Abstract
During the years 2005–2016, a total of 1067 samples for 24 types of herbal materia medica were investigated for the presence of aflatoxins (AFs) using immunoaffinity column cleanup and HPLC-coupled to a fluorescence detector after post-column derivatization. AFs were detected in 373 (35%) out of the total samples. Among them, Platycladi Semen (65% for total AFs and 79% for AFB1), Corydalis Rhizoma (53% for total AFs and 32% for AFB1), Corni Fructus (3% for total AFs), Coicis Semen (3% for total AFs and AFB1), Nelumbinis Semen (6% for total AFs and 9% for AFB1), Arecae Semen (18% for AFB1), Polygalae Radix (5% for total AFs and AFB1), and Cassiae Semen (25% for total AFs and 38% for AFB1) exceeded the official limits of 5 and 10 μg/kg, for AFB1 and total AFs (the sum of AFB1, AFB2, AFG1, and AFG2), respectively, set by the Taiwan government. We concluded that Platycladi Semen, Corydalis Rhizoma, and Cassiae Semen are the most commonly contaminated by AFs.
Keywords: Aflatoxins' contamination, Corydalis rhizoma, Herbal materia medica, Platycladi semen
1. Introduction
During the process of harvest, store, and transport, herbal materia medica could be contaminated by fungi leading to production of mycotoxins. Aflatoxins (AFs), a class of mycotoxins, are toxic secondary metabolites produced by Aspergillus parasiticus and Aspergillus flavus. Four AFs (AFB1, AFB2, AFG1, and AFG2) can be produced by A. parasiticus while two of them, AFB1 and AFB2, can be produced by A. flavus [1]. The official limits for the tolerance of AFs for certain herbal materia medica was established in some countries, including Korea [2] (15 μg/kg for total AFs and 10 μg/kg for AFB1), Italy [3] (10 μg/kg for total AFs and 5 μg/kg for AFB1), and Germany [3] and European Pharmacopeia [4] (4 μg/kg for total AFs and 2 μg/ kg for AFB1). In Taiwan, the official limit for total AFs, no more than 15 μg/kg for 14 items, was set in 2006. Moreover, this limit was reset to 10 μg/kg for total AFs and 5 μg/kg for AFB1 for 37 items in 2016. In this study, we surveyed AFs’ contamination in a total of 1067 samples for 24 monographs of herbal materia medica in our pharmaceutical factory using immunoaffinity column cleanup and HPLC-coupled to a fluorescence detector after post-column derivatization.
2. Materials and methods
2.1. Sample collection and preparation
All dried herbal herbal materia medica were imported from difference provinces of China. Herbal samples were prepared based on the research from Trucksess et al. [5] with minor modification. Briefly, 50 g of sample powder was placed into blender, added with 5 g of sodium chloride and 100 mL of 80% (v/v) methanol. After blended for 1 min, the mixture was filtrated by filter paper to obtain primary filtrate, and then 10 mL of primary filtrate was added with 40 mL of pure water followed by filtration using glass fiber filter paper to obtain secondary filtrate. 10 mL of the secondary filtrate was slowly passed through an Aflatest-P® immunoaffinity column (Vicam, MA, USA) at flow rate of 1 drop per sec. The column was washed twice with 10 mL of pure water and eluted with 1 mL of methanol. The 1 mL of eluent was collected and added with 1 mL of pure water to obtain sample solution.
2.2. Preparation of AFs standard solution
1 mL of AFs K-mix-M (Sigma–Aldrich, St. Louis MO, USA) containing 1 ppm of B1 and G1 as well as 0.3 ppm of B2 and G2 was added with 1 mL of 50% (v:v) methanol to obtain stock solution. 0.16 mL of stock solution was mixed with 50% (v:v) methanol to the final volume of 2 mL to obtain working solution. The different concentrations of standard solution were prepared by mixing working solution with 50% (v:v) methanol through serial dilution.
2.3. HPLC analysis
Analytical HPLC was performed on Hitachi D-7000 interface equipped with L-7485 fluorescence detector, L-7100 pump, L-7200 autosampler, L-7300 column oven, and post-column photochemical reaction system (Tokyo, Japan). Chromatographic separation was carried out on a Mightysil RP-18 column (250 × 4.6 mm, 5 μm). HPLC conditions were established according to the research from Trucksess et al. [5] with minor modification. Briefly, the wavelengths for excitation and emission were 360 nm and 440 nm, respectively. The mobile phase, flow rate, injection volume, column temperature, and stop time were set at 45% (v:v) methanol, 1.0 mL/min, 20 μL, 40°C, and 20 min, respectively.
