Abstract
Lycii Fructus, a solanaceous drug, is widely used as functional foods and in Traditional Chinese Medicine. Samples collected from different regions of China have been found to be not identical in chemical compositions which might affect the biological activities. Although many chromatographic and spectrometric methods have been reported to determine the concentration of betaine and other bioactive amino acids, disturbance resulted from other polar substances with low UV-absorbance and expensive mass facilities reduced the applicability of these techniques. In the present study, the strong cation exchange solid phase extraction procedure incorporated with 1H NMR was successfully developed as a rapid and reliable method that can simultaneously determine betaine, citric acid, threonine, alanine, and proline in various Lycii Fructus. In addition, ERETIC 2 method based on PULCON principle was also applied and compared with conventional method. This feasible and practical method offers a very powerful tool for the quality control of commercial Lycii Fructus from different sources.
Keywords: ERETIC 2, Betaine, 1H NMR, Lycii Fructus, PULCON
1. Introduction
Wolfberry fruits, a solanaceous origin, also known as Lycii Fructus are rich in polysaccharides, alkaloids, carotenoids, fatty acids, essential trace elements, and amino acids and have been used for centuries in Traditional Chinese Medicine to nourish the liver and kidney and protect the eyesight. Lycii Fructus had been demonstrated to display various biological functions, such as anti-inflammatory and hepatoprotective activities, and the prevention of tumor growth [1–3]. According to Taiwan Herbal Pharmacopeia, Lycium barbarum L. and L. chinense Mill. from Ningxia province of China are the authentic medicinal herbs of Lycii Fructus [4]. Different sources of Lycii Fructus had been reported to show different chemical compositions which might affect the biological activities. Therefore, establishment of composition fingerprint profile of Lycii Fructus for quality control is warranted. Currently, the quaternary ammonium cation betaine has been used as one of the biomarkers for Lycii Fructus identification and quality control of commercial products [1]. Betaine is one of the major components in the fruits of Lycium species and exhibits anti-inflammatory, hepatoprotective, and anti-tumor activities [5–7]. Although high performance liquid chromatography (HPLC) [8,9] had been reported to determine the components of Lycii Fructus, betaine was difficult to be distinguished from other amino acids or citric acid due to lack of UV-chromophore. In addition, liquid chromatography–mass spectrometry (LC-MS) method [10–12] which can solve the detection limit of low UV-absorbance substances still requires expensive mass facilities and tedious pretreatment procedures. High-resolution nuclear magnetic resonance (NMR) spectroscopy has become an increasingly important quantitative tool, providing high specificity and sensitivity for detecting natural products, even those without UV chromophore [13–17]. In our previous studies, 1H NMR spectroscopy was successfully used to quantify bioactive constituents from many natural products, including Coptidis Rhizoma, Codonopsis Radix, Ginkgo Folium, Phellodendri Cortex, and Nothapodytes foetida [18–22]. Herein we report a rapid quantitative 1H NMR method that can simultaneously quantify betaine, citric acid, and other amino acids with low UV-absorbance such as threonine, alanine, and proline in Lycii Fructus, respectively. To quantify these charged molecules with low UV-absorbance in Lycii Fructus, the strong cation exchange solid phase extraction (SCX-SPE) procedure was utilized to trap the desired molecules in the aqueous extracts of Lycium fruits from various sources and then the 1H NMR method was performed. With the aid of the developed method, the quantity of bioactive constituents in the commercial products of Lycii Fructus could be analyzed quickly and conveniently.
2. Materials and methods
2.1. Chemicals and materials
Deuterium oxide (D2O, 99.98%), maleic acid, and succinic acid were obtained from Sigma–Aldrich (Milwaukee, WI, US). The reference compounds (betaine, citric acid, proline, threonine, and alanine) were purchased from Merck (Darmstadt, Germany). The ultrapure water (H2O) was prepared with Milli-Q water purification system (Millipore, Bedford, MA, US). The analytical cartridge column was using Thermo Fisher Scientific (Waltham, MA, US) HyperSep SCX strong cation exchanger SPE columns (2000 mg). Lycium fruit samples 1–23 and 27–42 were purchased from the markets in China. Samples 24–26 were collected in Shanxi province of China in Oct, 2009. All samples were purchased and collected by Dr. Yong Peng. The materials were identified by Prof. C. S. Kuoh (Department of Life Science, National Cheng Kung University), and voucher specimen (TSWu 20100708-01-42) have been deposited in the Department of Chemistry, National Cheng Kung University, Tainan, Taiwan.
