Abstract
Flavonoids luteolin and quercetin can inhibit growth and metastasis of cancer cells. In our previous report, luteolin and quercetin was shown to block Akt/mTOR/c-Myc signaling. Here, we found luteolin and quercetin reduced protein level and transactivation activity of RPS19 in A431-III cells, which is isolated from parental A431 (A431-P) cell line. Further investigation the inhibitory mechanism of luteolin and quercetin on RPS19, we found c-Myc binding sites on RPS19 promoter. The Akt inhibitor LY294002, mTOR inhibitor rapamycin and c-Myc inhibitor 10058-F4 significantly suppressed RPS19 expression and transactivation activities. Overexpression and knockdown of c-Myc in cancer cells show RPS19 expression was regulated by c-Myc. Furthermore, Knockdown and overexpression of RPS19 was used to analyze of the function of RPS19 in cancer cells. The epithelial-mesenchymal transition (EMT) markers and metastasis abilities of cancer cells were also regulated by RPS19. These data suggest that luteolin and quercetin might inhibit metastasis of cancer cells by blocking Akt/mTOR/c-Myc signaling pathway to suppress RPS19-activated EMT signaling.
Keywords: Luteolin, Quercetin, c-Myc, RPS19, EMT
1. Introduction
Flavonoids are widely reported to be multiple kinase inhibitors which modulate cell signaling in tumor cell proliferation, invasion, angiogenesis and apoptosis [1–9]. The flavonoids luteolin (Lu) and quercetin (Qu) were suggested by our previous report and others as two of more effective inhibitory compounds among flavonoids [6,10,11]. According to the structure-activity-relationship (SAR) studies and molecular modeling, these two agents were found to dock in the src kinase active site. Recently, evidence indicated both Lu and Qu was inhibited metastasis of A431-III cancer cells through inhibition of Src/Cortatin, Akt/mammalian target of rapamycin (mTOR)/c-Myc signaling and reversing the EMT process [5].
The flavonoids are polyphenolic compounds found as integral components of the human diet. They are universally present as constituents of flowering plants, particularly of food plants. The flavonoids are phenyl substituted chromones (benzopyran derivatives) consisting of a 15-carbon basic skeleton (C6–C3–C6), composed of a chroman (C6–C3) nucleus (the benzo ring A and the heterocyclic ring C). The antioxidant and anti-inflammation activities of flavonoids extracted from plants are well studied [12–19]. An impressive body of information exists on the antitumor action of plant flavonoids [6,20]. Among the flavonoids examined, Lu and Qu were found to be the two most potent agents exert anticancer activities [6]. A wealthy of evidence demonstrated that both Lu and Qu have the capacity to nullify tumor promotion events [4,5,21]. In addition, recently studies have shown that dietary flavonoids Lu and Qu could inhibit tumor progression, invasion and angiogenesis. Both Lu and Qu could reverse cadherin expression, downregulate EMT markers, expunge cell-cell interactions, suppress secretion of matrix metalloproteinases (MMPs) and invasiveness of tumor cells [3,21,22]. Furthermore, Lu and Qu attenuate the phosphorylation of cortactin and Src in highly invasive A431-III subline. As a consequence, there ensues a disruption of invadopodia generation and the suppression of MMPs secretion [4]. Lu and Qu inhibited a specific action in hampering kinase activities and aforementioned inhibitory effects on cancer. However, it cannot be ruled out other mechanisms contributed to these effects and which were modulated by Lu and Qu. Taking together, a positive correlation between dietary flavonoid-rich diet and lower risk of colon, prostate and breast cancer lead to a conclusion that dietary flavonoids mediate the protective effects as chemopreventive agents, and can interact with different genes and proteins to play role in chemotherapy as well [23,24].
c-Myc is a regulator of ribosomal biogenesis and protein synthesis [25]. c-Myc-overexpressing in fibroblasts were known to activate several ribosomal proteins [26]. c-Myc is suggested a role in enhancement of ribosomal biogenesis. Abundant expression of specific Ribosomal proteins (RPs) may influent cell proliferation. For example, overexpression of RPS3A in mouse fibroblast cell line induced tumor formation [27]. Increasing expression of ribosomal protein S8 (RPS8), ribosomal protein S12 (RPS12), ribosomal protein L23A (RPL23A), ribosomal protein L27 (RPL27) and ribosomal protein L30 (RPL30) were reported in different tumor types [25,28]. Some of these RPs were known to be regulated by c-Myc induction. The ribosomal protein L11 (RPL11) was identified as a feedback inhibitor of c-Myc [29]. Ribosomal protein S14 (RPS14) is reported to directly inhibit c-Myc transcription and its degradation was mediated by microRNA 145 (miR-145) [30]. The RPS12 was highly expressed in A431-III cancer cell and is used as a clinical biomarker for cancer detection [31]. In our previous report, RPS12 was found highly expressed in A431-III cells [21]. The highly invasive A431-III tumor subline was derived from parental A431 (A431-P) tumor cells by three successive passages through a Boyden chamber with EHS matrigel-coated membrane support [1]. The enhanced metastasis potential of this highly invasive cancer subline was found through c-Myc activation [21]. RPS19 was demonstrated as a c-Myc target gene in hepatocellular carcinomas (HCC) and may contribute to hepatocyte transformation [32]. c-Myc was reported to regulate transcription of RPS19 by directly binding to the promoter region in HCC [32]. The singly-nucleotide polymorphism of RPS19 was correlated with risk of cervical intraepithelial neoplasia 3 cancer cases [33]. c-Myc was also reported to induce EMT signaling in endometrial cancer [34].
The flavonoids Lu and Qu could suppress the metastasis of A431-III cancer cells through reducing c-Myc expression [21]. We specifically undertook current studies with a view to further evaluating the anticancer effects of Lu and Qu individually on the modulation expression of RPS expression in both A431-P and A431-III cells. Secondly, Lu and Qu were also used to analyze the inhibitory effects on RPS19 through Akt/ mTOR/c-Myc signaling. Expression of EMT markers, and the migration and invasion of A431-III cells regulated by RPS19 were also explored.
2. Materials and methods
2.1. Chemicals and reagents
A431-P cells were obtained from ATCC (Manassas, VA, USA). A431-III cells were isolated from A431-P cells using a Boyden chamber [1]. RPMI-1640 and fetal bovine serum (FBS) were obtained from Gibco (Grand Island, NY, USA). Anti-RPS19 and anti-c-Myc antibodies were obtained from GeneTex (Irvine, TX, USA). The anti-phospho-Akt and anti-phospho-S6K antibodies were obtained from Cell Signaling (Beverly, MA, USA). Anti-GADPH and anti-β-actin antibodies were purchased from Santa Cruz (Capitola, CA, USA). Luteolin was purchased from Toronto Research Chemicals (North York, ON, Canada). Quercetin was purchased from Nacalai Tesque (Kyoto, Japan). Agarose, dimethyl sulfoxide (DMSO), and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) were purchased from Merck-Millipore (Darmstadt, Germany). Rapamycin, LY294002, and 10058-F4 were obtained from Sigma (St. Louis, MO, USA).
2.2. Cell culture
A431-P (A431, ATCC CRL-1555) and A431-III cells (sub-line of A431-P cells) were described in our previous report [1]. A431-P and A431-III cells were incubated in a 5% CO2 air atmosphere at 37 °C with RPMI-1640 medium (Gibco, NY, USA) containing 10% FBS (Gibco, NY, USA).
