Abstract
From several decades ago to now, cancer continues to be the leading cause of death worldwide, and metastasis is the major cause of cancer-related deaths. For health benefits, there is a great desire to use non-chemical therapy such as nutraceutical supplementation to prevent pathology development. Over 10,000 different natural bioactives or phytochemicals have been known that possessing potential preventive or supplementary effects for various diseases including cancer. Previously, the in vitro and in vivo anti-invasive and anti-metastatic activities of phenolic acids, monophenol, polyphenol and their derivatives and flavonoids and their derivatives have been reviewed. However, a vast number of natural dietary compounds other than phenolics have been demonstrated to potentially possess the ability to inhibit the invasion and metastasis of various cancers. In this review, we summarize the studies in recent decade on in vitro and in vivo effects and molecular mechanisms of natural bioactives, excluding the phenolics in food, in cancer invasion and metastasis. By combining this review of non-phenolics with the previous phenolics reviews, the puzzle for the contribution of natural dietary bioactives on cancer invasive or/and metastatic progress will be almost complete and more clear.
Keywords: Angiogenesis, Invasion, Metastasis, Natural dietary bioactives
1. Introduction
Cancer has continued to be the leading cause for lethality worldwide for several decades. The most common cancers accounting for death each year in order of fatality include lung, liver, colorectal, stomach, and breast cancer [1]. New statistics disclose that about 14.1 million new cancer cases and 8.2 million deaths occurred in 2012 worldwide; nearly half of people diagnosed with cancer have received treatment for invasive cancer and died from cancer or its treatment [2]. These data infer that cancer-related deaths are highly associated with metastasis. Metastasis symptoms are due to the spread of cancer from a primary neoplasm to other locations in the body and the formation of secondary tumors. Unexpected and undetectable tumor translocation causes treatments against cancer to become insignificant, eventually leading to death. Hence, the blockage or reduction of metastasis is believed to be an effective strategy for increasing the survival of patients with cancer. Metastasis occurs through several interconnected processes that begin with the entrance of cells from a primary tumor into the vasculature followed by their migration to distant organs, adhesion to endothelial cells lining blood vessels, and extravasation, which leads to infiltration into the underlying tissue. Following a cancer diagnosis, chemotherapy is used to prevent the local recurrence of a primary tumor and the spread of tumor cells. However, severe side effects may occur at the effective dose of many chemotherapeutics. For health benefits, there is great desire to use non-chemical therapies such as nutraceutical supplementation to prevent pathology development in developing or developed countries.
Natural bioactives or phytochemicals, which may be used as nutraceuticals, are generally referred to as compounds that lack essential nutrients and have specific biological activity in humans. There are estimated to be over 10,000 different varieties of phytochemicals, and each may potentially affect some type of disease or physiology. Numerous bioactives and phytochemicals derived from vegetative foods have been demonstrated to be effective in preventing metastasis [3]. These anti-metastatic natural compounds are roughly categorized into two classes i.e., phenolics and non-phenolics, and several subclasses are further included in each class. We previously reviewed the in vitro and in vivo anti-invasive and anti-metastatic bioactivities of phenolic compounds including phenolic acids, monophenol, polyphenol, and their derivatives (i.e., curcumin, resveratrol, gallic acid, chlorogenic acid, caffeic acid, carnosol, capsaicin, 6-shogaol, and 6-gingerol) [4] and flavonoids and their derivatives (i.e., EGCG, EGC, ECG, EC, genistein/genistin, silibilin, quercetin, anthocyanin, luteolin, apigenin, myricetin, tangeretin, kaempferol, glycitein, licoricidin, daidzein, and naringenin) [5]. However, a vast number of natural dietary compounds other than phenolics have also been demonstrated to potentially possess the capacity to inhibit the invasion and metastasis of various cancers. In this review, we summarize the in vitro and in vivo effects and underlying molecular mechanisms of the non-phenolic bioactives in food on cancer invasion and metastasis.
2. The molecular mechanisms of non-phenolic bioactives against in vitro cancer cell invasion
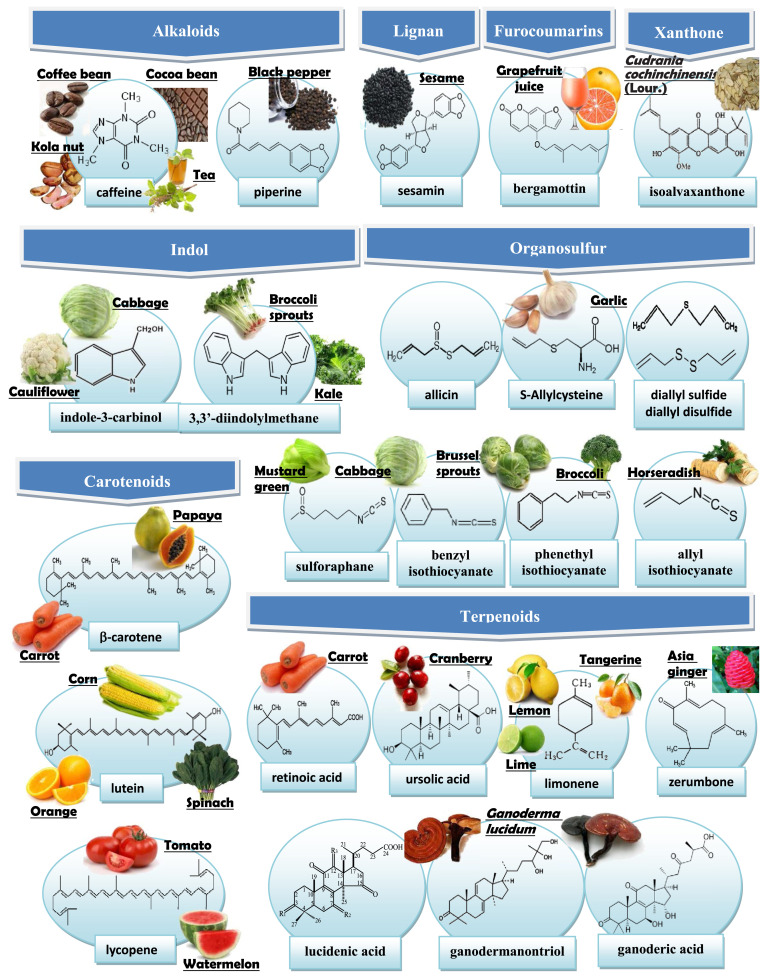
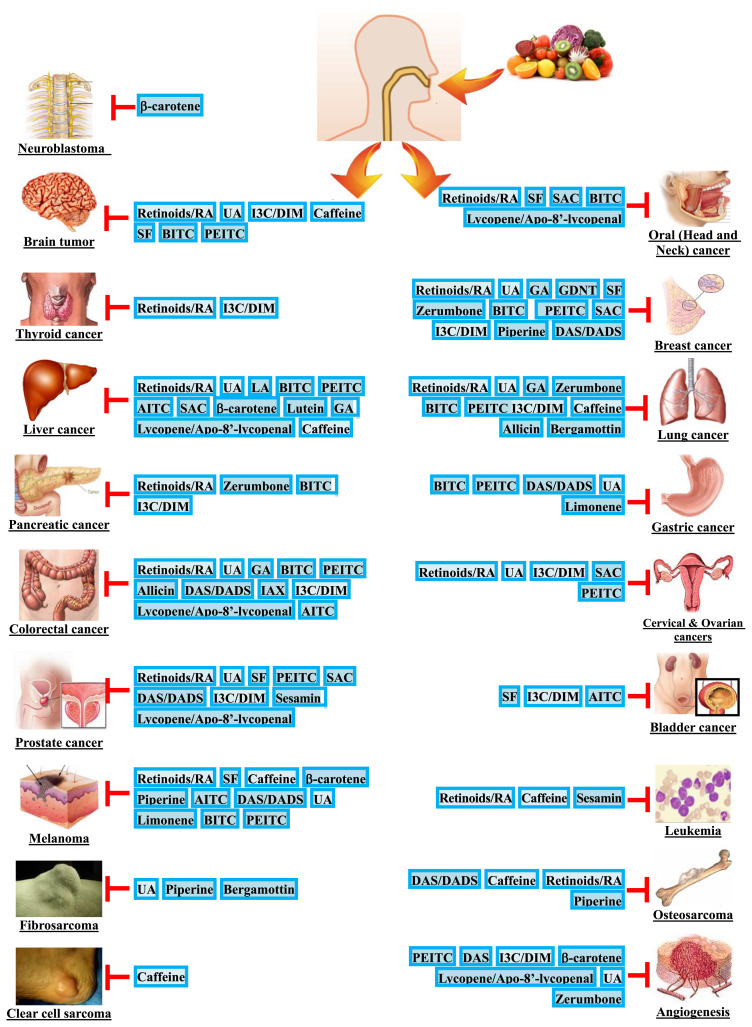
A large number of non-phenolic bioactives have been documented to possess in vitro anti-invasive and/or in vivo anti-metastatic activity. Fig. 1 shows the chemical structures of select non-phenolic bioactives that have an inhibitory effect on the invasion and/or metastasis of cancer and their representative dietary sources. Additionally, the anti-invasive and/or anti-metastatic activity of these non-phenolic bioactives have been tested and verified in a variety of cancer types, including brain, oral (head and neck), thyroid, breast, liver, lung, pancreatic, gastric, colorectal, cervical, ovarian, prostate, and bladder cancers; melanoma, leukemia, fibrosarcoma, osteosarcoma, neuroblastoma, and clear cell sarcoma are also inclusive (Fig. 2). In the following sections, we summarize the findings in recent decade for the anti-invasive and anti-metastatic properties of these bioactives on various cancer types.
Fig. 1.
Food sources and chemical structures of non-phenolic bioactives with anti-invasion and/or anti-metastasis activity.
Fig. 2.
Non-phenolic bioactives with potential anti-invasive and/or anti-metastatic activity against the most commonly diagnosed cancer sites. Abbreviations: AITC, Allyl isothiocyanate; BITC, benzyl isothiocyanate; DADS, Diallyl disulfide; DAS, Diallyl sulfide; DIM, 3,3-diindolylmethane; GA, Ganoderic acid; GDNT, Ganodermanontriol; IAX, Isoalavaxanthone; I3C, Indole-3-carbinol; LA, Lucidenic acid; PEITC, phenethyl isothiocyanate; RA: Retinoic acid; SAC, S-allycysteine; SF, Sulforaphane; UA: Ursolic acid.
2.1. Terpenoids
2.1.1. Retinoids/Retinoic acid
Retinoic acid (RA) is a metabolite of vitamin A (retinol) and is responsible for most of the vitamin A activity. The precursor of retinoic acid or vitamin A is present in large quantities in carrots. RA acts by binding to the intracellular retinoic acid receptor (RAR), which combines with the retinoid X receptor (RXR) as a heterodimer. This heterodimer binds to DNA at regions called retinoic acid response elements (RAREs) to regulate the transcrptional activity of retinoid target genes including genes that affect cell invasion and metastasis.
Against hepatoma: Cui et al. [6] demonstrated that the migration and invasion capability of hepa1–6 cells significantly inhibited by all trans-retinoic acid (ATRA) (0.1–10 μM) via decreasing mesenchymal markers (N-cadherin, vimentin, snail, and twist) and increasing epithelial marker (E-cadherin). Against lung cancer: In many studies, RA is an inhibitor on the invasive and migratory potential of lung cancer cells. Nevertheless, the use of ATRA is limited on lung cancer treatment. García-Regalado et al. [7] and Quintero Barceinas et al. [8] indicated that ATRA (5 μM) activates PI3k-Akt/ERK and also promotes invasion/migration of A549 cells through Rac-GTPase/RARα activation. Against breast cancer: It was observed that the epidermal growth factor receptor (EGFR) and integrin-α5, integrin-αv, integrin-β1, and integrin-β3 decreased, but TIMP-1 increased in ATRA-treated MDAMB231 cells. The invasive capacity of the MDAMB231 cells was attenuated by ATRA by regulating the focal adhesion kinase (FAK), ERK, PI3K, and transcription factor NF-kB signaling proteins [9]. Against melanoma: Retinoids (1 μM) causes a significant inhibitory effect on the invasion of B16–F10 cells [10]. Against glioblastoma (glioma): Bouterfa et al. [11] reported that most established glioma cell lines were insensitive to RA, but the migration of primary cultures of human glioblastoma multiforme was strongly inhibited by 10 μM retinoids. However, this proclaimation was soon refuted by Papi et al. [12], who found that RA may markedly decrease MMP-2/-9 and inhibit the migration and invasion of glioblastoma U87MG cells. Liang et al. [13] also identified that ATRA (5–40 μM) inhibit migration and invasion of U87 and SHG44 glioma cells may be partially associated with the effect of ATRA on the expression of MMP-2/-9. Against cervical and ovarian cancer: RA at 50 μM could inhibit MMP-2/-9 in the Hela and DoTc2-4510 cervical cancer cell lines and also inhibit MMP-2 in the SK-OV-3 ovarian cancer cell line [14]. Against thyroid carcinoma: ATRA reduced the invasive potential in the differentiated thyroid carcinoma cell lines FTC-133 and XTC.UC1, and the antiinvasive effect was more significant in the anaplastic thyroid cancer cell lines C643 and HTH74. By exploring the mechanism, it was found that ATRA diminished the urokinase plasminogen activator receptor (uPAR) activity of these four cell lines but increased the cell-ECM adhesion and E-cadherin expression and decreased urokinase plasminogen activator (uPA) expression in the C643 and HTH74 cell lines [15]. Against osteosarcoma: ATRA suppressed IL13-induced M2-polarized tumor-associated macrophages (TAM) and MMP-12, and then inhibited migration of K7M2 WT osteosarcoma cells [16].
2.1.2. Ursolic acid
Ursolic acid (UA) is a naturally occurring pentacyclic triterpene acid and is present in many vegetative foodstuffs and seasonings e.g., cranberries, apples, basil, rosemary, lavender, and hawthorn. This compound is capable of inhibiting various types of cancer cells by acting through the glucocorticoid receptor, reducing MMP-9 expression, and inhibiting the signal transducer and activator of transcription 3 (STAT3) activation pathway [17,18].
Against hepatoma: UA at 4 μM decreased the invasion and migration of Hep3B, Huh7, and HA22T cell lines; several metastasis-related factors, including hypoxia-inducible factor (HIF)-α, vascular endothelial growth factor (VEGF), inter-leukin (IL)-8, reactive oxygen species (ROS), nitric oxide (NO), and uPA were also reduced by the treatment [19]. Against lung cancer: UA dose-dependently (4–16 μM) reduced the cell invasion and migration of A549, H3255, and Calu-6 lung cancer cell models. The treatment also lowered the levels of VEGF, transforming growth factor (TGF)-β1, intercellular adhesion molecule (ICAM)-1, MMP-9/-2, and protein kinase C (PKC) in these cell lines [20]. Moreover, UA decreased expression of astrocyte-elevated gene-1 (AEG-1) accompanied by upregulation of E-cadherin and downregulation of N-cadherin and vimentin, correlating with inhibition of NF-κB in A549 cells [21]. Against breast cancer: UA dose-(2.5–10 μM) and time-dependently inhibited the migration and invasion of MDAMB231 cells, which may result from reductions in MMP-2 and uPA and an increase in TIMP-2 and plasminogen activator inhibitor (PAI)-1 through the suppression of VEGF, GRB2/Ras, RhoA, JNK, Akt/mTOR and NF-kB and AP-1 transcription factor signaling [22]. Against colorectal cancer: Treatment of 5–20 μM UA can significantly inhibit the constitutive NF-kB activation and downregulate metastatic proteins, such as MMP-9, VEGF, and ICAM-1, in HCT116, HT29, and Caco2 cells [23]. Against prostate cancer: PC-3 cells treated with 5 μM UA could inhibit cell invasion by downregulating MMP-9 and Akt [24]. The MMP-2/-9 activities in DU145 cell lines were also decreased by 1–10 μg/ml UA [25]. Shanmugam et al. [26] further revealed that UA is a potential agent for inhibiting the CXC Chemokine ligand 12 (CXCL12)-induced migration and invasion of DU145, PC-3, and LNCaP cell lines; this inhibition is associated with a reduction in the NF-kB-dependent Chemokine receptor 4 (CXCR4) expression. Against glioma: In IL-1β or tumor necrosis factor (TNF)-α-induced rat C6 glioma cells, UA (10–20 μM) could efficiently inhibit the interaction between ZIP/p62 and PKC-ζ and upregulate IkBα to suppress NF-kB activation; MMP-9 was sequentially decreased to block cell invasion [27]. Against ovarian carcinoma: The invasion and migration of HO-8910PM cells could be inhibited by UA by depressing the activity of gelatinase and the expression of MMP-2/-9 [28]. Against gastric cancer: UA (1–5 μM) inhibits the invasive phenotype of SNU484 gastric cancer cells, and MMP-2 may be responsible for the anti-invasive activity of UA in the cells [29].
