Abstract
Anemone raddeana Regel, a Traditional Chinese Medicine, has been demonstrated to possess cytotoxicity and anti-inflammatory activities. The purpose of this study is to establish analytical methods to identify and quantify the major active constituents in Anemone raddeana. A high-performance liquid chromatography coupled with electrospray ionization quadrupole time-of-flight tandem mass spectrometry (HPLC-ESI-Q/TOF-MS) was used to identify the components in the title plant material. To quantify the major components, a HPLC-UV method was developed and validated. The results showed that 37 compounds were identified based on the MS data and retention times. The contents of eight main bioactive compounds were determined by HPLC simultaneously. These methods could be used to effectively evaluate the quality of A. raddeana and provide a valuable reference for further study. In addition, the cytotoxicity activity of the different fractions of A. raddeana was determined. Hederacolchiside A1 (f) showed promising activity against ten human cancer cell lines with IC50 values from 0.29 to 3.48 μM.
Keywords: Anemone raddeana Regel, Quantification, Cytotoxicity
1. Introduction
Anemone raddeana Regel is a well-known Traditional Chinese Medicine (TCM) recorded in the Chinese Pharmacopoeia (2015 edition) for the treatment of rheumatism and pain [1]. This herbal is the major components in a few TCM formulas such as Huo-Luo-Wan and Zai-Zao-Wan [2]. Pharmacological and clinical results showed that the total triterpenoid saponins prepared from A. raddeana had cytotoxic and anti-inflammation activities [3]. Furthermore, chemical analysis demonstrated that triterpenoids were the major constituents in A. raddeana [4]. Some of the pure compounds have been tested using cell line or animal models. For example, raddeanoside A displayed significant cytotoxicity by inhibiting VEGFR2 signaling [5]. In the previous studies, we found that some triterpenoid isolated from A. raddeana exhibited promising effects on superoxide generation in human neutrophil, which was associated with anti-inflammation activity [6–9].
TCM plays an important role in public health due to its effectiveness [10]. However, quality control is challenge for TCM. A few methods, such as HPLC-UV, LC-MS/MS, and LC-NMR, have been developed and tested for the quality control of TCM [11–14]. For A. raddeana, a few analytic methods using HPLC have been reported. However, most of them only measured raddeanin A or raddeanin D [15–17]. The concern is that other components may also be active. To globally control the quality of the title plant material, we developed a HPLC-ESI-Q/TOF-MS method to identify the constituents in A. raddeana. In addition, we developed and validated an HPLC method to quantify eight bioactive triterpenoids. To provide scientific evidence to justify the traditional usage, we tested the activity of the major components in A. raddeana against several cancer cell lines.
2. Materials and methods
2.1. Chemicals and reagents
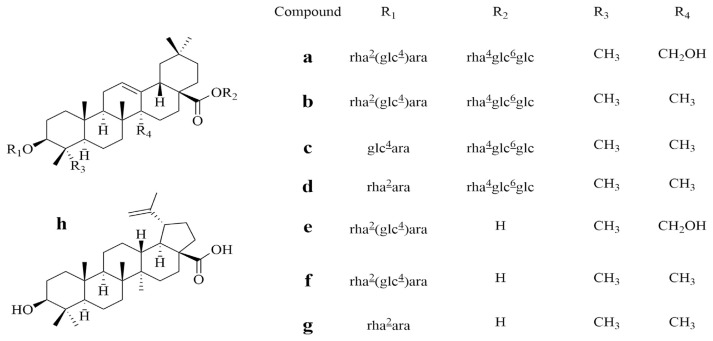
HPLC-grade methanol and acetonitrile were purchased from Yuwang Group Co., Ltd. (Shandong, China). The standard compounds, 3-O-α-l-rhamnopyranosyl (1 → 2)[β-d-glucopyr-anosyl (1 → 4)]-α-l-arabinopyranosyl-27-hydroxy-oleanolic acid 28-O-α-l-rhamnopyranosyl (1 → 4)-β-d-glucopyranosyl (1 → 6)-β-d-glucopyranoside (a), hederacholichiside E (b), raddeanoside R19 (c), hederacholichiside B (d), raddeanoside R20 (e), hederacolchiside A1 (f), eleutheroside K (g), and betulinic acid (h) were purified from the rhizome of A. raddeana. The purity of these standards was all above 98.0% by HPLC analysis. Their structures (Fig. 1) were elucidated by spectroscopic analysis (1D, 2D NMR) [4,18–22]. Other chemicals were analytical grade.
Fig. 1.
Chemical structures of the eight compounds.
2.2. Plants materials
Nineteen batches of A. raddeana (Sample 1–19) were collected from different regions in northeast of China in May 2014. These samples were authenticated by Professor Lu, (Department of Pharmacognosy, Shenyang Pharmaceutical University). The information was listed in Table 1.
Table 1.
Samples information of A. raddeana.
| Sample No. | Growth location | Sample No. | Growth location |
|---|---|---|---|
| 1 | Zuojia, Jilin | 11 | Yabuli, Heilongjiang |
| 2 | Huadian, Jilin | 12 | Heilongjiang |
| 3 | Tiangang, Jilin | 13 | Heilongjiang |
| 4 | Jingyu, Jilin | 14 | Qingyuan, Liaoning |
| 5 | Tonghua, Jilin | 15 | Benxi, Liaoning |
| 6 | Meihekou, Jilin | 16 | Fengcheng, Liaoning |
| 7 | Jilinshi, Jilin | 17 | Qianshan, Liaoning |
| 8 | Wuchang, Heilongjiang | 18 | Kuandian, Liaoning |
| 9 | Acheng, Heilongjiang | 19 | Aiyang, Liaoning |
| 10 | Yimianpo, Heilongjiang |
2.3. Preparation of sample solutions
2.3.1. Preparation of sample solutions for quantification
Nineteen batches of A. raddeana were grounded into powder. The powder (5.0 g) of each batch was extracted by reflux with 75% ethanol (3 × 50 mL, each 1 h). The combined extract was filtered and the solvent was removed using rotary vaporization under vacuum. The residue was dissolved in water and loaded to the HPD400 macroporous resin column (10.0 g), which was eluted with water till the eluate showed negative response to Molish reaction, followed by 70% ethanol till void of saponin. The saponin containing fractions were evaporated to remove ethanol, followed by in vacuo-drying at 40 °C to obtain the total saponin. An accurately weighed total saponin was dissolved in methanol and filtrated through 0.45 μm micropore membrane for analysis.
