detection algorithm (36) The number of GH pulses was calculated for a period of 6 hours. The amplitude of each pulse was defined as the difference between peak value and its nadir, and the average levels of the detected pulses were considered for the statistical analysis of pulse amplitude. Mean GH was calculated by averaging all GH values from each mouse. Commercially available ELISA kits were used to determine serum IGF-1 (R&D Systems; catalog No. MG100) and leptin (R&D Systems; catalog No. MOB00B) concentrations.
Quantitative Real-time Polymerase Chain Reaction
The entire hypothalamus, the pituitary gland and liver samples were collected to determine the gene expression by quantitative real-time PCR. Initially, total RNA was extracted with TRIzol (Invitrogen), followed by incubation in DNase I RNase-free (Roche Applied Science) and then reverse transcription using 2 µg of total RNA, SuperScript II Reverse Transcriptase (Invitrogen), and random primers p(dN)6 (Roche Applied Science). Real-time PCR was performed using the 7500TM Real-Time PCR System (Applied Biosystems), Power SYBR Green Gene Expression PCR Master Mix (Applied Biosystems), and specific primers for the following target genes: Actb (forward: gctccggcatgtgcaaag; reverse: catcacaccctggtgccta), Gh (forward: tgcccttgtccagtctgttt; reverse: atgtaggcacgctcgaactc), Ghr (forward: atcaatccaagcctggggac; reverse: acagctgaatagatcctgggg), Ghrh (forward: tatgcccgg aaagtgatccag; reverse: atccttgggaatccctgcaaga), Ghrhr (forward: gggtcctttcagggcttcat; reverse: gtcgtggccataccatttgc), Igf1 (forward: gtacttcctttccttctcctttgc; reverse: ccacactgaca tgcccaaga), Ppia (forward: tatctgcactgccaagactgagt; reverse: cttcttgctggtcttgccattcc), Sst (forward: ctgtcctgccgtctccagt; reverse: ctgcagaaactgacggagtct), Sstr1 (forward: catggaaga gcctggacgaa; reverse: atgagaatggcgctaccctg), Sstr2 (forward: tcctcacctatgccaacagc; reverse: ccgtaccactcaccttgacc), Sstr3 (forward: ctggctgtcagtggcatctt; reverse: cacgtagatcacca gcgagt), Sstr4 (forward: gccctggtcatcttcgtgat; reverse: atgaagagctcatcggcgac), and Sstr5 (forward: aacgcagaatgc tgtctcct; reverse: tgaactggttgatgccgtcc). Data were normalized to the geometric average of Actb and Ppia. Relative quantification of mRNA was calculated by 2–ΔΔCt.
Statistical Analysis
Statistical analyses were performed using Prism software (GraphPad). Differences between the groups were analyzed by one-way analysis of variance (ANOVA), followed by Newman-Keuls multiple comparisons test. Changes in body weight, body composition, and glycemia across time and 2DG-induced feeding response were determined by repeated-measures 2-way ANOVA. The description of the tests used in each experiment and the sample sizes are found in the figure legends. One VGAT GHR KO female was removed from the analysis of pulsatile GH secretion because it showed extremely high GH levels and the values were classified as outlier using the Rout or Grubb tests. We considered P values less than .05 to be statistically significant. All results are expressed as mean ± SEM.
Results
Distinct GABAergic and Glutamatergic Neurons are Responsive to Growth Hormone
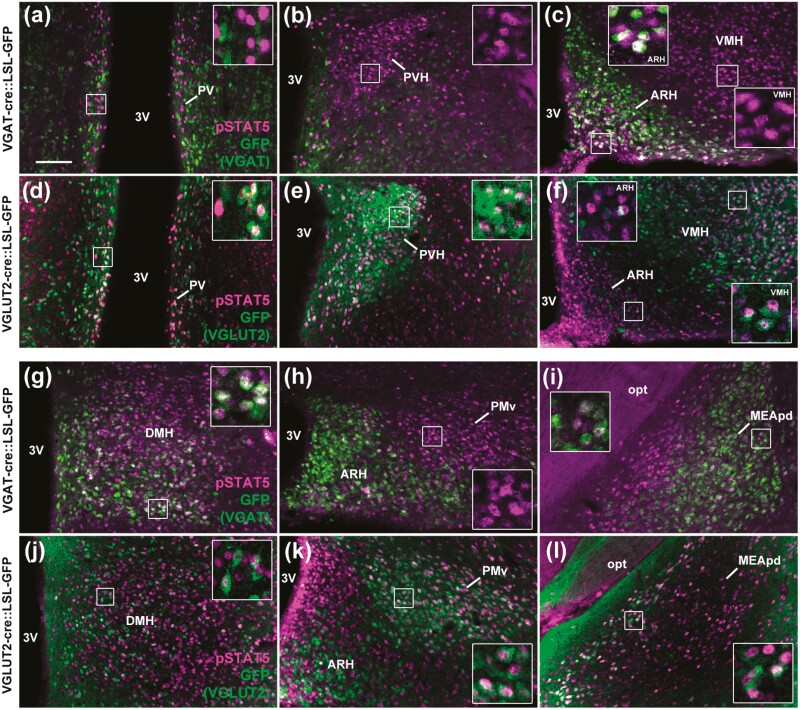
To identify the GABAergic and glutamatergic neuronal populations that are responsive to GH, GH-induced pSTAT5 was colocalized with GFP, as a marker of VGAT- or VGLUT2-expressing cells (Fig. 1). The majority of neurons in the ARH, ventral part of the DMH, and MEApd coexpressed GH-induced pSTAT5 and VGAT (Fig. 1A-1C and 1G-1I and Table 1). In contrast, GH-responsive neurons in the PV, PVH, lateral ARH, VMH, dorsal part of the DMH, PMv, and medial part of the MEApd colocalized with VGLUT2 (Fig. 1D-1F and 1J-1L and Table 2). Thus, distinct GABAergic and glutamatergic neuronal populations are responsive to GH.
Figure 1.
Different GABAergic and glutamatergic neuronal populations are responsive to GH. A to C and G to I, Several GABAergic neuronal populations (GFP staining in green representing VGAT-expressing cells) express GH-induced pSTAT5 (magenta nuclear staining; n = 4). D to F and J to L, Glutamatergic neurons (GFP staining in green representing VGLUT2-expressing cells) in different brain areas express GH-induced pSTAT5 (n = 6). Scale bar = 100 µm. The insets represent higher magnification images. 3V, third ventricle; ARH, arcuate nucleus; DMH, dorsomedial nucleus; GFP, green fluorescent protein; GH, growth hormone; MEApd, posterodorsal medial nucleus of the amygdala; opt, optic tract; PMv, ventral premammillary nucleus; PV, periventricular nucleus; PVH, paraventricular nucleus of the hypothalamus; VMH, ventromedial nucleus.
Table 1.
Distribution of GABAergic (vesicular inhibitory amino acid transporter–positive) neurons that express growth hormone–induced pSTAT5 in the mouse brain
| No. of pSTAT5 + cells | No. of doubled-labeled cells | GH responsive cells that express VGAT, % | |
|---|---|---|---|
| ARH | 208.4 ± 1.1 | 159.0 ± 1.1 | 76.3 ± 0.9 |
| DMH | 428.8 ± 12.5 | 146.3 ± 20.2 | 33.9 ± 3.9 |
| MEApd | 156.3 ± 26.2 | 61.0 ± 11.5 | 38.8 ± 1.6 |
| PMv | 226.1 ± 13.6 | 2.6 ± 1.2 | 1.3 ± 0.5 |
| PV | 62.7 ± 1.7 | 0.6 ± 0.6 | 1.1 ± 1.1 |
| PVH | 224.9 ± 2.4 | 0.2 ± 0.1 | 0.1 ± 0.1 |
| VMH | 573.0 ± 17.8 | 2.0 ± 1.0 | 0.3 ± 0.2 |
n = 4.
Abbreviations: ARH, arcuate nucleus; DMH, dorsomedial nucleus of the hypothalamus; MEApd, posterodorsal part of the medial nucleus of the amygdala; PMv, ventral premammillary nucleus; PV, periventricular nucleus; PVH, paraventricular nucleus of the hypothalamus; VGAT, vesicular inhibitory amino acid transporter; VMH, ventromedial nucleus of the hypothalamus.
Table 2.
Distribution of glutamatergic (vesicular glutamate transporter 2–positive) neurons that express growth hormone–induced pSTAT5 in the mouse brain
| No. of pSTAT5 + cells | No. of doubled-labeled cells | GH responsive cells that express VGLUT2, % | |
|---|---|---|---|
| ARH | 247.5 ± 2.5 | 7.0 ± 1.0 | 2.8 ± 0.4 |
| DMH | 405.0 ± 91.0 | 57.2 ± 20.2 | 13.7 ± 1.9 |
| MEApd | 232.5 ± 0.5 | 79.0 ± 21.0 | 33.9 ± 8.9 |
| PMv | 316.7 ± 26.9 | 288.3 ± 17.6 | 91.4 ± 2.4 |
| PV | 65.0 ± 5.0 | 26.0 ± 2.0 | 40.5 ± 6.2 |
| PVH | 230.6 ± 13.3 | 186.6 ± 11.1 | 80.9 ± 1.2 |
| VMH | 562.5 ± 18.8 | 516.5 ± 21.6 | 91.6 ± 0.7 |
n = 6.
Abbreviations: ARH, arcuate nucleus; DMH, dorsomedial nucleus of the hypothalamus; MEApd, posterodorsal part of the medial nucleus of the amygdala; PMv, ventral premammillary nucleus; PV, periventricular nucleus; PVH, paraventricular nucleus of the hypothalamus; VGAT, vesicular inhibitory amino acid transporter; VGLUT2, vesicular glutamate transporter 2; VMH, ventromedial nucleus of the hypothalamus.
