Abstract
Mulberry (Morus alba L.) leaves are widely used as herbal tea to prevent heat stroke. Potential chemical markers of the antioxidant properties and its correlation with harvesting times and leaf location were explored in this study. A 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical-scavenging assay guided isolation of mulberry leaves extract provided five phenolic compounds: 5-O-caffeoylquinic acid (1), 4-O-caffeoylquinic acid (2), gastrodin (3), isoquercetin (4) and rutin (5). The 50% radical-scavenging concentrations (SC50) of these compounds were 32.76 ± 0.27, 11.41 ± 0.48, 404.30 ± 4.92, 10.63 ± 0.96, and 10.57 ± 0.61 μg/mL, respectively. Chromatographic fingerprinting allowed content analysis of 1–5 in samples over a 12-month period. Compounds 1–5 were abundance in apical leaves (0–10 cm) in January and February at temperatures < 20 °C. Contents of 2 and 5 were highest in these months and were strongly correlated to the antioxidant property. Therefore, we suggested that the mulberry leaves harvested during January and February have high yield of 4-O-caffeoylquinic acid and this compound can be used as antioxidative marker in mulberry leaves.
Keywords: Morus alba, Leaves location, Antioxidant, 4-O-Caffeoylquinic acid, Harvest time
1. Introduction
Mulberry (Morus alba L.) is globally cultivated due to its economic importance as a food for silkworms (Bombyx mori L.) which dates 5000 years ago [1]. Besides of its agronomic value, leaves of mulberry are traditionally used in Asia and Europe either as food in the form of beverage or as decoction remedy for sore throat [2,3]. Several health benefits can be derived using leaves of M. alba. Previous studies reported the following biological activities; anti-oxidant, antibacterial, antiviral, anticancer, antifatigue, hypolipidemic, hepatoprotective, neuroprotective, inhibitors of α-glucosidase, tyrosinase, hypertension, and arteriosclerosis [4–6]. Phytochemicals in mulberry leaves such as phenolic acids, flavonol glycosides, chalcones, alkaloids, γ-aminobutyric acid, iminosugars, prenylated stilbenes, derivatives of aryl benzofuran and coumarins were responsible for the observed bioactivities [4–6]. The anti-oxidative property of M. alba was due to its ability to prevent lipid peroxidation and adipocytokine dysregulation [7]. Polyphenolic acids and glucosides were characterized from its leaves using HPLC-IT-TOF-MS techniques [8]. Furthermore, variation in flavonol glycosides was observed in different cultivars of mulberry in Japan [9], as much as significant variation in antioxidant potential were determined from three Morus species in Pakistan [10].
The surmounting evidence of the ameliorative properties against oxidative stress of the components of mulberry fruits and leaves when consumed as food, leads to increased attention of its application for the treatment of hyperglycemia [11]. Meanwhile as a medicinal material in China, the leaves were collected pass the winter frost [12]. This brings attention on the possible effects of environmental conditions on the content of bioactive substances in mulberry leaves. For an example, increased in applied nitrogen has an inverse effect on the chlorogenic acid but a positive significant effect on 1-deoxynojirimycin content [4]. Growing demand for this raw material as functional food sources due to its high antioxidant capacity would require mulberry farmers to address the good agricultural and collection practice (GACP) requirements to assure quality and effectiveness of harvested products. The aim of the present work was to identify potential chemical markers for the antioxidant properties of M. alba leaves using a bioassay guided isolation scheme. This study also determined the best harvest time and leaf location which provide most of these bioactive polyphenols. This method shall help ensure the quality of the material source. To the best of our knowledge, this is the first study conducted to monitor content changes of the antioxidant components in M. alba leaves for a period of 1 full year.
2. Materials and methods
2.1. Chemicals and reagents
2,2-Diphenyl-1-picrylhydrazyl (DPPH), Folin-Ciocalteu phenol reagent, trifluoroacetic acid (TFA), sulfuric acid, sodium carbonate, vanillin, gallic acid, l-ascorbic acid, (+)-catechin, deuterium oxide (D2O), and deuterated methanol (CD3OD) were purchased from Sigma–Aldrich (St. Louis, MO, USA). The Purospher STAR RP-18e column (4.0 mm i.d. × 250 mm, 5 μm) was purchased from Merck (Darmstadt, Germany). Methanol and acetonitrile were LiChrosolv and were purchased from Merck. Diaion HP-20 gel (Mitsubishi Chemical Industry, Tokyo, Japan), Sephadex LH-20 gel (GE Healthcare Biosciences, Uppsala, Sweden), LiChroprep RP-18 gel (40–63 μm, Merck), MCI CHP20P gel (Supelco, Bellefonte, PA, USA) and an Oasis HLB cartridge (12 mL, 500 mg, Waters, Milford, MA, USA) were used for the purification and isolation processes. Polyvinylidene difluoride (PVDF) 0.45-μm 13 mm syringe filters was purchased from IT’S Science Corporation (Taipei, Taiwan).
2.2. Plant materials
Commercial dry leaves of M. alba L. (locally known as Sang Ye) were purchased from a local traditional Chinese medicine (TCM) store in Taipei, Taiwan. Leaves were pulverized using an electric grinder. Fresh leaves of M. alba were collected from the mulberry tree at the botanical garden (N 25°01′30.340, E 121°33′36.287) located in the university campus of Taipei Medical University, from July 2014 to June 2015 (Fig. S1). This tree has a main tree trunk circumference of 57 cm and a crown height of about 450 cm. Leaves were collected from a branch located about 350 cm from the ground. During the 12-month sampling period, changes of the climatic factors over Taipei, Taiwan such as temperature (°C), the amount of precipitation (mm), and sunshine duration (h) were recorded using the Central Weather Bureau’s online database [13], Figs. S2–S4. Leaves were classified according to their relative position from the top of a branch. Apical leaves were collected from 0 to 10 cm, followed by middle leaves from 10 to 30 cm and bottom leaves from ≥30 cm. These were rinsed with reversed osmosis (R.O.) purified water and air dried at room temperature for 5–7 days while avoiding direct sunlight exposure. Only air-dried leaves with <15% moisture (AND MX-50, Tokyo, Japan) were pulverized using an electric grinder and kept in vacuum sealed containers avoiding direct sunlight until used for analysis. A voucher specimen was deposited in the School of Pharmacy, College of Pharmacy, Taipei Medical University (Taipei, Taiwan).