2.3. Collection of cell lysates
Cells were collected and washed three times using phosphate-buffered saline (PBS) and lysed in gold lysis buffer (20 mM Tris–HCl (pH 7.9), 1 mM EGTA, 0.8% NaCl, 0.1 mM b-glycerylphosphate, 1 mM sodium pyrophosphate, 10 mM NaF, 1 mM Na4P2O7, 1 mM Na3VO4, 10% glycerol, 1% Triton X-100) containing 1 mM PMSF, 10 μg/mL aprotinin, and 10 μg/mL leupeptin.
2.4. Western blot experiment
Total cell lysates were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and then incubated with a primary antibody overnight at 4 °C. A secondary antibody conjugated with horseradish peroxidase (HRP; Millipore, Darmstadt, Germany) was detected with an enhanced chemiluminescence (ECL) reagent kit (Millipore, Darmstadt, Germany) using the BioSpectrum® Imaging System (UVP, Upland, CA, USA). Relative quantification of ECL signals was carried out using ImageJ software (http://rsb.info.nih.gov/ij/index.html, National Institute of Health, Bethesda, MA, USA).
2.5. In vitro migration assay
A431-P and A431-III cells (1 × 106 cells per well) were plated onto 6-well culture plates overnight. A monolayer of cultural cells was wounded by manually scratching it with a pipette tip and then further incubation with culture medium and/or chemicals at 37 °C for 24 h. The wounding was captured using a phase-contrast Olympus IX70 microscope (Olympus, Tokyo, Japan) and a SPOT camera (Sterling Heights, MI, USA). Experiments were repeated three times for each treatment.
2.6. Amplification of full-length RPS19 complementary (c)DNA
Full-length RPS19 cDNA was obtained from A431-III cells via a PCR using a Marathon cDNA amplification kit (Clontech, Palo Alto, CA, USA) as previously described [35]. The following primer pairs were used for the PCR: RPS19-F (5′- GAT GCC TGG AGT TAC TGT AAA AGA CG -3′) and RPS19-R (5′- CTA ATG CTT CTT GTT GGC AGC TGC C -3′). The PCR products were cloned into the pGEM-T vector (Promega, Madison, WI, USA) for sequencing. Full-length RPS19 was inserted into a pcDNA3-HA plasmid to generate the pcDNA3-RPS19-HA plasmid.
2.7. Luciferase assay
The 5′-upstream 1162-bp long RPS19 (RPS19-pro) was identified and isolated from genomic DNA of A431-III cells using a PCR with specific primers as follows: RPS19-pro-F (5′- ACC CAA GTA CCT GGA GAT TTC CAG TTA GAC -3′) and RPS19-pro-R (5′- GGA ACA GTG GTG GAG AAT ACT ATG ATG GCG -3′). The amplified DNA fragment was then cloned into a pGEMT-Easy vector (Promega) following sequencing. The RPS19-pro fragment was then cloned into the pGL3-Basic vector as a pGL3-RPS19-pro plasmid. The pGL3-Basic and pGL3-RPS19-pro plasmids were transfected into A431-III cells with or without the pcDNA3-cMyc-HA plasmid [21] using the PolyJet transfection reagent (SignaGen Laboratories, Rockville, MD, USA) according to the manufacturer's instructions. Inhibitor-containing medium was replaced at 24 h post-transfection. Total cell lysates were harvested at 48 h post-transfection and luciferase activity was measured with a Luciferase Assay Reagent (Promega) using the Bio-Spectrum Imaging System (UVP).
2.8. Knockdown RPS19 by short hairpin (sh)RNA
RPS19 (shRPS19-1, clone ID: TRCN0000074913/ TRCN0000074915; shRPS19-2, clone ID: TRCN0000074916) and control (shGFP, clone ID: TrcN0000072178) shRNA vectors were obtained from the National RNAi core Facility at the Institute of Molecular Biology (Academia Sinica, Taipei, Taiwan). A431-III cells were transfected with shRNA using the Lipofectamine 2000 transfection reagent (Life Technology, Grand Island, NY, USA) following the manufacturer's instructions.
2.9. Transwell invasion assay
Transwells with polycarbonate filters (BD Biosciences, Franklin Lake, NJ, USA) were coated with extracellular matrix (ECM) for 1 h at 25 °C. Cultural medium was added to the lower compartment of the chamber. A431-III cells (5 × 104) transfected with shRNAs of RPS19 (shRPS19-1 and shRPS19-2), control shRNA (shGFP) or the pcDNA3-cMyc-HA, pcDNA3-RPS19-HA plasmids were added to the upper compartment of the chamber at 37 °C for 24 h, after which the filter was fixed with 3% glutaraldehyde in PBS and stained with crystal violet. Each experiment was repeated three times independently.
2.10. Statistical analysis
Three independent experiments were run to analyze the mean ± standard deviation (SD). For comparison between two groups, statistical analysis was determined by an unpaired Student's t-test. For comparison more than two groups, the one-way ANOVA following with Tukey's test was used. A probability of p < 0.05 is indicated by *; p < 0.01 is indicated by **; p < 0.001 is indicated by ***.
2.11. MTT assay
The A431-III cells (105 cells/well) were seeded in 48-well plates and incubated with different concentrations of flavonoids and inhibitors for 24 h. The medium was removed and the cells were washed with PBS 3 times. The 200 μL of cultural medium containing 20 μL of MTT (5 mg/mL) was added to each well and continued to culture at 37 °C for 3 h. The medium was then removed, and 200 μL of DMSO was added to dissolve the precipitate. The absorbance was measured using a microplate reader (Bio-Tec, Winooski, VT, USA) at a wavelength of 570 nm.
3. Results
3.1. RPS19 is highly expressed by A431-III cells
Our previous report indicated that highly invasive A431-III cells expressed higher amount of RPS12 through c-Myc activation which in turn increase the migratory and invasive abilities [21]. To determine whether RPS19, a ribosomal protein, was more highly expressed in A431-III cells, we then performed experiments and compared protein levels by Western blotting in A431-P and A431-III cells. Protein levels of RPS19 were higher in A431-III cells than A431-P cells (Fig. 1A and B). These data suggest that highly expression of RPS19 in A431-III cells might be related to the invasiveness of A431-III cells.
Fig. 1.
Protein levels of ribosomal protein S19 (RPS19) in A431-P and A431-III cells. A. Protein levels of RPS19 in A431-P and A431-III cells were measured by a Western blot experiment. B. The protein levels of RPS19 in A431-P and A431-III cells was analyzed by ImageJ software. Results from three independent experiments are expressed as the mean ± standard deviation (SD). * Indicates a significant difference compared to the control (p < 0.05).
