Abstract
To obtain the angiotension-I converting enzyme inhibitor (ACEI), a fusion ACEI polypeptide encoded with 8 DNA sequences of GPL, GPM, IKW, IVY, IRPVQ, IWHHT, IYPRY and IAPG, which were selected and designed and cloned into pGAPZαC and then transformed into Pichia pastoris SMD1168H. After 3 days induction, the fraction with highest ACEI activity was expressed and purified using a Ni Sepharose™ 6 Fast Flow. The IC50 of recombinant ACEI polypeptide was 88.2 μM. A 128-fold increase of ACEI activity (0.69 μM) was obtained after pepsin digestion, which was equivalent to 0.022 μM of captopril. Reverse phase HPLC indicated all the 8 peptides contained in ACEI-hydrolysate after pepsin digestion.
Keywords: Angiotesin I-converting enzyme inhibitory peptides, Cloning, Expression
1. Introduction
Hypertension, a common cardiovascular disease, is affecting nearly one billion patients worldwide. It is also regularly the cause of cardiac, renal and endothelial insufficiency, atherosclerosis, cerebral stroke and diabetes mellitus-related organ failure [1]. Renin–angiotensin system (RAS) is well known as the primary pathway for blood pressure and vascular tone regulation. In the RAS pathway, angiotensin I-converting enzyme acts as key enzyme to regulate the blood pressure as well as cardiovascular function. ACE is an enzyme of the hydrolase class that catalyzes cleavage of a dipeptide from the C-terminal end of angiotensin I (inactive) to form activated angiotensin II (highly active); also called peptidyl-dipeptidase A. Thus, the potent vasoconstrictor is secreted from the adrenal cortex including a sodium-retaining steroid and aldosterone, which is capable of increasing blood pressure. In addition, ACE inactivates a vasodilatory peptide, bradykinin. ACE is one of the zinc metallo-peptidases and activated by chloride. The ACE could be alternative transcribed in two isoforms from different initiation sites in a single gene. One expressed in most tissues is a larger ACE. The other one found in adult testis is smaller ACE [2]. Generally ACE is spread in mammalian tissues and also an ectoenzyme which has the membrane bound in vascular endothelial cells, absorptive epithelial, neuroepithelial, and male germinal cells [3,4]. From the previous studies, inhibition of ACE is a feasible strategy of hypertension therapy.
Various ACE inhibitors (ACEIs) have been investigated for palliating the hypertension. In current clinical treatment, synthetic ACEIs such as captopril, enalapril, lisinopril, ramipril and alacepril are effective cure for hypertension [5,6]. Unfortunately, treating hypertension with those synthetic ACEIs have shown some side effects, such as dried cough, headache, fever, renal impairment, taste disturbances, skin rashes, insomnia, and angioneurotic edema allergic reactions [6]. By contrast, ACE inhibiting peptides (ACEIP) are other alternative choices. Since the naturally occurring first ACEIP was isolated from snake venom [7,8] many ACEIPS have been identified from animals and plants such as chicken muscle [9], chicken egg, bovine milk [10], clam [11], jelly fish [12], maize and soybean [13] [14]. Due to encephalopathy crisis, foot and mouth disease, avian influenza, and religious reasons, the animal derived ACEIP was concerned about their safety [2].
To produce functional peptides with inhibitory ability against ACE with different expression system [15], a Pichia pastoris SMD1168H expression system was employed to produce a fusion ACEIP that peptide sequence has been proved the inhibition capacity in previous studies (Table 1). P. pastoris SMD1168H [16] has been applied for recombinant protein production for more than 20 years. The Pichia expression systems equipped with AOX1 promoter, a strong methanol-inducible promoter of alcohol oxidase I, are popular because they are easy grow to very high cell density in an inexpensive, non-complex and chemically defined medium [17]. As a eukaryote, P. pastoris has many advantages of higher eukaryotic expression systems such as protein processing, protein folding, and posttranslational modification. DNA encoded a fusion ACEI polypeptide could be hydrolyzed into individual ACEI peptides by pepsin. The nucleotide sequence consists of 830 was cloned into an AOX1 based expression vector, pGAPZαC. Through affinity chromatography and pepsin hydrolysis, various ACEI polypeptides could be cheaply obtained.