Neurochemical Phenotype of Neuronal Populations Possibly Involved in the Autoregulation of Growth Hormone Secretion
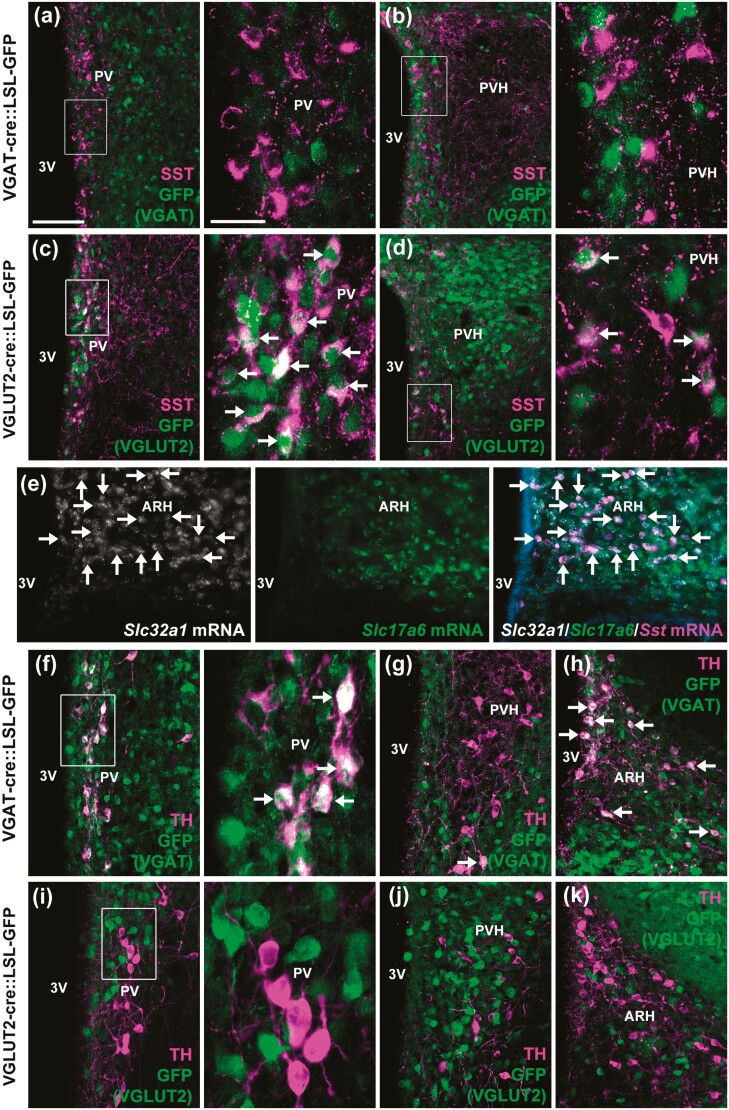
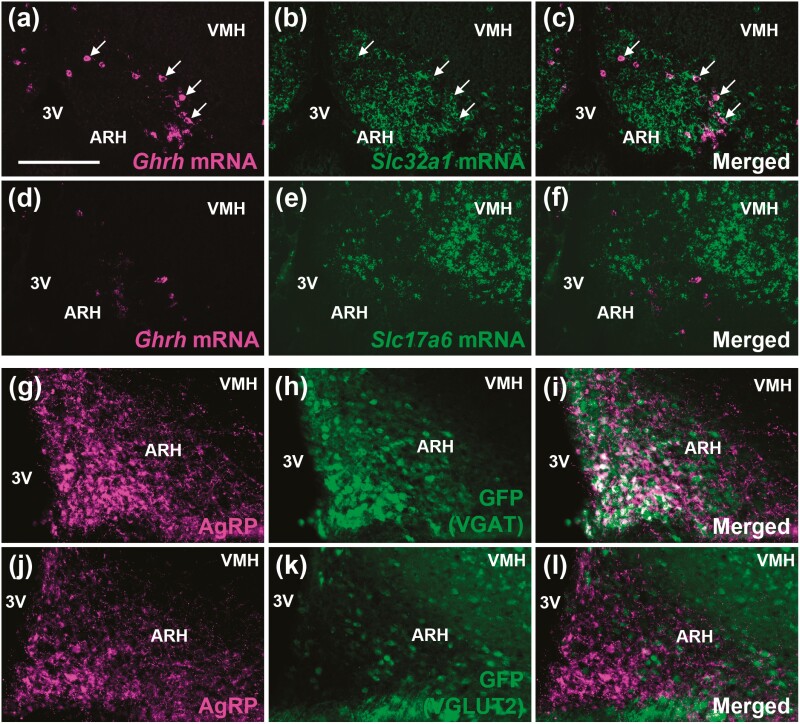
Several neuronal populations express the Ghr mRNA or are responsive to GH, and possibly regulate GH secretion via negative feedback loops. These include GHRH, AgRP/NPY, and TH neurons in the ARH (10, 20-23, 37, 38) and SST and TH neurons in the PV/PVH (9, 13, 23). Thus, we determined whether these cells present a GABAergic or glutamatergic phenotype (Fig. 2 and Fig. 3). No colocalization between SST and VGAT was observed in the PV and PVH, whereas VGLUT2 was expressed in 33.6 ± 0.1% of PVSST neurons and in 31.4 ± 1.0% of PVHSST neurons (Fig. 2A-2D). In contrast, approximately 93% of ARHSST neurons colocalized with Slc32a1 mRNA (which encodes VGAT), whereas Slc17a6 mRNA (which encodes VGLUT2) expression was found in 7% of ARHSST neurons (Fig. 2E). Regarding TH-expressing cells, 36.0 ± 3.2% of PVTH neurons (Fig. 2F) and 45.0 ± 12.0% of ARHTH neurons (Fig. 2H) coexpressed VGAT, whereas very few colocalizations with VGLUT2 were observed in PVTH (3.1 ± 0.3%; Fig. 2I) and ARHTH (0.6 ± 0.6%; Fig. 2K) neurons. PVHTH neurons show minor colocalization with both VGAT (2.2 ± 1.4%; Fig. 2G) and VGLUT2 (4.2 ± 1.6%; Fig. 2J). Approximately 41% of ARHGHRH neurons colocalized with Slc32a1 mRNA (Fig. 3A-3C), whereas virtually no ARHGHRH neuron coexpressed Slc17a6 mRNA (Fig. 3D-3F). In accordance with previous studies (39, 40), ARHAgRP/NPY neurons widely colocalized with VGAT (Fig. 3G-3I), whereas virtually no colocalization with VGLUT2 was observed (Fig. 3J-3L). Taken together, while a subgroup of PV/PVHSST neurons is glutamatergic, part of PV/ARHTH and ARHGHRH neurons, and the majority of ARHSST and ARHAgRP neurons, exhibit a GABAergic signature.
Figure 2.
Neurochemical phenotype of neuronal populations possibly involved in the autoregulation of GH secretion. A and B, SST cells (magenta staining) do not coexpress markers of GABAergic neurons (GFP staining in green representing VGAT-expressing cells) in the PV and PVH (n = 3). C and D, Abundant colocalizations of the SST in glutamatergic neurons (GFP staining representing VGLUT2-expressing cells) in the PV and PVH (n = 2). E, Colocalization between Slc32a1 mRNA (white staining), Slc17a6 mRNA (green staining), and Sst mRNA (magenta staining) in the ARH of C57BL/6 mice. F to H, Colocalizations of TH in GABAergic neurons of the PV, PVH, and ARH (n = 3). I to K, Absence of colocalization of TH in glutamatergic neurons of the PV, PVH, and ARH (n = 2). Scale bar = 100 µm (25 µm in higher magnification images). The arrows indicate double-labeled neurons. 3V, third ventricle; ARH, arcuate nucleus; GFP, green fluorescent protein; GH, growth hormone; mRNA, messenger RNA; PV, periventricular nucleus; PVH, paraventricular nucleus of the hypothalamus; SST, somatostatin; TH, tyrosine hydroxylase; VGAT, vesicular inhibitory amino acid transporter.
Figure 3.
Part of ARHGHRH neurons and practically all ARHAgRP neurons are GABAergic. A to C, Colocalization between Ghrh mRNA (magenta staining) and Slc32a1 mRNA (green staining) in the ARH (n = 4). The arrows indicate double-labeled neurons. D to F, No colocalization between Ghrh mRNA (magenta staining) and Slc17a6 mRNA (green staining) in the ARH (n = 4). G to I, ARHVGAT neurons (GFP staining in green representing VGAT-expressing cells) colocalize with AgRP peptide (magenta staining; n = 2). J to L, ARHVGLUT2 neurons (GFP staining in green representing VGLUT2-expressing cells) do not express AgRP (n = 2). Scale bar = 100 µm. 3V, third ventricle; AgRP, agouti-related protein; ARH, arcuate nucleus; GFP, green fluorescent protein; mRNA, messenger RNA; VGAT, vesicular inhibitory amino acid transporter; VMH, ventromedial nucleus.
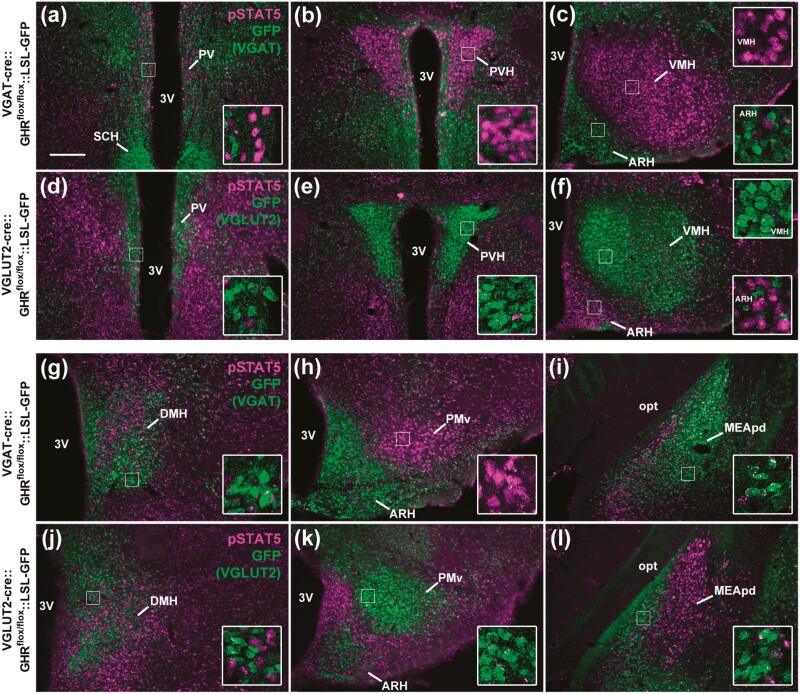
Generation of Vesicular Inhibitory Amino Acid Transporter Growth Hormone Receptor Knockout and Vesicular Glutamate Transporter 2 GHR KO Mice
To determine whether GHR signaling in GABAergic or glutamatergic neurons regulates GH secretion via a negative-feedback short loop, mice carrying ablation of GHR either in VGAT- or VGLUT2-expressing cells were produced (Fig. 4 and Table 3). As expected, virtually no colocalization between GFP (used as a marker of VGAT-expressing cells) and GH-induced pSTAT5 was observed in VGAT GHR KO mice, whereas a normal GH-induced pSTAT5 expression was observed in glutamatergic neuronal populations (Fig. 4A-4C and 4G-4I and Table 3). Conversely, GH responsiveness was nearly absent in glutamatergic neurons of VGLUT2 GHR KO mice, while GH-induced pSTAT5 was intact in GABAergic neuronal populations (Fig. 4D-4F and 4J-4L and Table 3). These findings validate the efficacy and specificity of our targeted deletions in the VGAT GHR KO and VGLUT2 GHR KO mouse models.
