Abstract
A series of 2-(1H-1,2,4-triazol-3-yl)acetates, as well as 4-mono- and 4,4-disubstituted 5-amino-2,4-dihydro-3H-pyrazol-3-ones (including spirocyclic derivatives) have been synthesized using the Pinner reaction strategy. α-Mono- and α,α-disubstituted ethyl cyanoacetates were converted into the corresponding carboxyimidate salts that served as the key intermediates. Their further reaction with formylhydrazide or hydrazine hydrate provided triazolylacetates or aminopyrazolones (including spirocyclic derivatives), depending on the structure of the starting Pinner salt and the nature of the nucleophile. The scope and limitations of the developed synthetic method have been established.
Keywords: cyclization, nitrogen heterocycles, imidates, spiro compounds, azoles
Graphical Abstract

Since 1877 when A. Pinner and Fr. Klein had discovered the synthesis of carboximidate hydrohalides (now referred to as the Pinner salts),1,2 this class of substances became an important part of the synthetic toolbox for organic chemists. These carboxylic acid derivatives have attracted much attention due to their remarkable reactivity towards carbo- and hetero-nucleophiles that has been widely exploited for the synthesis of numerous organic compounds including nitrogen heterocycles.3–5 Pyrazoles and 1,2,4-triazoles are examples of such heterocycles that have attracted much attention because of their remarkable applications in the pharmaceutical industry. In particular, these heterocycles have been incorporated into numerous marketed medicines such as diuretic Muzolimine for treatment of hypertension,6,7 antiviral agent Ribavirin for cure of chronic Hepatitis C virus (HCV),8–10 or alpha blocker Dapiprazole used to reverse mydriasis after an eye examination.11–13.
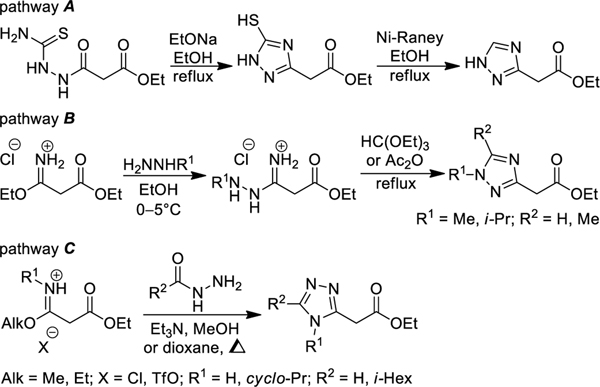
As a part of our ongoing efforts towards the synthesis of triazole derivatives for drug discovery,14–19 we have turned our attention to non-fused (1,2,4-triazol-3-yl)acetates – promising synthetic intermediates for organic and medicinal chemistry. Several synthetic approaches to these compounds have been developed to date. Among them are EtONa-mediated cyclizations of ethyl malonyl-3-thiosemicarbazide followed by desulfurization with Raney Ni (Scheme 1, pathway A);20,21 a reaction of ethyl 3-ethoxy-3-iminopropanoate with monosubstituted hydrazines and subsequent ring closure with HC(OEt)3 or Ac2O (pathway B);22,23 or the two-step condensation of ethyl 3-alkoxy- 3-iminopropanoates with hydrazides (pathways C).24,25 Surprisingly, α-mono-26 and α,α-disubstituted27,28 non-fused (1,2,4-triazol-3-yl)acetates 1 appeared to be largely underrepresented in the literature and have never been obtained via the Pinner reaction strategy. Herein, we have turned our attention to α-mono- and α,α-disubstituted ethyl cyanoacetates (2) as the possible starting materials for the preparation of the title compounds. A possible complication of the selected strategy can include an alternative reaction pathway including nucleophilic attack of the intermediate carboxyimidates 3 at the ester moiety (Scheme 2). Such reactions leading to 3-amino-1H-pyrazol-5(4H)-ones 4 have been known in the literature.29–35 This work is aimed at studying the scope and limitation of the aforementioned strategy for the synthesis of substituted triazolylacetates 1 or 3-amino-1H-pyrazol-5(4H)-ones 4, as well as factors affecting the chemoselectivity of this transformation.
Scheme 1.
Known approaches to 2-(1,2,4-triazol-3-yl) acetates
Scheme 2.
Reaction of carboxyimidate hydrochlorides with formylhydrazide
First of all, a series of appropriately substituted cyanoacetates 2a–f was converted into the corresponding carboximidate hydrochlorides 3a–f adopting the literature procedures.36–39 The latter, in turn, were allowed to react with formylhydrazide under base-mediated conditions (Table 1).
