Abstract
This paper presents an application of ultra high-performance liquid-chromatography-quadrupole-TOF high resolution mass spectrometry (UHPLC-Q-TOF HRMS) for simultaneous analysis of 23 illegal adulterated aphrodisiac type chemical ingredients in health foods and Chinese Traditional Patent Medicines (CTPMs). The mass spectrometer was operated in Information Dependent Acquisition (IDA) mode, which provides crucial information for the elemental composition analysis, structure elucidation and quantitative analysis simultaneously. Quantitative analysis was performed using the peak areas of the precursor ions in the XICs. The method validation included assessment of selectivity, sensitivity, calibration curve, accuracy, precision, recovery, matrix effect and stability. The results show good linear relationship with the concentrations of the analytes over wide concentration ranges (e.g., 0.05–10 μg/g for sildenafil) as all the fitting coefficients of determination r2 are >0.9984. The detection limits (LODs) were in the range of 0.002–0.1 μg/g. The recoveries were able to reach 82.5–103.6%, while the matrix effects ranged from 87.7 to 109.3%. The intra- and inter-day accuracies were in the range of 82.3–113.8%, while the intra- and inter-day precision ranged from 0.4 to 13.6%. Among 40 batches of health foods and 32 batches of CTPMs (including 28 capsules, 32 tablets, 10 liquid and 2 pills) samples, 28 batches of heath foods were positive. The detected chemical ingredients involved sildenafil, tadalafil, aildenafil and sulfoaildenafil. This method can be used for the screening, identification and quantification of illegal adulterated aphrodisiac chemical ingredients in health foods and CTPMs. Moreover, the LC-Q-TOF MS is very useful to structural elucidation of unknown compound.
Keywords: Q-TOF MS, Illegal adulteration, Aphrodisiac chemical ingredients, Qualitation, Quantitation
1. Introduction
In recent years, health foods and Chinese Traditional Patent Medicines (CTPMs) have been booming because of being believed as safer and healthier than synthetic drugs and free of side effects [1]. However, for some unscrupulous manufacturers, the deliberate addition of chemical ingredients into health foods and CTPMs is a profit-driven practice aiming to intensify the claimed natural health benefits of the products [2]. Over the past few years, many types of illegal adulterants are being detected in various forms of health foods and CTPMs without labeling, which can lead to potentially serious public health consequences [3–11].
Erectile dysfunction (ED) is a highly prevalent inability to achieve and maintain adequate erection and sexual performance. Synthetic phosphodiesterase type 5 enzyme (PDE-5) inhibitors (e.g. sildenafil, tadalafil, vardenafil) are a class of drugs clinically used for the treatment of ED. In addition, other drugs which mechanism of action is different from PDE-5 can also be used in clinic. These drugs include yohimbine (α-2 receptor antagonist), apomorphine (dopamine receptor agonist), phentolamine (α adrenergic receptor blocker), dapoxetine (selective serotonin reuptake inhibitor), testosterone (androgen), etc. However, clinically adverse side effects, such as headaches, gastrointestinal distress, tachycardia, facial flushing, hypertension, nasalcongestion, visual disorders, muscle aches, dizziness, and possibility of blindness and hearing loss have been reported [12–17]. Moreover, PDE-5 inhibitors may also cause potentially serious drug–drug interactions [18]. The patients taking nitrate medications should not use PDE-5 inhibitor, as this combination may result in severe hypotension and syncope [18,19]. Fatal cases caused by adulterated dietary supplements have been reported [20,21].
Therefore, it is dangerous for unknowing patients to be taking such health foods or CTPMs adulterated with PDE-5 inhibitors and other aphrodisiac chemical ingredients. Over the past few years, the approved PDE-5 inhibitors and their unapproved synthetic analogs have been routinely identified in “all-natural” health foods and CTPMs which claim to enhance sexual performance [22–31].
To escape regulatory detection and quality checking, the manufacturers of such illicit and adulterated sexual performance enhancement products are now using new analogs and other aphrodisiac drugs which are difficult to be detected by routine screening and inspection methods. A number of analytical methods have been developed for screening and confirmation of PDE-5 inhibitors in illicit sexual performance enhancer products, such as immunoassay [32], ion mobility spectrometry [33], thin-layer chromatography [34], high performance liquid chromatography (HPLC) with ultraviolet or fluorescence detection [35–38], gas chromatography–mass spectrometry (GC–MS) [39–42], liquid chromatography–mass spectrometry (LC–MS) [43–49]. Several literature have reported the detection of chemical substances in food or dietary supplements using high-resolution mass spectrometry (HRMS) with quadrupole-Orbitrap (Q-Orbitrap), atmospheric solids analysis probe (ASAP) or Fourier transform ion cyclotron resonance (FTICR) mass analyzers [8,50–54].
Time-of-flight (TOF) is one of the importance remembers of high resolution mass spectrometers analyzer. The development of TOF technology in high resolution mass spectrometers has enabled mass spectrometers to provide accurate mass up to 4–6 decimal places. This is importance for us to deduce elemental composition and the molecular formula of a compound [7,55]. In the previous reports, the methods based HRMS analyzers with TOF (such as LC-MS/TOF, LC-IT/TOF, LC-Q/TOF) have been adopted for screening, identification of PDE-5 inhibitors and deducing its fragmentation pathways [22,56–59].
However, these methods focus on PDE-5 inhibitors and its analogs. Since profit purpose and lack of test method, the possibility of other types of impotence drugs as adulterant in health foods and CTPMs is greatly increasing. The aim of the present study was to develop a rapid and effective multi-analyte method coupling ultrahigh-performance liquid chromatography (UHPLC) to Q-TOF HRMS for the detection of 23 illegal adulterated aphrodisiac chemical ingredients. This method was successfully applied to the screening, identification and quantification of 23 illegal adulterated aphrodisiac chemical ingredients. To the best of our knowledge, this is the first time to report the application of UPLC-Q-TOF/MS in screening of various types illegal adulterated synthetic chemicals in health foods and CTPMs.
2. Materials and methods
2.1. Chemicals and reagents
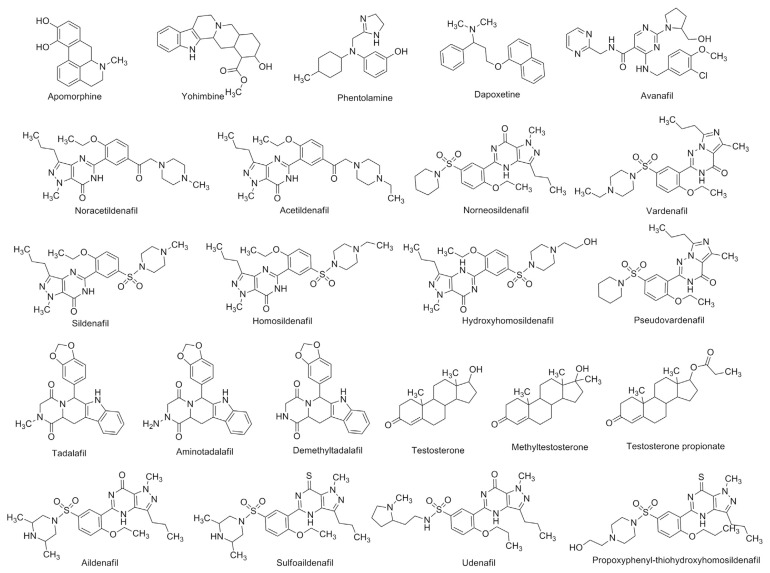
Noracetildenafil (99.8%), acetildenafil (99.7%), vardenafil hydrochloride (99.2%), hydroxyhomosildenafil (99.8%), sildenafil (99.6%), homosildenafil (99.2%), aminotadalafil (99.9%), tadalafil (99.5%), pseudovardenafil (99.5%) and norneosildenafil (99.9%) were purchased from TLC Pharmaceutical Standards Ltd. (Aurora, Canada). Sulfoaildenafil (98.0%), apomorphine hydrochloride hemihydrates (98.0%), yohimbine hydrochloride (98.0%), aildenafil (98.5%), avanafil (99.0%), N-desme-thyltadalafil (98.5%), udenafil (96.1%), propoxyphenyl-thiohydroxyhomosildenafil (96.0%), dapoxetine hydrochloride (98.0%) and phentolamine methanesulfonate salt (98.3%) were purchased from Toronto Research Chemicals Inc. (Toronto, Canada). Testosterone (99.5%) was purchased from Acros Organics Inc. (Geel, Belgium). Methyltestosterone (99.5%) and testosterone propionate (99.8%) were purchased from National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). Their chemical structures are displayed in Fig. 1. Chinese traditional patent medicines and health foods which claimed functions of aphrodisiac, enhancement of sexual performance, physical fatigue relief or immunity enhancement were bought from the local drug shops or markets. The samples without the studied 23 illegal adulterants were used as blank matrices. LC-MS grade acetonitrile, acetic acid and ammonium acetate were purchased from Thermo Fisher Scientific Inc. (MA, USA). Ultrapure water (18.2 M) was obtained from a Milli-Q Advantage A10 ultrapure water purification system.
Fig. 1.
Chemical structures of studied 23 analytes.
2.2. Instrumentation
The UHPLC-Q-TOF-MS/MS system consisted of two ExionLC AD pumps and an ExionLC AD autosampler coupled with a high resolution X500 Q-TOF mass spectrometer (Sciex, USA). The SCIEX OS 1.0 software from Sciex (Sciex, USA) contains instrument control, data acquisition, data processing, and reporting functionality, all in the one package. Chromatographic separation was achieved on a Agilent SB-C18 RRHD column (100 mm × 3.0 mm, 1.8 μm) (Agilent Technologies, USA). All centrifugation were performed on a Sigma 3–30 K refrigerated centrifuge (Sigma, Germany). Ultrasonic process was operated on a KQ-300 GDV Thermostat Ultrasonic Instrument (Kunshan, China).
