Abstract
Background and Objective
This was a multicenter study aimed at investigating the characteristics of cognitive decline, neuropsychiatric symptoms, and brain imaging in individuals with subjective cognitive decline (SCD) and subtle cognitive decline (pre–mild cognitive impairment [pre–MCI]).
Methods
Data were obtained from the Network-AD project (NET-2011-02346784). The included participants underwent baseline cognitive and neurobehavioral evaluation, FDG-PET, and amyloid PET. We used principal component analysis (PCA) to identify independent neuropsychological and neuropsychiatric dimensions and their association with brain metabolism.
Results
A total of 105 participants (SCD = 49, pre–MCI = 56) were included. FDG-PET was normal in 45% of participants and revealed brain hypometabolism in 55%, with a frontal-like pattern as the most frequent finding (28%). Neuropsychiatric symptoms emerging from the Neuropsychiatric Inventory and the Starkstein Apathy Scale were highly prevalent in the whole sample (78%). An abnormal amyloid load was detected in the 18% of the participants who underwent amyloid PET (n = 60). PCA resulted in 3 neuropsychological factors: (1) executive/visuomotor, correlating with hypometabolism in frontal and occipital cortices and basal ganglia; (2) memory, correlating with hypometabolism in temporoparietal regions; and (3) visuospatial/constructional, correlating with hypometabolism in frontoparietal cortices. Two factors emerged from the neuropsychiatric PCA: (1) affective, correlating with hypometabolism in orbitofrontal and cingulate cortex and insula; (2) hyperactive/psychotic, correlating with hypometabolism in frontal, temporal, and parietal regions.
Discussion
FDG-PET evidence suggests either normal brain function or different patterns of brain hypometabolism in SCD and pre–MCI. These results indicate that SCD and pre–MCI represent heterogeneous populations. Different neuropsychological and neuropsychiatric profiles emerged, which correlated with neuronal dysfunction in specific brain regions. Long-term follow-up studies are needed to assess the risk of progression to dementia in these conditions.
Neuropathologic changes leading to dementia start years before clinical onset of symptoms.1 A typical definition of “normal performance” is based on cutoff scores, set between 1 and 2 SDs of the comparison group (DSM-5 criteria for minor cognitive disorder suggest, as a guideline, that test performance in mild neurocognitive disorder should fall in the range of 1–2 SD below the normative mean, or between the 3rd and 16th percentiles on tests for which appropriate norms are available2). The most recent criteria for mild cognitive impairment (MCI)3 indicate that “scores on cognitive tests for individuals with MCI are typically 1 to 1.5 SDs below the mean for their age and education matched peers on culturally appropriate normative data (that is, for the impaired domain[s], when available)” but emphasize that “these ranges are guidelines and not cutoff scores.” The reason for this caution in referring to a psychometrically defined “normality” is that it does not consider the participant's baseline performance, which if known, may serve as a reference to denote the occurrence of a potential decline.
Subjective cognitive decline (SCD) defines a self-experienced decline in cognitive functions with performance within the normal range on standardized cognitive assessment.4 The subjective decline in memory of an individual irrespective of function in other domains, onset of SCD within the past 5 years in individuals aged 60 years or older, persistence of SCD over time, and worry associated with SCD such that the individual seeks medical help are features that increase the risk for future cognitive decline.5
Several studies have proposed the identification of an intermediate stage between normal cognition and MCI, labeled as pre–MCI.6,7 One definition6 of pre–MCI requires information about decline from a previous level of function. Another definition7 is based on a discrepancy between clinical judgment (MCI) and neuropsychological performance (normal).
Biomarker information plays a central role in defining a risk profile in these conditions. According to recent criteria, a normal cognitive profile, or the presence of subjective cognitive complaints or “subtle cognitive decline” associated with the positivity of physiopathologic biomarkers, lead to a diagnosis of preclinical Alzheimer disease (AD) in stages 1, 2, and 38 or 1 and 2.9 There is considerable evidence that a minority of participants fulfilling these criteria progress to dementia during follow-up (see reference 10 for a discussion). The assessment of biomarkers of neurodegeneration and pathology in subjective cognitive complaints/pre–MCI, combined with long follow-up, can be expected to provide useful information about the risk profile by identifying participants who are not on a trajectory to dementia.
The nuanced definition of pre–MCI including participants with very subtle or questionable cognitive impairment does not facilitate clinical and prognostic categorizations. An operational definition has been adopted in previous studies to include participants with a Clinical Dementia Rating (CDR) score of 0.5 and neuropsychological performance above −1.5 SD according to age and education norms, revealing subtle cognitive deficits involving the memory and executive domains.7 Again, cognitive impairment progression at 2–3 years has been reported for fewer than 30% of participants, confirming the need for a multimodal approach to identify the potential risk/protective factors for cognitive decline in this population. Concurrently, neuropsychiatric symptoms (NPS), including behavioral and psychiatric symptoms observed in cognitively normal persons, also increase the risk of incident MCI.11 Indeed, an emerging conceptual framework supports NPS as early noncognitive symptoms of dementia, as they are associated with metabolic dysfunction in the AD continuum, including preclinical, prodromal, and dementia stages of AD.12
Thus, biomarker investigation is crucial in individuals with subjective or subtle cognitive decline to reveal early biological changes and signs of neurodegeneration, helping to rule out AD pathology or other neurodegenerative diseases, and to stratify participants with different risks to progression. The current multicenter study aimed to investigate brain biological and functional changes in a sample of individuals with SCD and pre–MCI, searching for neuropsychological and neuropsychiatric correlates identifying distinct profiles already in the preclinical dementia phase.
Methods
Participants
We conducted the study with data from the clinical, prospective multicenter Network-AD project (AD-NET-02346784). The project involved 459 individuals in the dementia continuum, from the SCD/pre–MCI stage to the dementia phase. In the current study, we included individuals with SCD or in a pre–MCI stage according to clinical criteria4,7 and available data at baseline for different biomarkers (fluorodeoxyglucose [FDG-PET], n = 105; and amyloid PET, n = 60 out of 105). Another inclusion criterion was preserved ability to carry out everyday functions, measured by activities of daily living (ADLs) and instrumental ADLs.
Clinical and neuropsychological assessments performed at baseline discriminated between SCD and pre–MCI. Patients with SCD had self-reported cognitive complaints assessed by specialist clinicians through interviews. Participants with SCD (n = 49) had a self-experienced persistent decline in cognitive capacity but normal age/education-adjusted performances on standardized cognitive tests4 and a CDR score = 0. Patients with pre–MCI (n = 56) presented CDR scores = 0.5 and no impairment in objective neuropsychological tests or a CDR score = 0 but very mild impairment (within 1.5 SD of normal scores) on neuropsychological tests.7 See Table 1 for demographic and clinical information.