2.2. Sample preparation
Lycium fruit samples were air-dried at room temperature for three days and pulverized. Five grams of samples was extracted three times with 50 mL H2O by sonication for 30 min. The afforded solution was combined and filtered through a 0.45 μm membrane filter. The aqueous filtrate was transferred to a 250 mL volumetric flask and diluted to 250 mL with H2O. The strong cation exchanger SPE column was activated with 8 mL 0.1 N acetic acid and washed with 200 mL Milli-Q water to obtain pH 7. Then, 25 mL of diluted solution was passed through the SPE cartridge at a flow rate of 4 mL/min, and the cartridge was then washed with 30 mL H2O. The internal standard 0.5 mg maleic acid was added to the elution solvent, and the solvent was evaporated to dryness in vacuo to afford LYW. LYW was dissolved in 0.6 mL of D2O for NMR analysis. The SPE cartridge was then washed with 25 mL 5% ammonia water. The internal standard 0.5 mg succinic acid was added to the elution buffer and evaporated to dryness in vacuo to obtain LYN. LYN was also dissolved in 0.6 mL of D2O for NMR analysis.
2.3. 1H NMR spectrometric parameters
1H NMR spectra were recorded on a Bruker AVANCE III 400 MHz spectrometer in D2O solvent systems, and all chemical shifts are reported in parts per million (ppm, δ). For each sample, 100 scans were recorded with the following parameters: spectrum resolution 0.39 Hz/point; spectral width, 6393.862 Hz; A 90° pulse was used to obtain the maximum sensitivity; relaxation delay, 20 s; and acquisition time, 2.56 s. For quantitation the peak area was used, and the start and end points for the integration of each peak were selected manually. In addition, quantitative determination (qNMR) of targeted molecules in reference materials has been established using the ERETIC 2 methodology (electronic reference to access in vivo concentrations 2) based on the PULCON principle (pulse length based concentration determination). The NMR parameters for ERETIC 2 are the same as mentioned above. Bruker TopSpin version 3.0 software was used.
The amounts of citric acid were calculated by the following formula:
The amounts of betaine, threonine, alanine, and proline were calculated by the following formula:
A1: the area ratio of target signal to internal standard maleic acid;
A2: the area ratio of target signal to internal standard succinic acid;
N: the number of proton atom in target signal;
MW: molecular weight (maleic acid (IS): MW = 116; citric acid: MW = 192; succinic acid (IS): MW = 118; threonine: MW = 119; alanine: MW = 89; betaine: MW = 117; proline: MW = 115)
2.4. Recovery test and limit of detection
Known amounts of pure betaine and citric acid were spiked to samples to evaluate the % recovery. The recovery samples and a blank recovery sample were processed and analyzed as described above.
3. Results and discussion
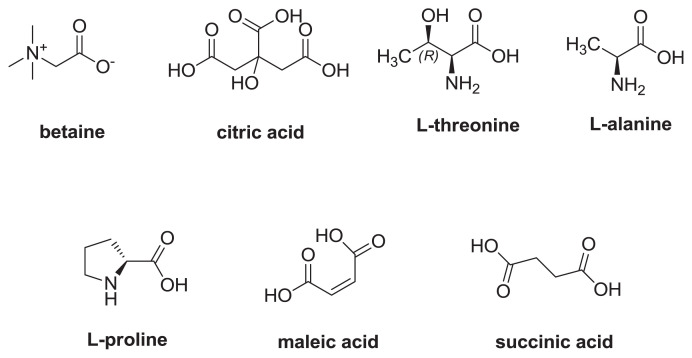
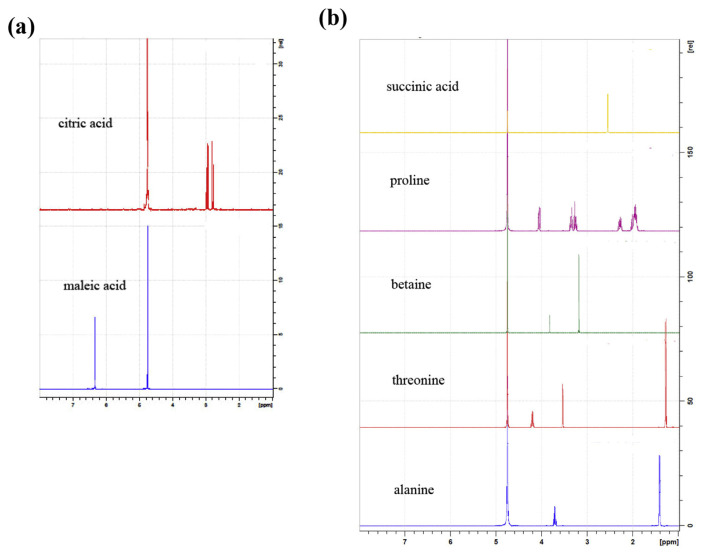
The structures of the target compounds including betaine, citric acid, proline, threonine, alanine, and internal standards including maleic acid, and succinic acid, are shown in Fig. 1. These target compounds were effectively extracted with water by sonication and then collected through SCX-SPE. Moreover, D2O (residue solvent peak δH 4.75 ppm) was used as NMR solvents to effectively dissolve all the target compounds. The analysis of their respective 1H NMR spectra revealed that the signals of betaine (δ 3.21, 9H), citric acid (δ 2.81, 2.99, 2H), proline (δ 4.08, 1H), threonine (δ 1.28, 3H), and alanine (δ 1.43, 3H) were well separated from the other signals, and thus these signals were selected as the target peaks for quantitation (Fig. 2). In addition, maleic acid (δ 6.36, 2H) and succinic acid (δ 2.37, 4H) in D2O solvent system were chosen as the internal standards in the samples except those detected by ERETIC 2 and the signals of internal standards were also well separated from the target signals.
