Abstract
In patients who undergo thrombectomy for acute ischemic stroke, the relationship between pre-admission antithrombotic (anticoagulation or antiplatelet) use and both radiographic and functional outcome is not well understood. We sought to explore the relationship between pre-admission antithrombotic use in patients who underwent thrombectomy for acute ischemic stroke at two medical centers in New York City between December 2018 and November 2020. Analyses were performed using analysis of variance and Pearson’s chi-squared tests. Of 234 patients in the analysis cohort, 65 (28%) were on anticoagulation, 64 (27%) were on antiplatelet, and 105 (45%) with no antithrombotic use pre-admission. 3-month Modified Rankin Scale (mRS) score of 3–6 was associated with pre-admission antithrombotic use (71% anticoagulation vs. 77% antiplatelet vs. 56% no antithrombotic, p = 0.04). There was no relationship between pre-admission antithrombotic use and Thrombolysis in Cerebral Iinfarction (TICI) score, post-procedure Alberta Stroke Program Early CT Score (ASPECTS) score, rate of hemorrhagic conversion, length of hospital admission, discharge NIH Stroke Scale (NIHSS), discharge mRS score, or mortality. When initial NIHSS score, post-procedure ASPECTS score, and age at admission were included in multivariate analysis, pre-admission antithrombotic use was still significantly associated with a 3-month mRS score of 3–6 (OR 2.36, 95% CI 1.03–5.54, p = 0.04). In this cohort of patients with acute ischemic stroke who underwent thrombectomy, pre-admission antithrombotic use was associated with 3-month mRS score, but no other measures of radiographic or functional outcome. Further research is needed on the relationship between use of specific anticoagulation or antiplatelet agents and outcome after acute ischemic stroke, but moreover, improve stroke prevention.
Keywords: Stroke, Anticoagulation, Antiplatelet, Antithrombotic, Neuroprognostication, Thrombectomy
Highlights
In a cohort of 234 patients with acute ischemic stroke who underwent thrombectomy, 55% were on antithrombotic (anticoagulation or antiplatelet) pre-admission
In patients with acute ischemic stroke who underwent thrombectomy, pre-admission antithrombotic use was not associated with TICI score, post-procedure ASPECTS score, hemorrhagic conversion, hospital length of stay, discharge NIHSS score, or discharge mRS score
In patients with acute ischemic stroke who underwent thrombectomy, pre-admission antithrombotic use was associated with 3-month mRS score
After thrombectomy for acute ischemic stroke, discharge antithrombotic use is not associated with 3-month functional outcome or complications
Further work is needed to elucidate the relationship between specific antithrombotic agents and outcome after thrombectomy for acute ischemic stroke
Introduction
Although antithrombotics (anticoagulation and antiplatelets) are used for primary and secondary stroke prophylaxis, patients taking these medications can still have acute ischemic strokes. In a cross-sectional study of over 1000 patients hospitalized with acute ischemic stroke, 40% were on antiplatelet pre-admission [1]. In a cohort of 534 patients with known atrial fibrillation who presented with acute ischemic stroke, 23% were on anticoagulation pre-admission [2]. In another large cross-sectional study of over 8000 patients hospitalized for acute ischemic stroke, 13% were on anticoagulation pre-admission [3].
The relationship between pre-admission antithrombotic use and outcome after ischemic stroke is unclear. While some studies showed no association between pre-admission antithrombotic use and clinical outcome [4–7], others demonstrated anticoagulation or antiplatelet use pre-admission was associated with improved outcome [2, 3, 8–10]. Haeusler et al. found that patients with atrial fibrillation on pre-admission anticoagulation use had better admission NIHSS scores, but did not have a lower mortality rate [2]. Meinel et al. found that pre-admission anticoagulation use was associated with lower admission NIHSS score and that use of direct oral anticoagulation (DOACs) was associated with better 3-month modified rankin scale (mRS) score compared with use of vitamin K antagonists or no anticoagulation [3]. Sanossian et al. found that pre-admission antiplatelet use was associated with a lower NIHSS score at presentation and a better discharge mRS score [9]. Würtz et al. noted that dual antiplatelet use pre-admission decreased 30-day mortality after ischemic stroke [10]. Dowlatshahi et al. found that pre-admission aspirin or clopidogrel use was associated with lower stroke severity on admission using the Canadian Neurological Scale, but there was no relationship between stroke severity on presentation and use of any other antiplatelets or anticoagulation [8]. Although no antiplatelets were associated with discharge outcome, pre-admission warfarin use was associated with milder disability on discharge, regardless of whether INR was < 2 or ≥ 2.
Of patients who undergo thrombectomy for acute ischemic stroke, 20–34% are on antiplatelets and 13–23% are on anticoagulation pre-admission [11–17]. Antithrombotics can alter clot composition, allowing for easier lysis, which could improve recanalization [7, 18, 19]. However, pre-admission antithrombotic use could worsen outcome after thrombectomy due to the increased risk for hemorrhagic conversion [20, 21]. Multiple studies have found that patients on pre-admission antiplatelet therapy have higher rates of successful recanalization with no increased risk of intracerebral hemorrhage (ICH) [11, 13]. While some studies have found no association between pre-admission antiplatelet use and functional outcomes or mortality [11, 12], Huo et al. reported better 3-month mRS scores and lower risk of 3-month mortality in patients taking dual antiplatelets. [13] In studies on the impact of pre-admission anticoagulation use in patients who underwent thrombectomy for acute ischemic stroke, some investigators found no increased risk of ICH and no difference in 3-month mRS scores or mortality [15, 16]. However, Černík et al. found that pre-admission anticoagulation use was associated with worse 3-month mRS score despite faster recanalization time [17]. In a systematic review and meta-analysis, Liu et al. reported anticoagulation use pre-admission was associated was lower likelihood of good functional outcome at 3-months after thrombectomy for acute ischemic stroke [22].
