Abstract
Fungal immunomodulatory protein (FIP-fve) is a potential functional food ingredient. However, undesirable component flammutoxin (FTX) would occur in the extracted fraction of FIP-fve. In this paper, an application of heating processing instead of the intensive separation process was employed in fractionation of FIP-fve, meanwhile, exclusion of FTX was reached. Contents of FIP-fve and FTX were monitored by HPLC-UV-ESI-MS. Both FIP-fve and FTX had higher thermal stability in a lower concentration solution. Cold water could effectively extract FIP-fve and FTX from fresh mushroom without acetic acid and disulfide-bond breaking agent β-mercaptoethanol commonly used in biochemical studies. Heating cold water extract contained 580 μg/mL FIP-fve and 452 μg/mL FTX at 60 °C for 5 min could effectively exclude FTX and remain 75% of FIP-fve. Adding 0.1 M trehalose or 20% ethanol did not significantly alter the stability of both proteins. The method developed is an applicable procedure for preparing FIP-fve solution free of FTX.
Keywords: FIP-fve, Flammulina velutipes, Flammutoxin, HPLC-UV-ESI-MS, Protein stability
1. Introduction
Flammulina velutipes (referred to as the golden needle mushroom) is an economical, popular edible mushroom. Various components of F. velutipes, such as polysaccharides and glycoproteins, are found to be bioactive. Among these functional proteins, FIP-fve, a novel bioactive polypeptide isolated from the fruiting bodies of F. velutipes [1], has been classified as a member of fungal immunomodulatory proteins (FIPs). FIPs such as LZ-8, FIP-vvo, and FIP-gts have been isolated and purified from Ganoderma lucidum (Lingzhi), Volvariella volvacea, and Ganoderma tsugae, respectively [2–4]. FIPs have been reported to assist the production of interleukin (IL)-2, interferon-gamma (IFN-γ), and tumor necrosis factor-alpha (TNF-α) in peripheral blood lymphocytes [1,3]. The oral administration of 10 mg/kg FIP-fve has been reported to significantly inhibit the size of tumors and increase the life span of BNL 1MEA.7R.1 (BNL) hepatoma-bearing mice. Other studies have reported that compared to FIP-gts, FIP-fve exhibits higher resistance to the digestion of simulated gastric fluid (SGF) and simulated intestinal fluid (SIF) [5]. Chu et al. [6] demonstrate that oral FIP-fve has an anti-inflammatory effect on house dust mite-induced airway inflammations. In addition, these results suggest that FIP-fve possesses a strong potential to be used as an alternative therapy in food or pharmaceutical products being commercially developed for allergic airway diseases such as asthma. Based on previous studies, FIP-fve is stable and demonstrates significant potential for commercial development into functional food or even pharmaceutical products.
Lin et al. [7] have isolated a cardiotoxic and cytolytic 22-kDa protein from the basidiocarps of F. velutipes and designated it as flammutoxin (FTX). FTX has been reported to cause the lysis of mammalian erythrocytes. Later, Bernheimer and Oppenheim [8] have purified a 32-kDa hemolytic protein from F. velutipes and referred to it as FTX assuming that the FTX isolated from the study of Lin et al. [7] was derived from their 32-kDa FTX via partial proteolysis. Furthermore, they have studied the susceptibility of erythrocytes of different mammals to FTX and have reported that hemolytic activity is inhibited by sucrose. Tomita et al. have isolated FTX as a single hemolysin of 31 kDa from F. velutipes, determined by the 28N terminal residues, and studied the molecular basis of the cytolytic action of this protein [9].
Classically, hemolysins have been defined as exotoxins capable of causing the lysis of red blood cells and nucleated cells. Hemolysins are pore-forming toxins, which interact with specific ligands on the surface of various target cells [10].
Pore-forming proteins have been proposed to be factors mainly responsible for gastrointestinal disorders, such as diarrhea and fluid accumulation [11]. Clinical reports about intestinal dysfunction caused by the excessive ingestion of F. velutipes have not been reported, possibly attributed to the fact that FTX is heat labile [12]. A system was developed to purify FIP-fve for commercial processed food products. Hence, it is crucial to consider the activity of FTX.
In general, proteins are heat-sensitive compounds. After heating, several food proteins can easily lose their biological activities. Hence, it is imperative to predict whether certain proteins can potentially remain bioactive during heating. This study examined the effects of heating on the stabilities of both FIP-fve and FTX by high-performance chromatography coupled with ultraviolet spectroscopy and electrospray ionization mass spectrometry (HPLC-UV-ESI-MS). UV was combined with ESI-MS for quantitative and qualitative analysis. In this study, UV and ESI-MS systems were coupled in tandem so as to obtain quantitative and qualitative data, respectively, in a single operation. Hence, the concentrations of FIP-fve and FTX are obtained from UV spectral analysis, while the molecular weights of the protein are calculated by ESI-MS.
2. Materials and methods
2.1. Materials
Golden needle mushrooms were obtained from local markets. HPLC-grade acetonitrile was purchased from Fisher Scientific (Pittsburgh, PA, USA). Trifluoroacetic acid (TFA) was purchased from Sigma (St. Louis, MO, USA). Ammonium sulfate and Tris base were purchased from JT-baker (Phillipsburg, NJ, USA). Milli-Q grade water was used in the mobile phase (Millipore, MA, USA). A Millipore Amicon® Ultra-15 centrifugal filter was purchased from Millipore (Bedford, MA, USA). All other chemicals were commercially purchased as analytical-grade reagents.