2.3. Standard solutions
All individual standard stock solutions were prepared in acetonitrile at 1mg/mL and stored at −20 °C. An intermediate standard mixture of the reference compounds was prepared by appropriate dilution of the individual stock solutions in acetonitrile. Matrix-matched working solutions were freshly prepared in blank sample extracts, which were extracted from the commercial products purchased from the local market. All of the stock and working solutions were stored at −20 °C in the dark when not in use.
2.4. Sample preparation
Chinese traditional patent medicines and health foods in this study were presented in four different oral forms including pellets, capsules, tablets or oral liquid. The tablets and pellets were smashed into fine powder; the capsules were cut open and the contents were mixed and then thoroughly homogenized. For oral liquids, three samples were evenly mixed and divided into fractions. Then a single oral dose of 0.2 g solid samples (for tablets, pills and capsules) or 2 mL oral liquid was accurately transferred to a 15 mL centrifuge tube and extracted with 10 mL ACN–H2O (8:2, v/v), followed by vortex for 1 min, sonication for 15 min, and centrifugation at 5000 rpm for 15 min, successively. The upper phase was immediately withdrawn and filtered through a 0.22 μm pore PTFE syringe filter. The subsequent filtrate was used for the UHPLC–MS analysis. Blank matrices samples were treated as samples described above. When the sample concentration was beyond the linear range, the sample solution was diluted to make the detection response within the linear ranges.
2.5. Chromatographic conditions
Chromatographic separation was performed on a SB-C18 RRHD column of Agilent (100 mm × 3.0 mm, 1.8 μm). A binary mobile solvent was used:mobile solvent A was 5 mmol/L ammonium acetate solution (adjusted pH to 3.4 with acetic acid), and mobile solvent B was acetonitrile. The mobile phase was delivered at a flow rate of 0.4 mL/min with a gradient elution profile. The gradient began at 25% B for 2min, and then linearly ramped to 55% B within 11min, then ramped to 90% B in 1 min and held at 90% B for 2.0 min, then the column was re-equilibrated at 25% B for 2min prior to the next injection. The autosampler tray temperature was set to 15 °C, while the column temperature was 40 °C. The injection volume was 5 μL.
2.6. Mass spectrometry conditions
The Q-TOF HRMS was equipped with a Turbo V™ ion source and the ESI+ mode was applied. The spray voltage and ion source temperature were set to 5.5 kV and 600 °C, respectively. The ion source gas 1, ion source gas 2, curtain gas and CAD gas were set to 55, 60, 35 and 7 psi, respectively. The analysis was executed in information dependent acquisition (IDA) mode. Under IDA mode, a TOF MS scan was performed firstly to generate “information” and a TOF MS/MS scan was then occurred based on predefined IDA criteria using information obtained in the TOF MS scan. The MS/MS spectra was generated in product ion scan mode at collision energies (CE) of 60 V with CE spread of 40 V.
2.7. Method validation
Quantitative analysis was performed using the peak areas of the precursor ions in the XICs. Method validation for assaying 23 aphrodisiac chemical drugs in traditional Chinese preparation and health food was done referring to the US Food and Drug Administration (FDA) guidelines on Bioanalytical Method Validation. The validation parameters included selectivity, sensitivity, calibration curve, accuracy and precision, recovery, matrix effect and stability.
2.7.1. Selectivity
The selectivity is the ability of an analytical method to differentiate and quantify the analyte in the presence of other components in the matrix. The selectivity of the method was evaluated by determining the level of interfering components in six individual sources of blank matrix.
2.7.2. Calibration curve
Calibration curve was constructed by plotting the analyte peak area (Y-axis) vs a series of analyte concentrations (X-axis). The regression equations were described as Y = a + bX, which was used to calculate the concentrations of QCs and samples. Linearity was assessed by the coefficient of determination (r2).
The limit of detection (LOD) was determined as the lowest concentration giving a signal-to-noise ratio of at least threefold (S/N ≥ 3). The lower limit of quantitation (LLOQ) was defined as the lowest concentration of the calibration curve, giving a signal-to-noise ratio of at least 10-fold (S/N ≥ 10), acceptable accuracy (80–120%) and sufficient precision (within 20%).
2.7.3. Accuracy and precision
The accuracy was calculated from the ratio of the mean values of the detected concentration (Cdet) and the nominal concentration (Cnom) as following: (Cdet/Cnom) × 100. The precision was expressed by relative standard deviation (RSD), which was calculated as RSD% = [standard deviation (SD)/Cdet] × 100.
2.7.4. Recovery
The recoveries of 23 aphrodisiac chemical drugs from matrices were investigated by comparing the response of 23 aphrodisiac chemical drugs after extraction from matrices with the response of the same concentration analytes spiked into the solution extracted from blank matrices.
2.7.5. Matrix effect
The matrix effect was evaluated by analyzing the response of analytes prepared in solvent and in extracted blank matrix at the same concentrations for three levels (low, medium and high). The value of matrix effect can be calculated as (Eq. (1)):
| (1) |
A refers to the peak areas obtained from neat solution standards, while B refers to the corresponding peak areas of standards spiked after extraction from matrix [60,61].
2.7.6. Stability
The post-preparative stability was conducted by repeatedly determining the processed QC samples which were kept in the autosampler (15 °C) for 24 h. The concentrations of the samples were calculated on the basis of original calibration standards.
2.8. Calculation
The chromatograms were processed using SCIEX OS 1.0 software developed by SCIEX. All calculations were completed in Microsoft Excel 2013 (Microsoft Co., Redmond, USA). The structures of chemicals were drawn in Chem & Bio Draw Ultra 12.0 (PerkinElmer, Inc., MA, USA).
2.9. Library-based qualitative screening and confirmation
The compound database could be imported and named by the Library Importer. Then, the exact mass library was generated in which the MS/MS spectra of each target parent ion were included.
Qualitative analysis is the identification of a target or untarget compound. In qualitative analysis, a sample can be processed with the searching library for screening out suspected positive samples and further reliable confirmation. Comparing acquired MS/MS spectra from unknown samples to a database of compounds with reference spectra is one of the most powerful tools in qualitative analysis. Library search algorithms compare the unknown spectra from the sample and then try to match the spectra to the known compounds and spectra in the database. The closer the match and the higher the reported score are, the more likely it is that the compound was identified.
In mass spectrometry, determining which compound is present is accomplished using mass accuracy, retention time, isotope pattern, library searching, and formula finding. Using all of these tools together can increase the confidence in identifying both targeted and non-targeted compounds in unknown samples. In general, the confidence levels for the qualitative rules were configured as: mass error less than 2 ppm, error in retention time less than 0.5 min, difference isotope ratio less than 5%, library hit score more than 80. If the confidence levels above were reached, the traffic light was green. Their weights are 30%, 30%, 10%, 30%, respectively. The combined score was obtained by synthesizing above four factors. The higher the score, the greater the possibility of positive was. The library search results, formula finding results, and other qualitative analysis results are available in the Results Table. Results Tables also include the calibration curves, the calculated concentration of each analyte, as well as statistics for the results.
3. Results and discussion
3.1. UHPLC-HRMS optimization
The peak shape and the chromatographic resolution were the main criteria of evaluation during optimization of the UHPLC method. Chromatographic conditions, such as the composition of mobile phase and the gradient condition, played a critical role in achieving good chromatographic behavior and appropriate ionization [8]. Different solvent compositions were investigated for the mobile phase consisting of methanol or acetonitrile as organic phase and water with formic acid, acetic acid, ammonium formate, ammonium acetate as aqueous phase. The pH of the mobile phase was optimized because most analytes contained one or more basic groups. Accordingly, water (containing 5 mM ammonium acetate and adjusted pH 3.4 with acetic acid)–acetonitrile was applied as the binary mobile solvents system. The gradient elution procedure was optimized and described in Section 2.5. Satisfactory results were obtained using the binary mobile solvents system and the gradient elution procedure described above, giving better peak shapes and excellent chromatographic separation of the 23 analytes within 18 min. Furthermore, no carryover could be detected.
Before the formal experiment started, a positive quick status check was performed to quickly verify the mass accuracy and resolution in TOF MS and MS/MS modes. If the mass accuracy did not meet the specification, the steps above could be repeated. If the resolution did not meet the specification, the TOF Tuning procedure could be performed to optimize the system. Thus, the satisfactory results were obtained with the mass errorless than 2 ppm and the resolution greater than 30,000.