We hypothesized that pre-admission antithrombotic use would negatively affect functional outcomes in patients with acute ischemic stroke who underwent thrombectomy. As a secondary aim, we sought to determine the relationship between discharge antithrombotic use and both functional outcome and complications.
Methods
Patient identification and data collection
We performed a retrospective chart review of the “Get with the Guidelines” database at the Tisch and Brooklyn campuses of NYU Langone Medical Center to identify patients who underwent thrombectomy for acute ischemic stroke between December 2018 and November 2020. Patients who underwent > 1 thrombectomies during this period were only included once. We abstracted data from our electronic medical record on sex, age, past medical history, pre-admission antithrombotic use, admission INR, SARS-CoV-2 polymerase chain reaction (PCR), intravenous tissue plasminogen activator (tPA) administration, clot location, time from last known well to groin puncture, hospital length of stay, and readmission within 3 months post-discharge. A board-certified neurologist determined initial and discharge NIHSS score, initial and post-procedure Alberta Stroke Program Early CT Score (ASPECTS) score, and discharge and 3-month mRS score. They also evaluated post-procedure imaging to assess for hemorrhagic conversion. An interventional radiologist determined post-procedure Thrombolysis in Cerebral Infarct (TICI) score.
Data analysis
We evaluated the relationship between pre-admission antithrombotic use and demographics, radiographic and functional outcome (TICI score, post-procedure ASPECTS score, discharge NIHSS score, discharge and 3-month mRS score, and discharge and 3-month mortality), hospital length of stay and hemorrhagic conversion. We performed this analysis comparing (1) patients on anticoagulation, antiplatelet, and no antithrombotic and (2) patients on either antithrombotic and no antithrombotic. We also explored the relationship between discharge antithrombotic use, defined as treatment on discharge or within 3 months of discharge, and 3-month mRS score, 3-month mortality, readmission, ischemic stroke 3-months post-discharge, and bleeding 3-months post-discharge. On analyses that compared patients on anticoagulation to patients on antiplatelet, patients on both anticoagulation and antiplatelet were only included in the anticoagulation group. mRS and ASPECTS scores were dichotomized (mRS 0–2 and 3–6 [23], ASPECTS ≥ 8 and < 8 [24]).
We performed a multivariate analysis assessing the relationship between dichotomized 3-month mRS score and pre-admission antithrombotic use which included initial NIHSS score, post-procedure ASPECTS score, and age at admission, given these are known to be related to functional outcome after stroke [25, 26]. This analysis was performed on the entire cohort as well as on patients with anterior circulation strokes only given that NIHSS and ASPECTS scores have less utility in posterior circulation strokes [27–30].
The study period overlapped with the height of the COVID-19 pandemic, so patients with a positive SARS-CoV-2 PCR during their admission were excluded from analyses due to the risk of coagulopathy in patients with COVID-19 [31–34] (all patients admitted after March 2020 were tested for COVID-19, and all patients who were not tested for COVID-19 were considered COVID-negative).
All analyses were performed using Welch’s two sample t-test, analysis of variance, Pearson’s chi-squared test, and logistical regression. All statistical analyses were completed using R Statistical Software (Foundation for Statistical Computing, Vienna, Austria).
Permissions
This study was approved by the IRB. Consent was waived.
Results
Patient demographics
During the study period, 248 patients underwent thrombectomy for acute ischemic stroke. Of these, 14 (6%) patients had a positive SARS-CoV-2 PCR, leaving 234 patients (median age 75 years, interquartile range (IQR) 61–84; 51% female, Table 1). Many patients in this cohort had conditions requiring antithrombotic use including atrial fibrillation (29%), coronary artery disease (CAD; 26%), prior ischemic stroke (20%), congestive heart failure (CHF; 16%), and deep vein thrombosis/pulmonary embolism (DVT/PE; 7%).
Table 1.
Demographics
| Parameter | All patients (n = 234) | Anticoagulation pre-admissiona (n = 65) | Antiplatelet pre-admissiona (n = 64) | None (n = 105) | p-value |
|---|---|---|---|---|---|
| Female, n (%) | 120 (51%) | 33 (51%) | 37 (58%) | 50 (48%) | 0.44 |
| Age, median (IQR) | 75 (61–84) | 75 (65–85) | 78 (71–84) | 70 (54–78) | < 0.001 |