2.3.2. Preparation of sample solutions for cytotoxic activity assay
Air-dried and powdered A. raddeana was refluxed with 75% ethanol. The dry extract was suspended in water and then successively partitioned with petroleum ether (PE), dichloromethane (CH2Cl2), ethyl acetate (EtOAc), and n-butanol (BuOH). Remove solvent under vacuum to afford PE, CH2Cl2, EtOAc, and BuOH fractions. These fractions were dissolved in DMSO to afford stock solutions used in MTT assay. Doxorubicin was used as the positive control.
2.4. Preparation of standard solutions
A standard solution of initial concentration was prepared by accurately weighing each standard sample (compound a–h) and dissolved with methanol, which was stored in a 10 mL volumetric flask. Then the standard solution was diluted to a series of different concentrations. Each standard working solution was filtered by 0.45 μm micropore membrane.
2.5. HPLC-ESI-Q/TOF-MS analysis
The experiment was performed on quadrupole time-of-flight tandem mass spectrometer coupled with an electrospray ionization source in the negative ion mode from m/z 50 to 1500 (Bruker Co., Karlsruhe, Germany). The instrumental parameters for the mass spectrometric analysis were set as follows: end plate offset, −500 V; capillary voltage, 3800 V; nebulizer gas pressure, 1.2 bar; temperature 180 °C and the flow rate was 8.0 L/min. All data were acquired and processed using Bruker Daltonics DataAnalysis 3.4. Software (Bruker Co., Germany).
2.6. HPLC analysis
2.6.1. HPLC conditions
HPLC analysis was operated on a Lab Alliance-Series III (SSI, USA). A C18 analytical column (250 mm × 4.6 mm, 5 μm, Alltech Associates Co., USA) was used. The separation was achieved by a linear gradient elution program of the two combined eluents: A (acetonitrile) and B [0.1% phosphoric acid (v/v) in water]. The detailed gradient elution was as follows: 0–28 min, 23%–36% A; 28–42 min, 36%–56% A; 42–52 min, 56%–90% A. The flow rate of the mobile phase was 1.0 mL/min. The wavelength was set at 203 nm. The column temperature was 30 °C. The injection volume was 10 μL.
2.6.2. HPLC method validation
The developed method was validated for its linearity, LODs (limit of detection), LOQs (limit of quantification), precision, repeatability, and sample stability. All calibration curves were plotted based on linear regression analysis of the integrated peak areas (y) versus concentrations (x) of identified constituents in the standard solutions to obtain a linear equation y = ax + b. The standard solution containing eight reference compounds was diluted to six different concentrations with methanol for HPLC injection. The experiment was performed in triplicate.
The LODs and LOQs were determined at signal-to-noise ratios (S/N) of 3 and 10, respectively.
Intra-day and inter-day variations were utilized to evaluate the precision. The intra-day variation was determined by successive analysis of the same sample solution six times within one day and inter-day variation was determined on three consecutive days. For testing stability, the same sample solution was analyzed after prepared for 0 h, 2 h, 4 h, 8 h, 12 h, and 24 h. To confirm the repeatability, six different working solutions from the same sample were injected. The recovery was determined by spiking the extracts with exact amount of each reference compound. Eight triterpenoids were spiked into the samples (the same as the known amounts), and then extracted, processed and quantified in accordance with the established method. The precision, repeatability and the stability were analyzed and variations expressed by RSD. The percent recovery rates for the analytes were presented as mean ± SD.
2.7. Cell culture
All of the cell lines, including Lung cancer (A549), human hepatocarcinoma (Hep-G2), human breast adenocarcinoma cell lines (MCF-7), human pancreatic cancer (CFPAC-1), human hepatocarcinoma (Hep 3B), human colon cancer (HT-29), human oral epidermoid carcinoma cells (KB), human esophageal cancer cell line (Eca-109), Lung cancer (SPC-A-1), gastric cancer cell line (SGC-7901), bladder cancer cell (5637), acute myeloid leukemia cells (HL-60), and Human glioma cells (U251), were cultured in Dulbecco’s Modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum and maintained at 37 °C with 5% CO2.
2.8. Viability assay
The cells were treated with the test compounds at different concentrations for 72 h. Then 10 μL MTT solution was added and the mixture was incubated for another 4 h at 37 °C. After the removal of the culture medium, 100 μL dimethyl sulfoxide (DMSO) was added. The absorbance was measured at 570 nm on Varioskan Flash Microplate Reader (Thermo Scientific, USA).
2.9. Statistical analysis
All values were represented by mean ± SD. The IC50 values were calculated by the SPSS 19.0 software.
3. Results and discussion
3.1. Identification of main chemical constituents of A. raddeana
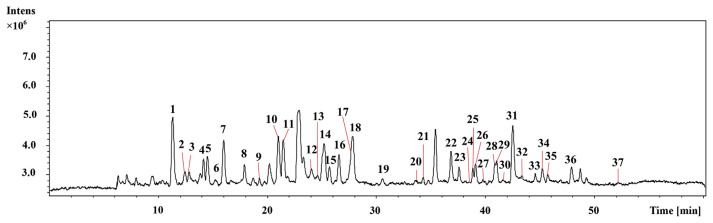
To identify the main chemical constituents in A. raddeana, a HPLC-ESI-Q/TOF-MS method was used in negative scan mode. A representative chromatogram was shown in Fig. 2. Based on the retention times, molecular formula, and the MS/MS data, 37 compounds were identified (Table 2).