Furthermore, another batch of fresh leaves of M. alba L. were collected from Yangmingshan National Park (N 25°09′34.230, E 121°32′46.392), a mountainous region at the northern part of Taiwan about 14.95 km away from Taipei on March 2017 (Fig. S5). This tree has a trunk circumference of 58.5 cm and a crown height of 500 cm. Leaves were collected from a branch about 350 cm from the ground. Two groups of leaves were classified as young (budding, translucent light green leaflets) and old (mature, opaque dark green to yellow leaves).
2.3. Extraction and bioassay-guided isolation
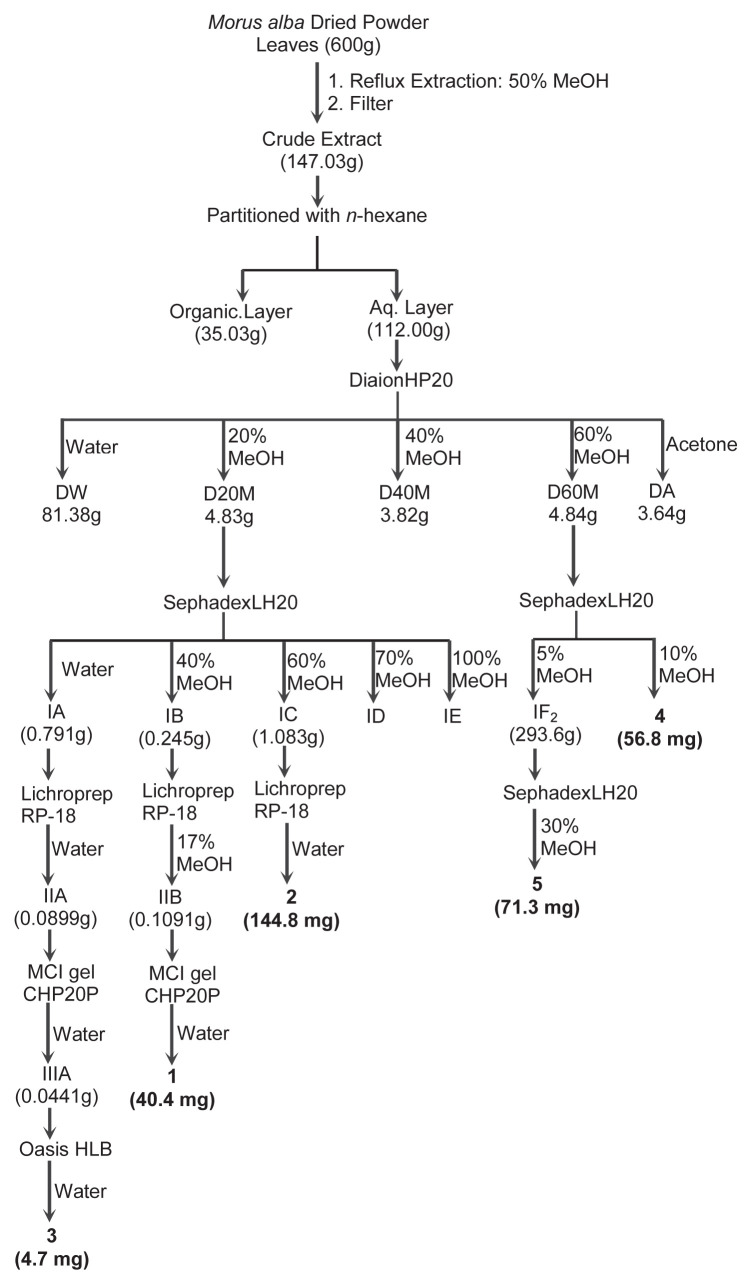
Commercial leaf powder (600 g) of M. alba was refluxed three times with 6 L of water-methanol (1:1, v/v) at 65 °C for 2 h. After filtration and concentration, the aqueous solution was lyophilized to obtain the 50% methanolic extract (147.03 g). The crude extract was re-suspended in 500 mL deionized water and partitioned with 500 mL n-hexane six times to provide an aqueous layer (112.00 g) and an organic layer (35.03 g). Following a DPPH radical scavenging activity-guided isolation, as described in the succeeding sections, the more bioactive aqueous layer (100 g) was chromatographed over a Diaion HP-20 (9.5 cm i.d. × 45 cm) column chromatography (CC), and eluted step-wise with deionized water, followed by methanol/water (2:8, 4:6, 6:4, v/v) and acetone. Resulting fractions were designated as DW (81.38 g), D20M (4.83 g), D40M (3.82 g), D60M (4.84 g), and DA (3.64 g). The most bioactive Diaion fraction D20M (4.00 g) was re-chromatographed on a Sephadex LH-20 (1.5 cm i.d. × 35.5 cm) CC and successively eluted with deionized water, methanol/water (4:6, 6:4, 7:3, v/v) and MeOH. The eluates were respectively assigned as IA–IE. Fractions IA–IC provided compounds 1–3. Briefly, fraction IB (0.245 g) was re-chromatographed with methanol/water (1.7:8.3, v/v) through a reversed-phase LiChroprep RP-18 (1.0 cm i.d. × 40.0 cm) CC and provided fraction IIB (0.1091 g). This was then passed through an MCI CHP20P gel (1.0 cm i.d. × 20 cm) CC using deionized water as an eluent to provide compound 1 (40.4 mg). Fraction IC (1.083 g) was loaded onto a LiChroprep RP-18 (1.0 cm i.d. × 40.0 cm) CC and eluted with deionized water to afford compound 2 (144.8 mg). Fraction IA (0.791 g) was passed through a LiChroprep RP-18 (1.0 cm i.d. × 40.0 cm) CC with deionized water to provide fraction IIA (89.9 mg). This was loaded onto an MCI CHP-20P gel (1.0 cm i.d. × 20 cm) CC and run with deionized water to afford fraction IIIA (44.1 mg). This was further purified using a solid-phase extraction Oasis HLB cartridge (1.5 cm i.d. × 1.0 cm) with deionized water as an eluent to afford compound 3 (4.7 mg). The other bioactive fraction D60M (4.84 g) was re-chromatographed on a Sephadex LH-20 (1.5 cm i.d. × 35.5 cm) CC, with successive elution with deionized water, methanol/water (0.5:9.5, 1.0:9.0, v/v) and then 10% increments of aqueous methanol until 100% MeOH was eluted out. Resulting fractions were designated IF1–12. The 10% aqueous methanol fraction (IF3) provided compound 4 (56.8 mg). The 5% aqueous methanol eluent provided fraction IF2 (293.6 mg) and was further purified and loaded onto a Sephadex LH-20 (1.0 cm i.d. × 37.5 cm) column. This was eluted with methanol/water (3.0:7.0, v/v) and provided compound 5 (71.3 mg). Structures of isolated compounds 1–5 were identified by nuclear magnetic resonance (1H NMR, 13C NMR, and 2D NMR) spectroscopic analyses (Bruker Avance DRX 500 MHz, Bruker Corp., Billerica, Massachusetts, USA), and their molecular weights were confirmed by a mass spectrometric analysis (API 2000TM System, AB SCIEX, Framingham, MA, USA) on the negative ESI mode. A summary of the isolation scheme is shown in Fig. 1.
