Abstract
Recent studies employing reporter gene technology indicate that the availabilities of the major nutrients nitrogen, phosphate, and iron to Pseudomonas are not severely limited in bulk soil. Indirect evidence has pointed to carbon limitation as a severe nutritional stress in this environment. We show that a plasmid (pGM115)-borne transcriptional fusion between the ςS-dependent Escherichia coli promoter Pfic and lacZ functions as a reliable reporter for carbon availability in Pseudomonas fluorescens. When P. fluorescens strain DF57(pGM115) was introduced into bulk soil, carbon-limiting conditions were indicated by citrate-repressible induction of β-galactosidase activity. To address carbon availability at the single-cell level, we developed an immunofluorescence double-staining procedure for individual DF57 cells expressing β-galactosidase from Pfic. Changes in cell size and expression of β-galactosidase were analyzed by flow cytometry. Cells extracted from soil microcosms reduced their size less than carbon-starved cells in pure culture and showed an increased tendency to aggregate. The single-cell analysis revealed that for cells residing in soil, the expression of β-galactosidase became heterogeneous and only a DF57 subpopulation appeared to be carbon limited. In soil amended with barley straw, limited nitrogen availability has been determined by use of the bioluminescent reporter strain P. fluorescens DF57-N3. We used strain DF57-N3(pGM115) as a double reporter for carbon and nitrogen limitation that allowed us to study the dynamics of carbon and nitrogen availabilities in more detail. In straw-amended soil β-galactosidase activity remained low, while nitrogen limitation-dependent bioluminescence appeared after a few days. Hence, nitrogen became limited under conditions where carbon resources were not completely exhausted.
The genus Pseudomonas comprises an important group of bacteria with environmental applications in bioremediation and biological control (4, 36). It is often assumed that bacteria inoculated into soil experience feast and famine conditions, where periods of apparent inactivity fluctuate with periods of sporadic growth due to small amounts of heterogeneously distributed nutrients and energy sources (39). During the transition from growth to inactivity, pseudomonads induce stress resistance (6, 31, 40) and increase the production of secondary metabolites active against plant pathogens (4, 31) as well as the ability to degrade xenobiotics (1, 34).
Indirect evidence suggests that carbon limitation affects pseudomonads in bulk soil. Hence, Van Overbeek et al. (40) found that Pseudomonas fluorescens strain R2f developed a stress-resistant state in the soil. This stress resistance was comparable to that developed by starved cells in culture, and it could be prevented by the addition of glucose to the soil. During recent years direct information on the availability of major nutrients to pseudomonads has been obtained by reporter gene technology. Studies exploiting whole-cell biosensors, which assess the biologically relevant nutrient pools in the soil, have revealed that the availabilities of phosphate, nitrogen, and iron to pseudomonads are not severely limited in natural bulk soil (13, 14, 20, 22). However, enrichment of the soil with plant residues may lead to nitrogen limitation of an introduced pseudomonad (14). Consequently, the proliferation of pseudomonads in the heterogeneous soil environment appears to be limited by dynamic changes in the carbon and nitrogen availabilities. Knowledge about the temporal and spatial variability in available carbon and nitrogen sources may thus improve our understanding of the fate of Pseudomonas inoculants in the soil. However, a reliable construct for determination of carbon availability is so far lacking, as the expression from the only published reporter is very weak and not entirely specific for carbon limitation (41).
In Pseudomonas the global gene regulator ςS is induced during entry into stationary phase (26, 31), and ςS influences, e.g., stress survival, antibiotic production (31), and alkane degradation (1). Hence, a reporter system for ςS-dependent gene expression might be useful for studies of feast and famine conditions encountered by Pseudomonas in the soil. The promoter of the Escherichia coli fic (for filamentation induced by cyclic AMP) gene appears to be involved in regulation of cell division (18). The fic promoter, hereafter referred to as Pfic, was later shown to be recognized preferentially by ςS and, apparently, does not require additional transcription factors (35, 38). This promoter is also reported to be ςS dependent in Pseudomonas (26).
In the present work we transferred the plasmid pGM115, carrying a transcriptional fusion between Pfic and lacZ, to P. fluorescens strain DF57. Pure-culture studies as well as soil experiments showed that this reporter responded specifically to carbon limitation. Moreover, an immunochemical double-staining procedure for DF57(pGM115) and analysis by flow cytometry provided data on the expression level in individual cells extracted from natural soil. To address dynamic changes in the carbon and nitrogen availabilities during straw degradation in the soil, we used a double reporter for carbon and nitrogen limitation. This was obtained by the transfer of pGM115 to strain DF57-N3, which harbors a chromosomal Tn5::luxAB reporter system induced by N limitation (14, 19).
MATERIALS AND METHODS
Bacterial strains and plasmids.
P. fluorescens DF57 (ampicillin resistant) was isolated from cucumber rhizosphere (32). P. fluorescens DF57-N3 carries a chromosomal Tn5::luxAB fusion to a promoter responding to nitrogen limitation (14, 19). The strain is resistant to ampicillin, kanamycin, and streptomycin. E. coli DH5α is the initial host for the broad-host-range plasmids pGM115 and pGM118 (23). pGM115 carries a lacZ gene controlled by Pfic, while pGM118 lacks a promoter region upstream of lacZ, and expression of β-galactosidase from this plasmid reflects the background transcriptional activity of the vector (23). Both plasmids carry resistance to kanamycin and chloramphenicol.
Media and growth conditions.
P. fluorescens strains were cultured at 20 or 28°C in Davis minimal medium (DMM) or Luria-Bertani broth (LB) as previously described (20), while E. coli DH5α was cultured in LB at 37°C. Growth of the cultures was measured as optical density at 600 nm (OD600) with a Shimadzu UV-160A spectrophotometer. For growth in liquid media, 25 μg of kanamycin ml−1 was added to cultures of DF57 and DH5α carrying pGM115 or pGM118, while 25 μg of kanamycin ml−1 and 30 μg of chloramphenicol ml−1 were added to cultures of DF57-N3(pGM115) and DF57-N3(pGM118). To obtain conditions of carbon, nitrogen, or phosphorus starvation, exponentially growing cells were harvested at an OD600 of 0.2 to 0.5, washed twice in the relevant starvation medium (DMM without either a carbon, nitrogen, or phosphate source as described previously [21]), and then resuspended in starvation medium at the original cell density. To obtain osmotic stress conditions, the relevant strain was cultured and harvested as described above. The cells were washed twice in DMM containing 2.5% (wt/vol) NaCl and then resuspended in this medium at an OD600 of 0.2 to 0.5. At this salt concentration DF57 was still able to grow, but at a reduced rate.