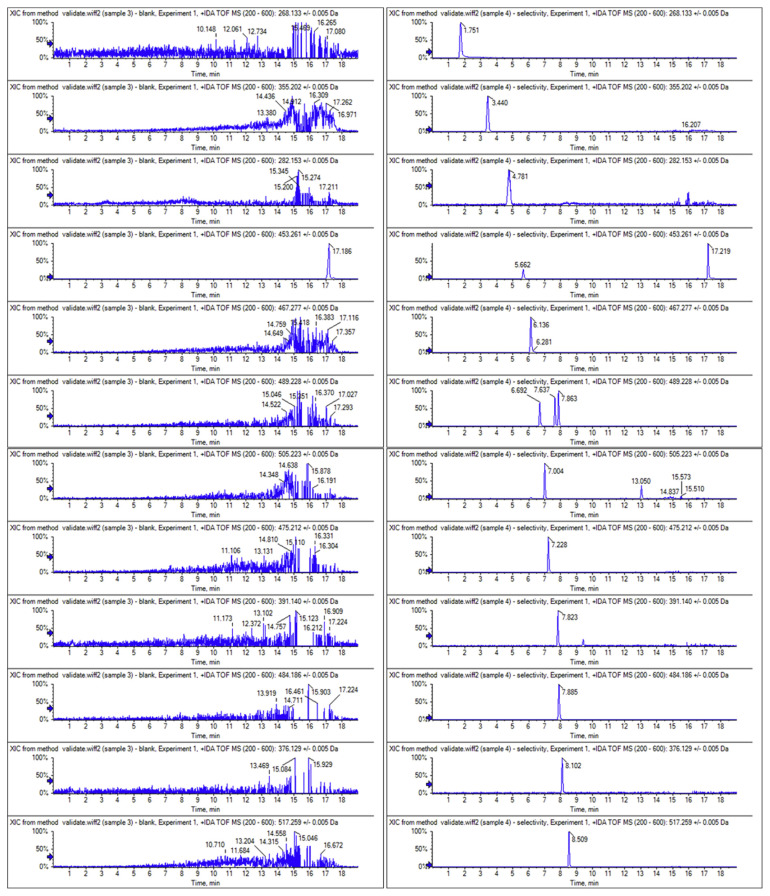
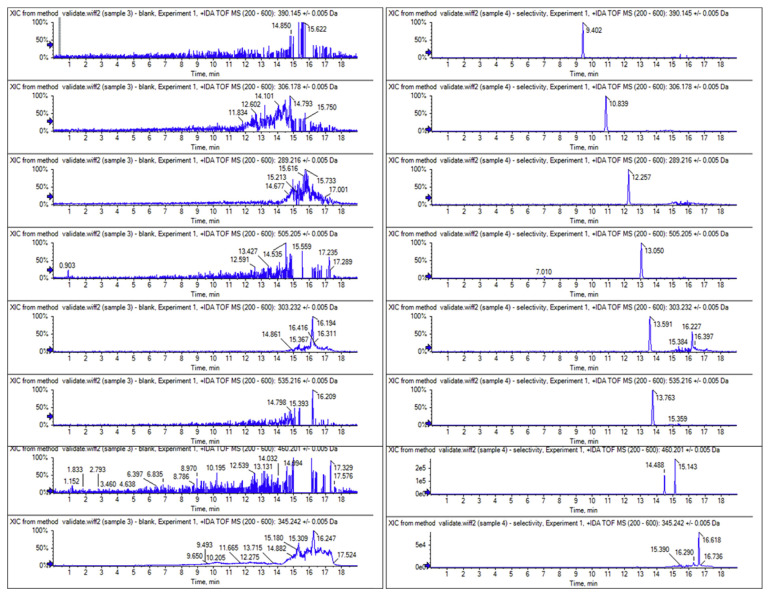
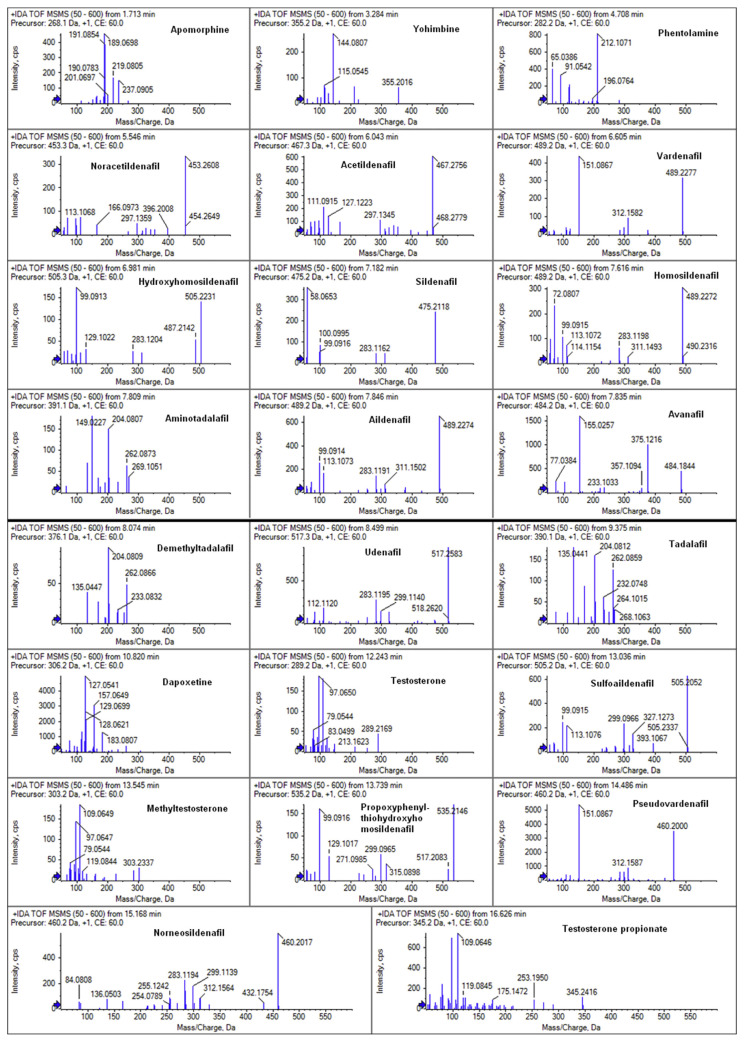
Under the optimized chromatographic conditions, acceptable chromatographic separation of 23 chemicals was achieved expect for aildenafil (7.87 min,m/z 489.2278) and avanafil (7.89 min, m/z 484.1858) (Fig. 2B). But this couple can be identified because of their m/z distinction. Furthermore, it can be noted that the baseline separation can be reached for two groups of isomers, as was the case of vardenafil, aildenafil and homosildenafil (m/z489.2278); and pseudovardenafil and norneosildenafil (m/z 460.2013). Therefore, all 23 compounds did not interfere with each other for qualitation and quantitation using the optimized methods in this assay. Therefore, the combination of RT, mass accuracy and MS2 fragment ions (Fig. 3) provide a suitable detection of the compounds. As showed in Table 1, all the mass errors (ppm) between theoretical and experimental m/z of analytes were below 2, indicating the highly reliable mass accuracy of Q-TOF.
Fig. 2.
Extracted ion chromatograms of 23 analytes in (A) blank matrices and (B) matrices spiked with 0.1 μg/g 23 analytes standards.
Fig. 3.
MS2 fragment ions of 23 analytes.
Table 1.
Retention time, accurate mass, correlation coefficients, linear ranges, LODs and LLOQs of 23 analytes.
| Analyte | Retention time (min) | Protonated ion mass [M + H]+ | Mass error (ppm) | Correlation coefficient (r2) | Linear range (μg/g) | LOD (μg/g) | LLOQ (μg/g) | |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Theoretical | Experimental | |||||||
| Apomorphine | 1.72 | 268.1332 | 268.1331 | −0.4 | 0.9995 | 0.15–10 | 0.05 | 0.15 |
| Yohimbine | 3.42 | 355.2016 | 355.2013 | −0.8 | 0.9994 | 0.15–10 | 0.05 | 0.15 |
| Phentolamine | 4.78 | 282.1601 | 282.1603 | 0.7 | 0.9991 | 0.05–8 | 0.02 | 0.05 |
| Noracetildenafil | 5.65 | 453.2609 | 453.2608 | −0.2 | 0.9991 | 0.05–10 | 0.02 | 0.05 |
| Acetildenafil | 6.12 | 467.2765 | 467.2761 | −0.9 | 0.9986 | 0.05–10 | 0.02 | 0.05 |
| Vardenafil | 6.70 | 489.2279 | 489.2274 | −1.0 | 0.9998 | 0.05–10 | 0.02 | 0.05 |
| Hydroxyhomosildenafil | 7.00 | 505.2228 | 505.2227 | −0.2 | 1.0000 | 0.05–10 | 0.02 | 0.05 |
| Sildenafil | 7.22 | 475.2122 | 475.2118 | −0.8 | 1.0000 | 0.05–10 | 0.02 | 0.05 |
| Homosildenafil | 7.63 | 489.2279 | 489.2274 | −1.0 | 1.0000 | 0.05–10 | 0.02 | 0.05 |
| Aminotadalafil | 7.83 | 391.1401 | 391.1403 | 0.5 | 0.9996 | 0.25–10 | 0.10 | 0.25 |
| Aildenafil | 7.87 | 489.2278 | 489.2274 | −0.8 | 1.0000 | 0.05–10 | 0.02 | 0.05 |
| Avanafil | 7.89 | 484.1858 | 484.1855 | −0.6 | 0.9993 | 0.05–10 | 0.02 | 0.05 |
| Demethyltadalafil | 8.10 | 376.1298 | 376.1294 | −1.1 | 0.9996 | 0.25–10 | 0.10 | 0.25 |
| Udenafil | 8.52 | 517.2592 | 517.2587 | −1.0 | 0.9992 | 0.05–10 | 0.02 | 0.05 |
| Tadalafil | 9.41 | 390.1448 | 390.145 | 0.5 | 0.9996 | 0.15–10 | 0.05 | 0.15 |
| Dapoxetine | 10.84 | 306.1852 | 306.1849 | −1.0 | 0.9984 | 0.005–2.5 | 0.002 | 0.005 |
| Testosterone | 12.25 | 289.2162 | 289.2161 | −0.3 | 0.9997 | 0.15–10 | 0.05 | 0.15 |
| Sulfoaildenafil | 13.05 | 505.2050 | 505.2048 | −0.4 | 0.9994 | 0.05–10 | 0.02 | 0.05 |
| Methyltestosterone | 13.58 | 303.2319 | 303.2321 | 0.7 | 0.9991 | 0.15–10 | 0.05 | 0.15 |
| Propoxyphenyl-thiohydroxyhomosildenafil | 13.76 | 535.2156 | 535.2152 | −0.7 | 0.9995 | 0.15–10 | 0.05 | 0.15 |
| Pseudovardenafil | 14.48 | 460.2013 | 460.2009 | −0.9 | 0.9991 | 0.05–10 | 0.02 | 0.05 |
| Norneosildenafil | 15.14 | 460.2013 | 460.2007 | −1.3 | 0.9994 | 0.05–10 | 0.02 | 0.05 |
| Testosterone propionate | 16.28 | 345.2424 | 345.2423 | −0.3 | 0.9998 | 0.25–10 | 0.10 | 0.25 |
3.2. Method validation
3.2.1. Selectivity
Representative XICs of 23 aphrodisiac chemicals in blank matrices and matrices spiked with 23 analytes were shown in Fig. 2A and B, respectively. The Q-TOF HRMS provided sufficient resolving power to distinguish analytes from the isobaric co-eluting sample matrix compounds. No interfering peaks were detected at the RT of all analytes in the matrix.
