Abstract
Curcumin (Cur), a polyphenolic compound extracted from spice and common food colourant turmeric, contains versatile bio-activities. Monoacetylcurcumin (MAC), a structural analogue of Cur, differs from Cur by acetyl modification, but retains enone groups. Comparative analysis revealed MAC effectively inhibited influenza virus infection (IAV) to a similar extent as, if not superior to, curcumin. Both compounds mildly reduced viral NA activity. Surprisingly, unlike Cur, the MAC inhibition of IAV did not occur through the blocking of HA activity. However, MAC strongly dampened Akt phosphorylation, the prerequisite signalling for efficient IAV propagation. A much stronger inhibition effect on IAV infection was observed when MAC treatment was in combination with Cur. Collectively, MAC demonstrated clear antiviral activity, and likely inhibited IAV via multiple mechanisms that were not identical to Cur. Importantly, Cur and MAC in combination synergistically inhibited IAV infection.
Keywords: Curcumin, Influenza virus, Antiviral, Haemagglutinin, Synergic
1. Introduction
Influenza A virus (IAV), an enveloped virus belonging to the family Orthomyxoviridae, is responsible for causing the human-to-human transmission of pandemic influenza, as well as cases of seasonal influenza. The World Health Organization (WHO) reports that of the estimated 5–10% of adults and 20–30% of children infected annually, 3–5 million cases of severe disease and 250,000–500,000 deaths occur from influenza each year worldwide. Recently, there has been a rise in the number of cases of bird-to-human transmission of avian influenza A viruses, including different HA subtypes (H5, and H7). H5N1 IAV first infected people in Hong Kong in 1997 and has subsequently infected more than 800 people with an approximate death rate of 60% [1]. In March 2013, a novel subtype of avian influenza A virus, identified as subtype H7N9, was transmitted from birds to human in China [2], and while H7N9 has lower mortality rate than H5N1, cases of H7N9 IAV continue to be reported in China. The most common antiviral drugs used to treat severe influenza target the viral neuraminidase (NA), despite the frequent emergence of viral resistance to these drugs [3]. In light of this, there is a clear need to develop drugs with more varied targets to treat influenza virus infections.
Natural products are potential sources of bioactive compounds, including some with antimicrobial activity [4,5]. Curcumin (Cur), a polyphenolic component of the spice and common food colourant turmeric (from the root of Curcuma longa), has gained much attention for its anti-oxidant and anti-inflammatory [6] activities, along with its anti-proliferative and anti-tumour activities [7]. It has been shown that the regulation of cellular signalling pathways, such as the activation of the transcription factor NF-κB, is central to providing curcumin with its versatile biological properties. Recently, Cur has also been shown to exhibit antiviral activity at non-cytotoxic concentrations against IAV [8–10], as well as against several enveloped viruses, including Japanese encephalitis virus, pseudorabies virus, dengue virus type 2, and vaccinia virus, but not against the non-enveloped enterovirus-71 [9]. The initial investigation into Cur’s anti-influenza activity was based on the importance of NF-κB activation in IAV replication [11] and Cur’s ability to act as a potent NF-κB inhibitor [12]. In addition to NF-κB, Cur also inhibits the phosphatidylinositol-3-kinase (PI3K)/AKT cellular pathway that leads to apoptosis in many tumour cells [13], while influenza virus infection activates PI3K/AKT [14], presumably in an attempt to prevent the cell from undergoing apoptosis and to benefit viral propagation at the initial stage of infection.
The anti-influenza activity of Cur was previously shown to be independent of one or both of the methoxyl groups found on its phenols [8] but is now thought to be dependent on the presence of two enone groups (α,β-unsaturated carbonyl groups) in the seven-carbon chain attached to its phenol rings (see Fig. 1A, dashed boxes). The enone groups of Cur could spontaneously form Michael adducts with sulfhydral (SH) groups [15], and the presence of glutathione (GSH), consisting of amino acids with an SH group, diminished Cur’s ability to inhibit IAV plaque formation [10].
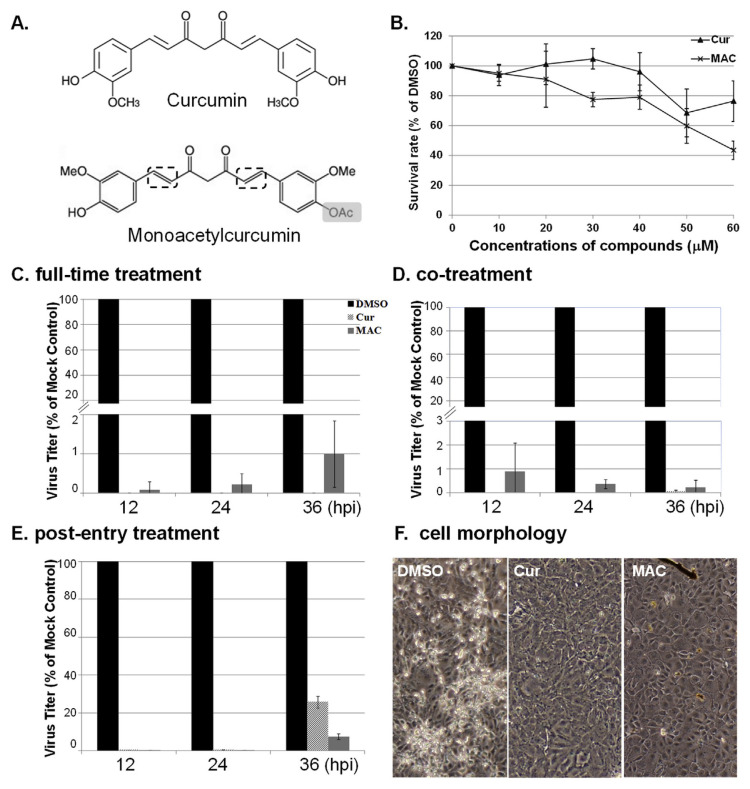
Fig. 1.