Plasmid purification and electroporation.
pGM115 and pGM118 were purified from E. coli DH5α using a plasmid purification kit (Qiagen GmbH, Hilden, Germany) and transferred to DF57 and DF57-N3 by electroporation as previously described (12). The presence of plasmids in transformants was confirmed on selective LB agar plates containing 0.01% (vol/vol) X-Gal (5-chloro-4-bromo-3-indolyl-β-d-galactopyranoside).
Soil microcosms.
The soil used was a loamy sand (pH 6.1) collected from a field cropped with barley at the Royal Veterinary and Agricultural University, Tåstrup, Denmark. The nutrient contents of the soil (in milligrams kilogram of dry matter−1) were as follows: NH4-N, 0.1; NO3-N, 34; total P, 5.8; and total organic C, 9,500. Surface soil (10 to 30 cm) was collected and stored in plastic bags at 4°C. Prior to use, soil was passed through a 4-mm mesh sieve.
The soil was either unamended or amended with carbon (720 mg of C kg−1 formulated as Na-citrate) or with 2.5% (wt/wt) ground barley straw (diameter, <2 mm). The nutrient contents of the straw (in milligrams kilogram of dry matter−1) for the water-soluble fraction were as follows: NH4-N, 49.8; NO3-N and NO2-N, 366; total N, 585; total P, 1,411; and total C, 31,142. The nutrient contents for the water-insoluble fraction were as follows: total N, 4,944; total P, 764; and total C, 458,550.
The soil was packed loosely in petri dishes and inoculated by spraying a cell suspension onto the soil, thereby bringing the water content to 15% (wt/wt), i.e., 60% of the field capacity of this soil. Bacterial inocula consisted of exponentially growing cells harvested at an OD600 of 0.3 to 0.5 and washed twice in 0.9% NaCl. The microcosms were incubated at 20°C.
Determination of β-galactosidase activity with o-nitrophenol β-galactoside as a substrate.
For pure cultures grown at 20°C, β-galactosidase activity was determined as described by Miller (24). Each β-galactosidase activity was calculated from four replicate samples and expressed in Miller units.
For soil microcosms, samples of 0.5 to 0.6 g soil were suspended in 5 to 6 ml of 0.9% NaCl. After vortexing, two samples (1 ml each) of the slurry were centrifuged (20,000 × g, 5 min, room temperature), and the pellets were resuspended in two 1-ml portions of Z-buffer (24). The cells were permeabilized with chloroform-sodium dodecyl sulfate as described by Miller (24), and o-nitrophenol β-galactoside was added to one sample while the other served as a negative control. After incubation at 30°C, the assays were terminated by the addition of 1 M Na2CO3. Subsequently, soil particles were removed by centrifugation as described above, and the OD420 of the supernatant was measured. In experiments where both β-galactosidase activity and bioluminescence were determined, soil particles were initially sedimented by an additional centrifugation at 500 × g for 1 min. The β-galactosidase activities from uninoculated or citrate-amended soils were below the detection limit of the assay. In straw-amended soil the background was approximately 0.02 A420 unit per h, and this background was subtracted when calculating the activities from DF57(pGM115) or DF57(pGM118).
Enzyme activities (A420 units) were related to DF57 cell numbers determined as CFU. In the equation for determining Miller units (24), the measured OD600 value was replaced by a calculated value based on the CFU in the sample and the conversion factor between cell numbers and OD600 for a DF57 pure culture (an OD600 of 1 equals 1.5 × 109 cells ml−1). Hence, in the modified equation CFU ml−1 × (1.5 × 109)−1 replaced the OD600 value. The cell-specific enzyme activities were consequently expressed as 1,000 × A420/hour × CFU × (6.7 × 10−10), referred to as modified Miller units. P. fluorescens DF57(pGM115) or DF57(pGM118) was plated on LB agar containing 25 μg of kanamycin ml−1. P. fluorescens DF57-N3(pGM115) or DF57-N3(pGM118) was plated on LB agar containing 25 μg of kanamycin ml−1 and 0.01% (vol/vol) X-Gal, as this medium allowed the stability of the plasmid to be assessed. Plasmid loss, indicated by the appearance of white colonies, was very rare, as DF57-N3 cells without plasmid always accounted for fewer than 1% of the culturable population. At the dilutions used in these experiments, no background from indigenous soil bacteria was observed on the plates.
Determination of β-galactosidase activity with Galactone-Plus as a substrate.
Determination of β-galactosidase activity with Galactone-Plus (Tropix, Inc., Bedford, Mass.) as a substrate was carried out for citrate-amended and unamended microcosms inoculated with ≈5 × 105 CFU g of soil−1. For soil experiments, samples of 0.7 g of soil were suspended in 7 ml of 0.9% NaCl. The slurry was vortexed for 1 min and centrifuged at 500 × g for 1 min to settle soil particles. The supernatant was then centrifuged (6,000 × g, 7 min), and the pellet containing bacterial cells was resuspended in 100 μl of Z-buffer and permeabilized with chloroform-sodium dodecyl sulfate as described above. The chemiluminescence assay and detection of chemiluminescence were carried out as recommended by the manufacturer. Chemiluminescence was measured using a Bio-Orbit 1252 (Struers Kebo Lab, Albertslund, Denmark) luminometer. β-Galactosidase activity from the indigenous soil bacteria was subtracted when calculating the cell-specific activity of DF57(pGM115).
Due to the lower inoculum, it was important to eliminate growth of indigenous bacteria on the selective media. This was obtained by determining CFU of DF57-N3(pGM115) and DF57-N3(pGM118) on LB agar plates containing 25 μg of kanamycin ml−1, 25 μg of streptomycin ml−1, 100 μg of ampicillin ml−1, and 0.01% (vol/vol) X-Gal. Nystatin (50 μg ml−1) was added as a fungicide. A control experiment showed that comparable cell counts of DF57-N3 were obtained with this medium and LB agar containing 25 μg of kanamycin ml−1 and 0.01% (vol/vol) X-Gal.
Purification of soil bacteria for flow cytometric analysis.