3.2.2. Calibration curve
The linear relationship between the chromatographic peak area and the concentration of the analytes was investigated and exhibited in Table 1. The linearity of calibration curve was assessed by the coefficient of determination (r2). As can be seen in Table 1, the calibration curves showed good linearity with r2 values higher than 0.9984 for all 23 analytes.
The LODs and LLOQs were determined as the analyte concentrations giving peak heights at least 3 and 10 times higher than the baseline noise, respectively. All the LOD and LLOQ values were in ranges of 0.002–0.1 μg/g and 0.005–0.25 μg/g, respectively.
3.2.3. Accuracy and precision
The results of intra- and inter-day accuracy and precision analyses performed at three spiking levels (low, medium and high) are presented in Table 2. The intra-day and inter-day accuracy of 23 analytes ranged from 82.8% to 113.0% and 80.0%–113.8%, respectively. The intra- and inter-day precision was in the range of 0.4–9.0% and 0.7–13.6%, respectively.
Table 2.
Accuracy, precision, recovery, matrix effect and stability for the determination of 23 analytes (n = 5).
| Analyte | QC concentration (μg/g) | Accuracy (%) | Precision (RSD, %) | Recovery (Mean ± SD, %) | Matrix effect (Mean ± SD, %) | Stability (Mean ± SD, %) | ||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Intra-day | Inter-day | Intra-day | Inter-day | |||||
| Apomorphine | 0.2 | 107.5 | 94.6 | 5.3 | 3.5 | 82.5 ± 7.6 | 95.7 ± 8.8 | 86 ± 5.3 |
| 2.5 | 96.1 | 95.7 | 3.4 | 4.5 | 94.1 ± 2.4 | 99.5 ± 3.1 | 94.7 ± 3.9 | |
| 8 | 99.6 | 99.1 | 5.6 | 2.8 | 94.3 ± 6.9 | 97.1 ± 1.2 | 97.2 ± 6.9 | |
| Yohimbine | 0.2 | 109.0 | 91.3 | 1.7 | 1.5 | 88.6 ± 5.4 | 100.8 ± 7.3 | 87.9 ± 7.6 |
| 2.5 | 100.0 | 99.2 | 1.1 | 2.0 | 97.1 ± 1.2 | 103.2 ± 1.4 | 94.2 ± 2.1 | |
| 8 | 99.3 | 93.9 | 1.6 | 2.1 | 93.9 ± 3.3 | 102.6 ± 2.3 | 91.7 ± 2.8 | |
| Phentolamine | 0.3 | 113.0 | 96.1 | 2.1 | 1.2 | 97.6 ± 3.4 | 109 ± 5.8 | 91.9 ± 2.2 |
| 2.5 | 98.7 | 90.7 | 1.3 | 6.7 | 97.2 ± 0.8 | 98.6 ± 0.7 | 100.9 ± 2.2 | |
| 8 | 99.4 | 89.1 | 0.6 | 1.6 | 97.5 ± 1.9 | 97.9 ± 1.7 | 101.1 ± 1.8 | |
| Noracetildenafil | 0.06 | 91.4 | 84.2 | 2.3 | 2.9 | 94.2 ± 4.3 | 102.4 ± 1.9 | 92.1 ± 2.1 |
| 0.5 | 106.8 | 102.4 | 1.4 | 1.1 | 98.7 ± 1.2 | 96.6 ± 1.5 | 96.7 ± 1.6 | |
| 8 | 101.7 | 100.0 | 0.7 | 1.6 | 98.7 ± 1.3 | 105.9 ± 0.8 | 98.4 ± 2.4 | |
| Acetildenafil | 0.06 | 83.0 | 83.4 | 1.6 | 1.9 | 99.2 ± 2.4 | 95 ± 1.1 | 100.4 ± 1 |
| 0.5 | 110.8 | 106.8 | 1.5 | 1.2 | 98.4 ± 1.5 | 101.2 ± 1.4 | 96.4 ± 1.9 | |
| 8 | 100.8 | 99.0 | 1.8 | 1.5 | 100 ± 2.8 | 106.3 ± 2.0 | 98.2 ± 2.1 | |
| Vardenafil | 0.06 | 91.9 | 90.7 | 2.6 | 4.4 | 101.3 ± 3.4 | 93.6 ± 2.2 | 98.7 ± 4.2 |
| 0.5 | 96.9 | 92.1 | 1.2 | 2.5 | 97.7 ± 1.7 | 95.2 ± 1.2 | 95.1 ± 2.6 | |
| 8 | 95.6 | 98.7 | 1.4 | 5.0 | 98.4 ± 1.2 | 96.7 ± 1.3 | 103.3 ± 6.2 | |
| Hydroxyhomosildenafil | 0.06 | 105.0 | 102.4 | 2.7 | 2.5 | 100.3 ± 4.3 | 103.2 ± 2.6 | 97.6 ± 3.6 |
| 0.5 | 97.9 | 91.4 | 2.1 | 0.8 | 96.3 ± 3.1 | 99.4 ± 2.1 | 93.4 ± 1.8 | |
| 8 | 97.1 | 94.7 | 1.1 | 2.9 | 98.4 ± 0.7 | 97.1 ± 1.0 | 97.6 ± 3.7 | |
| Sildenafil | 0.06 | 92.4 | 87.5 | 7.8 | 4.2 | 96.6 ± 8.6 | 94.4 ± 5.7 | 91.7 ± 6.4 |
| 0.5 | 97.5 | 92.7 | 1.5 | 2.0 | 96.5 ± 0.8 | 97.1 ± 1.4 | 95.1 ± 2.7 | |
| 8 | 96.9 | 94.5 | 0.6 | 1.2 | 97.2 ± 1.2 | 96.7 ± 0.7 | 97.5 ± 1.7 | |
| Homosildenafil | 0.06 | 94.0 | 90.6 | 1.8 | 1.0 | 99.1 ± 3.8 | 95.9 ± 1.5 | 96.4 ± 1.8 |
| 0.5 | 98.2 | 94.4 | 1.3 | 1.4 | 97.6 ± 2.0 | 96.8 ± 1.3 | 96.1 ± 1.0 | |
| 8 | 97.0 | 96.5 | 1.2 | 3.5 | 98.1 ± 1.7 | 97.3 ± 1.1 | 99.5 ± 4.2 | |
| Aminotadalafil | 0.3 | 86.0 | 86.5 | 4.9 | 3.5 | 98 ± 12.4 | 109.3 ± 11 | 100.8 ± 7.5 |
| 2.5 | 95.1 | 92.3 | 2.2 | 1.7 | 96.7 ± 1.7 | 97.8 ± 4.6 | 97.1 ± 3.2 | |
| 8 | 96.0 | 92.8 | 3.2 | 1.1 | 94.3 ± 1.7 | 100.1 ± 0.8 | 96.8 ± 3.5 | |
| Aildenafil | 0.06 | 96.9 | 97.7 | 5.9 | 1.0 | 103.6 ± 5.8 | 99.5 ± 5.4 | 101.1 ± 5.8 |
| 0.5 | 99.7 | 96.5 | 1.1 | 0.7 | 98.2 ± 1.2 | 97 ± 1.0 | 96.9 ± 0.7 | |
| 8 | 96.3 | 95.3 | 0.6 | 3.2 | 97.8 ± 0.7 | 99.5 ± 0.7 | 99 ± 3.5 | |
| Avanafil | 0.06 | 82.8 | 80.0 | 1.5 | 1.4 | 96.6 ± 1.0 | 98.1 ± 1.1 | 96.6 ± 1.7 |
| 0.5 | 107.2 | 105.0 | 0.4 | 1.2 | 99.1 ± 0.7 | 92.4 ± 0.4 | 97.9 ± 1.4 | |
| 8 | 96.5 | 99.7 | 0.9 | 3.3 | 99.9 ± 1.2 | 102.8 ± 1.0 | 103.3 ± 3.9 | |
| Demethyltadalafil | 0.3 | 92.8 | 88.3 | 6.3 | 5.2 | 97.4 ± 4.7 | 104.4 ± 6.2 | 95.3 ± 3.6 |
| 2.5 | 96.0 | 91.8 | 1.9 | 1.5 | 95.9 ± 2.2 | 99.3 ± 2.3 | 95.7 ± 2.4 | |
| 8 | 96.6 | 93.0 | 1.5 | 1.8 | 96.3 ± 1.8 | 100.8 ± 0.8 | 96.3 ± 2.1 | |
| Udenafil | 0.06 | 95.1 | 91.8 | 1.2 | 1.2 | 95.3 ± 2.2 | 100.8 ± 1.2 | 96.6 ± 2.1 |
| 0.5 | 103.8 | 98.4 | 0.4 | 1.3 | 98 ± 1.3 | 99.2 ± 0.4 | 94.8 ± 1.4 | |
| 8 | 99.9 | 100 | 0.4 | 1.5 | 99.6 ± 1.0 | 103.1 ± 0.6 | 100.1 ± 1.4 | |
| Tadalafil | 0.18 | 102.4 | 113.8 | 1.9 | 12.1 | 87.3 ± 7.3 | 87.7 ± 8.4 | 111 ± 11.8 |
| 2.5 | 100.3 | 96.8 | 3.4 | 2.6 | 98.6 ± 1.4 | 94.3 ± 5.1 | 96.5 ± 4.7 | |
| 8 | 97.7 | 89.3 | 1.7 | 1.7 | 98.3 ± 1.0 | 99.1 ± 1.6 | 91.4 ± 3.0 | |
| Dapoxetine | 0.02 | 107.9 | 99.4 | 3.8 | 1.9 | 101 ± 7.4 | 100.6 ± 4.9 | 97.8 ± 8.4 |
| 0.25 | 108.2 | 112.7 | 1.4 | 1.8 | 98.2 ± 1.6 | 99.4 ± 1.1 | 101.6 ± 1.9 | |
| 2.5 | 93.5 | 95 | 1.7 | 3.6 | 97.4 ± 2.3 | 93.1 ± 1.9 | 106.4 ± 1.9 | |
| Testosterone | 0.2 | 103 | 91.8 | 6.4 | 7.8 | 94.4 ± 11.3 | 104.1 ± 6.3 | 87 ± 16.4 |
| 2.5 | 100.9 | 104.6 | 3.3 | 1.6 | 100.6 ± 2.5 | 104.3 ± 5.5 | 96.5 ± 7 | |
| 8 | 101.7 | 96.1 | 4.1 | 1.3 | 94.4 ± 4.1 | 105.6 ± 4.5 | 89.3 ± 3.7 | |