2.4. Method validation
The linearity of the standard curve was established using different concentrations of the four AFs and three replications were conducted for each concentration. AFs-free sample, Astragli Radix, was used in recovery tests and in limit of quantification (LOQ) determination. Recovery tests and LOQ determination were determined using spike samples. For LOQ determination, AFB1, AFB2, AFG1, and AFG2 to a final concentration of 0.1547, 0.0956, 0.5438, and 0.1875 μg/kg, respectively, were added to Astragli Radix and conducted in triplicate. The LOQ for each AF were obtained using interpolation method according to a signal-to noise (S/N) ratio of 10:1. For recovery tests, low, medium, and high concentration of each AF were added to Astragli Radix and carried out in triplicate. The recovery rate was calculated as follows: (detection of value ÷ spike concentration) × 100%.
3. Results and discussion
3.1. Method validation
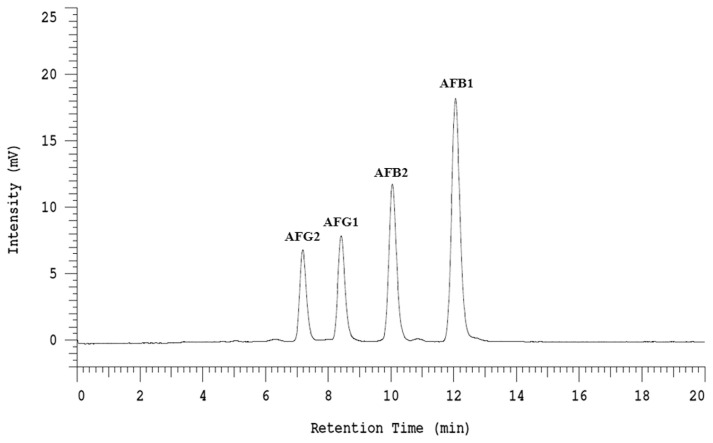
Each AF peak in the chromatogram was well identified by comparing the retention times with those of corresponding standard solution (Fig. 1). The levels of AFs in sample solution were determined by comparison to the respective standard curve. The coefficient of determination for each standard curve was higher than 0.995 (Table 1), indicating that the linearity is fine. As also shown in Table 1, the LOQ for AFB1, AFB2, AFG1, and AFG2 were 0.20, 0.10, 0.40, and 0.15 μg/kg, respectively. The ranges of recovery rate for four AFs are 87.47%–103.21% (Table 1), indicating this analytical method is valid. Herein, each value is the average from three replicated samples. It should be noted that several factors may affect the recovery rate, including the type of substrate [6,7], purification method [6], and derivatization device [6].
Fig. 1.
HPLC chromatogram of four aflatoxins (B1, B2, G1, and G2).
Table 1.
Linearity, LOQ, and recovery rate of four aflatoxins in Astragli Radix by HPLC.
| Linearity | ||||
|---|---|---|---|---|
|
| ||||
| Items | Concentration range (mg/kg) | Linear regression equation | R2 | |
| AFB1 | 0.309375e19.8 | Y = 83270X + 2748 | 0.99999 | |
| AFB2 | 0.095625e6.12 | Y = 181589X + 1490 | 0.99999 | |
| AFG1 | 0.54375e17.4 | Y = 34305X + 385 | 0.99994 | |
| AFG2 | 0.1875e6 | Y = 72147X + 3185 | 0.99871 | |
|
| ||||
| LOQ | ||||
|
| ||||
| Items | Spike value (mg/kg) | Detection value (mg/kg) | S/N ratio | S/N = 10 (mg/kg) |
|
| ||||
| AFB1 | 0.1547 | 0.1869 | 9.08 | 0.20 |
| AFB2 | 0.0956 | 0.1060 | 12.35 | 0.10 |
| AFG1 | 0.5438 | 0.5738 | 14.73 | 0.40 |
| AFG2 | 0.1875 | 0.1857 | 12.58 | 0.15 |
|
| ||||
| Recovery rate | ||||
|
| ||||
| Items | Spike value (mg/kg) | Detection value (mg/kg) | Recovery rate (%)a | |
|
| ||||
| AFB1 | 0.309375 | 0.2922 ± 0.02 | 94.45% | |
| 2.475 | 2.5072 ± 0.05 | 101.30% | ||
| 19.8 | 20.2128 ± 0.30 | 102.08% | ||
| AFB2 | 0.095625 | 0.0953 ± 0.01 | 99.66% | |
| 0.765 | 0.7853 ± 0.03 | 102.65% | ||
| 6.12 | 6.2072 ± 0.40 | 101.42% | ||
| AFG1 | 0.54375 | 0.5612 ± 0.03 | 103.21% | |
| 2.175 | 2.1856 ± 0.07 | 100.49% | ||
| 17.4 | 17.2867 ± 0.48 | 99.35% | ||
| AFG2 | 0.1875 | 0.1640 ± 0.02 | 87.47% | |
| 0.75 | 0.7519 ± 0.01 | 100.25% | ||
| 6 | 5.8828 ± 0.18 | 98.05% | ||
The recovery rate was calculated as follows: (detection of value ÷ spike concentration) × 100%.