Within 5 to 10 min of sampling, soil extracts prepared as described above were fixed for 1 h on ice with buffered formaldehyde (1% final concentration). Subsequently, bacteria were purified from the extracts by density gradient centrifugation essentially as described previously (37). Nycodenz (Nycomed Pharma, Oslo, Norway) with a density of 1.3 g ml−1 was injected below the fixed soil extracts. After a centrifugation step (10,000 × g, 30 min, 4°C) the bacteria at the top of the Nycodenz layer were carefully pipetted off and resuspended in sterile 0.9% NaCl. The purified bacteria were then centrifuged (6,000 × g, 10 min, 4°C), resuspended in buffered formaldehyde (1% final concentration), and incubated overnight at 5°C to further permeabilize the cells. On the next day, the formaldehyde was removed from the cells by three washes (6,000 × g, 5 min, room temperature) in phosphate-buffered saline.
Immunofluorescence double-labeling procedure.
Fixed cells were immunofluorescence labeled after a washing step according to the general approach and with the buffers described by Worm et al. (42). A mixture of primary antibodies (a polyclonal rabbit antibody toward E. coli β-galactosidase [Molecular Probes, Leiden, The Netherlands] and a polyclonal mouse antibody toward outer membrane lipopolysaccharide molecules of DF57 [7]) were added in final dilutions of 1:1,000 and incubated overnight at 37°C. Following a washing step, a mixture of secondary antibodies conjugated with fluorochromes was added at a 1:100 dilution and incubated for at least 3 h at 37°C. The secondary antibodies were F(ab)2 fragments of goat anti-rabbit immunoglobulin G conjugated with Alexa Fluor 488 (Molecular Probes) and F(ab)2 fragments of goat anti-mouse immunoglobulin G conjugated with R-phycoerythrin (Dako, Glostrup, Denmark).
Flow cytometry.
Immunolabeled DF57(pGM115) and DF57(pGM118) were analyzed with a FacsCalibur (Becton Dickinson, Paramus, N.J.) flow cytometer equipped with a 488-nm laser and detectors for forward scatter (FSC) and side scatter and for green (FL1), orange (FL2), and red (FL3) fluorescence. Cell surface staining of DF57 with R-phycoerythrin-conjugated antibodies (red) was detected in a plot of FL2 versus FSC. The intracellular β-galactosidase stained with Alexa Fluor 488-conjugated antibodies (green) was detected by the FL1 channel. The amplifications of the FL1 and FL2 detectors were adjusted to 800 and 600 mV, respectively. These levels of amplification were applied to measure green fluorescence with a high sensitivity and at the same time reduce crossover of fluorescence from R-phycoerythrin by compensating the FL1 signal for 15% of the FL2 signal.
Measurement of bioluminescence.
Bioluminescence from cells in soil suspensions was determined by luminometry as previously described (14).
RESULTS
Characterization of the Pfic in P. fluorescens DF57.
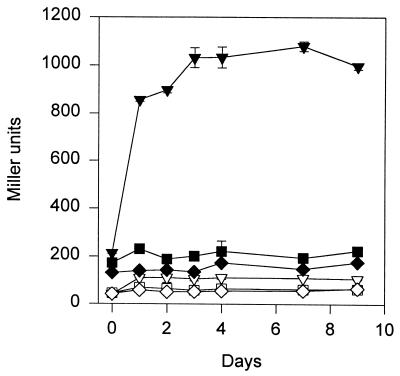
To generate a reporter system for ςS-dependent gene expression in P. fluorescens, the broad-host-range plasmid pGM115, carrying the lacZ gene controlled by Pfic, was transferred to P. fluorescens strain DF57 by electroporation. The Pfic-lacZ reporter system in DF57 was induced during entry into the stationary phase in both a rich medium (LB) (data not shown) and a defined medium (DMM) (Table 1). Bacteria enter the stationary phase if the medium is totally exhausted of an essential nutrient. We therefore examined the expression from Pfic during carbon, nitrogen, and phosphorous limitations. As shown in Fig. 1, a fivefold induction of β-galactosidase activity was seen after a shift to carbon-free medium, while no induction occurred during N or P limitation. Results for the control strain DF57(pGM118) demonstrated that the background transcriptional activity of the vector was low and relatively constant (Fig. 1). Our results further indicate that Pfic is not activated in growing DF57(pGM115) cells exposed to osmotic stress (Table 1). Hence, the expression of β-galactosidase from Pfic in P. fluorescens DF57(pGM115) seems to be a specific response to carbon limitation.
TABLE 1.
lacZ expression from the E. coli fic promoter in P. fluorescens DF57(pGM115) grown to stationary phase in minimal medium with and without osmotic stress
| Medium and time (h) | OD600 | Miller unitsa |
|---|---|---|
| DMM | ||
| 0b | 0.33 | 72 ± 3.4 |
| 3b | 0.71 | 163 ± 6.4 |
| 4.25b | 0.79 | 171 ± 6.4 |
| 24c | 3.27 | 828 ± 175.8 |
| DMM containing 2.5% NaCl | ||
| 0 | 0.36 | 59 ± 0.2 |
| 3 | 0.47 | 59 ± 4.9 |
| 4.25 | 0.53 | 63 ± 2.6 |
| 24 | 3.01 | 276 ± 43.0 |
Values represent the means of three replicates ± standard errors. The slightly lower enzyme activity at time zero in samples containing 2.5% NaCl compared to the control might be due to inhibition of β-galactosidase activity by NaCl present in the assay mixture.
Exponential phase.
Stationary phase.
FIG. 1.
Expression of β-galactosidase activity from the reporter strain P. fluorescens DF57(pGM115) (closed symbols) and the control strain P. fluorescens DF57(pGM118) (open symbols) during carbon (▾, ▿), nitrogen (▪, □), and phosphate (⧫, ◊) starvation. Data are mean values from a representative experiment performed in triplicate. Standard deviations are shown as bars. The experiment has been independently repeated twice.
The plasmids pGM115 and pGM118 were subsequently transferred to strain DF57-N3. This strain carries a chromosomal Tn5::luxAB reporter system induced by N limitation (14, 19). By transferring pGM115 to DF57-N3, we obtained a unique double-reporter system allowing us to address relative changes in the carbon and nitrogen availabilities to Pseudomonas by parallel measurements of β-galactosidase activity and bioluminescence. Expression of β-galactosidase activity from pGM115 and pGM118 in DF57-N3 during carbon starvation was the same as for the wild-type strain (data not shown).