Table 1.
The reaction of 3·HCl with formylhydrazide

| |||||
|---|---|---|---|---|---|
|
| |||||
| Entry | Starting compound 2 | Intermediate 3 (isolated yield, %) | Product 1 (yield by 1H NMR / isolated, %) | Product 4 (yield by 1H NMR / isolated, %) | R1/R2 |
| 1 | 2a | 3a·HCl (81) | 1a (100/78) | 4a (0/0) | H/H |
| 2 | 2b | 3b·HCl (75) | 1b (100/73) | 4b (0/0) | i-Pr/H |
| 3 | 2c | 3c·HCl (67) | 1c (0/0) | 4c (100/72) | Me/Me |
| 4 | 2d | 3d·HCl (40) | 1d (85/32) | 4d (15/5) | (CH2)2 |
| 5 | 2e | 3e·HCl (82) | 1e (40/31) | 4e (60/46) | (CH2)3 |
| 6 | 2f | 3f·HCl (77) | 1f (50/19)a | 4f (50/30)a | (CH2)2O(CH2)2 |
Compound 4f partially precipitated from the reaction mixture. The ratio is given for liquid reaction phase and overall yields are presented.
Intriguingly, while the unsubstituted (3a·HCl) and 2-i-Pr-substituted (3b·HCl) derivatives were completely converted into triazolylacetates 1a,b, the α,α-dimethylated counterpart 3c·HCl was exclusively turned into the corresponding aminopyrazolone 4c (Table 1, Entries 1–3). At the same time, the utilization of intermediates 3d–f·HCl bearing an annulated (hetero)cyclic substituent afforded mixtures of appropriately substituted triazolylacetates 1d–f and spirocyclic aminopyrazolones 4d–f at different ratios (Table 1, Entries 4–6).
These results might suggest that the nature of the α-substituent in 3 played a crucial role for the reaction direction with formylhydrazide. Apparently, a possibility of prototropy (or lack thereof) as well as conformational aspects of 3 could account for the selective formation of triazolylacetates 1a,b and 1d–f and aminopyrazolones 4c–f.
Thus, the deprotonation of carboximidate hydrochlorides (pKa ~ 540) with a base might lead to either imino ethers or imino enoles depending on the substitution at the α-carbon atom. Specifically, the salts of unsubstituted (3a·HCl) as well as 2-i-Pr- substituted (3b·HCl) imidates were likely converted into the corresponding ethyl 3-ethoxy-3-hydroxyacrylimidates 3a and 3b, while hydrochloride of α,α-dimethylated imidate 3c·HCl – into 3-ethoxy-3-iminopropanoate 3c (Scheme 3). The close proximity as well as coplanar position of imino group and enol functionalities in 3 allowed for the hydrogen bonding that further reinforced the adopted conformation (Figure 2).
Scheme 3.
Reaction of carboxyimidate hydrochlorides with formylhydrazine
Figure 2.
Proposed tautomeric forms and conformations of 3a–f
Next, ethyl 3-ethoxy-3-hydroxyacrylimidates 3a and 3b reacted with formylhydrazide as the Michael acceptors providing the interim amidine 5, that underwent the intramolecular condensation to give the corresponding triazolylacetates 4a,b (Scheme 4).
Scheme 4.
A proposed mechanism for the formation of 1a,b
Contrary to the above, 3c reacted with formylhydrazide at the carbonyl moiety first. There might be two possible reasons for this. On the one hand, a lone pair of electrons of the imine moiety precluded the formylhydrazide attack at the imidate fragment. On the other hand, hydrogen bonding increased the electrophilicity of the carbonyl group. The concerted impact of these factors resulted in the formation of intermediate 6 followed by intramolecular cyclization leading to aminopyrazolone 4c (Scheme 5).
Scheme 5.
The proposed mechanism for formation of 4c
At the same time, the annular atoms of the attached (hetero)cyclic substituent in 3e and 3f pushed both the imidate and carbonyl groups out of the plane due to the repulsive 1,3-interactions, thus disrupting the hydrogen bonding (Figure 2). This drived the imidate and carboxylate groups to possess comparable electrophilicity that led to the formation of both possible products (Scheme 6).
Scheme 6.