| Sulfoaildenafil | 0.06 | 96.1 | 94.4 | 6.6 | 7.6 | 101.3 ± 12.7 | 104.8 ± 4.4 | 98.6 ± 11.0 |
| 0.5 | 105.2 | 82.3 | 4.6 | 12.2 | 86.7 ± 4.4 | 96.2 ± 4.2 | 80.3 ± 6.0 | |
| 8 | 93.9 | 89.3 | 1.5 | 2.5 | 96.8 ± 1.3 | 96.7 ± 1.6 | 95.1 ± 3.5 | |
| Methyltestosterone | 0.2 | 101.6 | 95.9 | 6.4 | 5.3 | 99.4 ± 7.6 | 101.3 ± 2.2 | 96 ± 11.5 |
| 2.5 | 101.3 | 106.1 | 1.7 | 1.6 | 99.9 ± 3.5 | 100 ± 3.8 | 100 ± 3.8 | |
| 8 | 98.9 | 97.9 | 2.6 | 3.4 | 99.6 ± 2.5 | 101.2 ± 2.3 | 98.4 ± 3 | |
| Propoxyphenyl-thiohydroxyhomosildenafil | 0.2 | 105.4 | 91.6 | 8.2 | 13.6 | 97.3 ± 8.2 | 100.4 ± 5 | 87.8 ± 17.6 |
| 2.5 | 95.3 | 91.5 | 9 | 6.6 | 92.3 ± 9.4 | 97.1 ± 8.3 | 96.7 ± 11.1 | |
| 8 | 99 | 91.1 | 1.2 | 1.9 | 95.6 ± 2.9 | 101 ± 1.2 | 92.1 ± 2.5 | |
| Pseudovardenafil | 0.06 | 99.5 | 89.7 | 6.4 | 5.2 | 95.8 ± 5.2 | 108.9 ± 5.7 | 90.3 ± 5.8 |
| 0.5 | 102.4 | 97.3 | 2.2 | 2.7 | 97.9 ± 2.1 | 93.9 ± 1.9 | 95.1 ± 1.7 | |
| 8 | 91.6 | 94.9 | 2.6 | 4.2 | 98.6 ± 2.7 | 92.6 ± 2.4 | 103.6 ± 4.7 | |
| Norneosildenafil | 0.06 | 105.2 | 90.8 | 5.9 | 5.4 | 89.8 ± 6.4 | 105.8 ± 5.7 | 86.7 ± 9.4 |
| 0.5 | 100.9 | 95.3 | 1.5 | 3.1 | 95.7 ± 1.7 | 100.7 ± 1.5 | 94.4 ± 4.0 | |
| 8 | 98.5 | 95.2 | 1.9 | 1.3 | 97.9 ± 1.2 | 96.9 ± 1.9 | 96.7 ± 2.6 | |
| Testosterone propionate | 0.2 | 97.1 | 95.2 | 5.9 | 5.1 | 99.8 ± 9.8 | 95.4 ± 7.5 | 104.6 ± 5.5 |
| 2.5 | 103 | 96.8 | 1.7 | 3.1 | 99.6 ± 5.2 | 98.1 ± 3.7 | 96.5 ± 4.8 | |
| 8 | 103.4 | 96 | 1.8 | 3.3 | 101.5 ± 3.6 | 105.7 ± 2.8 | 97.2 ± 1.9 | |
These accuracy and precision results were within the acceptable criteria, showing that the method was reliable for the quantitative analysis of the 23 analytes in Chinese traditional patent medicines and health foods.
3.2.4. Recovery and matrix effect
As shown in Table 2, the extraction recoveries of the 23 analytes at three concentrations (low, medium and high) were over the range of 82.5–103.6%, which indicated that the selected extraction solvent ACN–H2O (8:2, v/v) could provide excellent extraction efficiency for all the 23 analytes from matrix.
The results of matrix effects evaluation are shown in Table 2. Signal suppression or enhancement effect was considered tolerable if the matrix effect value was in the range of 80%–120%. As shown in Table 2, the matrix effects of analytes ranged from 87.7% to 109.0% with SDs less than 8.8%. With such low level of matrix effects, this assay would be reliable for analysis in matrix.
3.2.5. Stability
As summarized in Table 2, the post-preparative was expressed as a percentage of the initial concentration (first analyzed batch) of the analytes in QC samples. The stability of all analytes in matrix was over the range of 80.3–111.0%, which indicated that all 23 analytes were stable in autosampler (24 h) at 15 °C. The results suggested that the developed method was reliable and suited for large scale sample screening.
3.3. Application of the Q-TOF/MSMS method to real samples
The application of the developed method for identifying the 23 aphrodisiac chemical ingredients in health foods or CTPMs was evaluated. Considering the fact that aphrodisiac chemical ingredients have been frequently detected in health foods or CTPMs in recent years, the significance of a novel high sensitive and high selective assay in this field is obvious.
Qualitative analysis is the identification of a target or unknown compound. In mass spectrometry, determining which compound is present is accomplished using mass accuracy, retention time, isotope pattern, library searching, and formula finding. Using all of these tools together can increase the confidence in identifying both targeted and non-targeted compounds in unknown samples. In general, the confidence levels for the qualitative rules were configured as: mass error less than 5 ppm, error in retention time less than 0.5min, difference isotope ratio less than 5%, library hit score more than 80. If the confidence levels above were reached, the traffic light was green. Their weights are 30%, 30%, 10%, 30%, respectively. The combined score was obtained by synthesizing above four factors. The higher the score, the greater the possibility of positive was. Quantitative analysis was performed using the peak areas of the precursor ions in the XICs.
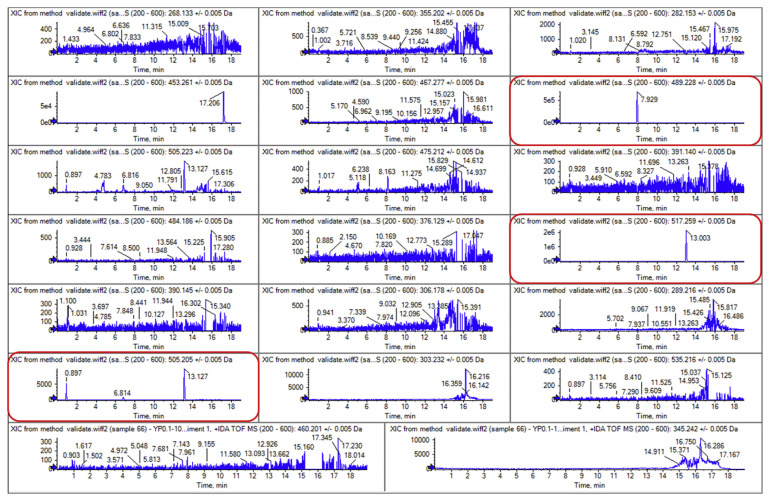
The developed UHPLC-Q-TOF/MSMS method was adopted for the routine screening of the 23 aphrodisiac chemical ingredients in 40 batches of health foods and 32 batches of CTPMs (including 28 capsules, 32 tablets, 10 liquid and 2 pills). These samples were collected from the local markets by Shandong Food and Drug Administration. The results showed that all the CTPMs were negative, while 28 batches of heath foods were positive. Sildenafil and tadalafil were detected simultaneously in 4 health foods, while aildenafil and sulfoaildenafil was detected simultaneously in 1 batch sample. Sildenafil was detected in 26 batches oral solid preparation with contents of 2.8–272.0 mg/g. Tadalafil was detected in 5 batches oral solid preparation with contents of 0.78–80.9 mg/g. Aildenafil and sulfoaildenafil was detected simultaneously in 1 batch liquid sample with contents of 102.3 μg/mL and 185.7 μg/mL respectively. As an example, Fig. 4 showed the extracted ion chromatograms of a representative positive sample which is used as a health wine product for tonifying kidney and improving sexual performance. This mass chromatography in Fig. 4 indicates that at least three main peaks (I, II, and III) appeared around 8–13 min, besides many other peaks from matrix interference.