Fig. 2.
Representative total ion chromatograms of A. raddeana obtained by HPLC-ESI-Q/TOF-MS in negative scan mode.
Table 2.
The compounds identified by HPLC-ESI-Q/TOF-MS.
| Peak | tR (min) | Formula M | [M–H]− | Fragmentation | Structurea | Reference | ||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| Measured mass | Calcd mass | R1 | R2 | R3 | R4 | |||||
| 1 | 11.371 | C65H106O31 | 1381.6645 | 1381.6645 | 911.5011 [M-H-rha-glc-glc] | rha1 → 2 [glc (1 →4)]ara | rha1 → 4glc1 →6glc | CH2OH | CH3 | [23,24] |
| 2 | 12.509 | C59H96O27 | 1235.6066 | 1235.6138 | 765.4435 [M-H-rha-glc-glc] | glc1 → 4ara | rha1 → 4glc1 →6glc | CH2OH | CH3 | [23] |
| 3 | 12.86 | C65H106O31 | 1381.6645 | 1381.665 | 911.4989 [M-H-rha-glc-glc] | rha1 → 2 [glc (1 →4)]ara | rha1 → 4glc1 →6glc | CH3 | CH2OH | [25] |
| 4 | 14.200 | C65H106O31 | 1381.6645 | 1381.6698 | 911.4986 [M-H-rha-glc-glc] | rha1 → 2glc1 → 2ara | rha1 → 4glc1 →6glc | CH3 | CH2OH | [20] |
| 5 | 14.568 | C59H96O26 | 1219.6117 | 1219.6134 | 749.4490 [M-H-rha-glc-glc] | rha1 → 2ara | rha1 → 4glc1 →6glc | CH2OH | CH3 | [24,26] |
| 6 | 15.455 | C53H86O22 | 1073.5538 | 1073.5523 | 603.3893 [M-H-rha-glc-glc] | ara | rha1 → 4glc1 →6glc | CH2OH | CH3 | [23,24] |
| 7 | 16.041 | C59H96O27 | 1235.6066 | 1235.6083 | 765.4467 [M-H-rha-glc-glc] | glc1 → 2ara | rha1 → 4glc1 →6glc | CH2OH | CH3 | [27] |
| 8 | 17.933 | C59H96O27 | 1235.6066 | 1235.6141 | – | glc1 → 4ara | rha1 → 4glc1 →6glc | CH3 | CH2OH | [25] |
| 9 | 19.322 | C59H96O26 | 1219.6117 | 1219.6166 | 749.4540 [M-H-rha-glc-glc] | rha1 → 2ara | rha1 → 4glc1 →6glc | CH3 | CH2OH | [21] |
| 10 | 21.013 | C65H106O31 | 1381.6645 | 1381.6715 | 911.5004 [M-H-rha-glc-glc] | rha1 → 2glc1 → 2ara | rha1 → 4glc1 →6glc | CH3 | CH2OH | [25] |
| 11 | 21.465 | C70H114O34 | 1497.7119 | 1497.7165 | 1027.549 [M-H-rha-glc-glc] | ara1 → 3rha1 →2 [glc (1 → 4)]ara | rha1 → 4glc1 →6glc | CH3 | CH3 | [25] |
| 12 | 24.027 | C65H106O30 | 1365.6696 | 1365.6686 | 895.5039 [M-H-rha-glc-glc] | rha1 → 2glc1 → 2ara | rha1 → 4glc1 →6glc | CH3 | CH3 | [25,26] |
| 13 | 24.579 | C65H106O30 | 1365.6696 | 1365.6678 | – | rha1 → 2 [glc (1 →4)]ara | rha1 → 4glc1 →6glc | CH3 | CH3 | [28] |
| 14 | 25.266 | C59H96O26 | 1219.6117 | 1219.6166 | 749.4502 [M-H-rha-glc-glc] | glc1 → 4ara | rha1 → 4glc1 →6glc | CH3 | CH3 | [19] |
| 15 | 25.701 | C59H96O26 | 1219.6117 | 1219.6155 | 749.4504 [M-H-rha-glc-glc] | glc1 → 2ara | rha1 → 4glc1 →6glc | CH3 | CH3 | [29] |
| 16 | 26.572 | C64H104O29 | 1335.6591 | 1335.6655 | 865.4963 [M-H-rha-glc-glc] | ara1 → 3rha1 →2ara | rha1 → 4glc1 →6glc | CH3 | CH3 | [25] |
| 17 | 27.559 | C65H106O31 | 1381.6645 | 1381.6715 | 911.5011 [M-H-rha-glc-glc] | glc1 → 2 [glc (1 →4)]ara | rha1 → 4glc1 →6glc | CH3 | CH3 | [25] |
| 18 | 27.76 | C59H96O25 | 1203.6168 | 1203.627 | 733.4583 [M-H-rha-glc-glc] | rha1 → 2ara | rha1 → 4glc1 →6glc | CH3 | CH3 | [25,30] |
| 19 | 30.573 | C53H86O21 | 1057.5589 | 1057.5684 | 587.4005 [M-H-rha-glc-glc] | ara | rha1 → 4glc1 →6glc | CH3 | CH3 | [23,26] |
| 20 | 33.603 | C47H76O18 | 927.4959 | 927.5085 | – | glc1 → 2 [glc (1 →4)]ara | H | CH2OH | CH3 | [27] |
| 21 | 34.205 | C52H84O21 | 1043.5432 | 1043.5538 | – | ara1 → 3rha1 →2 [glc (1 → 4)]ara | H | CH2OH | CH3 | [26] |
| 22 | 36.767 | C47H76O17 | 911.5010 | 911.5055 | – | rha1 → 2 [glc (1 →4)]ara | H | CH2OH | CH3 | [21,31] |