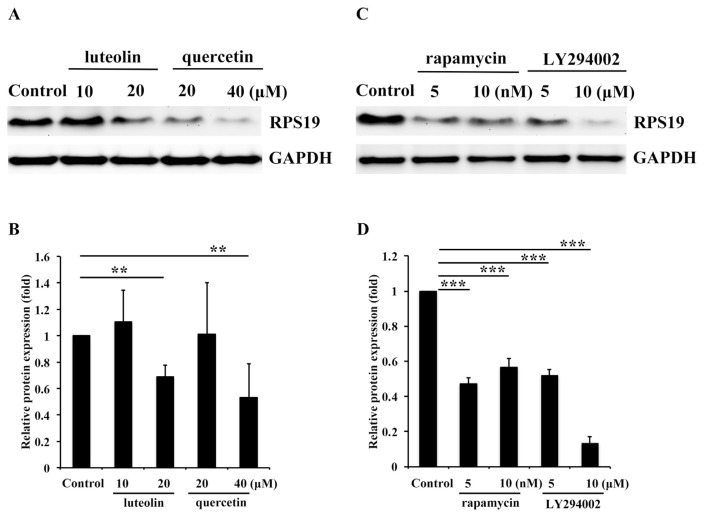
3.2. RPS19 expression might be inhibited by Lu and Qu through Akt/mTOR signaling
It is well known that Lu and Qu two most potent agents exert anticancer activities that could reduce the migratory and invasive abilities of cancer cells [4,5,21,23,36–39]. In our previous report, we found Akt/mTOR/c-Myc signaling was activated in A431-III cells and could be inhibited by Lu and Qu. Expression of ribosomal protein RPS12 was reduced by Lu and Qu through blocking activation of Akt/mTOR signaling. The phosphorylation of Akt and mTOR could be inhibited by Lu and Qu and protein level of c-Myc was also reduced [21]. The c-Myc expression was inhibited by Lu, Qu, rapamycin and LY294002 (Fig. S2). RPS19 is also a ribosomal protein. The cytotoxicity of LY294002, rapamycin, 10058-F4, Lu and Qu in A431-III cells was shown in Figure S1. To determine whether Lu and Qu can also reduce expression of RPS19 by blocking activation of Akt/mTOR/c-Myc signaling, we analyzed protein levels of RPS19 after treatment with Lu and Qu at the concentration of 10 and 20 μM, respectively. Both Lu and Qu were found significantly to reduce the activation of Akt/mTOR signaling [21] compared to the control. The protein level of RPS19 was significantly reduced by treatment with 20 μM of Lu and Qu and 40 μM of Qu compared to the control (Fig. 2A and B). To elucidate whether the reduction in RPS19 by Lu and Qu is the major cause of blocking Akt/mTOR signaling pathway, several experiments were performed. First, we treated A431-III cells with the Akt inhibitor, LY294002 at the concentration of 5 and 10 μM and the mTOR inhibitor rapamycin at the concentration of 5 and 10 nM. Both LY294002 and rapamycin were found to significantly reduce the activation of Akt/mTOR signaling pathway in A431-III cells [21]. Simultaneously, the protein level of RPS19 was significantly reduced by treated with 5 and 10 μM of LY294002 and also with 5 and 10 nM of rapamycin (Fig. 2C and D), respectively. These results suggest
Fig. 2.
Ribosomal protein S19 (RPS19) expression was inhibited by Qu, Qu through Akt/mammalian target of rapamycin (mTOR) signaling. A. The protein level of RPS19 in A431-III cells was measured by Western blotting after treatment with DMSO, 10 and 20 μM of Lu, or 20 and 40 μM of Qu for 24 h. B. Protein level changes of RPS19 after treatment with Lu or Qu were analyzed by ImageJ software. C. Protein levels of RPS19 in A431-III cells were measured by Western blotting after treatment with 5 and 10 nM of rapamycin or 5 and 10 μM of LY294002 for 24 h. D. Protein level changes of RPS19 after treatment with rapamycin or LY294002 were analyzed with ImageJ software. Data represent the mean (SD) of three different experiments. Statistical significance was determined by a one-way ANOVA with Tukey's test (*P < 0.05, **P < 0.01, and ***P < 0.001).
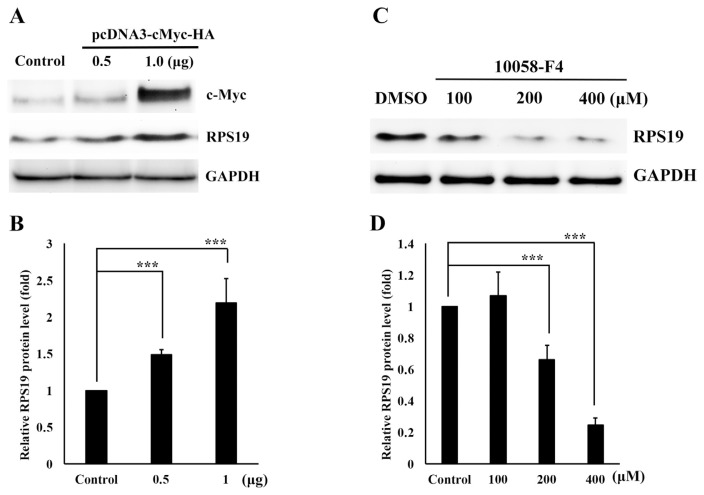
3.3. c-Myc activates RPS19 expression
Our previous report indicated that c-Myc exerted higher expression in A431-III cancer cells and this might regulate expression of RPS12, thus increase the invasiveness of cancer cells [21]. It's interesting to know whether c-Myc could also activates the expression of RPS19 in A431-III cancer cells in the same manner. We then analyzed the protein levels of RPS19 by overexpressing c-Myc in A431-P cells. The protein level of RPS19 significantly increased (Fig. 3A and B). By Treating of A431-III cells with the c-Myc inhibitor, 10058-F4, at the concentration of 100, 200 and 400 μM, we observed the protein level of RPS19 was significantly decreased (Fig. 3C and D). These data suggest that RPS19 expression is regulated by c-Myc.
Fig. 3.
c-Myc regulates ribosomal protein S19 (RPS19) expression. A. A431-P cells were transfected with 1 μg of pcDNA3-HA as the control, and 0.5 and 1.0 μg of pcDNA3-cMyc-HA. Protein levels of RPS19 was measured by Western blotting after 24 h. B. Protein level changes of RPS19 after transfection with the control or pcDNA3-cMyc-HA were analyzed with ImageJ software. C. A431-III cells were treated with DMSO, or 100, 200, and 400 μM of 10058-F4 for 24 h, and protein levels of RPS19 were measured by Western blotting. D. Protein level changes of RPS19 after treatment with DMSO or 10058-F4 were analyzed by ImageJ software. Data represent the mean (SD) of three different experiments. Statistical significance was determined by a one-way ANOVA with Tukey's test (*P < 0.05, **P < 0.01, and ***P < 0.001).