MAC inhibits influenza virus replication in a dose-dependent manner. Monoacetylcurcumin (MAC) is a structural analogue of curcumin (Cur). (A) As with Cur, MAC contains two enone functional groups (dashed boxes) and has an acetyl group where Cur has a free hydroxyl group (grey boxes). (B) The cytotoxicity of MAC was tested on MDCK cells. The survival rate was plotted as the percentage of cell numbers to that of cells treated with DMSO, the solvent control. The effect of MAC on influenza viral replication was analysed by time-of-drug addition, including Full-time treatment (C), Co-treatment (D), and Post-entry treatment (E). The titres of MAC or Cur treated cells were plotted as a percentage of the DMSO-treated cell controls (mock control). Data from C–E averaged from 3 independent experiments, ± SD. (F) No obvious cytopathic effects (CPE) were observed in full-time treatment cells treated with 30 μM Cur or MAC at 24 hpi.
It has been reported that application of Cur is limited by its intrinsic properties such as low aqueous solubility and poor stability, leading to poor bioavailability [16]. To circumvent this drawback, several strategies have been proposed, for instance, generation of curcumin structure analogues with bioactivity [17], and development of new formulation [18–20]. Several curcumin structural analogues have been reported and characterised. Among those analogues, tetrahydrocurcumin (THC), a Cur analogue that lacks the enones of Cur, markedly reduced anti-influenza activity compared with Cur [10]. Because THC is a major early metabolite of Cur in vivo [21], it is possible to render Cur less effective as an influenza therapeutic in patients. Monoacetylcurcumin (MAC) has identical enone groups and differs from Cur by only one acetyl group (Fig. 1A, grey box). MAC was first identified as an in vitro inhibitor of mammalian DNA polymerase λ [22], a polymerase involved in DNA repair; Cur also inhibits DNA polymerase λ [23]. More recently, MAC was shown to reduce NF-κB nuclear translocation induced by lipopolysaccharide (LPS) more effectively than curcumin [24], further strengthening its potential as an anti-influenza agent.
Hence, in this study, as MAC and Cur share similar structure, a series of experiments were performed to initially define whether MAC possess anti-influenza activity. Moreover, the underlying mechanisms were further investigated. Our results indicated that as with Cur, MAC acts as a potent inhibitor of influenza virus infection. However, MAC has no impact on viral HA function. As the treatment of cells with both MAC and Cur together demonstrated a synergistic effect, this implies that these two drugs may have different affinities for their targets and could be used in combination as an alternative influenza antiviral therapeutic.
2. Materials and methods
2.1. Cells and virus
Madin-Darby canine kidney (MDCK) cells were grown in minimal–essential medium (MEM) with 10% fetal bovine serum (FBS), and antibiotics (penicillin 100 U/ml, streptomycin 10 μg/ml). For infection, virus was diluted in infection medium (MEM without FBS) supplemented with antibiotics and 1 μg/ml of trypsin (Worthington, Freehold, NJ, USA) and inoculated to cells that have been washed with PBS.
Influenza Type A virus (IAV) strain PR8, A/Puerto Rico/8/34 (PR8, H1N1), was kindly provided by Paul Digard (University of Cambridge, UK).
2.2. Compounds
Curcumin and dimethyl sulfoxide (DMSO) were purchased from Sigma–Aldrich. MAC was synthesized as described in one previous study [22]. Reduced glutathione (GSH), purchased from Sigma-Aldrich, was dissolved in 10 mM of Tris (pH 7.5, adjusted using 5N KOH).
2.3. Cytotoxicity test
The cytotoxicity effect of curcumin and MAC was determined on the basis of cell survival rate [8]. Briefly, 7.5 × 104 MDCK cells seeded in 24-well plates were treated with compounds (DMSO, Cur and MAC) at the indicated concentrations. After 24 h, cells were stained with 0.4% trypan blue and total cell counts were measured. The survival rate was estimated as the ratio of living cells/total cell counts. The survival rate of DMSO-treatment control was arbitrarily set as 100%.
2.4. Time-of-drug addition test
Cur, MAC, or DMSO were included in the medium and added to cells at different time points of infection. Briefly, (1) full time treatment: the test compound was included in the cell culture medium for 8 h prior to and throughout the course of infection (2000 PFU/well); (2) co-treatment: the test compound mixed with virus in the infection medium was added simultaneously onto the cells and remained in the medium cells throughout the course of the experiment; (3) post-entry treatment: Cur and MAC were added to cells at 2 h post infection (hpi) and left with the cells throughout the time of infection.
2.5. Plaque assay
MDCK cells were infected with serial dilutions of IAV at 37 °C. Two hours after adsorption, unbound virus particles were removed and cells were then cultured with 1 ml/well MEM supplemented with 0.6% agarose at 37 °C, 5% CO2 for 2 days or until the plaques were visible. Viral plaques were then fixed with 100% methanol for 10 min and stained with crystal violet (Sigma).