3.2. Survey of AFs contamination in herbal materia medica
Several studies have conducted the survey of AFs contamination in herbal materia medica. The frequency of AF contamination was report by Rizzo et al. [8] to occur in 27 of 152 samples (17.8%) with the detected range of AFB1 from 10 to 2000 μg/kg; by Roy and Chourasia [9] to occur in 26 of 50 samples (52%) with the detected range of AFB1 from 170 to 670 μg/ kg; by Chourasia [10] to occur in 65 of 150 samples (43%) with the detected range of AFB1 from 50 to 910 μg/kg; by Lee et al. [2] to occur 124 of 729 samples (17%) with the detected range of AFB1 from 0.1 to 404.7 μg/kg; by Santos et al. [11] to occur 81 of 84 samples (96%) with the detected range of total AF from 1.4 to 855 μg/kg; and by Romagnoli et al. [12] to occur 0 of 27 samples. There are many differences in the amount and incidence rate of AFs contamination in herbal materia medica.
We summarized the survey of AFs contamination in herbal materia medica by Taiwan Food and Drug Administration (TFDA) from 2002 to 2013 [13–16]. A total of 1230 samples, belonging to 43 species, were collected from Chinese medicinal manufactory plants and drug stores and 107 (8.7%) AFs-contaminated samples were detected (Table 2). Among these AF-contaminated samples, 35 samples (2.8%) and 41 samples (3.3%) were above the current legislative level permissible in Taiwan (10 μg/kg for total AFs and 5 μg/kg for AFB1) (Table 2). Nelumbinis Semen (11.4% for total AFs and AFB1), Platycladi Semen (50% for total AFs and 60% for AFB1), and Corydalis Rhizoma (11.4% for total AFs and 14.3% for AFB1) are the most commonly contaminated by AFs and above the official limits (Table 2).
Table 2.
Survey of aflatoxins contamination in herbal materia medica by Taiwan Food and Drug Administration from 2002 to 2013 [13–16].
| Parts | Items | No. of samples | AF-contaminated samples | AFB1 detected value (mg/kg) | No. of out of specifications | |
|---|---|---|---|---|---|---|
|
| ||||||
| Total AF > 10 ppb | AFB1 > 5 ppb | |||||
| Semen | Coicis Semen | 120 | 24 (20%) | 0.2e45.3 | 3 (3%) | 2 (2%) |
| Nelumbinis Semen | 70 | 8 (11%) | 22.4e394.4 | 8 (11%) | 8 (11%) | |
| Platycladi Semen | 20 | 20 (100%) | 1.3e25.4 | 10 (50%) | 12 (60%) | |
| Rhizoma | Corydalis Rhizoma | 70 | 14 (20%) | 2.3e256.5 | 8 (11%) | 10 (14%) |
| Alismatis Rhizoma | 20 | 1 (5%) | 3.6 | e | e | |
| Batatatis Rhizoma | 50 | – | – | – | – | |
| Curcumae Longae Rhizoma | 20 | – | – | – | – | |
| Cimicifugae Rhizoma | 20 | – | – | – | – | |
| Atractylodis Rhizoma | 20 | – | – | – | – | |
| Acori Graminei Rhizoma | 20 | – | – | – | – | |
| Zingieris Rhizoma | 20 | – | – | – | – | |