The synthesis of 1e,f and 4e,f
Based on this, the interaction between cyclopropyl derivative 3d and formylhydrazide looks odd at the first glance since it should demonstrate the reactivity similar to that of 3c. A possible reason behind this might be the larger plane angle value between the carboximidate and the carboxylate moieties caused by the cyclopropane moiety, which hampered the formation of the intramolecular hydrogen bond in the structure of 3d and defined the somewhat preferred initial attack of the nucleophile at the carboximidate group. This assumption was confirmed by DFT calculations. In particular, the predicted Gibbs free energy value for the following hypothetic isodesmic reaction involving the conformers of 3c and 3d having intramolecular hydrogen bonds (Figure 3)
was ΔG = −3.6 kcal/mol, which reflected noticeable strain energy in the conformer of 3d (comparable to the stabilization gain typically provided by hydrogen bonding).
Figure 3.
Conformers of 3c and 3d having an intramolecular hydrogen bond according to DFT calculations
Since the above method appeared to be unsuitable for obtaining 4a, 4b and 4d, as well as 1c, we have considered other synthetic approaches to obtain these compounds. In this way, a series of pyrazolones 4a–f was synthesized by reaction of 3a–f·HCl with hydrazine hydrate (Table 2).
Table 2.
The reaction of 3·HCl with hydrazide hydrate

| |||
|---|---|---|---|
|
| |||
| Entry | Intermediate 3 | Product 4 (isolated yield, %) | R1/R2 |
| 1 | 3a·HCl | 4a (82) | H/H |
| 2 | 3b·HCl | 4b (66) | i-Pr/H |
| 3 | 3c·HCl | 4c (81) | Me/Me |
| 4 | 3d·HCl | 4d (70) | (CH2)2 |
| 5 | 3e·HCl | 4e (73) | (CH2)3 |
| 6 | 3f·HCl | 4f (74) | (CH2)2O(CH2)2 |
The inability of direct synthesis of 1c from 3c·HCl was also circumvented by an alternative synthetic pathway. Thus, ethyl triazolylacetate 1a was treated with BnCl and K2CO3 in DMF in order to install the protecting group at the heterocyclic core and prevent the methylation of the NH group at the later step. The obtained mixture of N-protected regioisomers 7 was exhaustively methylated at the active methylene group by using an excess of MeI and NaH as a base to give a mixture of regioisomeric α,α-dimethylated products 8. Eventually, Pd-catalyzed hydrogen-mediated cleavage of the benzyl protecting group afforded the target methyl 2-methyl 2-(1H-1,2,4-triazol-3-yl)acetate (1g) in 70% overall yield (Scheme 7).
Scheme 7.
The synthesis of 1g
Since the last step was carried out in methanolic media, transesterification took place. The use of ethanolic media was not fruitful and resulted in recovery of the starting material (possibly due to the suppressive effect of water in 96% EtOH on the catalyst efficiency).
Saponification of esters 1a,b,d–g proceeded smoothly; however, further isolation of the corresponding carboxylic acids was complicated because of partial decarboxylation. Therefore, the products were isolated as stable sodium (9a,b) or lithium (9c–f) salts (Scheme 8).
Scheme 8.
The synthesis of 9a–f
The structure of all products obtained was proven by standard spectroscopic methods. The solid state structure of 4a was additionally investigated by single crystal X-ray diffraction method (see the supporting information), which proved the tautomeric form given above. The analysis of the packing motif has shown that all the possibilities for hydrogen bonding were completely realized in the crystal. As the result, the crystal structure is described as a parallel packing of discrete 2D supramolecular networks.
In conclusion, an efficient approach to gram-scale synthesis of α-mono- and α,α-disubstituted 2-(1H-1,2,4-triazol-3-yl)acetates as well as 4-mono- and 4,4-disubstituted 5-amino-2,4-dihydro-3H-pyrazol-3-ones via the Pinner reaction strategy has been developed. It was found that 2-monosubstituted as well as cyclopropane-annulated 3-imino-3-alkoxy-2-methylpropanoates reacted with formylhydrazide affording the corresponding (1,2,4-triazol-3-yl)acetates. At the same time, 3-imino-3-alkoxy-2-methylpropanoates bearing an annulated carbo/heterocyclic ring (larger than cyclopropane) turned into approximately equimolar mixtures of the appropriately substituted triazolylacetates and spirocyclic aminopyrazolones. An analogous reaction with hydrazine hydrate gave the corresponding 5-amino-2,4-dihydro-3H-pyrazol-3-ones in all cases. An alternative approach to α,α-disubstituted 2-(1H-1,2,4-triazol-3-yl)acetates was also proposed based on the double alkylation of the methylene unit in the parent N-protected 2-(1H-1,2,4-triazol-3-yl)acetate. The saponification of (1,2,4-triazol-3-yl)acetates allowed for the preparation of the corresponding lithium or sodium salts; isolation of free carboxylic acids was accompanied with partial decarboxylation. The developed procedure provides access to promising building blocks and scaffolds that have a great potential for early drug discovery.41
Supplementary Material
Figure 1.