2.2. Sample preparation
2.2.1. Heat resistance of fruiting bodies
Unheated ammonium sulfate fractionation (UHA): Fresh mushrooms of F. velutipes were collected from local markets and rinsed with water. The protein extraction followed the procedure described by Ko et al. [1]. In brief, fresh mushrooms of F. velutipes (200 g) was pulverized and soaked in 200 mL of 5% acetic acid in the presence of 0.05 M β-mercaptoethanol for 24 h at 4 °C. The proteins in the supernatant were precipitated by the addition of ammonium sulfate until 95% saturation being attained and collected by centrifugation (14,000 × g for 30 min at 4 °C). The protein extract was transferred to a centrifugal filter device (with a molecular weight cut-off, MWCO, of 3 kDa) for desalting, concentration and purification. The UHA extract was filtered through a 0.45 μm pore-size nylon filter and stored at −25 °C until analysis. Ten microliters of each extract was used for on-line desalting HPLC-UV-ESI-MS and SDS-PAGE analysis. The UHA was used as the control group.
Heat treatment and ammonium sulfate fractionation (HA): First, the fruiting bodies of F. velutipes (200 g) were pulverized and boiled with 200 mL distilled water for 15 min. Next, the entire mixture was soaked with of 5% acetic acid in the presence of 0.05 M β-mercaptoethanol for 24 h at 4 °C. The proteins in supernatant were extracted in the same manner as described in the previous paragraph. The extract was designated as HA.
2.2.2. Temperature stability test of UHA
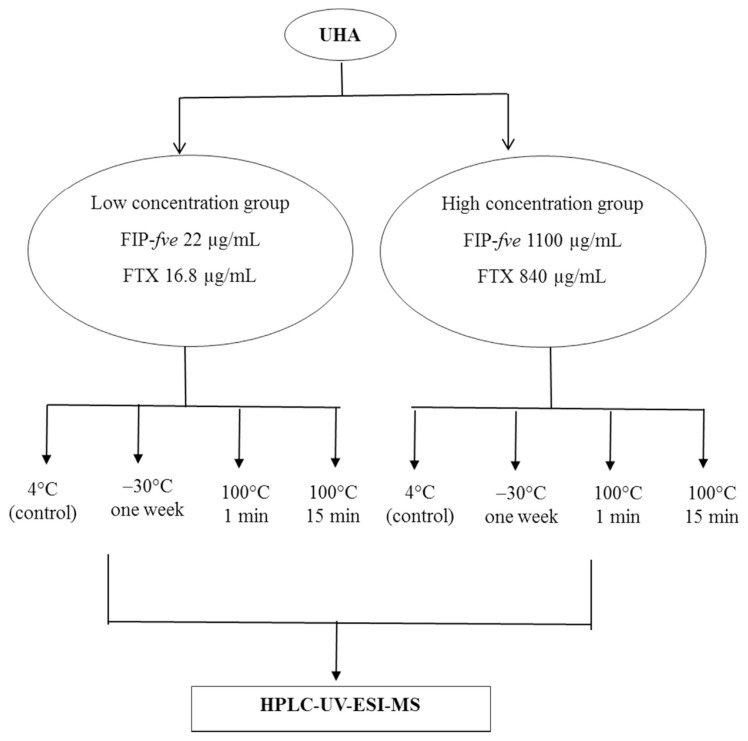
First, 1 mL of UHA extract was added to a microcentrifuge tube. Two UHA extracts of high and low concentrations, respectively, were subjected to heating at 100 °C at 1 or 5 min, or these samples were frozen for 1 week at −30 °C. In this study, 1100 μg/mL of FIP-fve in the crude extract was referred to “high” concentrations, while 22 μg/mL was referred to “low” concentration. After heat treatment, these samples were immediately cooled in ice water for 10 min. All further analyses were immediately performed by on-line desalting HPLC-UV-ESI-MS (Fig. 1).
Fig. 1.
Scheme for thermal stability test of FIP-fve and FTX proteins in Flammulina velutipes extract UHA.