Table 1.
Sequences, sources and IC50 of ACEI peptides.
2. Materials and methods
2.1. Experimental design
The experimental design for this study is shown in the flowchart. ACEI peptides with low IC50 value derived from different protein sources, thus far, reported were employed to design the primers. The ACEI peptides were synthesized by assembly polymerase chain reaction method. The PCR product was cloned into pGAPZαC expression vector. The plasmid was then transformed into Pichia pastoris SMD1168H to express ACEI protein. The recombinant ACEI protein was purified by Ni Sepharose™ 6 Fast Flow and hydrolyzed by pepsin. After hydrolysis, the inhibition activity of recombinant ACE peptides was determined.
2.2. Strains and vectors
P. pastoris SMD1168H and pGAPZαC vector were the product from Invitrogen Co. (Carlsbad, CA). ExSel, high-fidelity DNA polymerase, was the product of JMR Holdings, Inc. (London, U.K.). All restriction enzymes were the products of Promega Co. (Madison, WI). T4 DNA Ligase was from Thermo Scientific Inc. (Darmstadt, Germany). Zeocin was the product of InvivoGen (San Diego, California). The other chemicals were the products of Sigma–Aldrich Inc. (St. Louis, MO).
2.3. Construction of pGAPZαC-ACEI expression vector and electroporation into P. pastoris SMD1168H
Eight peptides with ACEI ability and appropriate protease cleavage site were selected according to the previous studies to design a recombinant sequences (Table 1). After amplification (Forward primer: 5′GCCGAATTCTATGGGACCAAT GC3′, Reverse primer: 5′-TGCGGCCGCTTAGTGATG3′), the PCR product and pGAPZαC expression vector had been digested with restriction enzyme and ligated into pGAPZαC expression vector using T4 DNA ligase. The plasmid was transformed into Escherichia coli Top 10F′, according to heat-shock procedure [18,19]. The restriction enzyme of EcoRI/NotI can be used to verify the plasmid and the sequence identification by α-factor and 3′AOX primers. Finally, the resulting construct was electroporated into P. pastoris SMD1168H competent cells. pGAPZαC vector is zeocin-resistant, P. pastoris SMD1168H with pGAPZαC can be screened with YPDS agar (1% yeast extract, 2% of peptone, 2% glucose and 1M sorbitol with 2% agar) containing 100, 200, 500, 1000 and 2000 μg/mL zeocin and incubated at 30 °C. After 3 days of incubation, it was confirmed by RT-PCR that using TRIzol® to extract their total RNA and synthesizing single-stranded cDNA by Avian Myeloblastosis Virus Reverse Transcriptase (AMV RT), thus perform PCR with amplification forward and reverse primers to verify whether ACEI RNA express in the pGAPZαC-ACEI-transformed SMD1168H.
2.4. Cultivation of P. pastoris transformant
P. pastoris SMD1168H containing pGAPZαC-ACEI was cultivated in 5 mL of YPD with 100 μg/mL zeocin at 30 °C overnight with 180 rpm shaking. The culture was transferred into 150 mL of YPD (10 g/L yeast extract, 20 g/L peptone and 20 g/L glucose) with 100 μg/mL zeocin under the same condition for 3 days. After 3 days induction at 30 °C, P. pastoris was harvested by 10 min of centrifugation at 3000 × g. The suspended cells were harvested by centrifugation and resuspended in 20 mL of 20 mM phosphate buffer (20 mM NaH2PO4, 0.5 M NaCl and 5 mM imidazole, pH 7.4). The suspended cells were sonicated (400 cycles with 15 s on/15 s off) in ice using a sonicator XL 2020 system (HEAT Systems Inc., Farmingdale, NY) and then centrifuged at 10,000 × g for 20 min at 4 °C.
2.5. Purification of recombinant ACEI from P. pastoris SMD1168H
The isolated recombinant ACEI was filtered through a 0.45 μm sterilized membrane (Supor® Membrane, Life Sciences). The filtrate was chromatographed on a Ni Sepharose™ 6 Fast Flow column (2.6 × 3.0 cm, Biorad), which was pre-equilibrated with 20 mM phosphate buffer, pH 7.4. After being washed with the same buffer, the recombinant ACEI was eluted with buffer containing 100–500 mM imidazole. Samples were analyzed by Spectrophotometer (Hitachi U-2800).