2.1.3. Ganoderic acid (GA)
Ganoderma lucidum (Leyss. ex Fr.) Karst, also called Lingzhi, has been used in traditional Chinese medicine to improve health and longevity. The observed anti-invasive activity is one of the medicinal characteristics of G. lucidum [30]. These activities may derive from the minor but manifold triterpenoid constitutes, such as ganoderic acids (GA), ganolucidic acids, ganolactone, ganodermanontriol (GDNT), lucidenic acids (LA), methyl lucidenate and hydroxylucidenic acid, in the Ganoderma species [31,32]. Against hepatoma: Wang et al. [33] indicated that the migration and invasion of HepG2 and SMMC7721 cells were suppressed by 75–100 μM of GA-A. Against lung cancer: GA–Me at a concentration of 16 μM or 10–20 μg/ml effectively inhibited the invasion of 95-D cells by suppressing MMP-2/-9 gene expression, cell migration, and cell adhesion to the ECM [34]. Against breast cancer: The inhibition of AP-1 and NF-kB, which results in the suppression of uPA secretion, may be attributed to the GA-A- and GA-H-inhibited invasive behaviors, including adhesion, migration, and invasion, of MDAMB231 cells [35]. Additionally, GA–Me inhibited invasion, MMP-9, angiogenesis, VEGF, and IL-6/-8 in MDAMB231 cells via suppressing NF-kB activity was also observed [36]. Against colorectal cancer: Several studies indicated that GA-T (8–16 μM) could inhibit the invasion, adhesion, and migration of HCT-116 cells. The inhibitory mechanism may involve the inhibition of IkBα degradation and NF-kB translocation, which leads to downregulated MMP-9, inducible nitric oxide synthase (iNOS), NO, and uPA in cells. In addition, p53 may be another important target for GA-T (16.3–32.6 μM)-inhibited HCT-116 cellular invasion [37,38].
2.1.4. Ganodermanontriol (GDNT)
GDNT is a Ganoderma alcohol that has an inhibitory effect on the adhesion, migration, and invasion of the highly invasive MDAMB231 human breast cancer cell line by suppressing uPA and uPAR [39].
2.1.5. Lucidenic acid (LA)
Four triterpenoid fractions, including lucidenic acids (LAs) A, B, C, and N, which were separated from a new Ganodema lucidum strain (YK-02), were used to treat phorbol 12-myristate 13-acetate (PMA)-induced HepG2 cells. The PMA-induced invasion of the cells was decreased by 50 μM LAs via the suppression of MMP-9 by inhibiting ERK phosphorylation and reducing the AP-1 and NF-kB DNA-binding activities [40,41].
2.1.6. Zerumbone
Zerumbone is a sesquiterpene constitute derive from the rhizome of Zingiber zerumbet (also called subtropical ginger, Asia ginger, or shampoo ginger). The anti-cancer activity of zerumbone has been revealed in several models. Recently, the antiinvasive activity of zerumbone has also been found. Against lung cancer: Zerumbone causes considerable suppression of osteopontin (OPN)-induced cell invasion through inhibiting the FAK/AKT/ROCK pathway in A549 cells [42]. Against breast and pancreatic cancers: The study of Han et al. [43] showed that IL-1β-induced cell migration, invasion, and MMP-3 were decreased by zerumbone in Hs578T and MDA-MB231 cells. Additionally, zerumbone (10 μM) dose-dependently suppressed the levels of TGF-β1-induced MMP-2/-9 expression and IL-1β-induced invasion in HCC1806 TNBC (triple-negative breast cancer) cells through the inhibition of smad3 and NF-κB activity [44,45]. Zerumbone (25 μM) also inhibited CXCL12-induced invasion of both breast (MCF7/HER2, HER2-overexpressing cell) and pancreatic cancer (AsPC-1) cells by downregulating the expression of CXCR4 and NF-kB [46].
The proposed target proteins and mechanisms for terpenoid compounds in the inhibition of cancer invasion in vitro are summarized in Table 1.
Table 1.
The proposed mechanisms of terpenoids compounds on the inhibition of cancers invasion in vitro.
| Bioactive (Effective dosage) | Cancer cell models | Biological effects | Molecular targets | References |
|---|---|---|---|---|
| Retinoids/Retinoic acid | ||||
| (0.1–10 μM) | Hepatoma: hepa1–6 | ↓: invasion; migration; vimentin; N-cadherin; snail; twist ↑: E-cadherin |
– | Cui et al. [6] |
| (5 μM) | Lung cancer: A549 | ↑: invasion; migration | ↑: PI3k-Akt; ERK; Rac-GTPase; RARα | García-Regalado et al. [7]; Quintero Barceinas et al. [8] |
| (20 μM) | Breast cancer: MDAMB231 | ↓: invasion; migration; MMP-9; EGFR; α5-/αv-/β1-/β3-integrin ↑: TIMP-1 |
↓: FAK; ERK; PI3K; NF-kB | Dutta et al. [9] |
| (1 μM) | Melanoma: B16F10 | ↓: invasion | – | Liu et al. [10] |
| (5–40 μM) | Glioblastoma (Glioma): U87MG/U87/SHG44 | ↓: invasion; migration; MMP-2/-9 | Papi et al. [12]; Liang et al. [13] | |
| (10–50 μM) | Cervical & Ovarian cancer: HeLa/DoTc2-4510/SK-OV-3 | ↓: invasion; MMP-2/-9 | – | Roomi et al. [14] |
| Thyroid cancer: FTC-133/XTC. | ↓: invasion; uPA/uPAR; MMP-2 | – | Lan et al. [15] | |
| UC1/C643/HTH74 | ↑: cell-ECM adhesion; E-cadherin | |||
| Osteosarcoma: K7M2 WT | ↓: migration; IL13-induced M2-polarized tumor-associated macrophages (TAM) and MMP-12 |
Zhou et al. [16] | ||
| Ursolic acid | ||||
| (4 μM) | Hepatoma: Hep3B/Huh7/HA22T | ↓: invasion; migration; uPA; VEGF; IL-8; ROS; NO | ↓: HIF-1α; | Lin et al. [19] |
| (4–16 μM) | Lung cancer: A549/H3255/Calu-6 | ↓: invasion; migration; VEGF; TGF-β1; ICAM-1; MMP-2/-9; AEG-1; N-cadherin; vimentin ↑: E-cadherin |
↓: PKC; NF-kB | Huang et al. [20]; Liu et al. [21] |
| (2.5–10 μM) | Breast cancer: MDAMB231 | ↓: invasion; migration; MMP-2; uPA; VEGF ↑: TIMP-2; PAI-1 |
↓: JNK; Akt/mTOR; RhoA; GRB2/Ras; NF-kB; AP-1 ↑: IkBα |
Yeh et al. [22] |
| (5–20 μM) | Colorectal cancer: HCT116/HT29/Caco2 | ↓:MMP-9; VEGF; ICAM-1 | ↓: NF-kB | Prasad et al. [23] |
| (1–10 μg/ml 5–50 μM) | Prostate cancer: DU145/PC-3/LNCaP | ↓: (CXCL12-induced) invasion/migration; MMP-2/-9; CXCR4 | ↓: Akt; NF-kB | Zhang et al. [24]; Kondo et al. [25]; Shanmugam et al. [26] |
| (10–20 μM) | Glioma: IL-1β or TNFα-induced C6 | ↓: invasion; MMP-9 | ↓: NF-kB; interaction of ZIP/p62 and PKC-ζ ↑: IkBα |
Huang et al. [27] |
| Ovarian cancer: HO-8910PM | ↓: invasion; migration; MMP-2/-9 | – | Yu et al. [28] | |
| (1–5 μM) | Gastric cancer: SNU484 | ↓: invasion; MMP-2 | Kim and Moon [29] | |
| Ganoderic acid | ||||
| (75–100 μM) | Hepatoma: HepG2/SMMC7721 | ↓: invasion; migration | Wang et al. [33] | |
| (10–20 μg/ml 16 μM) | Lung cancer: 95D | ↓: adhesion; migration; invasion; MMP-2/-9 | – | Chen et al. [34] |
| (0.1–0.5 mM) | Breast cancer: MDAMB231 | ↓: adhesion; migration; invasion; angiogenesis; uPA; MMP-9; VEGF; IL-6/-8 | ↓: AP-1; NF-kB | Jiang et al. [35]; Li et al. [36] |
| (8–32 μM) | Colorectal cancer: HCT116 | ↓: adhesion; migration; invasion; MMP-2/-9; uPA; iNOS/NOS2; NO ○: p53 is an important target |
↓: NF-kB; IkBα degradation | Chen et al. [37]; Chen and Zhong [38] |
| Ganodermanontriol | ||||
| Breast cancer: MDAMB231 | ↓: adhesion; migration; invasion; uPA/uPAR | – | Jiang et al. [39] | |
| Lucidenic acid | ||||
| (50 μM) | Hepatoma: PMA-induced HepG2 | ↓: invasion; MMP-9 | ↓: ERK; AP-1; NF-kB | Weng et al. [40,41] |
| Zerumbone | ||||
| (50 μM) | Lung cancer: A549 | ↓: invasion | ↓: FAK/Akt/ROCK | Kang et al. [42] |
| (10–25 μM) | Breast cancer: MCF7/HER2/HCC1806/Hs578T/MDA-MB231 | ↓: CXCL12-induced invasion; CXCR4; TGF-β1-induced MMP-2/-9; IL-1β-induced invasion, migration, and MMP-3 | ↓: NF-kB; smad3 | Sung et al. [46]; Han et al. [43]; Kim et al. [44]; Jeon et al. [45] |
| (25 μM) | Pancreatic cancer: AsPC-1 | ↓: CXCL12-induced invasion; CXCR4 | – | Sung et al. [46] |
2.2. Isothiocyanates
Sulforaphane (SF), benzyl isothiocyanate (BITC), and phenethyl isothiocyanate (PEITC) are isothiocyanate rich in cruciferous vegetables such as mustard green, cabbage, Brussels sprouts, broccoli, cauliflower, and radishes. These organosulfur compounds are generated by the hydrolysis of glucosinolate, a special compound found in cruciferous vegetables, through myrosinase catalysis. Many isothiocyanates exhibit multiple aspects of biological function that include anti-cancer and anti-metastasis [47,48].
2.2.1. Sulforaphane (SF)
Against breast cancer: SF inhibited the aggressive phenotype of untreated MDAMB231 cells through several different mechanisms including the reversal of the epithelial–mesenchymal transition (EMT) (e.g., the suppression of vimentin, Twist1 and POU5F1), a decrease in matrix degradation and extracellular proteolysis (e.g., MMP-7/-14), a reduction in pro-inflammatory cytokines [e.g., IL-1β/-6/-4, TNF-α, and inter-feron (INF)-γ], and the downregulation of pro-angiogenic growth factors [e.g., platelet derived growth factor (PDGF) and VEGF] [49]. Against oral cancer: The migration and invasion activity of YD10B oral cancer cells was decreased by 1 μM SF. At the molecular level, a reduction in the level of the secreted forms of MMP-1/-2 was also observed [50]. Against prostate cancer: Hahm et al. [51] found that the migration of PC-3 and LNCaP cells was decreased by 10 μM SF and the SF-inhibited migration was only marginally affected by Notch activation. In DU145 cells, SF inhibited invasion by activating ERK1/2 to upregulate E-cadherin and downregulate CD44v6, thereby reducing MMP-2 [52]. The inhibitions of DU145 and PC3 cells invasion by sulforaphane-cysteine (SFN-Cys), a metabolite of SF, were further identified that resulted from the down-regulation of galectin-1 [53]. Against bladder cancer: SF showed a significantly suppression on the adhesion, migration, and invasion of malignant transitional bladder cancer T24 cells. The SF-inhibited invasion and migration of T24 cells was mediated via MMP-2/-9 reduction and E-cadherin induction through reducing ZEB1 and Snail. Moreover, miR-200c was also the target for the inhibition by SF [54]. Against glioblastoma: Li et al. [55] treated SF (10–30 μM) on U87MG and U373MG cells and found that SF inhibited migration and invasion of cells via phosphorylating ERK1/2 in a sustained way, which contributed to the downregulation of MMP-2 and upregulation of CD44v6. While on U251MG cells, SF inhibited invasion via upregulating E-cadherin and downregulating MMP-2/-9 and galectin-3 [56]. SF (10 μM) has partial antitumor effects by inhibiting migration and invasion of C6 glioma cells through regulation of MMP-9 activation which associating with the suppression of FAK/JNK, NF-κB, and AP-1 [57].
2.2.2. Benzyl isothiocyanate (BITC)
Against hepatoma: By decreasing MMP-2/-9 and membrane type-1 (MT1)-MMP and increasing TIMP-2, BITC (5 μM) inhibited the metastatic activity of SK-Hep1 cells. Three major MAPKs signaling pathways i.e., ERK, JNK, and p38, were considered to be pivotal points for the BITC-mediated inhibition [58]. In Bel 7402 and HLE cells, BITC (40–80 μM) suppressed the invasive and migratory abilities and downregulated expressions of MMP-2/-9 and CXCR4 [59]. Against lung cancer: The migration and invasion of L9981 cells were inhibited by treatment with 5 μM BITC, and the metastasis-related genes MMP-2 and β-catenin were also modulated through the repression of the Akt signaling molecule and the Twist and NF-kB transcription factors [60]. Against breast cancer: BITC (4 μM) could profoundly inhibit both the basal and hepatocyte growth factor (HGF)-induced migration and invasion of MDAMB231 cells, which is associated with its suppressive activity on uPA through the upregulation of PAI-1. Moreover, the HGF-induced phosphorylation of c-met and Akt and NF-kB activity were all repressed by BITC [61]. Exposure of MDAMB231, SUM159, and MDAMB468 cells to 2.5 and 5 μM of BITC resulted in transcriptional repression of uPA and uPAR. BITC also suppressed Forkhead Box Q1 (FOXQ1), which mediating the induction of E-cadherin and inhibition of migration in MDAMB231 and SUM159 cells [62]. Against colorectal cancer: BITC (0.01–0.25 μM) inhibited the migration and invasion of HT29 cells by suppressing MMP-2/-9 and uPA, which may be involved in the BITC-repressed activities of PKC, ERK and JNK, and the levels of GRB2, PI3K, FAK, Ras, and AP-1 and the binding affinity of NF-kB-DNA [63]. Against gastric cancer: A distinguishing antiinvasive mechanism of BITC was demonstrated in the AGS human gastric cancer cell line by Ho et al. [64]. This team indicated that BITC exerted an inhibitory effect on Ras, ERK, GRB2, RhoA, FAK, ROCK1, iNOS, and cyclooxygenase-2 (COX)-2 by causing the inhibition of MMP-2/-7/-9 followed by the inhibition of the invasion and migration of AGS cells. Meanwhile, BITC also promoted the signaling of VEGF, mitogen-activated protein kinase kinase (MKK) 7, mitogen-activated protein kinase kinase kinase (MEKK or MAP3K) 3, JNK, PI3K, PKC, son of sevenless (SOS)-1 and the AP-1 and NF-kB transcription factors in AGS cells. This documented BITC molecular mechanism in AGS cells is quite different from that in other cancer cells. The result may need to be further verified by other tests. Against pancreatic cancer: By reducing RhoC (a signaling for cancer development), increasing RhoB (a tumor suppressor), and repressing STAT3, BITC (5–20 μM) demonstrated activity to suppress MMP-2, VEGF, VEGFR2, and HIF-α and substantially block the migration and invasion of the BxPC-3 and PanC-1 cell lines [65]. Against melanoma: BITC significantly inhibited cell mobility, migration, and invasion nature of B16F10 and A375.S2 cells. In these cells, BITC inhibited MMP-2, RhoA, Ras, and SOS-1 but increased TIMP, FAK, p-ERK1/2, p-p38, and p-JNK1/2 signaling [66,67]. Against head and neck squamous cell carcinoma: BITC (2.5–5 μM) decreased migration and invasion of HN12 cells resulted from its ability to inhibit vimentin [68]. Against glioma: Treatment of 10 μM BITC on C6 glioma cells showed a similar effect as SF [57].
2.2.3. Phenethyl isothiocyanate (PEITC)
Against lung cancer: Ten μM of PEITC exerted an inhibitory effect in L9981 cells that was similar to 5 μM BITC [60]. Against breast cancer: PEITC downregulated HIF-1α and ROS as well as retarded adhesion, aggregation, migration and invasion of the breast cancer cells (MCF-7/MDA-MB-231). Activities of MMP-2/-9 were also altered by PEITC [69]. Against colorectal cancer: PEITC (0.01–0.25 μM) inhibited the adhesion, migration, and invasion of HT29 cells by multiple signal transduction pathways. First, MMP-2/-9 was suppressed via PKC, ERK/JNK, RhoA, and SOS-1 signaling. Second, cell proliferation was inhibited via Ras, FAK, PI3K, GRB2, NF-kB, iNOS, and COX-2. Third, the MMP-7 expression and Akt activity were also repressed [70]. Against prostate cancer: Xiao and Singh [71] reported that the PEITC (1 μM)-mediated migration of PC-3 cells is correlated with the inactivation of Akt and the suppression of VEGF. Kim et al. [72] further demonstrated that PC-3 and LNCaP cells exposed to 5 μM PEITC activated Notch1/2, which may attenuate the PEITC inhibitory effect on cell migration. PEITC (2.5 μM) suppresses invasion of PC-3 and LNCaP cells through regulating miR-194 and downregulating expression of MMP-2/-9 by targeting bone morphogenetic protein 1 (BMP1) [73]. Against gastric cancer: Similar to BITC, PEITC also inhibited the migration and invasion of AGS cells by suppressing MMP-2/-7/-9, uPA, iNOS, and COX-2 and decreasing Ras, ERK, GRB2, RhoA, and FAK. Nevertheless, the levels of MKK7, MEKK3, PKC, SOS-1, and NF-kB, which were promoted by BITC, were attenuated by PEITC [74]. Against melanoma: On B16F10 and A375.S2 cells, PEITC showed a similar regulatory effect as BITC, except that TIMP, p-ERK1/2, p-p38, and p-JNK1/2 were decreased by PEITC treatment. PEITC further induced GRB2 and inhibited NF-κB protein level [66,67]. Against glioma and glioblastoma: According the results of Lee et al. [57], PEITC-treated C6 glioma cells obtained the same results as SF and BITC treatment. The study of Chou et al. [75] further identified that PEITC decreased the migration of GBM 8401 cells in a dose-dependent manner and the levels of proteins associated with migration and invasion (Ras, uPA, RhoA, GRB2, p-p38, p-JNK, p-ERK, p65, SOS1, MMP-2/-7/-9 and −13) were also depressed by PEITC. Against cervical carcinoma: PEITC exhibited an inhibitory effect on the adhesion and invasion of HeLa cells by reducing the expression of CDK1, MMP-2/9, CD44, and ICAM-1 as well as increasing the production of TGF-β, IL-6 and IL-8. The phosphorylation of Smad2 was also increased by PEITC [76]. Against ovarian cancer: PEITC suppresses the metastasis of ovarian cancer cells (SKOV3, HO8910, and EOC) associated with the inhibition of CRM1-mediated nuclear export and mTOR-STAT3 pathway [77].