5-Aminopyrazolone and 1,2,4-triazole-containing marketed drugs
Highlights.
2-(1,2,4-triazol-3-yl)acetates and 5-amino-2,4-dihydropyrazol-3-ones were synthesized.
Heterocyclization involving the Pinner salts was applied at the key step.
Its regioselectivity depended on the nature of the intermediate carboxyimidates.
Mono- and bifunctional heterocyclic (spirocyclic) building blocks were prepared.
Acknowledgments
The work was funded by Enamine Ldt. and NIH (Grant No. GM133836, to Y.S.M. and John Irwin). Additional funding from Ministry of Education and Science of Ukraine, Grant No. 19BF037-03 (A.V.D. and O.O.G.) is also acknowledged. The authors thank Dr. Andrii Kyrylchuk for DFT calculations, and Prof. Andrey A. Tolmachev for his encouragement and support.
Footnotes
Supplementary Material
Supplementary data associated with this article can be found in the online version.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Pinner A, Klein F. Ber. 1877, 10, 1889–1897. [Google Scholar]
- 2.Pinner A, Klein F. Ber. 1878, 11, 1475–1487. [Google Scholar]
- 3.Roger R, Neilson DG Chem. Rev. 1961, 61, 179–211. [Google Scholar]
- 4.Compr Wang Z.. Org. Name React. Reagents. 2010, 2237–2240. [Google Scholar]
- 5.Thakur R, Jaiswal Y, Kumar A. Org. Biomol. Chem. 2019, 17, 9829–9843. [DOI] [PubMed] [Google Scholar]
- 6.Wangemann P, Braitsch R, Greger R. Pflügers Arch Eur. J. Physiol. 1987, 410, 674–676. [DOI] [PubMed] [Google Scholar]
- 7.Reyes AJ, Leary WP Cardiovasc. Drugs Ther. 1993, 7, 23–28. [DOI] [PubMed] [Google Scholar]
- 8.Martin P, Jensen DM J. Gastroenterol. Hepatol. 2008, 23, 844–855. [DOI] [PubMed] [Google Scholar]
- 9.Rev Huggins J. W.. Infect. Dis. 1989, 11, 750–761. [Google Scholar]
- 10.Myers RP, Shah H, Burak KW, Cooper C, Feld JJ Can. J. Gastroenterol. Hepatol 2015, 29, 19–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nyman N, Keates EU Optom. Vis. Sci. 1990, 67, 705–709. [DOI] [PubMed] [Google Scholar]
- 12.Doughty MJ, Lyle WM Optom. Vis. Sci. 1992, 69, 358–368. [DOI] [PubMed] [Google Scholar]
- 13.Pharm Eltze M. J.. Pharmacol. 1997, 49, 1091–1095. [DOI] [PubMed] [Google Scholar]
- 14.Khomenko DM, Doroshchuk RO, Vashchenko OV, Lampeka RD Chem. Heterocycl. Compd. 2016, 52, 1–7. [Google Scholar]
- 15.Bogolyubsky AV, Savych O, Zhemera AV, Pipko SE, Grishchenko AV, Konovets AI, Doroshchuk RO, Khomenko DN, Brovarets VS, Moroz YS, Vybornyi M. ACS Comb. Sci. 2018, 20, 461–466. [DOI] [PubMed] [Google Scholar]
- 16.Pagacz-Kostrzewa M, Sałdyka M, Bil A, Gul W, Wierzejewska M, Khomenko DM, Doroschuk RO J. Phys. Chem. A. 2019, 123, 841–850. [DOI] [PubMed] [Google Scholar]
- 17.Khomenko DM, Doroschuk RO, Trachevskii VV, Shova S, Lampeka RD Tetrahedron Lett. 2016, 57, 990–992. [Google Scholar]
- 18.Khomenko DM, Doroshchuk RO, Raspertova IV, García López J, López Ortiz F, Shova S, Iegorov OA, Lampeka RD Tetrahedron Lett. 2019, 60, 151089. [Google Scholar]
- 19.Khomenko DM, Doroshchuk RO, Vashchenko OV, Lampeka RD Chem. Heterocycl. Compd. 2016, 52, 402–408. [Google Scholar]