| Past medical history, n (%) | |||||
| Atrial fibrillation | 69 (29%) | 46 (71%) | 14 (22%) | 9 (9%) | < 0.001 |
| Cancer | 39 (17%) | 16 (25%) | 10 (16%) | 13 (12%) | 0.11 |
| Congestive heart failure | 38 (16%) | 17 (26%) | 12 (19%) | 9 (9%) | 0.01 |
| Coronary artery disease | 61 (26%) | 22 (34%) | 26 (41%) | 13 (12%) | < 0.001 |
| Deep vein thrombosis/pulmonary embolism | 16 (7%) | 11 (17%) | 4 (6%) | 1 (1%) | < 0.001 |
| Stroke-hemorrhagic | 11 (5%) | 2 (3%) | 4 (6%) | 5 (5%) | 0.7 |
| Stroke-ischemic | 47 (20%) | 19 (29%) | 15 (23%) | 13 (12%) | 0.02 |
| Initial NIHSS score, median (IQR) | 17 (10–23) | 17 (8–23) | 16 (10–25) | 18 (10–23) | 0.99 |
| Initial ASPECTS score, n (%)c | 0.54 | ||||
| < 8 | 40 (17%) | 12 (18%) | 8 (13%) | 20 (19%) | |
| ≥ 8 | 193 (83%) | 53 (82%) | 55 (87%) | 85 (81%) | |
| Initial INR, median (IQR)b | 1.1 (1.0–1.2) | 1.2 (1.1–1.6) | 1.1 (1.0–1.1) | 1.0 (1.0–1.1) | < 0.001 |
| Intravenous tPA, n (%) | 74 (32%) | 6 (9%) | 20 (31%) | 48 (46%) | < 0.001 |
| Time from last known well to groin puncture (minutes), median (IQR)d | 299 (145–660) | 289 (136–572) | 302 (136–782) | 310 (172–583) | 0.14 |
NIHSS NIH stroke scale, tPA tissue plasminogen activator, IQR interquartile range, TICI Thrombolysis in Cerebral Infarct, ASPECTS Alberta Stroke Program Early CT Scores, mRS modified rankin scale
Bolded p-values are statistically significant (p < 0.05)
aPatients who were on both anticoagulation and antiplatelet pre-admission are listed only in the anticoagulation group
bData not available for 4 patients (1 in anticoagulation group, 1 in antiplatelet group, and 2 in none group)
cData not available for 1 patient in antiplatelet group
dData not available for 14 patients (4 in anticoagulation group, 4 in antiplatelet group, 6 in none group)
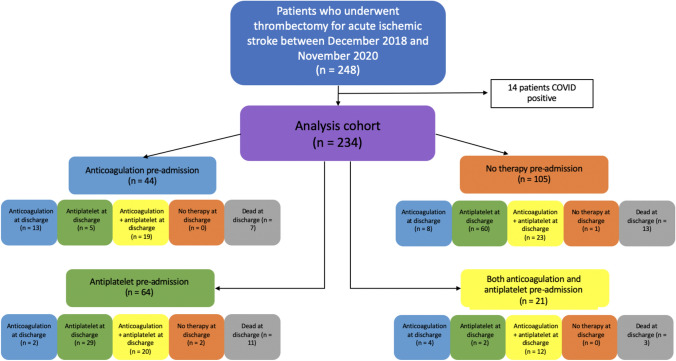
There were 65 (28%) patients with anticoagulation use pre-admission; 28 (43%) were on apixaban, 19 (29%) were on warfarin, 12 (18%) were on rivaroxaban, 4 (6%) were on enoxaparin or heparin, and 2 (3%) were on dabigatran. Of these, 21 (32%) were also taking antiplatelets. There were 64 (27%) patients with only antiplatelet use pre-admission; 43 (67%) were on only aspirin, 11 (17%) were on only plavix, and 10 (16%) were on both. There were 105 (45%) patients with no antithrombotic use pre-admission (Fig. 1).
Fig. 1.
Research cohort selection and pre-admission and discharge antithrombotic use
There were significant differences between patients taking pre-admission anticoagulation, antiplatelet, and neither in age (75 (IQR 65–85) years-old vs 78 (IQR 71–84) years-old vs 70 (IQR 54–78) years-old, p < 0.001), and rates of (1) atrial fibrillation (71% vs. 22% vs. 9%, p < 0.001), (2) CHF (26% vs. 19% vs. 9%, p = 0.01), (3) CAD (34% vs. 41% vs. 12%, p < 0.001), (4) DVT/PE (17% vs. 6% vs. 1%, p < 0.001), and (5) ischemic stroke (29% vs. 23% vs. 12%, p = 0.02; Table 1).
Stroke characteristics
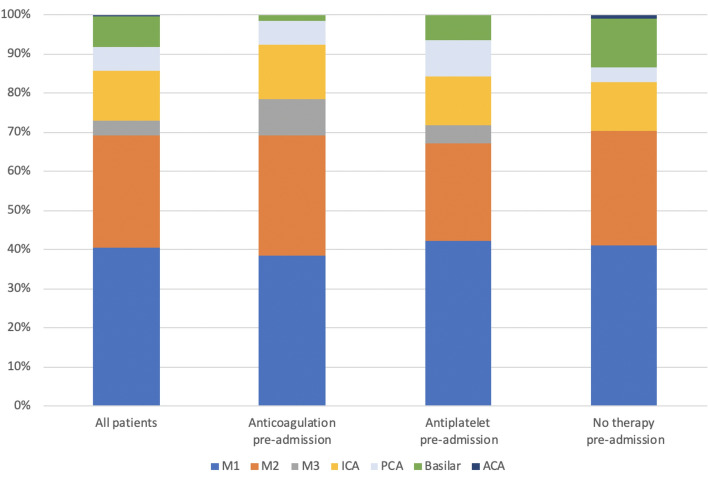
The median admission NIHSS score was 17 (IQR 10–23) and the median initial ASPECTS score was 9 (IQR 8–10). Admission NIHSS score and initial ASPECTS score did not significantly differ between patients based on antithrombotic use pre-admission. Most patients had a clot in the M1 or M2 segment of the middle cerebral artery (MCA; 41% and 29%, respectively, Fig. 2). Antithrombotic use was associated with initial INR (median 1.2 (IQR 1.1–1.6)) anticoagulation use versus (1.1 (1.0–1.1)) antiplatelet use versus (1.0 (1.0–1.1) no antithrombotic use, p < 0.001). Intravenous tPA was given to 32% of patients prior to thrombectomy; tPA administration rate was lower for patients with anticoagulation use than for patients with antiplatelet use or no antithrombotic use pre-admission (9% vs. 31% vs. 46%, p < 0.001). The time from last known well to groin puncture was similar between the groups, with a median of 299 min (4 h and 59 min; Table 1).
Fig. 2.
Clot location by pre-admission antithrombotic use
The relationship between pre-admission antithrombotic use and outcome
There were 135 (58%) patients who had TICI 3 recanalization and 50 (21%) who had TICI 2B recanalization. The median post-procedure ASPECTS score was 8 (IQR 5–9). There were 24 (11%) patients who had hemorrhagic conversion. The median hospital length of stay was 7 days (IQR 4–12). At discharge, the median NIHSS score was 6 (IQR 2–12) and there were 182 (78%) patients with a mRS score of 3–6. There were 34 patients (15%) who died prior to discharge; 30 (88%) had a do-not-resuscitate (DNR) order and 30 (88%) were made comfort care.