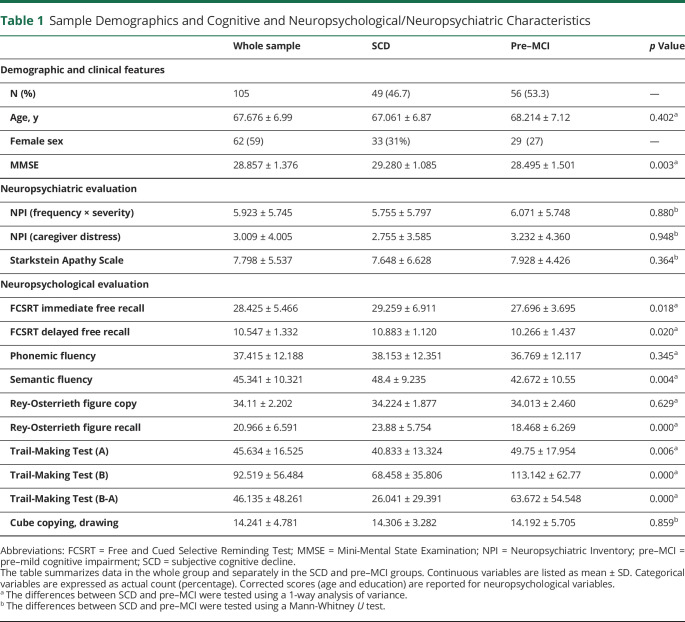
Table 1.
Sample Demographics and Cognitive and Neuropsychological/Neuropsychiatric Characteristics
Standard Protocol Approvals, Registrations, and Patient Consents
Written informed consent was obtained from participants. The study was approved by local ethics committees and conducted in compliance with the Declaration of Helsinki for the protection of human participants.
Clinical, Cognitive, and Neuropsychiatric Assessment
All participants underwent complete medical and neurologic assessment and neuropsychological and neuropsychiatric evaluations. Neuropsychological assessment is reported in Table 1. Neuropsychiatric profiles were assessed by the Neuropsychiatric Inventory (NPI) and the Starkstein Apathy Scale (SAS). For subsequent analysis, NPI scores were evaluated according to Aalten et al.,13 clustering 12 NPI symptoms into 4 independent dimensions as follows: (1) affective (anxiety and depression); (2) apathetic (apathy, eating, and appetite changes); (3) hyperactivity (agitation/aggression, irritability, euphoria/elation, aberrant motor behavior, and disinhibition); (4) psychotic (delusions, hallucinations, and nighttime sleep disturbances). The score for each neuropsychiatric dimension was defined as the sum score of the included NPI symptoms.14 The CDR and the Mini-Mental State Examination (MMSE) measured global status and staging. One-way analysis of variance (ANOVA) test for normally distributed variables and Mann-Whitney U test for nonparametric variables were used to test differences in neuropsychological and neuropsychiatric features between SCD and pre–MCI subgroups.
FDG-PET Imaging Acquisition, Preprocessing, and Analysis
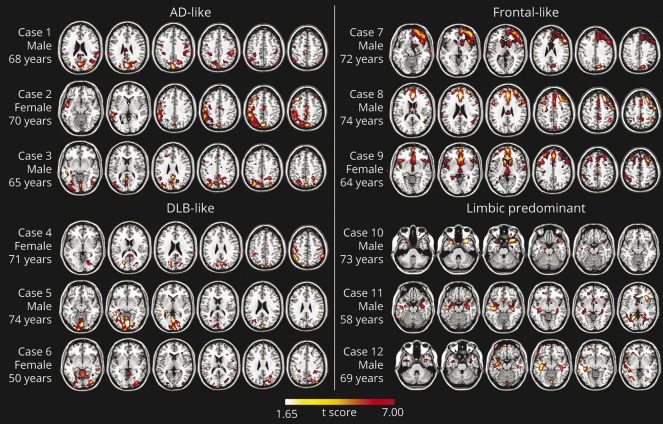
All participants underwent FDG-PET scans acquired in the participating centers. The FDG-PET acquisition procedures conformed to the European Association of Nuclear Medicine guidelines.15 Imaging from different scans showed high reproducibility of results.16 The subsequent analyses were all performed at the Nuclear Medicine Unit of the San Raffaele Hospital using validated procedures.17 Each patient scan was tested for relative hypometabolism based on statistical parametric mapping (SPM) procedures that include comparison with a large image database of FDG-PET scans from normal controls. The resulting single-participant SPM hypometabolic maps were visually inspected independently by 3 experts who excluded or classified hypometabolism patterns suggestive of specific neurodegenerative conditions, as previously described18,19 (Figure 1).
Figure 1. Examples of Single-Subject FDG-PET Hypometabolic Patterns Resulting From Statistical Parametric Mapping Single-subject analysis vs 112 controls; significance was set at uncorrected p <0.05 at the voxel level with k > 100 voxels.
AD = Alzheimer disease; DLB = dementia with Lewy bodies; pre–MCI = pre–mild cognitive impairment; SCD = subjective cognitive decline.
In addition, to provide a single-participant classification with an objective approach, we tested the group membership based on the adherence of the hypometabolism maps to predefined disease-specific anatomical templates, according to previously validated literature.18,19 The templates were generated using regions of interest (ROIs) selected from the automated anatomical labeling (AAL) atlas and set up with the Wake Forest University PickAtlas toolbox.20 Then, we compared the group membership obtained with the latter template-based method with the membership derived by the visual independent rating by means of Cohen kappa. A complete description of the classification procedure can be found in the supplementary materials (links.lww.com/WNL/B998).
[18F]-Florbetaben PET Image Acquisition, Preprocessing, and Analysis
Amyloid PET scan was performed in 60 participants using [18F]-Florbetaben (Neuraceq; Piramal). Data analysis was conducted at the nuclear medicine department of San Raffaele Hospital. A validated preprocessing pipeline allowed us to extract global cortical [18F]-Florbetaben standard uptake value ratio (SUVR) using normalization data based on the low-dose CT contextually acquired with the PET scan.21 To calculate cortical amyloid burden, 6 ROIs were defined using the AAL atlas, through the Wake Forest University PickAtlas toolbox for SPM,20 which included dorsolateral and medial frontal cortex, cingulum, precuneus, inferior and superior parietal lobules, lateral occipital cortex, and lateral temporal cortex. Images were scaled to the activity of the cerebellar gray matter, used as the reference region.22 The global cortical amyloid load was calculated as the average computed from the 6 ROIs. A cutoff of 1.45 was considered for classifying participants as amyloid-positive (Aβ+), with global cortical amyloid load higher than the chosen cutoff, or amyloid negative (Aβ–), with global cortical SUVR equal to or below the selected cutoff.23 To investigate the relationship between FDG-PET pattern expression and the amyloid status, we compared the prevalence of each hypometabolic pattern within the Aβ+ and Aβ– groups.