2.2.3. Heat resistance test of cold water extracts from fruiting bodies
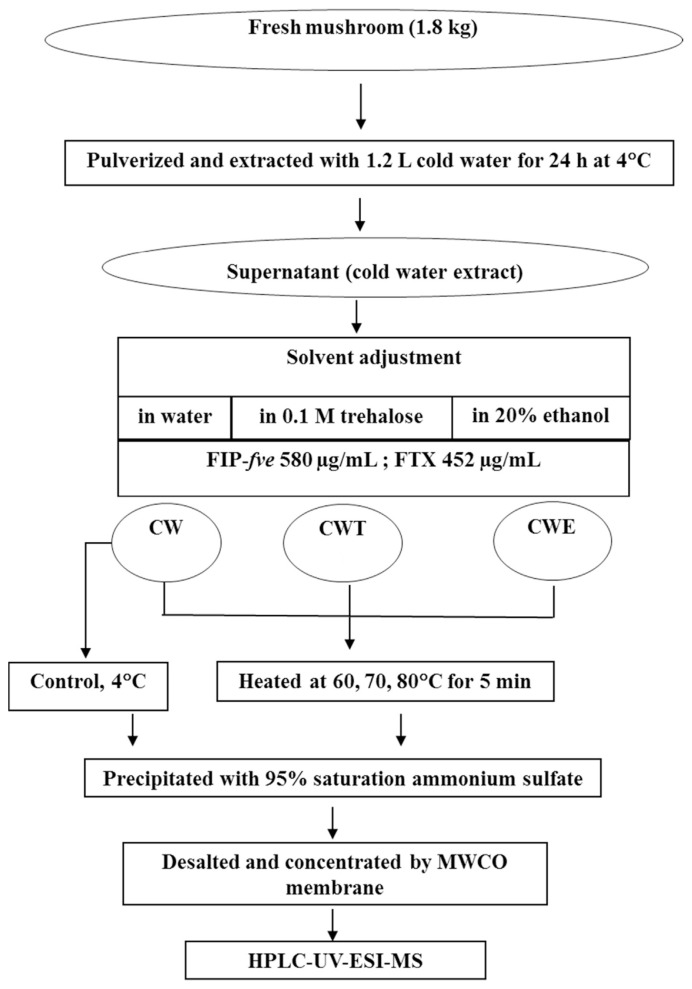
Cold water extract in water (CW): FIP-fve and FTX were purified from fresh mushroom of F. velutipes (1.8 kg). First, the fruiting bodies was pulverized and extraction with 1.2 L of 4 °C cold water, followed by standing for 24 h at 4 °C. After centrifugation at 11,300 × g at 4 °C for 30 min, the supernatant was collected and stored at 4 °C until analysis (Fig. 2). The extract was used as the control group. 20 mL of water were added to flasks containing 40 mL of the cold water extract. The extract was designated as CW. Second, the mixture was heated for 5 min at 60, 70, and 80 °C, respectively. Third, the proteins in supernatant were extracted by the addition of ammonium sulfate to 95% saturation. After centrifugation at 17,000 × g for 70 min at 4 °C, the extract was collected, followed by dialysis against distilled water (5 L) at 4 °C for 48 h. Finally, the dialy-zate was subjected to HPLC-UV-ESI-MS analysis.
Fig. 2.
Scheme for preparing cold water extract from Flammulina velutipes and thermal stability test of its proteins FIP-fve and FTX (CW: cold water extract; CWT: cold water extract in 0.1 M trehalose; CWE: cold water extract in 20% ethanol).
Cold water extract in trehalose (CWT): Twenty mL of 0.3 M trehalose were added to flasks containing 40 mL of the cold water extract. The extract was designated as CWT. The mixture was heated in the same manner as described in the previous paragraph.
Cold water extract in ethanol (CWE): Twenty mL of 60% ethanol were added to flasks containing 40 mL of the cold water extract. The extract was designated as CWE. The mixture was heated in the same manner as described in the previous paragraph.
2.3. SDS/PAGE
Samples were prepared for SDS-PAGE by mixing 15 μL of the sample with an equal volume of sample buffer. Second, all mixtures were boiled for 5 min and stored at 4 °C before electrophoresis. SDS-PAGE was conducted using a 15% poly-acrylamide gel and visualized by staining with Coomassie brilliant blue G250. A glycoprotein staining assay was conducted in SDS-PAGE using the periodic acid/Schiff technique.
2.4. Instruments
2.4.1. HPLC-UV-ESI-MS
HPLC analysis was performed on a Dionex UltiMate 3000 HPLC system, comprising a DGP 3600A dual gradient pump, a variable wavelength UV detector, and a WPS 3000SL autosampler with injection volume set at 10 μL. The UV detector was set at 280 nm for the real-time monitoring of the peak intensity. The analytical column was a Jupiter C-4 analytical column (300 Å, 5 μm, 250 mm × 4.6 mm I.D.) purchased from Phenomenex (Torrance, CA, USA). The mobile phase consisted of the combination of A (0.1% TFA in water) and B (0.1% TFA in ACN). The gradient was linearly varied from 10% B at 0 min and maintained constant at 10% B to 2.5 min, 10%–90% B (v/v) in 20 min, and finally to 10% B at 20.1 min and maintained constant at 10% B to 25 min at a flow rate of 1.0 mL/min. Two desalting procedures were performed: MWCO centrifugal filtration and on-line desalting solid-phase extraction SPE column (Inertsil C18, 5 μm, 50 mm × 4.6 mm I.D.).
Two ESI-MS analysis was use as their availability, one was API 3200™ (Applied Biosystems, Foster City, CA, USA) coupled to the HPLC system. Optimized MS parameters were utilized: capillary voltage, 5500 V; turbo heater temperature, 550 °C; nebulizer gas (air, GS1, 50 psi); heater gas (GS2, 50 psi); curtain gas, nitrogen (12 psi); declustering potential (DP), 50 V; dwell time, 150 ms; and pause time, 5 ms. All mass spectra were recorded in the continuous scan mode for positive ions in the scan range of m/z 500–1800. Reconstruction software (Bio-Analyst is used with API 3000) was required to generate parent mass. The other ESI-MS analysis was on Thermo Finnigan LXQ (Waltham, MA, USA). Optimized MS parameters were as follows: a sheath gas flow rate of 90 arbitrary units, an auxiliary gas flow rate of 50 arbitrary units, an ion spray voltage of 5 kV, a capillary temperature of 350 °C, a capillary voltage of 46 V, and a tube lens of 85 V. Reconstruction software (Protein Deconvolution 4.0) was required to generate parent mass.