2.6. Plaque reduction assay
Viruses were treated with Cur and MAC, and the remaining infectivity of IAV was determined based on the plaque formation ability [8]. In brief, IAV (100 plaque formation units, pfu) were incubated with test compounds for 1 h and then inoculated onto cell monolayers followed by standard plaque assay.
2.7. Glutathione competition test
The effect of glutathione (GSH) on anti-IAV activity mediated by MAC was evaluated in plaque formation ability. To do so, 60 μM Cur, MAC, THC (the curcumin analogue without enone group, as a negative control), or culture medium (the mock control) was mixed with an equal volume (150 μL) of 4-fold serially diluted (i.e. 20, 10, 5 μM) competitors, glutathione (GSH) for 1 h at room temperature. Then, the mixture of curcumin/competitor was incubated with 200 pfu of IAV for 1 h. The remaining viral infectivity were determined by plaque assay.
2.8. MUNANA assay
PR8 virus (1 × 105 pfu in 20 μL) was initially diluted with 1 μL of 10% NP-40 and 79 μL 1× assay buffer containing 32.5 mM MES (Sigma–Aldrich) and 4 mM CaCl2. To evaluate the effect of curcumoids on NA activity, the diluted viruses (1 μL) were pre-incubated with 49 μL of Cur, MAC, or DMSO (a negative control) at concentration of 120, 60, 30, 15 μM, or 0.3 nM, Tamiflu metabolite (a positive control for NA inhibition) for 60 min at room temperature. The reaction for measuring NA activity was initiated by the addition of 50 μL the fluorogenic substrate 300 μM 2′-(4-methylumbelliferyl)-alpha-D-N-acetylneuraminic acid (MUNANA, Sigma–Aldrich). After 1 h of incubation at 37 °C, the reaction was stopped by the addition of 100 μL of freshly prepared stop solution (0.138 N NaOH in 83% ethanol, pH 10.7).
2.9. Structural modelling of curcumin analogues
The molecular structures of Cur and MAC were constructed by Discovery Studio (DS) 2.5 modelling software (Accelrys Inc., San Diego, CA). Energy minimisation was done by using a salvation model and calculated by the GBSW parameter using Minimization and Dynamics protocols within DS. The calculation was achieved by a CHARMm (Chemistry at HARvard Macromolecular Mechanics) force-field [25].
2.10. Molecular modeling and docking
The crystal structure of PR8 HA (PDB code 1RU7) was obtained from Protein Data Bank and is equivalent in structure to that published previously [26]. Initially, water molecules in this complex structure were removed, and the modified HA after hydrogen saturation was applied to CHARMm force field [25] using the Discover Studio 2.1 package (http://accelrys.com/products/discovery-studio/). The 2D structures of sialic acid (SA), Cur, and MAC were constructed by using the ChemDraw program, and their corresponding 3D structures were converted by the Chem3D program (http://www.cambridgesoft.com/). The ligand binding site in the HA was defined as SA receptor binding site (RBS) in the LSTA-haemagglutinin complex structure (PDB code 1RVX) [26]. In the docking simulation of compounds the ligand-binding domain was defined as the region of the sphere with a 11 Å radius from the center of the binding pocket. Docking of compounds was performed in silico by employing the LibDock module in the Discover Studio 2.1 package, and further minimized by smart minimize algorithm with CHARMm force field in the Discover Studio 2.1 package [27]. The scores of -PLP2 for final docking poses were further calculated by Score Ligand poses module in the Discover Studio 2.1 package [28].
2.11. Detection of PI3K/AKT signaling by western blot analysis
MDCK cell were infected with IAV at an MOI (multiplicity of infection) of 5. At 1 h after infection, 30 μM Cur, MAC, or DMSO (negative control) were added in to cells. Infected cells were harvested in lysis buffer (25 mM Tris–HCl, 150 mM NaCl, 1% NP-40) at 4, 8, 12 hpi. The expression level of AKT, phospho-Akt (Thr308), NS1, β-actin were simultaneously detected by western blot analysis [8] using specific antibodies. Images were taken by ImageQuant LAS 4000 mini (GE Healthcare Life Sciences) and quantified using ImageJ.
2.12. Statistical analysis
All data were calculated with EXCEL (Microsoft Corp., Redmond, WA, USA) and analysed with SAS (SAS Institute, Cary, NC, USA). The results are reported as the mean ± SD.
3. Results and discussion
3.1. Monoacetylcurcumin inhibited influenza A virus replication and plaque formation in a dose-dependent manner
In a series of experiments aimed at testing the inhibitory effects of MAC on IAV replication, MAC (Fig. 1A, right panel), as well as Cur (Fig. 1A, left panel) in MDCK. A concentration of 30 μM was chosen for the compounds, as it showed low cytotoxicity in MDCK cells (Fig. 1B) and, as previously reported, represents an effective concentration of Cur for inhibiting virus replication and is close to the minimal inhibitory concentration of Cur that inhibits influenza viral haemagglutination [8].