3-month mRS scores were available for 188 patients; 51 (27%) with anticoagulation use, 52 (28%) with antiplatelet use, and 85 (45%) with no antithrombotic use pre-admission. Of these, 124 (66%) patients had mRS scores of 3–6. There were 17 patients who died between discharge and 3-months post-discharge, so of the 188 patients with 3-month mRS scores, there were 51 (27%) who died between admission and 3-month post-discharge; 37 (73%) had a DNR order and 36 (51%) were made comfort care.
3-month mRS score of 3–6 was associated with pre-admission antithrombotic use (71% anticoagulation versus 77% antiplatelet versus 56% no antithrombotic, p = 0.04; 74% antithrombotic versus 56% no antithrombotic, p = 0.02). There was no significant difference between other measures of radiographic and functional outcome including mortality (and mortality with code status of DNR or comfort care), hospital length of stay, or hemorrhagic conversion based on pre-admission antithrombotic use (Tables 2, 3).
Table 2.
Univariate analysis of outcome based on pre-admission anticoagulation or antiplatelet use
| Parameter | All patients (n = 234) | Anticoagulation pre-admissiona (n = 65) | Antiplatelet pre-admissiona (n = 64) | None (n = 105) | p-value |
|---|---|---|---|---|---|
| TICI score of 3, n (%) | 135 (58%) | 42 (65%) | 37 (58%) | 56 (53%) | 0.35 |
| Post-procedure ASPECTS score ≥ 8, n (%)d | 116 (52%) | 31 (50%) | 38 (60%) | 47 (48%) | 0.29 |
| Hemorrhagic conversion, n (%)b | 24 (11%) | 5 (8%) | 9 (15%) | 10 (10%) | 0.50 |
| Hospital length of stay (days), median (IQR) | 7 (4–12) | 7 (5–11) | 8 (4–15) | 5 (4–12) | 0.51 |
| Discharge NIHSS score, median (IQR)c | 6 (2–12) | 5 (2–13) | 6 (2–11) | 5 (1–11) | 0.61 |
| Discharge mRS score 3–6, n (%) | 182 (78%) | 54 (83%) | 53 (83%) | 75 (71%) | 0.11 |
| 3-month mRS score 3–6, n (%)e | 124 (66%) | 36 (71%) | 40 (77%) | 48 (56%) | 0.04 |
| Discharge mortality | 34 (15%) | 10 (15%) | 11 (17%) | 13 (12%) | 0.67 |
| 3-month mortalitye | 51 (27%) | 15 (29%) | 17 (33%) | 19 (22%) | 0.38 |
Bolded p-values are statistically significant (p < 0.05)
aPatients who were on both anticoagulation and antiplatelet pre-admission are listed only in the anticoagulation group
bData not available for 12 patients (3 in anticoagulation group, 2 in antiplatelet group, and 7 in none group)
cData not available for 34 patients (10 in anticoagulation group, 11 in antiplatelet group, 13 in none group)
dData not available for 11 patients (3 in anticoagulation group, 1 in antiplatelet group, and 7 in none group)
eData not available for 46 patients (14 in anticoagulation group, 12 in antiplatelet group, and 20 in none group)
Table 3.
Univariate analysis of outcome based on pre-admission antithrombotic use
| Parameter | Antithrombotics pre-admission (n = 129) | None (n = 105) | p-value |
|---|---|---|---|
| TICI score of 3, n (%) | 79 (61%) | 56 (53%) | 0.28 |
| Post-procedure ASPECTS score ≥ 8, n (%)a | 69 (55%) | 47 (48%) | 0.35 |
| Hemorrhagic conversion, n (%)b | 14 (11%) | 10 (10%) | 0.97 |
| Hospital length of stay (days), median (IQR) | 7 (5–13) | 5 (4–12) | 0.24 |
| Discharge NIHSS score, median (IQR)c | 6 (2–13) | 5 (1–11) | 0.61 |
| Discharge mRS score 3–6, n (%) | 107 (83%) | 75 (71%) | 0.05 |
| 3-month mRS score 3–6, n (%)d | 76 (74%) | 48 (56%) | 0.02 |
| Discharge mortality | 21 (16%) | 13 (12%) | 0.51 |
| 3-month mortalityd | 32 (31%) | 19 (22%) | 0.24 |
Bolded p-values are statistically significant (p < 0.05)
aData not available for 11 patients (4 in antithrombotic group and 7 in none group)
bData not available for 12 patients (5 in antithrombotic group and 7 in none group)
cData not available for 34 patients (21 in antithrombotic group 13 in none group)
dData not available for 46 patients (26 in antithrombotic group and 20 in none group)
When initial NIHSS score, post-procedure ASPECTS score, and age at admission were included in multivariate analysis, there were significant differences in 3-month mRS scores between patients on antithrombotics and those not (OR 2.36, 95% CI 1.03–5.54, p = 0.04); however, this relationship did not remain significant when only patients with anterior circulation strokes were included (p = 0.05). On multivariate analysis, there was no significant difference in 3-month mRS score between patients on anticoagulation and those not on anticoagulation and patients on antiplatelets and those not on antiplatelets (Table 4).
Table 4.