2.4.2. In-gel enzymatic protein digestion and identification
Selected protein spots were excised from the SDS-PAGE gel. After removing the ethanol solution, gel pieces were incubated in 100 μL distilled water for 10 min at room temperature and then in 40 μL of 50% ACN for 10 min. This step was repeated three times. Subsequently, the supernatants were removed, and the excised gel fragments were equilibrated with 40 μL of 50% ACN containing 25 mM ammonium bicarbonate for removing the Coomassie Blue stain. After removing the supernatants, gel pieces were dehydrated with 40 μL of 100% ACN and dried on a centrifugal evaporator under vacuum. For protein digestion, 20 μL of 10 μg/mL trypsin in 25 mM ammonium bicarbonate containing 2.5 mM CaCl2 (pH 7.8) was added to each sample and incubated overnight at 37 °C. The resulting tryptic fragments were identified from peptide mass fingerprinting. Peptides identified by Mascot database searching software.
3. Results
3.1. Heat resistance of FIP-fve and FTX in fruiting bodies
3.1.1. SDS-PAGE
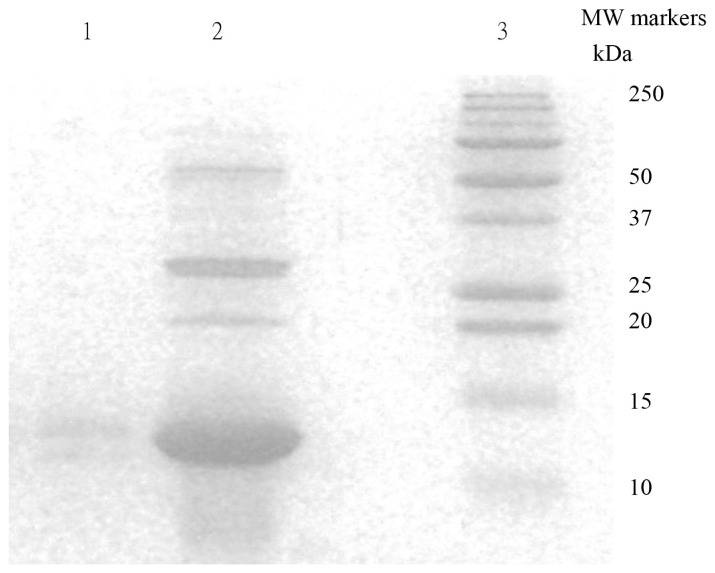
Different crude extracts were separated by SDS-PAGE (Fig. 3). The UHA extracts exhibited two major protein bands corresponding to molecular weights of 13 kDa and 30 kDa, and one faint band corresponding to a molecular weight of 22 kDa. This result is in agreement with the report of Ko et al. (1995) [1]. After bottom-up approach analysis, the database search resulted of molecular weights of 13 kDa is FIP-fve protein with sequence coverage of 43% and Mascot score of 1936. The heating treatment (HA) caused a protein denature as expected and the only traces amount of proteins was able to be extracted. The proteins present in the HA extracts only exhibited one faint band with a molecular weight of 13 kDa on SDS-PAGE gel. After bottom-up approach analysis, the database search resulted of molecular weights of 30 kDa is FTX protein with sequence coverage of 26% and Mascot score of 1413. The 22 kDa band on the gel contained two proteins and will discuss on next section.
Fig. 3.
SDS-PAGE analysis of crude protein extract of Flammulina velutipes. Electrophoresis was carried out on a 15% acrylamide gel (SDS-PAGE). Lane 1: Heat treatment and ammonium sulfate fractionation (HA) Lane 2: UnHeated Ammonium Sulfate fractionation (UHA) Lane 3: Molecular weight markers from Bio-Rad. Gel stained with Coomassie Blue G250.