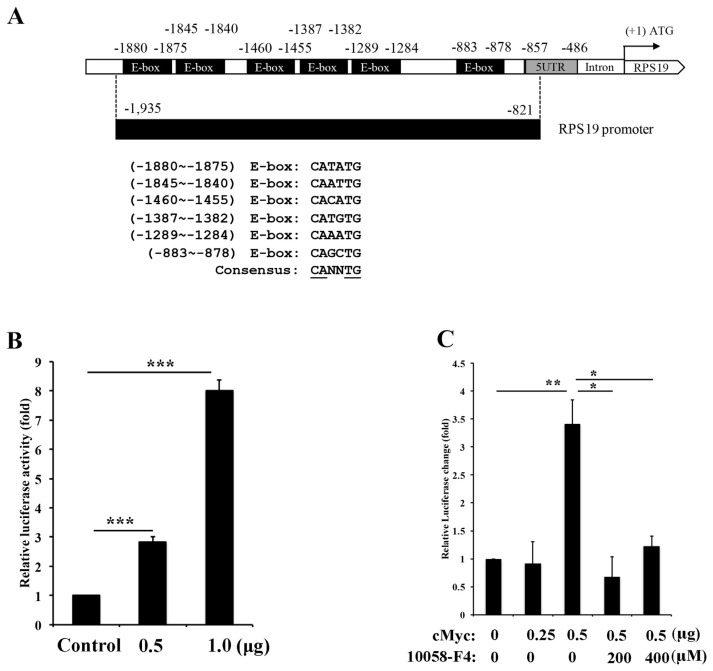
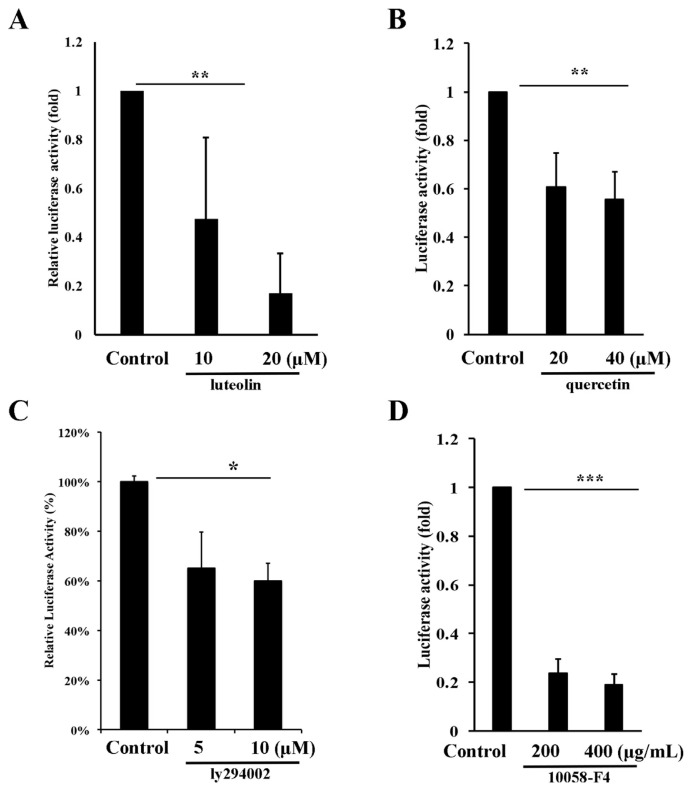
3.4. c-Myc activates RPS19 expression through Akt/ mTOR signaling pathway
Since c-Myc is a transcription factor and activates transcription of target gene through E-box (CANNTG) binding [40]. To test whether the expression of RPS19 is indeed activated by c-Myc through increasing transactivation activity of the RPS19 promoter, we set up a serial of experiments to explore this contention. We first analyzed and cloned the RPS19 promoter to the pGL3-basic vector to generate a pGL3-RPS19-Luc plasmid. There are six potential E-box motifs located in the 1115 bp upstream of the RPS19 promoter (Fig. 4A). After transfection of the pGL3-RPS19-Luc plasmid (0.5 and 1.0 μg) and pGL3-Basic plasmid (0.5 ug) as control into A431-P cells, the transactivation activity of the RPS19 promoter increased to 2.8-fold and 8.0-fold compared to the control and showed a dose-dependent manner (Fig. 4B). To elucidate if the transactivation activity of the RPS19 promoter was truly activated by c-Myc, we transfected 0.5 ug of pGL3-RPS19-Luc and pcDNA3-cMyc-HA plasmid (0.25 and 0.5 μg) into A431-P cells combined with or without treatment of 10058-F4 (200 and 400 μM) as measured transactivation activity by a luciferase assay. The transactivation activity increased to 0.9-fold and 3.4-fold after transfection with 0.25 and 0.5 μg of the pcDNA3-cMyc-HA plasmid compared to the control (Fig. 4C). This activation was reduced by treatment with 200 and 400 μM of 10058-F4 (Fig. 4C). Finally, in order to analyze the transactivation activity of RPS19 in A431-III cells, we transfected 0.5 μg of the pGL3-RPS19-Luc into A431-III cells and then treated them with DMSO (control), luteolin (10 and 20 μM), quercetin (20 and 40 μM), LY294002 (5 and 10 μM) or 10058-F4 (200 and 400 μM). The transactivation activity decreased after treatment with luteolin (Fig. 5A), quercetin (Fig. 5B), LY294002 (Fig. 5C) and 10058-F4 (Fig. 5D) as compared to the control. These data suggest that the transactivation activity of RPS19 is activated by Akt/mTOR/c-Myc signaling pathway in A431-III cells and reduced by luteolin and quercetin.
Fig. 4.
cMyc activates transactivation of ribosomal protein S19 (RPS19) in A431-P cells. A. The RPS19 promoter contains six potential E-box motifs. B. Transactivation of RPS19 was measured by a luciferase assay after transfection of A431-P cells with pcDNA3-HA (control), or 0.5 and 1.0 μg of pcDNA3-cMyc-HA for 24 h in A431-P cells. C. Transactivation of RPS19 was measured by transfection of A431-III cells with pcDNA3-HA (control), 0.25 and 0.5 μg of pcDNA3-cMyc-HA, and combined treated with or without 10058-F4 A431-III cells for 24 h in A431-III cells. Statistical significance between the groups was determined from three separate experiments by an unpaired Student's t-test. Results from three independent experiments are expressed as the mean ± standard deviation (SD). Statistical significance was determined by a one-way ANOVA with Tukey's test (*P < 0.05, **P < 0.01, and ***P < 0.001). that RPS19 expression might be reduced by both Lu and Qu through the Akt/mTOR signaling pathway.
Fig. 5.
Transactivation activity of ribosomal protein S19 (RPS19) is reduced by luteolin and quercetin through reduction of Akt/mTOR/cMyc signaling. Transactivation of RPS19 was measured by treatment of A431-III cells with 10 and 20 μM of luteolin (A), 20 and 40 μM of quercetin (B), 5 and 10 μM of LY294002 (C), 200 and 400 μM of 10058-F4 (D) for 24 h. Data represent the mean (SD) of three different experiments. Statistical significance was determined by a one-way ANOVA with Tukey's test (*P < 0.05, **P < 0.01, and ***P < 0.001).
3.5. RPS19 regulates EMT marker expression in cancer cells
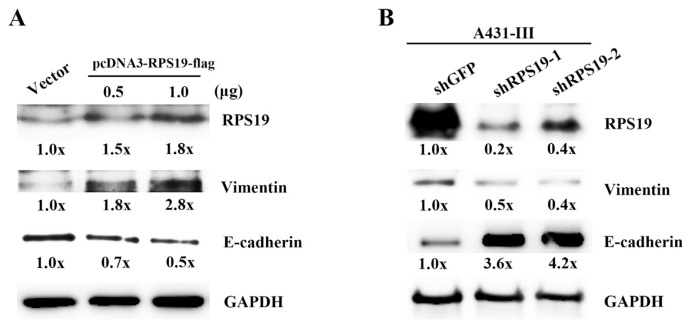
In our previous reports, the EMT markers in A431-P and A431-III cells were higher expressed and could be inhibited by Lu and Qu [5,21]. In this study, we hypothesized that RPs might be also play a key role responsible for induction/acceleration of EMT process. In order to gain insights into cellular events of RPS19 and EMT markers in A431 cells, we set to explore the link between RPS19 and EMT markers, especially E-cadherin and vimentin, to determine whether RPS19 contributed to the invasiveness of cancer cells. Therefore, we analyze the protein level of these two EMT markers using RPS19 overexpression and knockdown experiments. We cloned and constructed the pcDNA3-RPS19-flag plasmid for gene expression in A431-P cells and two shRNAs of RPS19 (shRPS19-1 and shRPS19-2) for a knockdown experiment in A431-III cells. The pcDNA3-RPS19-flag plasmid at 0.5 and 1.0 μg was transfected into A431-P cells, protein levels of RPS19, E-cadherin and vimentin were analyzed by a Western blotting experiment. Over-expression of RPS19 resulted in down-regulation of E-cadherin and up-regulation of vimentin (Fig. 6A and B). Knockdown RPS19 expression by the two shRNA (shRPS19-1 and shRPS19-2) in A431-III cells show opposite results (Fig. 6A and B). These data suggest that RPS19 regulates EMT markers expressions in A431-III cancer cells.