2.2.4. Allyl isothiocyanate (AITC)
Allyl isothiocyanate (AITC) is an organosulfur compound that comes from black mustard seeds (Brassica nigra) or brown Indian mustard (Brassica juncea). When these mustard seeds are broken, the myrosinase enzyme is released and acts on a glucosinolate known as sinigrin to produce AITC. AITC has demonstrated functions to inhibit metastasis and the colony formation of cancer cells [47]. Against colorectal cancer: AITC suppresses the invasion and migration of EGF-induced HT29 cells through downregulating MMP-2/-9 and MAPKs [78].
2.2.5. S-allycysteine (SAC)
Garlic (Allium sativum) is a member of the Allium vegetables and is widely applied for medicinal uses. The organosulfur compounds extracted from garlic are divided into two major types: one is a water-soluble type including S-allylcysteine (SAC) and S-allylmercaptocysteine (SAMC), and another is a lipid-soluble type and includes allicin, diallyl sulfide (DAS), diallyl disulfide (DADS), and diallyl trisulfide (DATS) [79]. Several garlic-derived organosulfur compounds have been found to be potentially preventive and therapeutic agents against cancers [80]. Against hepatoma: In MHCC97L hepatoma cells treated with 20–40 mM SAC, the migration and invasion were hindered and along with the increase and decrease of E-cadherin and VEGF, respectively [81]. Against breast cancer: Gapter et al. [82] provided evidence for a link between the induction of E-cadherin and reduction in MMP-2 with the inhibition of motility and invasion in MDAMB231 cells by SAC. Against nasopharyngeal carcinoma: Cho et al. [83] demonstrated that SAC reduced Slug and MMP-2/-9 involved in migration and invasion with the inhibition of Met-FAK signaling in HONE1 and HNE1 cells.
2.2.6. Allicin
Against colorectal cancer: Allicin at non-cytotoxic concentrations (i.e., 3–6 μg/ml) could significantly suppress the adhesion, migration, and invasion of LoVo colorectal cancer cells. Under the same treatment, the VEGF and uPAR mRNA levels were decreased in a dose-dependent manner [84]. Against lung cancer: Allicin inhibits the adhesion, invasion and migration of lung adenocarcinoma A549 and H1299 cells by altering TIMP (−1 & −2)/MMP (−2 & −9) balance, via reducing the activity of the PI3K/AKT signaling pathway [85].
2.2.7. Diallyl sulfide (DAS)/Diallyl disulfide (DADS)
Against breast cancer: DADS inhibited the invasion and migration of MCF-7 cells via downregulating the protein levels of vimentin and MMP-9 and upregulating E-cadherin expression [86]. DADS was demonstrated that suppresses SRC/Ras/ERK signaling-mediated metastasis by up-regulating miR-34a in MDA-MB-231 cells [87]. Against colorectal cancer: DADS inhibited the migration and invasion of Colo205 cells by suppressing MMP-2/-7/-9 and COX-2. In addition, PI3K, Ras, MEKK3, MKK7, ERK, JNK, p38, and NF-kB transcription factor signaling in DADS-treated cells were also decreased [88]. Treatment of DADS at a concentration of 45 mg/l on SW480 cells suppressed cell migration and invasion and downregulated the Rac1-ROCK1/PAK1-LIMK1-ADF/cofilin signaling pathway [89,90]. Against prostate cancer: DADS has anti-migratory and anti-invasive activities in LNCaP cells, and its inhibitory activities are associated with its suppressive potential on the claudin proteins (major components of tight junctions) and MMP-2/-9 [91]. Against gastric cancer: In AGS cells, migration and invasion were inhibited by DADS by reducing MMP-2/-9 and claudin-2/-3/-4 and increasing TIMP-1/-2 [92]. DADS suppressed cell migration and invasion of MGC803 cells that was coupled with decreased MMP-9, vimentin, and CD34 as well as increased TIMP-3 and E-cadherin. The Rac1-Pak1/Rock1-LIMK1 pathway may involve in the abovementioned regulation of DADS [93]. Moreover, DADS suppresses invasion of SGC-7901 cells by upregulation of miR-34a, via inhibition of the PI3K-Akt signaling pathway [94]. Against osteosarcoma: An in vitro transwell invasion assay indicated that the invasive activity of MG-63 cells was significantly declined by treating with 20–40 μg/ml DAS [95].
2.3. Indol
2.3.1. Indole-3-carbinol (I3C)/3,3-diindolylmethane (DIM)
Indole-3-carbinol (I3C) is a natural chemical found in cruciferous vegetables (Brassica plants) such as cabbage, cauliflower, broccoli, brussels sprouts, kale, and turnips. I3C readily dimerizes into 3,3-diindolylmethane (DIM), which is an acid-catalyzed stable compound and does not undergo further condensation reactions. Thus, DIM is thought to be the primary actor for the I3C chemopreventive effects. The I3C anti-invasive and anti-metastatic properties were also demonstrated by several studies.
Against hepatoma: DIM inhibited the invasion and migration of SMMC-7721 and MHCC-97H hepatocellular carcinoma cells. These inhibitory effects are through increasing pTEN expression and inhibiting FAK phosphorylation leading to decreased MMP-2/-9 [96]. Against lung cancer: DIM is a potential inhibitor of the invasion of H1650 and H1975 cells [97]. Against breast cancer: At 50–100 μM, I3C could significantly inhibit the migration and invasion of MCF-7 cells by diminishing MMP-2 expression via the suppression of ERK/Sp1-mediated gene [98]. At 10 μM, DIM could inhibit the invasion of MDAMB361 and 4T1 cells [97,99]. The migratory and invasive potential of the aggressive MDAMB231 breast cancer cells were inhibited by exposure to 10–50 μM DIM, which led to the inactivation of the uPA-uPAR system and the down-regulation of CXCR4 and CXCL12. In a low uPA-uPAR expressing cell line, MCF-7, DIM (10–50 μM) also inhibited the cellular migration and invasion by suppressing VEGF, MMP-9, CXCR4, and CXCL12 [100–102]. Against colorectal cancer: DIM inhibited the adhesion, migration, and invasion of HT-29 (50-TS) cells through the Akt and ERK pathways [103]. Against prostate cancer: I3C (100 μM) degrades β-catenin to attenuate the migration of basal and EGF-stimulated DU145 cells [104]. By repressing MMP-9 and uPA/uPAR through the inhibition of NF-kB-DNA binding activity and by decreasing the VEGF bioavailability, DIM (1–25 μM) effectively inhibited the invasion of LNCaP and C4–2B cells [105,106]. In PC3 PDGF-D cells, DIM (10–25 μM) significantly inhibited cell invasion by decreasing mTOR and Akt [107]. DIM (25 μM) also prevented bone metastasis and the progression of PC3 and C4–2B cells by downregulating miR-92a, which modulates a RANKL signaling-associated protein, expression, EMT, and cancer progression [108]. Against glioma: At 10 μM, DIM is effective for diminishing the invasion of H4 cells [97]. Against ovarian cancer: By downregulating CXCR4 and CXCL12, DIM (10–50 μM) could inhibit the migration and invasion of BG-1 cells [101,102]. Zou et al. [109] found that DIM inhibits adhesion, migration and invasion of SKOV3 and A2780 ovarian cancer cells, which was associated with down-regulation of the MMP-2/-9 and STAT3. Against pancreatic cancer: The treatment of Colo357 and Panc-1 cells with DIM increased miR-146a and decreased EGFR, metastasis-associated protein (MTA)-2, IRAK-1, and NF-kB, which resulted in an block in cell invasion [110]. Against thyroid cancer: DIM demonstrated anti-estrogenic like activity to evoke in vitro metastasis-associated events, including adhesion, migration, and invasion, in EG-stimulated BCRAP, 8505C, CGTHW-1, and ML-1 cells. Moreover, MMP-2 and MMP-9 were responsible for DIM-mediated inhibition [111]. Against bladder cancer: Sun et al. [112] revealed that the invasive characteristics including adhesion and migration in both types of bladder cancer cells, RT112 (E-cadherin positive bladder tumor cell line) and J82 (E-cadherin negative bladder tumor cell line), can be decreased by the treatment of DIM through inhibiting STAT signaling.
2.4. Carotenoids
2.4.1. Lycopene/apo-8′-lycopenal
Lycopene is a phytochemical in tomatoes, watermelon, pink grapefruit, pink guava, papaya, red bell peppers, and other red fruits and vegetables. Lycopene can be transformed into lycopenoids, such as apo-lycopenal, apo-lycopenol, and apo-lycopenoic acid, by carotene-oxygenase [113]. Apo-8′-lycopenal and apo-12′-lycopenal are two lycopene-transformed compounds that exist in lycopene-containing foods [114]. Lycopene may possess metastasis-inhibited capabilities in cancer cells. Against hepatoma: The meta-static phenotypes (i.e., adhesion, migration, and invasion) of SK-Hep1 cells were weakened by lycopene (1–10 μM) through the inhibition of MMP-2/-9, NF-kB, Sp-1, and NOX4 [115–117]. Compared to lycopene, apo-8′-lycopenal exerted a stronger inhibitory activity on the invasion and migration of SK-Hep1 cells, which mechanistically acts by decreasing MMP-2/-9, Rho small GTPase, ERK/p38, and PI3K/Akt and increasing TIMP-1/-2 and nm23-H1 [116]. Against colorectal cancer: Lycopene acts as a chemopreventive agent by inhibiting invasion and MMP-7 and augmenting the E-cadherin protein in leptin-stimulated HT29 cells. Lycopene also effectively inhibited the phosphorylation of Akt, glycogen synthase kinase-3β (GSK-3β), and ERK [118]. Against head and neck squamous cell carcinoma: Treatment with 25 μM lycopene may inhibit the invasive abilities of FaDu and Cal27 cells [119].
2.4.2. β-carotene
Beta-carotene is an antioxidative pigment found in vegetables (e.g., carrot and pumpkin), fruits (e.g., papaya and mango) and crustaceans (e.g., crab and lobster). The β-carotene structure is composed of two retinyl groups and is broken down in the mucosa of the human small intestine by β-carotene 15, 15′-monooxygenase to retinal to supplement vitamin A in humans and some other mammals. Against melanoma: β-Carotene downregulated and upregulated the expression of MMP-2/-9 and TIMP-1/-2, respectively, and inactivated several transcription factors including NF-kB, AP-1, activated transcription factor-2 (ATF2), and cyclic adenosine monophosphate response element-binding protein (CREB) in B16F10 cells [120]. Against neuroblastoma: By applying β-carotene to SK-N-BE (2)C cells, the migratory and invasive capabilities were attenuated and the MMP-2 and HIF-1α were also suppressed [121].
2.5. Alkaloids
2.5.1. Caffeine
The common sources of caffeine are coffee beans, kola nuts, tea, and cocoa beans. Caffeine may act as a central nervous system stimulant, temporarily warding off drowsiness and restoring alertness. Ordinary caffeine consumption has low health risk to humans, and caffeine consumption for years may even have a modest protective effect against some diseases including certain types of cancer. Against hepatoma: caffeine could significantly inhibit the migration and invasion of HepG2 and Huh7 cells at physiologically applicable concentration through Akt signaling pathway [122]. Against glioblastoma: In a series of glioblastoma cells, including primary human glioblastoma, U178MG, U87MG, T98G, U373MG, and M059K cells, caffeine-mediated the inhibition of the inositol 1,4,5-trisphosphate receptor subtype 3 (IP3R3, a calcium release channel), effectively blocking cell migration [123]. Caffeine was futher identified that reduced the invasion of U87MG, GBM8401, and LN229 glioma cells through ROCK-cathepsin B/FAK/ERK signaling pathway [124]. Against leukemia: Caffeine attenuated invasion and downregulated MMP-2/-9 in human leukemia U937 cells via Ca2+/ROS-mediated suppression of the ERK/c-Fos pathway and activation of p38MAPK/c-jun pathway [125].
2.5.2. Piperine
Piperine is an alkaloid that is responsible for the pungency of black pepper and long pepper, and it is also used in some forms of traditional medicine. Against breast cancer: Piperine significantly decreased the expression of MMP-9/-13 and inhibited the migration of 4T1 cells [126]. Against fibrosarcoma: In PMA-stimulated HT1080 cells, piperine depressed induced invasion, MMP-9, and MT1-MMP by downregulating the induced signaling of ERK and PKCα and the NF-kB and AP-1 transcription factors [127]. Against osteosarcoma: piperine inhibits migration and invasion of HOS and U2OS cells via increased expression of TIMP-1/-2 and down-regulation of MMP-2/-9 [128].
2.6. Furocoumarins
The natural furanocoumarin bergamottin, which was first isolated from bergamot oil, principally exists in grapefruit juice and to a lesser extent in the essential oils of other citrus fruits. At 5–50 μM, bergamottin suppresses the PMA-induced invasion and migration of HT1080 fibrosarcoma cells by depressing MMP-2/-9 and MT1-MMP. In addition, PMA-induced p38/JNK activation, NF-kB nuclear translocation, and IkBα degradation were also strongly repressed by bergamottin [129]. Bergamottin also suppressed invasion and migration of A549 lung adenocarcinoma cells [130].
2.7. Lignan
Sesamin, a lipid-soluble lignan isolated from the bark of Fagara plants and sesame oil, has been used as a vitamin E rich and fat-reduction dietary supplement for the defense against oxidation and healthcare. Recently, sesamin was found to have potent inhibitory activity on the metastatic phenotype (MMP-9, ICAM-1, and VEGF) of TNF-induced myeloid leukemia KBM-5 cells and LPS-induced prostate cancer PC-3 cells, which may be linked to the suppression of NF-kB activation, IkBα degradation, and p38 signaling. Nevertheless, this inhibition may occur only at high concentration (100 μM) treatment on both cells [131,132].
2.8. Xanthone
Isoalvaxanthone (IAX) is a bioactive xanthone isolated from Cudrania cochinchinensis (Lour.). At 0.1–5 μM, IAX inhibits the metastasis of SW620 colorectal cancer cells by suppressing migration and invasion. The antiinvasive activity of IAX may target MMP-2 via reducing Rac1 and AP-1 activity [133].
The proposed target proteins and mechanisms of the above-mentioned compounds for the inhibition of cancer invasion in vitro are summarized in Table 2.
Table 2.
The proposed mechanisms of dietary non-phenolic bioactives on the inhibition of cancers invasion in vitro.