2.6. Determination of ACE inhibition activity
Purification of recombinant ACEI was lyophilized by freeze dryer (Ilshin, TFD5505). The purified recombinant ACEI was digested by using pepsin (Pepsin/Protein = 1/25) in HCl (0.04 N, pH 1.65) at 37 °C for 16 h. The 5 kDa cut-off membrane was used to remove pepsin. The assay of ACE inhibition activity was performed with reverse-phase high-performance liquid chromatography. ACE (Angiotensin converting enzyme from rabbit lung, Sigma–Aldrich Inc.) was dissolved in 100 mM phosphate buffer (pH 8.3) containing 300 mM NaCl and prepared 5 mM hippuric acid-histidine-leucine (HHL) in the same condition. Both 75 μL of ACE and 75 μL of sample were incubated at 37 °C for 10 min and then added 75 μL of 5 mM HHL as a substrate. After incubation at 37 °C for 30 min, the reaction was stopped by adding 250 μL of 1 N HCl. After injecting 20 μL solution to Jupiter™ 5μ C18 300 Å column (250 × 4.60 mm 5 micron; phenomenex, USA), the column was eluted with 50% methanol containing 0.1% trifluoroacetic acid with a flow rate of 0.8 mL/min. Activity of ACE toward the hydrolysis of HHL synthetic peptide was confirmed by monitoring formation of hippuric acid (HA) at 228 nm. IC50 value was defined as the concentration of ACE inhibitor required to reduce half ACE activity [20].
2.7. Determination of protein concentration
Protein concentration was determined by using the Bio-Rad protein assay Kit. Bovine serum albumin was used as standard protein [21].
2.8. Determination of ACEI patterns after digestion with pepsin
The purified 20 μL ACEI and ACEI-hydrolysate were detected by a Jupiter™ 5μ C18 300 Å column (250 × 4.60 mm 5 micron; phenomenex, U.S.A.). They were eluted by a gradient of acetonitrile from 5% to 40% in 0.1% TFA solution at a flow rate of 1 mL/min. ACE enzyme can hydrolyze hippuric acid-histidine-leucine (HHL) to hippuric acid (HA). The formation of hippuric acid (HA) at 228 nm was used to determine the relative activity of ACEI peptides. The 8 synthetic peptides (IAPG, GPM, IRPVQ, IWHHT, GPL, IVY, IYPRY and IKW, synthesized by Bio-Protech, Taipei, Taiwan) were used as standard to identify if they were contained in the hydrolysate of recombinant ACEI, by comparing their retention time.
3. Results and discussion
3.1. Molecular cloning of ACEI in P. pastoris SMD1168H transformant and expression
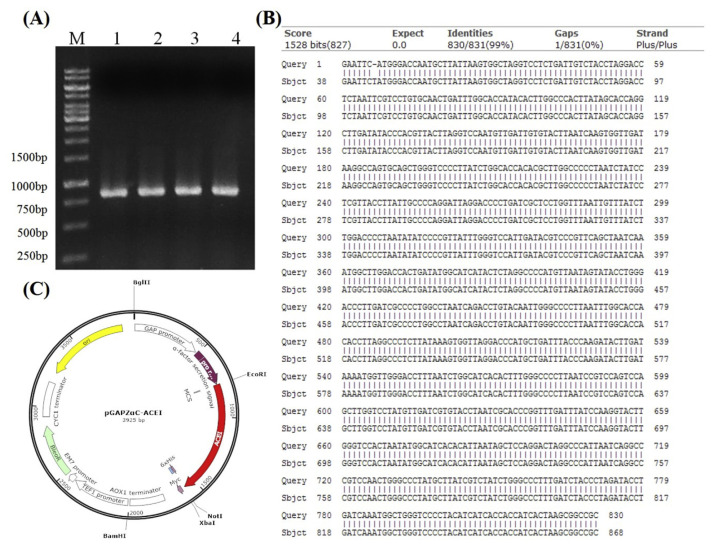
The full-length ACEI fragment was synthesized by assembly PCR method. The sequence of ACEI peptides from pUC-57 was amplified (Fig. 1-A). After the full sequence of recombinant ACEI was confirmed by sequencing (Fig. 1-B), the PCR product was ligated into pGAPZαC vector (Fig. 1-C). The confirmed pGAPZαC-ACEI plasmid was then electroporated into P. pastoris SMD1168H.