Effects of citrate amendment on expression of the Pfic-lacZ reporter system in soil.
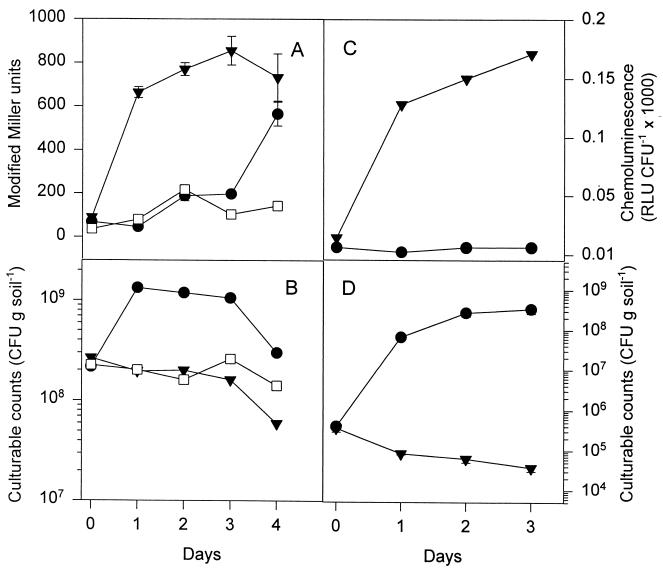
DF57(pGM115) and the control strain DF57(pGM118) were introduced into natural bulk soil at a density of ca. 108 CFU per g of soil. The reporter strain showed an approximately sevenfold induction of β-galactosidase activity (from ca. 100 modified Miller units to ca. 700 modified Miller units) within 2 days and maintained this high level throughout the experiment (Fig. 2A). The control strain DF57(pGM118) expressed a low and relatively stable β-galactosidase activity in the soil (50 to 100 modified Miller units) during the experiment. The populations of both strains showed a slight decline during the experimental period (Fig. 2B).
FIG. 2.
Expression of the Pfic-lacZ reporter system of P. fluorescens DF57 introduced into natural soil and natural soil amended with citrate. (A and C) β-Galactosidase activities from experiments performed with high and low cell densities of the reporter. (B and D) Population dynamics of strain DF57 in the same experiments. ▾, reporter strain DF57(pGM115) in natural soil; ●, DF57(pGM115) in citrate-amended soil; □, control strain DF57(pGM118) in natural soil. RLU, relative light units. Data are mean values from a representative experiment performed in triplicate; standard deviations are shown as bars. The experiment was repeated independently twice.
To test if the induction of the Pfic-lacZ reporter system in soil could be explained as a response to carbon limitation, the soil was amended with citrate prior to the introduction of DF57(pGM115). In citrate-amended soil, the increase in β-galactosidase activity was delayed (Fig. 2A) and occurred at a time when the cell population was declining after an initial growth response (Fig. 2B).
Strains carrying reporter gene constructs are often introduced into soil in very high cell numbers. To rule out artifacts caused by the large inocula, we determined β-galactosidase activity of DF57(pGM115) after introduction of ca. 5 × 105 CFU per g of soil, as this inoculum is comparable to the natural population of fluorescent pseudomonads in soil (17). To increase sensitivity, a chemiluminescent substrate was used for the β-galactosidase measurements. In these experiments we also observed an induction of β-galactosidase activity in soil which could be prevented (or delayed) by addition of citrate (Fig. 2C and D). Hence, we conclude that the ςS-dependent Pfic-lacZ reporter system in DF57 responds to carbon limitation even in the soil environment.
Flow cytometric analysis of expression of the Pfic-lacZ reporter system in individual cells.
To determine the heterogeneity in the carbon limitation response of DF57(pGM115) in soil, we developed an immunofluorescence double-staining procedure for individual DF57 cells expressing the Pfic-lacZ reporter system. Changes in cell size and in expression of β-galactosidase were then compared by flow cytometry for cells incubated in DMM without carbon and for cells extracted from natural soil by a density gradient centrifugation technique, which routinely recovered 50% ± 7% of the added cells (n = 4).
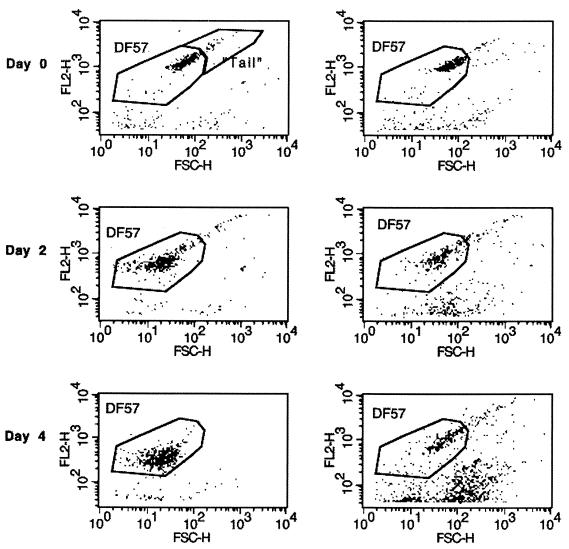
Immunofluorescence-labeled DF57 cells from pure cultures or extracted from soil microcosms were specifically detected by the flow cytometer. Figure 3 shows scatter plots of FL2-H (cell-specific immunostaining) versus FSC-H (FSC reflecting cell size [29]). For pure cultures, an approximately threefold reduction in the FSC-H signal indicated a decrease in cell size during incubation in DMM without carbon for 4 days. Note the region DF57, which is used to gate mainly for single cells in the subsequent analyses. The tail region comprises mainly cell aggregates. At day 0, 88% of the observations fell within the DF57 region, and this increased to 99% after 4 days, indicating a reduced tendency of the cells to form aggregates. In contrast, DF57 cells extracted from soil microcosms maintained a larger cell size and showed an increased tendency to form aggregates, as only ca. 65% of the observations fell within the DF57 region after 4 days in soil. Unspecific staining or autofluorescence of particles in the soil extracts was observed but could clearly be distinguished from cell staining due to a 10-fold difference in FL2-H fluorescence intensity (Fig. 3, right panels).
FIG. 3.