| Bioactive (Effective dosage) | Cancer cell models | Biological effects | Molecular targets | References |
|---|---|---|---|---|
| Isothiocyanates | ||||
| Sulforaphane | ||||
| Breast cancer: MDAMB231 | ↓: invasion; vimentin; MMP-7/-14; IL-1β/-4/-6; TNFα; IFNγ; PDGF; VEGF | ↓: Twist1; POU5F1 | Hunakova et al. [49] | |
| (1 μM) | Oral cancer: YD10B | ↓: migration; invasion; MMP-1/-2 | – | Jee et al. [50] |
| (10–15 μM) | Prostate cancer: PC-3/LNCaP/DU145/PC3 | ↓: migration; invasion; CD44v6; MMP-2; galectin-3 ↑: E-cadherin |
↑: ERK | Hahm et al. [51]; Peng et al. [52]; Tian et al. [53] |
| (5–20 μM) | Bladder cancer: T24 | ↓: adhesion; migration; invasion; MMP-2/-9; miR-200c ↑: E-cadherin |
↓: Snail; ZEB1 | Shan et al. [54] |
| (10–40 μM) | Glioblastoma and Glioma: U251MG/U87MG/U373MG/C6 | ↓: migration; invasion; MMP-2/-9; Galectin-3; CD44v6 ↑: E-cadherin |
↑: ERK ↓: FAK/JNK; NF-κB; AP-1 |
Li et al. [55]; Lee et al. [57]; Zhang et al. [56] |
| BITC | ||||
| (5–80 μM) | Hepatoma: SK-Hep1/Bel 7402/HLE | ↓: migration; invasion; MMP-2/-9; MT1-MMP; CXCR4 ↑: TIMP-2 |
↓: ERK/JNK/p38 | Hwang and Lee [58]; Zhu et al. [59] |
| (5 μM) | Lung cancer: L9981 | ↓:migration; invasion; MMP-2; β-catenin ↑: ROS |
↓: Akt; Twist; NF-kB | Wu et al. [60] |
| (2.5–5 μM) | Breast cancer: HGF-induced MDAMB231/MDAMB231/SUM159/MDAMB468 | ↓: migration; invasion; uPA/uPAR; c-met ↑: PAI-1; E-cadherin |
↓: Akt; NF-kB; FOXQ1 | Kim et al. [61]; Sehrawat et al. [62] |
| (0.01–0.25 μM) | Colorectal cancer: HT29 | ↓: migration; invasion; MMP-2/9; uPA | ↓: PKC; ERK/JNK; GRB2; PI3K; FAK; Ras; AP-1; NF-kB | Lai et al. [63] |
| Gastric cancer: AGS | ↓: migration; invasion; MMP-2/-7/-9; iNOS; COX-2 ↑: VEGF |
↓: Ras; ERK; GRB2; RhoA; FAK; ROCK1 ↑: MKK7; MEKK3; JNK; PI3K; PKC; SOS1; AP-1; NF-kB; |
Ho et al. [64] | |
| (5–20 μM) | Pancreatic cancer: BxPC-3/PanC-1 | ↓: migration; invasion; MMP-2; VEGF; VEGFR2; HIF-α | ↓: RhoC; STAT3 ↑: RhoB |
Boreddy et al. [65] |
| (1–5 μM) | Melanoma:B16F10/A375.S2 | ↓: migration; invasion; MMP-2; ↑: TIMP |
↓: RhoA; Ras; SOS-1; p38 ↑: ERK/JNK/p38; FAK |
Lai et al. [66]; Ma et al. [67] |
| (2.5–5 μM) | head and neck squamous cell carcinoma: HN12 | ↓: migration; invasion; vimentin | Wolf and Claudio [68] | |
| (10 μM) | Glioma: C6 | ↓: migration; invasion; MMP-9 | ↓: FAK/JNK; NF-κB; AP-1 | Lee et al. [57] |
| PEITC | ||||
| (10 μM) | Lung cancer: L9981 | ↓: migration; invasion; MMP-2; β-catenin ↑: ROS |
↓: Akt; Twist; NF-kB | Wu et al. [60] |
| Breast cancer: MCF-7/MDA-MB-231 | ↓: HIF-1α; ROS; adhesion; migration; invasion; MMP-2/-9 | Sarkar et al. [69] | ||
| (0.01–0.25 μM) | Colorectal cancer: HT29 | ↓: adhesion; migration; invasion; MMP-2/-7/-9; iNOS; COX-2 | ↓: PKC; ERK/JNK; RhoA; SOS1; Ras; PI3K/Akt; FAK; GRB2; NF-kB | Lai et al. [70] |
| (1–5 μM) | Prostate cancer: PC-3/LNCaP | ↓: migration; VEGF; MMP-2/-9; BMP1 ↑: Notch1/2; miR-194 |
↓: Akt | Xiao and Singh [71]; Kim et al. [72]; Zhang et al. [73] |
| Gastric cancer: AGS | ↓: migration; invasion; MMP-2/-7/-9; uPA; VEGF; iNOS; COX-2 | ↓: PKC; MEKK3; MKK7; ERK; NF-kB; RhoA; SOS1; Ras; GRB2; FAK | Yang et al. [74] | |
| (1–5 μM) | Melanoma: B16F10/A375.S2 | ↓: migration; invasion; MMP-2; TIMP | ↓: ERK/JNK/p38; RhoA; Ras; SOS-1; NF-kB ↑: FAK; GRB2; NF-kB |
Lai et al. [66]; Ma et al. [67] |
| (2–10 μM) | Glioblastoma and Glioma: C6/GBM8401 | ↓: migration; invasion; MMP-2/-7/-9/-13; uPA; | ↓: FAK/JNK/p38/ERK; NF-κB; AP-1; Ras, RhoA; GRB2; SOS1 | Lee et al. [57]; Chou et al. [75] |
| (5–10 μM) | cervical carcinoma: Hela | ↓: adhesion; invasion; CDK1; MMP-2/-9; CD44; ICAM-1 ↑: TGF-β; IL-6/-8 |
↑: smad2 | Zhang et al. [76] |
| ovarian cancer: SKOV3/HO8910/EOC | ↓: migration; invasion; CRM1 | ↓: mTOR | Shao et al. [77] | |
| AITC | ||||
| (5 μM) | Colorectal cancer: EGF-induced HT29 | ↓: migration; invasion; MMP-2/-9 | ↓: JNK/p38/ERK | Lai et al. [78] |
| S-allylcysteine | ||||
| (20–40 mM) | Hepatoma: MHCC97L | ↓: migration; invasion; VEGF ↑: E-cadherin |
– | Ng et al. [81] |
| (10–40 mM) | Breast cancer: MDAMB231 | ↓: adhesion; migration; invasion; MMP-2 ↑: E-cadherin |
– | Gapter et al. [82] |
| (10 mM) | Nasopharyngeal carcinoma: HONE1/HNE1 | ↓: migration; invasion; slug; MMP-2/-9 | ↓: Met/FAK/ERK | Cho et al. [83] |
| Allicin | ||||
| (3–6 μg/ml) | Colorectal cancer: LoVo | ↓: adhesion; migration; invasion; VEGF; uPAR | – | Gao et al. [84] |
| (5–10 μM) | Lung cancer: A549/H1299 | ↓: adhesion; invasion; migration; MMP-2/-9 ↑: TIMP-1/-2 |
↓: PI3K/AKT | Huang et al. [85] |
| Diallyl sulfide/Diallyl disulfide | ||||
| (100–400 μM) | Breast cancer: MCF-7/MDA-MB-231 | ↓: invasion; migration; MMP-9; Vimentin ↑: E-cadherin; miR-34a |
↓: SRC/Ras/ERK | Chen et al. [86]; Xiao et al. [87] |
| (10–25 μM 45 mg/l) | Colorectal cancer: Colo205/SW480 | ↓: migration; invasion; MMP-2/-7/-9; COX-2 | ↓: ERK/JNK/p38; Ras; PI3K; MEKK3; MKK7; NF-kB; Rac1-ROCK1/PAK1-LIMK1-ADF/cofilin | Zhou et al. [89]; Lai et al. [88]; Su et al. [90] |
| (100 μM) | Prostate cancer: LNCaP | ↓: migration; invasion; MMP-2/-9; claudin | – | Shin et al. [91] |
| (70–200 μM 30 mg/l) | Gastric cancer: AGS/MGC803/SGC-7901 | ↓: migration; invasion; MMP-2/-9; claudin-2/-3/-4; Vimentin; CD34 ↑: TIMP-1/-2/-3; E-cadherin; miR-34a |
↓: Rac1-ROCK1/PAK1-LIMK1-ADF/cofilin; PI3K/Akt | Park et al. [92]; Su et al. [93]; Wang et al. [94] |
| (20–40 μg/ml) | Osteosarcoma: MG-63 | ↓: invasion | – | Hu et al. [95] |
| Indole | ||||
| I3C/DIM | ||||
| (10–20 μM DIM) | Hepatoma: SMMC-7721/MHCC-97H | ↓: migration; invasion; MMP-2/-9 ↑: PTEN |
↓: FAK | Li et al. [96] |
| (20 μM DIM) | Lung cancer: H1650/H1975 | ↓: invasion | – | Rahimi et al. [97] |
| (50–100 μM I3C/2.5–25 μM DIM) | Breast cancer: MCF-7/MDAMB361/MDAMB231/4T1 | ↓: migration; invasion; MMP-2/-9; uPA/uPAR; VEGF; CXCR4; CXCL12 | ↓: ERK; Sp1 | Hung and Chang [98]; Rahimi et al. [97]; Hsu et al. [101,102]; Ahmad et al. [100]; Kim et al. [99] |
| (25 μM DIM) | Colorectal cancer: HT29 (50-TS) | ↓: adhesion; migration; invasion | ↓: Akt; ERK | Rajoria et al. [103] |
| (100 μM I3C | Prostate cancer: DU145/EGF-induced | ↓: migration; invasion; metastasis (to bone); | ↓: mTOR; Akt; NF-kB | Jeong et al. [104]; |
| /1–25 μM DIM) | DU145/PC3 PDGF-D/PC3/LNCaP/C4–2B | β-catenin; uPA/uPAR; VEGF; MMP-9; miR-92a | Kong et al. [105,107]; Ahmad et al. [106]; Li et al. [108] | |
| (10 μM DIM) | Glioma: H4 | ↓: invasion | – | Rahimi et al. [97] |
| (10–50 μM DIM) | Ovarian cancer: BG-1/SKOV3/A2780 | ↓: migration; invasion; CXCR4; CXCL12; MMP-2/-9 | ↓: STAT3 | Hsu et al. [101,102]; Zou et al. [109] |
| (25 μM DIM) | Pancreatic cancer: Colo357/Panc-1 | ↓: EGFR; MTA-2 ↑: miR-146a |
↓: IRAK-1; NF-kB | Li et al. [110] |
| (25 μM DIM) | Tyroid cancer: EG-mediated BCPAP/8505C/CGTHW-1/ML-1 | ↓: adhesion; migration; invasion; MMP-2/-9 | – | Rajoria et al. [111] |
| (1–50 μM DIM) | Bladder cancer: J82/RT112 | ↓: adhesion; migration | ↓: STAT | Sun et al. [112] |
| Carotenoids | ||||
| Lycopene/Apo-8′-lycopenal | ||||
| (1–10 μM) | Hepatoma: SK-Hep1/AH109A | ↓: adhesion; migration; invasion; MMP-2/-9; NOX4 ↑: nm23-H1; TIMP-1/-2 |
↓: Rho small GTPase; ERK/p38; PI3K/Akt; NF-kB; Sp-1 | Huang et al. [115]; Yang et al. [116]; Jhou et al. [117] |
| (0.5–2 μM) | Colorectal cancer: Leptin-stimulated HT29 | ↓: invasion; MMP-7 ↑: E-cadherin; |
↓: ERK; Akt; GSK-3β | Lin et al. [118] |
| (25 μM) | head and neck squamous cell carcinoma: FaDu/Cal27 | ↓: invasion | Ye et al. [119] | |
| β-carotene | ||||
| Melanoma: B16F10 | ↓: MMP-2/-9 ↑: TIMP-1/-2 |
↓: NF-kB; AP-1; ATF2; CREB | Guruvayoorappan and Kuttan [120] | |
| Neuroblastoma: SK-N-BE (2)C | ↓: migration; invasion; MMP-2; HIF-1α | Kim et al. [121] | ||
| Alkaloids | ||||
| Caffeine | ||||
| (50–600 μM) | Hepatoma: HepG2/Huh7 | ↓: migration; invasion | ↓: Akt | Dong et al. [122] |
| (1–10 mM) | Glioblastoma: primary human glioblastoma cells/U178MG/U87MG/T98G/U373MG/M059K/GBM8401/LN229 | ↓: migration; invasion | ↓: IP3R3-mediated Ca2+; ROCK-cathepsin B/FAK/ERK | Kang et al [123]]; Cheng et al. [124] |
| (10–100 μM) | Leukemia: U937 | ↓: invasion; MMP-2/-9 | ↓: Ca2+/ROS-mediated ERK/c-Fos ↑: Ca2+/ROS-mediated p38/c-jun |
Liu and Chang [125] |
| Piperine | ||||
| (140–280 μM) | Breast cancer: 4T1 | ↓: migration; MMP-9/-13 | – | Lai et al. [126] |
| (25 μM) | Fibrosarcoma: PMA-induced HT-1080 | ↓: invasion; MMP-9; MT1-MMP | ↓: ERK; PKCα; NF-kB; AP-1 | Hwang et al. [127] |
| Osteosarcoma: HOS/U2OS | ↓: migration; invasion; MMP-2/-9 ↑: TIMP-1/-2 |
Zhang et al. [128] | ||
| Furcoumarins | ||||
| Bergamottin | ||||
| (5–50 μM) | Fibrosarcoma: PMA-induced HT-1080 | ↓: invasion; migration; MMP-2/-9, MT1-MMP | ↓: p38; JNK; NF-kB; IkBα degradation | Hwang et al. [129] |
| (10–50 μM) | Lung cancer: A549 | ↓: invasion; migration | Wu et al. [130] | |
| Lignan | ||||
| Sesamin | ||||
| (100 μM) | Myeloid leukemia: TNF-induced KBM-5 | ↓: MMP-9; ICAM-1; VEGF | ↓: NF-kB; IkBα degradation | Harikumar et al. [131] |
| (100 μM) | Prostate cancer: LPS-induced PC-3 | ↓: MMP-9; ICAM-1; VEGF; TNF-α; IL-6 | ↓: NF-kB; p38 | Xu et al. [132] |
| Xanthone | ||||
| Isoalavaxanthone | ||||
| (0.1–5 μM) | Colorectal cancer: SW620 | ↓: invasion; migration; MMP-2 | ↓: Rac1; AP-1 | Wang et al. [133] |
3. The non-phenolic bioactives possess anti-angiogenic and/or in vivo anti-metastatic activity on cancer
3.1. Isothiocyanates
3.1.1. Sulforaphane (SF)
The administration of SF (25–50 mg/kg) may reduce the regional lymph node metastasis of orthotopically transplanted human breast cancer KPL-1 cells in athymic BALB/c mice [134]. SF (40 mg/kg) also inhibited the in vivo metastasis of the PC-3 human prostate cancer cells, which were orthotopically implanted in BALB/c nu/nu mice, by inhibiting angiogenesis and the activation of PI3K/Akt, ERK, and NF-kB and activating the FOXO3a transcription factor [135]. The administration of 6 μmole SF to transgenic adenocarcinomas in mouse prostate (TRAMP) mice effectively attenuated the risk of pulmonary metastasis [48].
3.1.2. Benzyl isothiocyanate (BITC)
In the murine mammary carcinoma 4T1 cell implanted BALB/c mice model, BITC (5–10 mg/kg) administration could reduce the number of pulmonary tumor nodules and the total pulmonary metastatic volume; the expression of CD31 and VEGF in the tumors and the level of MMP-2/-9, TIMP-1, and uPA in the animal sera and lung tissues were also reduced. However, the TIMP-2 and PAI-1 concentrations were increased in the sera and lungs of BITC-treated mice [136]. BITC at 12 μmol/kg exerted an inhibitory activity on tumor invasion in pancreatic cancer BxPC-3 cell implanted athymic nude mice. The inhibition may derive from BITC-inhibited angiogenesis, VEGF, VEGFR2, MMP-2, HIF-α, CD31, RhoC, and STAT3 [65].
3.1.3. Phenethyl isothiocyanate (PEITC)
The capillary-like tube structure formation and migration of human umbilical vein endothelial cells (HUVECs) were significantly inhibited by 1 μM PEITC. The PEITC-mediated inhibition of the angiongenic features of HUVECs was associated with the suppression of VEGF and VEGFR2 and the inactivation of Akt [71]. Oral administration of 10 μmol/kg PEITC suppressed the metastasis of EOC (epithelial ovarian cancer) cells in a xenograft mouse model in vivo. PEITC suppresses the metastasis of EOC through inhibiting CRM1-mediated nuclear export, MMP-2/-9, subsequently suppressing the mTOR-STAT3 pathway [77].
3.1.4. Allyl isothiocyanate (AITC)
Intraperitoneal AITC administration at 25 μg/dose or 1.1 mg/kg significantly inhibited tumor-directed capillary formation and tumor nodule formation in the lungs of B16F-10 melanoma cell implanted C57BL/6 mice. The NO and TNF-α levels in the mouse sera were also significantly downregulated by AITC [47,137]. Oral administration of 10 μmol/kg AITC inhibited VEGF secretion and metastasis (to muscle) in the AY27 bladder cancer cell implanted F344 rat model [138].
3.1.5. S-allycysteine (SAC)
SAC-inhibited hepatoma tumor cell lung metastasis was demonstrated by an in vivo xenograft liver tumor model, which contained a luciferase gene integrated in MHCC97L hepatoma cancer cells (MHCC97L-luc) implanted in athymic nude mice [81].
3.1.6. Diallyl sulfide (DAS)/Diallyl disulfide (DADS)
DAS (10 mg/kg) inhibited the production of proangiogenic factors, including IL-1β, IL-6, TNFα, and VEGF, and enhanced the production of anti-angiogenic factors, including IL-2 and TIMP in human melanoma B16F10 cell implanted in C57BL/6 mice. DAS (1–5 μg/ml) also retarded the proliferation, migration, invasion, and tube formation of HUVEC cells [139]. In an in vivo experiment, DAS at a concentration of 20–40 μg/ml significantly decreased microvessel density (MVD) of tissue in nude mice MG-63 cells tumor-bearing model was demonstrated by immunohistochemistry [95]. The metastatic markers, vimentin, CD34, and E-cadherin, changed by DADS administration were observed in MGC803 gastric cancer cells and SW480 colon cancer cells implanted BALB/c nude mice [90,93].
3.2. Terpenoids
3.2.1. Retinoids/Retinoic acid (RA)
In B16F10 melanoma cell implanted mice, the number of metastatic nodules formed in the lung was almost diminished by administrating 20 mg/ml RA to the mice [10]. Recent study by Li et al. [140] showed that ATRA treatment decreased the migration of esophageal squamous cell carcinoma EC1 cells and led to a marked decrease of Ang-1, Ang-2, Tie-2, VEGF, and VEGF receptors. In EC1 xenografted mice, the decrease of CD31, Ang-1, Ang-2, and Tie-2 expression by ATRA treatment were observed.
3.2.2. Ursolic acid (UA)
UA treatment could inhibit prostate cancer cell metastasized to lung and liver and suppress CXCR4 expression in the prostatic tissues of TRAMP mice [26]. Tumor-associated capillary formation in B16F10 melanoma cell-bearing C57BL/6 mice was inhibited by UA and a significant reduction in VEGF and NO and an elevation in TIMP-1 and IL-2 in the serum was also observed [141]. In in vitro HUVEC and rat aortic ring assays, UA was demonstrated to have the potential to inhibit cell migration, MMP-2/-9 expression, capillary formation, and vessel growth [141]. When examined in an HCT116 colon cancer cells orthotopic implanted nu/nu mice model, UA significantly inhibited distant organ metastasis and microvessel density (CD31). This effect was accompanied by the suppression of MMP-9, VEGF, NF-kB, STAT3, and β-catenin [23]. Administration of 12.5 mg/kg UA to HT29 colon cancer cells xenografted BALB/c athymic (nude) mice, the intratumoral microvessel density (MVD) reduction was observed. Several angiogenic factors, such as VEGF-A and bFGF, and signaling, such as sonic hedgehog (SHH), STAT3, Akt and p70S6K were suppressed by UA. Additionally, UA treatment also decreased the total number of blood vessels in the CAM model, and inhibited the proliferation, migration and tube formation of HUVECs [142]. Overall, these results implied that UA can inhibit metastasis of colorectal cancer through blocking multiple bio-markers linked to invasion, angiogenesis, and metastasis.