Principal Component Analyses
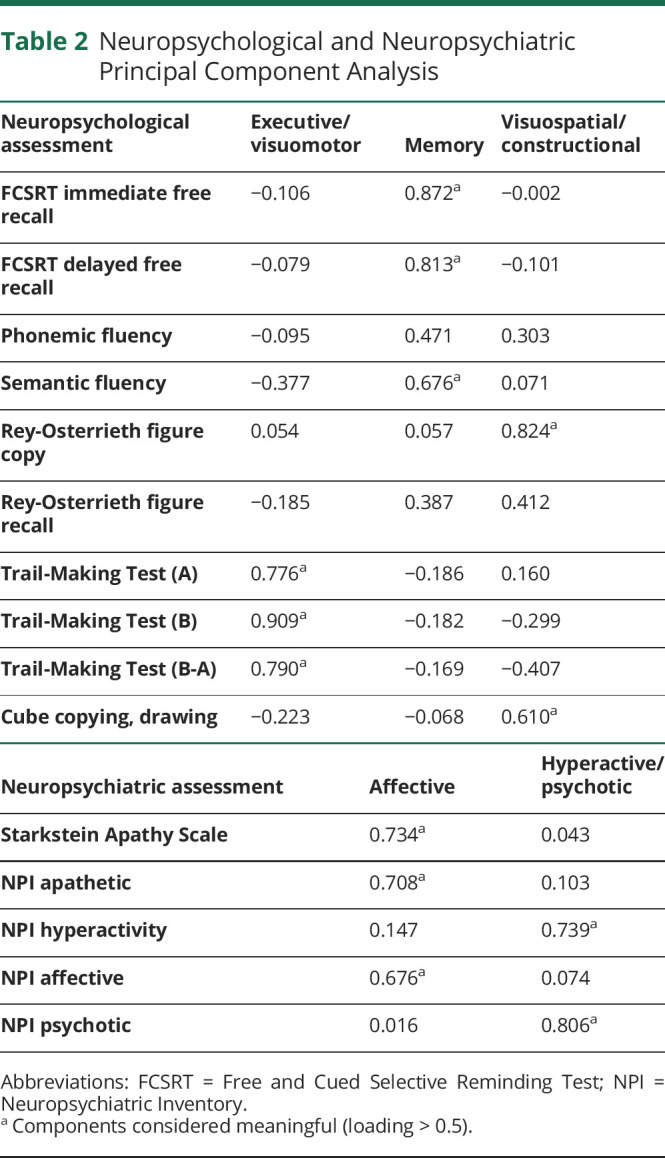
To explore the underlying dimensions of variation in the patients' neuropsychological and neuropsychiatric measures, 2 principal components analyses (PCAs) were applied in the whole sample using orthogonal varimax rotation. All the scores were converted to z scores based on the results of the whole cohort. The neuropsychological and neuropsychiatric measures entered 2 different PCAs to collapse the data into composite PCA scores. The correlation matrix was used for the extraction of components. The adequacy of the sample size of both PCAs was determined by means of Kaiser-Meyer-Olkin (KMO) measure of sampling adequacy and the Bartlett test of sphericity. Component loadings >0.5 were considered meaningful and component scores were computed using the regression method (see Table 2 for details). To investigate whether these factors were differentially expressed by SCD and pre–MCI groups, the regression scores of the PCA factors were compared by means of 1-way ANOVA. All statistical analyses were conducted with IBM SPSS Statistics for Windows, version 26.0.
Table 2.
Neuropsychological and Neuropsychiatric Principal Component Analysis
Correlation Analysis
To identify the correlations between brain metabolism and the PCA factors, the z scores of the tests grouped in the PCA factors were used as independent variables in voxel-wise multiple regression analyses together with global mean scaled metabolic rate as dependent variables (both in the whole participants' group and separately in SCD and the pre–MCI). Age was entered as a nuisance variable. For each PCA, a multivariate correlation matrix was computed using SPM12, running in MATLAB (MathWorks Inc.). The statistical threshold was set at p < 0.05, with Kep ≥100 voxels.
Data Availability
Anonymized data not provided in the article because of space limitations may be shared at the request of any qualified investigator for purposes of replicating procedures and results.
Results
Clinical, Neuropsychological, and Neuropsychiatric Characteristics
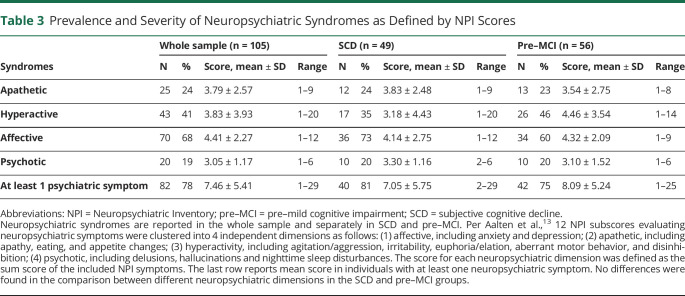
Detailed demographic, clinical, and neuropsychological information is shown in Table 1. Statistical comparison revealed, as expected, significant differences between SCD and pre–MCI groups in the neuropsychological evaluation. NPS were frequently reported in the whole sample, with 78% (n = 82) showing at least 1 symptom (defined as NPI total score ≥1). Regarding the neuropsychiatric subsyndromes, affective was the most frequent (68%). Table 3 reports the prevalence and severity of neuropsychiatric syndromes as defined by the NPI scores.
Table 3.