Flow cytometer analysis of P. fluorescens DF57(pGM115) incubated in DMM without carbon (left panels) or introduced into natural soil (right panels). DF57 was detected by indirect immunofluorescence labeling using an R-phycoerythrin-conjugated secondary antibody (orange fluorescence, FL2-H) and by FSC (FSC-H). The region DF57 encloses most registrations in DMM without carbon and is used to gate for single cells of DF57. The tail region contains cell aggregates. The experiment has been repeated independently twice.
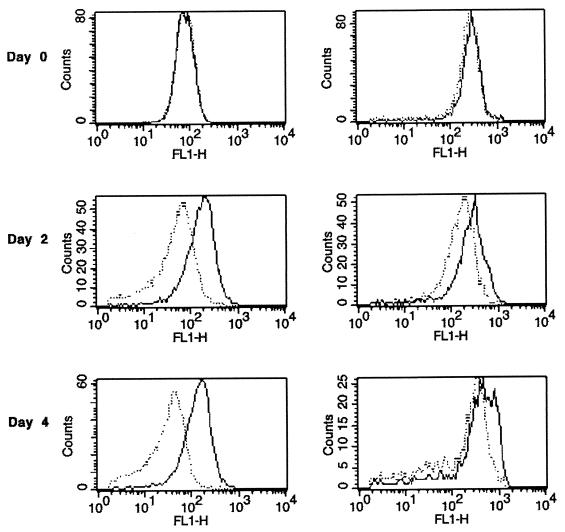
Just after transfer of exponentially growing cells to DMM without carbon, the distributions of immunofluorescence signals from β-galactosidase, referred to as FL1-H distributions below, were similar for the reporter strain DF57(pGM115) and the control strain DF57(pGM118). This is shown by a histogram presentation in Fig. 4, left panels. After 2 days or more of carbon starvation, however, the signal from DF57(pGM115) increased while that from DF57(pGM118) decreased, leading to a ca. fivefold difference in average expression level at day 4. A comparable analysis of cells extracted from natural soil revealed that the FL1-H distributions were comparable for strains DF57(pGM115) and DF57(pGM118) at day 0 (Fig. 4, right panels). With time the distributions separated, but less than in the pure-culture experiment (compare left and right panels of Fig. 4). At day 4 the expression of β-galactosidase from the reporter strain DF57(pGM115) became more heterogeneous. The FL1-H distribution for this strain became broader than that observed for the control strain and for both strains in pure culture. Hence, the single-cell analysis of the Pfic-lacZ reporter system showed that the expression of the carbon limitation response was more heterogeneous in natural soil than in pure culture and that a lower proportion of the DF57 cells appeared to be carbon limited in the soil than under pure culture conditions.
FIG. 4.
Flow cytometer analysis of expression of β-galactosidase by P. fluorescens DF57(pGM115) and DF57(pGM118) during incubation in DMM without carbon (left panels) and after inoculation into natural soil (right panels). DF57 was detected by indirect immunofluorescence labeling using an R-phycoerythrin-conjugated secondary antibody and by FSC. Only cells within the gated region DF57 are shown. β-Galactosidase was detected by indirect immunofluorescence labeling using an Alexa Fluor 488-conjugated secondary antibody (green fluorescence, FL1-H). Registrations were made for the reporter strain DF57(pGM115) (solid lines) and the control strain DF57(pGM118) (dotted lines). Data from representative experiments are shown. All experiments have been independently repeated twice.
Performance of a double reporter for carbon and nitrogen limitation in straw-amended soil.
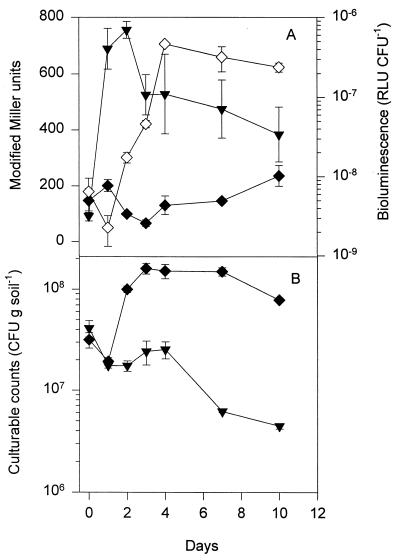
Straw contains a small fraction of soluble carbon compounds, including sugars and amino acids, while the majority of the carbon content is insoluble in water. When the double-reporter strain DF57-N3(pGM115) was introduced into natural soil amended with 2.5% barley straw at a density of 4 × 107 CFU g of soil−1, the carbon limitation signal (β-galactosidase activity) remained at the baseline level throughout the experiment, while the expression of bioluminescence, reporting nitrogen limitation, was induced ca. 100-fold after 2 days (Fig. 5). In unamended soil, the carbon limitation reporter was induced, while bioluminescence from the nitrogen limitation reporter was below the detection limit. The DF57-N3(pGM115) population declined slightly in natural soil, while it increased during the first 2 days in straw-amended soil and then declined (Fig. 5). These results clearly demonstrate that straw amendment leads to changes in the balance between the carbon and the nitrogen supplies to P. fluorescens DF57 and that the dual-reporter system in strain DF57 responds dynamically to such changes.
FIG. 5.
Population dynamics and starvation response of the double-reporter strain DF57-N3(pGM115) in natural soil and in natural soil amended with barley straw. (A) Expression of β-galactosidase activity (carbon limitation) and bioluminescence (nitrogen limitation). ▾, β-galactosidase activity in natural soil; ⧫, β-galactosidase activity in straw-amended soil; ◊, bioluminescence in straw-amended soil. Bioluminescence in natural soil was below the detection limit. (B) Population dynamics of strain DF57-N3(pGM115). ▾, unamended soil; ⧫, straw-amended soil. Data are mean values from a representative experiment performed in triplicate. Standard deviations are shown as bars. The experiment has been independently repeated twice.
DISCUSSION
Characterization of the Pfic in P. fluorescens DF57.
The physiological differentiation of a number of gram-negative bacteria during stationary phase has been linked to ςs-dependent gene expression (1, 5, 10, 25, 26, 31). In vitro studies have shown that Pfic is recognized preferentially by ςs in E. coli (35, 38) and apparently does not require additional transcription factors. β-Galactosidase activity expressed from Pfic on pGM115 is likewise ςS dependent in Pseudomonas putida (26).