Fig. 4.
Extracted ion chromatograms of the representative positive sample.
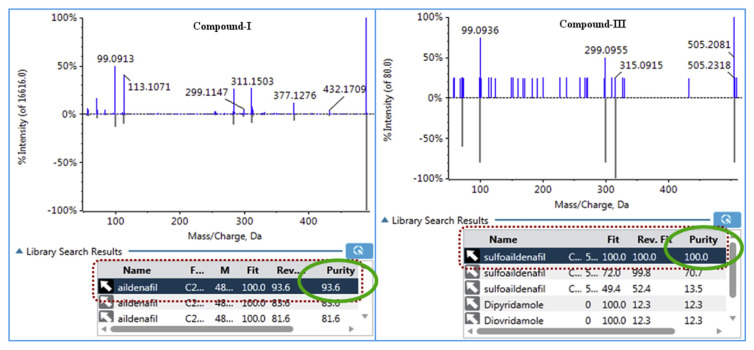
After processing the chromatograms using the method based on the qualitative and quantitative work flow of target compound, the results were shown in Results Table. As can be seen from the Results Table, the traffic lights of peaks I and III were green. Also, library research results show that the MS/MS spectra agree well with the library (purity 93.6 and 100, see Fig. 5.). Therefore, the compound-I and compound-III were assigned to aildenafil (m/z 489.2274, 7.87 min) and sulfoaildenafil (m/z 505.2048, 13.05 min).
Fig. 5.
The library research results of compound-I and III.
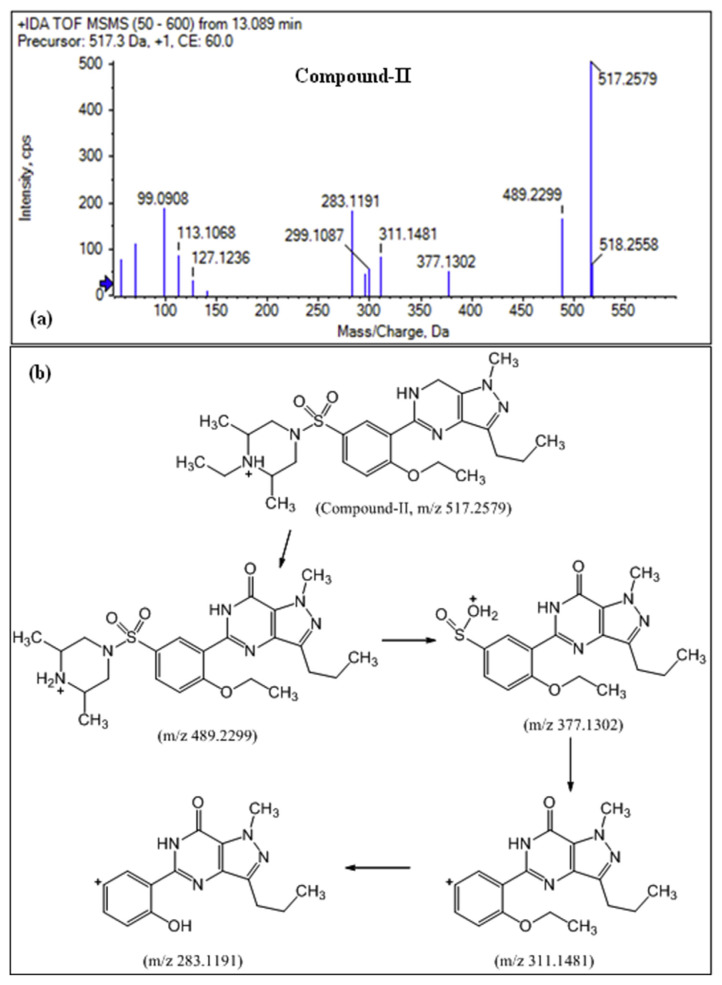
For compound-II (m/z 517.2579, 13.00 min), despite the accurate mass matched well with udenafil (m/z 517.2587, 8.52 min), a false positive identification was produced because its retention time and MS/MS spectra were totally different from udenafil. By analyzing the MS/MS spectrum of compound-II, it is very likely to belong to the family of sildenafil [50,57,62,63] because of the presence of fragment ions including m/z 489.2, 377.1, 311.1 and 283.1 (see Fig. 6). Among these fragment ions, the major product ion (m/z 489.2299) matched well with aildenafil (m/z 489.2274) with minor mass error (5 ppm). Moreover, we observed that the peak-II would disappear after a sample solution was diluted and placed for about one month, and no new peak arose. Therefore, it indicates that compound-II is a precursor of aildenafil (C23H32N6O4S), which may be generated by loss of C2H4 from the unknown molecule. Since the structural modifications of sildenafil analogs are commonly performed on the piperazine ring [5], it is a good indication of the insertion of one ethyl group attached to the nitrogen in the piperazine ring of aildenafil. These indicate that the compound-II is very likely to be a new synthetic analog of aildenafil, in which the 2,6-dimethyl piperazine moiety is replaced with N-ethyl-2,6-dimethyl piperazine. The chemical structure and the proposed fragmentation pathways forits main fragment ions are outlined in Fig. 6, which matches the findings reported by other authors [5,50,57,62,63].
Fig. 6.
(a) MS2 fragment ions of compound-II and (b) the proposed fragmentation pathways forits main fragment ions.
4. Conclusions
A rapid, sensitive and selective Q-TOF HRMS method was established and employed to screen, confirm, and quantitate 23 illegal adulterated aphrodisiac chemical ingredients in health foods and CTPMs. To the best of our knowledge, this is the first time to report the application of UPLC-Q-TOF/MS in screening of various types illegal adulterated synthetic chemicals in health foods and CTPMs. Simultaneous identification, confirmation and quantitation of analytes were achieved based on IDA mode of the Q-TOF/MS analyzer. The response showed good linear relationship with the analytes’ concentrations over wide ranges (e.g., 0.05–10 μg/g for sildenafil) with most the coefficient of determinations (r2) >0.9991. The detection limits (LODs) were in the range of 0.002–0.1 μg/g for different analytes. The recoveries ranged from 82.5% to 103.6%. The intra- and inter-day accuracies were in the range of 80.0%–113.8%, while the intra- and inter-day precision ranged from 0.4% to 13.6%.
Among 40 batches of health foods and 32 batches of CTPMs (including 28 capsules, 32 tablets, 10 liquid and 2 pills) samples, 28 batches of heath foods were positive. Sildenafil and tadalafil were detected simultaneously in 4 health foods, while aildenafil and sulfoaildenafil was detected simultaneously in 1 batch sample. The Q-TOF HRMS spectrometry has been proved to be a powerful tool for routine screening and quantitating of illegal adulterate in health foods and CTPMs. Moreover, the LC-Q-TOF method is very useful to structural elucidation of unknown compound.
Acknowledgement
This project was supported by Key Research and Development Project of Shandong Province (2016GSF120003) and Self-made Project of Shandong Institute for Food and Drug Control (2016ZNKT0015).
Funding Statement
This project was supported by Key Research and Development Project of Shandong Province (2016GSF120003) and Self-made Project of Shandong Institute for Food and Drug Control (2016ZNKT0015).