3.1.2. HPLC-UV data vs. HPLC-MS data
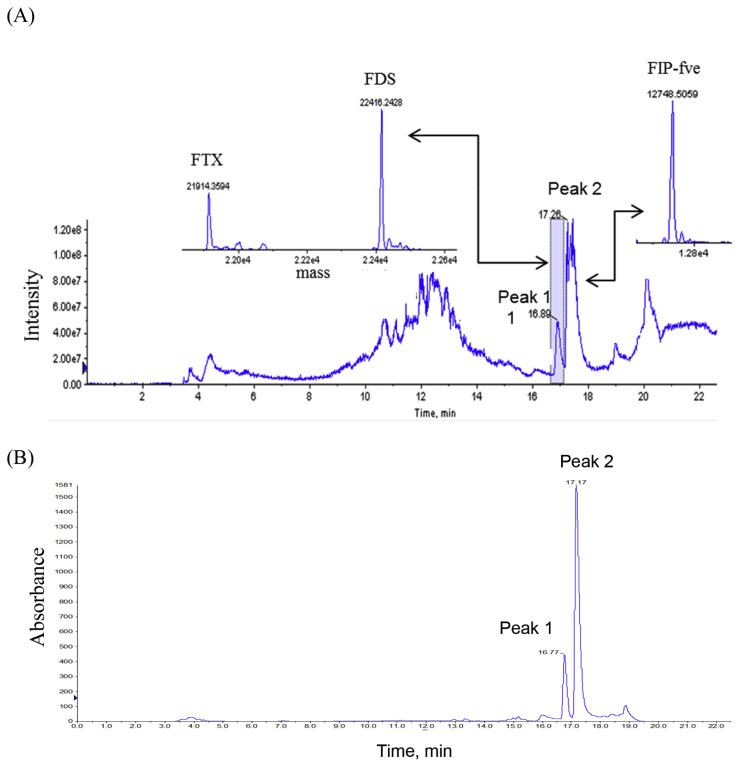
From the HPLC-MS and HPLC-UV results of UHA, two chromatographic peaks were observed. FIP-fve was resolved in the chromatogram with the retention time of 17.26 min and designated as peak 2 (Fig. 4). After deconvolution, the calculated molecular weight of FIP-fve was 12,748.5 Da by MS measurement (Fig. 4). With the same deconvolution approach, peak 1 exhibited two molecular weights of 22,416.2 Da and 21,914.4 Da, respectively (Fig. 4). The proteins in peak 1 were further fractionated and collected for protein identification analysis. After bottom-up approach analysis, the database results show that FTX and FDS (molecular weight: 20,087 Da, http://www.uniprot.org/uniprot/D2JY92, Last modified: February 9, 2010) were resolved from peak 1. From the results of in gel digestion, the molecular weight of 21,914.4 Da was attributed to the degradation products of FTX (Fig. 4), while that of 22,416.2 Da was presumed to be FDS (data not shown). FIP-fve and FTX concentration of UHA were 1100 and 840 μg/mL, respectively, and UV detection was performed at 280 nm using bovine serum albumin (BSA) as the calibration standard protein. Our results indicated that both FTX and FIP-fve are not detected from the HPLC-MS and HPLC-UV chromatogram of HA.
Fig. 4.
HPLC-UV-ESI-MS chromatograms, total ion chromatogram of MS (A) and UV chromatogram detected at 280 nm (B), of Flammulina velutipes extract UHA separated on a Jupiter C4-column. Reconstructed mass spectrum based on the multiple charge states. ABI Bioanalyst software was employed to generate the reconstructed parent mass.
3.2. Thermal stability of FIP-fve and FTX in UHA extracts
Table 1 summarizes the stabilities of FIP-fve and FTX to different temperature conditions. Based on the results obtained from chromatographic analysis, greater than 85.7% of FTX was degraded. After heating the high-concentration protein sample for 1 min at 100 °C, greater than 83.6% of FIP-fve was also lost. First, UHA extracts were diluted 50-fold (22 μg/mL, low concentration), followed by heating for 1 min at 100 °C in this solution, 50% of FTX (including FDS) and 85% of FIP-fve were detected. After this solution was heated for 15 min, FTX was not detected, but 24.1% of FIP-fve was still observed in the chromatogram of the solution.
Table 1.
Thermal stability of proteins in the extract from edible mushroom Flammulina velutipes.
| Temperature, time | UHAa | |||
|---|---|---|---|---|
|
| ||||
| Low concentration group | High concentration group | |||
|
|
|
|||
| FIP-fve (%) | FTX (%) (including FDS) | FIP-fve (%) | FTX (%) (including FDS) | |
| 4 °C, control | 100b | 100b | 100b | 100b |
| −30 °C, 1 week | 91.4 | 48.8 | 93.6 | 51.2 |
| 100 °C, 1 min | 85.0 | 50.0 | 16.4 | 14.3 |
| 100 °C, 15 min | 24.1 | N.D. | 5.5 | N.D. |
The proteins in UHA was extracted by 5% acetic acid and 0.05 M β-mercaptoethanol as solvent and fractionated by using 95% saturation of ammonium sulfate.
Concentrations of FIP-fve and FTX of the low concentration group were 22 and 16.8 μg/mL, respectively, and 1100 and 840 μg/mL, respectively, of the high concentration group.