Pure-culture experiments in rich media have shown that expression of Pfic is strongly growth phase dependent and is initiated during the transition to stationary phase in a number of gram-negative bacteria, including P. putida (23, 26, 38). However, expression studies performed under more defined conditions are scarce. In this study we found a high expression from Pfic in P. fluorescens strain DF57 during entry into stationary phase in both rich and defined media, and carbon limitation could be identified as a major inducer of expression from Pfic. In E. coli various ςS-dependent genes are induced by osmotic stress during growth (9, 11, 30), but our results indicate that Pfic is not activated by osmotic stress in P. fluorescens DF57, making the reporter system useful for studies of carbon availability to this strain.
Carbon limitation response in soil.
The physiological adaptations of bacteria to the physicochemical conditions relevant for natural soil environments have been addressed primarily by pure-culture experiments. In recent years reporter bacteria, or whole-cell biosensors, introduced into soil environments have proven to be very useful to determine the growth conditions for the bacteria in more realistic soil systems (13, 14, 20, 22). For Pseudomonas, studies employing reporters for the availability of specific nutrients have indicated that neither phosphate (20), nitrogen (13, 14), nor iron (22) appears to be severely limiting for P. fluorescens in the natural soils tested so far. In contrast, it is generally believed that microbial activity in agricultural soils is restricted by the small amount of available carbon (39).We here demonstrate carbon-limiting conditions for P. fluorescens DF57(pGM115) in bulk soil as supported by expression of the Pfic-lacZ reporter system and growth data. Another P. fluorescens biosensor (strain RA92 tagged with Tn5-B20 using lacZ as a reporter gene) responding primarily to carbon starvation has previously been introduced into soil (41). However, the response of RA92 was weak and transient (41). Furthermore, carbon-starved RA92 cells introduced into the soil also showed an increase in β-galactosidase activity, and no growth response was observed when the soil was amended with glucose (41), observations that complicate the interpretation of these earlier data.
The inocula of whole-cell biosensors are often quite high compared to the population of indigenous bacteria in soil (13, 14, 20, 22, 41), and the physicochemical conditions sensed by the introduced cells might differ from those sensed by the indigenous bacteria. The inoculum might affect the balance between substrates and nutrients in the soil, as predation on the introduced cells could lead to release of nutrients that increase metabolic activity and growth of the survivors (43). In the present study the conclusions concerning carbon limitation in soil are robust, as lacZ expression from the fic promoter in P. fluorescens DF57(pGM115) was induced both when the reporter was introduced into soil at a cell concentration comparable to the indigenous pseudomonad population in soil (17) and at a 700-fold-higher cell density.
Single-cell analysis of expression of the Pfic-lacZ reporter system
Soil consists of aggregates with various organic carbon contents forming diverse microenvironments for the bacterial populations (27). This complexity makes it important to address physiological responses of introduced reporter strains at the single-cell level. Recently, Joyner and Lindow (15) showed that iron availability was not uniform for cells of Pseudomonas syringae inoculated into simpler systems like leaves and artificial root systems. They identified the reporter organism by fluorescence in situ hybridization and monitored the activity of an iron-responsive promoter controlling the gene for green fluorescent protein. We used an immunochemical double-labeling procedure, employing specific antibodies for P. fluorescens DF57 and for β-galactosidase, to obtain sufficient sensitivity to monitor gene expression from a relatively weak promoter at the single-cell level.
The use of immunofluorescence techniques to detect β-galactosidase in single cells has been demonstrated previously for pure cultures (8, 16, 33). However, this study is the first to demonstrate cell-specific gene expression for bacteria in a complex soil environment and to address the heterogeneity of carbon availability to bacteria inoculated into soil. Our data indicate that DF57 responds differently to carbon limitation in pure culture and in soil. In pure culture a reduction of cell size occurs and a homogeneous population of cells develops. Cells extracted from the soil maintain a larger cell size and appear more heterogeneous. Also, with respect to the expression of the β-galactosidase reporter, the soil population of DF57 develops the largest heterogeneity, and only a subpopulation of the introduced Pseudomonas appears to encounter carbon-limiting conditions. The observed heterogeneity of individual cells residing in soil is thought to be due to differences in their microhabitats. The heterogeneity revealed by the single-cell analysis was not apparent from the bulk analyses for β-galactosidase activity, which gave comparable responses for pure-culture and soil experiments. We speculate that cell aggregation (Fig. 3) leads to an overestimation of β-galactosidase activity in the bulk assay for soil, as the activity was normalized by CFU. At the same time, the expression of β-galactosidase by cell aggregates has not been analyzed by flow cytometry, as signal intensities from aggregated cells are difficult to interpret.
Our results extend previous work by Van Overbeek and coworkers (40). They studied the changes in cell length and stress resistance, traits associated with starving cells, of Pseudomonas (6, 40). While the mean cell length was reduced by 45% after 5 days in liquid carbon starvation medium, the reductions of cell length in two soils were 10 and 32%. In accordance, the development of cellular stress resistance was less pronounced in soil than in the liquid starvation medium (40).
Carbon and nitrogen availabilities in straw-amended soil.
Although growth and activity of microbial communities in bulk soil can be carbon limited, the nitrogen availability appears to be significant under some circumstances. Hence, for the bioluminescent reporter strain P. fluorescens DF57-N3, we have previously shown that nitrogen limitation gradually developed during straw degradation in natural soil (14). Further, as DF57 is unable to degrade the cellulose in straw, we found that nitrogen limitation occurred only in the presence of a population of hydrolytic microorganisms. We proposed that the activity of this population increased the nitrogen demand for DF57-N3, by increasing the carbon availability through release of straw-derived carbon sources. The double-reporter strain presented here enabled us to analyze the dynamics of carbon and nitrogen availabilities to DF57 during straw mineralization in more detail. In the straw-amended soil the β-galactosidase activity of DF57-N3(pGM115) remained low, while nitrogen limitation-dependent lux expression appeared after a few days. This indicates that, relative to the demand for other essential nutrients, the DF57-N3 population received excessive amounts of carbon during these early stages of straw degradation. Hence, nitrogen became limited to the DF57-N3 population under conditions where the available carbon resources were not completely exhausted.
In addition to the less available polymers, straw contains a small fraction of water-soluble carbon compounds (3, 28). A rapid decomposition of these compounds and an increase in microbial biomass following straw amendment of soil has been demonstrated (2, 3). Copiotropic soil bacteria such as Pseudomonas spp. are geared to respond rapidly to increased availability of substrates. In accordance, we observed an increase in the culturable DF57-N3 population within the first few days after straw amendment. Further, this growth response occurred while the reporter signals for both C and N limitation were repressed, supporting our hypothesis that the reporter systems respond consistently to the physiological status of the bacteria.