Fig. 1.
The structures of analytical compounds and internal standards.
Fig. 2.
1H NMR spectra of (a) internal standard (maleic acid) and citric acid (b) internal standard (succinic acid), proline, betaine, threonine, and alanine. All compounds were detected in D2O system.
The calibration curve for each compound using the ratio of the peak area of the compound and the internal standards, were determined in the range of 0.0625–2.0 mg/mL to evaluate the accuracy of this method at different concentrations. Betaine, citric acid, proline, threonine, and alanine were found to show good linearity with r2 higher than 0.99 (0.9961, 0.9979, 0.9902, 0.9955 and 0.9956, respectively). In comparison, the previously reported chromatographic method [7,8] only showed linearity at the concentrations of 400–2000 ppm.
Relative recovery tests were conducted using three different quantities of the standards. The average relative recoveries were calculated as ratios by spiking known amounts of pure betaine and citric acid, and the experiments were performed in triplicate. The average relative recoveries of betaine and citric acid were determined as 99.34 ± 4.1% and 99.57 ± 3.5%, respectively. These data indicated that the reproducibility and recovery of the analysis process were acceptable. The precision and recovery examinations all displayed that the established 1H NMR methods were valid for the quantitative determination of the target compounds.
In addition, the Lycium fruit samples were only extracted, passed through SPE cartridge and re-dissolved in D2O for NMR analysis. The simple pre-treatment steps and brief experiment time also indicated this method as a feasible and practical tool for quality control and species authentication.
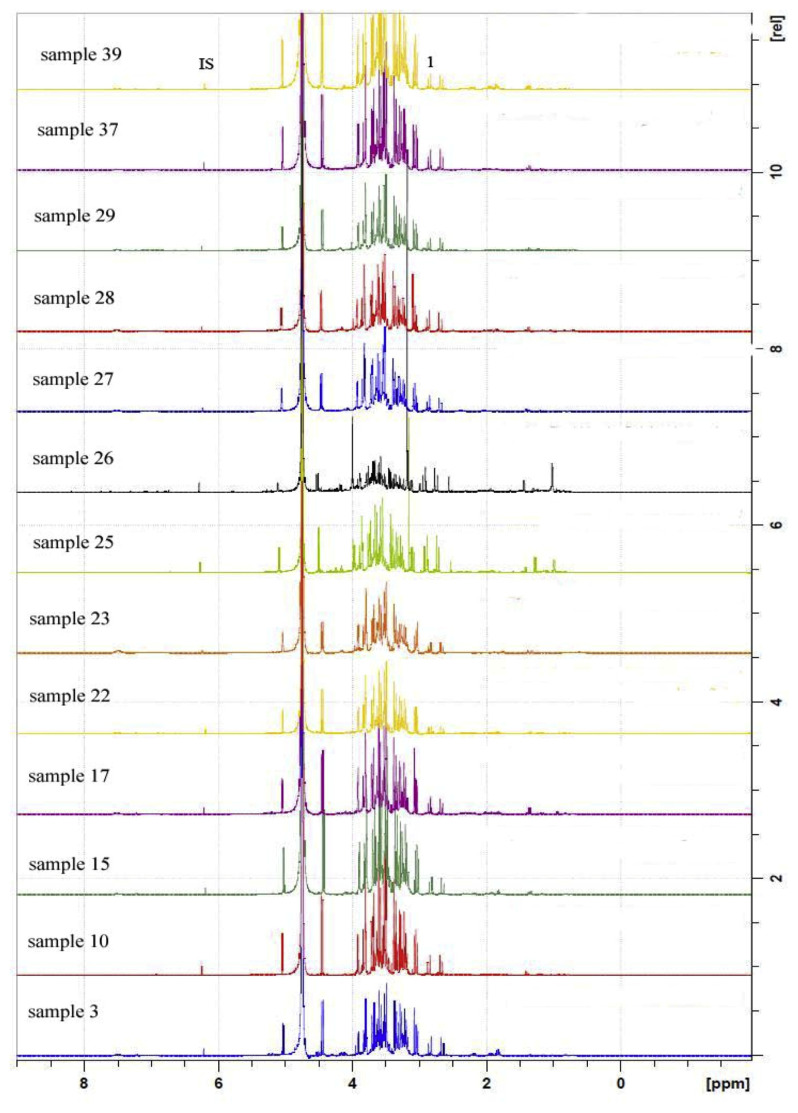
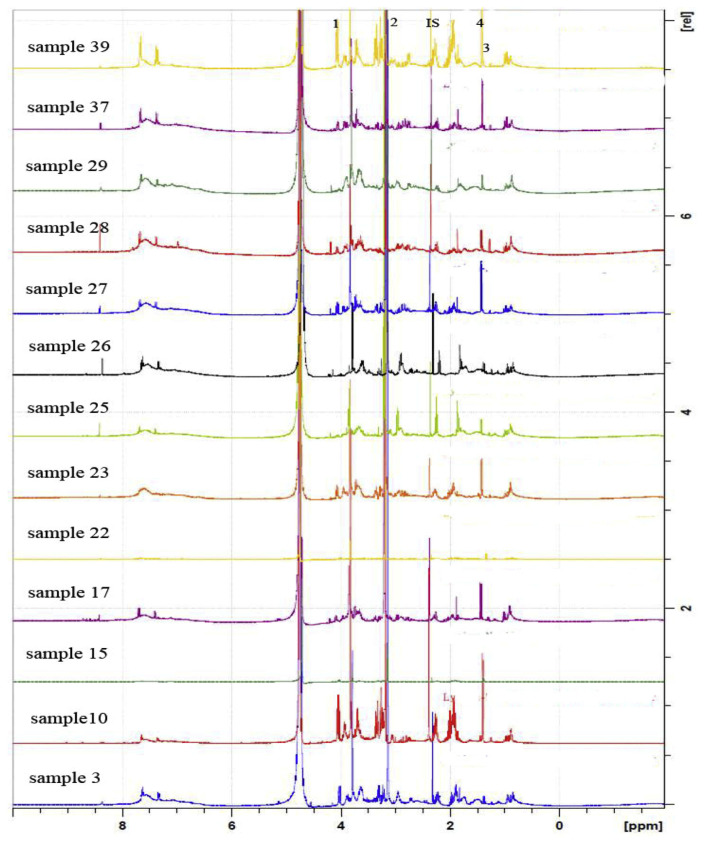
To demonstrate the practicality of the developed 1H NMR method, 39 commercial L. barbarum products purchased from different places in China and 3 wild samples of Lycium chinense collected from Shanxi were analyzed using 1H NMR, as reported in Table 1. Five grams of collected samples was extracted with water, passed through a strong cation exchanger SPE column and the cartridge was then washed with water. Maleic acid (0.5 mg) was added to the solution and the solvent was evaporated to dryness in vacuo to afford LYW fraction. The SPE cartridge was then washed with 25 mL 5% ammonia water. The internal standard 0.5 mg succinic acid was added to the elution buffer and evaporated to dryness in vacuo to obtain LYN fraction. All samples were analyzed in triplicates to determine the contents of five target compounds, and the analytical 1H NMR spectra of LYW and LYN from samples 3, 10, 15, 17, 22, 23, 25–29, 37 and 39 were shown in Figs. 3 and 4, respectively. The results obtained by the 1H NMR method were found to be highly accurate and reproducible for determining the target compounds with relative standard deviations (RSD) ≤ 8.80%. The percentages of betaine of 42 Lycium fruit samples were determined within the range between 0.21 and 1.17% (Table 1). Among them, sample 35 contained the highest amount of betaine.
Table 1.
