Abstract
Elevated levels of free fatty acids (FFAs) in the liver, resulting from either increased lipolysis or imbalanced FFAs flux, is a key pathogenic factor of hepatic steatosis. This study was conducted to examine the therapeutic effect of tetrahydrocurcumin (THC), a naturally occurring curcuminoid and a metabolite of curcumin, on oleic acid (OA)-induced steatosis in human hepatocellular carcinoma cells and to elucidate the underlying mechanism. HepG2 cells were incubated with OA to induce steatosis, and then treated with various concentrations of THC. The results showed that THC treatment significantly decreased lipid accumulation in OA-treated HepG2 cells, possibly, by inhibiting the expression of the lipogenic proteins, sterol regulatory element-binding protein 1 (SREBP-1c), peroxisome proliferator-activated receptor gamma (PPARγ), fatty acid synthase (FAS), and fatty acid-binding protein 4 (FABP4). Moreover, THC attenuated OA-induced hepatic lipogenesis in an adenosine monophosphate–activated protein kinase (AMPK)-dependent manner, which was reversed by pretreatment with an AMPK inhibitor. THC promoted lipolysis and upregulated the expression of genes involved in β-oxidation. Glucose uptake and insulin signaling impaired in HepG2 cells incubated with OA were abated by THC treatment, including phosphorylation of the insulin receptor substrate 1 (IRS-1)/phosphoinositide 3-kinase (PI3K)/Akt and downstream signaling pathways, forkhead box protein O1 (FOXO1) and glycogen synthase kinase 3 β (GSK3β), which are involved in gluconeogenesis and glycogen synthesis, respectively. Altogether, these results demonstrated the novel therapeutic benefit of THC against hepatic steatosis and, consequently, a potential treatment for non-alcoholic fatty liver disease (NAFLD).
Keywords: Tetrahydrocurcumin, Steatosis, HepG2, AMPK, Insulin resistance
1. Introduction
Hepatic steatosis is defined as the abnormal accumulation of hepatic triglyceride (TG) greater than 5%, by liver weight, and is the hallmark feature of non-alcoholic fatty liver disease (NAFLD) [1]. A plethora of epidemiological studies have shown that NAFLD is associated with cardiovascular disease, malignancy, and other metabolic disorders. Dysregulation of hepatic lipid metabolism results from a number of factors, such as an increased level of plasma free fatty acids (FFAs) from adipose tissue lipolysis or dietary intake, elevated hepatic de novo lipogenesis, decreased β-oxidation, and very-low-density lipoprotein (VLDL) export, causing abnormal hepatic TG accumulation [1,2]. Although the pathogenesis of NAFLD is not fully characterized, obesity and insulin resistance are highlighted as critical pathogenic factors, as they contribute to elevated lipolysis, increased levels and uptake of plasma FFAs in hepatocytes, altered fatty acid metabolism, and enhanced lipogenesis that altogether, ultimately, lead to hepatic stea-tosis [1,2]. The increase in FFAs influx, proinflammatory cytokines, and lipid intermediates in the liver exacerbate hepatic insulin resistance by interfering with the phosphorylation of insulin receptor substrates [3]. It has been suggested that hepatic insulin resistance is crucial for the metabolic dysregulation of NAFLD by aggravating hepatic steatosis, which is characterized by both reduced insulin-mediated hepatic glucose production and increased hepatic gluconeogenesis [4].
Adenosine monophosphate–activated protein kinase (AMPK) is a cellular nutrient sensor and a central regulator of energy homeostasis in most tissues and organs, including the liver, adipose tissue, and skeletal muscle. AMPK coordinates hepatic energy metabolism through transcriptional regulation of genes involved in lipid metabolism or direct phosphorylation of metabolic proteins or enzymes, such as sterol regulatory element-binding protein 1 (SREBP-1), carbohydrate-responsive element-binding protein (ChREBP), HMG-CoA reductase (HMGCR), acetyl-CoA carboxylase 1 (ACC1), and fatty acid synthase (FAS) [5]. Additionally, hepatic AMPK is responsible for maintaining whole-body glucose homeostasis by downregulating the expression of gluconeogenic genes, such as those encoding phosphoenolpyruvate carboxykinase (PEPCK) and glucose-6-phosphatase (G6Pase) [5,6]. Numerous studies have demonstrated that in vitro and in vivo AMPK activation protects against NAFLD and reverses insulin resistance, as it leads to increased fatty acid oxidation, inhibition of hepatic lipogenesis, cholesterol synthesis, and glucose production [7,8]. Thus, activation of AMPK has attracted attention as a therapeutic strategy against NAFLD [9].
Tetrahydrocurcumin (THC) was first discovered by Holder et al. in 1978 as a major reductive metabolite of curcumin that naturally exists in Zingiber mioga, Zingiber officinale, and Curcuma zedoaria [10–12]. THC has been shown to exhibit a wide spectrum of therapeutic and chemopreventive properties [13,14]. Although the pharmacological properties of THC are similar to those of curcumin, studies have demonstrated that THC is more active than curcumin as an antioxidant, anti-inflammatory, anti-cancer, antidiabetic, and neuroprotective agent [14]. More specifically, THC possesses superior anti-oxidative activity and upregulates levels of antioxidant enzymes and free radical scavenging activity, which are implicated against atherosclerosis [15], hypertension [16,17], diabetes [18], hyperlipidemia [19] and nephrotoxicity [20,21]. In addition, THC is more stable than curcumin in 0.1 M phosphate buffers at neutral pH and plasma [22]. Its half-life in cell culture medium and plasma is significantly longer than that of curcumin [23]. Structurally, THC is more hydrophilic than curcumin due to the compound’s additional hydrogen molecules [24]. Together, these properties suggest that THC may be more potent and efficacious against human diseases than curcumin, owing to its distinct chemical properties and stability.
In vivo studies demonstrated the potential protective effect of THC against chloroquine- and arsenic-induced hepatotoxicity [25,26]. The efficacy of THC on metabolic dysfunction was tested in streptozotocin-induced type 2 diabetic rats, decreasing levels of plasma insulin and gluconeogenic enzymes and improving blood glucose and hepatic carbohydrate metabolism [27,28]. In addition to its hypoglycemic activity, THC was reported to have hypolipidemic activity that reduced levels of serum and hepatic cholesterol, TG, FFAs, and HMGCR activity [18,19]. These studies illustrated the therapeutic effects of THC on metabolic diseases, such as diabetes mellitus and dyslipidemia, and more importantly, the higher potency of its antidiabetic and anti-hyperlipidemic effects in comparison to curcumin. Although evidence points to a potential protective role of THC against metabolic diseases, its efficacy on hepatic steatosis remains unknown. In the present study, we aimed to evaluate the inhibitory effect of THC on hepatic steatosis, by using a cellular steatosis model, and to elucidate the underlying mechanism.