Fig. 1.
Schematic diagram on the isolation of 1–5 from M. alba leaves.
2.4. Determination of condensed tannins content
Condensed tannins content was determined using a vanillin assay [14,15] with modifications. Briefly, vanillin (0.1 g) was dissolved in 10 mL of 80% H2SO4 in methanol. One milligram of each Diaion fractions was dissolved accordingly in 1 mL alcohol. An aliquot of 300 μL of each sample was mixed with 600 μL of the vanillin reagent and incubated for 15 min at 20 °C, after which the absorbance was measured at 530 nm in an MRX microplate reader (Dynex Technologies). Condensed tannins content was determined through a calibration curve, y = 10.28x + 0.036 of (+)-catechin in methanol at a range of 0.977–250 μg/mL (R2 = 0.999). Results are expressed as catechin equivalents (CE, μg catechin/mg sample).
2.5. Total phenolic content determination
The total phenolic content was determined following the Folin-Ciocalteu method [16,17] with modifications. The Diaion fractions DW, D20M, D40M, and D60M were dissolved in deionized water, while DA was dissolved in ethanol. Each test solutions were 1 mg/mL concentration. Then, 100 μL of each test fraction was incubated in the dark with 500 μL of Folin-Ciocalteu reagent and 400 μL of 7.5% aqueous Na2CO3 solution at 50 °C for 5 min. The absorbance at 600 nm was measured using an MRX microplate reader (Dynex Technologies). The total phenolic content was expressed as gallic acid equivalents (GAE, μg gallic acid/mg sample) using a calibration curve of y = 5.601x + 0.109 at a range of 15.63–500 μg/mL (R2 = 0.995) of standard gallic acid in ethanol.
2.6. DPPH radical scavenging assay
The antioxidant properties of the Diaion fractions and isolates were determined by evaluating their individual ability to scavenge DPPH free radical following previous methods [18,19] with modifications. The Diaion fractions DW, D20M, and DA were dissolved in ethanol, while the D40M and D60M fractions were dissolved in methanol. The Diaion fractions were treated similarly to the steps provided for the isolated compounds. A 100-μL aliquot of each stepwise diluted methanolic solution of each isolates was loaded, in triplicate, into a 96-well microtiter plate. This was performed over a concentration range of 3.91–62.50 μg/mL for compound 1; 1.95–15.63 μg/mL for compounds 2 and 4; 15.63–500 μg/mL for compound 3; and 3.91–15.63 μg/mL for compound 5. Meanwhile, ascorbic acid was used as a control at a concentration range of 8.87 μM to 0.567 mM. Moreover, 100 μL of a 200-μM ethanolic DPPH solution was added to each sample and incubated for 30 min at room temperature in the absence of light. The optical densities of the individual wells were recorded at 530 nm with an MRX microplate reader (Dynex Technologies, Guernsey, Channel Islands, UK). To evaluate, the DPPH radical-scavenging rate (%) = [1 − (ST/C)] × 100 was used, where ST and C are the values of the optical densities of the test sample and control, respectively.
Apical leaves were evaluated for its antioxidative property following the above method by dissolving the plant powders in methanol at a concentration range of 4.88–1.00 × 104 μg/mL. Plant powders were from the crude methanolic extract prepared following the extraction procedure for determining content variation of chemical markers in M. alba leaves.
2.7. Chromatographic fingerprints
The high-performance liquid chromatography (HPLC) apparatus used was LC-2010CHT (Shimadzu Scientific Instruments, Kyoto, Japan), with a serial dual plunger micro-volume pump and an auto-injector. The UV detector used a deuterium lamp source with a two-wavelength monitoring system at 190–370 nm or 371–600 nm. The stationary phase was a Purosphere STAR RP-18e reversed-phase column (Merck). The mobile phase was 0.05% TFA in water–CH3CN (v/v) with a gradient elution program (0–10 min, 99:1 to 92:8; 10–20 min, 92:8; 20–25 min, 92:8 to 88:12; 25–35 min, 88:12; 35–40 min, 88:12 to 84:16; 40–50 min, 84:16; 50–55 min, 84:16 to 80:20; and 55–65 min, 80:20). The flow rate was maintained at 1.0 mL/min, and the column temperature was maintained at 40 °C. The ultraviolet detector was set at 280 nm, to record the absorbance of the chemical markers, and generated fingerprint chromatograms.
Individual calibration curves of each chemical marker were constructed from the plot of the peak area against the concentration. Briefly, the isolated markers (1–5) were dissolved and serially diluted with methanol to provide a range of standard solutions as follows: 1, 0.24–500 μg/mL; 2, 3.91–2000 μg/mL; 3, 1.95–2000 μg/mL; and 4 and 5, 1.95–1000 μg/mL. The sample solutions were analyzed using the same HPLC conditions to generate the chromatographic fingerprint.
2.8. Intraday and interday variability and recovery
The precision and accuracy of the analytical method were evaluated by performing intraday and interday variability and recovery experiments using a commercially available sample. A spiked sample was prepared by adding a known concentration of each marker to the sample matrix solution. Meanwhile, a sample matrix was prepared and analyzed in accordance with the same added quantity as in the spiked sample. The extracted samples were then analyzed using the same HPLC conditions as described in the chromatographic fingerprinting, and the recovery rate (%) was calculated as follows:
where A is the concentration of the chemical markers in the un-spiked sample matrix, B is the concentration of the markers added in the standard diluent sample, and C is the concentration of the markers in the spiked sample. All analyses were in triplicate.