| 23 | 37.503 | C47H76O17 | 911.5010 | 911.5126 | – | rha1 → 2 [glc (1 →4)]ara | H | CH3 | CH2OH | [25] |
| 24 | 38.457 | C48H78O17 | 925.5166 | 925.5286 | 455.3650 [M-H-rha-glc-glc] | H | rha1 → 4glc1 →6glc | CH3 | CH3 | [32] |
| 25 | 38.809 | C41H66O12 | 749.4482 | 749.4586 | – | rha1 → 2ara | H | CH2OH | CH3 | [24] |
| 26 | 39.077 | C41H66O13 | 765.4431 | 765.4547 | 603.3992 [M-H-glc] | glc1 → 4ara | H | CH2OH | CH3 | [23,24] |
| 27 | 39.646 | C36H56O9 | 631.3852 | 631.3931 | 455.2545 [M-H-glcA] | glcA | H | CH3 | CH3 | [33] |
| 28 | 40.784 | C53H86O21 | 1057.5589 | 1057.5677 | – | glc1 → 2ara | rha1 → 4glc | CH3 | CH3 | [25,34] |
| 29 | 40.868 | C47H76O17 | 911.5010 | 911.5102 | 455.2526 [M-H-glc-glc-ara] | glc1 → 2 [glc (1 →4)]ara | H | CH3 | CH3 | [28] |
| 30 | 41.571 | C41H66O12 | 749.4482 | 749.4543 | – | rha1 → 2ara | H | CH3 | CH2OH | [22,25] |
| 31 | 42.459 | C47H76O16 | 895.5061 | 895.5156 | – | rha1 → 2 [glc (1 →4)]ara | H | CH3 | CH3 | [22,24] |
| 32 | 43.296 | C35H55O8 | 603.3891 | 603.3922 | 471.3511 [M-H-ara] | ara | H | CH2OH | CH3 | [26] |
| 33 | 44.518 | C47H76O16 | 895.5061 | 895.5102 | – | rha1 → 2glc1 → 2ara | H | CH3 | CH3 | [35] |
| 34 | 45.187 | C41H66O12 | 749.4482 | 749.4516 | 587.3973 [M-H-glc] | glc1 → 2ara | H | CH3 | CH3 | [25,35] |
| 35 | 45.639 | C46H74O15 | 865.4955 | 865.4979 | – | ara1 → 3rha1 →2ara | H | CH3 | CH3 | [18] |
| 36 | 47.849 | C41H66O11 | 733.4521 | 733.4602 | 587.4005 [M-H-rha] | rha1 → 2ara | H | CH3 | CH3 | [22,26] |
| 37 | 52.152 | C35H56O7 | 587.3953 | 587.3992 | – | ara | H | CH3 | CH3 | [25] |
The basic skeleton is the same as depicted in Fig. 1 for compounds a–g.
3.2. Optimization of HPLC method
The RP-HPLC condition for analyzing these triterpenoid glycosides was optimized. Acetonitrile and 0.1% phosphoric acid system as eluent using a C18 reversed-phase column and detection at 203 nm showed the best separation and peak shape.
3.3. Calibration and validation
The method was validated in terms of linearity, LODs, LOQs, precision, stability, repeatability and recovery test [36]. All calibration curves showed good linear regression (R2 > 0.999). The LODs and the LOQs for the analytes were less than 1.21 and 4.06 μg/mL (Table 3). The RSD values of precision, stability, and repeatability were less than 2.74%. The average recoveries of the analytes were 94.55%–103.57% and RSD values were less than 3.55% (Table 4). Therefore, the HPLC method was precise, accurate and sensitive enough for simultaneously quantitating eight compounds (a–h) in the extracts of A. raddeana.
Table 3.
Results of calibration curves, LOD and LOQ.
| Compound | Calibration curve | R2 | Linear range (μg/mL) | LOD (μg/mL) | LOQ (μg/mL) |
|---|---|---|---|---|---|
| a | y = 1.006 × 106x + 371 381 | 0.9990 | 34–270 | 1.05 | 4.06 |
| b | y = 1.174 × 106x + 4753 | 0.9991 | 86–688 | 0.33 | 0.79 |
| c | y = 6.916 × 105x + 92951 | 0.9997 | 42–338 | 1.21 | 3.86 |
| d | y = 1.088 × 106x + 1528223 | 0.9998 | 27–213 | 0.48 | 1.28 |
| e | y = 1.155 × 106x + 67640 | 0.9999 | 34–270 | 0.52 | 1.37 |
| f | y = 1.880 × 106x + 195490 | 0.9997 | 44–354 | 0.24 | 0.80 |
| g | y = 1.458 × 106x + 261874 | 0.9990 | 46–371 | 0.21 | 0.69 |
| h | y = 4.394 × 105x + 367245 | 0.9995 | 27–216 | 0.41 | 1.62 |
Table 4.