3.3. Heat resistance of FIP-fve and FTX in the cold water extracts of fruiting bodies
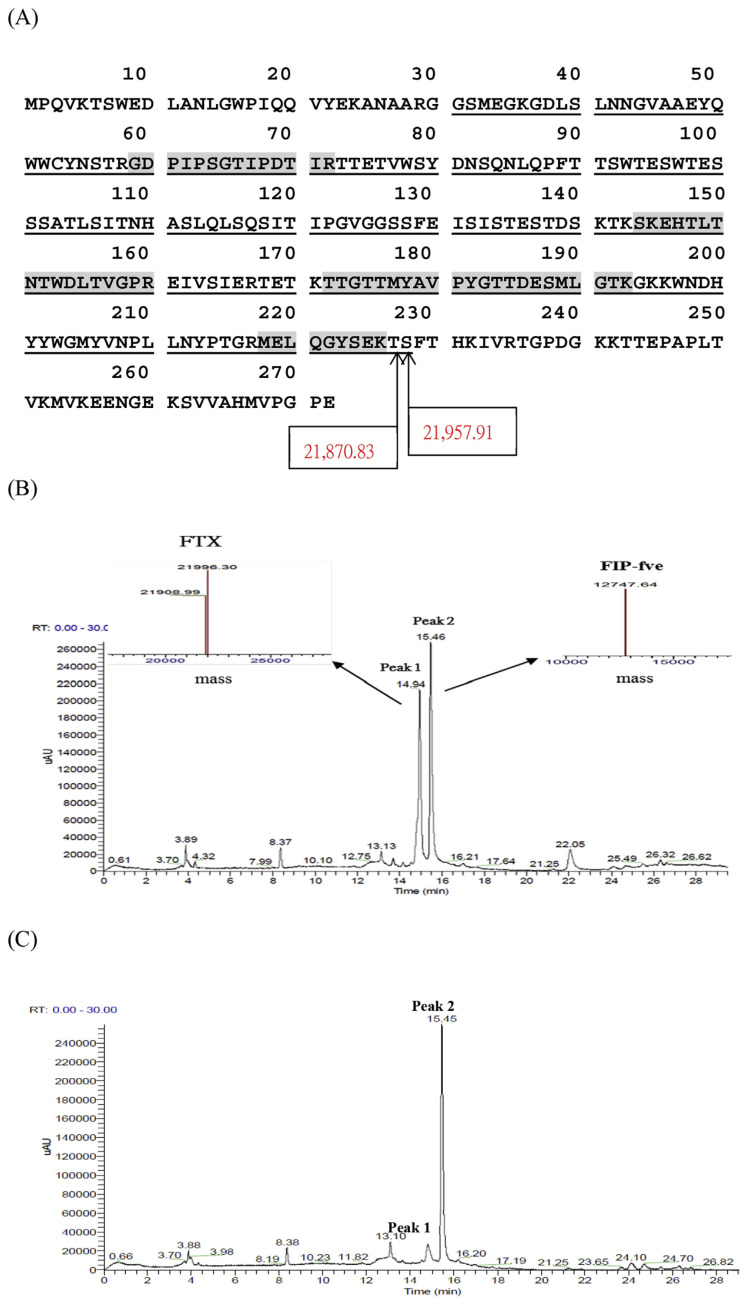
In the control group, FIP-fve and FTX contents were 580 μg/mL and 452 μg/mL, respectively. FDS was not detected from the HPLC-MS chromatogram of control group extracts. From the HPLC-MS results of the cold water extracts, peak 1 exhibited two molecular weights of 21,996.3 Da and 21,909.0 Da (Fig. 5), respectively. According to the results of in gel digestion, both molecular weight values were attributed to the degradation products of FTX. From the HPLC-MS results and in gel digestion results, the molecular weight of 21,909.0 Da was estimated and presumably with one residue truncated (Ser228) from the molecular weight of 21,996.3 Da. Hence, the molecular weight of 21,996.3 Da possibly have a N-terminal truncation of 30 amino acids and an C-terminal truncation of about 44 amino acids (Fig. 5). The difference between estimated and measured molecular weight indicated that modifications or substitution might occurred on the protein (http://www.unimod.org/modifications_list.php).
Fig. 5.
Amino acid sequence of the FTX (molecular weight: 30,095 Da, http://www.uniprot.org/uniprot/O74163 Last modified: November 1, 1998). The underlines indicate the regions of the molecular weight of 21,996.3 Da. The shadow sites indicate the matched protein after in-gel digestion. Peptides identified by Mascot database searching software (23% coverage) (A), HPLC-UV chromatograms of the cold water extracts from Flammulina velutipes without heating (control group) (B) and after heated at 60 °C for 5 min in 0.1 M trehalose solution (CWT group) (C). The separation was performed on a Jupiter C-4 column and peaks detected at 280 nm. The peak 1 and 2 were FTX, FIP-fve protein, respectively, confirmed on a Thermo Finnigan LXQ Mass Spectrometer and Protein Deconvolution 4.0 software was employed to generate the reconstructed parent mass.
As shown in Fig. 5 FIP-fve and FTX were detected in control group extracts, while FTX was hardly detected by UV chromatogram, but 75% of FIP-fve was detected after the CW extracts were heated for 5 min at 60 °C (Table 2).
Table 2.
Thermal stability of proteins in the cold water extract from edible mushroom Flammulina velutipes with trehalose and ethanol.
| Sample | Temperature (°C)b | Relative percentage (%) | |
|---|---|---|---|
|
| |||
| FIP-fve | FTX | ||
| CWa | 4 (control) | 100c | 100c |
| 60 | 75 | trace | |
| 70 | 23.1 | trace | |
| 80 | trace | trace | |
| CWTa | 60 | 78.8 | 1.1 |
| 70 | trace | trace | |
| 80 | trace | trace | |
| CWEa | 60 | trace | trace |
| 70 | trace | trace | |
| 80 | trace | trace | |
CW: cold water extract; CWT: cold water extract in 0.1 M trehalose solution; CWE: cold water extract in 20% ethanol.
The extracts were heated for 5 min at 60, 70, and 80 °C, respectively.
Concentrations of FIP-fve and FTX of all tested solution were 580 and 452 μg/mL, respectively.
As shown in Fig. 5, 1.1% of FTX and 78.8% FIP-fve were detected in the UV spectrum after CWT extracts by heating for 5 min at 60 °C (Table 2). However, after the extraction of F. velutipes, with or without the addition of heat stability modifiers, FTX and FIP-fve were not detected by heating at 70 and 80 °C for 5 min (Table 2). FTX and FIP-fve of CWE extracts were not detected at any of the investigated temperature.