Fig. 1.
(A) Agarose gel analysis of assembly PCR products using recombinant ACEI primer as template. Lane M: 1K bp DNA marker, lane 1–4: recombinant ACEI. (B) DNA sequencing of pGAPZαC vector comparison with recombinant ACEI gene. (C) Construction of the pGAPZαC-ACEI expression vector.
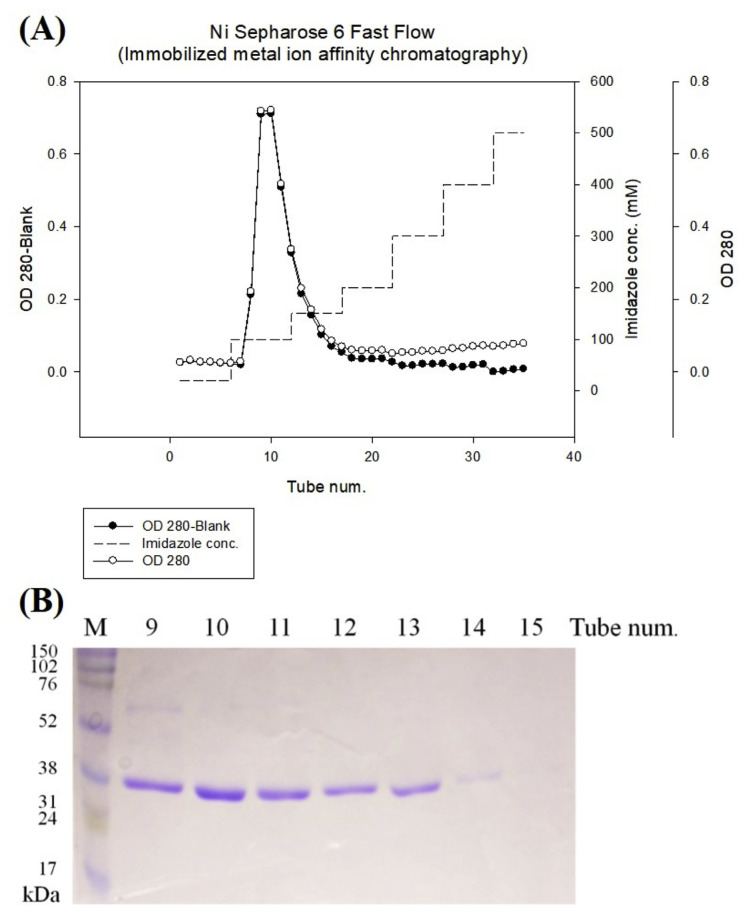
3.2. Purification of recombinant ACE inhibitors
Recombinant ACE inhibitors was purified to electrophoretic homogeneity using a Ni Sepharose 6 Fast Flow affinity chromatography (Fig. 2-A). According to SDS-PAGE gel stained with coomassie blue, the recombinant ACE inhibitor was highly purified (Fig. 2-B). These results indicated the recombinant ACE inhibitor was expressed successfully in P. pastoris expression system.
Fig. 2.
(A) The expression protein was purified from Ni Sepharose™ 6 Fast Flow column. The column was eluted with a linear gradient from 100 to 500 mM imidazole in 20 mM phosphate buffer, pH 7.4. (B) Profile of SDS-PAGE of recombinant ACEI by commassie blue staining.