2.9. HPLC quantitation of chemical markers in mulberry leaves
Two grams of leaf powder was dissolved in 5.0 mL of methanol and subjected to ultrasonic-assisted extraction (Delta Ultrasonic Cleaner DC200H, New Taipei City, Taiwan) set to 25 °C. The extraction was completed in a period of 1 h. The sample solutions were then centrifuged at 10,000 rpm, and the extracts were passed through a 0.45-μm PVDF 13 mm diameter filter. The chemical marker contents of the filtrates were quantified using the established reversed-phase HPLC analytical method.
2.10. Data analysis
All experiments performed and data collected were at least triplicate. These were analyzed using IBM SPSS V22.0 (IBM Corporation, New York, USA). Results are reported as mean ± standard deviation. Means were compared using ANOVA and followed by a Tukey posthoc test. Significance in difference was decided when p value < 0.05. Correlations between variables were analyzed using Pearson’s correlation coefficient. Multiple linear regression analysis with stepwise elimination method was used to establish the best regression model that predicted the antioxidant activity. The ratio between the variability explained by the regression model to the unexplained variability was computed with F-statistics.
3. Results and discussion
3.1. Isolated phenolic compounds as antioxidant chemical markers from M. alba leaves
Using a bioassay guided isolation scheme two phenolic acids, one phenolic-glycoside and two flavonol-glycosides were isolated from the 50% methanolic extract of M. alba leaves. The presence of these compounds in the respective fractions was supported by the phytochemical assay results, Table 1. The occurrence of flavonoids was identified at the higher methanolic fractions by determining their condensed tannin content. The 6–7-fold difference in the flavonoid content of the higher methanolic fractions compared with D20M suggested that the 20% methanolic fraction mostly contained phenolic acids and their derivatives. Total phenolic content in D20M was the highest followed by D60M, D40M, while no significance in the difference, p = 0.097, on the phenolic content of DW and DA. DPPH free radical scavenging assay showed that among the Diaion HP-20 column chromatography fractions, D20M (62.82%) was the most bioactive compared to D40M (37.18%) and D60M (35.04%), which the latter two do not differ significantly, p = 0.338. Followed by DW (16.00%) and DA (6.71%) being the least active. The potency of the partial fractions to scavenge 50% of the DPPH radicals (SC50) indicated that the D20M fraction was twice more effective compared to the D60M fraction, evaluated at a concentration of 39.06 μg/mL. These results were supported by a strong positive Pearson’s correlation, r = 0.924 (p < 0.001), between total phenolic content and their radical scavenging activity.
Table 1.
Phytochemical contents and DPPH radical scavenging activities of partial fractions of the 50% methanolic extract and chemical markers from M. alba leaves.
| Fractions/markers | Phytochemical assay | Antioxidant assay | ||
|---|---|---|---|---|
|
|
|
|||
| Total phenolic contenta (μg/mg) | Condensed tanninsb (μg/mg) | Radical scavengingc (%) | SC50d (μg/mL) | |
| DW | 23.04 ± 0.29 | 13.33 ± 0.52 | 16.35 ± 0.61 | 237.43 ± 7.73 |
| D20M | 316.91 ± 8.22 | 17.54 ± 0.42 | 62.82 ± 0.74 | 29.30 ± 0.22 |
| D40M | 239.60 ± 2.67 | 99.32 ± 3.41 | 37.18 ± 1.37 | 59.30 ± 0.96 |
| D60M | 259.00 ± 3.20 | 114.49 ± 1.43 | 35.04 ± 1.93 | 64.76 ± 2.08 |
| DA | 32.79 ± 1.45 | 297.56 ± 1.65 | 6.71 ± 1.47 | 287.96 ± 9.72 |
| 5CQA (1) | – | – | 23.47 ± 1.46 | 32.76 ± 0.27 |
| 4CQA(2) | – | – | 69.35 ± 3.44 | 11.41 ± 0.48 |
| Gastrodin (3) | – | – | 1.08 ± 0.74 | 404.30 ± 4.92 |
| Isoquercetin(4) | – | – | 73.49 ± 5.10 | 10.63 ± 0.96 |
| Rutin (5) | – | – | 62.88 ± 1.85 | 10.57 ± 0.61 |
| Ascorbic acide | – | – | – | 8.13 ± 0.05 |
Values in gallic acid μg/mg sample.
Values in catechin μg/mg sample.
Values in percentage, %.
Values in μg/mL sample.
As positive control.
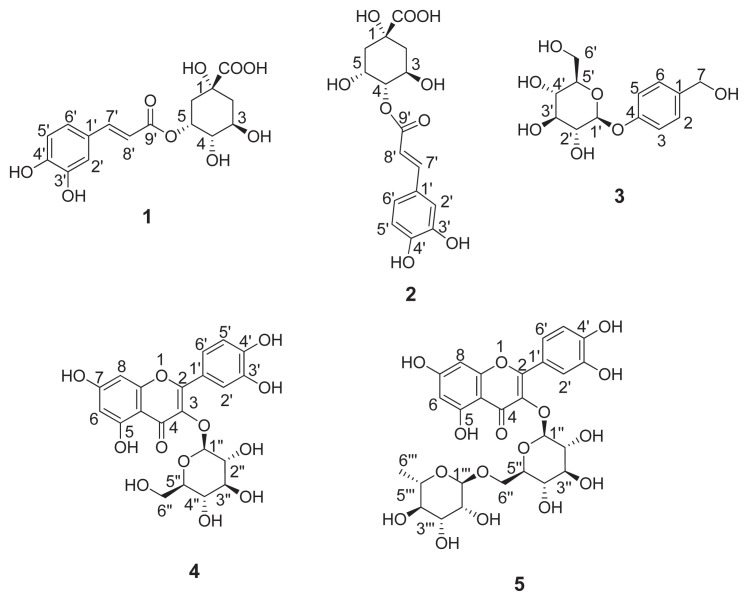
Structures of the isolated phenolic compounds are shown in Fig. 2. The isolated compounds were identified as 5-O-caffeoylquinic acid, 5CQA (1); 4-O-caffeoylquinic acid, 4CQA (2); gastrodin (3), isoquercetin (4) and rutin (5). All spectral data were provided in the supporting information. All assigned structure for the isolated compounds using 1D and 2D NMR spectrometric analyses were in good comparison with previous studies [20–24].
Fig. 2.
Structure of phenolic compounds isolated from M. alba leaves. 5-O-caffeoylquinic acid (1), 4-O-caffeoylquinic acid (2), gastrodin (3), isoquercetin (4) and rutin (5).