Contents of betaine, citric acid, threonine, alanine, and proline in 42 samples from the fruits of Lycium speciesa.
| NO. | Collection place | Betaine | Citric acid | Threonine | Alanine | Proline |
|---|---|---|---|---|---|---|
| 1 | Ningxia, China | 0.44 (1.56) | 1.17 (0.41)c | 0.03 (6.67) | 0.15 (0.34) | 0.61 (4.14) |
| 2 | Ningxia, China | 0.65 (0.11) | 1.27 (0.19) | 0.03 (3.94) | 0.19 (1.01) | 0.71 (1.12) |
| 3 | Ningxia, China | 0.59 (1.78) | 2.15 (6.43) | 0.01 (3.84) | 0.03 (6.14) | 0.41 (2.08) |
| 4 | Ningxia, China | 0.21 (4.15) | 1.42 (0.79) | 0.06 (6.59) | 0.06 (6.60) | 0.12 (2.43) |
| 5 | Ningxia, China | 0.84 (2.44) | 2.51 (2.00) | – | 0.20 (1.37) | 0.39 (7.36) |
| 6 | Ningxia, China | 0.70 (1.01) | 1.59 (1.01) | 0.02 (1.01) | 0.25 (1.01) | 0.44 (1.01) |
| 7 | Nei Mongol, China | 0.92 (7.35) | 1.83 (1.44) | 0.01 (6.40) | 0.16 (3.90) | 0.40 (6.14) |
| 8 | Nei Mongol, China | 0.67 (2.52) | 1.31 (0.80) | 0.02 (7.33) | 0.25 (3.55) | 0.32 (6.89) |
| 9 | Nei Mongol, China | 0.81 (1.85) | 1.07 (0.77) | 0.03 (4.51) | 0.15 (2.05) | 0.65 (1.22) |
| 10 | Nei Mongol, China | 0.48 (0.51) | 1.07 (0.10) | 0.02 (1.21) | 0.10 (1.12) | 0.46 (7.56) |
| 11 | Qinghai, China | 0.50 (8.64) | 1.14 (4.52) | 0.03 (5.58) | 0.15 (8.16) | 0.71 (8.80) |
| 12 | Qinghai, China | 0.61 (4.19) | 0.89 (0.11) | 0.01 (5.55) | 0.11 (3.98) | 0.46 (2.96) |
| 13 | Qinghai, China | 0.59 (0.34) | 1.41 (0.21) | 0.03 (6.53) | 0.22 (1.08) | 0.85 (0.86) |
| 14 | Qinghai, China | 0.52 (1.49) | 0.89 (0.53) | 0.01 (3.65) | 0.13 (2.09) | 0.85 (2.20) |
| 15 | Qinghai, China | 0.94 (0.73) | 1.41 (1.46) | 0.04 (2.81) | 0.15 (0.99) | 0.92 (1.65) |
| 16 | Gansu, China | 0.61 (8.48) | 1.31 (3.07) | 0.03 (0.87) | 0.15 (3.43) | 0.49 (8.47) |
| 17 | Gansu, China | 0.70 (2.34) | 1.08 (1.26) | 0.02 (4.80) | 0.14 (0.66) | 0.18 (6.77) |
| 18 | Gansu, China | 0.59 (3.42) | 1.40 (0.41) | 0.02 (3.01) | 0.12 (4.29) | 0.17 (7.90) |
| 19 | Gansu, China | 1.04 (1.50) | 0.79 (1.02) | 0.04 (7.80) | 0.20 (3.85) | 0.67 (3.04) |
| 20 | Xinjiang, China | 1.01 (3.94) | 1.24 (1.68) | 0.01 (8.30) | 0.20 (3.61) | 0.21 (2.43) |
| 21 | Xinjiang, China | 0.74 (1.80) | 1.33 (1.96) | 0.01 (0.71) | 0.14 (1.58) | 0.28 (4.66) |
| 22 | Xinjiang, China | 0.99 (3.47) | 0.81 (5.16) | 0.04 (4.94) | 0.22 (5.96) | 0.75 (7.22) |
| 23 | Shanxi, China | 1.09 (5.95) | 0.89 (4.63) | 0.02 (7.98) | 0.21 (2.75) | 0.47 (5.46) |
| 24b | Shanxi, China | 0.77 (8.41) | 1.95 (0.66) | 0.02 (7.14) | 0.06 (6.23) | 0.05 (3.17) |
| 25b | Shanxi, China | 0.80 (0.58) | 1.95 (0.45) | 0.04 (3.72) | 0.12 (3.91) | 0.03 (4.26) |
| 26b | Shanxi, China | 0.46 (1.42) | 1.25 (0.37) | 0.04 (6.42) | 0.08 (1.43) | – |
| 27 | Shaanxi, China | 0.62 (2.42) | 1.16 (0.57) | 0.01 (5.94) | 0.13 (7.06) | 0.21 (8.06) |
| 28 | Sichuan, China | 0.38 (2.80) | 1.59 (1.47) | 0.04 (8.03) | 0.08 (1.19) | 0.06 (7.25) |
| 29 | Hebei, China | 0.51 (6.26) | 0.94 (4.98) | 0.02 (6.79) | 0.06 (2.53) | 0.06 (7.36) |
| 30 | Beijing, China | 0.77 (2.03) | 1.50 (1.15) | 0.01 (3.74) | 0.12 (3.59) | 0.21 (5.13) |
| 31 | Beijing, China | 0.88 (1.93) | 1.60 (1.34) | 0.01 (7.59) | 0.16 (2.48) | 0.27 (8.76) |