2. Materials and Methods
2.1. Reagents and chemicals
THC (purity >98%, by HPLC) was kindly was provided by American Medical Holding, Inc. (New York, USA). DMEM, penicillin-streptomycin, and fetal bovine serum (FBS) were obtained from Gibco BRL (Grand Island, NY, USA). Insulin, β-actin was purchased from Sigma Chemical Co. (St. Louis, MO, USA). The p-AMPK (Thr 172), AMPK, p-ACC (Ser 79), ACC, PPARγ, FAS, FABP4, p-IRS1, p-PI3K, PI3K, p-Akt, Akt, p-FOXO1, FOXO1, p-GSK3β and GSK3β antibodies were purchased from Cell Signaling Technology (Beverly, MA, USA). The SREBP-1, PPARα antibody and compound C were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). 2-[N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino]-2-deoxyglucose (2-NBDG) was purchased from Cayman Chemical company (Ann Arbor, MI, USA). Sodium oleate was used to create an in vitro model for hepatic steatosis. Briefly, 30.4 mg sodium oleate was dissolved in 1 mL methanol to obtain 100 mM stock solution.
2.2. Cell culture and treatment
HepG2 cells purchased from the American Type Culture Collection (Rockville, MD, USA) were grown in DMEM supplemented with 2 mM glutamine (Gibco BRL), 1% penicillin/streptomycin (10,000 units of penicillin/mL and 10 mg streptomycin/mL), and 10% fetal bovine serum at 37 °C in a 5% CO2 humidified atmosphere. For induction of steatosis, HepG2 cells were incubated in FFA medium (0.2 mM oleic acid) for 12 h and cultured with DMEM medium (10% FBS) and then treated THC for indicated time.
2.3. Trypan blue assay
HepG2 cells were incubated in 24-well plate (2 × 105/mL) overnight and treated as described above. After incubation of THC for 24 h, cells were washed with sterile PBS, and treated with 0.25% trypsin–EDTA, and harvested. Cells were diluted in 0.4% trypan blue and then counted under a light microscope.
2.4. Oil red O staining
HepG2 cells were treated as described above. After 24 h, the cells were washed with sterile PBS, fixed with 10% formalin for 1 h, and stained with 0.5% Oil red O in isopropanol for 3 min at room temperature. The Oil red O stained cells were then rinsed with PBS few times to remove the excess stain. The stained lipid droplets within cells were visualized by light microscope and photographed with a digital camera. The stained lipid droplets were dissolved in 2-propanol and the absorbance was measured at 520 nm to quantify lipid accumulation.
2.5. Western blot analysis
The total proteins of HepG2 cells were extracted by adding lysis buffer to the cell pellets on ice for 60 min, followed by centrifugation at 10,000 × g for 30 min at 4 °C as described previously [29]. The protein concentration were measured by Bio-Rad Protein Assay (Bio-Rad Laboratories, Munich, Germany), and then 50 μg of protein samples were mixed with 5 × sample buffer and boiled at 100 °C for 10 min. The proteins were first separated on 8% polyacrylamide gels and electrophoretically transferred onto an immobile membrane (PVDF; Millipore Corp., Bedford, MA, USA) with transfer buffer composed of 25 mM Tris–HCl (pH 8.9), 192 mM glycine, and 20% methanol. The membranes were blocked with blocking solution and then immunoblotted with primary antibodies at 4 °C for overnight. The blots were rinsed with PBST buffer for three times. Then blots were incubated with 1:2000 dilution of the horseradish peroxide (HRP)-conjugated secondary antibody (Zymed Laboratories, San Francisco, CA, USA) and then washed again three times with PBST buffer. The transferred proteins were visualized with an enhanced chemiluminescence detection kit (ECL; Sigma Chemical Co., St Louis, MO, USA).
2.6. Reverse transcription polymerase chain reaction (RT-PCR)
Total RNA was extracted by using Trizol reagent according to the manufacturer’s instruction (Invitrogen, Carlsbad, CA, USA). 2 μg of total RNA was reverse transcribed into first strand cDNA, synthesized by using SuperScript III Reverse Transcriptase (Invitrogen, Renfrewshire, UK) in a final volume of 20 μL. RT reactions were performed at 56 °C for 50 min and 72 °C for 15 min in a GeneAmp® PCR System 9700 thermal cycler (Applied Biosystems). The thermal cycle conditions were initiated at 94 °C for 1 min, and 30 cycles of amplification (94 °C for 30 s, 56 °C for 12 s, and 72 °C for 1 min) were followed by extension at 72 °C for 3 min. The cDNA was amplified by PCR with following primers: Carnitine palmitoyltransferase 1a (CPT-1a), forward 5′-GGAATGAAATTCCCACTGTCTGTC-3′, reverse 5′-CAGTTCAGCCATCGCTGTTGTA-3′; Glucose transporter 4 (GLUT4), forward 5′–CCGCTACCTCTACATCATCCA-3′, reverse:5′-GCTTCCGCTTCTCATCCTTC-3′; β-actin forward 5′-AAGAGAGGCATCCTCACCCT-3′, reverse: 5′-TACATGGCTG-GGGTGTTGAA-3′. Each PCR reaction (10 μL) were separated by electrophoresis on 2% agarose gel and visualized by ethidium bromide staining.
2.7. Glycerol release assay
After treatment HepG2 cell with various concentration of THC in the presence of FFA medium for 24 h, the medium were collected and the glycerol contents in the culture medium was quantified using a colorimetric kit according to the manufacturer’s instructions (Cayman Chemical, Ann Arbor, MI, USA). Released glycerol was quantified at 540 nm on a 96-well plate reader.
2.8. Measurement of intracellular TG level
For TG quantification, the treatment HepG2 cells were washed with PBS and treated with 0.25% trypsin–EDTA, and harvested. Intracellular TG content was quantified using a colorimetric kit according to the manufacturer’s instructions (Cayman Chemical, Ann Arbor, MI, USA), and absorbance was measured at 540 nm.
2.9. Glucose uptake and flow cytometry
Glucose uptake assay was performed by using 2-NBDG. Briefly, HepG2 cells seed in 24-well and treated as described above and then the medium was removed. The wells were added 2-NBDG (100 μg/mL) in serum-free low glucose DMEM medium with 100 nM insulin for 20 min at 37 °C. The cells were examined and photographs were obtained with a fluorescence microscope (Axioscop, Carl Zeiss, Thomwood, NY, USA). Fluorescence intensity of 2-NBDG was recorded in the FL1 channel by using a FACScan laser flow cytometer. Data from 1000 single-cell events were collected.
2.10. Statistical analysis
Data are presented as means ± SE for the indicated number of independently performed experiments. Comparisons of statistical significance between groups were made by one-way analysis of variance (ANOVA) and Duncan’s Multiple Range Test. A P value < 0.05 was considered statistically significant. Analysis was performed using SAS v 9.1 software.