Results of time-of-drug addition strategy (Fig. 1C–E) indicated MAC reduced viral titres to approximately 1% or less of the DMSO solvent controls and to a similar extent as Cur in full-length treatment (Fig. 1C) and in co-treatment (Fig. 1D). Noticeably, the inhibitory effect of MAC was stronger than that of Cur at 36 hpi with post-entry treatment, where Cur-treated cells had slightly greater than 20% of the titres of the control cells (Fig. 1E). These data were corroborated by a microscopic examination of the cells (Fig. 1F). With MAC or Cur treatment, there were no visible signs of viral cytopathic effects (CPE) in the cells at 24 hpi (right panels), in contrast to the control cells (left panel). Taken together, MAC clearly demonstrates antiviral activity at each time point tested relative to IAV infection, and its antiviral activity is similar to curcumin.
3.2. MAC inhibited plaque formation ability
The mechanisms of anti-influenza virus activity mediated by MAC were further explored via a series of experiments. Because full-time and co-treatment of cells with MAC showed similar inhibitory levels on IAV production (Fig. 1C and D), MAC might be inhibiting an early step in viral replication and/or directly inactivating virus infectivity. To test whether MAC inhibits an early viral event, influenza virus was pre-treated with MAC at various concentrations for 1 h prior to infection, followed by plaque assays to evaluate the infectivity of IAV. MAC inhibited plaque formation to a similar degree as Cur at corresponding concentrations (Fig. 2A), and when compared with the control treatment, both MAC and Cur completely abrogated IAV plaque formation at concentrations of 10 μM and 30 μM, as well as demonstrating a clear dose–response inhibition. These data indicate that the incubation of MAC with virus is sufficient to inhibit viral infectivity and that it is possibly interfering with the function of the viral surface proteins, which in turn affects the infection of IAV.
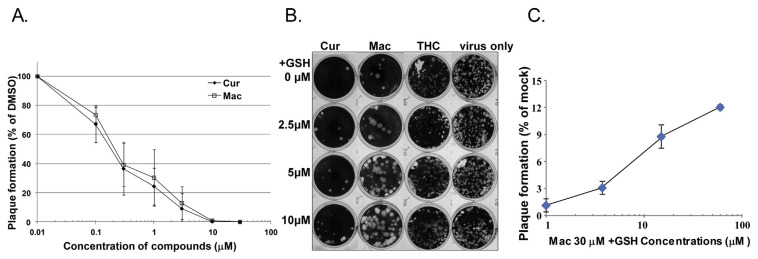
Fig. 2.
MAC and Cur inhibited IAV via multiple mechanisms. (A) MAC inhibits plaque formation ability of IAV and that is partially via Michael addition reactions (B–C). PR8 viruses were pre-incubated with various concentrations of curcumin, MAC, or DMSO for 1 h. The infectivity of treated IAV was estimated with plaque assay and the relative plaque reduction effect of MAC was plotted as a percentage of the DMSO control (A). To test whether reduced glutathione (GSH) attenuated the inhibitory effect of MAC on anti-IAV activity, 30 μM of MAC, THC, or DMSO (mock) was incubated with various concentrations (0–10 μM) of reduced glutathione (GSH, as a competitor) at room temperature followed by plaque reduction assay as described previously (B). The experiments were done three times and the effect of GSH on plaque reduction mediated by MAC was plotted (C).
3.3. Incubation of MAC with glutathione (GSH) impaired MAC’s plaque reduction ability and demonstrated the importance of enone groups for its antiviral activity
We previously hypothesised that the anti-influenza effects of Cur are mediated through its enone groups [10]. Enone groups are thought to participate in covalent reactions (Michael addition reactions) with proteins through the SH groups of cysteine residues within GSH [29]. MAC also contains two enone groups in the same position as Cur (Fig. 1A). Thus, whether MAC also inhibits the plaque formation ability of IAV through Michael addition reactions was worthy of investigation. As indicated in Fig. 2B, MAC (without GSH treatment) decreased the plaque formation units of IAV compared with those of the two control groups, DMSO and THC, another curcumin analogue lacking the enone group that has been shown to exert a lesser inhibitory effect on IAV [10]. However, upon the co-incubation of GSH at increasing concentrations, MAC lost its ability to reduce influenza virus plaque formation in a dose-dependent fashion (Fig. 2C). This result suggests that as with curcumin, MAC possibly modifies a key factor (cellular or/and viral protein) that is required for IAV infection via Michael addition reactions.
3.4. Monoacetylcurcumin did not inhibit viral haemagglutination
Previous experiments showed that Cur inhibits IAV haemagglutination [8–10]. In the current study, the direct incubation of MAC with IAV interfered with IAV’s ability to form plaques (Fig. 2). Therefore, attempts were made to identify the viral target(s) of MAC, in particular the viral surface glycoproteins, neuraminidase (NA) and haemagglutinin (HA).
NA, critical for IAV egress, is the target for a number of anti-IAV therapeutics, including oseltamivir. Because MAC is able to inhibit IAV replication later in replication, as evidenced by reduced viral titres when cells were treated with MAC post-viral entry (Fig. 2C), it is possible that MAC may interfere with viral NA. To test their effect on NA function, MAC or Cur at various concentrations was incubated with IAV for 1 h before performing MUNANA, an NA enzyme activity assay. The NA activity of MAC treatment was compared with that of Cur, the DMSO negative control. As shown in Fig. 3A, the NA inhibitor, oseltamivir metabolite (carboxylate), greatly reduced NA activity. Moreover, both Cur and MAC mildly reduced NA activity in a dose-dependent manner; at 25 μM, MAC treatment resulted in a 20% decrease in NA activity compared with DMSO, while Cur did not decrease NA activity at the same concentration (Fig. 3A). Nevertheless, neither MAC nor Cur was able to completely inhibit NA at concentrations tested to the extent that oseltamivir carboxylate did (Fig. 3A, inset).