Fig. 6.
Ribosomal protein S19 (RPS19) activates EMT signaling. Protein levels of RPS19, E-cadherin, and vimentin were measured by Western blotting after overexpression of RPS19 in A431-P cells (A) and RPS19 shRNA knockdown in A431-III cells (B). Protein levels of E-cadherin and vimentin from Western blotting was analyzed by ImageJ software. Data represent the mean (SD) of three different experiments. Statistical significance was determined by a one-way ANOVA with Tukey's test (*P < 0.05, **P < 0.01, and ***P < 0.001).
3.6. RPS19 promotes the migratory and invasiveness ability of cancer cells
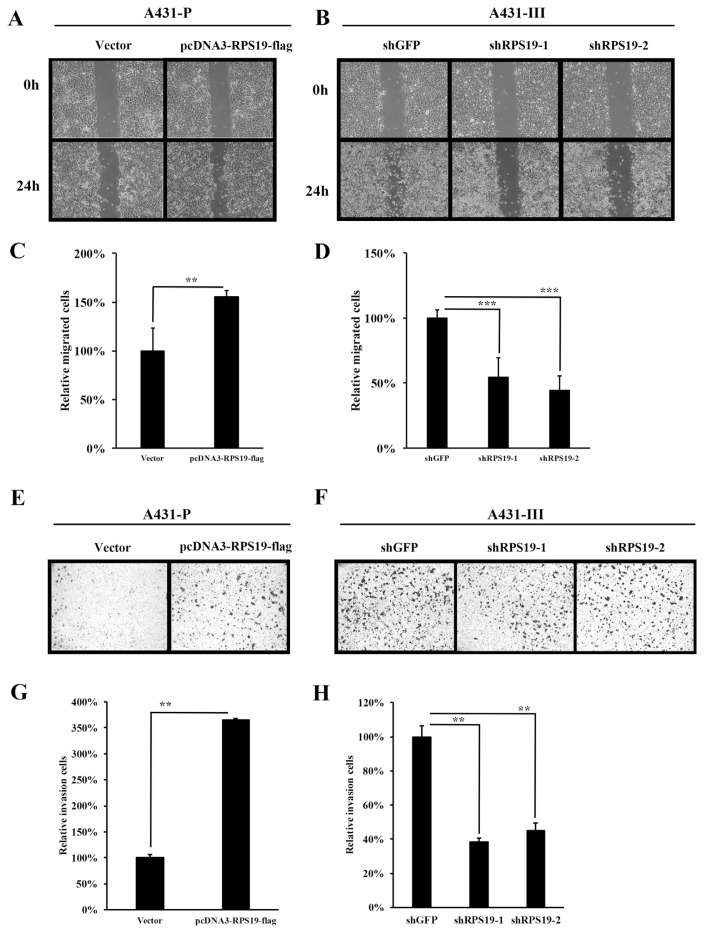
In our previous reports, the migration and invasion ability in A431-III cells was higher presented and could be inhibited by Lu and Qu [4,5,21]. In this study, we also present the inhibition of migration by Lu and Qu in A431-III cells (Fig. S3). To further analyze the migratory and invasive abilities of cancer cells regulated by RPS19, we prepared wound-healing and invasion assays using A431-P and A431-III cells. The migratory ability of A431-P cells after transfection with the pcDNA3-RPS19-flag plasmid increased to 1.55-fold compared to the control (Fig. 7A and C). Knockdown of RPS19 expression by the two shRNAs (shRPS19-1 and shRPS19-2) in A431-III cells decreased the migratory abilities by 0.55 and 0.44-fold compared to the control (Fig. 7B and D). The invasive abilities of A431-P cells after transfection with the pcDNA3-RPS19-flag increased by 3.65-fold compared to the control (Fig. 7E and G). Knockdown of RPS19 expression by the two shRNA (shRPS19-1 and shRPS19-2) in A431-III cells decreased the invasive abilities by 0.38 and 0.45-fold compared to the control (Fig. 7F and H). These data suggest that RPS19 promotes the migratory and invasive abilities of cancer cells.
Fig. 7.
Ribosomal protein S19 (RPS19) increases the migratory and invasive abilities of cancer cells. A wound-healing experiment was used to analyze cell migration after RPS19 overexpression in A431-P cells (A, C) and RPS19 shRNA knockdown in A431-III cells (B, D). A trans-well assay was used to analyze the invasive ability with RPS19 overexpression in A431-P cells (E, G) and RPS19 shRNA knockdown in A431-III cells (F, H). Migration and invasion cells were calculated and analyzed by ImageJ software. Statistical significance between the groups was determined from three separate experiments with an unpaired Student's t-test. Results from three independent experiments are expressed as the mean ± standard deviation (SD). Statistical significance was determined by a one-way ANOVA with Tukey's test (*P < 0.05, **P < 0.01, and ***P < 0.001).
4. Discussion
Flavonoids have been widely demonstrated to prevent cancer metastasis [41]. Lu and Qu show inherent potential as chemopreventive/anti-neoplastic agents. Previously, our laboratory furnished evidence to show that Lu and Qu are the two most potent agents and have the greater capacity to nullify tumor promotion events. Recently, we demonstrated that Lu and Qu were also found to inhibit the phosphorylation of cortactin and Src in A431-III cells. As a consequence, there ensues a disruption of invadopodia generation and the suppression of MMP secretion. These changes, in concert, bring about a reduction in metastasis [5].
In our and other previous reports, it was suggested that Lu and Qu possess the same abilities to bind to ATP-binding sites in PTK [6,36,42–45]. Inhibition of migration and invasion of A431-III cells by Lu and Qu occurs through inhibition of EMT signaling [5], inhibition of Src/FAK/Cortactin signaling [4], and mTOR/c-Myc signaling [21]. Lu and Qu are also inhibited UBE2S expression and contributes to reduce migration and invasion abilities of cancer cells [22]. These inhibitions by both Lu and Qu seems to have similar structure of flavonoids. Both luteolin and quercetin processes a double bond between C2 and C3 in ring C, and −OH groups on C3′ and C4’ in its B-ring. However, quercetin have an additional −OH substitution on C3 [6]. We suggest that both Lu and Qu might contribute to inhibit RPS19 expression through Akt/mTOR/c-Myc signaling to reduce metastasis abilities of cancer cells.
In a previous report, ribosomal proteins in a human metastasis model were analyzed using coupled 2-D liquid chromatography and mass spectrometry. In a comparison of the N4A4 metastasis subline and NM2C5 non-metastatic subline. Both sublines were originally isolated from the MDA-MB-435 breast tumor cell line. Highly expression of RPS19 was detected in the N4A4 but not in the NM2C5 cell lines, which suggests that some ribosomal proteins are associated with a metastatic phenotype [46]. This metastasis model is similar to our metastasis model of cancer cells. We obtained a highly invasive A431-III sunline from A431-P cells and compared the messenger (m)RNA and protein levels between these two cell lines. Previously, we reported the metastasis ability of A431-III cells was reduced by the treatment with Lu and Qu and exhibited a dose-dependent manner [4,5,21]. These results suggest that our metastasis model was useful in analyzing metastasis inhibition by flavonoids.