| 32 | Beijing, China | 0.62 (2.47) | 1.32 (3.62) | 0.01 (7.86) | 0.09 (3.27) | 0.04 (8.66) |
| 33 | Beijing, China | 0.95 (3.13) | 2.10 (1.48) | 0.01 (4.29) | 0.17 (2.53) | 0.17 (7.55) |
| 34 | Beijing, China | 0.77 (6.29) | 1.83 (0.43) | 0.02 (7.17) | 0.14 (2.19) | 0.15 (8.67) |
| 35 | Beijing, China | 1.17 (3.91) | 1.77 (0.58) | 0.02 (5.83) | 0.25 (2.33) | 0.28 (7.26) |
| 36 | Beijing, China | 0.54 (1.21) | 1.25 (1.61) | 0.01 (6.29) | 0.14 (1.16) | 0.29 (7.98) |
| 37 | Beijing, China | 0.78 (1.55) | 0.98 (1.57) | 0.02 (4.08) | 0.19 (3.16) | 0.11 (3.42) |
| 38 | Beijing, China | 0.81 (2.60) | 1.25 (1.11) | 0.03 (4.63) | 0.24 (5.73) | 0.58 (5.03) |
| 39 | Beijing, China | 0.83 (8.06) | 1.28 (1.45) | 0.05 (6.78) | 0.27 (6.17) | 1.51 (5.80) |
| 40 | Beijing, China | 0.64 (5.84) | 1.32 (2.25) | 0.01 (2.46) | 0.15 (7.74) | 0.48 (7.99) |
| 41 | Beijing, China | 0.57 (5.74) | 1.26 (4.37) | 0.01 (5.55) | 0.13 (7.27) | 0.32 (1.12) |
| 42 | Beijing, China | 0.77 (2.67) | 1.06 (1.85) | – | 0.14 (1.20) | 0.09 (6.70) |
Recorded on % (w/w) of Lycium species.
Samples 24–26 were L. chinense samples, and others were L. barbarum.
% RSD, all experiments were based on triplicate measurements.
Fig. 3.
1H NMR spectra of LYW from samples 3, 10, 15, 17, 22–23, 25–29, 37, and 39. IS: maleic acid (δ 6.36, 2H). 1: citric acid (δ 2.81, 2H).
Fig. 4.
1H NMR spectra of LYN from samples 3, 10, 15, 17, 22–23, 25–29, 37, and 39. IS: succinic acid (δ 2.37, 4H); 1: proline (δ 4.08, 1H); 2: betaine (δ 3.21, 9H); 3: threonine (δ 1.28, 3H); 4: alanine (δ 1.43, 3H).
The advantages of the developed analytical method are discussed as follows. The SCX-SPE pre-processing method can collect charged compounds and separate out other interfering compounds. 1H NMR can record compounds that are poorly detected by measuring UV absorbance, including betaine and amino acids. Moreover, 1H NMR method provides a quantitative method of analyzing Lycii Fructus that does not require preparation of compound derivatives, control of pH values, or addition of NMR shifting reagents. In addition, several compounds can be simultaneously quantitated in a highly specific quality analysis. The analytical solvent system uses only water without any other organic solvents, which is very safe, convenient, uncomplicated, and green.
ERETIC 2 based on PULCON (Pulse Length-based Concentration determination) principle is a new fully automated concentration determination technology based on NMR without addition of any internal standards [23]. A known compound of interest is detected and defined as an ERETIC reference. The analysis is performed using the same parameters. Then the amounts of target compounds are determined by software processing. For example, samples 10 and 13 were analyzed by ERETIC 2 software and compared to the original method based on the addition of the internal standard to samples. The results for the contents of betaine, citric acid, proline, threonine, and alanine between the two methods were very similar (Table 2), indicating that the NMR ERETIC 2 method can be conveniently applied to the quantification of bioactive compounds in foods, plants, and medicines.
Table 2.