Figure 4.
GHR ablation in GABAergic and glutamatergic neurons. A to C and G to I, GABAergic neurons (VGAT-positive cells; GFP staining in green) are no longer responsive to GH in VGAT GHR KO mice, whereas GH-induced pSTAT5 (magenta nuclear staining) is still observed in non-GABAergic neurons (n = 3). D to F and J to L, Glutamatergic neurons (VGLUT2-positive cells) are no longer responsive to GH in VGLUT2 GHR KO mice, whereas GH-induced pSTAT5 is still observed in nonglutamatergic neurons (n = 3). Scale bar = 200 µm. Insets represent higher magnification images. 3V, third ventricle; ARH, arcuate nucleus; DMH, dorsomedial nucleus; GH, growth hormone; GHR, GH receptor; KO, knockout; MEApd, posterodorsal medial nucleus of the amygdala; opt, optic tract; PMv, ventral premammillary nucleus; PV, periventricular nucleus; PVH, paraventricular nucleus of the hypothalamus; SCH, suprachiasmatic nucleus; VGAT, vesicular inhibitory amino acid transporter; VMH, ventromedial nucleus.
Table 3.
Validation of cell-specific ablation of growth hormone receptor (GHR) in vesicular inhibitory amino acid transporter GHR knockout (KO) and vesicular glutamate transporter 2 GHR KO mice
| VGAT GHR KO mice | VGLUT2 GHR KO mice | |||
|---|---|---|---|---|
| No. of pSTAT5+ cells | No. of VGAT/pSTAT5+ cells | No. of pSTAT5+ cells | No. of VGLUT2/pSTAT5+ cells | |
| ARH | 25.7 ± 3.7 | 0.0 ± 0.0 | 134.3 ± 6.7 | 0.0 ± 0.0 |
| DMH | 297.0 ± 26.3 | 0.0 ± 0.0 | 240.6 ± 30.9 | 0.0 ± 0.0 |
| MEApd | 59.8 ± 29.1 | 0.0 ± 0.0 | 139.3 ± 5.4 | 0.0 ± 0.0 |
| PMv | 188.7 ± 10.0 | 0.0 ± 0.0 | 1.8 ± 1.0 | 0.2 ± 0.2 |
| PV | 72.0 ± 11.8 | 0.0 ± 0.0 | 5.3 ± 1.7 | 0.0 ± 0.0 |
| PVH | 296.3 ± 5.4 | 0.0 ± 0.0 | 2.7 ± 1.4 | 0.0 ± 0.0 |
| VMH | 879.0 ± 50.5 | 0.0 ± 0.0 | 4.7 ± 0.6 | 0.0 ± 0.0 |
n = 3/group.
Abbreviations: ARH, arcuate nucleus; DMH, dorsomedial nucleus of the hypothalamus; GHR, growth hormone receptor; MEApd, posterodorsal part of the medial nucleus of the amygdala; PMv, ventral premammillary nucleus; PV, periventricular nucleus; PVH, paraventricular nucleus of the hypothalamus; VGAT, vesicular inhibitory amino acid transporter; VGLUT2, vesicular glutamate transporter 2; VMH, ventromedial nucleus of the hypothalamus.
Opposite Effects on Body Growth of Growth Hormone Receptor Ablation in GABAergic and Glutamatergic Neurons
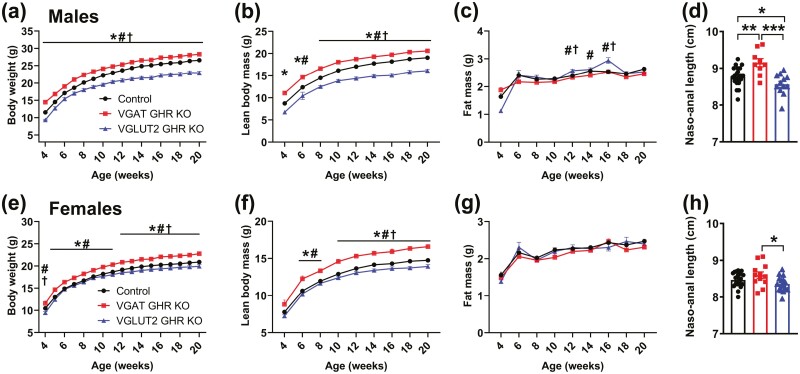
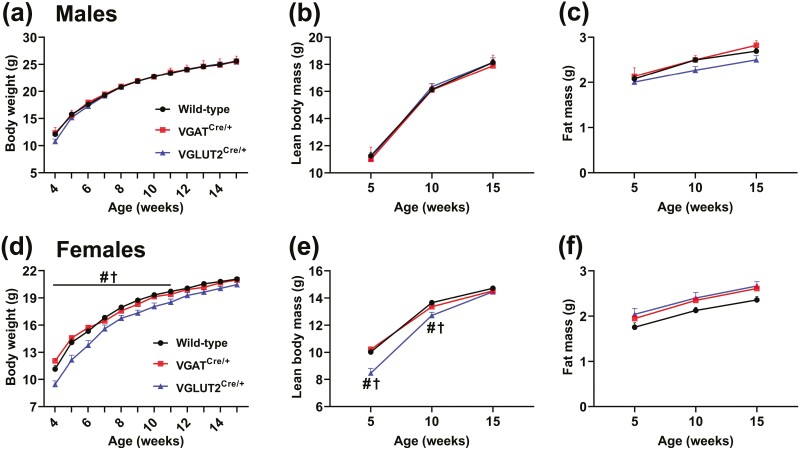
To determine possible consequences of GHR ablation in GABAergic and glutamatergic neurons on somatic growth, body weight and body composition were longitudinally assessed in VGAT GHR KO and VGLUT2 GHR KO mice (Fig. 5). Remarkably, VGAT GHR KO mice showed increased body weight and lean mass, compared to control mice, both in males and females (Fig. 5A and 5B, and 5E and 5F). No statistically significant change in fat mass was observed in VGAT GHR KO mice (Fig. 5C and 5G). In contrast, male and female VGLUT2 GHR KO mice showed a reduction in body weight and lean mass compared to control and VGAT GHR KO mice. Male VGLUT2 GHR KO mice also exhibited a slight and transitory increase in body fat mass between ages 12 and 16 weeks (Fig. 5C), whereas no differences in body adiposity were observed in females (Fig. 5G). Naso-anal length was increased in VGAT GHR KO mice (Fig. 5D and 5H), whereas VGLUT2 GHR KO male mice exhibited decreased naso-anal length compared to control mice (see Fig. 5D). In accordance with the changes in lean body mass, VGAT GHR KO mice showed increases in the mass of the liver, heart, and kidneys, compared to control mice, whereas VGLUT2 GHR KO mice exhibited reduced mass of these tissues (data not shown). Conclusively, GHR ablation in GABAergic neurons leads to increased body growth, whereas VGLUT2 GHR KO mice display decreased somatic growth.
Figure 5.
Opposite effects on body growth of GHR ablation in GABAergic and glutamatergic neurons. A to C, Changes along time in body weight, lean body mass, and body fat mass in male control (n = 41), VGAT GHR KO (n = 41), and VGLUT2 GHR KO (n = 15) mice. *P < .05, VGAT GHR KO vs control. #P < .05, VGAT GHR KO vs VGLUT2 GHR KO. † P < .05, VGLUT2 GHR KO vs control (repeated-measures 2-way ANOVA and Newman-Keuls multiple comparisons test). D, Naso-anal length of male control (n = 22), VGAT GHR KO (n = 9), and VGLUT2 GHR KO (n = 12) mice. *P < .05; **P < .01; ***P < .001 (1-way ANOVA and Newman-Keuls multiple comparisons test). E to G, Changes along time in body weight, lean body mass, and body fat mass in female control (n = 43), VGAT GHR KO (n = 15), and VGLUT2 GHR KO (n = 15) mice. H, Naso-anal length of female control (n = 23), VGAT GHR KO (n = 12), and VGLUT2 GHR KO (n = 16) mice. ANOVA, analysis of variance; GHR, growth hormone receptor; KO, knockout; VGAT, vesicular inhibitory amino acid transporter.