The development of bacteria with multiple reporter systems enables simultaneous monitoring of various physiological responses of a model strain. It is our expectation that this technology may help us to approach a more complete understanding of the dynamic conditions experienced by bacteria in soil.
ACKNOWLEDGMENTS
This work was supported by the Danish Agricultural and Veterinary Research Council (grant 9313839).
We thank G. Miksch for providing the plasmids pGM115 and pGM118, May-Britt Prahm and Elisabeth Koluda for excellent technical assistance, and Lena Nilson for helpful discussions.
REFERENCES
- 1.Canosa I, Yuste L, Rojo F. Role of the alternative sigma factor ςS in expression of the AlkS regulator of the Pseudomonas oleovorans alkane degradation pathway. J Bacteriol. 1999;181:1748–1754. doi: 10.1128/jb.181.6.1748-1754.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cogle A L, Saffigna P G, Strong W M. Carbon transformation during wheat straw decomposition. Soil Biol Biochem. 1989;21:367–372. [Google Scholar]
- 3.Cohran V L, Horton K A, Cole C V. An estimation of microbial death rate and limitations of N or C during wheat straw decomposition. Soil Biol Biochem. 1988;20:293–298. [Google Scholar]
- 4.Dowling D N, O'Gara F. Metabolites of Pseudomonas involved in the biocontrol of plant disease. Trends Biochem Technol. 1994;12:133–141. [Google Scholar]
- 5.Flavier A B, Schell M A, Denny T P. An RpoS (sigmaS) homologue regulates acylhomoserine lactone-dependent autoinduction in Ralstonia solanacearum. Mol Microbiol. 1998;28:475–486. doi: 10.1046/j.1365-2958.1998.00804.x. [DOI] [PubMed] [Google Scholar]
- 6.Givskov M, Eberl L, Møller S, Poulsen L K, Molin S. Responses to nutrient starvation in Pseudomonas putida KT2442: analysis of general cross-protection, cell shape, and macromolecular content. J Bacteriol. 1994;176:7–14. doi: 10.1128/jb.176.1.7-14.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hansen M, Kragelund L, Nybroe O, Sørensen J. Early colonization of barley roots by Pseudomonas fluorescens studied by immunofluorescence technique and confocal laser scanning microscopy. FEMS Microbiol Ecol. 1997;23:353–360. [Google Scholar]
- 8.Harry E J, Pogliano K, Losick R. Use of immunofluorescence to visualize cell-specific gene expression during sporulation in Bacillus subtilis. J Bacteriol. 1995;177:3386–3393. doi: 10.1128/jb.177.12.3386-3393.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hengge-Aronis R. Back to log phase: sigmaS as a global regulator in the osmotic control of gene expression in Escherichia coli. Mol Microbiol. 1996;21:887–893. doi: 10.1046/j.1365-2958.1996.511405.x. [DOI] [PubMed] [Google Scholar]
- 10.Hengge-Aronis R. Regulation of gene expression during entry into stationary phase. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C.: ASM Press; 1996. pp. 1497–1513. [Google Scholar]
- 11.Hengge-Aronis R, Lange R, Henneberg N, Fischer D. Osmotic regulation of RpoS-dependent genes in Escherichia coli. J Bacteriol. 1993;175:259–265. doi: 10.1128/jb.175.1.259-265.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Højberg O, Schnider U, Winteler H V, Sørensen J, Haas D. Oxygen-sensing reporter strain of Pseudomonas fluorescens for monitoring the distribution of low-oxygen habitats in soil. Appl Environ Microbiol. 1999;65:4085–4093. doi: 10.1128/aem.65.9.4085-4093.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jensen L E, Kragelund L, Nybroe O. Expression of a nitrogen regulated lux gene fusion in Pseudomonas fluorescens DF57 studied in pure culture and in soil. FEMS Microbiol Ecol. 1998;25:23–32. [Google Scholar]
- 14.Jensen L E, Nybroe O. Nitrogen availability to Pseudomonas fluorescens DF57 is limited during decomposition of barley straw in bulk soil and in the barley rhizosphere. Appl Environ Microbiol. 1999;65:4320–4328. doi: 10.1128/aem.65.10.4320-4328.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joyner D C, Lindow S E. Heterogeneity of iron bioavailability on plants assessed with a whole-cell GFP-based bacterial biosensor. Microbiology. 2000;146:2435–2445. doi: 10.1099/00221287-146-10-2435. [DOI] [PubMed] [Google Scholar]
- 16.Kang Y, Saile E, Schell M A, Denny T P. Quantitative immunofluorescence of regulated eps gene expression in single cells of Ralstonia solanacearum. Appl Environ Microbiol. 1999;65:2356–2362. doi: 10.1128/aem.65.6.2356-2362.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kirchner M J, Wollum A G, King L D. Soil microbial populations and activities in reduced chemical input agroecosystems. Soil Sci Soc Am J. 1993;57:1289–1295. [Google Scholar]
- 18.Komano T, Utsumi R, Kawamukai M. Functional analysis of the fic gene involved in regulation of cell division. Res Microbiol. 1991;142:269–277. doi: 10.1016/0923-2508(91)90040-h. [DOI] [PubMed] [Google Scholar]
- 19.Kragelund L, Christoffersen B, Nybroe O, de Bruijn F J. Isolation of lux reporter gene fusions in Pseudomonas fluorescens DF57 inducible by nitrogen or phosphorus starvation. FEMS Microbiol Ecol. 1995;17:95–106. [Google Scholar]