Multivariate analysis of dichotomized 3-month mRS score based on pre-admission antithrombotic use
| All patients (n = 178)a | Patients with anterior circulation strokes (n = 155)b | |||
|---|---|---|---|---|
| Predictors | OR (95% CI) | p-value | OR (95% CI) | p-value |
| Pre-admission anticoagulation or antiplatelet use | ||||
| Anticoagulation or antiplatelet use | 2.36 (1.03–5.54) | 0.04 | 2.40 (1.00–5.96) | 0.05 |
| Initial NIHSS score | 1.12 (1.06–1.19) | < 0.001 | 1.10 (1.03–1.17) | 0.004 |
| Post-procedure ASPECTS score | 0.84 (0.71–0.97) | 0.02 | 0.78 (0.64–0.92) | 0.007 |
| Age at admission | 1.06 (1.03–1.08) | < 0.001 | 1.06 (1.03–1.10) | < 0.001 |
| Pre-admission anticoagulation use | ||||
| Anticoagulation use | 1.36 (0.56–3.44) | 0.50 | 1.67 (0.66–4.47) | 0.29 |
| Initial NIHSS score | 1.12 (1.06–1.18) | < 0.001 | 1.10 (1.03–1.17) | 0.004 |
| Post-procedure ASPECTS score | 0.86 (0.73–0.99) | 0.04 | 0.80 (0.66–0.94) | 0.01 |
| Age at admission | 1.06 (1.04–1.09) | < 0.001 | 1.07 (1.04–1.10) | < 0.001 |
| Pre-admission antiplatelet use | ||||
| Antiplatelet use | 1.92 (0.83–4.62) | 0.14 | 1.57 (0.64–3.98) | 0.33 |
| Initial NIHSS score | 1.12 (1.06–1.19) | < 0.001 | 1.09 (1.03–1.17) | 0.004 |
| Post-procedure ASPECTS score | 0.84 (0.72–0.98) | 0.03 | 0.79 (0.66–0.93) | 0.01 |
| Age at admission | 1.06 (1.03–1.09) | < 0.001 | 1.07 (1.04–1.10) | < 0.001 |
Odds ratios for ASPECTS and NIHSS scores are based on a 1-point increase
Bolded p-values are statistically significant (p < 0.05)
a46 patients without 3-month mRS scores were excluded from analysis. An additional 10 patients without post-procedure ASPECTS score were excluded
b39 patients without 3-month mRS scores were excluded from analysis. An additional 8 patients without post-procedure ASPECTS score were excluded
The relationship between discharge antithrombotic use and outcome
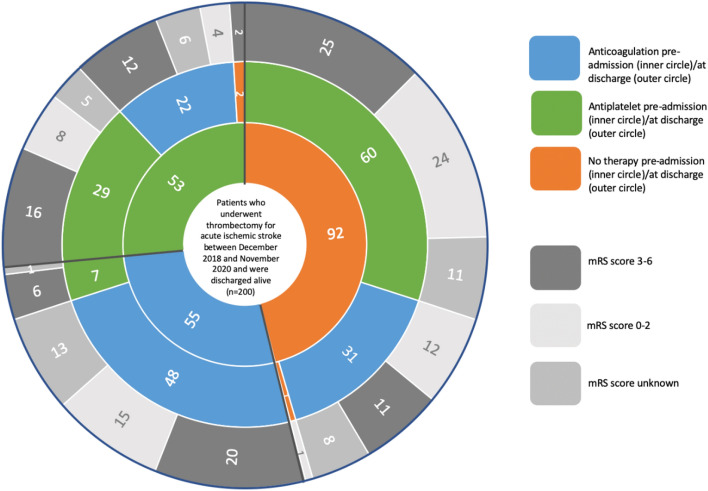
Of the 200 patients discharged alive, three were not discharged on antithrombotics (two were discharged to hospice and one had no indication for antithrombotic because their stroke was due to fungal endocarditis). Of the remaining 197 patients, 101 (51%) were discharged on anticoagulation; 74 (73%) of these patients were discharged on both anticoagulation and antiplatelet (Fig. 1). Of these 101 patients, 48 (48%) were on anticoagulation pre-admission, 22 (22%) were on antiplatelet pre-admission, and 31 (31%) were on neither. Of the 96 patients discharged on antiplatelet, 7 (7%) were taking anticoagulation, 29 (30%) were taking antiplatelet, and 60 (63%) were on neither pre-admission.
3-month mRS scores were available for 156 patients discharged alive; 74 (47%) were discharged on anticoagulation, 79 (51%) were discharged on antiplatelet, and 3 (2%) were discharged on no antithrombotic. Of the 74 patients discharged on anticoagulation, 43 (58%) had a 3-month mRS score of 3–6, and of the 79 patients discharged on antiplatelet, 47 (59%) had a 3-month mRS score of 3–6 (Fig. 3).
Fig. 3.
3-month modified rankin scale score based on pre-admission and discharge antithrombotic use
There were 38 patients (19%) readmitted within 3-months of discharge; 20 (53%) were on anticoagulation and 18 (39%) were on antiplatelet at discharge. There was only one patient with ischemic stroke and one patient with bleeding in the 3-months post-discharge; both were discharged on anticoagulation. The other 36 patients were readmitted for infections (15), cardiovascular problems (9), gastrointestinal problems (3), social issues (2), additional stroke workup (2), recrudescence of stroke symptoms (1), deep vein thrombosis (1), metabolic derangement (1), respiratory symptoms (1), and orthopedic problems (1).
Discharge antithrombotic use was not associated with 3-month mRS score, 3-month mortality, readmission rate, or ischemic stroke or bleeding in the 3-months post-discharge (Table 5).
Table 5.
Univariate analysis of outcome based on discharge anticoagulation or antiplatelet use
| Parameter | All patients (n = 200)a | Anticoagulation at dischargeb (n = 101) | Antiplatelet at dischargeb (n = 96) | p-value |
|---|---|---|---|---|
| 3-month mRS score 3–6, n (%)c | 92 (59%) | 43 (58%) | 47 (59%) | 1 |
| Readmission within 3 months post-discharge, n (%) | 38 (19%) | 20 (20%) | 18 (19%) | 0.97 |
| Ischemic stroke 3-months post-discharge, n (%) | 1 (0.5%) | 1d (1%) | 0 (0%) | 1 |
| Bleeding 3-months post-discharge, n (%) | 1 (0.5%) | 1e (1%) | 0 (0%) | 1 |
| 3-month mortalityc | 19 (12%) | 8 (11%) | 9 (11%) | 1 |
Bolded p-values are statistically significant (p < 0.05)
aThere were 200 patients discharged alive
bPatients who were discharged on both anticoagulation and antiplatelet are listed only in the anticoagulation group. There were three patients who were not discharged on either therapy
cData not available for 44 patients (27 in anticoagulation group and 17 in antiplatelet group)
dOne patient who was discharged on apixaban was readmitted with an ischemic stroke and found to have lung cancer
eOne patient who was discharged on apixaban and aspirin was readmitted with a gastrointestinal bleed due to rectal ulcers
Discussion
In this cohort of 234 patients who underwent thrombectomy for acute ischemic stroke, over half were on antithrombotics pre-admission, consistent with prior reported rates of anticoagulation and antiplatelet use pre-admission in this population [11–17]. Pre-admission antithrombotic use was associated with worse 3-month mRS scores even when controlling for initial NIHSS score, post-procedure ASPECTS score, and age at admission, but did not affect any other measure of radiographic or functional outcome. Additionally, there was no relationship between discharge antithrombotic use and functional outcome or complications.