3. Results
3.1. THC decreased intracellular lipid accumulation in oleic acid-induced HepG2 cells
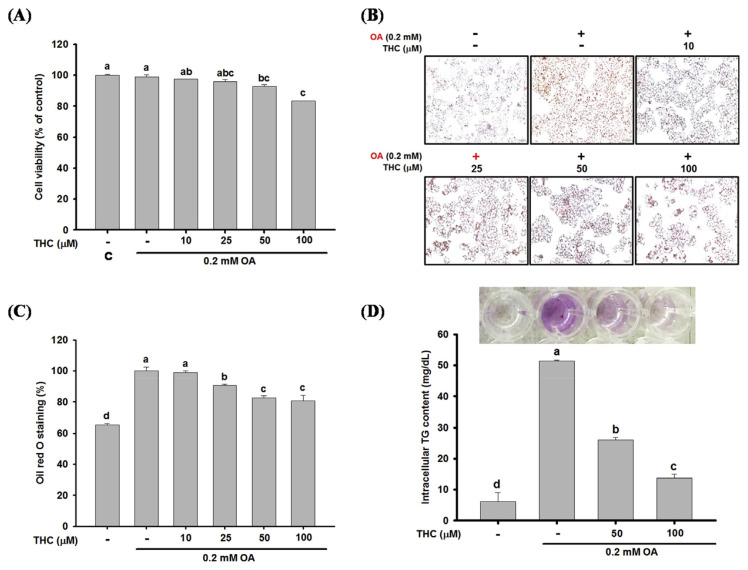
HepG2 cells were initially stimulated with oleic acid (OA) to induce steatosis, followed by treatment with THC to examine its therapeutic effect on steatosis. The cytotoxic effect of THC on OA-treated HepG2 cells was determined by trypan blue assay. HepG2 cells were incubated with OA for 12 h and then treated with various concentrations of THC (10–100 μM) for 24 h. As shown in Fig. 1A, cell viability was not significantly affected by THC at concentrations by 10 and 25 μM. However, 100 μM THC decreased cell viability by 17%, but there were no visible changes to the morphological features of the cells under microscopic examination. Next, the effect of THC on OA-induced cellular steatosis was examined by Oil Red O staining. Incubating HepG2 cells with OA induced steatosis, which was marked by a significant accumulation of intracel-lular lipid droplets in comparison to untreated control cells (Fig. 1B–1C). Steatosis in these HepG2 cells was significantly attenuated by THC treatment, indicated by a visible decrease in the accumulation of lipid droplets and intracellular TG content (Fig. 1B–1D). This was demonstrative of the potency of THC suppression of OA-induced cellular steatosis.
Fig. 1.
Effect of THC on OA-induced lipid accumulation in HepG2 cells. HepG2 cells were stimulated OA for 12 h and then cultured in DMEM medium with or without 10–100 μM of THC for 24 h. (A) Cell viability were measured by the trypan blue assay. (B) Cells were stained with Oil Red O and photographed ( × 200 magnification). (C) Lipid content was extracted from Oil Red O stained cells by isopropanol and quantified by spectrophotometric analysis at 520 nm. (D) Intracellular TGs were extracted and analyzed as described in Materials and Methods. Data were presented as the mean ± SE (n = 3). Means with different letters (a–d) above the bars are significantly different (P < 0.05).
3.2. Effect of THC on expression of lipogenenic factors and AMPK signaling in oleic acid-induced steatosis
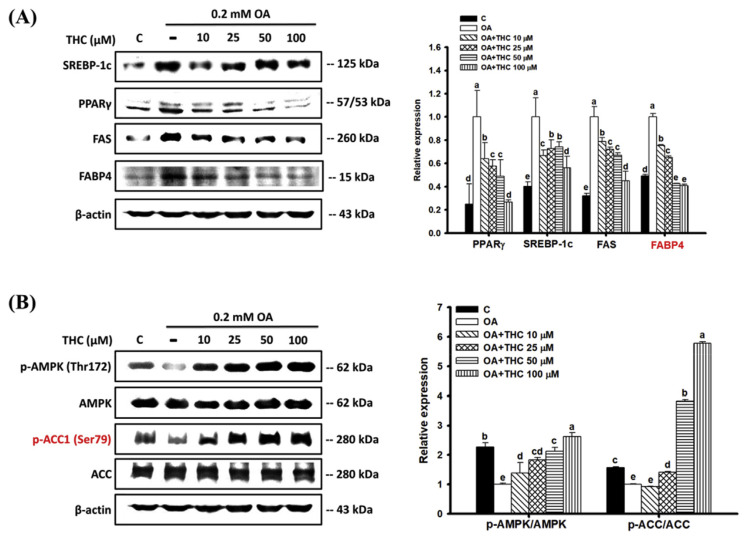
To investigate whether THC attenuated OA-induced cellular steatosis by downregulating lipogenic factors, the expression of SREBP-1c, peroxisome proliferator-activated receptor gamma (PPARγ), and downstream targets, FAS and fatty acid-binding protein 4 (FABP4) was detected by western blot analysis. Fig. 2A shows that protein levels of SREBP-1c, PPARγ, FAS, and FABP4 significantly increased in cells treated with OA compared to those in untreated control cells. However, these protein levels were significantly reduced by THC. OA-induced steatosis abated phosphorylation of AMPK in HepG2 cells, leading to the decreased phosphorylation of ACC at Ser79 and, consequently, its increased activation (Fig. 2B). Following THC treatment, there was a significant increase in phosphorylation of AMPK and, thereby, AMPK-mediated inactivation of ACC. These observations suggested that THC attenuated steatosis, possibly through the downregulation of lipogenesis and upregulation of AMPK signaling in HepG2 cells.
Fig. 2.
Effect of THC on protein expression of lipogenenic factors and AMPK signaling in OA-treated HepG2 cells. Cells were treated as described above. Total protein were extracted and the protein expression of (A) lipogenenic factors, PPARγ, SREBP-1c, FAS and FABP4, (B) p-AMPK (Thr172), AMPK, p-ACC (Ser79) and ACC were determined by Western Blot analysis with specific antibodies. The figures represent one of three experiments with similar results. Mean values within each column with different labels (a–e) are significantly different (P < 0.05).