Results of the antioxidant assay of the isolated chemical markers evaluated at a concentration 15.63 μg/mL, Table 1, showed that the highest DPPH radical-scavenging activities (%) were for isoquercetin, 4CQA and rutin. However, no significance in difference of activity between 4CQA with rutin (p = 0.128) and 4CQA with isoquercetin (p = 0.471). Furthermore, SC50 of 4CQA do not significantly differ from rutin (p = 0.990) and isoquercetin (p = 0.992). Results suggested that the effectiveness of the three compounds was comparable with each other. Our experimental results showed a 3-fold significantly larger difference in the percent radical-scavenging activity between 4CQA and 5CQA, p < 0.001. The high antioxidant activity of 4CQA is attributed to the higher possibility of forming hydrogen bonds by the equatorial hydroxyl groups with the carbonyl oxygen of the equatorially situated caffeoyl group [25,26]. The loss of antioxidant ability over DPPH radicals of gastrodin was attributed to the absence of a catechol moiety. This result is supported by previously published structural relationships of antioxidant properties of the catechol moiety using density functional theory [27,28].
3.2. Chromatographic fingerprint, precision and accuracy of analytical method
The HPLC fingerprint identified occurrence of 5CQA, 4CQA, gastrodin, isoquercetin, and rutin, at their respective retention times of 15.0, 23.3, 9.0, 49.6 and 47.9 min, Table 2. A good linear regression was shown for the calibration curves used over the concentration ranges of 1.95–2000 μg/mL and 0.24–500 μg/mL, and R2 ranged 0.992–0.999. Intraday and interday variability for the analysis of all chemical markers exhibited relative standard deviations (RSDs) of <4.23% and <3.38% respectively, Table 3. These results indicated good repeatability of the analytical method employed. Results of the recovery experiments showed 92.27–115.22% recovery and all RSDs were <9.67%, which indicated good accuracy of the analytical method.
Table 2.
Calibration curves for each chemical markers using HPLC analytical method.
| Markers | Linear regression | Concentration range (μg/mL) | R 2 | Retention time (min) |
|---|---|---|---|---|
| 5CQA (1) | y = 3,582,159.99x − 4931.95 | 0.24–500 | 0.999 | 15.0 |
| 4CQA (2) | y = 12,513,294.19x − 404,619.22 | 3.91–2000 | 0.996 | 23.3 |
| Gastrodin (3) | y = 340,963.23x + 296.19 | 1.95–2000 | 0.999 | 9.0 |
| Isoquercetin (4) | y = 6,893,310.84x − 95,753.27 | 1.95–1000 | 0.992 | 49.6 |
| Rutin (5) | y = 3,483,559.92x − 21,240.95 | 1.95–1000 | 0.993 | 47.9 |
Table 3.
Precision and accuracy of the HPLC analytical method.
| Markers | Intraday | Interday | Recovery | ||||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| Mean ± SD (μg/g) | RSD (%) | Mean ± SD (μg/g) | RSD (%) | Qty. added (μg/mL) | Mean ± SD (%) | RSD (%) | |
| 5CQA (1) | 408.25 ± 7.03 | 1.72 | 412.71 ± 13.97 | 3.38 | 3.1 | 92.27 ± 6.14 | 6.66 |
| 4CQA (2) | 512.17 ± 7.89 | 1.54 | 516.65 ± 14.20 | 2.74 | 41.8 | 115.22 ± 9.89 | 8.59 |
| Gastrodin (3) | 558.48 ± 23.66 | 4.23 | 570.08 ± 10.13 | 1.77 | 5.4 | 108.57 ± 5.51 | 5.07 |
| Isoquercetin (4) | 369.38 ± 5.11 | 1.38 | 371.71 ± 9.87 | 2.65 | 14.7 | 102.24 ± 5.47 | 5.35 |
| Rutin (5) | 579.44 ± 8.78 | 1.51 | 585.46 ± 17.59 | 3.00 | 16.5 | 110.60 ± 10.70 | 9.67 |
3.3. Content variation of chemical markers in M. alba leaves
The chromatographic fingerprint allowed monitoring of variation in content of chemical markers for the 12 months duration of this study, Fig. 3. Mean contents of each marker in mg/g dry mass (DM) from leaves sampled at different location of a branch (apical, middle, and bottom) for 12-months are shown in Table S1. The mean contents for each location-sampled leaves were compared and showed that marker contents in apical leaves were significantly (p < 0.005) higher as compared to both lower located leaves, Table 4. Moreover, the mean marker contents of middle leaves were not significantly different (p = 0.904) from bottom leaves. Means for each marker in apical leaves showed highest content for gastrodin and 4CQA, followed by isoquercetin, 5CQA and rutin. Rutin was found to be least in content at all locations. However, decrease in content of markers was observed and tend to be more homogenous as leaves location shifts towards the bottom. Significance in the difference of the means is summarized in Table 4.
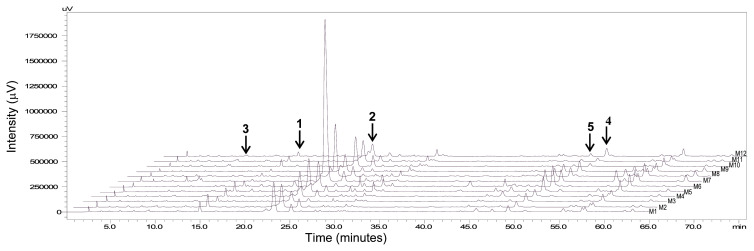
Fig. 3.
Chromatographic profile of 1–5 of M. alba apical leaves collected from the mulberry tree in the botanical garden of Taipei Medical University and monitored from July 2014 to June 2015, indicated as M1–M12 respectively. The UV absorption detector was set at 280 nm.
Table 4.
Mean content of chemical markers of leaves collected from different locations on a tree branch of a mulberry tree in the botanical garden of Taipei Medical University.
| Markers | Location | Mean (each marker) | ||
|---|---|---|---|---|
|
|
||||
| Apical | Middle | Bottom | ||
| 5CQA (1) | 0.50 ± 0.24A | 0.33 ± 0.12Aa | 0.31 ± 0.12ABa | 0.40 ± 0.20A |
| 4CQA (2) | 1.55 ± 1.64B | 0.51 ± 0.16BCa | 0.38 ± 0.13Ba | 0.91 ± 1.18B |
| Gastrodin (3) | 1.67 ± 1.44B | 0.56 ± 0.20Ca | 0.47 ± 0.28Ba | 0.99 ± 1.09B |
| Isoquercetin (4) | 0.59 ± 0.37Aa | 0.39 ± 0.27ABb | 0.42 ± 0.30Bab | 0.48 ± 0.33A |
| Rutin (5) | 0.47 ± 0.38A | 0.16 ± 0.09a | 0.15 ± 0.07Aa | 0.29 ± 0.29A |
| Content of all markers | 0.96 ± 1.13 | 0.39 ± 0.22a | 0.35 ± 0.23a | |
Contents are expressed as mg/g DW. Similar uppercase letters for each marker in a column and similar lowercase letters in a row show no significance in the difference between determinations using Tukey test at 5% probability.