Prevalence and Severity of Neuropsychiatric Syndromes as Defined by NPI Scores
Brain Hypometabolism Patterns
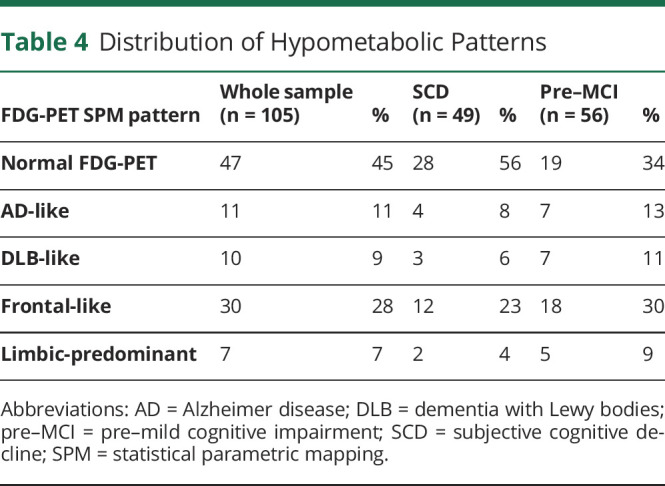
Considering the whole sample, 45% of participants did not reveal brain metabolic abnormalities, while 55% showed brain hypometabolism, with the frontal-like pattern as the most frequent (28%) finding. The assessment was conducted by 3 experts, showing near-perfect agreement in the SPM hypometabolism map classification (Cohen kappa >0.95). The visual rating of SPM maps allowed us to classify participants into 1 of 5 patterns: (1) normal brain metabolism; (2) temporoparietal hypometabolism, namely AD-like pattern24; (3) temporoparietal and occipital hypometabolism, namely dementia with Lewy bodies (DLB)–like pattern18; (4) hypometabolism in the frontal cortex, specifically frontal-like pattern25; and (5) limbic-predominant pattern19 (see Figure 1 for examples of single-participant FDG-PET hypometabolism patterns). Table 4 shows the rate of hypometabolism patterns in the whole sample and separately in the SCD and pre–MCI subgroups.
Table 4.
Distribution of Hypometabolic Patterns
Applying the template-based procedure, the classification was coherent with the visual SPM single-participant rating in 80 participants out of 105. Cohen kappa was used to measure the agreement between 2 classifications (visual and template-based) revealing a substantial agreement (κ = 0.670; p = 0.000). The template-based approach failed to classify individuals showing hypometabolism both in disease-specific and overlapping ROIs, in particular participants with AD- and DLB-like pattern (4 participants classified as AD-like pattern with the visual rating were classified in the DLB-like group with the template-based approach) and participants with frontal and limbic-predominant pattern (6 participants classified as frontal-like pattern with the visual rating were classified in the limbic-predominant group with the template-based approach), due to overlap between these templates.
The Free and Cued Selective Reminding Test (FCSRT) (delayed free recall) scores significantly differed between subgroups with different hypometabolic patterns (Kruskal-Wallis H = 12.72, p = 0.013); however, this result did not survive Bonferroni correction for multiple comparisons. Performances at the semantic fluency test significantly differed between subgroups with different hypometabolic patterns (F = 2.77, p = 0.031). Bonferroni post hoc test showed a significant difference between normal FDG-PET and the limbic-predominant pattern subgroups (p = 0.044), where the latter subgroup showed poor performance in semantic fluency compared with the normal FDG-PET subgroup. NPI scores (both frequency × severity total scores and caregiver distress scores) significantly differed between subgroups with different hypometabolic patterns (Kruskal-Wallis H = 12.92, p = 0.012; Kruskal-Wallis H = 11.93, p = 0.018). Bonferroni post hoc test showed a significant difference between normal FDG-PET and frontal-like subgroups (p = 0.011; p = 0.008), the latter subgroup presenting higher scores in NPI compared with the normal FDG-PET subgroup. Analyzing the NPI subscore differences among the groups defined on the basis of different hypometabolism patterns, the frontal-like group showed higher scores in the irritability (p = 0.001), disinhibition (p = 0.027), and sleep disturbances (p = 0.001) items compared with the normal FDG-PET subgroup, and the limbic-predominant group showed a higher score in the disinhibition (p = 0.004) scale than the normal FDG-PET subgroup; no specific neuropsychiatric disturbances as detected by the NPI were observed in the AD-like or the DLB-like subgroups.
[18F]-Florbetaben PET and FDG-PET Correlation
Based on the selected cutoff for global cortical SUVR, 18% (n = 11) of 60 participants were classified as Aβ+ and 82% (n = 49) as Aβ–. We performed a comparison between the hypometabolism patterns expressed in the Aβ+ and the Aβ–group. The comparison showed no significant differences (χ2 = 4,526; p = 0.339). In detail, 7 out of 11 Aβ+ participants also had an abnormal FDG-PET hypometabolism pattern: 3/7 presented an FDG-PET AD-like hypometabolism pattern and 4/7 showed a frontal-like pattern. Four participants showed abnormal cortical amyloid load and no signs of neurodegeneration at the FDG-PET scan. No participants with the DLB-like pattern or the limbic-predominant pattern were present in the Aβ+ group. In the case of Aβ– participants, 18/49 also had a normal FDG-PET scan, while 31/49 showed an altered FDG-PET hypometabolism pattern, and 25/31 had a non-AD hypometabolic pattern.
Principal Component Analysis
The neuropsychological variables entered a PCA to collapse the test z scores into composite PCA factors (Table 2). Sample size was acceptable (KMO = 0.561) and correlations were sufficiently large (Bartlett test = 553.115; p < 0.000). According to the PCA, 3 components captured 62.69% of the variance and they were interpreted as the best dimensional representation of the full dataset. Three neuropsychological PCA components emerged: (1) executive/visuomotor, (2) memory, and (3) visuospatial/constructional (Table 2). Only the Trail-Making Test (TMT) scores (collapsed in factor 1, executive/visuomotor) run in a different direction (high scores representing poor performance) as compared with other test scores.
The neuropsychiatric variables entered a PCA to collapse the test z scores into composite PCA factors (Table 2). Sample size was acceptable (KMO = 0.622) and correlations were sufficiently large (Bartlett test = 27.39; p < 0.002). Two components captured 54.65% of the variance. Two neuropsychiatric PCA components were evident: (1) affective and (2) hyperactive/psychotic (see Table 2 for variables included in each component).
In the SCD vs pre–MCI analysis, the memory factor and executive/visuomotor factor significantly differ between SCD and pre–MCI participants (F = 9.47, p = 0.003; F = 12.59, p = 0.001). Pre–MCI participants showed lower scores at memory and executive tests compared with the SCD group. Of note, the frontal-like subgroup showed greater hyperactive/psychotic features compared with the normal FDG-PET subgroup.