Increased Pulsatile Growth Hormone (GH) Secretion in Vesicular Inhibitory Amino Acid Transporter GH Receptor Knockout Mice
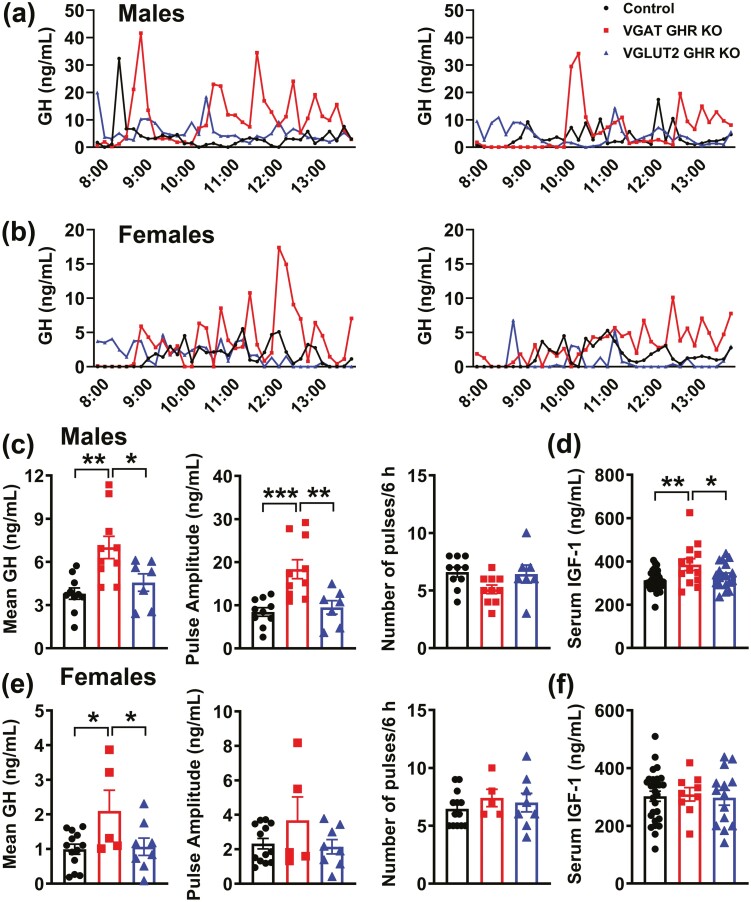
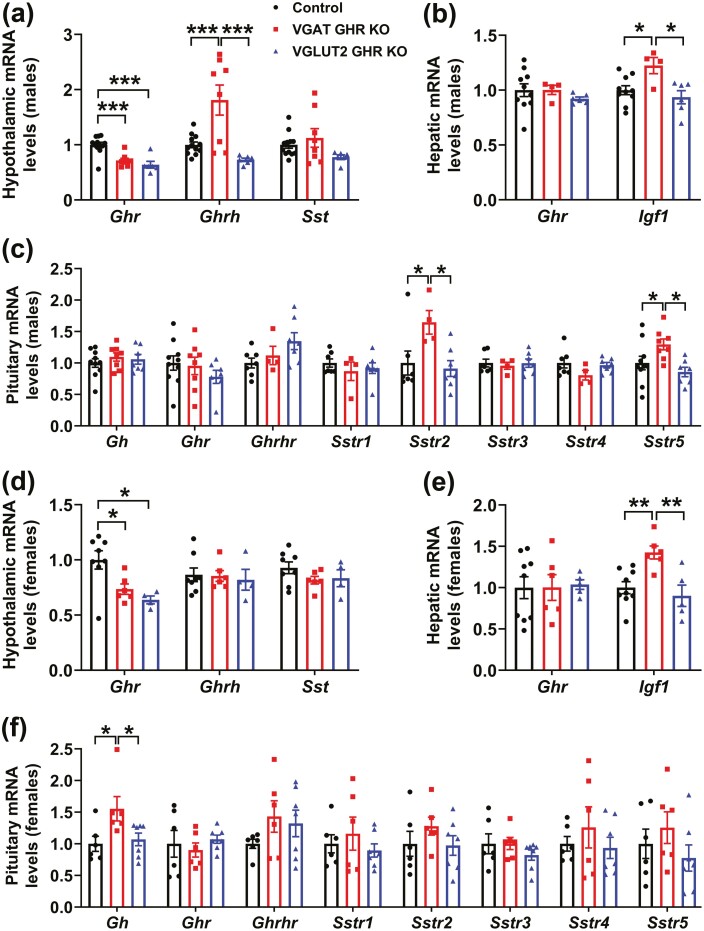
Pulsatile GH secretion was determined in male and female VGAT GHR KO and VGLUT2 GHR KO mice. Notably, VGAT GHR KO male mice displayed an increase in mean GH levels and pulse amplitude, whereas a normal GH pulse frequency was observed, as compared to control and VGLUT2 GHR KO mice (Fig. 6A and 6B). Female VGAT GHR KO mice showed increased mean GH levels compared to the other groups (Fig. 6B and 6E). In contrast, VGLUT2 GHR KO mice showed a similar pulsatile GH secretion compared to control animals (Fig. 6A-6C and 6E). Serum IGF-1 levels were increased in VGAT GHR KO male mice in comparison with the other groups (Fig. 6D), whereas no changes in serum IGF-1 levels were observed in the females (Fig. 6F). Interestingly, ablation of GHR in either VGAT- or VGLUT2-expressing cells decreased hypothalamic Ghr mRNA levels in approximately 35% each (Fig. 7A and 7D). In addition, hypothalamic Ghrh mRNA levels were increased in VGAT GHR KO male mice, whereas no statistically significant changes in Sst mRNA levels were observed between the groups (Fig. 7A and 7D). In the liver, Ghr mRNA levels were unchanged, but a statistically significant increase in Igf1 mRNA expression was observed in the VGAT GHR KO mice (Fig. 7B and 7E). Gene expression was also analyzed in the pituitary gland and while VGAT GHR KO male mice exhibited increased Sstr2 and Sstr5 mRNA levels compared to other groups, VGAT GHR KO female mice showed a significant increase in Gh mRNA levels in comparison with control and VGLUT2 GHR KO females (Fig. 7C and 7F). Therefore, the elevated GH secretion in VGAT GHR KO mice explains their increased lean body mass.
Figure 6.
Increased pulsatile GH secretion in VGAT GHR KO mice. A and B, Representative examples of the pulsatile pattern of GH secretion in 8-week-old control, VGAT GHR KO, and VGLUT2 GHR KO mice both in A, males, and B, females. C and D, Mean GH levels, GH pulse amplitude, GH pulse frequency, and serum IGF-1 levels in 8-week-old control (n = 10-32), VGAT GHR KO (n = 10-13), and VGLUT2 GHR KO (n = 7-19) male mice. E and F, Mean GH levels, GH pulse amplitude, GH pulse frequency, and serum IGF-1 levels in 8-week-old control (n = 13-27), VGAT GHR KO (n = 5-9), and VGLUT2 GHR KO (n = 8-14) female mice. *P < .05; **P < .01; ***P < .001 (1-way ANOVA and Newman-Keuls multiple comparisons test). ANOVA, analysis of variance; GH, growth hormone; GHR, GH receptor; IGF-1, insulin-like growth factor 1; KO, knockout; VGAT, vesicular inhibitory amino acid transporter.
Figure 7.
Increased hypothalamic Ghrh and hepatic Igf1 mRNA levels in VGAT GHR KO mice. A, Hypothalamic gene expression in 8-week-old control (n = 12), VGAT GHR KO (n = 8), and VGLUT2 GHR KO (n = 6) male mice. B, Hepatic gene expression in 8-week-old control (n = 10), VGAT GHR KO (n = 4), and VGLUT2 GHR KO (n = 6) male mice. C, Pituitary gene expression in control (n = 7-10), VGAT GHR KO (n = 4-8), and VGLUT2 GHR KO (n = 7) male mice. D, Hypothalamic gene expression in 8-week-old control (n = 8), VGAT GHR KO (n = 6), and VGLUT2 GHR KO (n = 4) female mice. E, Hepatic gene expression in 8-week-old control (n = 9), VGAT GHR KO (n = 6), and VGLUT2 GHR KO (n = 5) female mice. F, Pituitary gene expression in control (n = 6), VGAT GHR KO (n = 6), and VGLUT2 GHR KO (n = 7) female mice. *P < .05; **P < .01; ***P < .001 (1-way ANOVA and Newman-Keuls multiple comparisons test). ANOVA, analysis of variance; GH, growth hormone; GHR, GH receptor; IGF-1, insulin-like growth factor 1; mRNA, messenger RNA; KO, knockout; VGAT, vesicular inhibitory amino acid transporter.
Growth Hormone Receptor Ablation in Vesicular Inhibitory Amino Acid Transporter– and Vesicular Glutamate Transporter 2–expressing Cells Leads to Different Metabolic Changes
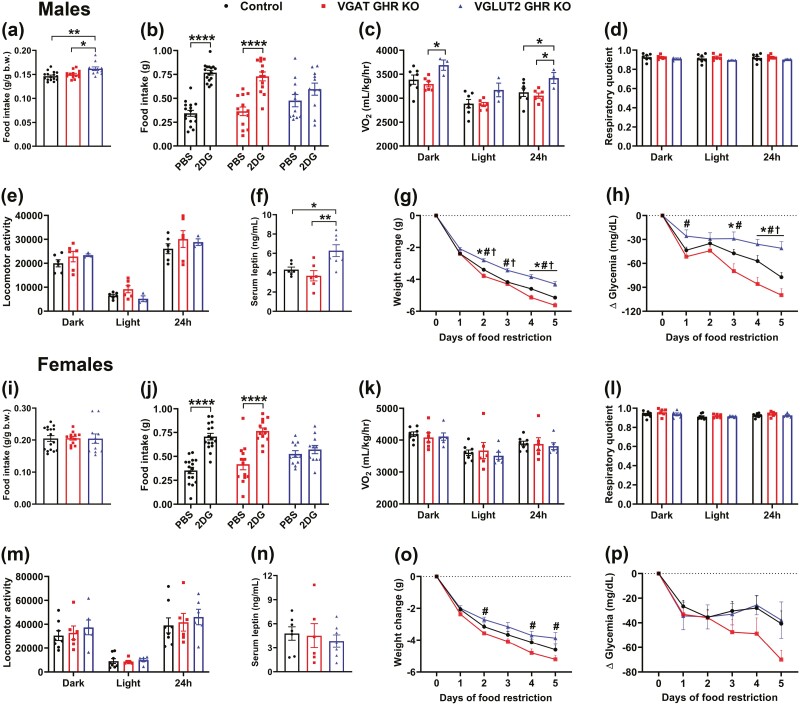
In addition to the evaluation of the somatotropic axis, possible metabolic alterations were assessed in VGAT GHR KO and VGLUT2 GHR KO mice. VGLUT2 GHR KO male mice showed increased relative food intake (Fig. 8A). No changes in food intake were observed in VGAT GHR KO male mice (see Fig. 8A) or between the females (Fig. 8I). GHR ablation either in proopiomelanocortin (POMC) or AgRP neurons attenuates the glucoprivic hyperphagia induced by 2DG injection (22, 41). Thus, the feeding response to 2DG was determined in VGAT GHR KO and VGLUT2 GHR KO mice. Remarkably, whereas VGAT GHR KO mice exhibited a normal 2DG-induced hyperphagia compared to the control group, VGLUT2 GHR KO mice displayed a blunted feeding response to 2DG both in males (Fig. 8B) and females (Fig. 8J). VGLUT2 GHR KO male mice also showed higher VO2 compared to control and VGAT GHR KO mice (Fig. 8C), whereas no difference in VO2 was found between the females (Fig. 8K). No statistically significant differences between the groups were observed in the respiratory quotient (Fig. 8D and 8L) and ambulatory activity (Fig. 8E and 8M). In males, VGLUT2 GHR KO mice exhibited increased serum leptin levels compared to control and VGAT GHR KO mice (Fig. 8F). No difference in serum leptin levels was found in the females (Fig. 8N). When animals were subjected to 5 days of 60% food restriction, VGAT GHR KO male mice showed a greater weight loss on days 2, 4, and 5 of food restriction compared to control mice (Fig. 8G). In contrast, GHR ablation in VGLUT2 cells reduced the weight loss in male mice (see Fig. 8G). A reduction in glycemia was also observed in VGAT GHR KO male mice during the last days of food restriction, whereas VGLUT2 GHR KO male mice were able to sustain a higher glycemia compared to the other groups (Fig. 8H). In females, VGAT GHR KO mice exhibited increased weight loss during food restriction compared to VGLUT2 GHR KO mice (Fig. 8O). In addition, the drop in blood glucose levels tended to be accentuated in VGAT GHR KO female mice during food restriction, although it did not reach statistical significance (Fig. 8P). Taken together, VGAT GHR KO and VGLUT2 GHR KO mice exhibit distinct metabolic alterations.