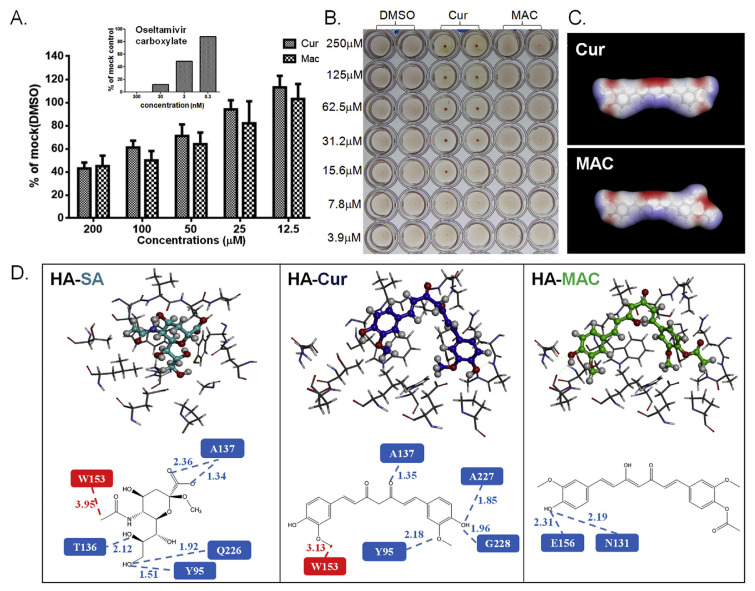
Fig. 3.
The effect of MAC on two viral surface proteins. Effect of Mac on viral HA and NA activity was measured by the MUNANA assay (A) and the haemagglutination inhibitory (HI) test (B), respectively. IAV was incubated with various concentrations of the tested compounds (MAC, Cur, or DMSO control) for 1 h, followed by the MUNANA assay to evaluate the residual viral NA activity after treatment. The fluorescence (indicating NA activity) relative to the DMSO control was estimated based on three independent experiments. (B) IAV was incubated with 2-fold serially diluted MAC, Cur (positive control), or DMSO (vehicle control), and the HI effect was tested by incubation with chicken RBC for 30 min. The energy-minimized 3-dimensional structures of Cur and MAC were analysed computationally (C). The electrostatic potential at each point on a constant electronic density surface is represented graphically in red and corresponds to regions where the electrostatic potential is most negative, while blue corresponds to the most positive regions, and the yellow arrow indicates the acetyl group on MAC. Simulation of the interaction of curcumin analogues with HA (D). The interaction interface of sialic acid (SA), Cur, and MAC on HA (upper panel), as well as the interactive amino acid residues of HA (lower panel), were predicted by docking simulation. Amino acid residues around the binding pocket of HA and the ligand compounds (SA, Cur, and MAC) are shown in stick- and ball-stick structures, respectively (D, upper panel). Detailed intermolecular interactions are illustrated between the HA RBS and ligands, of which the formation of H-bonding and hydrophobic interaction are shown in green and red squares, respectively. Distances (Å) of H-bonding and hydrophobic interaction are shown by green dashes and red dashes, respectively (D, lower panel).
Next, the effect of MAC on IAV haemagglutination was determined via a haemagglutination inhibition assay (HI) assay. Surprisingly, unlike curcumin, MAC did not inhibit HA at any concentration tested (Fig. 3B), indicating that MAC does not inhibit IAV binding to its host cell early in IAV infection.
To determine a possible explanation for their distinct HA inhibition abilities, a computational analysis of Cur and MAC structures was then conducted by using molecular simulation analysis software. The three-dimensional structures of these two compounds were similar. However, the acetyl group produced a bulge structure on one side of the MAC structure (Fig. 3C). Subsequently, molecular docking was performed to predict the interaction of HA with these two compounds in comparison with SA, a positive control for the binding pocket definition in HA.
Consistent with one previous finding [10], SA fits strongly inside the receptor binding site (RBS) of HA (Fig. 3D, HA-SA, top panel), and two types of intermolecular interactions, including hydrogen (H)-bonding and hydrophobic interaction, were observed (Fig. 3D, lower panel). Five H-bonds were formed within the RBS pocket: two between the carboxyl group at C-1 and residue A136, two between C-9 and Y95/Q226, and one between the hydroxyl group at C-8 and T136. In addition, one hydrophobic interaction was found between the N-acetyl group at C-5 and W153 (Fig. 3D, HA-SA). Similar to SA, curcumin also fits adequately inside the binding pocket of HA (Fig. 3D, HA-Cur), which is consistent with a previous finding [10]. Four H-bonds were formed within the RBS pocket: two between the hydroxyl group at C-2 and A227/G228, one between the ketone group at C-9 and A137, and one between C-1 and Y95. One hydrophobic interaction was found between C-11 and W153 (Fig. 3D, HA-Cur, lower panel). Unlike SA and Cur, a different interaction profile was observed between MAC and HA. Only two H-bonds were formed between C-2 and E156/N131 (Fig. 3D, HA-MAC). The theoretical binding affinity scores (PLP2 values) of SA, Cur, and MAC to HA were −75.97, −89.21, and −68.93, respectively. Nevertheless, an overlap plot of docking structures indicated that the fitness of MAC to the SA binding groove was lower than that of Cur.