An increasing ribosomal protein level was reported to be associated with human cancers [25]. These ribosomal proteins are RPS12 in A431-III cancer cells [31,47]; RPL37 and RPL7a in prostate cancer [48]; RPL15 in oesophageal cancer [49]; RPL13 in gastrointestinal cancer [50]; RPL13, RPL36a, RPs8, RPL12, RPL23a, RPL27 and RPL30 in hepatocellular cancer [28]; and RPs3, RPs6, RPs8, RPs12, RPL5, RPL22, RPL26 and RPL35 in colorectal cancer [51]. Myc is a regulator of ribosomal biogenesis and protein synthesis [25]. The Myc protein as a transcription factor cooperates with the max protein to activate transcription of ribosomal proteins. RPL11 was reported to be activated by c-Myc in human cancers [52,53]. In our previous report, RPS12 was activated by Akt/mTOR/c-Myc signaling in A431-III cancer cells and increased the metastasis of cancer cells. This activation could be reduced by treatment with Lu and Qu [21]. Using this metastasis cell model, we further compared protein levels of RPS19 in A431-III and A431-P cells. Higher expression of RPS19 was detected in A431-III cells compared to A431-P cells and contributed to the metastasis of cancer cells. Treatment with Lu and Qu reduced protein levels of RPS19. We suggest that Lu and Qu can reduce metastasis by inhibiting RPS19 expression.
RSP12 and RPS19 are both ribosomal protein and contributes to cancer proliferation and metastasis. Knockdown RPS12 expression in gastric cancer inhibits the proliferation and metastasis of gastric cancer [54]. Breast cancer samples analyzed by Two-dimensional polyacrylamide gel electrophoresis (2D-PAGE) and matrix-assisted laser desorption/ ionization tandem time-of-flight mass spectrometry (MALDI-TOF/TOF-MS) show that RPS12 was involved in the pathological process of breast cancer with changed expression and may be as a useful biomarker [55]. RPS12 expression was significantly increased in metastasis stage of gastric cancer rather than premalignant stage or cancer stage [56]. The protein level of RPS12 in late stage of cervical cancer biopsies were increased comparison with normal tissue [57]. RPS12 was contributed to metastasis of cervical cancer cells activated by Akt/mTOR/c-Myc signaling [21]. RPS19 was also higher expressed in cancers and related to malignant potential. The RPS19 cDNA was higher expressed in primary colon carcinomas tissue and associated with cancer progression and differentiation [58]. Both RPS12 and RPS19 was shown to differential expressed in primary cervical cancers [59]. The c-Myc was reported to activate cancer malignancy by regulating ribosome biogenesis [25,53,60]. In our previous report and present data, we found RPS12 and RPS19 might be activated by Akt/mTOR/c-Myc signaling. These results suggest that RPS12 and RPS19 were potential targets for cancer therapy.
In this study, we confirmed that RPS19 was highly expressed in A431-III cells. The RPS19 contributes to migratory and invasive abilities of A431-III cells. Expression of RPS19 is transcriptionally regulated by c-Myc. Lu and Qu significantly reduced RPS19 expression. This reduction was due to the blocking Akt/mTOR/c-Myc signaling pathway by treatment of Lu and Qu. Our finding provided evidence of the functional role of Lu and Qu in metastasis inhibition of cervix cancer cells.
In conclusion, in addition to inhibit tumor progression, invasion, angiogenesis and reverse EMT, these two flavonoids not only ablate the RPS expression, but also block Akt/mTOR/ c-Myc signaling pathway. Our study provides a reliable model to investigate and confirm the importance of RPS expression and Akt/mTOR/c-Myc signaling pathway in cancer invasion. Lu and Qu seem to have inherent potential to attenuate tumor metastasis.
Appendix A. Supplementary data
Supplementary data related to this article can be found at https://doi.org/10.1016/j.jfda.2018.01.012.
Funding Statement
This work was supported by Taipei Medical University-Wan Fang Hospital (104TMU-WFH-08) and the Ministry of Science and Technology, Taiwan (MOST106-2314-B-038-028).
Footnotes
Funding
This work was supported by Taipei Medical University-Wan Fang Hospital (104TMU-WFH-08) and the Ministry of Science and Technology, Taiwan (MOST106-2314-B-038-028).
Conflicts of interest statement
The authors declare that they have no conflicts of interest.
References
- 1. Kao WT, Lin CY, Lee LT, Lee PP, Hung CC, Lin YS, et al. Investigation of MMP-2 and -9 in a highly invasive A431 tumor cell sub-line selected from a Boyden chamber assay. Anticancer Res. 2008;28:2109–20. [PubMed] [Google Scholar]
- 2. Lin CY, Tsai PH, Kandaswami CC, Chang GD, Cheng CH, Huang CJ, et al. Role of tissue transglutaminase 2 in the acquisition of a mesenchymal-like phenotype in highly invasive A431 tumor cells. Mol Cancer. 2011;10:87. doi: 10.1186/1476-4598-10-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lin CY, Tsai PH, Kandaswami CC, Lee PP, Huang CJ, Hwang JJ, et al. Matrix metalloproteinase-9 cooperates with transcription factor Snail to induce epithelial-mesenchymal transition. Cancer Sci. 2011;102:815–27. doi: 10.1111/j.1349-7006.2011.01861.x. [DOI] [PubMed] [Google Scholar]
- 4. Lin YC, Tsai PH, Lin CY, Cheng CH, Lin TH, Lee KP, et al. Impact of flavonoids on matrix metalloproteinase secretion and invadopodia formation in highly invasive A431-III cancer cells. PLoS One. 2013;8:e71903. doi: 10.1371/journal.pone.0071903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lin YS, Tsai PH, Kandaswami CC, Cheng CH, Ke FC, Lee PP, et al. Effects of dietary flavonoids, luteolin, and quercetin on the reversal of epithelial-mesenchymal transition in A431 epidermal cancer cells. Cancer Sci. 2011;102:1829–39. doi: 10.1111/j.1349-7006.2011.02035.x. [DOI] [PubMed] [Google Scholar]
- 6. Kandaswami C, Lee LT, Lee PP, Hwang JJ, Ke FC, Huang YT, et al. The antitumor activities of flavonoids. In Vivo. 2005;19:895–909. [PubMed] [Google Scholar]