Precision, Stability, Reproducibility and Recovery (n = 6) in quantitation.
| Compound | Precision (RSD, %) | Stability | Repeatability | Recovery | ||
|---|---|---|---|---|---|---|
|
|
|
|
|
|||
| Intra-day | Inter-day | (RSD, %) | (RSD, %) | Average (%) | RSD (%) | |
| a | 1.12 | 2.59 | 1.45 | 1.45 | 98.3 ± 1.78 | 1.81 |
| b | 1.64 | 1.68 | 2.45 | 2.20 | 101.03 ± 2.92 | 2.89 |
| c | 1.02 | 2.05 | 1.10 | 1.10 | 95.92 ± 3.41 | 3.55 |
| d | 2.22 | 2.20 | 1.60 | 0.88 | 94.55 ± 2.95 | 3.12 |
| e | 1.81 | 3.18 | 1.81 | 1.81 | 96.67 ± 3.25 | 3.36 |
| f | 2.49 | 2.74 | 1.80 | 1.88 | 99.13 ± 2.57 | 2.59 |
| g | 1.07 | 1.21 | 0.70 | 2.74 | 103.57 ± 2.45 | 1.90 |
| h | 1.02 | 1.97 | 2.05 | 2.44 | 96.77 ± 2.91 | 3.01 |
3.4. Quality evaluation of the eight compounds
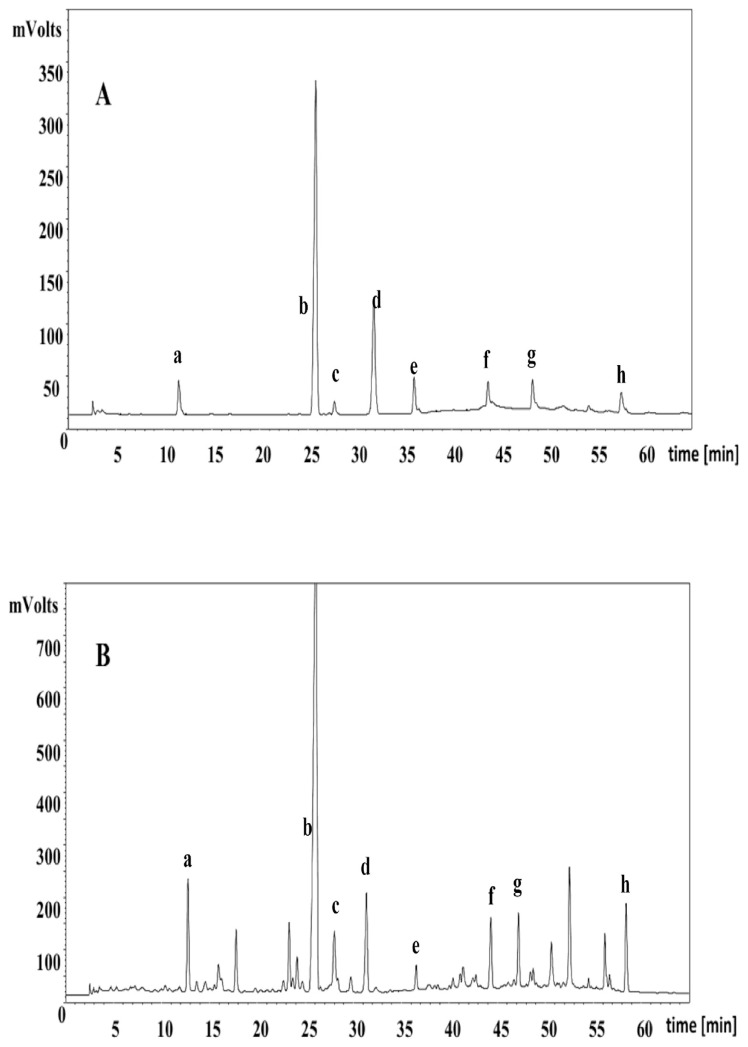
The proposed method was applied to simultaneously determine the eight compounds in 19 batches of A. raddeana samples. A representative HPLC chromatogram was shown in Fig. 3A. The content of eight analytes in 19 samples were listed in Table 5 and that of hederacholichiside B (d) (0.68–5.94 mg/g) showed the most remarkable difference and that of hederacholichiside E (b) (8.20–14.12 mg/g) had the highest amount. Based on this result, the content of each compound varied to a great extent among different sources. The relationship between the bioactivity and the content of these major glycosides should be further studied.
Fig. 3.
HPLC chromatogram of standard chemicals (A) and sample extract (B).
Table 5.
Contents of the eight components in different batches of A. raddeana samples (mg/g, n = 3).
| Samples.NO. | compound a | compound b | compound c | compound d | compound e | compound f | compound g | compound h |
|---|---|---|---|---|---|---|---|---|
| 1 | 3.48 ± 0.080 | 10.65 ± 0.269 | 1.25 ± 0.030 | 2.16 ± 0.042 | 0.61 ± 0.008 | 1.66 ± 0.037 | – | 0.18 ± 0.004 |
| 2 | 3.06 ± 0.080 | 10.73 ± 0.178 | 1.96 ± 0.036 | 2.19 ± 0.039 | 0.58 ± 0.007 | 2.18 ± 0.039 | 0.46 ± 0.012 | – |
| 3 | 3.59 ± 0.090 | 12.25 ± 0.239 | 1.98 ± 0.041 | 1.86 ± 0.022 | 0.81 ± 0.015 | 2.16 ± 0.029 | 0.35 ± 0.004 | 0.17 ± 0.005 |
| 4 | 3.63 ± 0.100 | 12.13 ± 0.232 | 1.40 ± 0.036 | 0.68 ± 0.009 | 0.42 ± 0.006 | 1.16 ± 0.023 | 0.19 ± 0.004 | – |
| 5 | 4.17 ± 0.060 | 12.58 ± 0.308 | 1.92 ± 0.021 | 1.59 ± 0.016 | 0.32 ± 0.008 | 0.74 ± 0.014 | 0.22 ± 0.006 | 0.10 ± 0.001 |