Correlation Analysis
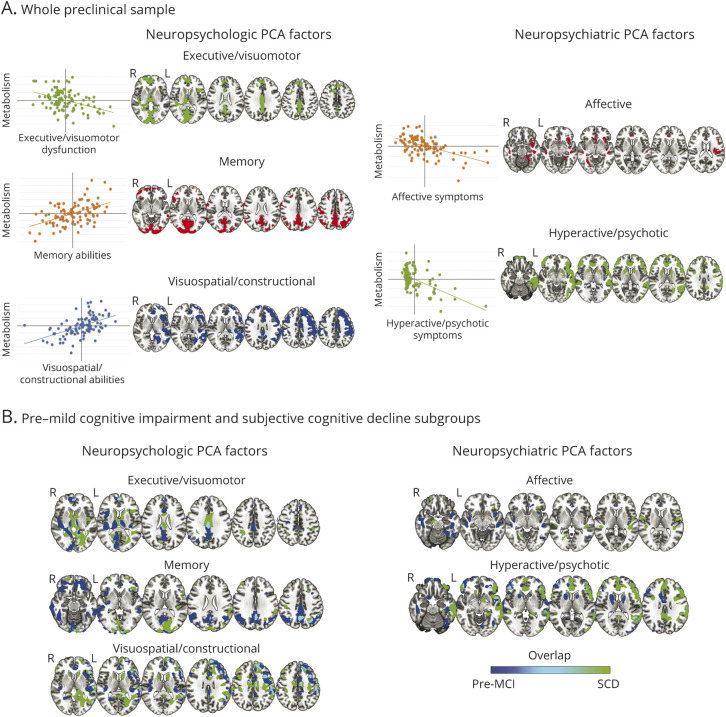
Figure 2 shows the results of voxel-wise multivariate regression models between PCA neuropsychological/neuropsychiatric factors and brain metabolism. Significant correlations emerged both in the whole sample and in the SCD and pre–MCI groups separately. The significant correlations between the PCA factors and brain metabolism were observed in several brain regions, with reduced metabolism correlating with lower performances in neuropsychological tests or higher disturbances in neuropsychiatric assessment (Figure 2).
Figure 2. Results of Voxel-wise Multivariate Regression Models Between the Grouped Test in Neuropsychological PCA and Neuropsychiatric PCA Factors and Brain Glucose Metabolism, After Factoring out the Effect of Age (p < 0.05).
(A) Results in the whole preclinical group. On the left of each result, the scatterplots display the correlations between factors and glucose metabolism in the significant clusters in the whole sample. Significant correlations among the 3 neuropsychological principal component analysis (PCA) factors and brain metabolism and the 2 neuropsychiatric PCA factors and brain metabolism are reported on the left and the right, respectively. The executive/visuomotor factor showed a significant negative correlation with metabolism (i.e., linear decrease in glucose metabolism together with impaired performance, thus higher scores) in the superior and the middle frontal gyri, lingual gyrus, cuneus, precuneus, and middle cingulate cortex, plus the caudate nuclei and thalamus, bilaterally. The memory factor showed a significant positive correlation with metabolism (i.e., linear decrease in glucose metabolism together with decreasing scores) in the precuneus, cuneus, superior and inferior parietal lobules, the posterior and middle cingulate cortices, and the superior and the middle frontal gyri. The visuospatial/constructional factor showed a significant positive correlation with right-lateralized metabolism (i.e., linear decrease in glucose metabolism together with decreasing scores of grouped tests), specifically in the angular gyrus, the anterior and middle cingulate cortex, and the dorsolateral frontal cortex. The affective factor showed a significant negative correlation with brain metabolism (i.e., linear decrease in glucose metabolism together with increasing symptoms) mainly in the right hemisphere, insula, temporal pole, anterior cingulate cortex, superior temporal gyrus, and inferior frontal gyrus (pars orbitalis). The hyperactive/psychotic factor showed a significant negative correlation with metabolism (i.e., linear decrease in glucose metabolism together with increasing symptoms) in the orbitofrontal cortex and prefrontal cortex, anterior cingulate cortex, left lateral temporal cortex, insula, and caudate. (B) Results of voxel-wise multivariate regression models (run separately for pre–mild cognitive impairment [pre–MCI] and subjective cognitive decline [SCD] subgroups). Results obtained in pre–MCI and SCD are blue and green, respectively (overlap areas are light blue). The executive/visuomotor factor negatively correlated with metabolism in extended temporoparietal and occipital regions in the pre–MCI group, whereas in the SCD group, this factor correlated mainly with subcortical structures. The positive correlations with the memory factor were prevalently represented by the pre–MCI group in frontal-temporal-parietal cortex. Correlations in the visuospatial/constructional factor were more represented in the right hemisphere and broadly similar in the 2 groups. The affective factor correlated with insular and temporal medial metabolism in both groups, whereas the hyperactive/psychotic factor correlated more extensively with frontal, temporal, and insular regions in the SCD group.
Discussion
The current study demonstrates the presence of patterns of brain hypometabolism indicative of dysfunctional or neurodegenerative brain changes in participants with normal or modestly impaired neuropsychological performance complaining of cognitive changes. Notably, a number of patients did not show signs of abnormal brain metabolism, and amyloid PET results were negative in the majority of the sample. This evidence indicates the heterogeneity within the sample and various possible underlying conditions.
FDG-PET with validated and standardized procedures for quantification of brain metabolism has shown high accuracy in identifying hypometabolic patterns specific for AD or other conditions of neurodegeneration and, crucially, even in excluding the presence of neurodegeneration.18,24 The limited available FDG-PET data in SCD and pre–MCI were not conclusive, also because they were analyzed only at a group level, with lack of individual specificity.26,27 Different from the voxel-wise approach applied here, allowing evaluation of the dysfunctional characteristics of the MCI condition in single individuals,17 the group analysis lacks diagnostic and prognostic precision, mainly because it focuses only on brain hypometabolism in AD-related regions.26,27
In our study, single-participant SPM analysis applied in single individuals with SCD or pre–MCI indicates normal brain metabolism, thus the absence of neurodegeneration, in 45% of the participants, while a specific hypometabolic pattern was observed in 55% of the sample. A frontal-like pattern was present in 28% of the whole sample. In the latter, FDG-PET showed the involvement of regions typically affected in the frontotemporal dementia spectrum,28-30 including the anterior cingulate cortex, frontal medial, dorsolateral, and orbitofrontal cortex, temporal lateral cortex, insula, and thalamus25 (Figure 1). These individuals in the frontal-like group showed a specific neuropsychiatric profile, namely a higher NPI global score, indicating the presence of NPS. Only a minority of participants (11%) showed a pattern of hypometabolism indicative for AD and involving temporoparietal areas and posterior cingulate cortex. This finding is intriguing because SCD and pre–MCI are usually considered as prodromal stages of AD, preceding the MCI condition.31 Seven percent of participants had temporal medial, limbic-predominant hypometabolism pattern, recently reported in long-lasting amnestic MCI not progressing to dementia.19 These participants had a specific neuropsychological profile, showing lower performances in semantic word fluency, as previously reported.32 Lastly, few participants (9%) were classified as having a DLB-like pattern, showing hypometabolism in temporal-parietal and occipital cortices, variably associated with frontal and subcortical hypometabolism.18 These findings confirm that the SCD and pre–MCI labels include an extremely heterogeneous population. The template-based approach confirmed the heterogeneity of the sample. The classification provided by this approach was largely congruent with the results of the single-participant SPM analysis, failing only in the case of individuals showing a pattern of hypometabolism overlapping with the following templates (i.e., the AD and the DLB templates and the frontal and the limbic-predominant templates).