3.3. Inhibition activity of recombinant ACEI
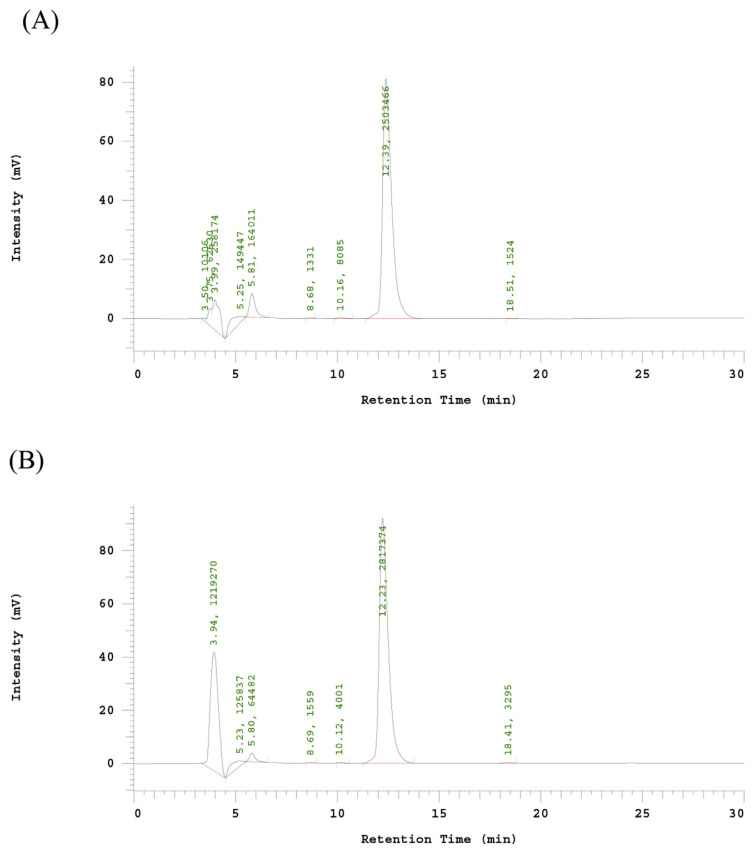
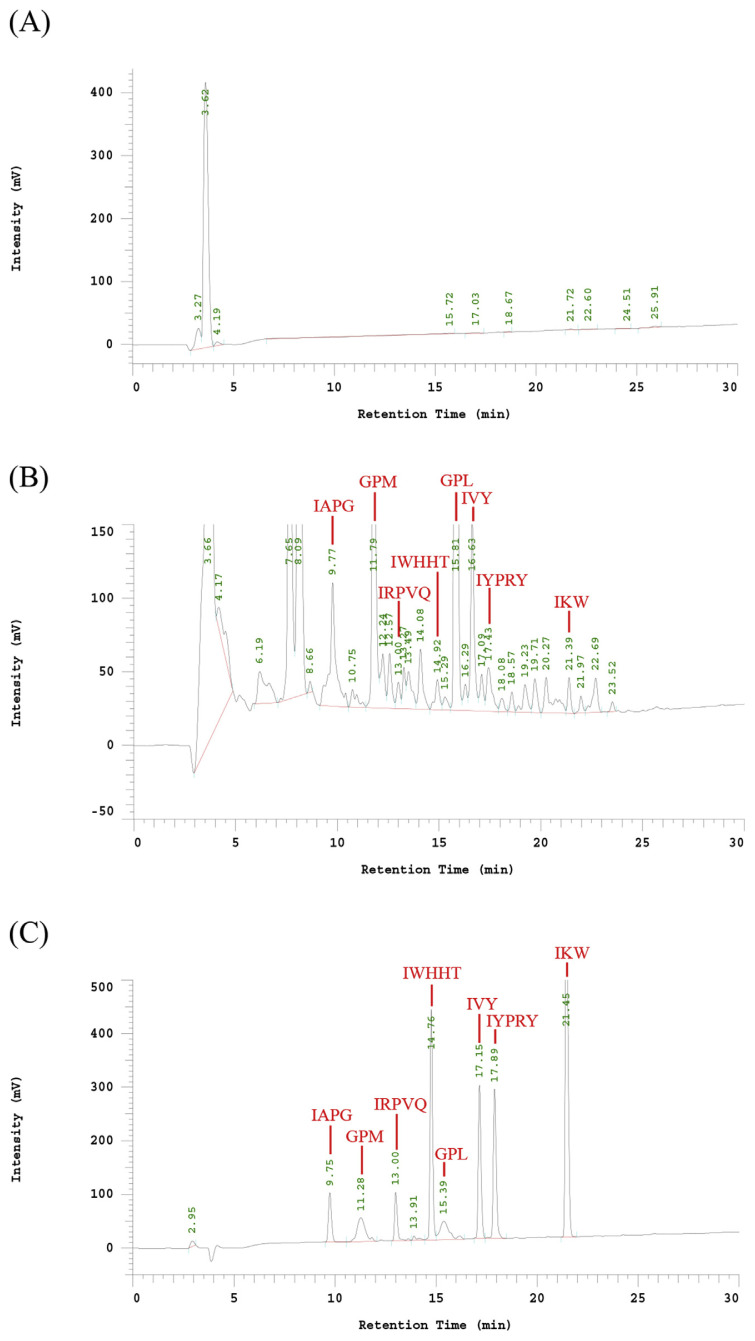
The liquid chromatography peaks as recombinant ACE inhibitors were collected and lyophilized by freeze dryer. One gram of the lyophilized powder was dissolved in 1 mL borate buffer, and then was diluted to 5%, 25%, 50%, 75% and 100% to determine ACEI activity. A 56.93 mg/mL of protein concentration of ACEI lyophilized powder can inhibit 50% of ACE activity. The peak area of HA was 103039 at 5.97 min by HPLC chromatogram (data not show). The IC50 of recombinant ACE inhibitor without hydrolysis with pepsin was 2684.9 μg/mL (88.2 μM) (Table 2). Fig. 3-A indicated the HPLC chromatogram of ACE hydrolysis HHL (hippuric acid-histidine-leucine) without ACE inhibitor. After hydrolyzed the recombinant ACE inhibitors by pepsin, the peak area with a retention time of 5.80 min of HA decreased to 64482 (Fig. 3-B). The inhibition ratio increased to 73.8%. The IC50 of digested recombinant ACE inhibitors was 21 μg/mL (0.69 μM) much better than ostrich egg white peptide (46.7 μg/mL) [22], about 128-fold increase after pepsin digestion (Table 2). As shown in Fig. 4, the retention time of purified recombinant ACE inhibitor was 3.62 min, only had one specific peak. After cleavage of purified recombinant ACE inhibitor with pepsin, the HPLC elution patterns of 8 fractions were with the expected pepsin cleavage pattern (IAPG, GPM, IRPVQ, IWHHT, GPL, IVY, IYPRY and IKW). The retention time of each peptide fractions were compare with the synthetic