| 6 | 4.21 ± 0.090 | 11.71 ± 0.253 | 1.27 ± 0.014 | 1.87 ± 0.028 | 0.82 ± 0.018 | 2.33 ± 0.046 | 0.52 ± 0.009 | – |
| 7 | 2.95 ± 0.050 | 10.88 ± 0.185 | 1.46 ± 0.038 | 1.99 ± 0.044 | 0.49 ± 0.006 | 1.50 ± 0.017 | 0.36 ± 0.006 | 0.10 ± 0.001 |
| 8 | 3.06 ± 0.060 | 10.73 ± 0.145 | 1.96 ± 0.030 | 2.19 ± 0.037 | 0.58 ± 0.016 | 2.18 ± 0.054 | 0.46 ± 0.011 | – |
| 9 | 2.39 ± 0.040 | 8.70 ± 0.114 | 1.20 ± 0.020 | 3.41 ± 0.080 | 0.64 ± 0.010 | 2.45 ± 0.026 | 0.62 ± 0.017 | 0.31 ± 0.003 |
| 10 | 3.93 ± 0.070 | 9.01 ± 0.105 | 1.64 ± 0.028 | 2.13 ± 0.052 | 0.78 ± 0.020 | 1.92 ± 0.020 | 0.47 ± 0.013 | 0.10 ± 0.002 |
| 11 | 3.60 ± 0.070 | 10.38 ± 0.274 | 2.78 ± 0.064 | 2.14 ± 0.037 | 0.48 ± 0.006 | 1.58 ± 0.037 | 0.25 ± 0.004 | 0.10 ± 0.001 |
| 12 | 3.93 ± 0.100 | 9.29 ± 0.252 | 2.09 ± 0.058 | 4.32 ± 0.055 | 0.72 ± 0.011 | 2.41 ± 0.045 | 0.88 ± 0.020 | 0.13 ± 0.002 |
| 13 | 1.96 ± 0.030 | 12.14 ± 0.243 | 1.25 ± 0.032 | 1.88 ± 0.040 | 0.86 ± 0.015 | 2.87 ± 0.068 | 0.42 ± 0.008 | 0.18 ± 0.002 |
| 14 | 2.66 ± 0.050 | 8.20 ± 0.216 | 2.39 ± 0.057 | 1.39 ± 0.024 | 0.73 ± 0.010 | 2.58 ± 0.050 | 0.27 ± 0.006 | 0.27 ± 0.004 |
| 15 | 2.94 ± 0.070 | 11.65 ± 0.308 | 0.48 ± 0.009 | 2.18 ± 0.047 | 0.69 ± 0.016 | 2.29 ± 0.062 | 0.56 ± 0.010 | 0.60 ± 0.011 |
| 16 | 2.73 ± 0.030 | 10.20 ± 0.141 | 1.11 ± 0.026 | 5.94 ± 0.163 | 0.68 ± 0.009 | 1.86 ± 0.042 | 0.84 ± 0.016 | 0.53 ± 0.006 |
| 17 | 4.06 ± 0.110 | 14.12 ± 0.387 | 2.85 ± 0.048 | 2.03 ± 0.049 | 0.55 ± 0.011 | 1.55 ± 0.018 | 0.16 ± 0.004 | – |
| 18 | 3.31 ± 0.050 | 13.93 ± 0.299 | 1.61 ± 0.042 | 1.83 ± 0.027 | 0.46 ± 0.012 | 1.30 ± 0.028 | 0.14 ± 0.003 | 0.17 ± 0.002 |
| 19 | 3.69 ± 0.050 | 12.25 ± 0.272 | 1.70 ± 0.026 | 2.09 ± 0.037 | 0.66 ± 0.016 | 1.81 ± 0.034 | 0.26 ± 0.006 | 0.27 ± 0.005 |
3.5. MTT cytotoxicity assay
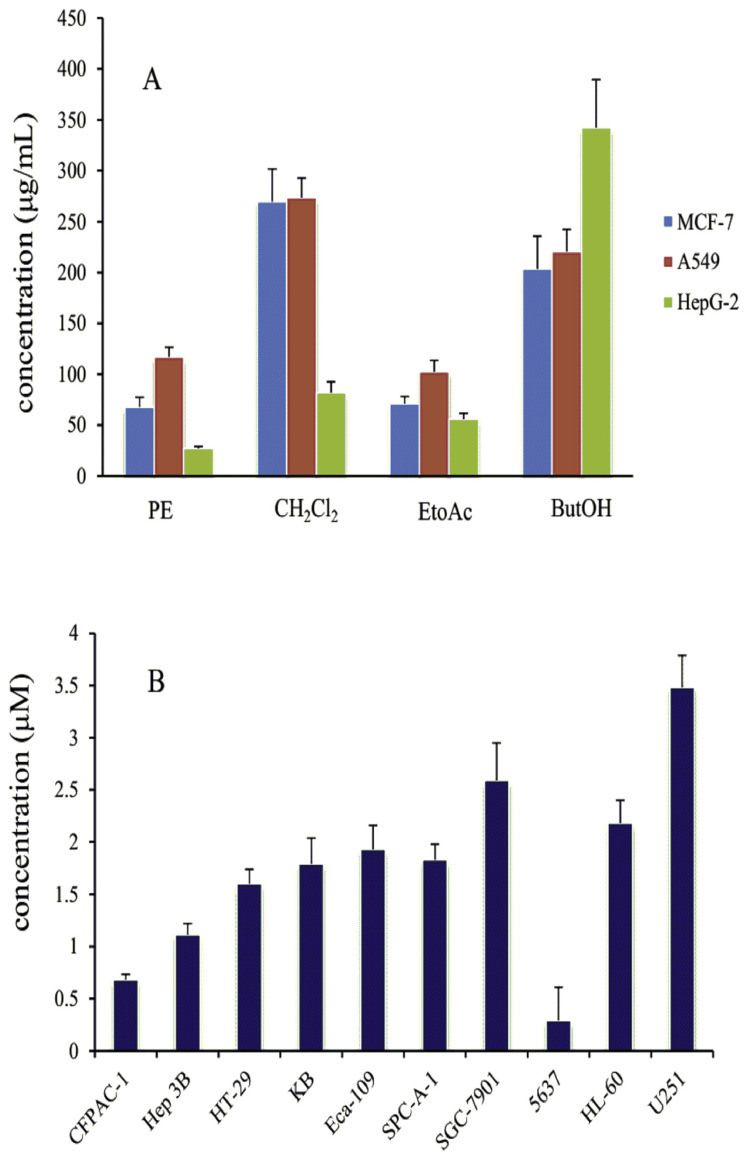
The results of different fraction of A. raddeana were shown in Fig. 4A. Petroleum ether and ethyl acetate fraction showed the potential cytotoxicity. The anti-proliferation activity of hederacolchiside A1 (f) against ten cancer cells lines was evaluated. As compound f exhibited a strong cytotoxicity with IC50 values from 0.29 to 3.48 μM (Fig. 4B) and was isolated from the ethyl acetate fraction, this fraction contained the cytotoxic ingredients of A. raddeana.