REFERENCES
- 1. Yuan R, Lin Y. Traditional Chinese medicine: an approach to scientific proof and clinical validation. Pharmacol Ther. 2000;86:191–8. doi: 10.1016/s0163-7258(00)00039-5. [DOI] [PubMed] [Google Scholar]
- 2. Vaclavik L, Krynitsky AJ, Rader JI. Targeted analysis of multiple pharmaceuticals, plant toxins and other secondary metabolites in herbal dietary supplements by ultra-high performance liquid chromatography-quadrupole-orbital ion trap mass spectrometry. Anal Chim Acta. 2014;810:45–60. doi: 10.1016/j.aca.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 3. Aqai P, Cevik E, Gerssen A, Haasnoot W, Nielen MW. High-throughput bioaffinity mass spectrometry for screening and identification of designer anabolic steroids in dietary supplements. Anal Chem. 2013;85:3255–62. doi: 10.1021/ac3036052. [DOI] [PubMed] [Google Scholar]
- 4. Hird SJ, Lau BPY, Schuhmacher R, Krska R. Liquid chromatography-mass spectrometry for the determination of chemical contaminants in food. Trends Anal Chem. 2014;59:59–72. [Google Scholar]
- 5. Kee CL, Koh HL, Bloodworth BC, Zeng Y, Kiang KH, Low MY, et al. Structural elucidation of propoxyphenyl isobutyl aildenafil, adulterant in a health supplement using high-resolution Orbitrap mass spectrometry. J Pharm Biomed Anal. 2014;98:153–9. doi: 10.1016/j.jpba.2014.05.029. [DOI] [PubMed] [Google Scholar]
- 6. Lebel P, Gagnon J, Furtos A, Waldron KC. A rapid, quantitative liquid chromatography-mass spectrometry screening method for 71 active and 11 natural erectile dysfunction ingredients present in potentially adulterated or counterfeit products. J Chromatogr A. 2014;1343:143–51. doi: 10.1016/j.chroma.2014.03.078. [DOI] [PubMed] [Google Scholar]
- 7. Patel DN, Li L, Kee CL, Ge X, Low MY, Koh HL. Screening of synthetic PDE-5 inhibitors and their analogues as adulterants: analytical techniques and challenges. J Pharm Biomed Anal. 2014;87:176–90. doi: 10.1016/j.jpba.2013.04.037. [DOI] [PubMed] [Google Scholar]
- 8. Shi F, Guo C, Gong L, Li J, Dong P, Zhang J, et al. Application of a high resolution benchtop quadrupole-Orbitrap mass spectrometry for the rapid screening, confirmation and quantification of illegal adulterated phosphodiesterase-5 inhibitors in herbal medicines and dietary supplements. J Chromatogr A. 2014;1344:91–8. doi: 10.1016/j.chroma.2013.12.030. [DOI] [PubMed] [Google Scholar]
- 9. Song F, El-Demerdash A, Lee SJ. Screening for multiple phosphodiesterase type 5 inhibitor drugs in dietary supplement materials by flow injection mass spectrometry and their quantification by liquid chromatography tandem mass spectrometry. J Pharm Biomed Anal. 2012;70:40–6. doi: 10.1016/j.jpba.2012.05.017. [DOI] [PubMed] [Google Scholar]
- 10. Campbell N, Clark JP, Stecher VJ, Thomas JW, Callanan AC, Donnelly BF, et al. Adulteration of purported herbal and natural sexual performance enhancement dietary supplements with synthetic phosphodiesterase type 5 inhibitors. J Sex Med. 2013;10:1842–9. doi: 10.1111/jsm.12172. [DOI] [PubMed] [Google Scholar]
- 11. Schramek N, Wollein U, Eisenreich W. Pyrazolopyrimidines in ‘all-natural’ products for erectile dysfunction treatment: the unreliable quality of dietary supplements. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2015;32:127–40. doi: 10.1080/19440049.2014.992980. [DOI] [PubMed] [Google Scholar]
- 12. Aschenbrenner DS. Serious adverse effects of testosterone abuse. Am J Nurs. 2017;117:20–1. doi: 10.1097/01.NAJ.0000512295.65275.66. [DOI] [PubMed] [Google Scholar]
- 13. Kearney T, Tu N, Haller C. Adverse drug events associated with yohimbine-containing products: a retrospective review of the California Poison Control System reported cases. Ann Pharmacother. 2010;44:1022–9. doi: 10.1345/aph.1P060. [DOI] [PubMed] [Google Scholar]
- 14. Laties AM. Vision disorders and phosphodiesterase type 5 inhibitors: a review of the evidence to date. Drug Saf. 2009;32:1–18. doi: 10.2165/00002018-200932010-00001. [DOI] [PubMed] [Google Scholar]
- 15. Shindel AW. 2009 update on phosphodiesterase type 5 inhibitor therapy part 2: updates on optimal utilization for sexual concerns and rare toxicities in this class. J Sex Med. 2009;6:2352–64. doi: 10.1111/j.1743-6109.2009.01447.x. Quiz 65–66. [DOI] [PubMed] [Google Scholar]
- 16. Wespes E, Amar E, Hatzichristou D, Montorsi F, Pryor J, Vardi Y. European Association of U. Guidelines on erectile dysfunction. Eur Urol. 2002;41:1–5. doi: 10.1016/s0302-2838(01)00008-2. [DOI] [PubMed] [Google Scholar]
- 17. Yue FG, Dong L, Hu TT, Qu XY. Efficacy of Dapoxetine for the treatment of premature ejaculation: a meta-analysis of randomized clinical trials on intravaginal ejaculatory latency time, patient-reported outcomes, and adverse events. Urology. 2015;85:856–61. doi: 10.1016/j.urology.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 18. Gur S, Kadowitz PJ, Gokce A, Sikka SC, Lokman U, Hellstrom WJ. Update on drug interactions with phosphodiesterase-5 inhibitors prescribed as first-line therapy for patients with erectile dysfunction or pulmonary hypertension. Curr Drug Metab. 2013;14:265–9. [PubMed] [Google Scholar]
- 19. Stief CG, Uckert S, Becker AJ, Truss MC, Jonas U. The effect of the specific phosphodiesterase (PDE) inhibitors on human and rabbit cavernous tissue in vitro and in vivo. J Urol. 1998;159:1390–3. [PubMed] [Google Scholar]
- 20. Patel DN, Low WL, Tan LL, Tan MM, Zhang Q, Low MY, et al. Adverse events associated with the use of complementary medicine and health supplements: an analysis of reports in the Singapore Pharmacovigi-lance database from 1998 to 2009. Clin Toxicol (Phila) 2012;50:481–9. doi: 10.3109/15563650.2012.700402. [DOI] [PubMed] [Google Scholar]
- 21. Poon WT, Lam YH, Lai CK, Chan AY, Mak TW. Analogues of erectile dys-function drugs: an under-recognised threat. Hong Kong Med J. 2007;13:359–63. [PubMed] [Google Scholar]
- 22. Damiano F, Silva C, Gregori A, Vacondio F, Mor M, Menozzi M, et al. Analysis of illicit dietary supplements sold in the Italian market: identification of a sildenafil thioderivative as adulterant using UPLC-TOF/MS and GC/MS. Sci Justice. 2014;54:228–37. doi: 10.1016/j.scijus.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 23. Ge X, Li L, Koh HL, Low MY. Identification of a new sildenafil analogue in a health supplement. J Pharm Biomed Anal. 2011;56:491–6. doi: 10.1016/j.jpba.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 24. Gilard V, Balayssac S, Tinaugus A, Martins N, Martino R, Malet-Martino M. Detection, identification and quantification by 1H NMR of adulterants in 150 herbal dietary supplements marketed for improving sexual performance. J Pharm Biomed Anal. 2015;102:476–93. doi: 10.1016/j.jpba.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 25. Kee CL, Ge X, Koh HL, Low MY. Isolation and characterization of propoxyphenyl linked sildenafil and thiosildenafil analogues in health supplements. J Pharm Biomed Anal. 2012;70:265–72. doi: 10.1016/j.jpba.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 26. Kee CL, Ge X, Low MY, Koh HL. Structural elucidation of a new sildenafil analogue using high-resolution Orbitrap mass spectrometry. Rapid Commun Mass Spectrom. 2013;27:1380–4. doi: 10.1002/rcm.6590. [DOI] [PubMed] [Google Scholar]
- 27. Lee HM, Kim CS, Jang YM, Kwon SW, Lee BJ. Separation and structural elucidation of a novel analogue of vardenafil included as an adulterant in a dietary supplement by liquid chromatography-electrospray ionization mass spectrometry, infrared spectroscopy and nuclear magnetic resonance spectroscopy. J Pharm Biomed Anal. 2011;54:491–6. doi: 10.1016/j.jpba.2010.09.022. [DOI] [PubMed] [Google Scholar]
- 28. Lee JH, Park HN, Ganganna B, Jeong JH, Park SK, Lee J, et al. Isolation and structural elucidation of a new tadalafil analogue in health supplements: bisprenortadalafil. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2016;33:945–52. doi: 10.1080/19440049.2016.1179134. [DOI] [PubMed] [Google Scholar]
- 29. Mustazza C, Borioni A, Rodomonte AL, Bartolomei M, Antoniella E, Di Martino P, et al. Characterization of Sildenafil analogs by MS/MS and NMR: a guidance for detection and structure elucidation of phosphodiesterase-5 inhibitors. J Pharm Biomed Anal. 2014;96:170–86. doi: 10.1016/j.jpba.2014.03.038. [DOI] [PubMed] [Google Scholar]
- 30. Shang NN, Shao YX, Cai YH, Guan M, Huang M, Cui W, et al. Discovery of 3-(4-hydroxybenzyl)-1-(thiophen-2-yl) chromeno[2,3-c]pyrrol-9(2H)-one as a phosphodiesterase-5 inhibitor and its complex crystal structure. Biochem Pharmacol. 2014;89:86–98. doi: 10.1016/j.bcp.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 31. Toomey VM, Litzau JJ, Flurer CL. Isolation and structural characterization of two tadalafil analogs found in dietary supplements. J Pharm Biomed Anal. 2012;59:50–7. doi: 10.1016/j.jpba.2011.09.038. [DOI] [PubMed] [Google Scholar]
- 32. Guo JB, Xu Y, Huang ZB, He QH, Liu SW. Development of an immunoassay for rapid screening of vardenafil and its potential analogues in herbal products based on a group specific monoclonal antibody. Anal Chim Acta. 2010;658:197–203. doi: 10.1016/j.aca.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 33. Mans DJ, Callahan RJ, Dunn JD, Gryniewicz-Ruzicka CM. Rapid-screening detection of acetildenafils, sildenafils and avanafil by ion mobility spectrometry. J Pharm Biomed Anal. 2013;75:153–7. doi: 10.1016/j.jpba.2012.11.031. [DOI] [PubMed] [Google Scholar]