Contents of betaine, citric acid, threonine, alanine, and proline in samples 10 and 13 by conventional (adding internal standards) method and NMR Digital ERETIC.a
| Samples no. | Betaine | Citric acid | Threonine | Alanine | Proline |
|---|---|---|---|---|---|
| 10c | 0.48 (0.50)b | 1.14 (0.27) | 0.02 (1.53) | 0.10 (1.05) | 0.46 (7.52) |
| 13c | 0.59 (0.36) | 1.42 (0.09) | 0.03 (6.10) | 0.22 (1.11) | 0.86 (0.76) |
| 10d | 0.48 (0.51) | 1.07 (0.10) | 0.02 (1.21) | 0.10 (1.12) | 0.46 (7.56) |
| 13d | 0.59 (0.34) | 1.41 (0.21) | 0.03 (6.53) | 0.22 (1.08) | 0.85 (0.86) |
Recorded on % (w/w) of Lycium species.
% RSD, all experiments were based on triplicate measurement.
Analysis by Digital ERETIC 2.
Analysis by addition of internal stands.
The developed 1H NMR method was successfully applied for the rapid and reliable simultaneous determination of betaine, citric acid, threonine, alanine, and proline in various sources of Lycium fruits as well as those in commercial products. It offers a short analysis time and can serve as a useful tool for the routine analysis of betaine and four other compounds. Furthermore, application of the ERETIC 2 method is more conveniently for quality control. Therefore, the ERETIC 2 1H NMR method is highly applicable in the quantitative analysis of bioactive principles in the various samples of Lycii Fructus.
Acknowledgment
Thanks are also given to Rezware Technology Inc., Taiwan for supporting Bruker TopSpin version 3.0 software.
Abbreviations used
- ERETIC 2
Electronic Reference To access In vivo Concentration 2
- SCX-SPE
strong cation exchange solid phase extraction
- PULCON
PUlse Length-based CONcentration determination
Appendix A. Supplementary data
Supplementary data related to this article can be found at https://doi.org/10.1016/j.jfda.2018.01.001.
Funding Statement
This study was supported by the Ministry of Science and Technology, Taiwan, to T-S. Wu.
Footnotes
Funding information
This study was supported by the Ministry of Science and Technology, Taiwan, to T-S. Wu.
REFERENCES
- 1. Chang RC, So KF. Use of anti-aging herbal medicine, Lycium barbarum, against aging-associated diseases. What do we know so far? Cell Mol Neurobiol. 2008;28:643–52. doi: 10.1007/s10571-007-9181-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Luo Q, Li Z, Huang X, Yan J, Zhang S, Cai YZ. Lycium barbarum polysaccharides: protective effects against heat-induced damage of rat testes and H2O2-induced DNA damage in mouse testicular cells and beneficial effect on sexual behavior and reproductive function of hemicastrated rats. Life Sci. 2006;79:613–21. doi: 10.1016/j.lfs.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 3. Wu HT, He XJ, Hong YK, Ma T, Xu YP, Li HH. Chemical characterization of Lycium barbarum polysaccharides and its inhibition against liver oxidative injury of high-fat mice. Int J Biol Macromol. 2010;46:540–3. doi: 10.1016/j.ijbiomac.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 4.Taiwan Ministry of Health and Welfare. Taiwan herbal pharmacopeia. 2nd ed. Ministry of Health and Welfare; 2016. 2016/12/06. [Google Scholar]
- 5. Lee SY, Ko KS. Effects of S-adenosylmethionine and its combinations with taurine and/or betaine on glutathione homeostasis in ethanol-induced acute hepatotoxicity. J Cancer Prev. 2016;21:164–72. doi: 10.15430/JCP.2016.21.3.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ge CX, Yu R, Xu MX, Li PQ, Fan CY, Li JM, et al. Betaine prevented fructose-induced NAFLD by regulating LXRα/PPARα pathway and alleviating ER stress in rats. Eur J Pharmacol. 2016;770:154–64. doi: 10.1016/j.ejphar.2015.11.043. [DOI] [PubMed] [Google Scholar]
- 7. Kim DH, Sung B, Chung HY, Kim ND. Modulation of colitis-associated colon tumorigenesis by baicalein and betaine. J Cancer Prev. 2014;19:153–60. doi: 10.15430/JCP.2014.19.3.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mar MH, Ridky TW, Garner SC, Zeisel SH. A method for the determination of betaine in tissues using high performance liquid chromatography. J Nutr Biochem. 1995;6:392–8. doi: 10.1016/0955-2863(95)80008-z. [DOI] [PubMed] [Google Scholar]