3.3. THC attenuated oleic acid-induced steatosis through an AMPK-dependent pathway
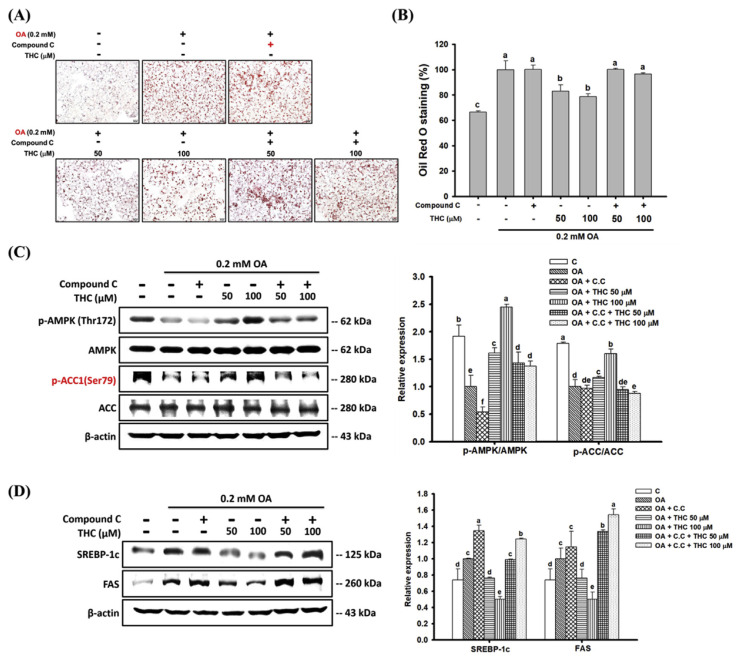
To validate whether the reductive effects of THC on OA-induced steatosis was AMPK-dependent, we pretreated cells with the AMPK inhibitor, compound C, and measured the intracellular lipid accumulation. Consistent with previous observations, Oil Red O staining showed that OA-induced lipid accumulation in HepG2 cells, whereas THC significantly retarded lipid accumulation. However, pretreatment with compound C reversed the THC-induced reduction of steatosis (Fig. 3A and B). In addition, THC-induced phosphorylation of AMPK and ACC was abated in the presence of compound C (Fig. 3C). These results supported the hypothesis that THC-induced activation of AMPK was causative of its inhibitory effect on lipid accumulation in steatosis. Also, THC significantly diminished the protein expression of lipogenic factors, SREBP-1c and FAS, in OA-treated cells whereas was reversed by pre-incubated with compound C (Fig. 3D). Therefore, THC attenuated OA-induced steatosis in HepG2 cells through an AMPK-dependent mechanism.
Fig. 3.
Effects of compound C on THC attenuated lipogenesis and AMPK signaling in OA-treated HepG2 cells. HepG2 cells were pretreated 2 μM compound C for 1 h followed by stimulation of OA for 12 h, and then treated with 10–100 μM of THC for another 24 h. (A) Oil Red O stained HepG2 cells were photographed ( × 200 magnification) and (B) lipid content was extracted and measured by spectrophotometric analysis at 520 nm. Protein extracts were prepared and the protein levels of (C) p-AMPKα (Thr172), AMPKα, p-ACC (Ser79) and ACC, (D) SREBP-1c and FAS were examined by Western Blot analysis. Data were presented as the mean ± SE (n = 3). Means with different letters (a–d) above the bars are significantly different (P < 0.05).
3.4. THC elevated lipolysis and increased fatty acid oxidation in oleic acid-treated HepG2 cells
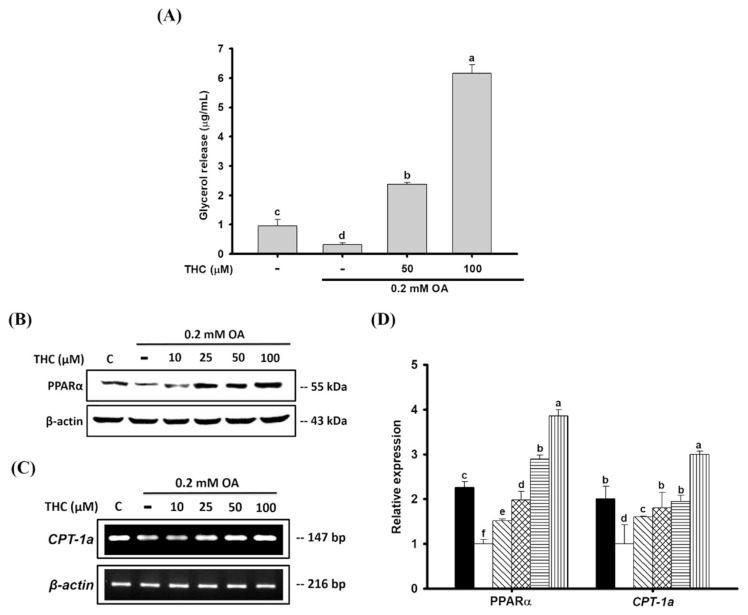
We investigated whether THC’s reductive effects on OA-induced steatosis was associated with lipolysis by measuring the concentration of released glycerol metabolite in cell culture medium. Lipolysis decreased in cells incubated with OA compared to that in untreated control cells (Fig. 4A). On the other hand, THC stimulated the release of glycerol into the culture medium in a dose-dependent manner. Moreover, protein expression of PPARα, a critical regulator of mitochondrial β-oxidation of fatty acids, was decreased by OA treatment, but was prominently upregulated by THC in a dose-dependent manner (Fig. 4B). A similar observation was made regarding the expression of carnitine palmitoyltransferase 1 (CPT1), which is a known target of PPARα involved in the mitochondrial transport of long-chain fatty acids [30]. CPT-1a expression was downregulated in OA-treated cells, whereas THC treatment significantly elevated CPT-1a expression (Fig. 4C). These findings indicated that THC stimulated lipolysis and fatty acid oxidation in HepG2 cells, thus leading to the reduction in OA-induced lipid accumulation.
Fig. 4.
Effects of THC on lipolysis and fatty acid oxidation in OA-treated HepG2 cells. HepG2 cells were stimulated OA for 12 h and then cultured in DMEM medium with or without THC for 24 h. (A) Release glycerol into the culture medium were quantified by its OD value at 540 nm. (B) Total protein was extracted and the protein level of PPARα was analyzed by Western Blot analysis. (C) Total RNA was also isolated, and the mRNA expressions of CPT-1a were determined by RT-PCR. (D) Densitometry of PPARα protein and CPT-1a mRNA content normalized to β-actin. Data were presented as the mean ± SE (n = 3). Means with different letters (a–e) above the bars are significantly different (P < 0.05).
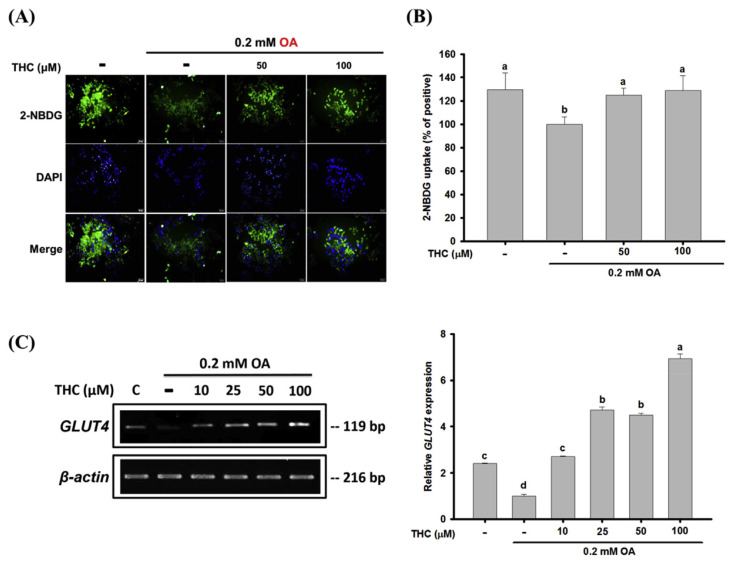
3.5. THC increased glucose uptake in oleic acid-treated HepG2 cells
Increased FFA influx has been shown to impair insulin signaling and glucose metabolism in the liver [3]. To determine the effect of THC on glucose uptake in steatosis, the 2-NBDG glucose uptake assay was performed in OA-treated HepG2 cells. 2-NBDG is a fluorescent glucose analog, and the results showed that the level of fluorescent-labeled glucose significantly decreased in response to OA compared to that in the untreated control cells (Fig. 5A). Flow cytometric analysis validated this result (Fig. 5B). Cells treated with THC had significantly higher 2-NBDG uptake, compared to cells treated with OA only. In addition, the expression of GLUT4 decreased in OA-treated cells, but substantially increased in response to THC (Fig. 5C). These results indicated that the dysregulated glucose consumption observed in cellular steatosis induced by OA could be reversed by THC.