peptides. Most importantly, GPL was repeated for most times (18 times) in this recombinant ACE inhibitor, the others were repeated for only 6 times. GPL had the highest intensity in ACEI-hydrolysate after digestion with pepsin. According to Y. Li et al. report, The Tuna AI peptide monomer was released by fusion protein cleavage, resulted in a significant decrease of systolic blood pressure [23]. From these results, we can make sure that the recombinant ACE inhibitor can be successfully digested into functional ACEI peptides in human stomach in vitro. The recombinant ACE inhibitor could be used for the mass production of ACEI for health foods or drugs.
Table 2.
The inhibition rate and IC50 of recombinant ACEI.
| Inhibition rate (%) | IC50 | |
|---|---|---|
| Control | 0% | – |
| Recombinant ACEI protein | 50% | 2684.9 μg/mL |
| (without hydrolysis with pepsin) | 88.2 μM | |
| Recombinant ACEI protein | 73.8% | 21 μg/mL |
| (hydrolyzed by pepsin) | 0.69 μM | |
| Captopril | – | 0.022 μM |
Fig. 3.
HPLC chromatogram of ACE inhibitor activity determination eluted (1 mL/min) with 50% aqueous methanol (v/v, containing 0.1% trifluoroactic acid) on Jupiter 5μ C18 300 Å (250 × 4.60 mm) column, at 228 nm. (A) Control of ACE hydrolysis HHL (hippuric acid-histidine-leucine) without ACE inhibitor. (B) The ACE inhibitor activity of the ACEI peptides. The peak area with a retention time of 5.80 min of HA decreased to 64482.
Fig. 4.
Reverse phase HPLC pattern of (A) purified ACEI; (B) ACEI-hydrolysate after digestion with pepsin; (C) the synthetic 8 peptides as standard.