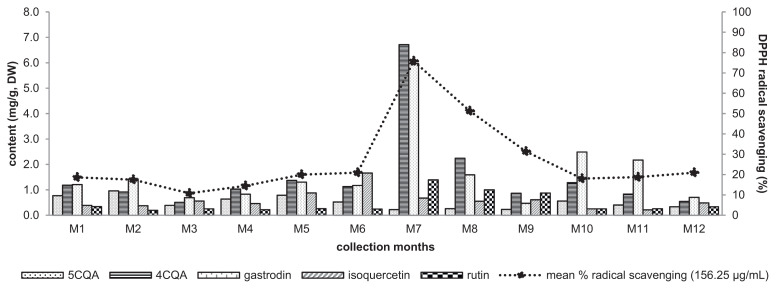
It was also observed that the leaves entered dormancy from November 2014 to March 2015. By this period the isoquercetin was highest in December (apical leaves) and November (middle and bottom leaves), but its content began to drop in all parts as the temperature fell below 20 °C with less sunshine exposure at 60.5 h but a higher rain volume of 86.8 mm. Bottom leaves were abscissed in December while middle leaves in January to March, which explains why there were no samples collected for these parts on those months, Table S1. Antioxidative markers 4CQA (6.72 mg/g DM) and rutin (1.40 mg/g DM), and the least active gastrodin (5.97 mg/g DM), were highest in content on January 2015. It was observed that January 2015 was the driest month over Taipei City with precipitation of only 20 mm, and sunshine exposure of 95.7 h but with the coldest temperature of 16 °C for the entire period of study. These conditions might be favorable for energy conservation and concentrating 4CQA in leaves during the dormancy period [29,30]. Furthermore, the content of 4CQA in apical leaves decreased from January by 3.0- and 7.7-fold in February and March where increasing amount of rainfall was observed but with temperatures still remained below 20 °C. Increased in rainfall relieves drought stress responsible for the increased phenolic acid content in plants [30]. Rutin decreased in content by 1.4- and 1.6-fold for the same months. Isoquercetin content was also decreased by 1.2- and 1.1-fold while gastrodin by 3.7- and 12.7-fold. Reduced in precipitation volume in April with almost the same amount as in February increased the content of 4CQA to 1.28 mg/g DM, however the content was still significantly lower (p < 0.001) than February. This discrepancy might have been due to an observed increase in temperature to >20 °C in April [31]. A further increase in precipitation in May was accompanied by an increase in temperature to 25 °C until it plateaued at 30 °C as the season approached summer, and 4CQA exhibited a decreased in concentration until around 0.55 mg/g DM. However, gastrodin was generally highest in content among all markers during the hot months of April to September. Even though this was the least active against DPPH radical scavenging, however previous research showed neuroprotective and anti-Alzheimer’s properties of gastrodin [32]. Furthermore, the contents of 4CQA, gastrodin, and rutin were >1.000 mg/g DM in the months of January and February 2015, Table S1. Results for these months corresponded to a reference [33] quality level of rutin in mulberry leaves as herbal materials.
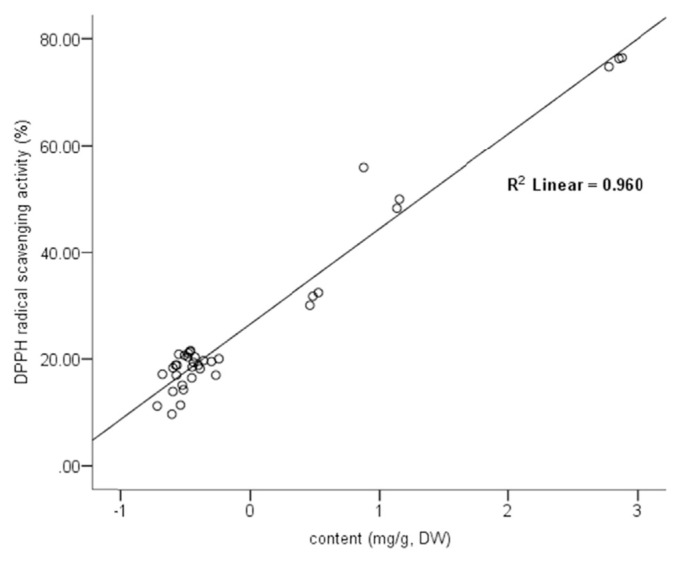
Apical leaves were a major contributor to the overall chemical marker content for all leaves at varying location. Furthermore, 12-month apical leaves samples were evaluated for their antioxidative property against DPPH radicals and the results are shown in Fig. 4. It can be seen that January exhibited the highest radical scavenging activity (%) followed by February and March. Regression analysis identified strong positive Pearson’s correlation between the % radical scavenging activity and the content distribution of antioxidative markers rutin, r = 0.949 (p < 0.001), 4CQA, r = 0.908 (p < 0.001) and also gastrodin, r = 0.769 (p < 0.001). While the antioxidant isoquercetin, r = 0.091 (p > 0.05) had a very weak Pearson’s correlation and the lesser active 5CQA, r = −0.542 (p < 0.05) was negatively correlated. A multiple linear regression model containing all markers showed strong positively correlated (r = 0.980) with an R2 = 0.961, F(5,30) = 148.57, p < 0.001. A backward elimination of predictors was performed to optimize the model. The first marker excluded was 5CQA, provided F(4, 31) = 191.48, p < 0.001. Gastrodin was the second marker removed, F(3, 32) = 258.74, p < 0.001. The third marker excluded was isoquercetin, providing the final model, y = 5.661 + 4.525X1 + 29.380X2, with only 4CQA(X1) and rutin(X2) as significant predictors (r = 0.980), R2 = 0.960, F(2, 33) = 392.35, p < 0.001. All R2-change were less than 0.1%, showing that all excluded markers have no significant contribution to the predictability on bioactivity of the model. A scatter plot of this correlation is shown in Fig. 5.