Fig. 5.
Effects of THC on glucose uptake in OA-treated HepG2 cells. OA-stimulated HepG2 cells were treated with or without THC for 24 h and then were incubated with a fluorescent glucose derivative, 2-NBDG, for 20 min. (A) The intracellular level of 2-NBDG was examined by fluorescence microscopy (400 × magnification, 50 μm). Representative images of cells from three independent experiments were shown. (B) Fluorescence intensity of 2-NBDG was measured and quantified by FASC analysis. (C) The mRNA level of GLUT4 was determined by semiquantitative RT-PCR. Values are the mean ± SE of three independent experiments. The bars with different letters are significantly different (P < 0.05).
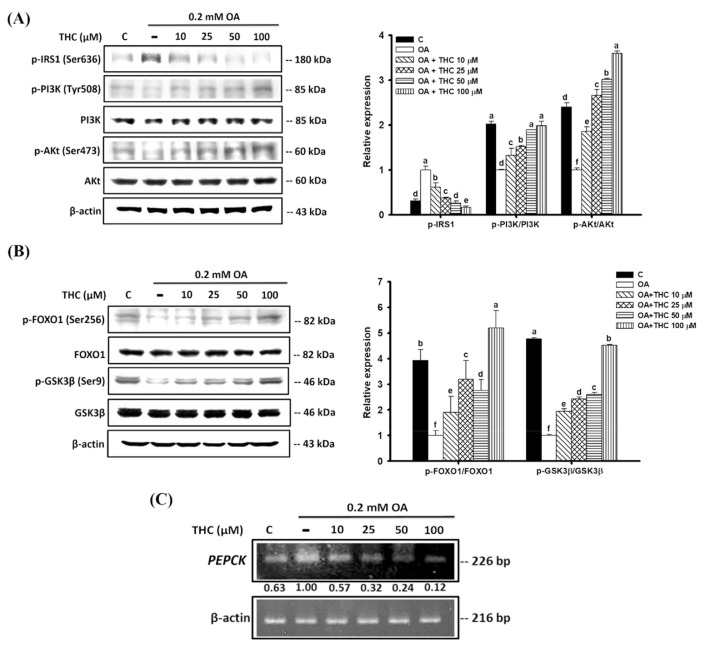
3.6. THC improved insulin signaling in oleic acid-treated HepG2 cells
OA has been shown to impair insulin signaling in hepatocytes [31]. To elucidate the mechanistic action of THC on the modulation of glucose metabolism in OA-treated HepG2 cells, insulin signaling was measured. Fig. 6A demonstrates that incubating HepG2 cells with OA significantly increased phosphorylation of insulin receptor substrate 1 (IRS-1) at Ser636 compared to that in untreated control cells, indicative of inhibited insulin signaling. This was accompanied by the downregulation of downstream phosphoinositide 3-kinase (PI3K) and Akt signaling. However, THC attenuated the increased phosphorylation of IRS-1 at Ser636 and subsequently activated PI3K/Akt signaling. The upregulation of PI3K/Akt signaling may have been caused by the increase in glucose uptake in response to THC (Fig. 5). Insulin-mediated phosphorylation of forkhead box protein O1 (FOXO1) at Ser256 by Akt is important for the repression of FOXO1-dependent transcription of gluconeogenic genes [32,33]. Here we found the phosphorylation of FOXO1 was reduced by oleic acid which correlated with elevated gene expression of its downstream target PEPCK (Fig. 6B and C). In addition, phosphorylation of glycogen synthase kinase 3 β (GSK3β) at Ser9 by Akt, involved in the stimulation of glycogen synthase, was attenuated by OA. However, THC treatment restored these phosphorylation levels, suggesting that THC may be able to reverse insulin resistance in steatosis (Fig. 6B).
Fig. 6.
Effects of THC attenuated OA-induced insulin resistance in HepG2 cells. OA-stimulated HepG2 cells were treated with or without THC for 24 h. The phosphorylation states of (A) IRS1, PI3K and Akt, (B) FOXO1 and GSK3β were evaluated by Western Blot analysis. Values are the mean ± SE of three independent experiments. The bars with different letters are significantly different (P < 0.05). (C) The mRNA level of PEPCK was determined by semiquantitative RT-PCR. The values under each lane indicated relative density of the band normalized to β-actin.
4. Discussion
In the current study, we demonstrated for the first time that THC attenuated oleic acid-induced steatosis in HepG2 cells through several targets. First, THC significantly attenuated lipid accumulation in HepG2 cells stimulated with OA, indicating its potential therapeutic benefit against hepatic steatosis. Second, THC dramatically elevated lipolysis in HepG2 cells incubated with OA. Lastly, THC simultaneously increased glucose uptake and activated insulin signaling in OA-treated HepG2 cells.
Elevated FFAs in obese individuals is considered a critical pathogenic factor of metabolic diseases, including NAFLD and diabetes [2,3,34]. In the present study, an unsaturated fatty acid, oleic acid, was used to induce steatosis in HepG2 cells to investigate the therapeutic benefit of THC and characterize the underlying mechanism of action. We observed that although THC was cytotoxic to OA-treated HepG2 cells at the highest concentration (100 μM), it effectively reduced intra-cellular lipid accumulation. Our data suggested that this reduction in lipid accumulation may contribute to several mechanisms to combat hepatic steatosis, including the attenuation of lipogenesis and the promotion of lipolysis and β-oxidation.