3.2.3. Ganoderic acid (GA)
Lewis lung carcinoma (LLC) implanted C57B/6 mouse model experiments demonstrated that the administration of 28 mg/kg GA-T suppressed LLC lung metastasis and downregulated MMP-2/-9 expression [37].
3.2.4. Zerumbone
Oral administration of 20 mg/kg zerumbone to MDA-MB231 cells xenografted Balb/c nude mice, the growth and metastatic potential of TNBC xenograft tumors were effectively suppressed [44]. Zerumbone blocked the pancreatic cancer PaCa cells-associated angiogenesis through the inhibition of NF-κB and NF-κB-dependent proangiogenic gene products [143].
3.3. Alkaloids
3.3.1. Caffeine
Caffeine-potentiated chemotheraphy consisting of 1.5 g caffeine/m2/day has been demonstrated to potentially prevent local recurrence or metastasis in patients with metastatic lung adenocarcinoma and prolong the survival rate of osteosarcoma patients with pulmonary metastasis [144,145]. Moreover, caffeine-potentiated chemotheraphy (1.5 g caffeine/m2/day) applied to five patients with clear cell sarcorma also decreased the risk for metastasis (distant metastasis newly developed in only one patient) [146].
3.3.2. Piperine
Piperine exerted an inhibitory effect on lung metastasis in the spontaneously metastasizing 4T1 mouse mammary carcinoma model through the administration with 5 mg/kg piperine [126].
3.4. Indole
3.4.1. Indole-3-carbinol (I3C)/3,3-diindolylmethane (DIM)
The oral administration of DIM (5–10 mg/kg) resulted in a marked reduction in the number of pulmonary tumor nodules in 4T1 breast cancer cell implanted BALB/c mice; the reduced levels of MMP-2/-9, TIMP-1, VCAM-1, IL-1β/IL-6, and TNFα and elevated levels of TIMP-2 were detected in the mouse sera and lungs [99]. The anti-angiogenic activity of DIM has been demonstrated by in vitro HUVEC and in vivo angiogenesis assays in C57BL/6 mice treated with 1–10 μM and 5 mg/kg, respectively [105,147]. Oral administration of 10 mg/kg DIM to SMMC-7721 hepatoma cells implanted BALB/c mice can inhibit the cells metastasized to lung and the anti-metastasis effect of DIM could be resulted from its down-regulated expression and activation of MMP-2/-9 partly induced by up-regulation of pTEN and inhibition of phospho-FAK [96].
3.5. Carotenoids
3.5.1. Lycopene
Huang et al. [148] found that high-lycopene supplementation (20 mg/kg) could mediate several metastasis-associated markers in SK-Hep1 hepatoma cell implanted athymic nude mice including a decrease in proliferating cellular nuclear antigen (PCNA), VEGF, and MMP-2/-9 and an increase in nm23-H1, which may contribute to the attenuation of tumor invasion and metastasis. Lycopene at a concentration of 1–10 μM was observed to decrease the capillary-like tube length, tube formation, migration, and invasion of HUVEC in a dose-dependent manner. Such actions were accompanied by the downregulation of MMP-2, uPA, and Rac1 and the upregulation of TIMP-2 and PAI-1. Moreover, VEGFR2-mediated PI3K/Akt and ERK/p38 signaling pathways in HUVECs were also attenuated by lycopene [149,150]. The anti-angiogenic activity of lycopene was further confirmed in ex vivo rat aortic ring, in vivo chorioallantoic membrane, and in vivo matrigel plug assays by using 2.5–10 μM, 1–15 μg, and 400 μg/plug, respectively [150].
3.5.2. β-Carotene
Through a HUVEC tube formation assay, a rat aortic ring assay, and tumor-directed capillaries in C57BL/6 mice; β-carotene demonstrated potential inhibitory effects on angiogenesis [120]. Oral administration of β-carotene to immunodeficient nude mice which injected with neuroblastoma SK-N-BE (2)C cells via the tail vein can decrease the incidence of liver metastasis. Furthermore, mRNA levels of MMPs, membrane-type (MT) 2 MMP and TIMPs in liver tumor tissues were also lower following β-carotene treatment [121].
The proposed target proteins and mechanisms of the above-mentioned compounds for the inhibition of cancer metastasis in vivo are summarized in Table 3.
Table 3.
The anti-angiogenesis and in vivo anti-metastasis of dietary non-phenolic bioactives.
| Bioactive (Effective dosage) | Cell or animal models | Biological effects | References |
|---|---|---|---|
| Isothiocyanates | |||
| Sulforaphane | |||
| (25–50 mg/kg) | KPL-1 breast cancer cell implanted athymic BALB/c mice | ↓: regional lymph node metastasis | Kanematsu et al. [134] |
| (40 mg/kg) | PC-3 prostate tumor implanted BALB/c nu/nu mice | ↓: angiogenesis; metastasis; PI3K/Akt; ERK; NF-kB ↑: FOXO3a |
Shankar et al. [135] |
| (6 μmole) | transgenic adenocarcinoma of mouse prostate (TRAMP) mice | ↓: pulmonary metastasis | Singh et al. [48] |
| BITC | |||
| (5–10 mg/kg) | 4T1 breast cancer cells implanted BALB/c mice | ↓: pulmonary metastasis; MMP-2/-9; TIMP-1; uPA; CD31; VEGF ↑: TIMP-2; PAI-1 |
Kim et al. [136] |
| (12 μmol/kg) | BxPC-3 pancreatic cancer cells implanted athymic nude mice | ↓: angiogenesis; MMP-2; VEGF; VEGFR2; HIF-α; CD31; RhoC; STAT3; | Boreddy et al. [65] |
| PEITC | |||
| (1 μM) | HUVEC | ↓: angiogenesis; migration; VEGF; VEGFR2; Akt | Xiao and Singh [71] |
| (10 μmol/kg) | EOC cells xenograft mouse | ↓: metastasis; CRM1; mTOR; STAT3; MMP-2/-9 | Shao et al. [77] |
| AITC | |||
| (25 μg/dose) (1.1 mg/kg) | B16F-10 melanoma cells implanted C57BL/6 mice | ↓: metastasis (to lung); NO; TNF-α; angiogenesis | Manesh and Kuttan [47]; Thejass and Kuttan [137] |
| (10 μmol/kg) | AY27 bladder cancer cells implanted F344 rat | ↓: VEGF; metastasis (to muscle) | Bhattacharya et al. [138] |
| S-allylcysteine | |||
| Luciferase gene integrated MHCC97L hepatoma cancer cells (MHCC97L -luc) implanted athymic nude mice |
↓: metastasis (to lung) | Ng et al. [81] | |
| Diallyl sulfide/Diallyl disulfide | |||
| (10 mg/kg) | B16F-10 melanoma cells implanted C57BL/6 mice | ↓: angiogenesis; IL-1β/IL-6; TNFα; VEGF ↑: TIMP; IL-2 |
Thejass and Kuttan [139] |
| (1–5 μg/ml) | HUVEC | ↓: proliferation; migration; invasion; tube formation | Thejass and Kuttan [139] |
| (20–40 μg/ml) | MG-63 osteosarcoma cells bearing nude mice | ↓: microvessel density (angiogenesis) | Hu et al. [95] |
| (100 mg/kg) | MGC803 gastric cancer cells implanted BALB/c nude mice |
↓: Vimentin; CD34 ↑: E-cadherin |
Su et al. [93] |
| (100 mg/kg) | SW480 colon cancer cells implanted BALB/c nude mice | ↓: Vimentin; CD34 ↑: E-cadherin |
Su et al. [90] |
| Terpenoids | |||
| Retinoids/Retinoic acid | |||
| (20 mg/ml) | B16F10 melanoma cells implanted mice | ↓: metastasis (to lung) | Liu et al. [10] |
| (0.1–10 μM) | EC1 esophageal squamous cell carcinoma/EC1 xenografted mice | ↓: migration; CD31; Ang-1; Ang-2; Tie-2; VEGF; VEGFR | Li et al. [140] |
| Ursolic acid | |||
| Transgenic adenocarcinoma of mouse prostate (TRAMP) mice | ↓: CXCR4; metastasis (to lung and liver) | Shanmugam et al. [26] | |
| (50 mg/kg) | B16 melanoma cells implanted mice | ↓: metastasis (to lung) | Kanjoormana and Kuttan [141] |
| HUVEC/rat aortic ring assay | ↓: angiogenesis; MMP-2/-9 | Kanjoormana and Kuttan [141] | |
| (250 mg/kg) | HCT116 colon cancer cells orthotopic implanted nu/nu mice | ↓: metastasis; micerovessel density (CD31); MMP-9; VEGF; NF-kB; STAT3; β-catenin | Prasad et al. [23] |
| (12.5 mg/kg) | HT29 colon cancer cells xenografted BALB/c athymic (nude) mice | ↓: cancer growth; intratumoral microvessel density (MVD); VEGF-A; bFGF; sonic hedgehog (SHH); STAT3; Akt; p70S6K | Lin et al. [142] |
| (0.25 mg) | Chorioallantoic membrane assay | ↓: angiogenesis | Lin et al. [142] |
| (40 μM) | HUVEC | ↓: proliferation; migration; tube formation | Lin et al. [142] |
| Ganoderic acid | |||
| (28 mg/kg) | Lewis Lung Carcinoma implanted C57B/6 mice | ↓: metastasis (to lung); MMP-2/-9 | Chen et al. [37] |
| Zerumbone | |||
| (20 mg/kg) | MDA-MB231 cells xenografted Balb/c nude mice | ↓: metastasis | Kim et al. [44] |
| HUVEC | ↓: tube formation; pancreatic cancer (PaCa) cells-associated angiogenesis | Shamoto et al. [143] | |
| Alkaloids | |||
| Caffeine | |||
| (1.5 g/m2/day) | Lung adenocarcinoma patients | ↓: local recurrence or metastasis | Hayashi et al. [144] |
| (1.5 g/m2/day) | Osteosarcoma patients with pulmonary metastasis | ↑: survival rate | Kimura et al. [145] |
| (1.5 g/m2/day) | Caffeine-potentiated chemotherapy for clear cell sarcoma in five patients | ↓: metastasis (distal metastasis newly developed in only one patient) | Karita et al. [146] |
| Piperine | |||
| (5 mg/kg) | Spontaneously metastasizing 4T1 mouse mammary carcinoma model | ↓: metastasis (to lung) | Lai et al. [126] |
| Indole | |||
| I3C/DIM | |||
| (5–10 mg/kg) | 4T1 breast cancer cells implanted BALB/c mice | ↓: metastasis (to lung); MMP-2/-9; TIMP-1; VCAM-1; IL-1β/IL-6; TNFα ↑: TIMP-2 |
Kim et al. [61] |
| (1–10 μM) | HUVEC | ↓: angiogenesis | Chang et al. [147]; Kong et al. [105] |
| (5 mg/kg) | In vivo angiogenesis assay in C57BL/6 mice | ↓: angiogenesis | Chang et al. [147] |
| (10 mg/kg) | SMMC-7721 hepatoma cells implanted BALB/c mice | ↓: metastasis (to lung); MMP-2/-9; FAK ↑: PTEN |
Li et al. [96] |
| Carotenoids | |||
| Lycopene | |||
| (20 mg/kg) | SK-Hep1 hepatoma cells implanted athymic nude mice | ↓: PCNA; VEGF; MMP-2/-9 ↑: nm23-H1 |
Huang et al. [148] |
| (1–10 μM) | HUVEC | ↓: angiogenesis; MMP-2; uPA; Rac1; VEGF R2- mediated ERK/p38/Akt/PI3K ↑: TIMP-2; PAI-1 |
Sahin et al. [149]; Chen et al. [150] |
| (2.5–10 μM) | Rat aortic ring | ↓: angiogenesis | Chen et al. [150] |
| (1–15 μg) | Chorioallantoic membrane assay (CAM) | ||
| (400 mg/plug) | Matrigel plug assay in mice | ||
| β-carotene | |||
| HUVEC/rat aortic ring assay/tumor-directed capillaries in C57BL/6 mice | ↓: angiogenesis | Guruvayoorappan and Kuttan [120] | |
| immunodeficient nude mice injected with SK-N-BE (2)C cells via the tail vein | ↓: metastasis (to liver); MMPs/MT-MMP; TIMP; HIF-1α | Kim et al. [121] | |
4. Conclusions
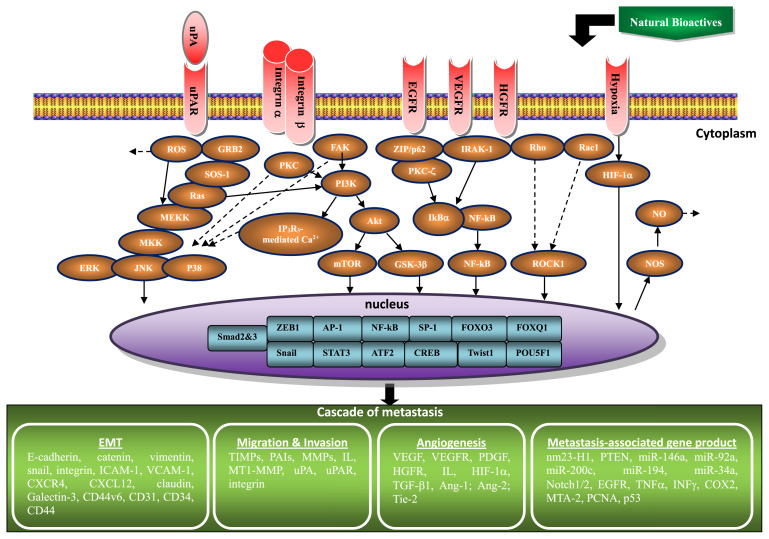
This review summarized the studies describing the in vitro and in vivo anti-invasive, anti-metastatic, and anti-angiogenic activities, including the related molecular mechanisms and effective doses, of the individual natural dietary non-phenolic bioactives. From this review, several pieces of hidden information from past and current studies of natural dietary non-phenolic compounds against metastasis are disclosed. Several suggestions for future research are therefore raised: (a) In the non-phenolic class of natural dietary compounds, terpenoids and isothiocyanantes are thus far the most studied for inhibiting cancer invasion/metastasis in vitro and in vivo. Isothiocyanantes also contribute the most diverse derivatives for the studies on anti-angiogenic and anti-metastatic potential. The clinical tries for these types of compounds should be further carried out to develop anti-invasive supplement of cancer. (b) One of the terpenoids compounds, retinoic acid/retinoid, usually depresses invasive/migratory capability of various cancers. Nevertheless, the treatment of retinoic acid/retinoid occasionally promotes the invasion and migration of A549 lung cancer cells. The specific effect of retinoic acid/retinoid on lung cancer needs to be clarified. (c) The effective in vitro antiinvasive dosage for most of the non-phenolic bioactives is in 1–100 μM but in 10–40 mM for SAC. Obviously, application of SAC should be low efficacy for this purpose. (d) The minimum and maximum dose of these compounds used in animal models for suppressing angiogenesis and metastasis are 5 and 250 mg/kg, respectively. While converting the dosage from experiment animal (mouse) to a human with 60 kg body weight, physiological achievable doses (0.033 and 1.65 g per day) are obtained. (e) Many proteins are involved in the anti-metastatic effect of these compounds including the upregulation of nm23-H1, TIMP-1/-2/-3, PAI-1, E-cadherin, ROS, VEGF, PTEN, miR-146a, miR-194, miR-34a, Notch1/2, TGF-β, and IL-2/-6/-8, the downregulation of vimentin, MMP-1/-2/-3/-7/-9/-13/-14, MT1-MMP, uPA, uPAR, α5-/αv-/β1-/β3-integrin, EGFR, VEGF, VEGFR2, PDGF, c-met (HGFR), IL-1β/-4/-6/-8, ROS, iNOS/NOS2, NOX4, NO, TGF-β1, ICAM-1, CXCR4, CXCL12, TNFα, IFNγ, COX-2, snail, claudin-2/-3/-4, miR-92a, miR-200c, TIMP-1 (in sera and lung of BITC-mediated and DIM-mediated 4T1 cell-injected mice), VCAM-1, MTA-2, PCNA, Galectin-3, CD44v6, CD31, CD34, CD44, Ang-1, Ang-2, Tie-2, and the modulation of p53. (f) Variations in the level or expression of these candidate proteins may result from promoting the signaling of RhoB, IkBα, ERK/JNK/p38, FAK, GRB2, smad2, and Ca2+/ROS-mediated p38/c-jun and the transcription factor of FOXO3 and NF-kB; suppressing pathways implicated in HIF-1α, smad3, RhoA/RhoC/Rac1/ROCK1/PAK1-LIMK1-ADF/cofilin, IRAK-1, ZIP/p62/PKC-ζ, FAK/PKC/PI3K/Akt/mTOR, GSK-3β, IP3R3-mediated Ca2+, GRB2/SRC/SOS-1/Ras/MEKK3/MKK7/ERK/JNK/p38, and Ca2+/ROS-mediated ERK/c-Fos and the AP-1, NF-kB, Sp-1, ATF2, CREB, Twist1, POU5F1, FOXQ1, ZEB1, Snail, and STAT3 transcription factor signaling. These cytosolic signaling molecules, nuclear transcription factors, and metastasis-associated proteins involved in the modulation of key steps for inhibiting invasion and metastasis of various cancer cells by natural non-phenolic bioactive compounds are represented schematically in Fig. 3.