- 34. Hu X, Fang G, Han A, Fu Y, Tong R, Wang S. Rapid detection of six phosphodiesterase type 5 enzyme inhibitors in healthcare products using thin-layer chromatography and surface enhanced Raman spectroscopy combined with BP neural network. J Sep Sci. 2017;40:2506–14. doi: 10.1002/jssc.201700024. [DOI] [PubMed] [Google Scholar]
- 35. Cheng CL, Kang GJ, Chou CH. Development and validation of a high-performance liquid chromatographic method using fluorescence detection for the determination of vardenafil in small volumes of rat plasma and bile. J Chromatogr A. 2007;1154:222–9. doi: 10.1016/j.chroma.2007.03.077. [DOI] [PubMed] [Google Scholar]
- 36. Fejos I, Neumajer G, Beni S, Jankovics P. Qualitative and quantitative analysis of PDE-5 inhibitors in counterfeit medicines and dietary supplements by HPLC-UV using sildenafil as a sole reference. J Pharm Biomed Anal. 2014;98:327–33. doi: 10.1016/j.jpba.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 37. Park M, Ahn S. Quantitative analysis of sildenafil and tadalafil in various fake drugs recently distributed in Korea. J Forensic Sci. 2012;57:1637–40. doi: 10.1111/j.1556-4029.2012.02164.x. [DOI] [PubMed] [Google Scholar]
- 38. Sacre PY, Deconinck E, Chiap P, Crommen J, Mansion F, Rozet E, et al. Development and validation of a ultra-high-performance liquid chromatography-UV method for the detection and quantification of erectile dysfunction drugs and some of their analogues found in counterfeit medicines. J Chromatogr A. 2011;1218:6439–47. doi: 10.1016/j.chroma.2011.07.029. [DOI] [PubMed] [Google Scholar]
- 39. Man CN, Noor NM, Lajis R. Identification of thioketone analogues of sildenfil using gas chromatography-mass spectrometry. J Chromatogr A. 2011;1218:7055–60. doi: 10.1016/j.chroma.2011.08.037. [DOI] [PubMed] [Google Scholar]
- 40. Man CN, Nor NM, Lajis R, Harn GL. Identification of sildenafil, tadalafil and vardenafil by gas chromatography-mass spectrometry on short capillary column. J Chromatogr A. 2009;1216:8426–30. doi: 10.1016/j.chroma.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 41. Mokhtar SU, Chin ST, Kee CL, Low MY, Drummer OH, Marriott PJ. Rapid determination of sildenafil and its analogues in dietary supplements using gas chromatography-triple quadrupole mass spectrometry. J Pharm Biomed Anal. 2016;121:188–96. doi: 10.1016/j.jpba.2016.01.034. [DOI] [PubMed] [Google Scholar]
- 42. Nikolaou P, Papoutsis I, Athanaselis S, Alevisopoulos G, Khraiwesh A, Pistos C, et al. Development and validation of a GC/MS method for the determination of tadalafil in whole blood. J Pharm Biomed Anal. 2011;56:577–81. doi: 10.1016/j.jpba.2011.05.036. [DOI] [PubMed] [Google Scholar]
- 43. Hadwiger ME, Trehy ML, Ye W, Moore T, Allgire J, Westenberger B. Identification of amino-tadalafil and rimonabant in electronic cigarette products using high pressure liquid chromatography with diode array and tandem mass spectrometric detection. J Chromatogr A. 2010;1217:7547–55. doi: 10.1016/j.chroma.2010.10.018. [DOI] [PubMed] [Google Scholar]
- 44. Jeong JH, Lee JH, Kim HJ, Park HJ, Hwang IS, Han KM, et al. LC-ESI-MS/MS analysis of phosphodiesterase-5 inhibitors and their analogues in foods and dietary supplements in Korea. Food Addit Contam Part B Surveill. 2016;9:1–8. doi: 10.1080/19393210.2014.968220. [DOI] [PubMed] [Google Scholar]
- 45. Lanzarotta A, Crowe JB, Witkowski M, Gamble BM. A multidisciplinary approach for the analysis of an adulterated dietary supplement where the active pharmaceutical ingredient was embedded in the capsule shell. J Pharm Biomed Anal. 2012;67–68:22–7. doi: 10.1016/j.jpba.2012.04.023. [DOI] [PubMed] [Google Scholar]
- 46. Patterson R, Mabe P, Mitchell EN, Cory W. Lifestyle illicit drug seizures: a routine ESI-LC-MS method for the identification of sildenafil and vardenafil. Forensic Sci Int. 2012;222:83–8. doi: 10.1016/j.forsciint.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 47. Proenca P, Mustra C, Marcos M, Franco JM, Corte-Real F, Vieira DN. Validated UPLC-MS/MS assay for the determination of synthetic phosphodiesterase type-5 inhibitors in postmortem blood samples. J Forensic Leg Med. 2013;20:655–8. doi: 10.1016/j.jflm.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 48. Strano-Rossi S, Odoardi S, Castrignano E, Serpelloni G, Chiarotti M. Liquid chromatography-high resolution mass spectrometry (LC-HRMS) determination of stimulants, anorectic drugs and phosphodiesterase 5 inhibitors (PDE5I) in food supplements. J Pharm Biomed Anal. 2015;106:144–52. doi: 10.1016/j.jpba.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 49. Zhang Y, Huang Z, Ding L, Yan H, Wang M, Zhu S. Simultaneous determination of yohimbine, sildenafil, vardenafil and tadalafil in dietary supplements using high-performance liquid chromatography-tandem mass spectrometry. J Sep Sci. 2010;33:2109–14. doi: 10.1002/jssc.200900841. [DOI] [PubMed] [Google Scholar]
- 50. Zhong WF, Tong WS, Zhou SS, Yip KM, Li SL, Zhao ZZ, et al. Qualitative and quantitative characterization of secondary metabolites and carbohydrates in Bai-Hu-Tang using ultraperformance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry and ultraperformance liquid chromatography coupled with photodiode array detector. J Food Drug Anal. 2017;25:946–59. doi: 10.1016/j.jfda.2016.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Guo C, Shi F, Gong L, Tan H, Hu D, Zhang J. Ultra-trace analysis of 12 beta(2)-agonists in pork, beef, mutton and chicken by ultrahigh-performance liquid-chromatography-quadrupole-orbitrap tandem mass spectrometry. J Pharm Biomed Anal. 2015;107:526–34. doi: 10.1016/j.jpba.2015.01.048. [DOI] [PubMed] [Google Scholar]
- 52. Chang CS, Yeh TS. Detection of 10 sweeteners in various foods by liquid chromatography/tandem mass spectrometry. J Food Drug Anal. 2014;22:318–28. doi: 10.1016/j.jfda.2014.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tsai CF, Kuo CH, Shih DY. Determination of 20 synthetic dyes in chili powders and syrup-preserved fruits by liquid chromatography/tandem mass spectrometry. J Food Drug Anal. 2015;23:453–62. doi: 10.1016/j.jfda.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Vaclavik L, Schmitz JR, Halbardier JF, Mastovska K. Single-Laboratory validation study of a method for screening and identification of phosphodiesterase type 5 inhibitors in dietary ingredients and supplements using liquid chromatography/quadrupole-orbital ion trap mass spectrometry: first Action 2015.12. J AOAC Int. 2016;99:55–72. doi: 10.5740/jaoacint.15-0202. [DOI] [PubMed] [Google Scholar]
- 55. Singh S, Handa T, Narayanam M, Sahu A, Junwal M, Shah RP. A critical review on the use of modern sophisticated hyphenated tools in the characterization of impurities and degradation products. J Pharm Biomed Anal. 2012;69:148–73. doi: 10.1016/j.jpba.2012.03.044. [DOI] [PubMed] [Google Scholar]
- 56. Do JA, Noh E, Yoon SB, Park HJ, Cho S, Park SK, et al. Identification and evaluation of fragmentation pathways of PDE-5 inhibitor analogues using LC-QTOF-MS. Anal Sci Technol. 2015;28:278–87. [Google Scholar]
- 57. Guo B, Wang M, Liu Y, Zhou J, Dai H, Huang Z, et al. Wide-scope screening of illegal adulterants in dietary and herbal supplements via rapid polarity-switching and multistage accurate mass confirmation using an LC-IT/TOF hybrid instrument. J Agric Food Chem. 2015;63:6954–67. doi: 10.1021/acs.jafc.5b02222. [DOI] [PubMed] [Google Scholar]
- 58. Roh SH, Kang YP, Park S, Huh Y, Lee J, Park JH, et al. Determination of tadalafil and N-desmethylsibutramine in health and dietary supplements using ultra-performance liquid chromatography (UPLC) coupled with quadrupole-time-of-flight mass spectrometry (Q-TOF MS) Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2011;28:1475–82. doi: 10.1080/19440049.2011.601280. [DOI] [PubMed] [Google Scholar]
- 59. Savaliya AA, Shah RP, Prasad B, Singh S. Screening of Indian aphrodisiac ayurvedic/herbal healthcare products for adulteration with sildenafil, tadalafil and/or vardenafil using LC/PDA and extracted ion LC-MS/TOF. J Pharm Biomed Anal. 2010;52:406–9. doi: 10.1016/j.jpba.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 60. Cappiello A, Famiglini G, Palma P, Pierini E, Termopoli V, Trufelli H. Overcoming matrix effects in liquid chromatography-mass spectrometry. Anal Chem. 2008;80:9343–8. doi: 10.1021/ac8018312. [DOI] [PubMed] [Google Scholar]
- 61. Matuszewski BK, Constanzer ML, Chavez-Eng CM. Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC-MS/MS. Anal Chem. 2003;75:3019–30. doi: 10.1021/ac020361s. [DOI] [PubMed] [Google Scholar]
- 62. Lee SK, Kim DH, Yoo HH. Comparative metabolism of sildenafil in liver microsomes of different species by using LC/MS-based multivariate analysis. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879:3005–11. doi: 10.1016/j.jchromb.2011.08.037. [DOI] [PubMed] [Google Scholar]
- 63. Venhuis BJ, de Kaste D. Towards a decade of detecting new analogues of sildenafil, tadalafil and vardenafil in food supplements: a history, analytical aspects and health risks. J Pharm Biomed Anal. 2012;69:196–208. doi: 10.1016/j.jpba.2012.02.014. [DOI] [PubMed] [Google Scholar]