THC attenuated lipid accumulation by decreasing the protein expression of SREBP-1, a transcription factor important in the regulation of lipogenic genes [35], and its downstream target, FAS, a key enzyme in de novo fatty acid synthesis [36]. PPARγ, a transcription factor central to lipid synthesis, was upregulated by OA, in addition to its target, FABP4. FABP4 is a member of the FABP family in adipocyte tissue, and studies have demonstrated that elevated hepatic PPARγ is associated with increased expression of FABP4 in hepatocytes [37,38]. Further, inactivation of ACC enzymes by direct phosphorylation at Ser79 is known to inhibit its enzymatic activity, thus inhibiting the conversion of acetyl-CoA to malonyl-CoA in fatty acid biosynthesis [39,40]. Two isoforms of ACC are present in the liver, ACC1 and ACC2, and each has a distinct metabolic function. ACC1 is cytoplasmic and is an important regulator of de novo hepatic lipogenesis, whereas ACC2 is localized in the mitochondria and is involved in fatty acid oxidation [41]. THC treatment induced ACC1 inactivation in OA-treated HepG2 cells, which may have subsequently decreased fatty acid synthesis. The molecular mechanism by which THC attenuated lipogenesis was likely through the activation of AMPK, a master regulator of cellular metabolism. AMPK regulates lipid metabolism by directly affecting enzyme activity via rapid phosphorylation and regulation of transcription factors responsible for lipid synthesis, such as SREBP-1 [5]. Li et al. showed that hepatic activation of AMPK reduced diet-induced steatosis through the suppression of SREBP-1c-dependent lipogenesis [42]. In the current study, THC treatment activated AMPK signaling. Moreover, our results demonstrated that in the presence of the AMPK inhibitor, compound C, THC failed to suppress lipid accumulation in OA-treated HepG2 cells, and the inhibition of AMPK further led to increased activation of ACC1, SREBP-1c, and FAS. These results highlighted the importance of AMPK in the therapeutic effect of THC on steatosis. In fact, previous studies reported that curcumin, the parent compound of THC, inhibited steatosis through the upregulation of AMPK [43,44]. Although additional research is needed, this is the first study that clearly demonstrated that THC activated AMPK signaling.
Another possible mechanism by which THC attenuated steatosis in OA-treated HepG2 cells was through the stimulation of lipolysis and β-oxidation. Our results showed that THC treatment increased levels of released glycerol in the culture medium of HepG2 cells, indicative of lipolysis. Moreover, THC treatment increased the protein levels of PPARα, a transcription factor vital to fatty acid oxidation, and its downstream target, CPT1 [45]. Although β-oxidation was not measured in the present study, we proposed that THC may have stimulated fatty acid oxidation, but further investigation is required to make a definitive conclusion. In addition, AMPK has been reported to positively regulate fatty acid oxidation by upregulating PPARα and CPT1 [46]. We demonstrated that THC induced AMPK activation; therefore, this may have contributed to the upregulation of PPARα in response to THC treatment. However, again, additional studies are needed to fully understand this mechanism. Taken together, these results demonstrated that THC exhibited a positive effect on lipolysis and fatty acid oxidation in steatosis, which may point to a potential therapeutic strategy in the treatment of obesity-induced hepatic steatosis.
An increase in FFA flux to the liver has been linked to hepatic insulin resistance, which is strongly associated with the development of NAFLD [47]. Insulin binds to and activates the insulin receptor, a tyrosine kinase receptor, which subsequently activates a complex cascade of downstream signaling pathways involved in the regulation of glucose metabolism, such as the IRS-1/PI3K/Akt signaling pathway [48]. FFAs and intracellular lipid metabolites cause aberrant glucose metabolism in the liver by blocking hepatic insulin, leading to defects in insulin signaling and, consequently, failure to stimulate glucose uptake, activate glycogen synthesis, and inhibit gluconeogenesis [49]. Here, we observed a decrease in glucose uptake and GLUT4 expression in OA-induced HepG2 cells, which was indicative of defective glucose metabolism [49]. After treatment with THC, glucose uptake was effectively restored and GLUT4 expression was increased. Moreover, OA-induced inhibition of IRS-1 and downregulation of PI3K/Akt signaling was reversed by THC treatment. Defective hepatic insulin signaling induced by elevated FFAs is associated with increased gluconeogenesis [50–52] and reduce glycogen synthesis [53]. Akt has been reported to normally contribute to hepatic insulin signaling by regulating hepatic glucose production. Akt directly phosphorylates FOXO1 and mediates the nuclear exclusion and downregulation of FOXO1, thereby decreasing the transcription of gluconeogenic genes, such as PEPCK [32]. In addition, Akt phosphorylation inactivates GSK3β, thus activating glycogen synthase and facilitating glycogen accumulation [54]. In our study, Akt signaling impaired by OA was associated with reduced phosphorylation of FOXO1 and elevated PEPCK gene expression in HepG2 cells. THC treatment reversed the inhibitory effects of OA on Akt signaling, phosphorylating FOXO-1 and repressing PEPCK expression. Moreover, we demonstrated that THC reversed the decreased phosphorylation of GSK3β in OA-treated stea-tosis, suggesting that THC may have activated glycogen syn-thase, but this was not further examined in the present study. Altogether, our data demonstrated that THC restored impaired insulin signaling in OA-treated HepG2 cells, which implicated its therapeutic benefit in improving glucose metabolism in steatosis.
In summary, our findings suggested that THC exhibited potential therapeutic effect against hepatic steatosis, which we proposed was likely through the mechanisms of the downregulation of lipogenesis, promotion of lipolysis, and activation of insulin signaling. In addition, we demonstrated that THC activated AMPK signaling, which could be essential to the mechanism underlying its anti-lipogenic effects. Although further investigation is required to validate the role and mechanism of THC against hepatic steatosis, our data were sufficient to implicate THC as an attractive candidate for the treatment of NAFLD.
Acknowledgements
This study was supported by the Ministry of Science and Technology (MOST 105-2320-B-022-001), Taiwan.
Funding Statement
This study was supported by the Ministry of Science and Technology (MOST 105-2320-B-022-001), Taiwan.