Fig. 4.
Cytotoxic activity of different part of extract (A) and hederacolchiside A1 (B).
4. Conclusions
Thirty seven compounds were identified from A. raddeana primarily by HPLC-ESI-Q/TOF-MS for the first time. Eight tri-terpenoid glycosides were simultaneously quantified by HPLC for the quality control. The cytotoxic activity of the extract of A. raddeana was tested. The result indicated that the ethyl acetate soluble fraction was the most potent. Further separation from this fraction yielded hederacolchiside A1, a tri-terpenoid glycoside, which showed good inhibitory activity against ten human cancer cells lines, providing scientific evidence for the potential of A. raddeana as an anti-cancer traditional medicine.
Acknowledgments
This work was supported by National Natural Science Foundation of China [No.81373900]; the Special Fund for TCM supported by State Administration of Traditional Chinese Medicine of China [No. 201407002]; and National Natural Science Foundation of China [No. U15082201009120].
Funding Statement
This work was supported by National Natural Science Foundation of China [No.81373900]; the Special Fund for TCM supported by State Administration of Traditional Chinese Medicine of China [No. 201407002]; and National Natural Science Foundation of China [No. U15082201009120].
Footnotes
Conflicts of interest statement
The authors declare that there is no conflict of interest.
REFERENCES
- 1.National Commission of Chinese Pharmacopoeia. Pharmacopoeia of People’s Republic of China. Beijing, China: China: Medical Science and Technology Press; 2015. [Google Scholar]
- 2. Xu Z, Mou X, Gao XZ, Tian JK, Liu A. Progress in chemical and pharmacological studies of Anemone raddeana Regel. Res Pract Chin Med. 2001;15:53–4. [In Chinese, English abstract] [Google Scholar]
- 3. Zhang YF, Liu D, Zhang L, Li LK, Gong JY. Determination of raddeanin A from Anemone raddeana rhizoma by HPLC-ELSD. Chin Tradit Pat Med. 2014;36:1930–2. [In Chinese, English abstract] [Google Scholar]
- 4.Zhou HL. Regel [PhD thesis] Changchun: Changchun University of Chinese Medicine; 2007. A preliminary study on the chemical constituents and biological activities of Anemone raddeana. [Google Scholar]
- 5. Guan YY, Liu HJ, Luan X, Xu JR, Lu Q, Liu YR, et al. Raddeanin A, a triterpenoid saponin isolated from Anemone raddeana, suppresses the angiogenesis and growth of human colorectal tumor by inhibiting VEGFR2 signaling. Phytomedicine. 2015;22:103–10. doi: 10.1016/j.phymed.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 6. Lu JC, Sun QS, Sugahara K, Sagara Y, Kodama H. Effect of six compounds isolated from rhizome of Anemone raddeana on the superoxide generation in human neutrophil. Biochem Bioph Res Co. 2001;280:918–22. doi: 10.1006/bbrc.2000.4183. [DOI] [PubMed] [Google Scholar]
- 7. Yamashita K, Lu HW, Lu JC, Chen G, Yokoyama T, Sagara Y, et al. Effect of three triterpenoids, lupeol, betulin, and betulinic acid on the stimulus-induced superoxide generation and tyrosyl phosphorylation of proteins in human neutrophils. Clin Chim Acta. 2002;325:91–6. doi: 10.1016/s0009-8981(02)00252-8. [DOI] [PubMed] [Google Scholar]
- 8. Chen X, Lu JC, He WF, Chi HD, Yamashita K, Manabe M, et al. Antiperoxidation activity of triterpenoids from rhizome of Anemone raddeana. Fitoterapia. 2009;80:105–11. doi: 10.1016/j.fitote.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 9. Wei SH, He WF, Lu JC, Wang ZH, Yamashita K, Yokoyama M, et al. Effects of five oleanolic acid triterpenoid saponins from the rhizome of Anemone raddeana on stimulus-induced superoxide generation, phosphorylation of proteins and translocation of cytosolic compounds to cell membrane in human neutrophils. Fitoterapia. 2012;83:402–7. doi: 10.1016/j.fitote.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 10. Hung HY, Wu TS. Recent progress on the traditional Chinese medicines that regulate the blood. J Food Drug Anal. 2016;24:221–38. doi: 10.1016/j.jfda.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization. General guidelines for methodologies on research and evaluation of traditional medicines. Geneva, Switzerland: World Health Organization; 2002. [Google Scholar]
- 12. Perumal SS, Ekambaram SP, Raja S. Analytical method development and validation of simultaneous estimation of rabeprazole, pantoprazole, and itopride by reverse-phase high-performance liquid chromatography. J Food Drug Anal. 2014;22:520–6. doi: 10.1016/j.jfda.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fan JS, Lee IJ, Lin YL. Flavone glycosides from commercially available Lophatheri Herba and their chromatographic fingerprinting and quantitation. J Food Drug Anal. 2015;23:821–7. doi: 10.1016/j.jfda.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang L, Jiang ZZ, Yang J, Li YY, Wang YF, Chai X. Chemical material basis study of Xuefu Zhuyu decoction by ultra-performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry. J Food Drug Anal. 2015;23:811–20. doi: 10.1016/j.jfda.2015.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao ZK. Regel [PhD thesis] Hangzhou: Hangzhou Normal University; 2013. The quality control and toxicity studies of Anemone raddeana. [Google Scholar]