This study adds to the existing literature on the relationship between pre-admission antithrombotic use and outcome after thrombectomy for ischemic stroke. To date, pre-admission antiplatelet use has been shown to be safe, with no increase in ICH, but with the exception of one study that demonstrated dual antiplatelet use was associated with improved 3-month functional outcome and mortality, most existing data do not suggest pre-admission antiplatelet use impacts functional outcome [11–13]. Pre-admission anticoagulation use has also been shown to be safe; however, some studies demonstrated worse functional outcomes in this population [15–17, 22]. Consistent with this literature, we found no increase in adverse events in patients on antithrombotics pre-admission. Further, we found pre-admission antithrombotic use was associated with worse 3-month mRS score, but no other measure of functional or radiographic outcomes. The worse post-discharge functional outcome in patients on antithrombotics pre-admission may stem from differences in baseline characteristics in this population, including older age, more comorbidities, and higher rates of atrial fibrillation, which have been independently associated with worse outcomes [22]. Distinct from prior studies, we grouped all patients on anticoagulation and all patients on antiplatelets together regardless of specific therapies within these classes [5, 16, 19]. In addition, while other studies focused only on anticoagulation or antiplatelet therapy, our analyses included both cohorts of patients [11–17]. Additionally, most analyses on pre-admission anticoagulation use compared patients taking anticoagulation pre-admission to patients not taking anticoagulation, the latter of which included both patients taking antiplatelet and patients taking neither [15–17]. Similarly, some studies on antiplatelet use pre-admission did not exclude patients taking anticoagulation [12, 14]. Some of the existing studies on the relationship between pre-admission anticoagulation and antiplatelet use and outcome after thrombectomy for ischemic stroke also excluded important cohorts of patients, such as those with prior severe disability, fatal malignancy, uncompensated hypertension, renal failure, recent prior stroke, posterior circulation strokes, or those who were given intravenous tPA or intra-arterial thrombolysis. [12, 13, 15–17]
Our findings also add to the existing literature on the relationship between secondary stroke prophylaxis on discharge and both functional outcome and complications. We found that 19% of patients were readmitted within 3-months of discharge, consistent with prior estimates [35, 36]. We noted no association between discharge anticoagulation or antiplatelet use and readmission. This is consistent with the findings reported by Won Han et al., in which no association between outcomes and secondary stroke prophylaxis were found during a 90-day period [36]. However, the efficacy of secondary stroke prophylaxis is evident in larger studies over longer time periods. A large meta-analysis found a reduction in vascular events of 37 per 1000 among patients with history of transient ischemic attack (TIA) or stroke on antiplatelet compared to controls [37]. Similarly, a meta-regression analysis of eleven randomized control trials of secondary stroke prevention found aspirin reduced risk of stroke by 15% [38]. Aspirin has thus been designated a Class 1 therapy to prevent future strokes after TIA or ischemic stroke [39]. There is also evidence for anticoagulation use for secondary prophylaxis in patients with non-valvular atrial fibrillation. In the European Atrial Fibrillation Trial (EAFT), warfarin reduced the annual risk of stroke from 12 to 4%, and reduced the primary composite outcome (vascular death, myocardial infarction, stroke, or systemic embolism, HR 0.53; 95% CI 0.36–0.79) [40]. Thus, while we did not find benefits of secondary prophylaxis to outcomes over a 3-month period, meta-analyses and studies looking over longer timescales have found anticoagulation and antiplatelet use to be efficacious.
There are some limitations to this study. The cohort only includes patients from one city and is relatively small. Data were collected retrospectively. The follow-up timeframe to assess outcome was only 3-months, and 3-month mRS score was not available for all patients. Readmission data was only available for our hospital system. We did not evaluate the relationship between use of individual antithrombotic agents and outcome. In addition, comorbidities that necessitate treatment with antithrombotics, such as atrial fibrillation, CAD, CHF, ischemic stroke, and DVT, were significantly associated with antithombotic use and could themselves potentially confound or mediate the relationship between antithrombotic therapy and outcomes. While all patients who tested positive for COVID-19 were excluded from analysis, it is possible that some patients included in our cohort who were not tested prior to April 2020 had COVID-19.
Conclusion
Despite antithrombotic use, patients can have acute ischemic strokes necessitating treatment with thrombectomy. Our findings demonstrate that in patients who underwent thrombectomy for acute ischemic stroke, (1) pre-admission antithrombotic use is associated with worse 3-month mRS score, but no other measures of radiographic or functional outcome; and (2) discharge antithrombotic use is not associated with 3-month functional outcome or complications. Further research is needed on the relationship between use of specific anticoagulation or antiplatelets and outcome after acute ischemic stroke, but moreover, improve stroke prevention.
Declarations
Conflict of interest
The authors report no competing interests or funding.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Penina Krieger, Email: peninakrieger@gmail.com.
Ariane Lewis, Email: Ariane.Kansas.Lewis@gmail.com.