Figure 8.
GHR ablation in VGAT- and VGLUT2-expressing cells leads to different metabolic changes. A, Food intake relative to body weight in approximately 20-week-old control (n = 16), VGAT GHR KO (n = 13), and VGLUT2 GHR KO (n = 11) male mice. *P < .05; **P < .01 (1-way ANOVA and Newman-Keuls multiple comparisons test). B, 2-Deoxy-D-glucose (2DG)-induced feeding response in control (n = 16), VGAT GHR KO (n = 13), and VGLUT2 GHR KO (n = 11) male mice. ****P < .0001 (repeated-measures 2-way ANOVA and Bonferroni multiple comparisons test). C to E, VO2, respiratory quotient and locomotor activity in control (n = 7), VGAT GHR KO (n = 6), and VGLUT2 GHR KO (n = 3) male mice. F, Serum leptin levels in control (n = 6), VGAT GHR KO (n = 6), and VGLUT2 GHR KO (n = 7) male mice. *P < .05; **P < .01 (1-way ANOVA and Newman-Keuls multiple comparisons test). G and H, Changes in body weight and blood glucose levels during 5 days of 60% food restriction in control (n = 16), VGAT GHR KO (n = 13), and VGLUT2 GHR KO (n = 11) male mice. *P < .05, VGAT GHR KO vs control. #P < .05, VGAT GHR KO vs VGLUT2 GHR KO. †P < .05, VGLUT2 GHR KO vs control (repeated-measures 2-way ANOVA and Newman-Keuls multiple comparisons test). I, Food intake relative to body weight in approximately 20-week-old control (n = 16), VGAT GHR KO (n = 12), and VGLUT2 GHR KO (n = 11) female mice. J, 2DG-induced feeding response in control (n = 16), VGAT GHR KO (n = 13), and VGLUT2 GHR KO (n = 11) female mice. K to M, VO2, respiratory quotient, and locomotor activity in control (n = 6), VGAT GHR KO (n = 6), and VGLUT2 GHR KO (n = 6) female mice. N, Serum leptin levels in control (n = 7), VGAT GHR KO (n = 6), and VGLUT2 GHR KO (n = 7) female mice. O and P, Changes in body weight and blood glucose levels during 5 days of 60% food restriction in control (n = 8), VGAT GHR KO (n = 7), and VGLUT2 GHR KO (n = 5) female mice. ANOVA, analysis of variance; GH, growth hormone; GHR, GH receptor; IGF-1, insulin-like growth factor 1; mRNA, messenger RNA; KO, knockout; VGAT, vesicular inhibitory amino acid transporter; VO2, oxygen consumption.
Vesicular Glutamate Transporter 2–cre Mice Exhibit a Previously Unreported Phenotype of Mild Reduction in Body Weight
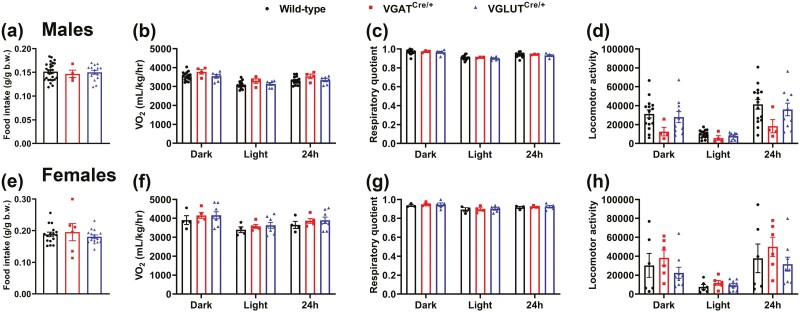
Because VGLUT2 GHR KO mice displayed normal GH secretion, but decreased body weight, we decided to investigate whether the expression of the Cre transgenes, independently of GHR ablation, affects body growth. Thus, VGAT-cre, VGLUT2-cre, and wild-type littermate mice were compared. No statistically significant changes in body weight, lean mass, and fat mass were observed between wild-type, VGAT-cre, and VGLUT2-cre male mice (Fig. 9A-9C). However, in females, VGLUT2-cre mice showed a reduction in body weight and lean mass until age 11 weeks, whereas body fat mass was similar between the groups (Fig. 9D-9F). Thus, the Cre insertion into the 3′ untranslated region of the Slc17a6 gene may cause slight reductions in body weight and lean mass in females, which may partially explain the phenotype observed in the VGLUT2 GHR KO mice. We also determined possible changes in energy balance. However, no differences in food intake, VO2, respiratory quotient, or locomotor activity were observed in VGAT-cre and VGLUT2-cre mice compared to wild-type littermate mice, in males (Fig. 10A-10D) or females (Fig. 10E-10H).
Figure 9.
VGLUT2-cre mice exhibit a mild body weight reduction. A to C, Changes across time in body weight, lean body mass, and body fat mass in male wild-type (n = 27), VGATCre/+ (n = 4), and VGLUT2Cre/+ (n = 15) mice. D to F, Changes across time in body weight, lean body mass, and body fat mass in female control (n = 14), VGATCre/+ (n = 6), and VGLUT2Cre/+ (n = 15) mice. #P < .05, VGATCre/+ vs VGLUT2Cre/+. †P < .05, VGLUT2Cre/+ vs wild-type (repeated-measures 2-way ANOVA and Newman-Keuls multiple comparisons test). ANOVA, analysis of variance; VGAT, vesicular inhibitory amino acid transporter.
Figure 10.
No changes in energy balance of VGATCre/+ and VGLUT2Cre/+ mice. A, Food intake relative to body weight in wild-type (n = 27), VGATCre/+ (n = 4), and VGLUT2Cre/+ (n = 15) male mice. B to D, VO2, respiratory quotient and locomotor activity in wild-type (n = 15), VGATCre/+ (n = 3-4), and VGLUT2Cre/+ (n = 6-10) male mice. E, Food intake relative to body weight in wild-type (n = 16), VGATCre/+ (n = 6), and VGLUT2Cre/+ (n = 15) female mice. F to H, VO2, respiratory quotient, and locomotor activity in wild-type (n = 3-6), VGATCre/+ (n = 4-6), and VGLUT2Cre/+ (n = 5-9) female mice. VGAT, vesicular inhibitory amino acid transporter; VO2, oxygen consumption.
Discussion
Hypothalamic neurons are able to sense changes in GH levels to regulate pituitary GH secretion via negative-feedback loops (3, 4). However, only a few studies so far have investigated whether GHR expression in specific neuronal populations is required for the autoregulation of pulsatile GH secretion. In the present study we show that GHR expression in GABAergic, but not in glutamatergic, neurons is necessary for the autoregulation of GH secretion. The disruption of GHR signaling in VGAT-expressing cells leads to increased GH secretion, liver Igf1 expression, and consequently, higher body weight and lean mass. We also revealed different metabolic consequences of GHR ablation in VGAT and VGLUT2 cells. These differences probably reflect the distinct neuronal populations affected by these targeted deletions (22, 23, 41-43).
The use of the Cre-loxP system presents shortfalls, including “Cre toxicity” that has been described in several strains (44, 45). Herein, we disclosed a previously unreported phenotype of mild body weight reduction in VGLUT2-cre female mice. This is a popular mouse strain and according to the Jackson Laboratory website (https://www.jax.org/strain/028863), it has been used in hundreds of publications. Thus, investigators aiming to use this model, especially in the metabolism field, should be aware of a possible growth alteration. In our case, since the control group was composed of animals expressing only Ghrflox/flox alleles, part of the reduction in body weight observed in VGLUT2 GHR KO mice may be related to the Cre transgene, independently of GHR ablation. However, it remains unknown why reduced body weight was observed in male and female VGLUT2 GHR KO mice, whereas only VGLUT2-cre females exhibited a lower body weight.
Several neurochemically defined neuronal populations express VGAT or VGLUT2 in the hypothalamus (39). Although it was not in the scope of the present study to exhaustively identify the specific group of neurons that explain the phenotype exhibited by VGAT GHR KO or VGLUT2 GHR KO mice, we showed that PV/ARHTH, ARHGHRH, ARHSST, and ARHAgRP neurons express markers of GABAergic cells, whereas PV/PVHSST neurons are mostly glutamatergic. Consequently, the loss of GH-negative feedback presented by VGAT GHR KO mice is not explained by GHR ablation in PV/PVHSST neurons, even though these cells contain all the prerequisites necessary to sense and respond to variations in GH levels and consequently regulate pituitary GH secretion (9, 11, 13, 16). Accordingly, a recent study showed that GHR ablation in SST-expressing cells does not affect body growth or GH secretion in male and female mice (46). GHR ablation in ARHAgRP neurons causes no changes in body growth (22, 47, 48), indicating that although Ghr mRNA is highly enriched in ARHAgRP/NPY neurons (20-22, 25, 49), this GABAergic neuronal population is probably not responsible for the alterations in the somatotropic axis observed in VGAT GHR KO mice.
In the present study we demonstrated that a subset of TH neurons in the PV and ARH coexpressed VGAT, whereas colocalizations with VGLUT2 were minimal. In accordance with our findings, Brown et al (27) showed that approximately 50% of ARHTH neurons exhibit markers of GABAergic cells. A recent study has shown that GHR ablation in TH neurons leads to the loss of GH-negative feedback and increased body growth in mice (23). Similar to GHR ablation in TH cells, VGAT GHR KO male mice showed increased GH pulse amplitude, without alterations in GH pulse frequency, which suggests a common neuroendocrine alteration in these mouse models. Both VGAT GHR KO and TH GHR KO male mice also exhibited increased hypothalamic Ghrh mRNA levels (23), indicating that GHRH neurons are not receiving the negative-feedback information of GH circulating levels; otherwise hypothalamic Ghrh mRNA expression should be suppressed (11, 18, 19). A subgroup of ARHGHRH neurons coexpresses TH (50, 51) and here we showed that part of these cells is GABAergic. Thus, although GHR ablation in VGAT/GHRH cells could lead to the loss of GH-negative feedback, less than 10% of ARHGHRH neurons express Ghr mRNA (38) and only a few ARHGHRH/TH neurons exhibit GH-induced pSTAT5 (23). Therefore, it is likely that hypophysiotropic ARHGHRH neurons are indirectly regulated by another population of GH-responsive neurons, possibly by TH neurons (23). This regulation may involve either GABA or dopamine neurotransmission. In this regard, optogenetic stimulation of ARHTH neurons induces postsynaptic GABA currents in neighboring cells (52), which may include hypophysiotropic ARHGHRH neurons. As evidence of a dopamine role regulating the hypothalamic-GH axis, mice carrying a neuron-specific D2 dopamine receptor ablation exhibit reduction in hypothalamic Ghrh mRNA expression, serum IGF-1 levels and, consequently, body growth (53, 54). Future studies are still necessary to determine whether the control of GH secretion requires GHR, GABA, or dopamine signaling in ARHGHRH neurons.