4. Discussion
In this study, a semi-preparative protocol was developed for maintaining FIP-fve in solution and for removing FTX. Hence, it is essential to compare the effects of various conventional food processing operations on the stabilities of FIP-fve and FTX. Tong et al. (2008) have investigated processing tolerances of FIP-fve proteins, and the results obtained from SDS-PAGE indicated that even after autoclaving (121 °C, 15 min) and boiling (100 °C, 30 min) showing that there was little degradation through the foregoing processes [13]. On the other hand, Tomita et al. have reported that FTX partially aggregates upon heating at 100 °C [9]. In this study, the heat stabilities of FIP-fve and FTX were compared. Next, the effect of trehalose (a protecting agent) and ethanol (a denaturing agent) on the stabilities of FIP-fve and FTX was also investigated.
HPLC-UV-ESI-MS was employed to monitor the stability of FIP-fve after mimicking some food processing conditions. In the experiments, FIP-fve and FTX were not detected after heating the mushroom at 100 °C for 15 min. In contrast, a previous study has reported that autoclaving (121 °C, 15 min) and boiling (100 °C, 30 min) do not degrade the conformations and properties of FIP-fve [13]. The results obtained from the above two experiments were inconsistent. Hence, these results were further investigated. Tong et al. [13] have reported the thermal tolerance of purified FIP-fve. In this study, the thermal tolerance of FIP-fve from fruiting bodies was investigated. The experimental results indicated that heating can cause some interactions between FIP-fve and other compounds. Hence, it is possibly difficult to extract FIP-fve from fruiting bodies. Experiments were conducted to examine the heat resistance of extractions by heat treatment at 100 °C for 1 or 15 min. The result obtained is consistent with those obtained from the study of Ou et al. [5]. The immunomodulatory activity of FIP-fve is based on its ability to stimulate human peripheral blood lymphocytes to release IFN-γ. The immunomodulatory activity of FIP-fve was considerably decreased upon heat treatment at 100 °C for 2–8 min. However, these activities were stable upon heating at 100 °C for 1 min [5]. In this study, 85% of FTX and 83% of FIP-fve aggregated after the high-concentration protein solution was heated for 1 min at 100 °C. However, 50% of FTX (including FDS) and 85% of FIP-fve were detected after the low-concentration protein solution was heated for 1 min at 100 °C. The results show that thermal stability of low concentrations proteins was better than high concentrations proteins, but has not yet achieved the goal of removing FTX.
FIP-fve proteins were extracted with 5% acetic acid containing 0.1% β-mercaptoethanol in our laboratory. Although β-mercaptoethanol can stabilize proteins during extraction, it is considered to be toxic with an unpleasant odor. Hence, β-mercaptoethanol cannot be used in the food industry. Instead, acetic acid (food-additive grade) can be used to enhance extraction efficiency, but its acidity might decrease the stability of proteins in case commercialized products manufactured for protein extraction. On the other hand, the FIP-fve from fruiting bodies can be extracted with cold water, and cold water extractions can be treated by various methods in the food industry, including heating, as well as the addition of ethanol and trehalose (as a modifier for protein stability), for retaining FIP-fve while simultaneously removing FTX in solution.
The results of this study indicated that FIP-fve, with a high solution concentration of 580 μg/mL, was not detected for 5 min at low temperatures (70 and 80 °C). These results demonstrate that it is relatively easy to cause protein aggregation and precipitation with a “high” concentration FIP-fve extraction. After extraction, the solutions were heated for 5 min at 60 °C without the addition of heat stability modifiers: FTX was hardly detected in the UV spectra, albeit 75% of FIP-fve was detected.
Previous studies indicated that proteins are unstable in a majority of polar solvents, such as ethanol, and their solubility decreases more than 20-fold in the presence of 20% ethanol [14]. Our results indicated that both FTX and FIP-fve are not detected after the addition of ethanol into extractions. These results demonstrate that FIP-fve and FTX exhibit minimal tolerance in ethanol even at 60 °C for 5 min. Trehalose has been reported to inhibit the aggregation of denatured proteins (e.g., bovine glutamate dehydrogenase) following heat shock [15]. As shown in Table 2, the stability of proteins provided no significant contribution with the addition of trehalose. Hence, experimental results permit the determination of optimum processing conditions.
5. Conclusion
FIP-fve is a potential natural ingredient for functional food or pharmaceutical products. However, purification of FIP-fve is a time-consuming task because the present of FTX and it became a major obstacle for further bioactivity study and product development. This study illustrated a simple and feasible processing method for preparing FIP-fve that the heating cold water extract of the fresh mushroom at 60 °C for 5 min could effectively exclude FTX and remain 75% of FIP-fve. The thermal unstable properties of FTX also provided the explanation of the discrepancy of its molecular-weight determination in the literature [7–9].
Acknowledgments
The analytical instrument was supported by Joint Center for Instruments and Researches, College of Bioresources and Agriculture, National Taiwan University.
This research was financially supported by Ministry of Science and Technology, Taiwan, ROC (project: NSC 100-2221-E-034-001, MOST 103-2815-C-002-173-B).