It is possible that the modification of a bulky acetyl group at the C-2 position on MAC causes steric hindrance to the interaction with the HA binding pocket, which in turn lowers the fitness with HA and leads to the failure of HA inhibition by MAC.
3.5. Monoacetylcurcumin-treated influenza virus-infected cells exhibited decreased levels of AKT phosphorylation
Influenza viruses hijack intracellular signalling pathways, such as NF-κB, PI3K/Akt, and MAPK signalling, for their own benefits [30]. IAV activates the PI3K/AKT pathway, which is required for multiple functions in the course of viral infection [14]. Curcumin has been demonstrated to inhibit tumour growth via the suppression of the PI3K/AKT signal transduction pathway [31], a key player in cell-growth signalling. Because the effect of MAC on AKT activation has not yet been reported, we first investigated whether MAC would block the activation of the PI3K/AKT pathway and, in turn, dampen IAV replication.
It has been shown that Cur interferes with viral entry [8,10], and thus lowered virus load could further reduce the induction of cellular signalling. To rule out this possible bias, a post-entry protocol was used to address the net effect of Cur/MAC in infected cells. Indeed, the amount of phosphorylated AKT (Thr308) increased from 4 to 12 hpi in each group, with the virus only (PR8) control displaying the largest increase, while the basal level of AKT protein did not change in either treatment group over time (Fig. 4A). Furthermore, both the MAC-and Cur-treated infected cells had decreased amounts of phosphorylated AKT, which was coincident with a decrease in NS1 accumulation at all time points compared with that of IAV-infected cells without treatment; normalized AKT-pi and NS1 levels are shown in Fig. 4A in the lower panels. Noticeably, the AKT phosphorylation level in MAC-treated cells was lower than that in the Cur-treated group, suggesting that MAC was acting as a strong suppressor of PI3K/AKT pathway activation. It has been shown that the PI3K/AKT pathway, a cellular signalling pathway for the anti-viral response, is misused by the IAV to support its own replication [14]. Hence, it was suggested that a fully functional PI3K/AKT pathway is critical for efficient viral entry. In our study, MAC was given after viral adsorption, and it is thus possible that the suppression of PI3K/AKT activation likely affects membrane fusion, which is worthy of further investigation.
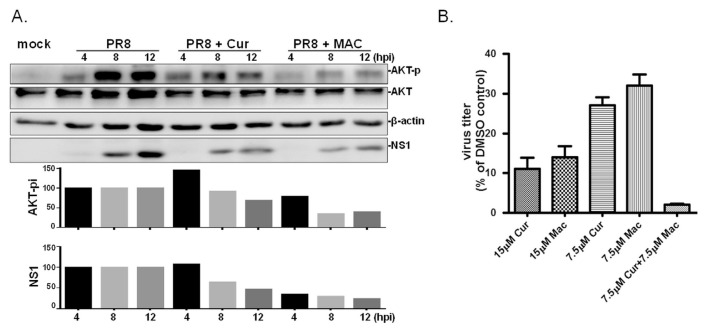
Fig. 4.
MAC dampens influenza virus-induced activation of AKT signalling pathway and works in synergy with Cur. (A) MDCK cells were treated with MAC or Cur at 1 h post IAV infection (at MOI of 5 viruses per cell); PR8 indicates cells infected with PR8 without treatment. The total proteins were harvested at 4, 8, 12 hpi. Basal level of AKT, the phosphorylated AKT (AKT-p), viral NS1 protein (NS1), and β-actin were simultaneously detected by Western blot analysis. This experiment was conducted twice and results presented with similar trends. The relative level of phosphorylated AKT and NS1 was normalized with basal AKT, or actin, respectively, in each sample. Subsequently, the relative level of AKT-p and NS1 in infected cells treated with MAC or Cur was estimated by comparing with those in mock treatment at the same time point. (B) MDCK cells were treated with MAC alone, or in combination with Cur at concentrations indicated at 8 h prior to IAV infection (2000 PFU). The tested compounds were present in the medium throughout the time of infection. The yield of viral progenies was determined at 24 hpi by standard plaque assay. The experiment was conducted three times and the relative viral titer was plotted as the percentage of plaque number to that of cells treated with DMSO, the solvent control.
3.6. Synergistic effect of curcumin and MAC on inhibition of IAV infectivity
Results in current study indicated both MAC and Cur inhibit IAV; possibly via non-identical modes of action. Attempts was then made to investigate whether these two compounds have the synergistic effect on anti-influenza activity. Because treatment with 30 μM curcumin or MAC strongly abrogated IAV infection, a lower concentration (15 μM) was then used to evaluate the possible synergistic effects. Under full-time treatment conditions, the cells that were MAC-treated at 15 μM produced approximately 14% of the amount of virus compared with DMSO-treated cells and yielded approximately 32% of the amount of virus at 7.5 μM compared with the control (Fig. 4B), demonstrating a dose response to MAC. Interestingly, when the cells were treated with 7.5 μM MAC in combination with 7.5 μM Cur, the virus yield was reduced to 2% of that of the control group. It is worth noting that this inhibition strength was significantly stronger than treatment with 15 μM of the individual compounds, either MAC or Cur (11% or 15%, respectively), indicating that MAC and Cur inhibit IAV in a synergistic fashion and possibly possess different modes of anti-influenza action.