Although ARHTH neurons regulate several biological functions, part of these cells controls prolactin secretion (27, 55, 56). Notably, prolactin receptor ablation in VGAT neurons does not affect the feedback regulation of prolactin secretion, indicating that VGAT/TH neurons play a distinct functional role compared to the classical tuberoinfundibular dopamine neurons (27). Thus, given the similar consequences on the somatotropic axis caused by GHR ablation in VGAT and TH cells (23) and the unknown function of VGAT/TH neurons (27), we hypothesize that VGAT/TH neurons are involved in the control of GH secretion through short-loop negative feedback, possibly via the regulation of ARHGHRH neurons. Of note, Ghrh mRNA expression was increased only in VGAT GHR KO male mice. VGAT GHR KO male mice also showed increased serum IGF-1 levels, whereas no difference was observed in females. The hypothalamic-GH-liver axis exhibits marked sex differences, which leads to distinct patterns of hepatic gene expression between males and females (3, 4, 45, 57). A previous study has shown that female mice exposed to GH hypersecretion exhibit a lower proportional increase in serum IGF-1 levels compared to male mice (58). In addition, GHR signaling in the liver causes sexually differentiated effects in the circulating levels of IGF-binding proteins (59). Even GHRH neurons may present sex differences since estrogen receptor α signaling in this neuronal population plays sex-specific roles in growth and sexual maturation (60). Thus, sex differences in GHRH neurons, hepatic gene expression profile, and IGF-binding proteins levels may explain the sex-specific contrasts observed in the present study.
We and others have recently shown that central GHR signaling regulates metabolism (22, 41, 43, 47-49, 61, 62). Our findings indicate that both GABAergic and glutamatergic neuronal populations are involved in the central effects of GH-modulating energy homeostasis. VGLUT2 GHR KO male mice exhibited increased relative food intake. The dwarf GHR KO mice also display higher relative food intake (63). The causes of the increased food intake in dwarf GHR KO mice are unknown, but they are possibly associated with their low blood glucose and insulin levels, or due to increased serum levels of corticosterone (63). Independently of the mechanisms behind the increased food intake in GHR KO mice, our findings indicate that the hyperphagia induced by the absence of GHR signaling is possibly mediated by glutamatergic neurons. In addition, the increased energy expenditure exhibited by VGLUT2 GHR KO mice is possibly secondary to their higher food intake since both changes were observed only in male mice. VGLUT2 GHR KO male and female mice showed a blunted orexigenic response to 2DG. Although the higher basal food intake may cause a ceiling effect, POMC-specific GHR ablation also reduces the 2DG-induced feeding response without changing basal food intake (41). Considering that POMC neurons can express markers of either GABAergic or glutamatergic cells (64, 65), the present findings support that GHR ablation in VGLUT2-expressing POMC neurons is likely responsible for mediating the effects of GH on 2DG-induced hyperphagia.
GHR deletion in AgRP neurons prevents the neuroendocrine adaptations that save energy during prolonged food deprivation (22). Consequently, AgRP GHR KO mice exhibit increased energy expenditure and weight loss during food restriction. This effect is partially mediated by the STAT5 intracellular signaling pathway (66). Additionally, previous studies have shown that GH secretion is required to sustain glycemia during prolonged food restriction (22, 67, 68). GH maintains glycemia in starved mice by increasing hepatic gluconeogenesis (68). Although this effect can be directly in the liver, hypothalamic neurons including GABAergic AgRP neurons are able to indirectly regulate hepatic glucose production (69, 70). Mice carrying GHR ablation in AgRP neurons also exhibit decreased glycemia during food restriction (22). In the present study we observed increased weight loss and lower glycemia in VGAT GHR KO mice during food deprivation. These findings are in accordance with the fact that ARHAgRP neurons express VGAT, resulting in similar phenotypes between VGAT GHR KO and AgRP GHR KO mice during food restriction. Surprisingly, VGLUT2 GHR KO mice were able to partially prevent the weight loss and drop in glycemia during food restriction. Additional studies are necessary to understand how GHR signaling in glutamatergic neurons regulates metabolism during food deprivation. Thus, the absence of GHR signaling in GABAergic and glutamatergic neuronal populations causes opposite metabolic effects during food deprivation.
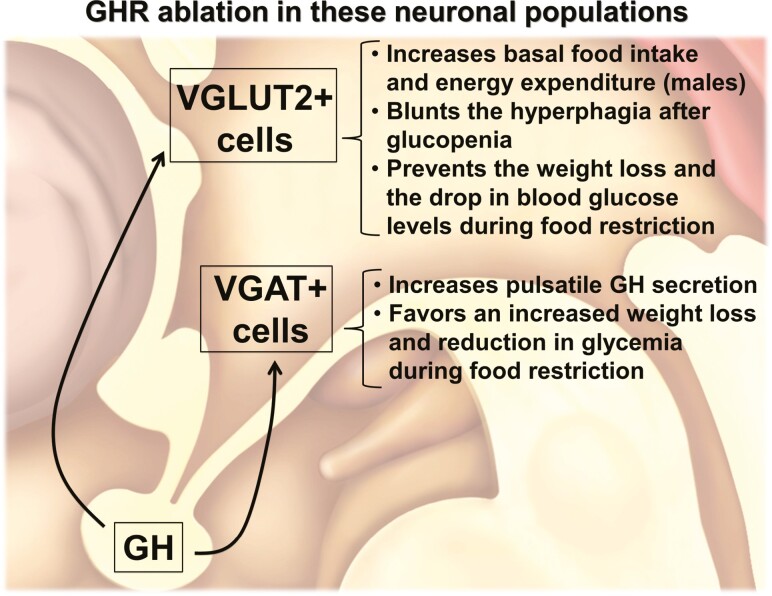
In summary, we reveal that distinct GABAergic and glutamatergic neuronal populations are responsive to GH. The autoregulation of GH secretion via short-loop feedback mechanisms is mediated by VGAT-expressing cells. In contrast, GHR signaling in VGAT and VGLUT2 neuronal populations modulates different aspects of metabolism. These findings advance the knowledge about the central components of the hypothalamic-GH neuroendocrine axis and help to identify the neurons involved in the biological effects of GH (Fig. 11).
Figure 11.
Diagram summarizing the major findings of the present study.
Acknowledgments
We thank Emily Henson from the Michigan Diabetes Center (NIH P30 DK020572, MICPC In Situ Hybridization Laboratory) for technical assistance, and Prof Martin Metzger for the critical reading of the article.
Glossary
Abbreviations
- 2DG
2-deoxy-D-glucose
- ACD
Advanced Cell Diagnostics
- AgRP
agouti-related protein
- ANOVA
analysis of variance
- ARH
arcuate nucleus
- DMH
dorsomedial nucleus of the hypothalamus
- ELISA
enzyme-linked immunosorbent assay
- GABA
γ-aminobutyric acid
- GFP
green fluorescent protein
- GH
growth hormone
- GHR
growth hormone receptor
- GHRH
growth hormone–releasing hormone
- IGF-1
insulin-like growth factor 1
- KO
knockout
- KPBS
potassium phosphate-buffered saline
- MEApd
posterodorsal part of medial nucleus of the amygdala
- mRNA
messenger RNA
- NIDDK-NHPP
National Institute of Diabetes and Digestive and Kidney Diseases–National Hormone and Pituitary Program
- NPY
neuropeptide Y
- PBS
phosphate-buffered saline
- PBS-T
PBS with 0.05% Tween-20
- PCR
polymerase chain reaction
- PMv
ventral premammillary nucleus
- POMC
proopiomelanocortin
- PV
periventricular nucleus
- PVH
paraventricular nucleus of the hypothalamus
- SST
somatostatin
- TH
tyrosine hydroxylase
- VGAT
vesicular inhibitory amino acid transporter
- VGLUT2
vesicular glutamate transporter 2
- VMH
ventromedial nucleus of the hypothalamus
- VO2
O2 consumption
Contributor Information
Willian O dos Santos, Department of Physiology and Biophysics, Instituto de Ciencias Biomedicas, Universidade de São Paulo, São Paulo, 05508-000, Brazil.
Frederick Wasinski, Department of Physiology and Biophysics, Instituto de Ciencias Biomedicas, Universidade de São Paulo, São Paulo, 05508-000, Brazil.
Mariana R Tavares, Department of Physiology and Biophysics, Instituto de Ciencias Biomedicas, Universidade de São Paulo, São Paulo, 05508-000, Brazil.
Ana M P Campos, Department of Physiology and Biophysics, Instituto de Ciencias Biomedicas, Universidade de São Paulo, São Paulo, 05508-000, Brazil.
Carol F Elias, Department of Molecular and Integrative Physiology, University of Michigan, Ann Arbor, Michigan, 48109-5622, USA.
Edward O List, Edison Biotechnology Institute and Heritage College of Osteopathic Medicine, Ohio University, Athens, Ohio, 45701, USA.
John J Kopchick, Edison Biotechnology Institute and Heritage College of Osteopathic Medicine, Ohio University, Athens, Ohio, 45701, USA.
Raphael E Szawka, Department of Physiology and Biophysics, Instituto de Ciencias Biologicas, Universidade Federal de Minas Gerais, Belo Horizonte, Minas Gerais, 31270-901, Brazil.
Jose Donato, Jr, Department of Physiology and Biophysics, Instituto de Ciencias Biomedicas, Universidade de São Paulo, São Paulo, 05508-000, Brazil.
Financial Support
This work was supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP/Brazil grant Nos. 2016/20897-3 to F.W., 2020/10102-9 to M.R.T., and 2020/01318-8 to J.D.J.); the Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG/Brazil grant No. CBB-APQ-03308-16 to R.E.S.); and the National Institutes of Health (NIH grant No. R01AG059779 to J.J.K. and E.O.L.; National Institute of Child Health and Human Development grant No. R01HD069702 to C.F.E.).
Disclosures
The authors have nothing to disclose.
Data Availability
The data that support the findings of this study are available from the corresponding author on reasonable request.