- 20.Kragelund L, Hosbond C, Nybroe O. Distribution of metabolic activity and phosphate starvation response of lux-tagged Pseudomonas fluorescens reporter bacteria in the barley rhizosphere. Appl Environ Microbiol. 1997;63:4920–4928. doi: 10.1128/aem.63.12.4920-4928.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kragelund L, Nybroe O. Culturability and expression of outer membrane proteins during carbon, nitrogen, or phosphorus starvation of Pseudomonas fluorescens DF57 and Pseudomonas putida DF14. Appl Environ Microbiol. 1994;60:2944–2948. doi: 10.1128/aem.60.8.2944-2948.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loper J E, Henkels M D. Availability of iron to Pseudomonas fluorescens in rhizosphere and bulk soil evaluated with an ice nucleation reporter gene. Appl Environ Microbiol. 1997;63:99–105. doi: 10.1128/aem.63.1.99-105.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miksch G, Dobrowolski P. Growth phase-dependent induction of stationary-phase promoters of Escherichia coli in different gram-negative bacteria. J Bacteriol. 1995;177:5374–5378. doi: 10.1128/jb.177.18.5374-5378.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 25.Mukherjee A, Cui Y, Ma W, Liu Y, Ishihama A, Eisenstark A, Chatterjee A K. RpoS (sigma-S) controls expression of rsmA, a global regulator of secondary metabolites, hairpin, and extracellular proteins in Erwinia carotovora. J Bacteriol. 1998;180:3629–3634. doi: 10.1128/jb.180.14.3629-3634.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramos-Gonzalez M I, Molin S. Cloning, sequencing, and phenotypic characterization of the rpoS gene from Pseudomonas putida KT2440. J Bacteriol. 1998;180:3421–3431. doi: 10.1128/jb.180.13.3421-3431.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ranjard L, Poly F, Combrisson J, Richaume A, Gourbiere F, Thioulouse J, Nazaret S. Heterogeneous cell density and genetic structure of bacterial pools associated with various soil microenvironments as determined by enumeration and DNA fingerprinting approach (RISA) Microb Ecol. 2000;39:263–272. [PubMed] [Google Scholar]
- 28.Reber H, Schara A. Degradation sequences in wheat straw extracts inoculated with soil suspensions. Soil Biol Biochem. 1971;3:381–383. [Google Scholar]
- 29.Robertson B R, Button D K, Koch A L. Determination of the biomasses of small bacteria at low concentrations in a mixture of species with forward light scatter measurements by flow cytometry. Appl Environ Microbiol. 1998;64:3900–3909. doi: 10.1128/aem.64.10.3900-3909.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Santos J M, Freire P, Vicente M, Arraiano C M. The stationary-phase morphogene bolA from Escherichia coli is induced by stress during early stages of growth. Mol Microbiol. 1999;32:789–798. doi: 10.1046/j.1365-2958.1999.01397.x. [DOI] [PubMed] [Google Scholar]
- 31.Sarniguet A, Kraus J, Henkels M D, Muehlchen A M, Loper J E. The sigma factor sigma ςs affects antibiotic production and biological control activity of Pseudomonas fluorescens Pf-5. Proc Natl Acad Sci USA. 1995;92:12255–12259. doi: 10.1073/pnas.92.26.12255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sørensen J, Skouv J, Jørgensen A, Nybroe O. Rapid identification of environmental isolates of Pseudomonas aeruginosa, P. fluorescens and P. putida by SDS-PAGE analysis of whole-cell protein patterns. FEMS Microbiol Ecol. 1992;101:41–50. [Google Scholar]
- 33.Sun L, Jacobson B J, Dien B S, Srienc F, Fuchs J A. Cell cycle regulation of the Escherichia coli nrd operon: requirement for a cis-acting upstream AT-rich sequence. J Bacteriol. 1994;176:2415–2426. doi: 10.1128/jb.176.8.2415-2426.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sze C C, Moore T, Shingler V. Growth phase-dependent transcription of the ς54-dependent Po promoter controlling the Pseudomonas-derived (methyl)phenol dmp operon of pVI150. J Bacteriol. 1996;178:3727–3735. doi: 10.1128/jb.178.13.3727-3735.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tanaka K, Takayanagi Y, Fujita N, Ishihama A, Takahashi H. Heterogeneity of the principal sigma factor in Escherichia coli: the rpoS gene product, sigma 38, is a second principal sigma factor of RNA polymerase in stationary-phase Escherichia coli. Proc Natl Acad Sci USA. 1993;90:3511–3515. doi: 10.1073/pnas.90.8.3511. . (Erratum, 90:8303.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Timmis K N. Designing microorganisms for the treatment of toxic wastes. Annu Rev Microbiol. 1994;48:525–557. doi: 10.1146/annurev.mi.48.100194.002521. [DOI] [PubMed] [Google Scholar]
- 37.Unge A, Tombolini R, Mølbak L, Jansson J K. Simultaneous monitoring of cell number and metabolic activity of specific bacterial populations with a dual gfp-luxAB marker system. Appl Environ Microbiol. 1999;65:813–821. doi: 10.1128/aem.65.2.813-821.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Utsumi R, Kusafuka S, Nakayama T, Tanaka K, Takayanagi Y, Takahashi H, Noda M, Kawamukai M. Stationary phase-specific expression of the fic gene in Escherichia coli K-12 is controlled by the rpoS gene product (sigma 38) FEMS Microbiol Lett. 1993;113:273–278. doi: 10.1111/j.1574-6968.1993.tb06526.x. [DOI] [PubMed] [Google Scholar]
- 39.Van Elsas J D, Van Overbeek L S. Bacterial response to soil stimuli. In: Kjelleberg S, editor. Starvation in bacteria. New York, N.Y: Plenum Press; 1993. pp. 55–79. [Google Scholar]
- 40.Van Overbeek L S, Eberl L, Givskov M, Molin S, Van Elsas J D. Survival of, and induced stress resistance in, carbon-starved Pseudomonas fluorescens cells residing in soil. Appl Environ Microbiol. 1995;61:4202–4208. doi: 10.1128/aem.61.12.4202-4208.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van Overbeek L S, Van Elsas J D, Van Veen J A. Pseudomonas fluorescens Tn5–B20 mutant RA92 responds to carbon limitation in soil. FEMS Microbiol Ecol. 1997;24:57–71. [Google Scholar]
- 42.Worm J, Jensen L E, Hansen T S, Søndergård M, Nybroe O. Interactions between proteolytic and non-proteolytic Pseudomonas fluorescens affect protein degradation in a model community. FEMS Microbiol Ecol. 2000;32:103–109. doi: 10.1111/j.1574-6941.2000.tb00704.x. [DOI] [PubMed] [Google Scholar]
- 43.Wright D A, Killham K, Glover L A, Prosser J I. Role of pore size location in determining bacterial activity during predation by protozoa in soil. Appl Environ Microbiol. 1995;61:3537–3543. doi: 10.1128/aem.61.10.3537-3543.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]