- 7. Tu SH, Chen LC, Ho YS. An apple a day to prevent cancer formation: reducing cancer risk with flavonoids. J Food Drug Anal. 2017;25:119–24. doi: 10.1016/j.jfda.2016.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li W, He N, Tian L, Shi X, Yang X. Inhibitory effects of polyphenol-enriched extract from Ziyang tea against human breast cancer MCF-7 cells through reactive oxygen species-dependent mitochondria molecular mechanism. J Food Drug Anal. 2016;24:527–38. doi: 10.1016/j.jfda.2016.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang C-C, Ho C-T, Lee S-C, Way T-D. Isolation of eugenyl β-primeveroside from Camellia sasanqua and its anticancer activity in PC3 prostate cancer cells. J Food Drug Anal. 2016;24:105–11. doi: 10.1016/j.jfda.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Taliou A, Zintzaras E, Lykouras L, Francis K. An open-label pilot study of a formulation containing the anti-inflammatory flavonoid luteolin and its effects on behavior in children with autism spectrum disorders. Clin Ther. 2013;35:592–602. doi: 10.1016/j.clinthera.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 11. Taube C, Weng Z, Zhang B, Asadi S, Sismanopoulos N, Butcher A, et al. Quercetin is more effective than cromolyn in blocking human mast cell cytokine release and inhibits contact dermatitis and photosensitivity in humans. PLoS One. 2012;7:e33805. doi: 10.1371/journal.pone.0033805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Do QD, Angkawijaya AE, Tran-Nguyen PL, Huynh LH, Soetaredjo FE, Ismadji S, et al. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J Food Drug Anal. 2014;22:296–302. doi: 10.1016/j.jfda.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liao DY, Chai YC, Wang SH, Chen CW, Tsai MS. Antioxidant activities and contents of flavonoids and phenolic acids of Talinum triangular extracts and their immunomodulatory effects. J Food Drug Anal. 2015;23:294–302. doi: 10.1016/j.jfda.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hyun TK, Song SC, Song C-K, Kim J-S. Nutritional and nutraceutical characteristics of Sageretia theezans fruit. J Food Drug Anal. 2015;23:742–9. doi: 10.1016/j.jfda.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kalidindi N, Thimmaiah NV, Jagadeesh NV, Nandeep R, Swetha S, Kalidindi B. Antifungal and antioxidant activities of organic and aqueous extracts of Annona squamosa Linn. leaves. J Food Drug Anal. 2015;23:795–802. doi: 10.1016/j.jfda.2015.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mathew LE, Sindhu G, Helen A. Dolichos biflorus exhibits anti-inflammatory and antioxidant properties in an acute inflammatory model. J Food Drug Anal. 2014;22:455–62. doi: 10.1016/j.jfda.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Engida AM, Faika S, Nguyen-Thi BT, Ju YH. Analysis of major antioxidants from extracts of Myrmecodia pendans by UV/ visible spectrophotometer, liquid chromatography/tandem mass spectrometry, and high-performance liquid chromatography/UV techniques. J Food Drug Anal. 2015;23:303–9. doi: 10.1016/j.jfda.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Denardin CC, Hirsch GE, da Rocha RF, Vizzotto M, Henriques AT, Moreira JCF, et al. Antioxidant capacity and bioactive compounds of four Brazilian native fruits. J Food Drug Anal. 2015;23:387–98. doi: 10.1016/j.jfda.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chiang H-M, Chen H-C, Wu C-S, Wu P-Y, Wen K-C. Rhodiola plants: chemistry and biological activity. J Food Drug Anal. 2015;23:359–69. doi: 10.1016/j.jfda.2015.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang Y, Li W, Ning J, Hong R, Wu H. Major flavonoid constituents and short-term effects of Chun Mee tea in rats. J Food Drug Anal. 2015;23:93–8. doi: 10.1016/j.jfda.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lin C-W, Lai G-M, Chen K-C, Lin T-H, Fan J-J, Hsu R-L, et al. RPS12 increases the invasiveness in cervical cancer activated by c-Myc and inhibited by the dietary flavonoids luteolin and quercetin. J Funct Foods. 2015;19:236–47. [Google Scholar]
- 22. Lin T-H, Hsu W-H, Tsai P-H, Huang Y-T, Lin C-W, Chen K-C, et al. Dietary flavonoids, luteolin and quercetin, inhibit invasion of cervical cancer by reduction of UBE2S through epithelial–mesenchymal transition signaling. Food Funct. 2017;8:1558–68. doi: 10.1039/c6fo00551a. [DOI] [PubMed] [Google Scholar]
- 23. Batra P, Sharma AK. Anti-cancer potential of flavonoids: recent trends and future perspectives. 3 Biotech. 2013;3:439–59. doi: 10.1007/s13205-013-0117-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yang K. Chemoprevention studies of the flavonoids quercetin and rutin in normal and azoxymethane-treated mouse colon. Carcinogenesis. 2000;21:1655–60. doi: 10.1093/carcin/21.9.1655. [DOI] [PubMed] [Google Scholar]
- 25. van Riggelen J, Yetil A, Felsher DW. MYC as a regulator of ribosome biogenesis and protein synthesis. Nat Rev Cancer. 2010;10:301–9. doi: 10.1038/nrc2819. [DOI] [PubMed] [Google Scholar]
- 26. Guo QM, Malek RL, Kim S, Chiao C, He M, Ruffy M, et al. Identification of c-myc responsive genes using rat cDNA microarray. Cancer Res. 2000;60:5922–8. [PubMed] [Google Scholar]
- 27. Naora H, Takai I, Adachi M, Naora H. Altered cellular responses by varying expression of a ribosomal protein gene: sequential coordination of enhancement and suppression of ribosomal protein S3a gene expression induces apoptosis. J Cell Biol. 1998;141:741–53. doi: 10.1083/jcb.141.3.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kondoh N, Shuda M, Tanaka K, Wakatsuki T, Hada A, Yamamoto M. Enhanced expression of S8, L12, L23a, L27 and L30 ribosomal protein mRNAs in human hepatocellular carcinoma. Anticancer Res. 2001;21:2429–33. [PubMed] [Google Scholar]
- 29. Dai MS, Arnold H, Sun XX, Sears R, Lu H. Inhibition of c-Myc activity by ribosomal protein L11. EMBO J. 2007;26:3332–45. doi: 10.1038/sj.emboj.7601776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhou X, Hao Q, Liao JM, Liao P, Lu H. Ribosomal protein S14 negatively regulates c-Myc activity. J Biol Chem. 2013;288:21793–801. doi: 10.1074/jbc.M112.445122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cheng Q, Lau WM, Chew SH, Ho TH, Tay SK, Hui KM. Identification of molecular markers for the early detection of human squamous cell carcinoma of the uterine cervix. Br J Cancer. 2002;86:274–81. doi: 10.1038/sj.bjc.6600038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hunecke D, Spanel R, Langer F, Nam SW, Borlak J. MYC-regulated genes involved in liver cell dysplasia identified in a transgenic model of liver cancer. J Pathol. 2012;228:520–33. doi: 10.1002/path.4059. [DOI] [PubMed] [Google Scholar]