References
- 1. Hindmarsh P, Smith PJ, Brook CG, Matthews DR. The relationship between height velocity and growth hormone secretion in short prepubertal children. Clin Endocrinol (Oxf). 1987;27(5):581-591. Doi: 10.1111/j.1365-2265.1987.tb01188.x [DOI] [PubMed] [Google Scholar]
- 2. Finkelstein BS, Imperiale TF, Speroff T, Marrero U, Radcliffe DJ, Cuttler L. Effect of growth hormone therapy on height in children with idiopathic short stature: a meta-analysis. Arch Pediatr Adolesc Med. 2002;156(3):230-240. Doi: 10.1001/archpedi.156.3.230 [DOI] [PubMed] [Google Scholar]
- 3. Steyn FJ, Tolle V, Chen C, Epelbaum J. Neuroendocrine regulation of growth hormone secretion. Compr Physiol. 2016;6(2):687-735. Doi: 10.1002/cphy.c150002 [DOI] [PubMed] [Google Scholar]
- 4. Murray PG, Higham CE, Clayton PE. 60 years of neuroendocrinology: the hypothalamo-GH axis: the past 60 years. J Endocrinol. 2015;226(2):T123-T140. Doi: 10.1530/JOE-15-0120 [DOI] [PubMed] [Google Scholar]
- 5. Bertherat J, Bluet-Pajot MT, Epelbaum J. Neuroendocrine regulation of growth hormone. Eur J Endocrinol. 1995;132(1):12-24. Doi: 10.1530/eje.0.1320012 [DOI] [PubMed] [Google Scholar]
- 6. Müller EE, Locatelli V, Cocchi D. Neuroendocrine control of growth hormone secretion. Physiol Rev. 1999;79(2):511-607. Doi: 10.1152/physrev.1999.79.2.511 [DOI] [PubMed] [Google Scholar]
- 7. Ranke MB, Wit JM. Growth hormone—past, present and future. Nat Rev Endocrinol. 2018;14(5):285-300. Doi: 10.1038/nrendo.2018.22 [DOI] [PubMed] [Google Scholar]
- 8. Fodor M, Kordon C, Epelbaum J. Anatomy of the hypophysiotropic somatostatinergic and growth hormone-releasing hormone system minireview. Neurochem Res. 2006;31(2):137-143. Doi: 10.1007/s11064-005-9017-3 [DOI] [PubMed] [Google Scholar]
- 9. Burton KA, Kabigting EB, Clifton DK, Steiner RA. Growth hormone receptor messenger ribonucleic acid distribution in the adult male rat brain and its colocalization in hypothalamic somatostatin neurons. Endocrinology. 1992;131(2):958-963. Doi: 10.1210/endo.131.2.1353444 [DOI] [PubMed] [Google Scholar]
- 10. Huang L, Tan HY, Fogarty MJ, et al. Actions of NPY, and its Y1 and Y2 receptors on pulsatile growth hormone secretion during the fed and fasted state. J Neurosci. 2014;34(49):16309-16319. Doi: 10.1523/JNEUROSCI.4622-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bertherat J, Timsit J, Bluet-Pajot MT, et al. Chronic growth hormone (GH) hypersecretion induces reciprocal and reversible changes in mRNA levels from hypothalamic GH-releasing hormone and somatostatin neurons in the rat. J Clin Invest. 1993;91(4):1783-1791. Doi: 10.1172/JCI116389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nass R, Toogood AA, Hellmann P, et al. Intracerebroventricular administration of the rat growth hormone (GH) receptor antagonist G118R stimulates GH secretion: evidence for the existence of short loop negative feedback of GH. J Neuroendocrinol. 2000;12(12):1194-1199. Doi: 10.1046/j.1365-2826.2000.00586.x [DOI] [PubMed] [Google Scholar]
- 13. Pellegrini E, Bluet-Pajot MT, Mounier F, Bennett P, Kordon C, Epelbaum J. Central administration of a growth hormone (GH) receptor mRNA antisense increases GH pulsatility and decreases hypothalamic somatostatin expression in rats. J Neurosci. 1996;16(24):8140-8148. Doi: 10.1523/JNEUROSCI.16-24-08140.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Minami S, Kamegai J, Sugihara H, Suzuki N, Wakabayashi I. Growth hormone inhibits its own secretion by acting on the hypothalamus through its receptors on neuropeptide Y neurons in the arcuate nucleus and somatostatin neurons in the periventricular nucleus. Endocr J. 1998;45(Suppl):S19-S26. Doi: 10.1507/endocrj.45.suppl_s19 [DOI] [PubMed] [Google Scholar]
- 15. Wagner C, Caplan SR, Tannenbaum GS. Genesis of the ultradian rhythm of GH secretion: a new model unifying experimental observations in rats. Am J Physiol. 1998;275(6):E1046-E1054. Doi: 10.1152/ajpendo.1998.275.6.E1046 [DOI] [PubMed] [Google Scholar]
- 16. Kamegai J, Minami S, Sugihara H, Higuchi H, Wakabayashi I. Growth hormone induces expression of the c-fos gene on hypothalamic neuropeptide-Y and somatostatin neurons in hypophysectomized rats. Endocrinology. 1994;135(6):2765-2771. Doi: 10.1210/endo.135.6.7988469 [DOI] [PubMed] [Google Scholar]
- 17. Rogers KV, Vician L, Steiner RA, Clifton DK. The effect of hypophysectomy and growth hormone administration on pre-prosomatostatin messenger ribonucleic acid in the periventricular nucleus of the rat hypothalamus. Endocrinology. 1988;122(2):586-591. Doi: 10.1210/endo-122-2-586 [DOI] [PubMed] [Google Scholar]
- 18. Chomczynski P, Downs TR, Frohman LA. Feedback regulation of growth hormone (GH)-releasing hormone gene expression by GH in rat hypothalamus. Mol Endocrinol. 1988;2(3): 236-241. Doi: 10.1210/mend-2-3-236 [DOI] [PubMed] [Google Scholar]
- 19. de Gennaro Colonna V, Fidone F, Cocchi D, Müller EE. Feedback effects of growth hormone on growth hormone-releasing hormone and somatostatin are not evident in aged rats. Neurobiol Aging. 1993;14(5):503-507. Doi: 10.1016/0197-4580(93)90109-o [DOI] [PubMed] [Google Scholar]
- 20. Chan Y, Steiner R, Clifton D. Regulation of hypothalamic neuropeptide-Y neurons by growth hormone in the rat. Endocrinology. 1996;137(4):1319-1325. Doi: 10.1210/en.137.4.1319 [DOI] [PubMed] [Google Scholar]
- 21. Kamegai J, Minami S, Sugihara H, Hasegawa O, Higuchi H, Wakabayashi I. Growth hormone receptor gene is expressed in neuropeptide Y neurons in hypothalamic arcuate nucleus of rats. Endocrinology. 1996;137(5):2109-2112. Doi: 10.1210/endo.137.5.8612554 [DOI] [PubMed] [Google Scholar]
- 22. Furigo IC, Teixeira PDS, de Souza GO, et al. Growth hormone regulates neuroendocrine responses to weight loss via AgRP neurons. Nat Commun. 2019;10(1):662. Doi: 10.1038/s41467-019-08607-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wasinski F, Pedroso JAB, Dos Santos WO, et al. Tyrosine hydroxylase neurons regulate growth hormone secretion via short-loop negative feedback. J Neurosci. 2020;40(22):4309-4322. Doi: 10.1523/JNEUROSCI.2531-19.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Romanov RA, Zeisel A, Bakker J, et al. Molecular interrogation of hypothalamic organization reveals distinct dopamine neuronal subtypes. Nat Neurosci. 2017;20(2):176-188. Doi: 10.1038/nn.4462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Campbell JN, Macosko EZ, Fenselau H, et al. A molecular census of arcuate hypothalamus and median eminence cell types. Nat Neurosci. 2017;20(3):484-496. Doi: 10.1038/nn.4495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Henry FE, Sugino K, Tozer A, Branco T, Sternson SM. Cell type-specific transcriptomics of hypothalamic energy-sensing neuron responses to weight-loss. Elife. 2015;4:e09800. Doi: 10.7554/eLife.09800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Brown RS, Kokay IC, Phillipps HR, et al. Conditional deletion of the prolactin receptor reveals functional subpopulations of dopamine neurons in the arcuate nucleus of the hypothalamus. J Neurosci. 2016;36(35):9173-9185. Doi: 10.1523/JNEUROSCI.1471-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Toossi H, Del Cid-Pellitero E, Stroh T, Jones BE. Somatostatin varicosities contain the vesicular GABA transporter and contact orexin neurons in the hypothalamus. Eur J Neurosci. 2012;36(10):3388-3395. Doi: 10.1111/j.1460-9568.2012.08253.x [DOI] [PubMed] [Google Scholar]
- 29. Kiss J, Csaba Z, Csáki A, Halász B. Glutamatergic innervation of growth hormone-releasing hormone-containing neurons in the hypothalamic arcuate nucleus and somatostatin-containing neurons in the anterior periventricular nucleus of the rat. Brain Res Bull. 2006;70(4-6):278-288. Doi: 10.1016/j.brainresbull.2006.05.006 [DOI] [PubMed] [Google Scholar]
- 30. Hrabovszky E, Turi GF, Liposits Z. Presence of vesicular glutamate transporter-2 in hypophysiotropic somatostatin but not growth hormone-releasing hormone neurons of the male rat. Eur J Neurosci. 2005;21(8):2120-2126. Doi: 10.1111/j.1460-9568.2005.04076.x [DOI] [PubMed] [Google Scholar]
- 31. Farhy LS, Veldhuis JD. Joint pituitary-hypothalamic and intrahypothalamic autofeedback construct of pulsatile growth hormone secretion. Am J Physiol Regul Integr Comp Physiol. 2003;285(5):R1240-R1249. Doi: 10.1152/ajpregu.00086.2003 [DOI] [PubMed] [Google Scholar]
- 32. List EO, Berryman DE, Funk K, et al. The role of GH in adipose tissue: lessons from adipose-specific GH receptor gene-disrupted mice. Mol Endocrinol. 2013;27(3):524-535. Doi: 10.1210/me.2012-1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Furigo IC, Metzger M, Teixeira PD, Soares CR, Donato J Jr. Distribution of growth hormone-responsive cells in the mouse brain. Brain Struct Funct. 2017;222(1):341-363. Doi: 10.1007/s00429-016-1221-1 [DOI] [PubMed] [Google Scholar]
- 34. Wasinski F, Klein MO, Bittencourt JC, Metzger M, Donato J Jr. Distribution of growth hormone-responsive cells in the brain of rats and mice. Brain Res. 2021;1751:147189. Doi: 10.1016/j.brainres.2020.147189 [DOI] [PubMed] [Google Scholar]
- 35. Steyn FJ, Huang L, Ngo ST, et al. Development of a method for the determination of pulsatile growth hormone secretion in mice. Endocrinology. 2011;152(8):3165-3171. Doi: 10.1210/en.2011-0253 [DOI] [PubMed] [Google Scholar]