Fig. 3.
Schematic representation of the signaling pathways and effectual proteins involved in the inhibition of metastasis cascade in various cancer cells by non-phenolic bioactives.
Combining this review of non-phenolics with previous phenolics (phenolic acids, monophenol, polyphenol, andflavonoids) reviews [4,5], the puzzle for the contribution of natural dietary bioactives to cancer invasion or/and metastasis is almost complete. These lines of scientific evidence suggest that the daily consumption of natural foods containing adequate amounts of the bioactives mentioned above may be beneficial for the prevention of metastasis and could improve cancer prognosis.
Acknowledgement
This research work was partly supported by a research grant from Ministry of Science and Technology (MOST 103-2313-B-165-001-MY3), Taiwan, Republic of China.
Abbreviations
- AEG
astrocyte-elevated gene
- AITC
Allyl isothiocyanate
- ATF2
activated transcription factor-2
- ATRA
all trans-retinoic acid
- BITC
Benzyl isothiocyanate
- BMP
bone morphogenetic protein
- COX-2
cyclooxygenase-2
- CREB
cyclic adenosine monophosphate response element-binding protein
- CXCL
CXC Chemokine ligand
- CXCR
CXC Chemokine receptor
- DADS
diallyl disulfide
- DAS
diallyl sulfide
- DATS
diallyl trisulfide
- DIM
Diindolylmethane
- ECM
extracellular matrix
- EGFR
epidermal growth factor receptor
- EMT
epithelial-mesenchymal transition
- FAK
focal adhesion kinase
- GA
ganoderic acids
- GDNT
ganodermanontriol
- GSK
glycogen synthase kinase
- HGF
hepatocyte growth factor
- HGFR
hepatocyte growth factor receptor
- HIF
hypoxia-inducible factor
- HUVEC
human umbilical vein endothelial cell
- IAX
isoalavaxanthone
- I3C
indole-3-carbinol
- ICAM intercellular adhesion molecule; IL
interleukin
- INF
interferon
- iNOS
inducible nitric oxide synthase
- IP3R3
inositol 1,4,5-trisphosphate receptor subtype 3
- LA
lucidenic acid
- MEKK
mitogen-activated protein kinase kinase kinase
- MKK
mitogen-activated protein kinase kinase
- MMP
matrix metalloproteinases
- MT-1 MMP membrane type-1 MMP; MTA
metastasis-associated protein
- MVD
microvessel density
- NO
nitric oxide
- OPN
osteopontin
- PAI
plasminogen activator inhibitor
- PCNA
proliferating cellular nuclear antigen
- PDGF
platelet derived growth factor
- PEITC
phenethyl isothiocyanate
- PMA
phorbol 12-myristate 13-acetate
- PKC
protein kinase C
- RA
retinoic acid
- RAR
retinoic acid receptor
- RARE
retinoic acid response element
- ROS
reactive oxygen species
- RXR
retinoid X receptor
- SAC
S-allylcysteine
- SAMC
S-allylmercaptocysteine
- SF
sulforaphane
- SHH
sonic hedgehog
- SOS
son of sevenless
- STAT
signal transducer and activator of transcription
- TAM
tumor-associated macrophages
- TGF
transforming growth factor
- TIMP
tissue inhibitor metalloproteinase protein
- TNF
tumor necrosis factor
- TRAMP
transgenic adenocarcinoma of mouse prostate
- UA
ursolic acid
- uPA
urokinase plasminogen activator
- uPAR
urokinase plasminogen activator receptor
- VEGF
vascular endothelial growth factor
Funding Statement
This research work was partly supported by a research grant from Ministry of Science and Technology (MOST 103-2313-B-165-001-MY3), Taiwan, Republic of China.
Footnotes
Conflicts of interest
None declared.
REFERENCES
- 1.Stewart BW, Wild CP, editors. World cancer report 2014. Lyon: International Agency for Research on Cancer; 2014. [Google Scholar]
- 2. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 3. Sliva D. Suppression of cancer invasiveness by dietary compounds. Mini Rev Med Chem. 2008;8:677–88. doi: 10.2174/138955708784567412. [DOI] [PubMed] [Google Scholar]
- 4. Weng CJ, Yen GC. Chemopreventive effects of dietary phytochemicals against cancer invasion and metastasis: phenolic acids, monophenol, polyphenol, and their derivatives. Cancer Treat Rev. 2012a;38:76–87. doi: 10.1016/j.ctrv.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 5. Weng CJ, Yen GC. Flavonoids, a ubiquitous dietary phenolic subclass, exert extensive in vitro anti-invasive and in vivo anti-metastatic activities (review) Cancer Metastasis Rev. 2012b;31:323–51. doi: 10.1007/s10555-012-9347-y. [DOI] [PubMed] [Google Scholar]
- 6. Cui J, Gong M, He Y, Li Q, He T, Bi Y. All-trans retinoic acid inhibits proliferation, migration, invasion and induces differentiation of hepa1–6 cells through reversing EMT in vitro. Int J Oncol. 2016;48:349–57. doi: 10.3892/ijo.2015.3235. [DOI] [PubMed] [Google Scholar]
- 7. García-Regalado A, Vargas M, García-Carrancá A, Aréchaga-Ocampo E, González-De la Rosa CH. Activation of Akt pathway by transcription-independent mechanisms of retinoic acid promotes survival and invasion in lung cancer cells. Mol Cancer. 2013;12:44. doi: 10.1186/1476-4598-12-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Quintero Barceinas RS, García-Regalado A, Aréchaga-Ocampo E, Villegas-Sepúlveda N, González-De la Rosa CH. All-trans retinoic acid induces proliferation, survival, and migration in a549 lung cancer cells by activating the erk signaling pathway through a transcription-independent mechanism. Biomed Res Int. 2015;2015:404368. doi: 10.1155/2015/404368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dutta A, Sen T, Chatterjee A. All-trans retinoic acid (ATRA) downregulates MMP-9 by modulating its regulatory molecules. Cell Adh Migr. 2010;4:409–18. doi: 10.4161/cam.4.3.11682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu X, Chan SY, Ho PC. Comparison of the in vitro and in vivo effects of retinoids either alone or in combination with cisplatin and 5-fluorouracil on tumor development and metastasis of melanoma. Cancer Chemother Pharmacol. 2008;63:167–74. doi: 10.1007/s00280-008-0763-1. [DOI] [PubMed] [Google Scholar]
- 11. Outerfa H, Picht T, Kess D, Herbold C, Noll E, Black PM, et al. Retinoids inhibit human glioma cell proliferation and migration in primary cell cultures but not in established cell lines. Neurosurgery. 2000;46:419–30. doi: 10.1097/00006123-200002000-00029. [DOI] [PubMed] [Google Scholar]
- 12. Papi A, Bartolini G, Ammar K, Guerra F, Ferreri AM, Rocchi P, et al. Inhibitory effects of retinoic acid and IIF on growth, migration and invasiveness in the U87MG human glioblastoma cell line. Oncol Rep. 2007;18:1015–21. doi: 10.3892/or.18.4.1015. [DOI] [PubMed] [Google Scholar]
- 13. Liang C, Yang L, Guo S. All-trans retinoic acid inhibits migration, invasion and proliferation, and promotes apoptosis in glioma cells in vitro. Oncol Lett. 2015;9:2833–8. doi: 10.3892/ol.2015.3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Roomi MW, Monterrey JC, Kalinovsky T, Rath M, Niedzwiecki A. In vitro modulation of MMP-2 and MMP-9 in human cervical and ovarian cancer cell lines by cytokines, inducers and inhibitors. Oncol Rep. 2010;23:605–14. doi: 10.3892/or_00000675. [DOI] [PubMed] [Google Scholar]
- 15. Lan L, Cui D, Luo Y, Shi BY, Deng LL, Zhang GY, et al. Inhibitory effects of retinoic acid on invasiveness of human thyroid carcinoma cell lines in vitro. J Endocrinol Invest. 2009;32:731–8. doi: 10.1007/BF03346528. [DOI] [PubMed] [Google Scholar]
- 16. Zhou Q, Xian M, Xiang S, Xiang D, Shao X, Wang J, et al. All-trans retinoic acid prevents osteosarcoma metastasis by inhibiting M2 polarization of tumor-associated macrophages. Cancer Immunol Res. 2017;5:547–59. doi: 10.1158/2326-6066.CIR-16-0259. [DOI] [PubMed] [Google Scholar]
- 17. Shishodia S, Majumdar S, Banerjee S, Aggarwal BB. Ursolic acid inhibits nuclear factor-kappaB activation induced by carcinogenic agents through suppression of IkappaBalpha kinase and p65 phosphorylation: correlation with down-regulation of cyclooxygenase 2, matrix metalloproteinase 9, and cyclin D1. Cancer Res. 2003;63:4375–83. [PubMed] [Google Scholar]
- 18. Pathak AK, Bhutani M, Nair AS, Ahn KS, Chakraborty A, Kadara H, et al. Ursolic acid inhibits STAT3 activation pathway leading to suppression of proliferation and chemosensitization of human multiple myeloma cells. Mol Cancer Res. 2007;5:943–55. doi: 10.1158/1541-7786.MCR-06-0348. [DOI] [PubMed] [Google Scholar]
- 19. Lin CC, Huang CY, Mong MC, Chan CY, Yin MC. Antiangiogenic potential of three triterpenic acids in human liver cancer cells. J Agric Food Chem. 2011a;59:755–62. doi: 10.1021/jf103904b. [DOI] [PubMed] [Google Scholar]
- 20. Huang CY, Lin CY, Tsai CW, Yin MC. Inhibition of cell proliferation, invasion and migration by ursolic acid in human lung cancer cell lines. Toxicol In Vitro. 2011;25:1274–80. doi: 10.1016/j.tiv.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 21. Liu K, Guo L, Miao L, Bao W, Yang J, Li X, et al. Ursolic acid inhibits epithelial-mesenchymal transition by suppressing the expression of astrocyte-elevated gene-1 in human nonsmall cell lung cancer A549 cells. Anticancer Drugs. 2013;24:494–503. doi: 10.1097/CAD.0b013e328360093b. [DOI] [PubMed] [Google Scholar]
- 22. Yeh CT, Wu CH, Yen GC. Ursolic acid, a naturally occurring triterpenoid, suppresses migration and invasion of human breast cancer cells by modulating c-Jun N-terminal kinase, Akt and mammalian target of rapamycin signaling. Mol Nutr Food Res. 2010;54:1285–95. doi: 10.1002/mnfr.200900414. [DOI] [PubMed] [Google Scholar]
- 23. Prasad S, Yadav VR, Sung B, Reuter S, Kannappan R, Deorukhkar A, et al. Ursolic acid inhibits growth and metastasis of human colorectal cancer in an orthotopic nude mouse model by targeting multiple cell signaling pathways: chemosensitization with capecitabine. Clin Cancer Res. 2012;18:4942–53. doi: 10.1158/1078-0432.CCR-11-2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhang Y, Kong C, Zeng Y, Wang L, Li Z, Wang H, et al. Ursolic acid induces PC-3 cell apoptosis via activation of JNK and inhibition of Akt pathways in vitro. Mol Carcinog. 2010;49:374–85. doi: 10.1002/mc.20610. [DOI] [PubMed] [Google Scholar]
- 25. Kondo M, MacKinnon SL, Craft CC, Matchett MD, Hurta RA, Neto CC. Ursolic acid and its esters: occurrence in cranberries and other Vaccinium fruit and effects on matrix metalloproteinase activity in DU145 prostate tumor cells. J Sci Food Agric. 2011;91:789–96. doi: 10.1002/jsfa.4330. [DOI] [PubMed] [Google Scholar]
- 26. Shanmugam MK, Manu KA, Ong TH, Ramachandran L, Surana R, Bist P, et al. Inhibition of CXCR4/CXCL12 signaling axis by ursolic acid leads to suppression of metastasis in transgenic adenocarcinoma of mouse prostate model. Int J Cancer. 2011;129:1552–63. doi: 10.1002/ijc.26120. [DOI] [PubMed] [Google Scholar]
- 27. Huang HC, Huang CY, Lin-Shiau SY, Lin JK. Ursolic acid inhibits IL-1beta or TNF-alpha-induced C6 glioma invasion through suppressing the association ZIP/p62 with PKC-zeta and downregulating the MMP-9 expression. Mol Carcinog. 2009;48:517–31. doi: 10.1002/mc.20490. [DOI] [PubMed] [Google Scholar]
- 28. Yu LB, Wang J, Ma BZ, Sun WZ. Inhibitive effect of ursolic acid on the invasion and metastasis of ovarian carcinoma cells HO-8910PM. Sichuan Da Xue Xue Bao Yi Xue Ban. 2010;41:986–8. 1038. [Chinese] [PubMed] [Google Scholar]
- 29. Kim ES, Moon A. Ursolic acid inhibits the invasive phenotype of SNU-484 human gastric cancer cells. Oncol Lett. 2015;9:897–902. doi: 10.3892/ol.2014.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Paterson RR. Ganoderma–a therapeutic fungal biofactory. Phytochemistry. 2006;67:1985–2001. doi: 10.1016/j.phytochem.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 31. Akihisa T, Nakamura Y, Tagata M, Tokuda H, Yasukawa K, Uchiyama E, et al. Ani-inflammatory and anti-tumor-promoting effects of triterpene acids and sterols from the fungus Ganoderma lucidum. Chem Biodivers. 2007;4:224–31. doi: 10.1002/cbdv.200790027. [DOI] [PubMed] [Google Scholar]
- 32. Chen NH, Liu JW, Zhong JJ. Ganoderic acid Me inhibits tumor invasion through down-regulating matrix metalloproteinases 2/9 gene expression. J Pharmacol Sci. 2008;108:212–6. doi: 10.1254/jphs.sc0080019. [DOI] [PubMed] [Google Scholar]
- 33. Wang X, Sun D, Tai J, Wang L. Ganoderic acid A inhibits proliferation and invasion, and promotes apoptosis in human hepatocellular carcinoma cells. Mol Med Rep. 2017;16:3894–900. doi: 10.3892/mmr.2017.7048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chen Y, Yan Y, Xie MY, Nie SP, Wei L, Gong XF, et al. Development of a chromatographic fingerprint for the chloroform extracts of Ganoderma lucidum by HPLC and LC–MS. J Pharm Biomed Anal. 2008;47:469–77. doi: 10.1016/j.jpba.2008.01.039. [DOI] [PubMed] [Google Scholar]
- 35. Jiang J, Grieb B, Thyagarajan A, Sliva D. Ganoderic acids suppress growth and invasive behavior of breast cancer cells by modulating AP-1 and NF-kappaB signaling. Int J Mol Med. 2008;21:577–84. [PubMed] [Google Scholar]
- 36. Li F, Wang Y, Wang X, Li J, Cui H, Niu M. Ganoderic acids suppress growth and angiogenesis by modulating the NF-κB signaling pathway in breast cancer cells. Int J Clin Pharmacol Ther. 2012a;50:712–21. doi: 10.5414/CP201663. [DOI] [PubMed] [Google Scholar]
- 37. Chen NH, Liu JW, Zhong JJ. Ganoderic acid T inhibits tumor invasion in vitro and in vivo through inhibition of MMP expression. Pharmacol Rep. 2010;62:150–63. doi: 10.1016/s1734-1140(10)70252-8. [DOI] [PubMed] [Google Scholar]
- 38. Chen NH, Zhong JJ. p53 is important for the anti-invasion of ganoderic acid T in human carcinoma cells. Phytomedicine. 2011;18:719–25. doi: 10.1016/j.phymed.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 39. Jiang J, Jedinak A, Sliva D. Ganodermanontriol (GDNT) exerts its effect on growth and invasiveness of breast cancer cells through the down-regulation of CDC20 and uPA. Biochem Biophys Res Commun. 2011;415:325–9. doi: 10.1016/j.bbrc.2011.10.055. [DOI] [PubMed] [Google Scholar]
- 40. Weng CJ, Chau CF, Chen KD, Chen DH, Yen GC. The anti-invasive effect of lucidenic acids isolated from a new Ganoderma lucidum strain. Mol Nutr Food Res. 2007;51:1472–7. doi: 10.1002/mnfr.200700155. [DOI] [PubMed] [Google Scholar]
- 41. Weng CJ, Chau CF, Hsieh YS, Yang SF, Yen GC. Lucidenic acid inhibits PMA-induced invasion of human hepatoma cells through inactivating MAPK/ERK signal transduction pathway and reducing binding activities of NF-kappaB and AP-1. Carcinogenesis. 2008;29:147–56. doi: 10.1093/carcin/bgm261. [DOI] [PubMed] [Google Scholar]
- 42. Kang CG, Lee HJ, Kim SH, Lee EO. Zerumbone suppresses osteopontin-induced cell invasion through inhibiting the FAK/AKT/ROCK pathway in human non-small cell lung cancer A549 cells. J Nat Prod. 2016;79:156–60. doi: 10.1021/acs.jnatprod.5b00796. [DOI] [PubMed] [Google Scholar]
- 43. Han J, Bae SY, Oh SJ, Lee J, Lee JH, Lee HC, et al. Zerumbone suppresses IL-1β-induced cell migration and invasion by inhibiting IL-8 and MMP-3 expression in human triple-negative breast cancer cells. Phytother Res. 2014;28:1654–60. doi: 10.1002/ptr.5178. [DOI] [PubMed] [Google Scholar]
- 44. Kim S, Lee J, Jeon M, Lee JE, Nam SJ. Zerumbone suppresses the motility and tumorigenecity of triple negative breast cancer cells via the inhibition of TGF-β1 signaling pathway. Oncotarget. 2016;7:1544–58. doi: 10.18632/oncotarget.6441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jeon M, Han J, Nam SJ, Lee JE, Kim S. Elevated IL-1β expression induces invasiveness of triple negative breast cancer cells and is suppressed by zerumbone. Chem Biol Interact. 2016;258:126–33. doi: 10.1016/j.cbi.2016.08.021. [DOI] [PubMed] [Google Scholar]