4. Conclusions
In our study, we demonstrated for the first time that MAC possesses a potent inhibitory effect on IAV infection. Despite being a structural analogue of curcumin, it abrogates IAV propagation via different mechanisms of action from curcumin. MAC causes the suppression of PI3K/AKT activation, which is likely one of the mechanisms that accounts for the lowered replication of IAV in cells. A synergistic antiviral effect was observed when Cur in combination with MAC. It suggests an alternative strategy for the clinical application of Cur.
Acknowledgment
The authors would like to acknowledge Professor S.R. Shih, Chang Gung University, Taiwan, for technique support. This study was partial funded by the National Science Council, Taiwan (MOST-105-2321-B-005-012), and by Biotechnology center, National Chung Hsing University (NCHU-CSMU-10511).
Funding Statement
This study was partial funded by the National Science Council, Taiwan (MOST-105-2321-B-005-012), and by Biotechnology center, National Chung Hsing University (NCHU-CSMU-10511).
Footnotes
Conflicts of interest
The authors declare that there are no conflicts of interest.
REFERENCES
- 1. Mertz D, Kim TH, Johnstone J, Lam PP, Science M, Kuster SP, et al. Populations at risk for severe or complicated Avian Influenza H5N1: a systematic review and meta-analysis. PLoS One. 2014;9:e89697. doi: 10.1371/journal.pone.0089697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gao R, Cao B, Hu Y, Feng Z, Wang D, Hu W, et al. Human infection with a novel avian-origin influenza a (H7N9) virus. NEJM. 2013;368:1888–97. doi: 10.1056/NEJMoa1304459. [DOI] [PubMed] [Google Scholar]
- 3. Gubareva LV. Molecular mechanisms of influenza virus resistance to neuraminidase inhibitors. Virus Res. 2004;103:199–203. doi: 10.1016/j.virusres.2004.02.034. [In eng] [DOI] [PubMed] [Google Scholar]
- 4. Chen GH, Lin YL, Hsu WL, Hsieh SK, Tzen JTC. Significant elevation of antiviral activity of strictinin from Pu’er tea after thermal degradation to ellagic acid and gallic acid. J Food Drug Anal. 2015;23:116–23. doi: 10.1016/j.jfda.2014.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wu PW. A review on the analysis of ingredients with health care effects in health food in Taiwan. J Food Drug Anal. 2015;23:343–50. doi: 10.1016/j.jfda.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brouet I, Ohshima H. Curcumin, an anti-tumour promoter and anti-inflammatory agent, inhibits induction of nitric oxide synthase in activated macrophages. BBRC (Biochem Biophys Res Commun) 1995;206:533–40. doi: 10.1006/bbrc.1995.1076. [In eng] [DOI] [PubMed] [Google Scholar]
- 7. Aggarwal BB, Kumar A, Bharti AC. Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res. 2003;23:363–98. [In eng] [PubMed] [Google Scholar]
- 8. Chen DY, Shien JH, Tiley L, Chiou SS, Wang SY, Chang TJ, et al. Curcumin inhibits influenza virus infection and haemagglutination activity. Food Chem. 2010;119:1346–51. [In English] [Google Scholar]
- 9. Chen TY, Chen DY, Wen HW, Ou JL, Chiou SS, Chen JM, et al. Inhibition of enveloped viruses infectivity by curcumin. PLoS One. 2013;8:1–11. doi: 10.1371/journal.pone.0062482. [In English] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ou JL, Mizushina Y, Wang SY, Chuang DY, Nadar M, Hsu WL. Structure-activity relationship analysis of curcumin analogues on anti-influenza virus activity. FEBS J. 2013;280:5829–40. doi: 10.1111/febs.12503. [In English] [DOI] [PubMed] [Google Scholar]
- 11. Nimmerjahn F, Dudziak D, Dirmeier U, Hobom G, Riedel A, Schlee M, et al. Active NF-kappaB signalling is a prerequisite for influenza virus infection. J Gen virol. 2004;85:2347–56. doi: 10.1099/vir.0.79958-0. [DOI] [PubMed] [Google Scholar]
- 12. Bharti AC, Donato N, Singh S, Aggarwal BB. Curcumin (diferuloylmethane) down-regulates the constitutive activation of nuclear factor-kappa B and IkappaBalpha kinase in human multiple myeloma cells, leading to suppression of proliferation and induction of apoptosis. Blood. 2003;101:1053–62. doi: 10.1182/blood-2002-05-1320. [DOI] [PubMed] [Google Scholar]
- 13. Deeb D, Jiang H, Gao X, Al-Holou S, Danyluk AL, Dulchavsky SA, et al. Curcumin [1,7-bis(4-hydroxy-3-methoxyphenyl)-1-6-heptadine-3,5-dione; C21H20O6] sensitizes human prostate cancer cells to tumor necrosis factor-related apoptosis-inducing ligand/Apo2L-induced apoptosis by suppressing nuclear factor-kappaB via inhibition of the prosurvival Akt signaling pathway. J Pharmacol Exp Therapeut. 2007;321:616–25. doi: 10.1124/jpet.106.117721. [In eng] [DOI] [PubMed] [Google Scholar]