| Pinelliae Rhizoma | 20 | – | – | – | – | |
| Fructus | Setariae Germinatus Fructus | 20 | 11 (55%) | 0.2–8.6 | 1 (5%) | 1 (5%) |
| Piperis Fructus | 20 | 1 (5%) | – | – | – | |
| Ligustri Fructus | 20 | – | – | – | – | |
| Foeniculi Fructus | 20 | – | – | – | – | |
| Crataegi Fructus | 20 | – | – | – | – | |
| Jujubae Fructus | 20 | – | – | – | – | |
| Lycii Fructus | 20 | – | – | – | – | |
| Corni Fructus | 20 | – | – | – | – | |
| Anisi Stellati Fructus | 20 | – | – | – | – | |
| Citri Immaturus Fructus | 20 | – | – | – | – | |
| Gardeniae Fructus | 20 | – | – | – | – | |
| Schisandrae Fructus | 20 | – | – | – | – | |
| Radix | Astragli Radix | 140 | 9 (6%) | 4.3–64.3 | 3 (2%) | 7 (5%) |
| Polygalae Radix | 20 | 11 (55%) | 0.7–8.1 | 1 (5%) | – | |
| Puerariae Radix | 20 | 1 (5%) | – | – | – | |
| Glycyrrhizae Radix | 20 | – | – | – | – | |
| Scutellariae Radix | 20 | – | – | – | – | |
| Paeoniae Alba Radix | 20 | – | – | – | – | |
| Gentianae Macrophyllae Radix | 20 | – | – | – | – | |
| Gentianae Rhizoma et Radix | 20 | – | – | – | – | |
| Ginseng Radix | 20 | – | – | – | – | |
| Others | Citri Reticulatae Pericarpium | 40 | 6 (15%) | – | – | – |
| Massa Medicata Fermentata | 20 | 1 (5%) | 6.7 | 1 (5%) | 1 (5%) | |
| Arecae Pericarpium | 20 | – | – | – | – | |
| Farfarae Flos | 20 | – | – | – | – | |
| Polyporus | 20 | – | – | – | – | |
| Fritillariae Cirrhosae Bulbus | 20 | – | – | – | – | |
| Poria | 20 | – | – | – | – | |
| Clematidis Caulis | 20 | – | – | – | – | |
| Cinnamomi Ramulus | 20 | – | – | – | – | |
| Moutan Radicis Cortex | 20 | – | – | – | – | |
| Total | 1230 | 107 (9%) | – | 35 (3%) | 41 (3%) | |
During 2005 to 2016, the amount and incidence rate in a total of 1067, belonging to 24 species, herbal materia medica were examined for the presence of AFs. 373 (35%) AFs-contaminated samples were detected and the amount of AFB1 in AFs-contaminated samples was in the range of 0.2052–693.38 μg/kg (Table 3). Eight herbal samples, including Platycladi Semen, Corydalis Rhizoma, Polygalae Radix, Nelumbinis Semen, Coicis Semen, Arecae Semen, Cassiae Semen, and Corni Fructus, exceeded the official limits of 5 and 10 μg/kg established by the Taiwan government for AFB1 and total AFs, respectively, accounted for 214 (20%) for total AFs and 259 (24%) for AFB1 (Table 3). Among them, Platycladi Semen (65% for total AFs and 79% for AFB1), Corydalis Rhizoma (53% for total AFs and 32% for AFB1), and Cassiae Semen (25% for total AFs and 38% for AFB1) are the most commonly contaminated by AFs and are above the official limits (Table 3).
Table 3.
Investigation of aflatoxin contamination in herbal materia medica in our factory from 2005 to 2016.