Abbreviations
- FIP-fve
fungal immunomodulatory proteins from Flammulina velutipes
- FTX
flammutoxin
- FDS
putative uncharacterized protein
- UHA
Unheated ammonium sulfate fraction
- HA
heat treatment and ammonium sulfate fractionation
- CW
cold water extract in water
- CWT
cold water extract in 0.1 M trehalose
- CWE
cold water extract in 20% ethanol
Funding Statement
This research was financially supported by Ministry of Science and Technology, Taiwan, ROC (project: NSC 100-2221-E-034-001, MOST 103-2815-C-002-173-B).
REFERENCES
- 1. Ko JL, Hsu CI, Lin RH, Kao CL, Lin JY. A new fungal immunomodulatory protein, FIP-fve isolated from the edible mushroom, Flammulina velutipes and its complete aminoacid-sequence. Eur J Biochem. 1995;228:244–9. [PubMed] [Google Scholar]
- 2. Kino K, Yamashita A, Yamaoka K, Watanabe J, Tanaka S, Ko K, et al. Isolation and characterization of a new immunomodulatory protein, ling zhi-8 (Lz-8), from Ganoderma lucidium. J Biol Chem. 1989;264:472–8. [PubMed] [Google Scholar]
- 3. Hsu HC, Hsu CI, Lin RH, Kao CL, Lin JY. Fip-vvo, a new fungal immunomodulatory protein isolated from Volvariella volvacea. Biochem J. 1997;323:557–65. doi: 10.1042/bj3230557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lin WH, Hung CH, Hsu CI, Lin JY. Dimerization of the N-terminal amphipathic alpha-helix domain of the fungal immunomodulatory protein from Ganoderma tsugae (Fip-gts) defined by a yeast two-hybrid system and site-directed mutagenesis. J Biol Chem. 1997;272:20044–8. doi: 10.1074/jbc.272.32.20044. [DOI] [PubMed] [Google Scholar]
- 5. Ou CC, Hsiao YM, Wang WH, Ko J, Lin MY. Stability of fungal immunomodulatory protein, FIP-gts and FIP-fve, in IFN-production. Food Agric Immunol. 2009;20:319–32. [Google Scholar]
- 6. Chu PY, Sun HL, Ko JL, Ku MS, Lin LJ, Lee YT, et al. Oral fungal immunomodulatory protein-Flammulina velutipes has influence on pulmonary inflammatory process and potential treatment for allergic airway disease: a mouse model. J Microbiol Immunol Infect. 2017;50:297–306. doi: 10.1016/j.jmii.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 7. Lin JY, Lin YJ, Chen CC, Wu HL, Shi GY, Jeng TW. Cardiotoxic protein from edible mushrooms. Nature. 1974;252:235–7. doi: 10.1038/252235a0. [DOI] [PubMed] [Google Scholar]
- 8. Bernheimer AW, Oppenheim JD. Some properties of flammutoxin from the edible mushroom Flammulina velutipes. Toxicon. 1987;25:1145–52. doi: 10.1016/0041-0101(87)90132-2. [DOI] [PubMed] [Google Scholar]
- 9. Tomita T, Ishikawa D, Noguchi T, Katayama E, Hashimoto Y. Assembly of flammutoxin, a cytolytic protein from the edible mushroom Flammulina velutipes, into a pore-forming ring-shaped oligomer on the target cell. Biochem J. 1998;333:129–37. doi: 10.1042/bj3330129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gonzalez MR, Bischofberger M, Pernot L, van der Goot FG, Freche B. Bacterial pore-forming toxins: the (w)hole story? Cell Mol Life Sci. 2008;65:493–507. doi: 10.1007/s00018-007-7434-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Watanabe H, Narai A, Shimizu M. Purification and cDNA cloning of a protein derived from Flammulina velutipes that increases the permeability of the intestinal Caco-2 cell monolayer. Eur J Biochem. 1999;262:850–7. doi: 10.1046/j.1432-1327.1999.00440.x. [DOI] [PubMed] [Google Scholar]
- 12. Narai A, Watanabe H, Iwanaga T, Tomita T, Shimizu M. Effect of a pore-forming protein derived from Flammulina velutipes on the Caco-2 intestinal epithelial cell monolayer. Biosci Biotechnol Biochem. 2004;68:2230–8. doi: 10.1271/bbb.68.2230. [DOI] [PubMed] [Google Scholar]
- 13. Tong MH, Chien PJ, Chang HH, Tsai MJ, Sheu F. High processing tolerances of immunomodulatory proteins in Enoki and Reishi mushrooms. J Agric Food Chem. 2008;56:3160–6. doi: 10.1021/jf800205g. [DOI] [PubMed] [Google Scholar]
- 14. Pace CN, Trevino S, Prabhakaran E, Scholtz JM. Protein structure, stability and solubility in water and other solvents. Philos Trans R Soc London Ser B. 2004;359:1225–35. doi: 10.1098/rstb.2004.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hottiger T, De Virgilio C, Hall MN, Boller T, Wiemken A. The role of trehalose synthesis for the acquisition of thermotolerance in yeast. II. Physiological concentrations of trehalose increase the thermal stability of proteins in vitro. FEBS J. 1994;219:187–93. doi: 10.1111/j.1432-1033.1994.tb19929.x. [DOI] [PubMed] [Google Scholar]