- 46. Sung B, Jhurani S, Ahn KS, Mastuo Y, Yi T, Guha S, et al. Zerumbone down-regulates chemokine receptor CXCR4 expression leading to inhibition of CXCL12-induced invasion of breast and pancreatic tumor cells. Cancer Res. 2008;68:8938–44. doi: 10.1158/0008-5472.CAN-08-2155. [DOI] [PubMed] [Google Scholar]
- 47. Manesh C, Kuttan G. Effect of naturally occurring allyl and phenyl isothiocyanates in the inhibition of experimental pulmonary metastasis induced by B16F-10 melanoma cells. Fitoterapia. 2003;74:355–63. doi: 10.1016/s0367-326x(03)00055-8. [DOI] [PubMed] [Google Scholar]
- 48. Singh SV, Warin R, Xiao D, Powolny AA, Stan SD, Arlotti JA, et al. Sulforaphane inhibits prostate carcinogenesis and pulmonary metastasis in TRAMP mice in association with increased cytotoxicity of natural killer cells. Cancer Res. 2009;69:2117–25. doi: 10.1158/0008-5472.CAN-08-3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hunakova L, Sedlakova O, Cholujova D, Gronesova P, Duraj J, Sedlak J. Modulation of markers associated with aggressive phenotype in MDA-MB-231 breast carcinoma cells by sulforaphane. Neoplasma. 2009;56:548–56. doi: 10.4149/neo_2009_06_548. [DOI] [PubMed] [Google Scholar]
- 50. Jee HG, Lee KE, Kim JB, Shin HK, Youn YK. Sulforaphane inhibits oral carcinoma cell migration and invasion in vitro. Phytother Res. 2011;25:1623–8. doi: 10.1002/ptr.3397. [DOI] [PubMed] [Google Scholar]
- 51. Hahm ER, Chandra-Kuntal K, Desai D, Amin S, Singh SV. Notch activation is dispensable for D, L-sulforaphane-mediated inhibition of human prostate cancer cell migration. PLoS One. 2012;7:e44957. doi: 10.1371/journal.pone.0044957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Peng X, Zhou Y, Tian H, Yang G, Li C, Geng Y, et al. Sulforaphane inhibits invasion by phosphorylating ERK1/2 to regulate E-cadherin and CD44v6 in human prostate cancer DU145 cells. Oncol Rep. 2015;34:1565–72. doi: 10.3892/or.2015.4098. [DOI] [PubMed] [Google Scholar]
- 53. Tian H, Zhou Y, Yang G, Geng Y, Wu S, Hu Y, et al. Sulforaphane-cysteine suppresses invasion via downregulation of galectin-1 in human prostate cancer DU145 and PC3 cells. Oncol Rep. 2016;36:1361–8. doi: 10.3892/or.2016.4942. [DOI] [PubMed] [Google Scholar]
- 54. Shan Y, Zhang L, Bao Y, Li B, He C, Gao M, et al. Epithelial-mesenchymal transition, a novel target of sulforaphane via COX-2/MMP2, 9/Snail, ZEB1 and miR-200c/ZEB1 pathways in human bladder cancer cells. J Nutr Biochem. 2013;24:1062–9. doi: 10.1016/j.jnutbio.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 55. Li C, Zhou Y, Peng X, Du L, Tian H, Yang G, et al. Sulforaphane inhibits invasion via activating ERK1/2 signaling in human glioblastoma U87MG and U373MG cells. PLoS One. 2014;9:e90520. doi: 10.1371/journal.pone.0090520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zhang Z, Li C, Shang L, Zhang Y, Zou R, Zhan Y, et al. Sulforaphane induces apoptosis and inhibits invasion in U251MG glioblastoma cells. SpringerPlus. 2016;5:235. doi: 10.1186/s40064-016-1910-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lee CS, Cho HJ, Jeong YJ, Shin JM, Park KK, Park YY, et al. Isothiocyanates inhibit the invasion and migration of C6 glioma cells by blocking FAK/JNK-mediated MMP-9 expression. Oncol Rep. 2015;34:2901–8. doi: 10.3892/or.2015.4292. [DOI] [PubMed] [Google Scholar]
- 58. Hwang ES, Lee HJ. Benzyl isothiocyanate inhibits metalloproteinase-2/-9 expression by suppressing the mitogen-activated protein kinase in SK-Hep1 human hepatoma cells. Food Chem Toxicol. 2008;46:2358–64. doi: 10.1016/j.fct.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 59. Zhu M, Li W, Dong X, Chen Y, Lu Y, Lin B, et al. Benzyl-isothiocyanate induces apoptosis and inhibits migration and invasion of hepatocellular carcinoma cells in vitro. J Cancer. 2017;8:240–8. doi: 10.7150/jca.16402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wu X, Zhu Y, Yan H, Liu B, Li Y, Zhou Q, et al. Isothiocyanates induce oxidative stress and suppress the metastasis potential of human non-small cell lung cancer cells. BMC Cancer. 2010;10:269. doi: 10.1186/1471-2407-10-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kim EJ, Eom SJ, Hong JE, Lee JY, Choi MS, Park JH. Benzyl isothiocyanate inhibits basal and hepatocyte growth factor-stimulated migration of breast cancer cells. Mol Cell Biochem. 2012;359:431–40. doi: 10.1007/s11010-011-1039-3. [DOI] [PubMed] [Google Scholar]
- 62. Sehrawat A, Kim SH, Vogt A, Singh SV. Suppression of FOXQ1 in benzyl isothiocyanate-mediated inhibition of epithelial-mesenchymal transition in human breast cancer cells. Carcinogenesis. 2013;34:864–73. doi: 10.1093/carcin/bgs397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lai KC, Huang AC, Hsu SC, Kuo CL, Yang JS, Wu SH, et al. Benzyl isothiocyanate (BITC) inhibits migration and invasion of human colon cancer HT29 cells by inhibiting matrix metalloproteinase-2/-9 and urokinase plasminogen (uPA) through PKC and MAPK signaling pathway. J Agric Food Chem. 2010a;58:2935–42. doi: 10.1021/jf9036694. [DOI] [PubMed] [Google Scholar]
- 64. Ho CC, Lai KC, Hsu SC, Kuo CL, Ma CY, Lin ML, et al. Benzyl isothiocyanate (BITC) inhibits migration and invasion of human gastric cancer AGS cells via suppressing ERK signal pathways. Hum Exp Toxicol. 2011;30:296–306. doi: 10.1177/0960327110371991. [DOI] [PubMed] [Google Scholar]
- 65. Boreddy SR, Sahu RP, Srivastava SK. Benzyl isothiocyanate suppresses pancreatic tumor angiogenesis and invasion by inhibiting HIF-α/VEGF/Rho-GTPases: pivotal role of STAT-3. PLoS One. 2011;6:e25799. doi: 10.1371/journal.pone.0025799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lai KC, Hsiao YT, Yang JL, Ma YS, Huang YP, Chiang TA, et al. Benzyl isothiocyanate and phenethyl isothiocyanate inhibit murine melanoma B16F10 cell migration and invasion in vitro. Int J Oncol. 2017;51:832–40. doi: 10.3892/ijo.2017.4084. [DOI] [PubMed] [Google Scholar]
- 67. Ma YS, Hsiao YT, Lin JJ, Liao CL, Lin CC, Chung JG. Phenethyl isothiocyanate (PEITC) and benzyl isothiocyanate (BITC) inhibit human melanoma A375.S2 cell migration and invasion by affecting MAPK signaling pathway in vitro. Anticancer Res. 2017;37:6223–34. doi: 10.21873/anticanres.12073. [DOI] [PubMed] [Google Scholar]
- 68. Wolf MA, Claudio PP. Benzyl isothiocyanate inhibits HNSCC cell migration and invasion, and sensitizes HNSCC cells to cisplatin. Nutr Cancer. 2014;66:285–94. doi: 10.1080/01635581.2014.868912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sarkar R, Mukherjee S, Biswas J, Roy M. Phenethyl isothiocyanate, by virtue of its antioxidant activity, inhibits invasiveness and metastatic potential of breast cancer cells: HIF-1α as a putative target. Free Radic Res. 2016;50:84–100. doi: 10.3109/10715762.2015.1108520. [DOI] [PubMed] [Google Scholar]
- 70. Lai KC, Hsu SC, Kuo CL, Ip SW, Yang JS, Hsu YM, et al. Phenethyl isothiocyanate inhibited tumor migration and invasion via suppressing multiple signal transduction pathways in human colon cancer HT29 cells. J Agric Food Chem. 2010b;58:11148–55. doi: 10.1021/jf102384n. [DOI] [PubMed] [Google Scholar]
- 71. Xiao D, Singh SV. Phenethyl isothiocyanate inhibits angiogenesis in vitro and ex vivo. Cancer Res. 2007;67:2239–46. doi: 10.1158/0008-5472.CAN-06-3645. [DOI] [PubMed] [Google Scholar]
- 72. Kim SH, Sehrawat A, Sakao K, Hahm ER, Singh SV. Notch activation by phenethyl isothiocyanate attenuates its inhibitory effect on prostate cancer cell migration. PLoS One. 2011a;6:e26615. doi: 10.1371/journal.pone.0026615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Zhang C, Shu L, Kim H, Khor TO, Wu R, Li W, et al. Phenethyl isothiocyanate (PEITC) suppresses prostate cancer cell invasion epigenetically through regulating microRNA-194. Mol Nutr Food Res. 2016;60:1427–36. doi: 10.1002/mnfr.201500918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Yang MD, Lai KC, Lai TY, Hsu SC, Kuo CL, Yu CS, et al. Phenethyl isothiocyanate inhibits migration and invasion of human gastric cancer AGS cells through suppressing MAPK and NF-kappaB signal pathways. Anticancer Res. 2010;30:2135–43. [PubMed] [Google Scholar]
- 75. Chou YC, Chang MY, Wang MJ, Yu FS, Liu HC, Harnod T, et al. PEITC inhibits human brain glioblastoma GBM 8401 cell migration and invasion through the inhibition of uPA, Rho A, and Ras with inhibition of MMP-2, -7 and -9 gene expression. Oncol Rep. 2015;34:2489–96. doi: 10.3892/or.2015.4260. [DOI] [PubMed] [Google Scholar]
- 76. Zhang L, Hao Q, Bao L, Liu W, Fu X, Chen Y, et al. Phenethyl isothiocyanate suppresses cervical carcinoma metastasis potential and its molecular mechanism. Mol Med Rep. 2014;10:2675–80. doi: 10.3892/mmr.2014.2565. [DOI] [PubMed] [Google Scholar]
- 77. Shao WY, Yang YL, Yan H, Huang Q, Liu KJ, Zhang S. Phenethyl isothiocyanate suppresses the metastasis of ovarian cancer associated with the inhibition of CRM1-mediated nuclear export and mTOR-STAT3 pathway. Cancer Biol Ther. 2017;18:26–35. doi: 10.1080/15384047.2016.1264540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Lai KC, Lu CC, Tang YJ, Chiang JH, Kuo DH, Chen FA, et al. Allyl isothiocyanate inhibits cell metastasis through suppression of the MAPK pathways in epidermal growth factor stimulated HT29 human colorectal adenocarcinoma cells. Oncol Rep. 2014;31:189–96. doi: 10.3892/or.2013.2865. [DOI] [PubMed] [Google Scholar]
- 79. Herman-Antosiewicz A, Singh SV. Signal transduction pathways leading to cell cycle arrest and apoptosis induction in cancer cells by Allium vegetable derived organosulfur compounds: a review. Mutat Res. 2004;555:121–31. doi: 10.1016/j.mrfmmm.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 80. Powolny AA, Singh SV. Multitargeted prevention and therapy of cancer by diallyl trisulfide and related Allium vegetable-derived organosulfur compounds. Cancer Lett. 2008;269:305–14. doi: 10.1016/j.canlet.2008.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Ng KT, Guo DY, Cheng Q, Geng W, Ling CC, Li CX, et al. A garlic derivative, S-allylcysteine (SAC), suppresses proliferation and metastasis of hepatocellular carcinoma. PLoS One. 2012;7:e31655. doi: 10.1371/journal.pone.0031655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Gapter LA, Yuin OZ, Ng KY. S-Allylcysteine reduces breast tumor cell adhesion and invasion. Biochem Biophys Res Commun. 2008;367:446–51. doi: 10.1016/j.bbrc.2007.12.175. [DOI] [PubMed] [Google Scholar]
- 83. Cho O, Hwang HS, Lee BS, Oh YT, Kim CH, Chun M. Met inactivation by S-allylcysteine suppresses the migration and invasion of nasopharyngeal cancer cells induced by hepatocyte growth factor. Radiat Oncol J. 2015;33:328–36. doi: 10.3857/roj.2015.33.4.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Gao Y, Liu YQ, Cao WK, Chen XF, Wan YY, Heng C, et al. Effects of allicin on invasion and metastasis of colon cancer LoVo cell line in vitro. Zhonghua Yi Xue Za Zhi. 2009;89:1382–6. [Chinese] [PubMed] [Google Scholar]
- 85. Huang L, Song Y, Lian J, Wang Z. Allicin inhibits the invasion of lung adenocarcinoma cells by altering tissue inhibitor of metalloproteinase/matrix metalloproteinase balance via reducing the activity of phosphoinositide 3-kinase/AKT signaling. Oncol Lett. 2017;14:468–74. doi: 10.3892/ol.2017.6129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Chen XX, Liu XW, Zhou ZG, Chen XY, Li LD, Xiong T, et al. Diallyl disulfide inhibits invasion and metastasis of MCF-7 breast cancer cells in vitro by down-regulating p38 activity. Nan Fang Yi Ke Da Xue Xue Bao. 2016;36:814–8. [PubMed] [Google Scholar]
- 87. Xiao X, Chen B, Liu X, Liu P, Zheng G, Ye F, et al. Diallyl disulfide suppresses SRC/Ras/ERK signaling-mediated proliferation and metastasis in human breast cancer by up-regulating miR-34a. PLoS One. 2014;9:e112720. doi: 10.1371/journal.pone.0112720. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 88. Lai KC, Hsu SC, Kuo CL, Yang JS, Ma CY, Lu HF, et al. Diallyl sulfide, diallyl disulfide, and diallyl trisulfide inhibit migration and invasion in human colon cancer colo 205 cells through the inhibition of matrix metalloproteinase-2, -7, and -9 expressions. Environ Toxicol. 2013;28:479–88. doi: 10.1002/tox.20737. [DOI] [PubMed] [Google Scholar]
- 89. Zhou Y, Su J, Shi L, Liao Q, Su Q. DADS downregulates the Rac1-ROCK1/PAK1-LIMK1-ADF/cofilin signaling pathway, inhibiting cell migration and invasion. Oncol Rep. 2013;29:605–12. doi: 10.3892/or.2012.2168. [DOI] [PubMed] [Google Scholar]
- 90. Su J, Zhou Y, Pan Z, Shi L, Yang J, Liao A, et al. Downregulation of LIMK1-ADF/cofilin by DADS inhibits the migration and invasion of colon cancer. Sci Rep. 2017;7:45624. doi: 10.1038/srep45624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Shin DY, Kim GY, Kim JI, Yoon MK, Kwon TK, Lee SJ, et al. Anti-invasive activity of diallyl disulfide through tightening of tight junctions and inhibition of matrix metalloproteinase activities in LNCaP prostate cancer cells. Toxicol In Vitro. 2010;24:1569–76. doi: 10.1016/j.tiv.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 92. Park HS, Kim GY, Choi IW, Kim ND, Hwang HJ, Choi YW, et al. Inhibition of matrix metalloproteinase activities and tightening of tight junctions by diallyl disulfide in AGS human gastric carcinoma cells. J Food Sci. 2011;76:T105–11. doi: 10.1111/j.1750-3841.2011.02114.x. [DOI] [PubMed] [Google Scholar]
- 93. Su B, Su J, Zeng Y, Liu F, Xia H, Ma YH, et al. Diallyl disulfide suppresses epithelial-mesenchymal transition, invasion and proliferation by downregulation of LIMK1 in gastric cancer. Oncotarget. 2016;7:10498–512. doi: 10.18632/oncotarget.7252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Wang G, Liu G, Ye Y, Fu Y, Zhang X. Upregulation of miR-34a by diallyl disulfide suppresses invasion and induces apoptosis in SGC-7901 cells through inhibition of the PI3K/Akt signaling pathway. Oncol Lett. 2016;11:2661–7. doi: 10.3892/ol.2016.4266. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 95. Hu Y, Chen L, Yi C, Yang F, Chen J. Experimental study on inhibitory effects of diallyl sulfide on growth and invasion of human osteosarcoma MG-63 cells. J Huazhong Univ Sci Technol Med Sci. 2012;32:581–5. doi: 10.1007/s11596-012-1000-z. [DOI] [PubMed] [Google Scholar]
- 96. Li WX, Chen LP, Sun MY, Li JT, Liu HZ, Zhu W. 3′3-Diindolylmethane inhibits migration, invasion and metastasis of hepatocellular carcinoma by suppressing FAK signaling. Oncotarget. 2015;6:23776–92. doi: 10.18632/oncotarget.4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Rahimi M, Huang KL, Tang CK. 3,3′-Diindolylmethane (DIM) inhibits the growth and invasion of drug-resistant human cancer cells expressing EGFR mutants. Cancer Lett. 2010;295:59–68. doi: 10.1016/j.canlet.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Hung WC, Chang HC. Indole-3-carbinol inhibits Sp1-induced matrix metalloproteinase-2 expression to attenuate migration and invasion of breast cancer cells. J Agric Food Chem. 2009;57:76–82. doi: 10.1021/jf802881d. [DOI] [PubMed] [Google Scholar]