- 36. Vidal A, Zhang Q, Médigue C, Fabre S, Clément F. DynPeak: an algorithm for pulse detection and frequency analysis in hormonal time series. PLoS One. 2012;7(7):e39001. Doi: 10.1371/journal.pone.0039001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wasinski F, Chaves FM, Pedroso JAB, et al. Growth hormone receptor in dopaminergic neurones regulates stress-induced prolactin release in male mice. J Neuroendocrinol. 2021;33(3):e12957. Doi: 10.1111/jne.12957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Burton KA, Kabigting EB, Steiner RA, Clifton DK. Identification of target cells for growth hormone’s action in the arcuate nucleus. Am J Physiol. 1995;269(4 Pt 1):E716-E722. Doi: 10.1152/ajpendo.1995.269.4.E716 [DOI] [PubMed] [Google Scholar]
- 39. Vong L, Ye C, Yang Z, Choi B, Chua S, Lowell Bradford B. Leptin action on GABAergic neurons prevents obesity and reduces inhibitory tone to POMC neurons. Neuron. 2011;71(1):142-154. Doi: 10.1016/j.neuron.2011.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pu S, Jain MR, Horvath TL, Diano S, Kalra PS, Kalra SP. Interactions between neuropeptide Y and gamma-aminobutyric acid in stimulation of feeding: a morphological and pharmacological analysis. Endocrinology 1999;140(2):933-940. Doi: 10.1210/endo.140.2.6495 [DOI] [PubMed] [Google Scholar]
- 41. Quaresma PGF, Teixeira PDS, Furigo IC, et al. Growth hormone/STAT5 signaling in proopiomelanocortin neurons regulates glucoprivic hyperphagia. Mol Cell Endocrinol. 2019;498:110574. Doi: 10.1016/j.mce.2019.110574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Quaresma PGF, Dos Santos WO, Wasinski F, Metzger M, Donato J Jr. Neurochemical phenotype of growth hormone-responsive cells in the mouse paraventricular nucleus of the hypothalamus. J Comp Neurol. 2021;529(6):1228-1239. Doi: 10.1002/cne.25017 [DOI] [PubMed] [Google Scholar]
- 43. Furigo IC, de Souza GO, Teixeira PDS, et al. Growth hormone enhances the recovery of hypoglycemia via ventromedial hypothalamic neurons. FASEB J. 2019;33(11):11909-11924. Doi: 10.1096/fj.201901315R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Declercq J, Brouwers B, Pruniau VPEG, et al. Metabolic and behavioural phenotypes in Nestin-Cre mice are caused by hypothalamic expression of human growth hormone. PLoS One. 2015;10(8):e0135502. Doi: 10.1371/journal.pone.0135502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Viollet C, Simon A, Tolle V, et al. Somatostatin-IRES-Cre mice: between knockout and wild-type? Front Endocrinol (Lausanne). 2017;8:131. Doi: 10.3389/fendo.2017.00131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chaves FM, Wasinski F, Tavares MR, et al. Effects of the isolated and combined ablation of growth hormone and IGF-1 receptors in somatostatin neurons. Endocrinology. 2022;163(5):bqac045. Doi: 10.1210/endocr/bqac045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Teixeira PDS, Couto GC, Furigo IC, List EO, Kopchick JJ, Donato J Jr. Central growth hormone action regulates metabolism during pregnancy. Am J Physiol Endocrinol Metab. 2019;317(5): E925-E940. Doi: 10.1152/ajpendo.00229.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wasinski F, Barrile F, Pedroso JAB, et al. Ghrelin-induced food intake, but not GH secretion, requires the expression of the GH receptor in the brain of male mice. Endocrinology. 2021;162(7):bqab097. Doi: 10.1210/endocr/bqab097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. de Lima JBM, Debarba LK, Rupp AC, et al. ARCGHR neurons regulate muscle glucose uptake. Cells. 2021;10(5):1093. Doi: 10.3390/cells10051093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Phelps CJ, Romero MI, Hurley DL. Growth hormone-releasing hormone-producing and dopaminergic neurones in the mouse arcuate nucleus are independently regulated populations. J Neuroendocrinol. 2003;15(3):280-288. Doi: 10.1046/j.1365-2826.2003.01009.x [DOI] [PubMed] [Google Scholar]
- 51. Bouyer K, Loudes C, Robinson ICAF, Epelbaum J, Faivre-Bauman A. Multiple co-localizations in arcuate GHRH-eGFP neurons in the mouse hypothalamus. J Chem Neuroanat. 2007;33(1):1-8. Doi: 10.1016/j.jchemneu.2006.10.002 [DOI] [PubMed] [Google Scholar]
- 52. Zhang X, van den Pol AN. Dopamine/Tyrosine hydroxylase neurons of the hypothalamic arcuate nucleus release GABA, communicate with dopaminergic and other arcuate neurons, and respond to dynorphin, Met-enkephalin, and oxytocin. J Neurosci. 2015;35(45):14966-14982. Doi: 10.1523/JNEUROSCI.0293-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Díaz-Torga G, Feierstein C, Libertun C, et al. Disruption of the D2 dopamine receptor alters GH and IGF-I secretion and causes dwarfism in male mice. Endocrinology. 2002;143(4):1270-1279. Doi: 10.1210/endo.143.4.8750 [DOI] [PubMed] [Google Scholar]
- 54. Noaín D, Pérez-Millán MI, Bello EP, et al. Central dopamine D2 receptors regulate growth-hormone-dependent body growth and pheromone signaling to conspecific males. J Neurosci. 2013;33(13):5834-5842. Doi: 10.1523/JNEUROSCI.5673-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lamberts SW, Macleod RM. Regulation of prolactin secretion at the level of the lactotroph. Physiol Rev. 1990;70(2):279-318. Doi: 10.1152/physrev.1990.70.2.279 [DOI] [PubMed] [Google Scholar]
- 56. Grattan DR. 60 years of neuroendocrinology: the hypothalamo-prolactin axis. J Endocrinol. 2015;226(2):T101-T122. Doi: 10.1530/JOE-15-0213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Oshida K, Vasani N, Waxman DJ, Corton JC. Disruption of STAT5b-regulated sexual dimorphism of the liver transcriptome by diverse factors is a common event. PLoS One. 2016;11(3):e0148308. Doi: 10.1371/journal.pone.0148308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Liu Z, Han T, Fishman S, et al. Ablation of hepatic production of the acid-labile subunit in bovine-GH transgenic mice: effects on organ and skeletal growth. Endocrinology. 2017;158(8):2556-2571. Doi: 10.1210/en.2016-1952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. List EO, Berryman DE, Funk K, et al. Liver-specific GH receptor gene-disrupted (LiGHRKO) mice have decreased endocrine IGF-I, increased local IGF-I, and altered body size, body composition, and adipokine profiles. Endocrinology. 2014;155(5):1793-1805. Doi: 10.1210/en.2013-2086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Garcia-Galiano D, Cara AL, Tata Z, et al. ERα signaling in GHRH/Kiss1 dual-phenotype neurons plays sex-specific roles in growth and puberty. J Neurosci. 2020;40(49):9455-9466. Doi: 10.1523/JNEUROSCI.2069-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Donato J Jr, Wasinski F, Furigo IC, Metzger M, Frazão R. Central regulation of metabolism by growth hormone. Cells. 2021;10(1):129. Doi: 10.3390/cells10010129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Cady G, Landeryou T, Garratt M, et al. Hypothalamic growth hormone receptor (GHR) controls hepatic glucose production in nutrient-sensing leptin receptor (LepRb) expressing neurons. Mol Metab. 2017;6(5):393-405. Doi: 10.1016/j.molmet.2017.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Egecioglu E, Bjursell M, Ljungberg A, et al. Growth hormone receptor deficiency results in blunted ghrelin feeding response, obesity, and hypolipidemia in mice. Am J Physiol Endocrinol Metab. 2006;290(2):E317-E325. Doi: 10.1152/ajpendo.00181.2005 [DOI] [PubMed] [Google Scholar]
- 64. Hentges ST, Otero-Corchon V, Pennock RL, King CM, Low MJ. Proopiomelanocortin expression in both GABA and glutamate neurons. J Neurosci. 2009;29(43):13684-13690. Doi: 10.1523/JNEUROSCI.3770-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wittmann G, Hrabovszky E, Lechan RM. Distinct glutamatergic and GABAergic subsets of hypothalamic pro-opiomelanocortin neurons revealed by in situ hybridization in male rats and mice. J Comp Neurol. 2013;521(14):3287-3302. Doi: 10.1002/cne.23350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Furigo IC, Teixeira PDS, Quaresma PGF, Mansano NS, Frazão R, Donato J. STAT5 ablation in AgRP neurons increases female adiposity and blunts food restriction adaptations. J Mol Endocrinol. 2020;64(1):13-27. Doi: 10.1530/JME-19-0158 [DOI] [PubMed] [Google Scholar]
- 67. Zhao TJ, Liang G, Li RL, et al. Ghrelin O-acyltransferase (GOAT) is essential for growth hormone-mediated survival of calorie-restricted mice. Proc Natl Acad Sci U S A. 2010;107(16):7467-7472. Doi: 10.1073/pnas.1002271107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Li RL, Sherbet DP, Elsbernd BL, Goldstein JL, Brown MS, Zhao TJ. Profound hypoglycemia in starved, ghrelin-deficient mice is caused by decreased gluconeogenesis and reversed by lactate or fatty acids. J Biol Chem. 2012;287(22):17942-17950. Doi: 10.1074/jbc.M112.358051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Könner AC, Janoschek R, Plum L, et al. Insulin action in AgRP-expressing neurons is required for suppression of hepatic glucose production. Cell Metab. 2007;5(6):438-449. Doi: 10.1016/j.cmet.2007.05.004 [DOI] [PubMed] [Google Scholar]
- 70. Farias Quipildor G, Mao K, Beltran PJ, Barzilai N, Huffman DM. Modulation of glucose production by central insulin requires IGF-1 receptors in AgRP neurons. Diabetes. 2021;70(10):2237-2249. Doi: 10.2337/db21-0028 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author on reasonable request.