Regarding amyloid status, the reported rate of amyloid positivity in SCD ranges from 12% to 43%.33 Amyloid PET studies in the preclinical phase of dementia showed that cortical amyloid load is frequently associated with SCD, even years before the onset of objective cognitive deficit.34 In our study, an abnormal brain amyloid load was evident in 18% of participants who underwent amyloid PET. In about one-third of Aβ+ cases, there was an AD-like hypometabolism pattern, confirming the neurobiology of AD and the risk to develop AD dementia in a minority of participants. Interestingly, no participants with the DLB-like and limbic-predominant patterns were Aβ+, while one-third of participants with an abnormal amyloid load showed a frontal-like hypometabolism pattern, suggesting an atypical AD or the possibility of copathology, including TDP-43 proteinopathy and cerebral amyloid angiopathy, which can coexist with AD pathologic changes. The final one-third of participants with abnormal amyloid accumulation showed no brain metabolism abnormalities. These findings feed the debate on the role of amyloid pathology, as a considerable percentage of participants with normal cognition may show brain amyloid deposition, and individuals with abnormal amyloid load and normal FDG-PET pattern are unlikely to progress to AD dementia, as revealed in a large MCI population.35 On the other hand, individuals with abnormal brain amyloid load and no actual signs of neurodegeneration on brain imaging may still represent a very early stage along the dementia continuum, as it has been reported that a long time span separates the preclinical dementia phase and the occurrence of the earliest manifestations of objective cognitive decline.36
The single-subject SPM evaluation found about 45% of cases with a normal FDG-PET scan. In these individuals, FDG-PET has a fundamental exclusionary role for actual signs of neurodegeneration. This is associated with a favorable prognosis and long-term stability.37 As confirmation, a recent longitudinal study investigating CSF biomarker positivity in SCD showed a favorable prognosis associated with the absence of biomarkers of pathology and neurodegeneration.38
Parallel to the categorical framework approach, we applied a data-driven dimensional approach capturing the neuropsychological and neuropsychiatric differences and relating them to the participants' brain metabolism. Different neuropsychological profiles at this stage correlate with neuronal dysfunction in specific brain regions (Figure 2), with crucial differences between SCD and pre–MCI. Three different neuropsychological dimensions, revealed by PCA, correlated with specific metabolic dysfunction: (1) executive/visuomotor, with metabolism in frontal, occipital cortex, and basal ganglia; (2) episodic memory, with metabolism in temporal-parietal regions; (3) visuospatial/constructional, with metabolism in right frontoparietal cortices. These components are in line with the profile of subtle neuropsychological deficits, involving especially memory and executive functions, recently identified in a large SCD cohort.39 In our study, the executive/visuomotor dimension included the TMT subtests, evaluating attentive, executive, and visuomotor functions. Higher scores in the TMT subtests indicate possible attentive, executive, or visuomotor deficit and were inversely correlated with metabolism in regions known to be involved in executive functions, visuospatial skills, and motor control; that is, the frontal cortex, occipital cortex, and subcortical structures such as basal ganglia.40 The memory dimension involved participants whose memory performance correlated with metabolism in the precuneus, cuneus, temporoparietal cortices, cingulate cortex, and the superior and middle frontal regions. These data parallel the distribution of brain hypometabolism in typical AD cases, with additional slight occipital and frontal involvement, confirming that monitoring of the memory domain is of utmost importance in identifying participants with possible AD-like risk of progression.5 Finally, the visuospatial/constructional dimension includes participants whose test scores correlate with metabolism in widespread right frontotemporal and parietal areas.41
The correlation of neuropsychiatric profiles with brain glucose metabolism is of particular interest. By using NPI subsyndromes and SAS as inputs, PCA provides 2 dimensions: affective, including apathy, anxiety, depressive, and eating disturbances; and hyperactive/psychotic, including agitation, irritability, euphoria, aberrant motor behavior, disinhibition, delusions, hallucinations, and nighttime sleep disturbances.13,42 The affective dimension is inversely correlated with metabolism in the orbitofrontal cortex, cingulate cortex, and insula. These findings are in line with previous reports in participants with AD dementia, where the presence of apathy correlated with hypometabolism in the orbitofrontal cortices, and the occurrence of anxiety and depression with metabolism in the anterior cingulate and frontal and prefrontal cortices14 (see reference 12 for a review). However, despite strong epidemiologic evidence that affective NPS are significant predictors of progression from preclinical to early clinical stages of AD, experimental data on the metabolic profile in preprodromal participants with affective symptoms are scarce. Hypometabolism in regions that undergo accelerated atrophy in preclinical and clinical stages of AD (i.e., bilateral temporal, parietal, and posterior cingulate cortices) is associated in cognitively normal adults with subthreshold symptoms of depression, particularly those related to apathy and anhedonia.43 Because these associations can be independent of cortical amyloid burden43 or cognitive symptoms and can be longitudinally, but not cross-sectionally, associated with amyloid PET AD biomarkers,44,45 the neurobiology of apathy/anhedonia and depressive symptoms may not be mediated solely by AD proteinopathies. A pattern of hypometabolism in the absence of high amyloid burden at the preclinical stage may suggest that apathy and anhedonia symptoms are signals of neuronal injury driven by other mechanisms, or conferring increased vulnerability to AD pathophysiology and clinical decline. Given the robust relationship between cerebrovascular disease and affective symptoms, and the vulnerability of cognitive control and affective networks to either age-related or vascular changes and frontotemporal dementia,46 combined etiopathologies may contribute to affective NPS in predementia syndromes. The hyperactive/psychotic dimension had significant inverse correlation with the frontotemporal, insular, and caudate metabolism, again in line with previous reports.14 Findings of metabolic dysfunctions in hyperactivity/psychotic subsyndromes are mostly limited to participants in the clinical phase of AD (in particular, delusion, hallucinations, and nighttime disturbances are infrequently reported in early stages). However, sleep disturbances and irritability (which emerges in the dementia prodrome and is associated with similar metabolic changes to agitation) predicted posterior cingulate hypometabolism at 2 years.47 This finding, and the fact that agitation and irritability are associated with metabolic changes reflecting neurodegeneration in regions associated with core AD pathology, suggest that at least the hyperactivity subsyndrome constitutes an early clinical manifestation of AD pathophysiology.11,47
When evaluating SCD and pre–MCI separately, some differences in neuropsychological/neuropsychiatric profiles emerged (Figure 2). Of note, participants with SCD drove the correlations between the hyperactive/psychotic factor and widespread metabolism in frontal-temporal regions, suggesting a possible dysfunctional correlate of these neuropsychiatric disturbances.48 This is in line with literature data on the preclinical dementia stages reporting a high prevalence of NPS in SCD populations.48,49 The occurrence of neuropsychiatric disturbances may represent a clinical feature per se or a precipitating factor in dementia progression,11 pointing out the importance of an extensive clinical, neuropsychological and neurobehavioral, and instrumental approach for the correct identification of different clinical prognostic patterns.