- 16. Li Y, Zhang H, Liu DY. Quantitative analysis of raddeanoside D in radde Anemone rhizome (Anemone raddeana) from different habitats by HPLC. Chin Tradit Herbal Drugs. 2000;31:15–6. [In Chinese, English abstract] [Google Scholar]
- 17. Zhang YF, Li ZZ, Zhao LL, Gong JY, Cai GZ. Study on the HPLC fingerprint of Anemone raddeana. China Pharm. 2016;27:399–401. [In Chinese, English abstract] [Google Scholar]
- 18.Fan L. Regel [MSD thesis] Shenyang: Shenyang Pharmaceutical University; 2010. Separation and preparation of saponins from Anemone raddeana. [Google Scholar]
- 19. Lu JC, Xu BB, Gao S, Kodama H. A new superoxide-generation inhibitor from rhizome of Anemone raddeana Regel. Chin Chem Lett. 2009;20:694–7. [Google Scholar]
- 20. Wu FE, Koike K, Ohmoto T, Chen WX. Saponins from Chinese folk medicine, “Zhu jie xiang fu”, Anemone raddeana Regel. Chem Pharm Bull. 1989;37:2445–7. doi: 10.1248/cpb.37.2445. [DOI] [PubMed] [Google Scholar]
- 21. Lu JC, Xu BB, Gao S, Fan L, Zhang HF, Liu RX, et al. Structure elucidation of two triterpenoid saponins from rhizome of Anemone raddeana Regel. Fitoterapia. 2009;80:345–8. doi: 10.1016/j.fitote.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 22. Lu JC, Xu BB, Zhang XY, Sun QS. Study on chemical constituents of rhizome of Anemone raddeana. Acta Pharm Sin. 2002;37:709–12. [In Chinese, English abstract] [PubMed] [Google Scholar]
- 23. Zhou Y, Fu Y, Peng SL, Liang J, Ding LS. HPLC-MSn analysis of triterpenoid saponins from the whole plants of Anemone begoniifolia. J Instrum Anal. 2005;24:121–2. [In Chinese, English abstract] [Google Scholar]
- 24. Zhou Y, Li R, Wang XM, Peng SL, Ding LS. HPLC/MSn analysis of triterpenoid saponins from Anemone rupestris ssp. gelida. Chin J Org Chem. 2006;26:116–9. [In Chinese, English abstract] [Google Scholar]
- 25. Li F, Xu KJ, Ding LS, Wang MK. Rapid analysis of triterpenoidic saponins in Anemone raddeana using electrospray ionization multi-stage mass spectrometry combined with silica gel column chromatography. Chin J Anal Chem. 2011;39:219–24. [In Chinese, English abstract] [Google Scholar]
- 26. Guo YC, Ouyang H, He MZ, Liang QD, Song YG, Rao XY, et al. Identification of saponins in rhizomes of Anemone davidii by UPLC/Q-TOF-MS/MS. Chin Tradit Herbal Drugs. 2014;45:1378–87. [In Chinese, English abstract] [Google Scholar]
- 27. Li XC, Wang DZ, Wu SG, Yang CR. Triterpenoid saponins from Pulsatilla campanella. Phytochemistry. 1990;29:595–9. [Google Scholar]
- 28. Fan L, Lu JC, Xu BB, Gao S, Zhang HF, Liu RX. Oleanane saponins from rhizome of Anemone raddeana. Helv Chim Acta. 2010;93:58–63. [Google Scholar]
- 29. Liao X, Li BG, Ding LS, Pan YJ, Chen YZ. New triterpenoid saponins from Anemone begoniifolia. Acta Pharm Sin. 2000;35:821–5. [In Chinese, English abstract] [PubMed] [Google Scholar]
- 30. Wang XY, Liu DY, Xia ZT, Liu KF, Li J. Chemical constituents of rhizome of Anenone raddeana. Chin J Anal Chem. 2004;32:587–92. [In Chinese, English abstract] [Google Scholar]
- 31. Kim Y, Bang SC, Lee JH, Ahn BZ. Pulsatilla saponin D: the antitumor principle from Pulsatilla koreana. Arch Pharm Res. 2004;27:915–8. doi: 10.1007/BF02975843. [DOI] [PubMed] [Google Scholar]
- 32. Ye WC, Zhang QW, Zhou SX, Che CT. Four new oleanane saponins from Anemone anhuiensis. Chem Pharm Bull. 2001;49:632–4. doi: 10.1248/cpb.49.632. [DOI] [PubMed] [Google Scholar]
- 33. Zhang LT, Takaishi Y, Zhang YW, Duan HQ. Studies on chemical constituents from rhizome of Anemone flaccida. Chin J Chin Mater Med. 2008;33:1696–9. [In Chinese, English abstract] [PubMed] [Google Scholar]
- 34. Wu FE, Zhu ZQ. Study on the chemical constituents of the Chinese medicinal herb Anemone raddeana Regel. Acta Chim Sin. 1985;43:692–7. [In Chinese, English abstract] [Google Scholar]
- 35. Wu FE, Zhu ZQ. Study on the chemical constituents of the Chinese medicinal herb Anemone raddeana Regel. Acta Chim Sin. 1984;42:253–8. [In Chinese, English abstract] [Google Scholar]
- 36. Zhao YD, Chen SF, Wang YD, Lv CN, Wang J, Lu JC. Effect of drying processes on prenylflavonoid content and antioxidant activity of Epimedium koreanum Nakai. J Food Drug Anal. 2017:1–11. doi: 10.1016/j.jfda.2017.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]