References
- 1.Qureshi AI, Kirmani JF, Safdar A, et al. High prevalence of previous antiplatelet drug use in patients with new or recurrent ischemic stroke: buffalo metropolitan area and Erie county stroke study. Pharmacotherapy. 2006;26(4):493–498. doi: 10.1592/phco.26.4.493. [DOI] [PubMed] [Google Scholar]
- 2.Haeusler KG, Konieczny M, Endres M, Villringer A, Heuschmann PU. Impact of anticoagulation before stroke on stroke severity and long-term survival. Int J Stroke. 2012;7(7):544–550. doi: 10.1111/j.1747-4949.2011.00672.x. [DOI] [PubMed] [Google Scholar]
- 3.Meinel TR, Branca M, De Marchis GM, et al. Prior anticoagulation in patients with ischemic stroke and atrial fibrillation. Ann Neurol. 2021;89(1):42–53. doi: 10.1002/ana.25917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sivenius J, Cunha L, Diener HC, et al. Antiplatelet treatment does not reduce the severity of subsequent stroke. Neurology. 1999;53(4):825–825. doi: 10.1212/WNL.53.4.825. [DOI] [PubMed] [Google Scholar]
- 5.De Keyser J, Herroelen L, De Klippel N. Early outcome in acute ischemic stroke is not influenced by the prophylactic use of low-dose aspirin. J Neurol Sci. 1997;145(1):93–96. doi: 10.1016/S0022-510X(96)00250-X. [DOI] [PubMed] [Google Scholar]
- 6.Nowak K, Włodarczyk E, Porębska K, et al. Mechanical thrombectomy for acute ischaemic stroke during therapeutic anticoagulation: long-term outcomes. Neurol Neurochir Pol. 2020;54(6):538–543. doi: 10.5603/PJNNS.a2020.0088. [DOI] [PubMed] [Google Scholar]
- 7.Wang C, Hang Y, Cao Y, et al. Association between prior anticoagulation and thrombus composition in mechanical thrombectomy patients with atrial fibrillation. J Stroke Cerebrovasc Dis. 2022;31(4):106347. doi: 10.1016/j.jstrokecerebrovasdis.2022.106347. [DOI] [PubMed] [Google Scholar]
- 8.Dowlatshahi D, Hakim A, Fang J, Sharma M. Pre admission antithrombotics are associated with improved outcomes following ischaemic stroke: a cohort from the registry of the Canadian stroke network. Int J Stroke. 2009;4(5):328–334. doi: 10.1111/j.1747-4949.2009.00331.x. [DOI] [PubMed] [Google Scholar]
- 9.Sanossian N, Saver JL, Rajajee V, et al. Premorbid antiplatelet use and ischemic stroke outcomes. Neurology. 2006;66(3):319–323. doi: 10.1212/01.wnl.0000195889.05792.f1. [DOI] [PubMed] [Google Scholar]
- 10.Würtz M, Schmidt M, Grove EL, et al. Pre-admission use of platelet inhibitors and short-term stroke mortality: a population-based cohort study. Euro Heart J Cardiovasc Pharmacotherap. 2018;4(3):158–165. doi: 10.1093/ehjcvp/pvy010. [DOI] [PubMed] [Google Scholar]
- 11.Merlino G, Sponza M, Gigli GL, et al. Prior use of antiplatelet therapy and outcomes after endovascular therapy in acute ischemic stroke due to large vessel occlusion: a single-center experience. J Clin Med. 2018;7(12):E518. doi: 10.3390/jcm7120518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van de Graaf RA, Zinkstok SM, Chalos V, et al. Prior antiplatelet therapy in patients undergoing endovascular treatment for acute ischemic stroke: results from the MR CLEAN registry. Int J Stroke. 2021;16(4):476–485. doi: 10.1177/1747493020946975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huo X, Raynald JJ, et al. Safety and efficacy of oral antiplatelet for patients who had acute ischaemic stroke undergoing endovascular therapy. Stroke Vasc Neurol. 2021;6(2):230–237. doi: 10.1136/svn-2020-000466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pandhi A, Tsivgoulis G, Krishnan R, et al. Antiplatelet pretreatment and outcomes following mechanical thrombectomy for emergent large vessel occlusion strokes. J NeuroIntervent Surg. 2018;10(9):828–833. doi: 10.1136/neurintsurg-2017-013532. [DOI] [PubMed] [Google Scholar]
- 15.Zapata-Wainberg G, Ximénez-Carrillo Á, Trillo S, et al. Mechanical thrombectomy in orally anticoagulated patients with acute ischemic stroke. J NeuroIntervent Surg. 2018;10(9):834–838. doi: 10.1136/neurintsurg-2017-013504. [DOI] [PubMed] [Google Scholar]
- 16.Wong JWP, Churilov L, Dowling R, et al. Safety of endovascular thrombectomy for acute ischaemic stroke in anticoagulated patients ineligible for intravenous thrombolysis. Cerebrovasc Dis. 2018;46(5–6):193–199. doi: 10.1159/000493801. [DOI] [PubMed] [Google Scholar]
- 17.Černík D, Šaňák D, Divišová P, et al. Mechanical thrombectomy in patients with acute ischemic stroke on anticoagulation therapy. Cardiovasc Intervent Radiol. 2018;41(5):706–711. doi: 10.1007/s00270-018-1902-7. [DOI] [PubMed] [Google Scholar]
- 18.Ajjan RA, Standeven KF, Khanbhai M, et al. Effects of aspirin on clot structure and fibrinolysis using a novel in vitro cellular system. ATVB. 2009;29(5):712–717. doi: 10.1161/ATVBAHA.109.183707. [DOI] [PubMed] [Google Scholar]
- 19.Seiffge DJ, Hooff RJ, Nolte CH, et al. Recanalization therapies in acute ischemic stroke patients: impact of prior treatment with novel oral anticoagulants on bleeding complications and outcome. Circulation. 2015;132(13):1261–1269. doi: 10.1161/CIRCULATIONAHA.115.015484. [DOI] [PubMed] [Google Scholar]
- 20.Álvarez-Sabín J, Maisterra O, Santamarina E, Kase CS. Factors influencing haemorrhagic transformation in ischaemic stroke. The Lancet Neurology. 2013;12(7):689–705. doi: 10.1016/S1474-4422(13)70055-3. [DOI] [PubMed] [Google Scholar]