Fig. 4.
Content of 1–5 of apical leaves corresponding DPPH radical scavenging activity (%) at their respective month. The antioxidant activity was evaluated at 156.25 μg/mL. Leaves samples were collected in the botanical garden of Taipei Medical University from July 2014 to June 2015, described as M1–M12 respectively and content are mean ± SD (mg/g DW) of triplicate determinations.
Fig. 5.
Scatter plot showing correlation between the content of chemical markers (mg/g, DW) and DPPH radical scavenging activity (%), with 4CQA and rutin as predictors.
Content analysis of the young leaflets collected from Yangmingshan National Park showed increased 4CQA (22.97 ± 0.02 mg/g DM) and rutin (0.68 ± 0.00 mg/g DM) levels compared to older leaves, for the same markers at 1.48 ± 0.00 mg/g DM and 0.04 ± 0.00 mg/g DM, respectively. The data supported the 4CQA and rutin were rich in apical leaves. However, isoquercetin content was identified higher in older leaves (2.33 ± 0.00 mg/g DM) than in younger leaves (0.82 ± 0.29 mg/g DM). Furthermore, 4CQA, and rutin can be used as a chemical marker to identify relative tender leaves of mulberry.
4. Conclusion
In conclusion, 4CQA and isoquercetin exhibited the strongest antioxidant activities, and were comparable to rutin, when evaluated at their isolated forms. However, only 4CQA and rutin contributed significantly to the predictability of the antioxidant property in the 12-month crude extracts using DPPH free radical-scavenging assay. Furthermore, the significantly higher content of 4CQA among the isolated active compounds qualifies it as a good antioxidant chemical marker. It is evident that the relative location on the tree branch and age of leaves affect the contents of their bioactive compounds. Apical leaves were proven to contain the greatest amounts of these antioxidant compounds. January and February were the best months to harvest mulberry leaves, since they contained the highest concentrations of 4CQA, gastrodin, and rutin while exhibited the highest radical scavenging activity for the duration of this study. Moreover, the isoquercetin content was highest in December when the temperature had just begun to drop below 20 °C. Thus, the suggested climatic conditions for harvesting mulberry leaves with high contents of antioxidant compounds were identified as a critical temperature of <20 °C, a long sunshine exposure of 80–100 h, and dry weather, with precipitation of 50 mm. Furthermore, using the chromatographic fingerprint, the above isolated compounds are good candidates as chemical markers for quality control monitoring of antioxidant compounds in mulberry leaves. The results of this study provide evidence-based guidelines for mulberry producers when harvesting leaves with high polyphenol contents which can be developed as functional foods.
Appendix A. Supplementary data
Supplementary data related to this article can be found at https://doi.org/10.1016/j.jfda.2017.11.011.
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
REFERENCES
- 1.Huo YK. Mulberry cultivation and utilization in China. In: Sánchez MD, editor. Mulberry for animal production. Vol. 147. Rome: Food and Agriculture Organization of the United Nations; 2002. [Google Scholar]
- 2. Pieroni A, Quave CL, Santoro RF. Folk pharmaceutical knowledge in the territory of the Dolomiti Lucane, inland southern Italy. J Ethnopharmacol. 2004;95:373–84. doi: 10.1016/j.jep.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 3. Chau CF, Wu SH. The development of regulations of Chinese herbal medicines for both medicinal and food uses. Trends Food Sci Technol. 2006;17:313–23. [Google Scholar]
- 4. Sugiyama M, Takahashi M, Katsube T, Koyama A, Itamura H. Effects of applied nitrogen amounts on the functional components of mulberry (Morus alba L.) leaves. J Agric Food Chem. 2016;64:6923–9. doi: 10.1021/acs.jafc.6b01922. [DOI] [PubMed] [Google Scholar]
- 5. Chen H, He X, Liu Y, Li J, He Q, Zhang C, et al. Extraction, purification and anti-fatigue activity of γ-aminobutyric acid from mulberry (Morus alba L.) leaves. Sci Rep. 2016;6:18933. doi: 10.1038/srep18933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gryn-Rynko A, Bazylak G, Olszewska-Slonina D. New potential phytotherapeutics obtained from white mulberry (Morus alba L.) leaves. Biomed Pharmacother. 2016;84:628–36. doi: 10.1016/j.biopha.2016.09.081. [DOI] [PubMed] [Google Scholar]
- 7. Sugimoto M, Arai H, Tamura Y, Murayama T, Khaengkhan P, Nishio T, et al. Mulberry leaf ameliorates the expression profile of adipocytokines by inhibiting oxidative stress in white adipose tissue in db/db mice. Atherosclerosis. 2009;204:388–94. doi: 10.1016/j.atherosclerosis.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 8. Dugo P, Donato P, Cacciola F, Germano MP, Rapisarda A, Mondello L. Characterization of the polyphenolic fraction of Morus alba leaves extracts by HPLC coupled to a hybrid IT-TOF MS system. J Sep Sci. 2009;32:3627–34. doi: 10.1002/jssc.200900348. [DOI] [PubMed] [Google Scholar]
- 9. Sugiyama M, Katsube T, Koyama A, Itamura H. Varietal differences in the flavonol content of mulberry (Morus spp.) leaves and genetic analysis of quercetin 3-(6-malonylglucoside) for component breeding. J Agric Food Chem. 2013;61:9140–7. doi: 10.1021/jf403136w. [DOI] [PubMed] [Google Scholar]
- 10. Iqbal S, Younas U, Sirajuddin, Chan KW, Sarfraz RA, Uddin K. Proximate composition and antioxidant potential of leaves from three varieties of mulberry (Morus sp.): a comparative study. Int J Mol Sci. 2012;13:6651–64. doi: 10.3390/ijms13066651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zheng Y-c, He H, Wei X, Ge S, Lu Y-H. Comparison of regulation mechanisms of five mulberry ingredients on insulin secretion under oxidative stress. J Agric Food Chem. 2016;64:8763–72. doi: 10.1021/acs.jafc.6b03845. [DOI] [PubMed] [Google Scholar]