We acknowledge some limitations of the study. The lack of longitudinal assessment does not allow the investigation of the value of PET biomarkers in predicting clinical outcomes. This is particularly important in participants showing inconclusive biomarker alterations and individuals with subtle cognitive impairment and without brain imaging changes. Due to the multicenter design of our study, we could not perform a fully data-driven approach on both clinical and imaging data. However, we chose the single-participant SPM method to classify participants based on brain hypometabolism maps, which is not affected by the acquisition of PET imaging from different scans.16 In addition, to test the single-participant independent rating, we employed an objective approach based on the adherence of the hypometabolism maps to disease-specific templates, which largely confirmed the SPM method classification. Furthermore, we included information on the amyloid status in individuals who underwent amyloid PET, but we could not include CSF analysis and measures of tau pathology, which were available only in a minority of participants. Lastly, we did not include MRI data and white matter damage analysis, which could have clarified the vascular burden in our sample. Further studies should investigate other biological characteristics to provide a detailed description of participants with SCD and pre–MCI, allowing the consideration of additional mechanisms that could be involved in the interpretation of our findings.
Acknowledgment
The authors thank the individuals participating in the study and their families. Data used in preparation of this article were obtained from the Network-AD database. The authors thank the coinvestigators within the Network-AD who contributed to the design and implementation of Network-AD or provided data. A complete listing of Network-AD investigators can be found in appendix 2 (links.lww.com/WNL/B997).
Glossary
- AAL
automated anatomical labeling
- Aβ
β-amyloid
- AD
Alzheimer disease
- ADL
activities of daily living
- ANOVA
analysis of variance
- CDR
Clinical Dementia Rating
- DLB
dementia with Lewy bodies
- DSM-5
Diagnostic and Statistical Manual of Mental Disorders, 5th edition
- FCSRT
Free and Cued Selective Reminding Test
- KMO
Kaiser-Meyer-Olkin
- MCI
mild cognitive impairment
- MMSE
Mini-Mental State Examination
- NPI
Neuropsychiatric Inventory
- NPS
neuropsychiatric symptoms
- PCA
principal component analysis
- pre–MCI
pre–mild cognitive impairment
- ROI
region of interest
- SAS
Starkstein Apathy Scale
- SCD
subjective cognitive decline
- SPM
statistical parametric mapping
- SUVR
standard uptake value ratio
- TMT
Trail-Making Test
Appendix. Authors
Study Funding
This work was supported by the Italian Ministry of Health (Ricerca Finalizzata Progetto Reti Nazionale AD NET-2011-02346784).
Disclosures
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Jack CR, Knopman DS, Jagust WJ, et al. Tracking pathophysiological processes in Alzheimer's disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12(2):207-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sachdev PS, Blacker D, Blazer DG, et al. Classifying neurocognitive disorders: the DSM-5 approach. Nat Rev Neurol Nat. 2014;10(11):634-642. [DOI] [PubMed] [Google Scholar]
- 3.Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):270-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jessen F, Amariglio RE, Van Boxtel M, et al. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer's disease. Alzheimers Dement. 2014;10(6):844-852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jessen F, Amariglio RE, Buckley RF, et al. The characterisation of subjective cognitive decline. Lancet Neurol. 2020;19(3):271-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Storandt M, Grant EA, Miller JP, Morris JC. Longitudinal course and neuropathologic outcomes in original vs revised MCI and in pre-MCI. Neurology. 2006;67(3):467-473. [DOI] [PubMed] [Google Scholar]
- 7.Duara R, Loewenstein DA, Greig MT, et al. Pre-MCI and MCI: neuropsychological, clinical, and imaging features and progression rates. Am J Geriatr Psychiatry. 2011;19(11):951-960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):280-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jack CR Jr, Bennett DA, Blennow K, et al. NIA-AA Research Framework: toward a biological definition of Alzheimer's disease. Alzheimers Dement. 2018;14(4):535-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dubois B, Villain N, Frisoni GB, et al. Clinical diagnosis of Alzheimer’s disease: recommendations of the International Working Group. Lancet Neurol. 2021;20:484-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ismail Z, Smith EE, Geda Y, et al. Neuropsychiatric symptoms as early manifestations of emergent dementia: provisional diagnostic criteria for mild behavioral impairment. Alzheimers Dement. 2016;12(2):195-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ng KP, Chiew HJ, Rosa-Neto P, Kandiah N, Ismail Z, Gauthier S. Brain metabolic dysfunction in early neuropsychiatric symptoms of dementia. Front Pharmacol. 2019;10:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aalten P, Verhey FRJ, Boziki M, et al. Neuropsychiatric syndromes in dementia: results from the European Alzheimer Disease Consortium: part I. Dement Geriatr Cogn Disord. 2007;24(6):457-463. [DOI] [PubMed] [Google Scholar]
- 14.Ballarini T, Iaccarino L, Magnani G, et al. Neuropsychiatric subsyndromes and brain metabolic network dysfunctions in early onset Alzheimer's disease. Hum Brain Mapp. 2016;37(12):4234-4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Varrone A, Asenbaum S, Vander Borght T, et al. EANM procedure guidelines for PET brain imaging using [18F]FDG, version 2. Eur J Nucl Med Mol Imaging. 2009;36(12):2103-2110. [DOI] [PubMed] [Google Scholar]
- 16.Presotto L, Ballarini T, Caminiti SP, Bettinardi V, Gianolli L, Perani D. Validation of 18 F–FDG-PET single-subject optimized SPM procedure with different PET scanners. Neuroinformatics. 2017;15(2):151-163. [DOI] [PubMed] [Google Scholar]
- 17.Perani D, Anthony P, Rosa D, et al. Validation of an optimized SPM procedure for FDG-PET in dementia diagnosis in a clinical setting. Neuroimage Clin. 2014;6:445-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caminiti SP, Ballarini T, Sala A, et al. FDG-PET and CSF biomarker accuracy in prediction of conversion to different dementias in a large multicentre MCI cohort. Neuroimage Clin. 2018;18:167-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tondo G, Carli G, Santangelo R, et al. Biomarker-based stability in limbic-predominant amnestic mild cognitive impairment. Eur J Neurol. 2021;28(4):1123-1133. [DOI] [PubMed] [Google Scholar]