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1. Fabbrini E, Magkos F. Hepatic steatosis as a marker of metabolic dysfunction. Nutrients. 2015;7:4995–5019. doi: 10.3390/nu7064995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fabbrini E, Sullivan S, Klein S. Obesity and nonalcoholic fatty liver disease: biochemical, metabolic, and clinical implications. Hepatology. 2010;51:679–89. doi: 10.1002/hep.23280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Karpe F, Dickmann JR, Frayn KN. Fatty acids, obesity, and insulin resistance: time for a reevaluation. Diabetes. 2011;60:2441–9. doi: 10.2337/db11-0425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Utzschneider KM, Kahn SE. Review: the role of insulin resistance in nonalcoholic fatty liver disease. J Clin Endocrinol Metab. 2006;91:4753–61. doi: 10.1210/jc.2006-0587. [DOI] [PubMed] [Google Scholar]
- 5. Srivastava RA, Pinkosky SL, Filippov S, Hanselman JC, Cramer CT, Newton RS. AMP-activated protein kinase: an emerging drug target to regulate imbalances in lipid and carbohydrate metabolism to treat cardio-metabolic diseases. J Lipid Res. 2012;53:2490–514. doi: 10.1194/jlr.R025882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Koo SH, Flechner L, Qi L, Zhang X, Screaton RA, Jeffries S, et al. The CREB coactivator TORC2 is a key regulator of fasting glucose metabolism. Nature. 2005;437:1109–11. doi: 10.1038/nature03967. [DOI] [PubMed] [Google Scholar]
- 7. Woods A, Williams JR, Muckett PJ, Mayer FV, Liljevald M, Bohlooly Y, et al. Liver-specific activation of AMPK prevents steatosis on a high-fructose diet. Cell Rep. 2017;18:3043–51. doi: 10.1016/j.celrep.2017.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Henriksen BS, Curtis ME, Fillmore N, Cardon BR, Thomson DM, Hancock CR. The effects of chronic AMPK activation on hepatic triglyceride accumulation and glycerol 3-phosphate acyltransferase activity with high fat feeding. Diabetol Metab Syndr. 2013;5:29. doi: 10.1186/1758-5996-5-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Smith BK, Marcinko K, Desjardins EM, Lally JS, Ford RJ, Steinberg GR. Treatment of nonalcoholic fatty liver disease: role of AMPK. Am J Physiol Endocrinol Metab. 2016;311:E730–40. doi: 10.1152/ajpendo.00225.2016. [DOI] [PubMed] [Google Scholar]
- 10. Holder GM, Plummer JL, Ryan AJ. The metabolism and excretion of curcumin (1,7-bis-(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione) in the rat. Xenobiotica. 1978;8:761–8. doi: 10.3109/00498257809069589. [DOI] [PubMed] [Google Scholar]
- 11. Yang L, Zhou C, Huang K, Song L, Zheng Q, Yu R, et al. Antioxidative and cytotoxic properties of diarylheptanoids isolated from Zingiber officinale. Zhongguo Zhong Yao Za Zhi. 2009;34:319–23. [PubMed] [Google Scholar]
- 12. Han JS, Lee S, Kim HY, Lee CH. Ms-based metabolite profiling of aboveground and root components of zingiber mioga and officinale. Molecules. 2015;20:16170–85. doi: 10.3390/molecules200916170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wu JC, Tsai ML, Lai CS, Wang YJ, Ho CT, Pan MH. Chemopreventative effects of tetrahydrocurcumin on human diseases. Food Funct. 2014;5:12–7. doi: 10.1039/c3fo60370a. [DOI] [PubMed] [Google Scholar]
- 14. Aggarwal BB, Deb L, Prasad S. Curcumin differs from tetrahydrocurcumin for molecular targets, signaling pathways and cellular responses. Molecules. 2014;20:185–205. doi: 10.3390/molecules20010185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Naito M, Wu X, Nomura H, Kodama M, Kato Y, Kato Y, et al. The protective effects of tetrahydrocurcumin on oxidative stress in cholesterol-fed rabbits. J Atheroscler Thromb. 2002;9:243–50. doi: 10.5551/jat.9.243. [DOI] [PubMed] [Google Scholar]
- 16. Nakmareong S, Kukongviriyapan U, Pakdeechote P, Kukongviriyapan V, Kongyingyoes B, Donpunha W, et al. Tetrahydrocurcumin alleviates hypertension, aortic stiffening and oxidative stress in rats with nitric oxide deficiency. Hypertens Res. 2012;35:418–25. doi: 10.1038/hr.2011.180. [DOI] [PubMed] [Google Scholar]
- 17. Sangartit W, Kukongviriyapan U, Donpunha W, Pakdeechote P, Kukongviriyapan V, Surawattanawan P, et al. Tetrahydrocurcumin protects against cadmium-induced hypertension, raised arterial stiffness and vascular remodeling in mice. PLoS One. 2014;9:e114908. doi: 10.1371/journal.pone.0114908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Murugan P, Pari L. Effect of tetrahydrocurcumin on lipid peroxidation and lipids in streptozotocin-nicotinamide-induced diabetic rats. Basic Clin Pharmacol Toxicol. 2006;99:122–7. doi: 10.1111/j.1742-7843.2006.pto_447.x. [DOI] [PubMed] [Google Scholar]
- 19. Pari L, Murugan P. Antihyperlipidemic effect of curcumin and tetrahydrocurcumin in experimental type 2 diabetic rats. Ren Fail. 2007;29:881–9. doi: 10.1080/08860220701540326. [DOI] [PubMed] [Google Scholar]
- 20. Gao Y, Li J, Wu L, Zhou C, Wang Q, Li X, et al. Tetrahydrocurcumin provides neuroprotection in rats after traumatic brain injury: autophagy and the PI3K/AKT pathways as a potential mechanism. J Surg Res. 2016;206:67–76. doi: 10.1016/j.jss.2016.07.014. [DOI] [PubMed] [Google Scholar]
- 21. Gao Y, Zhuang Z, Gao S, Li X, Zhang Z, Ye Z, et al. Tetrahydrocurcumin reduces oxidative stress-induced apoptosis via the mitochondrial apoptotic pathway by modulating autophagy in rats after traumatic brain injury. Am J Transl Res. 2017;9:887–99. [PMC free article] [PubMed] [Google Scholar]
- 22. Pfeiffer E, Hoehle SI, Walch SG, Riess A, Solyom AM, Metzler M. Curcuminoids form reactive glucuronides in vitro. J Agric Food Chem. 2007;55:538–44. doi: 10.1021/jf0623283. [DOI] [PubMed] [Google Scholar]
- 23. Vijaya Saradhi UV, Ling Y, Wang J, Chiu M, Schwartz EB, Fuchs JR, et al. A liquid chromatography-tandem mass spectrometric method for quantification of curcuminoids in cell medium and mouse plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878:3045–51. doi: 10.1016/j.jchromb.2010.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Anand P, Thomas SG, Kunnumakkara AB, Sundaram C, Harikumar KB, Sung B, et al. Biological activities of curcumin and its analogues (Congeners) made by man and Mother Nature. Biochem Pharmacol. 2008;76:1590–611. doi: 10.1016/j.bcp.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 25. Pari L, Amali DR. Protective role of tetrahydrocurcumin (THC) an active principle of turmeric on chloroquine induced hepatotoxicity in rats. J Pharm Pharm Sci. 2005;8:115–23. [PubMed] [Google Scholar]
- 26. Muthumani M. Tetrahydrocurcumin potentially attenuates arsenic induced oxidative hepatic dysfunction in rats. J Clinic Toxicol. 2013;3:168. doi: 10.4172/2161-0495.1000168. [DOI] [Google Scholar]