- 12.Songyu C, Decai T, Yingzhi Y. Science of Chinese Materia Medica. China: Shanghai Pujiang Education Press; 2003. [Google Scholar]
- 13.Central Weather Bureau. Climate statistics monthly data. Taipei, Taiwan: 2015. [accessed 01, 07, 15]. Available at: http://www.cwb.gov.tw/V7e/climate/monthlyData/mD.htm. [Google Scholar]
- 14. Sun B, Ricardo-da-Silva JM, Spranger I. Critical factors of vanillin assay for catechins and proanthocyanidins. J Agric Food Chem. 1998;46:4267–74. [Google Scholar]
- 15. Ouerghemmi I, Bettaieb Rebey I, Rahali FZ, Bourgou S, Pistelli L, Ksouri R, et al. Antioxidant and antimicrobial phenolic compounds from extracts of cultivated and wild-grown Tunisian Ruta chalepensis. J Food Drug Anal. 2017;25(2):350–9. doi: 10.1016/j.jfda.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dudonné S, Vitrac X, Coutière P, Woillez M, Mérillon J-M. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays. J Agric Food Chem. 2009;57:1768–74. doi: 10.1021/jf803011r. [DOI] [PubMed] [Google Scholar]
- 17. Wannes WA, Marzouk B. Characterization of myrtle seed (Myrtus communis var. baetica) as a source of lipids, phenolics, and antioxidant activities. J Food Drug Anal. 2016;24(2):316–23. doi: 10.1016/j.jfda.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tseng SH, Chien TY, Tzeng CF, Lin YH, Wu CH, Wang CC. Prevention of hepatic oxidative injury by Xiao-Chen-Chi-Tang in mice. J Ethnopharmacol. 2007;111(2):232–9. doi: 10.1016/j.jep.2006.11.030. [DOI] [PubMed] [Google Scholar]
- 19. Tian S, Hao C, Xu G, Yang J, Sun R. Optimization conditions for extracting polysaccharide from Angelica sinensis and its antioxidant activities. J Food Drug Anal. 2017;25(4):766–75. doi: 10.1016/j.jfda.2016.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Forino M, Tenore GC, Tartaglione L, Carmela D, Novellino E, Ciminiello P. (1S,3R,4S,5R)5-O-Caffeoylquinic acid: isolation, stereo-structure characterization and biological activity. Food Chem. 2015;178:306–10. doi: 10.1016/j.foodchem.2015.01.109. [DOI] [PubMed] [Google Scholar]
- 21. Nakatani N, Kayano S-i, Kikuzaki H, Sumino K, Katagiri K, Mitani T. Identification, quantitative determination, and antioxidative activities of chlorogenic acid isomers in prune (Prunus domestica L.) J Agric Food Chem. 2000;48:5512–6. doi: 10.1021/jf000422s. [DOI] [PubMed] [Google Scholar]
- 22. Shimoda K, Katsuragi H. Enzymatic resolution of (RS)-1-phenylalkyl β-d-glucosides to (R)-1-phenylalkyl β-primeverosides and (S)-1-phenylalkyl β-d-glucosides via plant xylosyltransferase. Tetrahedron: Asymmetry. 2010;21:2060–5. [Google Scholar]
- 23. Shokoohinia Y, Rashidi M, Hosseinzadeh L, Jelodarian Z. Quercetin-3-O-beta-d-glucopyranoside, a dietary flavonoid, protects PC12 cells from H(2)O(2)-induced cytotoxicity through inhibition of reactive oxygen species. Food Chem. 2015;167:162–7. doi: 10.1016/j.foodchem.2014.06.079. [DOI] [PubMed] [Google Scholar]
- 24. Forino M, Tartaglione L, Dell’Aversano C, Ciminiello P. NMR-based identification of the phenolic profile of fruits of Lycium barbarum (goji berries). Isolation and structural determination of a novel N-feruloyl tyramine dimer as the most abundant antioxidant polyphenol of goji berries. Food Chem. 2016;194:1254–9. doi: 10.1016/j.foodchem.2015.08.129. [DOI] [PubMed] [Google Scholar]
- 25. Kost D, Peor N, Sod-Moriah G, Sharabi Y, Durocher DT, Raban M. Conformationally controlled intramolecular charge transfer complexes. J Org Chem. 2002;67:6938–43. doi: 10.1021/jo020164t. [DOI] [PubMed] [Google Scholar]
- 26. Bonnet A, Chisholm J, Motherwell WDS, Jones W. Hydrogen bonding preference of equatorial versus axial hydroxyl groups in pyran and cyclohexane rings in organic crystals. CrystEngComm. 2005;7:71. [Google Scholar]
- 27. Kozlowski D, Trouillas P, Calliste C, Marsal P, Lazzaroni R, Duroux JL. Density functional theory study of the conformational, electronic, and antioxidant properties of natural chalcones. J Phys Chem A. 2007;111:1138–45. doi: 10.1021/jp066496+. [DOI] [PubMed] [Google Scholar]
- 28. Anouarel H, Raweh S, Bayach I, Taha M, Baharudin MS, Di Meo F, et al. Antioxidant properties of phenolic Schiff bases: structure–activity relationship and mechanism of action. J Comput Aided Mol Des. 2013;27:951–64. doi: 10.1007/s10822-013-9692-0. [DOI] [PubMed] [Google Scholar]
- 29. Verelst W, Skirycz A, Inzé D. Abscisic acid, ethylene and gibberellic acid act at different developmental stages to instruct the adaptation of young leaves to stress. Plant Signal Behav. 2010;5:473–5. doi: 10.4161/psb.5.4.11421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liang Z, Ma Y, Xu T, Cui B, Liu Y, Guo Z, et al. Effects of abscisic acid, gibberellin, ethylene and their interactions on production of phenolic acids in Salvia miltiorrhiza Bunge hairy roots. PLos One. 2013;8:e72806. doi: 10.1371/journal.pone.0072806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Michaeli R, Philosoph-Hadas S, Riov J, Meir S. Chilling-induced leaf abscission of Ixora coccinea plants. I. Induction by oxidative stress via increased sensitivity to ethylene. Physiol Plant. 1999;107:166–73. doi: 10.1034/j.1399-3054.2001.1130306.x. [DOI] [PubMed] [Google Scholar]
- 32. Zhang JS, Zhou SF, Wang Q, Guo JN, Liang HM, Deng JB, et al. Gastrodin suppresses BACE1 expression under oxidative stress condition via inhibition of the PKR/eIF2alpha pathway in Alzheimer’s disease. Neuroscience. 2016;325:1–9. doi: 10.1016/j.neuroscience.2016.03.024. [DOI] [PubMed] [Google Scholar]
- 33.Chiu W-T, editor. Taiwan herbal pharmacopeia. Republic of China: Ministry of Health and Welfare Taiwan; 2015. [Google Scholar]