- 20.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19(3):1233-1239. [DOI] [PubMed] [Google Scholar]
- 21.Presotto L, Iaccarino L, Sala A, et al. Low-dose CT for the spatial normalization of PET images: a validation procedure for amyloid-PET semi-quantification. Neuroimage Clin. 2018;20:153-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Catafau AM, Bullich S, Seibyl JP, et al. Cerebellar amyloid-β plaques: how frequent are they, and do they influence 18F-florbetaben SUV ratios? J Nucl Med Soc Nucl Med. 2016;57(11):1740-1745. [DOI] [PubMed] [Google Scholar]
- 23.Ong K, Villemagne VL, Bahar-Fuchs A, et al. 18 F-florbetaben Aβ imaging in mild cognitive impairment. Alzheimers Res Ther. 2013;5(1):1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perani D, Cerami C, Caminiti SP, et al. Cross-validation of biomarkers for the early differential diagnosis and prognosis of dementia in a clinical setting. J Nucl Med Mol Imaging. 2016;43(3):499-508. [DOI] [PubMed] [Google Scholar]
- 25.Cerami C, Della Rosa PA, Magnani G, et al. Brain metabolic maps in mild cognitive impairment predict heterogeneity of progression to dementia. Neuroimage Clin. 2015;7:187-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vannini P, Hanseeuw B, Munro CE, et al. Hippocampal hypometabolism in older adults with memory complaints and increased amyloid burden. Neurology. 2017;88(18):1759-1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scheef L, Spottke A, Daerr M, et al. Glucose metabolism, gray matter structure, and memory decline in subjective memory impairment. Neurology. 2012;79(13):1332-1339. [DOI] [PubMed] [Google Scholar]
- 28.Teune LK, Bartels AL, De Jong BM, et al. Typical cerebral metabolic patterns in neurodegenerative brain diseases. Mov Disord. 2010;25(14):2395-2404. [DOI] [PubMed] [Google Scholar]
- 29.Armstrong MJ, Litvan I, Lang AE, et al. Criteria for the diagnosis of corticobasal degeneration. Neurology. 2013;80(5):496-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rascovsky K, Hodges JR, Knopman D, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134(pt 9):2456-2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rabin LA, Smart CM, Amariglio RE. Subjective cognitive decline in preclinical Alzheimer's disease. Annu Rev Clin Psychol. 2017;13:369-396. [DOI] [PubMed] [Google Scholar]
- 32.Marra C, Villa G, Quaranta D, Valenza A, Vita MG, Gainotti G. Probable Alzheimer's disease patients presenting as “focal temporal lobe dysfunction” show a slow rate of cognitive decline. J Int Neuropsychol Soc. 2012;18(1):144-150. [DOI] [PubMed] [Google Scholar]
- 33.Jansen WJ, Ossenkoppele R, Knol DL, et al. Prevalence of cerebral amyloid pathology in persons without dementia: a meta-analysis. JAMA. 2015;313(19):1924-1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang X, Huang W, Su L, et al. Neuroimaging advances regarding subjective cognitive decline in preclinical Alzheimer's disease. Mol Neurodegener. 2020;15(1):1-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perani D, Iaccarino L, Lammertsma AA, et al. A new perspective for advanced positron emission tomography–based molecular imaging in neurodegenerative proteinopathies. Alzheimers Dement. 15(8):1081-1103. [DOI] [PubMed] [Google Scholar]
- 36.Insel PS, Weiner M, Scott MacKin R, et al. Determining clinically meaningful decline in preclinical Alzheimer disease. Neurology. 2019;93(4):E322–E333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iaccarino L, Sala A, Perani D. Predicting long-term clinical stability in amyloid-positive participants by FDG-PET. Ann Clin Transl Neurol. 2019;6(6):1113-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ebenau JL, Timmers T, Wesselman LMP, et al. ATN classification and clinical progression in subjective cognitive decline: the SCIENCe project. Neurology. 2020;95(1):46-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wolfsgruber S, Kleineidam L, Guski J, et al. Minor neuropsychological deficits in patients with subjective cognitive decline. Neurology. 2020;95(9):e1134–e1143. [DOI] [PubMed] [Google Scholar]
- 40.Varjacic A, Mantini D, Demeyere N, Gillebert CR. Neural signatures of Trail Making Test performance: evidence from lesion-mapping and neuroimaging studies. Neuropsychologia. 2018;115:78-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gainotti G, Trojano L. Constructional apraxia. Handb Clin Neurol. 2018;151:331-348. [DOI] [PubMed] [Google Scholar]
- 42.Frisoni G, Rozzini L, Gozzetti A, et al. Behavioral syndromes in Alzheimer's disease: description and correlates. Dement Geriatr Cogn Disord. 1999;10(2):130-138. [DOI] [PubMed] [Google Scholar]
- 43.Donovan NJ, Hsu DC, Dagley AS, et al. Depressive symptoms and biomarkers of Alzheimer's disease in cognitively normal older adults. J Alzheimer Dis. 2015;46(1):63-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fjell AM, McEvoy L, Holland D, Dale AM, Walhovd KB, Initiative ADN. What is normal in normal aging? Effects of aging, amyloid and Alzheimer's disease on the cerebral cortex and the hippocampus. Prog Neurobiol. 2014;117:20-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Babulal GM, Ghoshal N, Head D, et al. Mood changes in cognitively normal older adults are linked to Alzheimer disease biomarker levels. Am J Geriatr Psychiatry. 2016;24(11):1095-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee J-Y, Park S, Mackin S, et al. Differences in prefrontal, limbic, and white matter lesion volumes according to cognitive status in elderly patients with first-onset subsyndromal depression. PloS One. 2014;9(1):e87747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ng KP, Pascoal TA, Mathotaarachchi S, et al. Neuropsychiatric symptoms predict hypometabolism in preclinical Alzheimer disease. Neurology. 2017;88(19):1814-1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Slavin MJ, Brodaty H, Kochan NA, et al. Prevalence and predictors of “subjective cognitive complaints” in the Sydney Memory and Ageing Study. Am J Geriatr Psychiatry. 2010;18(8):701-710. [DOI] [PubMed] [Google Scholar]
- 49.Braam AW, Copeland JRM, Delespaul PAEG, et al. Depression, subthreshold depression and comorbid anxiety symptoms in older Europeans: results from the EURODEP concerted action. J Affect Disord. 2014;155:266-272. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data not provided in the article because of space limitations may be shared at the request of any qualified investigator for purposes of replicating procedures and results.