| Parts | Items | No. of samples | AF-contaminated samples | AFB1 detected value (μg/kg) | Out of specifications | |
|---|---|---|---|---|---|---|
|
| ||||||
| Total AFs > 10 ppb | AFB1 > 5 ppb | |||||
| Semen | Platycladi Semen | 233 | 216 (93%) | 0.25–592.0 | 151 (65%) | 183 (79%) |
| Coicis Semen | 37 | 7 (19%) | 0.40–12.0 | 1 (3%) | 1 (3%) | |
| Nelumbinis Semen | 32 | 4 (13%) | 0.31–163.3 | 2 (6%) | 3 (9%) | |
| Arecae Semen | 17 | 5 (29%) | 1.54–38.5 | – | 3 (18%) | |
| Cassiae Semen | 8 | 4 (50%) | 2.67–12.9 | 2 (25%) | 3 (38%) | |
| Ziziphi Spinosae Semen | 8 | 1 (13%) | 1.03 | – | – | |
| Persicae Semen | 7 | – | – | – | – | |
| Rhizoma | Corydalis Rhizoma | 200 | 98 (49%) | 0.21–693.4 | 53 (27%) | 63 (32%) |
| Belamacanae Rhizoma | 10 | – | – | – | – | |
| Fructus | Jujubae Fructus | 99 | – | – | – | |
| Corni Fructus | 66 | 23 (35%) | 0.25–0.73 | 2 (3%) | – | |
| Crataegi Fructus | 35 | – | – | – | – | |
| Lycii Fructus | 34 | – | – | – | – | |
| Ligustri Fructus | 27 | – | – | – | – | |
| Foeniculi Fructus | 25 | – | – | – | – | |
| Radix | Astragli Radix | 75 | – | – | – | – |
| Hedysari Radix | 28 | – | – | – | – | |
| Polygalae Radix | 16 | 6 (38%) | 2.26–118.1 | 3 (19%) | 3 (19%) | |
| Saposhnikoviae Radix | 6 | – | – | – | – | |
| Scrophulariae Radix | 5 | – | – | – | – | |
| Glycyrrhizae Radix | 5 | – | – | – | – | |
| Others | Citri Reticulatae Pericarpium | 65 | 2 (3%) | 0.30–0.53 | – | – |
| Massa Medicata Fermentata | 15 | 7 (5%) | 0.38–2.03 | – | – | |
| Arecae Pericarpium | 14 | – | – | – | – | |
| Total | 1067 | 373 (35%) | – | 214 (20%) | 259 (24%) | |
Based on the different parts, AF-contaminated samples were detected in semen of 237 samples (69%), in rhizome of 98 samples (47%), in fructus of 23 samples (8%), in radix of 6 samples (4%), and in others of 9 samples (10%). The samples exceeded the official limits of Taiwan regulations in semen of 156 samples (46%) for total AFs and 193 samples (56%) for AFB1, in rhizome of 53 samples (25%) for total AFs and 63 samples (30%) for AFB1, in fructus of 2 samples (0.7%) for total AFs, in radix of 3 samples (2%) for total AFs and 3 samples (2%) for AFB1. These results were similar with the finding that semen is the most commonly contaminated by AFs, as indicated by previous studies [2,13–16].
It should be noted that several differences exist between TFDA surveys and our study. One is that Taiwan government establish the official limits for AFs in herbal materia medica (15 μg/kg for total AFs for 14 items was set in 2006 and 10 μg/kg for total AFs and 5 μg/kg for AFB1 for 37 items in 2016) based on the TFDA surveys from 2002 to 2013. Our study applied the current regulations to summary and to compare the results between TFDA surveys and our findings. Another is that the incidence of AF-contaminated samples and maximum quantity of AFB1 in Platycladi Semen and Corydalis Rhizoma were quite different. The possible explanation for this difference may attribute the source and origin of herbal materia medica. In TFDA surveys, herbal samples were collected from Chinese medicinal manufactory plants and drug stores in Taiwan which have been pre-tested for AFs contamination, by contrast, our herbal samples were collected from different provinces of China.
Notably, AFB1 is well known carcinogen and exhibited the strongest toxicity among these four AFs [5] and the LD50 of AFB1 in mouse is 9 mg/kg [17]. A formula is available for converting animal dose to human equivalent dose (HED) in mg/kg, i.e., multiply the mouse dose in mg/kg/day by 0.08 [18]. By calculation, the LD50 of AFB1 for the 60-kg healthy person consumption is around 44 mg/day. In spite of the ingested level, the cumulative effect of AFB1 is to increase cancer risk. Therefore, how to reduce AFs contamination in herbal materia medica is an important issue. The treatments of AFs-contaminated herbal materia medica in our factory can be divided into two ways. One is that AFs-detected value is higher than 5 μg/kg for AFB1 and 10 μg/kg for total AFs, we must reject or destroy. Another is that AFs-detected value is under current official limits, we will store herbal materia medica at −20 °C freezer to avoid the growth of A. parasiticus and A. flavus. In addition, several innovative technologies, such as biological control, sorting technology, electromagnetic radiation, ozone fumigation, chemical agents, and improved packaging materials, have been used to minimize AFs contamination in agricultural products [19].
4. Conclusion
This survey provides useful information about the AFs-contaminated herbal materia medica and hopes to boost the awareness of crisis among farmers, manufacturer, and consumers. To ensure the quality of final products, the most important task is to control each herbal materia medica before manufacturing. We concluded that Platycladi Semen, Corydalis Rhizoma, and Cassiae Semen are the most commonly contaminated by AFs.
Footnotes
Conflicts of interest
We declare no conflict of interest involved in this study.