- 33. Safaeian M, Hildesheim A, Gonzalez P, Yu K, Porras C, Li Q, et al. Single nucleotide polymorphisms in the PRDX3 and RPS19 and risk of HPV persistence and cervical precancer/ cancer. PLoS One. 2012;7:e33619. doi: 10.1371/journal.pone.0033619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Liu L, Zhang J, Yang X, Fang C, Xu H, Xi X. SALL4 as an epithelial-mesenchymal transition and drug resistance inducer through the regulation of c-Myc in endometrial cancer. PLoS One. 2015;10:e0138515. doi: 10.1371/journal.pone.0138515. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 35. Cheng CH, Chen GD, Yeh MS, Chu CY, Hsu YL, Hwang PP, et al. Expression and characterization of the JAK kinase and STAT protein from brine shrimp, Artemia franciscana. Fish Shellfish Immunol. 2010;28:774–82. doi: 10.1016/j.fsi.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 36. Huang YT, Hwang JJ, Lee PP, Ke FC, Huang JH, Huang CJ, et al. Effects of luteolin and quercetin, inhibitors of tyrosine kinase, on cell growth and metastasis-associated properties in A431 cells overexpressing epidermal growth factor receptor. Br J Pharmacol. 1999;128:999–1010. doi: 10.1038/sj.bjp.0702879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lin Y, Shi R, Wang X, Shen HM. Luteolin, a flavonoid with potential for cancer prevention and therapy. Curr Cancer Drug Targets. 2008;8:634–46. doi: 10.2174/156800908786241050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chowdhury AR, Sharma S, Mandal S, Goswami A, Mukhopadhyay S, Majumder HK. Luteolin, an emerging anti-cancer flavonoid, poisons eukaryotic DNA topoisomerase I. Biochem J. 2002;366:653–61. doi: 10.1042/BJ20020098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Seelinger G, Merfort I, Wölfle U, Schempp C. Anti-carcinogenic effects of the flavonoid luteolin. Molecules. 2008;13:2628–51. doi: 10.3390/molecules13102628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Walhout AJ, Gubbels JM, Bernards R, van der Vliet PC, Timmers HT. c-Myc/Max heterodimers bind cooperatively to the E-box sequences located in the first intron of the rat ornithine decarboxylase (ODC) gene. Nucleic Acids Res. 1997;25:1493–501. doi: 10.1093/nar/25.8.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Weng C-J, Yen G-C. Flavonoids, a ubiquitous dietary phenolic subclass, exert extensive in vitro anti-invasive and in vivo anti-metastatic activities. Cancer Metastasis Rev. 2012;31:323–51. doi: 10.1007/s10555-012-9347-y. [DOI] [PubMed] [Google Scholar]
- 42. Lee LT, Huang YT, Hwang JJ, Lee PP, Ke FC, Nair MP, et al. Blockade of the epidermal growth factor receptor tyrosine kinase activity by quercetin and luteolin leads to growth inhibition and apoptosis of pancreatic tumor cells. Anticancer Res. 2002;22:1615–27. [PubMed] [Google Scholar]
- 43. Lee LT, Huang YT, Hwang JJ, Lee AY, Ke FC, Huang CJ, et al. Transinactivation of the epidermal growth factor receptor tyrosine kinase and focal adhesion kinase phosphorylation by dietary flavonoids: effect on invasive potential of human carcinoma cells. Biochem Pharmacol. 2004;67:2103–14. doi: 10.1016/j.bcp.2004.02.023. [DOI] [PubMed] [Google Scholar]
- 44. Ferriola PC, Cody V, Middleton E., Jr Protein kinase C inhibition by plant flavonoids. Kinetic mechanisms and structure-activity relationships. Biochem Pharmacol. 1989;38:1617–24. doi: 10.1016/0006-2952(89)90309-2. [DOI] [PubMed] [Google Scholar]
- 45. Agullo G, Gamet-Payrastre L, Manenti S, Viala C, Remesy C, Chap H, et al. Relationship between flavonoid structure and inhibition of phosphatidylinositol 3-kinase: a comparison with tyrosine kinase and protein kinase C inhibition. Biochem Pharmacol. 1997;53:1649–57. doi: 10.1016/s0006-2952(97)82453-7. [DOI] [PubMed] [Google Scholar]
- 46. Kreunin P, Yoo C, Urquidi V, Lubman DM, Goodison S. Differential expression of ribosomal proteins in a human metastasis model identified by coupling 2-D liquid chromatography and mass spectrometry. Cancer Genomics Proteomics. 2007;4:329–39. [PMC free article] [PubMed] [Google Scholar]
- 47. Fjeldbo CS, Aarnes EK, Malinen E, Kristensen GB, Lyng H. Identification and Validation of reference genes for RT-qPCR studies of hypoxia in squamous cervical cancer patients. PLoS One. 2016;11:e0156259. doi: 10.1371/journal.pone.0156259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Vaarala MH, Porvari KS, Kyllonen AP, Mustonen MV, Lukkarinen O, Vihko PT. Several genes encoding ribosomal proteins are over-expressed in prostate-cancer cell lines: confirmation of L7a and L37 over-expression in prostate-cancer tissue samples. Int J Cancer. 1998;78:27–32. doi: 10.1002/(sici)1097-0215(19980925)78:1<27::aid-ijc6>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 49. Wang Q, Yang C, Zhou J, Wang X, Wu M, Liu Z. Cloning and characterization of full-length human ribosomal protein L15 cDNA which was overexpressed in esophageal cancer. Gene. 2001;263:205–9. doi: 10.1016/s0378-1119(00)00570-9. [DOI] [PubMed] [Google Scholar]
- 50. Kobayashi T, Sasaki Y, Oshima Y, Yamamoto H, Mita H, Suzuki H, et al. Activation of the ribosomal protein L13 gene in human gastrointestinal cancer. Int J Mol Med. 2006;18:161–70. [PubMed] [Google Scholar]
- 51. Pogue-Geile K, Geiser JR, Shu M, Miller C, Wool IG, Meisler AI, et al. Ribosomal protein genes are overexpressed in colorectal cancer: isolation of a cDNA clone encoding the human S3 ribosomal protein. Mol Cell Biol. 1991;11:3842–9. doi: 10.1128/mcb.11.8.3842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Dai MS, Sears R, Lu H. Feedback regulation of c-Myc by ribosomal protein L11. Cell Cycle. 2007;6:2735–41. doi: 10.4161/cc.6.22.4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Dai MS, Lu H. Crosstalk between c-Myc and ribosome in ribosomal biogenesis and cancer. J Cell Biochem. 2008;105:670–7. doi: 10.1002/jcb.21895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Chen D, Zhang R, Shen WEI, Fu HAO, Liu S, Sun K, et al. RPS12-specific shRNA inhibits the proliferation, migration of BGC823 gastric cancer cells with S100A4 as a downstream effector. Int J Oncol. 2013;42:1763–9. doi: 10.3892/ijo.2013.1872. [DOI] [PubMed] [Google Scholar]
- 55. Deng S-S, Xing T-Y, Zhou H-Y, Xiong R-H, Lu Y-G, Wen B, et al. Comparative proteome analysis of breast cancer and Adjacent normal breast tissues in human. Genomics Proteomics Bioinformatics. 2006;4:165–72. doi: 10.1016/S1672-0229(06)60029-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sun X-J, Sun K-L, Zheng Z-H, Hao D-M, Fu W-N, Xu H-M, et al. Analysis of gene expression profiles for distinct stages of intestinal-type gastric cancer using suppression subtractive hybridization and cDNA microarray. Scand J Gastroenterol. 2009;40:1244–5. doi: 10.1080/00365520500206426. [DOI] [PubMed] [Google Scholar]
- 57. Cheng Q, Lau WM, Tay SK, Chew SH, Ho TH, Hui KM. Identification and characterization of genes involved in the carcinogenesis of human squamous cell cervical carcinoma. Int J Cancer. 2002;98:419–26. doi: 10.1002/ijc.10177. [DOI] [PubMed] [Google Scholar]
- 58. Kondoh N, Schweinfest CW, Henderson KW, Papas TS. Differential expression of S19 ribosomal protein, laminin-binding protein, and human lymphocyte antigen class I messenger RNAs associated with colon carcinoma progression and differentiation. Cancer Res. 1992;52:791–6. [PubMed] [Google Scholar]
- 59. Lee SH, Shim CS, Lee JH. Profiling of differentially expressed genes in human cervical carcinoma. Anim Cell Syst. 2009;13:381–9. [Google Scholar]
- 60. Luft F. The rise of a ribosomopathy and increased cancer risk. J Mol Med (Berl) 2010;88:1–3. doi: 10.1007/s00109-009-0570-0. [DOI] [PubMed] [Google Scholar]