- 9. Gorham J. Separation and quantitative estimation of betaine esters by high-performance liquid chromatography. J Chromatogr A. 1986;361:301–10. [Google Scholar]
- 10. Shin YG, Cho KH, Kim JM, Park MK, Park JH. Determination of betaine in Lycium chinense fruits by liquid chromatography-electrospray ionization mass spectrometry. J Chromatogr A. 1999;857:331–5. doi: 10.1016/s0021-9673(99)00720-7. [DOI] [PubMed] [Google Scholar]
- 11. Beale R, Airs R. Quantification of glycine betaine, choline and trimethylamine N-oxide in seawater particulates: minimisation of seawater associated ion suppression. Anal Chim Acta. 2016;938:114–22. doi: 10.1016/j.aca.2016.07.016. [DOI] [PubMed] [Google Scholar]
- 12. Guiraud SP, Montoliu I, Da Silva L, Dayon L, Galindo AN, Corthesy J, et al. High-throughput and simultaneous quantitative analysis of homocysteine-methionine cycle metabolites and co-factors in blood plasma and cerebrospinal fluid by isotope dilution LC-MS/MS. Anal Bioanal Chem. 2017;409:295–305. doi: 10.1007/s00216-016-0003-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bonneau N, Cynober T, Jullian JC, Champy P. 1H qNMR quantification of Annonaceous acetogenins in crude extracts of Annona muricata L. Fruit Pulp. Phytochem Anal. 2017;28:251–6. doi: 10.1002/pca.2668. [DOI] [PubMed] [Google Scholar]
- 14. Clendinen CS, Stupp GS, Wang B, Garrett TJ, Edison AS. 13C metabolomics: NMR and IROA for unknown identification. Curr Metabolomics. 2016;4:116–20. doi: 10.2174/2213235X04666160407212156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Petrakis EA, Cagliani LR, Tarantilis PA, Polissiou MG, Consonni R. Sudan dyes in adulterated saffron (Crocus sativus L.): identification and quantification by 1H NMR. Food Chem. 2017;217:418–24. doi: 10.1016/j.foodchem.2016.08.078. [DOI] [PubMed] [Google Scholar]
- 16. Beyer T, Diehl B, Holzgrabe U. Quantitative NMR spectroscopy of biologically active substances and excipients. Bioanal Rev. 2010;2:1–22. [Google Scholar]
- 17. Malet-Martino M, Holzgrabe U. NMR techniques in biomedical and pharmaceutical analysis. J Pharm Biomed Anal. 2011;55:1–15. doi: 10.1016/j.jpba.2010.12.023. [DOI] [PubMed] [Google Scholar]
- 18. Li CY, Lin CH, Wu CC, Lee KH, Wu TS. Efficient 1H nuclear magnetic resonance method for improved quality control analyses of Ginkgo constituents. J Agric Food Chem. 2004;52:3721–5. doi: 10.1021/jf049920h. [DOI] [PubMed] [Google Scholar]
- 19. Li CY, Lin CH, Wu TS. Quantitative analysis of camptothecin derivatives in Nothapodytes foetida using 1H-NMR method. Chem Pharm Bull (Tokyo) 2005;53:347–9. doi: 10.1248/cpb.53.347. [DOI] [PubMed] [Google Scholar]
- 20. Li CY, Tsai SI, Damu AG, Wu TS. A rapid and simple determination of protoberberine alkaloids in Rhizoma Coptidis by 1H NMR and its application for quality control of commercial prescriptions. J Pharm Biomed Anal. 2009;49:1272–6. doi: 10.1016/j.jpba.2009.02.028. [DOI] [PubMed] [Google Scholar]
- 21. Li CY, Lu HJ, Lin CH, Wu TS. A rapid and simple determination of protoberberine alkaloids in Cortex Phellodendri by 1H NMR and its application for quality control of commercial traditional Chinese medicine prescriptions. J Pharm Biomed Anal. 2006;40:173–8. doi: 10.1016/j.jpba.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 22. Li CY, Xu HX, Han QB, Wu TS. Quality assessment of Radix Codonopsis by quantitative nuclear magnetic resonance. J Chromatogr A. 2009;1216:2124–9. doi: 10.1016/j.chroma.2008.10.080. [DOI] [PubMed] [Google Scholar]
- 23. Watanabe R, Sugai C, Yamazaki T, Matsushima R, Uchida H, Matsumiya M, et al. Quantitative nuclear magnetic resonance spectroscopy based on PULCON methodology: application to quantification of invaluable marine toxin, okadaic acid. Toxins. 2016;8:294. doi: 10.3390/toxins8100294. [DOI] [PMC free article] [PubMed] [Google Scholar]