References
- 1. Amare MG, Keller NP. Molecular mechanisms of Aspergillus flavus secondary metabolism and development. Fungal Genet Biol. 2014;66:11–8. doi: 10.1016/j.fgb.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 2. Lee SD, Yu IS, Jung K, Kim YS. Incidence and level of aflatoxins contamination in medicinal plants in Korea. Mycobiology. 2014;42:339–45. doi: 10.5941/MYCO.2014.42.4.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bennett JW, Klich M. Mycotoxins. Clin Microbiol Rev. 2003;16:497–516. doi: 10.1128/CMR.16.3.497-516.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.European Directorate for the Quality of Medicines and Health Care (EDQM) European pharmacopoeia. 7th ed. Strasbourg: Council of Europe; 2011. [Google Scholar]
- 5. Trucksess MW, Stack ME, Nesheim S, Page SW, Albert RH, Hansen TJ, et al. Immunoaffinity column coupled with solution fluorometry or liquid chromatography postcolumn derivatization for determination of aflatoxins in corn, peanuts, and peanut butter: collaborative study. J Assoc Off Anal Chem. 1991;74:81–8. [PubMed] [Google Scholar]
- 6. Reiter E, Zentek J, Razzazi E. Review on sample preparation strategies and methods used for the analysis of aflatoxins in food and feed. Mol Nutr Food Res. 2009;53:508–24. doi: 10.1002/mnfr.200800145. [DOI] [PubMed] [Google Scholar]
- 7. Ip SP, Che CT. Determination of aflatoxins in Chinese medicinal herbs by high-performance liquid chromatography using immunoaffinity column cleanup improvement of recovery. J Chromatogr A. 2006;1135:241–4. doi: 10.1016/j.chroma.2006.10.025. [DOI] [PubMed] [Google Scholar]
- 8. Rizzo I, Vedoya G, Maurutto S, Haidukowski M, Varsavsky E. Assessment of toxigenic fungi on Argentinean medicinal herbs. Microbiol Res. 2004;159:113–20. doi: 10.1016/j.micres.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 9. Roy A, Chourasia H. Mycoflora, mycotoxin producibility and mycotoxins in traditional herbal drugs from India. J Gen Appl Microbiol. 1990;36:295–302. [Google Scholar]
- 10. Chourasia HK. Mycobiota and mycotoxins in herbal drugs of Indian pharmaceutical industries. Mycol Res. 1995;99:697–703. [Google Scholar]
- 11. Santos L, Marín S, Sanchis V, Ramos A. Screening of mycotoxin multicontamination in medicinal and aromatic herbs sampled in Spain. J Sci Ind Res. 2009;89:1802–7. [Google Scholar]
- 12. Romagnoli B, Menna V, Gruppioni N, Bergamini C. Aflatoxins in spices, aromatic herbs, herb-teas and medicinal plants marketed in Italy. Food Control. 2007;18:697–701. [Google Scholar]
- 13. Chin L, Chang-Chien IF, Cheng RB, Huang CY, Lin JH. An investigation on aflatoxin contamination in Chinese herb. Ann Rep BFDA Taiwan ROC. 2006;24:143–50. [In Chinese, English abstract] [Google Scholar]
- 14. Chin L, Chen YH, Tseng MC, Lo CF, Lin JH. Investigation on the aflatoxins contamination in Chinese herbal materials. Ann Rep BFDA Taiwan RO C. 2009;27:65–70. [In Chinese, English abstract] [Google Scholar]
- 15. Chen YH, Chin L, Liu YC, Shih YC, Lo CF. Investigation on the aflatoxins contamination in Chinese herbal materials (III) Ann Rept Food Drug Res. 2012;3:397–403. [In Chinese, English abstract] [Google Scholar]
- 16. Chin L, Chen YH, Lin MC, Liu YC, Cheng HF, Shih YC. Investigation aflatoxin contamination in Chinese herbal materials (IV): a report. Ann Rep Food Drug Res. 2014;5:197–203. [In Chinese, English abstract] [Google Scholar]
- 17. Patterson DS. Metabolism as a factor in determining the toxic action of the aflatoxins in different animal species. Food Cosmet Toxicol. 1973;11:287–94. doi: 10.1016/s0015-6264(73)80496-1. [DOI] [PubMed] [Google Scholar]
- 18. Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:659–61. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 19. Udomkun P, Wiredu AN, Nagle M, Müller J, Vanlauwe B, Bandyopadhyay R. Innovative technologies to manage aflatoxins in foods and feeds and the profitability of application-A review. Food Control. 2017;76:127–38. doi: 10.1016/j.foodcont.2017.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]