- 14. Ehrhardt C, Wolff T, Pleschka S, Planz O, Beermann W, Bode JG, et al. Influenza A virus NS1 protein activates the PI3K/Akt pathway to mediate antiapoptotic signaling responses. J Virol. 2007;81:3058–67. doi: 10.1128/JVI.02082-06. [In eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Awasthi S, Pandya U, Singhal SS, Lin JT, Thiviyanathan V, Seifert WE, Jr, et al. Curcumin-glutathione interactions and the role of human glutathione S-transferase P1-1. Chem Biol Interact. 2000;128:19–38. doi: 10.1016/s0009-2797(00)00185-x. [In eng] [DOI] [PubMed] [Google Scholar]
- 16. Goel A, Kunnumakkara AB, Aggarwal BB. Curcumin as “Curecumin”: from kitchen to clinic. Biochem Pharmacol. 2008;75:787–809. doi: 10.1016/j.bcp.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 17. Prasad S, Tyagi AK. Curcumin and its analogues: a potential natural compound against HIV infection and AIDS. Food Funct. 2015;6:3412–9. doi: 10.1039/c5fo00485c. [In eng] [DOI] [PubMed] [Google Scholar]
- 18. Hu B, Liu XX, Zhang CL, Zeng XX. Food macromolecule based nanodelivery systems for enhancing the bioavailability of polyphenols. J Food Drug Anal. 2017;25:3–15. doi: 10.1016/j.jfda.2016.11.004. [In English] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pathakoti K, Manubolu M, Hwang HM. Nanostructures: current uses and future applications in food science. J Food Drug Anal. 2017;25:245–53. doi: 10.1016/j.jfda.2017.02.004. [In English] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lin CH, Chen CH, Lin ZC, Fang JY. Recent advances in oral delivery of drugs and bioactive natural products using solid lipid nanoparticles as the carriers. J Food Drug Anal. 2017;25:219–34. doi: 10.1016/j.jfda.2017.02.001. [In English] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pan MH, Huang TM, Lin JK. Biotransformation of curcumin through reduction and glucuronidation in mice. Drug Metab Dispos. 1999;27:486–94. [In eng] [PubMed] [Google Scholar]
- 22. Mizushina Y, Ishidoh T, Takeuchi T, Shimazaki N, Koiwai O, Kuramochi K, et al. Monoacetylcurcumin: a new inhibitor of eukaryotic DNA polymerase lambda and a new ligand for inhibitor-affinity chromatography. BBRC (Biochem Biophys Res Commun) 2005;337:1288–95. doi: 10.1016/j.bbrc.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 23. Mizushina Y, Hirota M, Murakami C, Ishidoh T, Kamisuki S, Shimazaki N, et al. Some anti-chronic inflammatory compounds are DNA polymerase lambda-specific inhibitors. Biochem Pharmacol. 2003;66:1935–44. doi: 10.1016/s0006-2952(03)00551-3. [DOI] [PubMed] [Google Scholar]
- 24. Nishida M, Nishiumi S, Mizushina Y, Fujishima Y, Yamamoto K, Masuda A, et al. Monoacetylcurcumin strongly regulates inflammatory responses through inhibition of NF-kappaB activation. Int J Mol Med. 2010;25:761–7. doi: 10.3892/ijmm_00000402. [In eng] [DOI] [PubMed] [Google Scholar]
- 25. Brooks BRBRE, Olafson BD, States DJ, Swaminathan S, Karplus M. CHARMM: a program for macromolecular energy minimization and dynamics calculations. J Comp Chem. 1983;4:187–217. [Google Scholar]
- 26. Gamblin SJ, Haire LF, Russell RJ, Stevens DJ, Xiao B, Ha Y, et al. The structure and receptor binding properties of the 1918 influenza hemagglutinin. Science. 2004;303:1838–42. doi: 10.1126/science.1093155. [DOI] [PubMed] [Google Scholar]
- 27. Dixon SL, Merz KM., Jr One-dimensional molecular representations and similarity calculations: methodology and validation. J Med Chem. 2001;44:3795–809. doi: 10.1021/jm010137f. [DOI] [PubMed] [Google Scholar]
- 28.Gehlhaar DK, Bouzida D, Rejto PA. ACS symposium series. In: Parrill LRRM, editor. Rational drug design: novel methodology and practical applications. Vol. 719. Washington, DC: American Chemical Society; 1999. pp. 292–311. [Google Scholar]
- 29. Narayan V, Ravindra KC, Chiaro C, Cary D, Aggarwal BB, Henderson AJ, et al. Celastrol inhibits Tat-mediated human immunodeficiency virus (HIV) transcription and replication. J Mol Biol. 2011;410:972–83. doi: 10.1016/j.jmb.2011.04.013. [In eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gaur P, Munjhal A, Lal SK. Influenza virus and cell signaling pathways. Med Sci Monit. 2011;17:RA148–R154. doi: 10.12659/MSM.881801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wu JC, Lai CS, Badmaev V, Nagabhushanam K, Ho CT, Pan MH. Tetrahydrocurcumin, a major metabolite of curcumin, induced autophagic cell death through coordinative modulation of PI3K/Akt-mTOR and MAPK signaling pathways in human leukemia HL-60 cells. Mol Nutr Food Res. 2011;55:1646–54. doi: 10.1002/mnfr.201100454. [DOI] [PubMed] [Google Scholar]