- 99. Kim EJ, Shin M, Park H, Hong JE, Shin HK, Kim J, et al. Oral administration of 3,3′-diindolylmethane inhibits lung metastasis of 4T1 murine mammary carcinoma cells in BALB/c mice. J Nutr. 2009;139:2373–9. doi: 10.3945/jn.109.111864. [DOI] [PubMed] [Google Scholar]
- 100. Ahmad A, Kong D, Wang Z, Sarkar SH, Banerjee S, Sarkar FH. Down-regulation of uPA and uPAR by 3,3′-diindolylmethane contributes to the inhibition of cell growth and migration of breast cancer cells. J Cell Biochem. 2009b;108:916–25. doi: 10.1002/jcb.22323. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 101. Hsu EL, Chen N, Westbrook A, Wang F, Zhang R, Taylor RT, et al. CXCR4 and CXCL12 down-regulation: a novel mechanism for the chemoprotection of 3,3′-diindolylmethane for breast and ovarian cancers. Cancer Lett. 2008;265:113–23. doi: 10.1016/j.canlet.2008.02.033. [DOI] [PubMed] [Google Scholar]
- 102. Hsu EL, Chen N, Westbrook A, Wang F, Zhang R, Taylor RT, et al. Modulation of CXCR4, CXCL12, and tumor cell invasion potential in vitro by phytochemicals. J Oncol. 2009;1985;49 doi: 10.1155/2009/491985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Rajoria S, Suriano R, Wilson YL, Schantz SP, Moscatello A, Geliebter J, et al. 3,3′-diindolylmethane inhibits migration and invasion of human cancer cells through combined suppression of ERK and AKT pathways. Oncol Rep. 2011b;25:491–7. doi: 10.3892/or.2010.1076. [DOI] [PubMed] [Google Scholar]
- 104. Jeong YM, Li H, Kim SY, Yun HY, Baek KJ, Kwon NS, et al. Indole-3-carbinol inhibits prostate cancer cell migration via degradation of beta-catenin. Oncol Res. 2011;19:237–43. doi: 10.3727/096504011x12970940207922. [DOI] [PubMed] [Google Scholar]
- 105. Kong D, Li Y, Wang Z, Banerjee S, Sarkar FH. Inhibition of angiogenesis and invasion by 3,3′-diindolylmethane is mediated by the nuclear factor-kappaB downstream target genes MMP-9 and uPA that regulated bioavailability of vascular endothelial growth factor in prostate cancer. Cancer Res. 2007;67:3310–9. doi: 10.1158/0008-5472.CAN-06-4277. [DOI] [PubMed] [Google Scholar]
- 106. Ahmad A, Kong D, Sarkar SH, Wang Z, Banerjee S, Sarkar FH. Inactivation of uPA and its receptor uPAR by 3,3′-diindolylmethane (DIM) leads to the inhibition of prostate cancer cell growth and migration. J Cell Biochem. 2009a;107:516–27. doi: 10.1002/jcb.22152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Kong D, Banerjee S, Huang W, Li Y, Wang Z, Kim HR, et al. Mammalian target of rapamycin repression by 3,3′-diindolylmethane inhibits invasion and angiogenesis in platelet-derived growth factor-D-overexpressing PC3 cells. Cancer Res. 2008;68:1927–34. doi: 10.1158/0008-5472.CAN-07-3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Li Y, Kong D, Ahmad A, Bao B, Sarkar FH. Targeting bone remodeling by isoflavone and 3,3′-diindolylmethane in the context of prostate cancer bone metastasis. PLoS One. 2012b;7:e33011. doi: 10.1371/journal.pone.0033011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Zou M, Zhang X, Xu C. IL6-induced metastasis modulators p-STAT3, MMP-2 and MMP-9 are targets of 3,3′-diindolylmethane in ovarian cancer cells. Cell Oncol (Dordr) 2016;39:47–57. doi: 10.1007/s13402-015-0251-7. [DOI] [PubMed] [Google Scholar]
- 110. Li Y, Vandenboom TG, 2nd, Wang Z, Kong D, Ali S, Philip PA, et al. miR-146a suppresses invasion of pancreatic cancer cells. Cancer Res. 2010;70:1486–95. doi: 10.1158/0008-5472.CAN-09-2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Rajoria S, Suriano R, George A, Shanmugam A, Schantz SP, Geliebter J, et al. Estrogen induced metastatic modulators MMP-2 and MMP-9 are targets of 3,3′-diindolylmethane in thyroid cancer. PLoS One. 2011a;6:e15879. doi: 10.1371/journal.pone.0015879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Sun Y, Cheng MK, Griffiths TR, Mellon JK, Kai B, Kriajevska M, et al. Inhibition of STAT signalling in bladder cancer by diindolylmethane-relevance to cell adhesion, migration and proliferation. Curr Cancer Drug Targets. 2013;13:57–68. [PubMed] [Google Scholar]
- 113. Lian F, Wang XD. Enzymatic metabolites of lycopene induce Nrf2-mediated expression of phase II detoxifying/antioxidant enzymes in human bronchial epithelial cells. Int J Cancer. 2008;123:1262–8. doi: 10.1002/ijc.23696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Kopec RE, Riedl KM, Harrison EH, Curley RW, Jr, Hruszkewycz DP, Clinton SK, et al. Identification and quantification of apo-lycopenals in fruits, vegetables, and human plasma. J Agric Food Chem. 2010;58:3290–6. doi: 10.1021/jf100415z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Huang CS, Fan YE, Lin CY, Hu ML. Lycopene inhibits matrix metalloproteinase-9 expression and down-regulates the binding activity of nuclear factor-kappa B and stimulatory protein-1. J Nutr Biochem. 2007;18:449–56. doi: 10.1016/j.jnutbio.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 116. Yang CM, Hu TY, Hu ML. Antimetastatic effects and mechanisms of apo-8′-lycopenal, an enzymatic metabolite of lycopene, against human hepatocarcinoma SK-Hep-1 cells. Nutr Cancer. 2012;64:274–85. doi: 10.1080/01635581.2012.643273. [DOI] [PubMed] [Google Scholar]
- 117. Jhou BY, Song TY, Lee I, Hu ML, Yang NC. Lycopene inhibits metastasis of human liver adenocarcinoma SK-Hep-1 cells by downregulation of NADPH Oxidase 4 protein expression. J Agric Food Chem. 2017;65:6893–903. doi: 10.1021/acs.jafc.7b03036. [DOI] [PubMed] [Google Scholar]
- 118. Lin MC, Wang FY, Kuo YH, Tang FY. Cancer chemopreventive effects of lycopene: suppression of MMP-7 expression and cell invasion in human colon cancer cells. J Agric Food Chem. 2011b;59:11304–18. doi: 10.1021/jf202433f. [DOI] [PubMed] [Google Scholar]
- 119. Ye M, Wu Q, Zhang M, Huang J. Lycopene inhibits the cell proliferation and invasion of human head and neck squamous cell carcinoma. Mol Med Rep. 2016;14:2953–8. doi: 10.3892/mmr.2016.5597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Guruvayoorappan C, Kuttan G. Beta-carotene inhibits tumor-specific angiogenesis by altering the cytokine profile and inhibits the nuclear translocation of transcription factors in B16F-10 melanoma cells. Integr Cancer Ther. 2007;6:258–70. doi: 10.1177/1534735407305978. [DOI] [PubMed] [Google Scholar]
- 121. Kim YS, Lee HA, Lim JY, Kim Y, Jung CH, Yoo SH, et al. β-Carotene inhibits neuroblastoma cell invasion and metastasis in vitro and in vivo by decreasing level of hypoxia-inducible factor-1α. J Nutr Biochem. 2014;25:655–64. doi: 10.1016/j.jnutbio.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 122. Dong S, Kong J, Kong J, Shen Q, Kong F, Sun W, et al. Low concentration of caffeine inhibits the progression of the hepatocellular carcinoma via Akt signaling pathway. Anticancer Agents Med Chem. 2015;15:484–92. doi: 10.2174/1871520615666150209110832. [DOI] [PubMed] [Google Scholar]
- 123. Kang SS, Han KS, Ku BM, Lee YK, Hong J, Shin HY, et al. Caffeine-mediated inhibition of calcium release channel inositol 1,4,5-trisphosphate receptor subtype 3 blocks glioblastoma invasion and extends survival. Cancer Res. 2010;70:1173–83. doi: 10.1158/0008-5472.CAN-09-2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Cheng YC, Ding YM, Hueng DY, Chen JY, Chen Y. Caffeine suppresses the progression of human glioblastoma via cathepsin B and MAPK signaling pathway. J Nutr Biochem. 2016;33:63–72. doi: 10.1016/j.jnutbio.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 125. Liu WH, Chang LS. Caffeine induces matrix metalloproteinase-2 (MMP-2) and MMP-9 down-regulation in human leukemia U937 cells via Ca2+/ROS-mediated suppression of ERK/c-fos pathway and activation of p38 MAPK/c-jun pathway. J Cell Physiol. 2010;224:775–85. doi: 10.1002/jcp.22180. [DOI] [PubMed] [Google Scholar]
- 126. Lai LH, Fu QH, Liu Y, Jiang K, Guo QM, Chen QY, et al. Piperine suppresses tumor growth and metastasis in vitro and in vivo in a 4T1 murine breast cancer model. Acta Pharmacol Sin. 2012;33:523–30. doi: 10.1038/aps.2011.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Hwang YP, Yun HJ, Kim HG, Han EH, Choi JH, Chung YC, et al. Suppression of phorbol-12-myristate-13-acetate-induced tumor cell invasion by piperine via the inhibition of PKCα/ERK1/2-dependent matrix metalloproteinase-9 expression. Toxicol Lett. 2011;203:9–19. doi: 10.1016/j.toxlet.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 128. Zhang J, Zhu X, Li H, Li B, Sun L, Xie T, et al. Piperine inhibits proliferation of human osteosarcoma cells via G2/M phase arrest and metastasis by suppressing MMP-2/-9 expression. Int Immunopharmacol. 2015;24:50–8. doi: 10.1016/j.intimp.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 129. Hwang YP, Yun HJ, Choi JH, Kang KW, Jeong HG. Suppression of phorbol-12-myristate-13-acetate-induced tumor cell invasion by bergamottin via the inhibition of protein kinase Cdelta/p38 mitogen-activated protein kinase and JNK/nuclear factor-kappaB-dependent matrix metalloproteinase-9 expression. Mol Nutr Food Res. 2010;54:977–90. doi: 10.1002/mnfr.200900283. [DOI] [PubMed] [Google Scholar]
- 130. Wu HJ, Wu HB, Zhao YQ, Chen LJ, Zou HZ. Bergamottin isolated from Citrus bergamia exerts in vitro and in vivo antitumor activity in lung adenocarcinoma through the induction of apoptosis, cell cycle arrest, mitochondrial membrane potential loss and inhibition of cell migration and invasion. Oncol Rep. 2016;36:324–32. doi: 10.3892/or.2016.4833. [DOI] [PubMed] [Google Scholar]
- 131. Harikumar KB, Sung B, Tharakan ST, Pandey MK, Joy B, Guha S, et al. Sesamin manifests chemopreventive effects through the suppression of NF-kappaB-regulated cell survival, proliferation, invasion, and angiogenic gene products. Mol Cancer Res. 2010;8:751–61. doi: 10.1158/1541-7786.MCR-09-0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Xu P, Cai F, Liu X, Guo L. Sesamin inhibits lipopolysaccharide-induced proliferation and invasion through the p38-MAPK and NF-κB signaling pathways in prostate cancer cells. Oncol Rep. 2015;33:3117–23. doi: 10.3892/or.2015.3888. [DOI] [PubMed] [Google Scholar]
- 133. Wang L, Kuang L, Pan X, Liu J, Wang Q, Du B, et al. Isoalvaxanthone inhibits colon cancer cell proliferation, migration and invasion through inactivating Rac1 and AP-1. Int J Cancer. 2010;127:1220–9. doi: 10.1002/ijc.25119. [DOI] [PubMed] [Google Scholar]
- 134. Kanematsu S, Yoshizawa K, Uehara N, Miki H, Sasaki T, Kuro M, et al. Sulforaphane inhibits the growth of KPL-1 human breast cancer cells in vitro and suppresses the growth and metastasis of orthotopically transplanted KPL-1 cells in female athymic mice. Oncol Rep. 2011;26:603–8. doi: 10.3892/or.2011.1311. [DOI] [PubMed] [Google Scholar]
- 135. Shankar S, Ganapathy S, Srivastava RK. Sulforaphane enhances the therapeutic potential of TRAIL in prostate cancer orthotopic model through regulation of apoptosis, metastasis, and angiogenesis. Clin Cancer Res. 2008;14:6855–66. doi: 10.1158/1078-0432.CCR-08-0903. [DOI] [PubMed] [Google Scholar]
- 136. Kim EJ, Hong JE, Eom SJ, Lee JY, Park JH. Oral administration of benzyl-isothiocyanate inhibits solid tumor growth and lung metastasis of 4T1 murine mammary carcinoma cells in BALB/c mice. Breast Cancer Res Treat. 2011b;130:61–71. doi: 10.1007/s10549-010-1299-8. [DOI] [PubMed] [Google Scholar]
- 137. Thejass P, Kuttan G. Allyl isothiocyanate (AITC) and phenyl isothiocyanate (PITC) inhibit tumour-specific angiogenesis by downregulating nitric oxide (NO) and tumour necrosis factor-alpha (TNF-alpha) production. Nitric Oxide. 2007a;16:247–57. doi: 10.1016/j.niox.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 138. Bhattacharya A, Li Y, Geng F, Munday R, Zhang Y. The principal urinary metabolite of allyl isothiocyanate, N-acetyl-S-(N-allylthiocarbamoyl) cysteine, inhibits the growth and muscle invasion of bladder cancer. Carcinogenesis. 2012;33:394–8. doi: 10.1093/carcin/bgr283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Thejass P, Kuttan G. Antiangiogenic activity of diallyl sulfide (DAS) Int Immunopharmacol. 2007b;7:295–305. doi: 10.1016/j.intimp.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 140. Li N, Lu Y, Li D, Zheng X, Lian J, Li S, et al. All-trans retinoic acid suppresses the angiopoietin-Tie2 pathway and inhibits angiogenesis and metastasis in esophageal squamous cell carcinoma. PLoS One. 2017;12:e0174555. doi: 10.1371/journal.pone.0174555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Kanjoormana M, Kuttan G. Antiangiogenic activity of ursolic acid. Integr Cancer Ther. 2010;9:224–35. doi: 10.1177/1534735410367647. [DOI] [PubMed] [Google Scholar]
- 142. Lin J, Chen Y, Wei L, Hong Z, Sferra TJ, Peng J. Ursolic acid inhibits colorectal cancer angiogenesis through suppression of multiple signaling pathways. Int J Oncol. 2013;43:1666–74. doi: 10.3892/ijo.2013.2101. [DOI] [PubMed] [Google Scholar]
- 143. Shamoto T, Matsuo Y, Shibata T, Tsuboi K, Nagasaki T, Takahashi H, et al. Zerumbone inhibits angiogenesis by blocking NF-κB activity in pancreatic cancer. Pancreas. 2014;43:396–404. doi: 10.1097/MPA.0000000000000039. [DOI] [PubMed] [Google Scholar]
- 144. Hayashi M, Tsuchiya H, Yamamoto N, Karita M, Shirai T, Nishida H, et al. Caffeine-potentiated chemotherapy for metastatic carcinoma and lymphoma of bone and soft tissue. Anticancer Res. 2005;25:2399–405. [PubMed] [Google Scholar]
- 145. Kimura H, Tsuchiya H, Shirai T, Nishida H, Hayashi K, Takeuchi A, et al. Caffeine-potentiated chemotherapy for metastatic osteosarcoma. J Orthop Sci. 2009;14:556–65. doi: 10.1007/s00776-009-1372-5. [DOI] [PubMed] [Google Scholar]
- 146. Karita M, Tsuchiya H, Yamamoto N, Shirai T, Hayashi K, Nishida H. Caffeine-potentiated chemotherapy for clear cell sarcoma: a report of five cases. Int J Clin Oncol. 2011;18:33–7. doi: 10.1007/s10147-011-0337-9. [DOI] [PubMed] [Google Scholar]
- 147. Chang X, Tou JC, Hong C, Kim HA, Riby JE, Firestone GL, et al. 3,3′-Diindolylmethane inhibits angiogenesis and the growth of transplantable human breast carcinoma in athymic mice. Carcinogenesis. 2005;26:771–8. doi: 10.1093/carcin/bgi018. [DOI] [PubMed] [Google Scholar]
- 148. Huang CS, Liao JW, Hu ML. Lycopene inhibits experimental metastasis of human hepatoma SK-Hep-1 cells in athymic nude mice. J Nutr. 2008;138:538–43. doi: 10.1093/jn/138.3.538. [DOI] [PubMed] [Google Scholar]
- 149. Sahin M, Sahin E, Gümüşlü S. Effects of lycopene and apigenin on human umbilical vein endothelial cells in vitro under angiogenic stimulation. Acta Histochem. 2012;114:94–100. doi: 10.1016/j.acthis.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 150. Chen ML, Lin YH, Yang CM, Hu ML. Lycopene inhibits angiogenesis both in vitro and in vivo by inhibiting MMP-2/uPA system through VEGFR2-mediated PI3K-Akt and ERK/p38 signaling pathways. Mol Nutr Food Res. 2012;56:889–99. doi: 10.1002/mnfr.201100683. [DOI] [PubMed] [Google Scholar]