- 21.Prodan CI, Stoner JA, Cowan LD, Dale GL. Lower coated-platelet levels are associated with early hemorrhagic transformation in patients with non-lacunar brain infarction: coated-platelets and early hemorrhagic transformation. J Thromb Haemost. 2010;8(6):1185–1190. doi: 10.1111/j.1538-7836.2010.03851.x. [DOI] [PubMed] [Google Scholar]
- 22.Liu M, Zheng Y, Li G. Safety of recanalization therapy in patients with acute ischemic stroke under anticoagulation: a systematic review and meta-analysis. J Stroke Cerebrovasc Dis. 2018;27(9):2296–2305. doi: 10.1016/j.jstrokecerebrovasdis.2018.04.012. [DOI] [PubMed] [Google Scholar]
- 23.Powers WJ, Derdeyn CP, Biller J, et al. 2015 American Heart Association/American Stroke Association focused update of the 2013 guidelines for the early management of patients with acute ischemic stroke regarding endovascular treatment: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015;46(10):3020–3035. doi: 10.1161/STR.0000000000000074. [DOI] [PubMed] [Google Scholar]
- 24.Pexman JH, Barber PA, Hill MD, et al. Use of the alberta stroke program early CT score (ASPECTS) for assessing CT scans in patients with acute stroke. AJNR Am J Neuroradiol. 2001;22(8):1534–1542. [PMC free article] [PubMed] [Google Scholar]
- 25.Le Bouc R, Clarençon F, Meseguer E, et al. Efficacy of endovascular therapy in acute ischemic stroke depends on age and clinical severity. Stroke. 2018;49(7):1686–1694. doi: 10.1161/STROKEAHA.117.020511. [DOI] [PubMed] [Google Scholar]
- 26.Davoli A, Motta C, Koch G, et al. Pretreatment predictors of malignant evolution in patients with ischemic stroke undergoing mechanical thrombectomy. J NeuroIntervent Surg. 2018;10(4):340–344. doi: 10.1136/neurintsurg-2017-013224. [DOI] [PubMed] [Google Scholar]
- 27.Nouh A, Remke J, Ruland S. Ischemic posterior circulation stroke: a review of anatomy, clinical presentations, diagnosis, and current management. Front Neurol. 2014 doi: 10.3389/fneur.2014.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sato S, Toyoda K, Uehara T, et al. Baseline NIH stroke scale score predicting outcome in anterior and posterior circulation strokes. Neurology. 2008;70(24):2371–2377. doi: 10.1212/01.wnl.0000304346.14354.0b. [DOI] [PubMed] [Google Scholar]
- 29.Linfante I, Llinas RH, Schlaug G, Chaves C, Warach S, Caplan LR. Diffusion-weighted imaging and national institutes of health stroke scale in the acute phase of posterior-circulation stroke. Arch Neurol. 2001 doi: 10.1001/archneur.58.4.621. [DOI] [PubMed] [Google Scholar]
- 30.De Marchis GM, Kohler A, Renz N, et al. Posterior versus anterior circulation strokes: comparison of clinical, radiological and outcome characteristics. J Neurol Neurosurg Psychiatry. 2011;82(1):33–37. doi: 10.1136/jnnp.2010.211151. [DOI] [PubMed] [Google Scholar]
- 31.Iba T, Levy JH, Levi M, Thachil J. Coagulopathy in COVID-19. J Thromb Haemost. 2020;18(9):2103–2109. doi: 10.1111/jth.14975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levi M, Thachil J, Iba T, Levy JH. Coagulation abnormalities and thrombosis in patients with COVID-19. The Lancet Haematology. 2020;7(6):e438–e440. doi: 10.1016/S2352-3026(20)30145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Katsoularis I, Fonseca-Rodríguez O, Farrington P, Lindmark K, Fors Connolly AM. Risk of acute myocardial infarction and ischaemic stroke following COVID-19 in Sweden: a self-controlled case series and matched cohort study. The Lancet. 2021;398(10300):599–607. doi: 10.1016/S0140-6736(21)00896-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shakil SS, Emmons-Bell S, Rutan C, et al. stroke among patients hospitalized with COVID-19: results from the American Heart Association COVID-19 cardiovascular disease registry. Stroke. 2021 doi: 10.1161/STROKEAHA.121.035270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bravata DM, Ho SY, Meehan TP, Brass LM, Concato J. Readmission and death after hospitalization for acute ischemic stroke: 5-year follow-up in the medicare population. Stroke. 2007;38(6):1899–1904. doi: 10.1161/STROKEAHA.106.481465. [DOI] [PubMed] [Google Scholar]
- 36.Won Han S, Bushnell CD. stroke secondary medication persistence and risk for hospital readmission within 90 days after discharge. J Neurol Neurosci. 2016 doi: 10.21767/2171-6625.100087. [DOI] [Google Scholar]
- 37.Antiplatelet Trialists Collaboration Collaborative overview of randomised trials of antiplatelet therapy prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. BMJ. 1994;308(6921):81–106. doi: 10.1136/bmj.308.6921.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johnson ES, Lanes SF, Wentworth CE, Satterfield MH, Abebe BL, Dicker LW. A metaregression analysis of the dose-response effect of aspirin on stroke. Arch Intern Med. 1999;159(11):1248. doi: 10.1001/archinte.159.11.1248. [DOI] [PubMed] [Google Scholar]
- 39.Kernan WN, Ovbiagele B, Black HR, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American heart Association/American stroke association. Stroke. 2014;45(7):2160–2236. doi: 10.1161/STR.0000000000000024. [DOI] [PubMed] [Google Scholar]
- 40.EAFT (European Atrial Fibrillation Trial) Study Group Secondary prevention in non-rheumatic atrial fibrillation after transient ischaemic attack or minor stroke. The Lancet. 1993;342(8882):1255–1262. doi: 10.1016/0140-6736(93)92358-Z. [DOI] [PubMed] [Google Scholar]