- 20.Ainsworth C, Jones RG J. Am. Chem. Soc. 1954, 76, 5651–5654. [Google Scholar]
- 21.Nara H, Kaieda A, Sato K, Naito T, Mototani H, Oki H, Yamamoto Y, Kuno H, Santou T, Kanzaki N, Terauchi J, Uchikawa O, Kori MJ Med. Chem. 2017, 60, 608–626. [DOI] [PubMed] [Google Scholar]
- 22.Moderhack D, Schneider JC J. Heterocycl. Chem. 2007, 44, 393–401. [Google Scholar]
- 23.Kettle JG, Anjum R, Barry E, Bhavsar D, Brown C, Boyd S, Campbell A, Goldberg K, Grondine M, Guichard S, Hardy CJ, Hunt T, Jones RDO, Li X, Moleva O, Ogg D, Overman RC, Packer MJ, Pearson S, Schimpl M, Shao W, Smith A, Smith JM, Stead D, Stokes S, Tucker M, Ye YJ Med. Chem. 2018, 61, 8797–8810. [DOI] [PubMed] [Google Scholar]
- 24.Kotoku M, Maeba T, Fujioka S, Yokota M, Seki N, Ito K, Suwa Y, Ikenogami T, Hirata K, Hase Y, Katsuda Y, Miyagawa N, Arita K, Asahina K, Noguchi M, Nomura A, Doi S, Adachi T, Crowe P, Tao H, Thacher S, Hashimoto H, Suzuki T, Shiozaki MJ Med. Chem. 2019, 62, 2837–2842. [DOI] [PubMed] [Google Scholar]
- 25.Kiselyov AS, Piatnitski Chekler EL, Chernisheva NB, Salamandra LK, Semenov VV Tetrahedron Lett. 2009, 50, 3809–3812. [Google Scholar]
- 26.Capuano L, Djokar K. Liebigs Ann. Chem. 1985, 1985, 2305–2312. [Google Scholar]
- 27.Bronner S. M., Crawford J. J., Cridland A., Cyr P., Fauber B., Gancia E., Gobbi A., Hurley C., Killen J., Lee W., Rene O., Van Niel M. B., Ward S., Winship P., Zbieg J. WO2018083105A1, 2018.
- 28.Murakami T., Kawano T., Shiraki R., Ishii H., Yoshimura S., Ohkawa T., Hosaka M., Fukudome H., Inoki Y. EP1790641A1, 2007.
- 29.Gong H, Krische MJ Angew. Chem. Int. Ed. 2005, 44, 7069–7071. [DOI] [PubMed] [Google Scholar]
- 30.Stasevych MV, Zvarych VI, Lunin VV, Khomyak SV, Vovk MV, Novikov VP Chem. Heterocycl. Compd. 2017, 53, 927–929. [Google Scholar]
- 31.El-Gamal KM, El-Morsy AM, Saad AM, Eissa IH, Alswah MJ Mol. Struct. 2018, 1166, 15–33. [Google Scholar]
- 32.Kadam A, Dawane B, Pawar M, Shegokar H, Patil K, Meshram R, Gacche R. Bioorg Chem. 2014, 53, 67–74. [DOI] [PubMed] [Google Scholar]
- 33.Mohi El-Deen EM, Anwar MM, Hasabelnaby SM Res. Chem. Intermed. 2016, 42, 1863–1883. [Google Scholar]
- 34.Salem MS, Errayes AO J. Chem. Res. 2016, 40, 299–304. [Google Scholar]
- 35.Gagnon PE, Nolin B, Jones RN Can. J. Chem. 1951, 29, 843–847. [DOI] [PubMed] [Google Scholar]
- 36.Scheithauer S, Mayer R. Chem. Ber. 1967, 100, 1413–1427. [Google Scholar]
- 37.Muller J, Ramuz H, Wagner H-P Helv. Chim. Acta. 1983, 66, 809–813. [Google Scholar]
- 38.Chiba T, Takahashi T, Sakaki J, Kaneko C. Chem. Pharm. Bull. 1985, 33, 3046–3049. [Google Scholar]
- 39.Kobayashi T, Inoue T, Kita Z, Yoshiya H, Nishino S, Oizumi K, Kimura T. Chem. Pharm. Bull. 1995, 43, 788–796. [DOI] [PubMed] [Google Scholar]
- 40.Kaválek J, Panchartek J, Potěšil T, Štěrba V. Collect. Czech. Chem. Commun. 1986, 51, 677–683. [Google Scholar]
- 41.Grygorenko OO, Volochnyuk DM, Ryabukhin SV, Judd DB Chem. Eur. J. 2020, 26, 1196–1237. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.