- 27. Murugan P, Pari L. Influence of tetrahydrocurcumin on hepatic and renal functional markers and protein levels in experimental type 2 diabetic rats. Basic Clin Pharmacol Toxicol. 2007;101:241–5. doi: 10.1111/j.1742-7843.2007.00109.x. [DOI] [PubMed] [Google Scholar]
- 28. Pari L, Murugan P. Effect of tetrahydrocurcumin on blood glucose, plasma insulin and hepatic key enzymes in streptozotocin induced diabetic rats. J Basic Clin Physiol Pharmacol. 2005;16:257–74. doi: 10.1515/jbcpp.2005.16.4.257. [DOI] [PubMed] [Google Scholar]
- 29. Lai CS, Liao SN, Tsai ML, Kalyanam N, Majeed M, Majeed A, et al. Calebin-A inhibits adipogenesis and hepatic steatosis in high-fat diet-induced obesity via activation of AMPK signaling. Mol Nutr Food Res. 2015;59:1883–95. doi: 10.1002/mnfr.201400809. [DOI] [PubMed] [Google Scholar]
- 30. Brandt JM, Djouadi F, Kelly DP. Fatty acids activate transcription of the muscle carnitine palmitoyltransferase I gene in cardiac myocytes via the peroxisome proliferator-activated receptor alpha. J Biol Chem. 1998;273:23786–92. doi: 10.1074/jbc.273.37.23786. [DOI] [PubMed] [Google Scholar]
- 31. Svedberg J, Bjorntorp P, Smith U, Lonnroth P. Free-fatty acid inhibition of insulin binding, degradation, and action in isolated rat hepatocytes. Diabetes. 1990;39:570–4. doi: 10.2337/diab.39.5.570. [DOI] [PubMed] [Google Scholar]
- 32. Zhang W, Patil S, Chauhan B, Guo S, Powell DR, Le J, et al. FoxO1 regulates multiple metabolic pathways in the liver: effects on gluconeogenic, glycolytic, and lipogenic gene expression. J Biol Chem. 2006;281:10105–17. doi: 10.1074/jbc.M600272200. [DOI] [PubMed] [Google Scholar]
- 33. Guo S, Rena G, Cichy S, He X, Cohen P, Unterman T. Phosphorylation of serine 256 by protein kinase B disrupts transactivation by FKHR and mediates effects of insulin on insulin-like growth factor-binding protein-1 promoter activity through a conserved insulin response sequence. J Biol Chem. 1999;274:17184–92. doi: 10.1074/jbc.274.24.17184. [DOI] [PubMed] [Google Scholar]
- 34. Zhang J, Zhao Y, Xu C, Hong Y, Lu H, Wu J, et al. Association between serum free fatty acid levels and nonalcoholic fatty liver disease: a cross-sectional study. Sci Rep. 2014;4:5832. doi: 10.1038/srep05832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yang J, Goldstein JL, Hammer RE, Moon YA, Brown MS, Horton JD. Decreased lipid synthesis in livers of mice with disrupted Site-1 protease gene. Proc Natl Acad Sci U S A. 2001;98:13607–12. doi: 10.1073/pnas.201524598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest. 2002;109:1125–31. doi: 10.1172/JCI15593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gao M, Ma Y, Alsaggar M, Liu D. Dual outcomes of rosiglitazone treatment on fatty liver. AAPS J. 2016;18:1023–31. doi: 10.1208/s12248-016-9919-9. [DOI] [PubMed] [Google Scholar]
- 38. Schadinger SE, Bucher NL, Schreiber BM, Farmer SR. PPARgamma2 regulates lipogenesis and lipid accumulation in steatotic hepatocytes. Am J Physiol Endocrinol Metab. 2005;288:E1195–205. doi: 10.1152/ajpendo.00513.2004. [DOI] [PubMed] [Google Scholar]
- 39. Merrill GF, Kurth EJ, Hardie DG, Winder WW. AICA riboside increases AMP-activated protein kinase, fatty acid oxidation, and glucose uptake in rat muscle. Am J Physiol. 1997;273:E1107–12. doi: 10.1152/ajpendo.1997.273.6.E1107. [DOI] [PubMed] [Google Scholar]
- 40. Ha J, Daniel S, Broyles SS, Kim KH. Critical phosphorylation sites for acetyl-CoA carboxylase activity. J Biol Chem. 1994;269:22162–8. [PubMed] [Google Scholar]
- 41. Bianchi A, Evans JL, Iverson AJ, Nordlund AC, Watts TD, Witters LA. Identification of an isozymic form of acetyl-CoA carboxylase. J Biol Chem. 1990;265:1502–9. [PubMed] [Google Scholar]
- 42. Li Y, Xu S, Mihaylova MM, Zheng B, Hou X, Jiang B, et al. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab. 2011;13:376–88. doi: 10.1016/j.cmet.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kang OH, Kim SB, Seo YS, Joung DK, Mun SH, Choi JG, et al. Curcumin decreases oleic acid-induced lipid accumulation via AMPK phosphorylation in hepatocarcinoma cells. Eur Rev Med Pharmacol Sci. 2013;17:2578–86. [PubMed] [Google Scholar]
- 44. Um MY, Hwang KH, Ahn J, Ha TY. Curcumin attenuates diet-induced hepatic steatosis by activating AMP-activated protein kinase. Basic Clin Pharmacol Toxicol. 2013;113:152–7. doi: 10.1111/bcpt.12076. [DOI] [PubMed] [Google Scholar]
- 45. Burri L, Thoresen GH, Berge RK. The role of PPARalpha activation in liver and muscle. PPAR Res. 20102010 doi: 10.1155/2010/542359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lee WJ, Kim M, Park HS, Kim HS, Jeon MJ, Oh KS, et al. AMPK activation increases fatty acid oxidation in skeletal muscle by activating PPARalpha and PGC-1. Biochem Biophys Res Commun. 2006;340:291–5. doi: 10.1016/j.bbrc.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 47. Perry RJ, Samuel VT, Petersen KF, Shulman GI. The role of hepatic lipids in hepatic insulin resistance and type 2 diabetes. Nature. 2014;510:84–91. doi: 10.1038/nature13478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Boucher J, Kleinridders A, Kahn CR. Insulin receptor signaling in normal and insulin-resistant states. Cold Spring Harb Perspect Biol. 2014;6 doi: 10.1101/cshperspect.a009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ragheb R, Medhat AM. Mechanisms of fatty acid-Induced insulin resistance in muscle and liver. J Diabetes Metab. 2011;2:127. doi: 10.4172/2155-6156.1000127. [DOI] [Google Scholar]
- 50. Boden G. Role of fatty acids in the pathogenesis of insulin resistance and NIDDM. Diabetes. 1997;46:3–10. [PubMed] [Google Scholar]
- 51. Chen X, Iqbal N, Boden G. The effects of free fatty acids on gluconeogenesis and glycogenolysis in normal subjects. J Clin Invest. 1999;103:365–72. doi: 10.1172/JCI5479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Williamson JR, Kreisberg RA, Felts PW. Mechanism for the stimulation of gluconeogenesis by fatty acids in perfused rat liver. Proc Natl Acad Sci U S A. 1966;56:247–54. doi: 10.1073/pnas.56.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lam TK, Carpentier A, Lewis GF, van de WG, Fantus IG, Giacca A. Mechanisms of the free fatty acid-induced increase in hepatic glucose production. Am J Physiol Endocrinol Metab. 2003;284:E863–73. doi: 10.1152/ajpendo.00033.2003. [DOI] [PubMed] [Google Scholar]
- 54. Fang X, Yu SX, Lu Y, Bast RC, Jr, Woodgett JR, Mills GB. Phosphorylation and inactivation of glycogen synthase kinase 3 by protein kinase A. Proc Natl Acad Sci U S A. 2000;97:11960–5. doi: 10.1073/pnas.220413597. [DOI] [PMC free article] [PubMed] [Google Scholar]