4. Conclusion
In our study, a P. pastoris expression system was used to substitute the enzymatic digestion from natural food to prepare ACEI peptides. It can simply and quickly purify ACEI recombinant protein by using Ni Sepharose ™ 6 Fast Flow affinity chromatography. The ACE inhibitory activity has increased about 128-fold activity after hydrolysis. The advantages of the study to express ACEI peptides include high protein expression level and low cost. The animal test will be performed to evaluate the regulation of blood pressure. Further commercial scale production of these ACEIP will be also carried out to confirm their application in the health foods.
REFERENCES
- 1. Tanaka M, Tokuyasu M, Matsui T, Matsumoto K. Endothelium-independent vasodilation effect of di- and tri-peptides in thoracic aorta of Sprague-Dawley rats. Life Sci. 2008;82:869–75. doi: 10.1016/j.lfs.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 2. Ketnawa S, Rawdkuen S. Angiotensin converting enzyme inhibitory peptides from aquatic and their processing byproducts: a review. IJSID. 2012;2:185–99. [Google Scholar]
- 3. Stevens BR, Fernandez A, Kneer C, Cerda JJ, Phillips MI, Woodward ER. Human intestinal brush border angiotensin-converting enzyme activity and its inhibition by antihypertensive Ramipril. Gastroenterology. 1988;94:942–7. doi: 10.1016/0016-5085(88)90551-3. [DOI] [PubMed] [Google Scholar]
- 4. Sibony M, Gasc JM, Soubrier F, Alhenc-Gelas F, Corvol P. Gene expression and tissue localization of the two isoforms of angiotensin I converting enzyme. Hypertension. 1993;21:827–35. doi: 10.1161/01.hyp.21.6.827. [DOI] [PubMed] [Google Scholar]
- 5. Wijesekara I, Kim SK. Angiotensin-I-converting enzyme (ACE) inhibitors from marine resources: prospects in the pharmaceutical industry. Mar Drugs. 2010;8:1080–93. doi: 10.3390/md8041080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vermeirssen V, Van Camp J, Verstraete W. Bioavailability of angiotensin I converting enzyme inhibitory peptides. Br J Nutr. 2004;92:357–66. doi: 10.1079/bjn20041189. [DOI] [PubMed] [Google Scholar]
- 7. Ferreira SH, Bartelt DC, Greene LJ. Isolation of bradykinin-potentiating peptides from Bothrops jararaca venom. Biochemistry. 1970;9:2583–93. doi: 10.1021/bi00815a005. [DOI] [PubMed] [Google Scholar]
- 8. Ondetti MA, Williams NJ, Sabo EF, Pluscec J, Weaver ER, Kocy O. Angiotensin-converting enzyme inhibitors from the venom of Bothrops jararaca. Isolation, elucidation of structure, and synthesis. Biochemistry. 1971;10:4033–9. doi: 10.1021/bi00798a004. [DOI] [PubMed] [Google Scholar]
- 9. Iroyukifujita H, Eiichiyokoyama K, Yoshikawa M. Classification and antihypertensive activity of angiotensin I-Converting enzyme inhibitory peptides derived from food proteins. J Food Sci. 2000;65:564–9. [Google Scholar]
- 10. Miguel M, Contreras MM, Recio I, Aleixandre A. ACE-inhibitory and antihypertensive properties of a bovine casein hydrolysate. Food Chem. 2009;112:211–4. [Google Scholar]
- 11. Shi L, Wu T, Sheng N, Yang L, Wang Q, Liu R, et al. Characterization of angiotensin-I converting enzyme inhibiting peptide from Venerupis philippinarum with nanoliquid chromatography in combination with orbitrap mass spectrum detection and molecular docking. J Ocean Univ China. 2017;16:473–8. [Google Scholar]
- 12. Liu X, Zhang M, Zhang C, Liu C. Angiotensin converting enzyme (ACE) inhibitory, antihypertensive and antihyperlipidaemic activities of protein hydrolysates from Rhopilema esculentum. Food Chem. 2012;134:2134–40. doi: 10.1016/j.foodchem.2012.04.023. [DOI] [PubMed] [Google Scholar]
- 13. Chen J-R, Yang S-C, Suetsuna K, Chao JCJ. Soybean protein-derived hydrolysate affects blood pressure in spontaneously hypertensive rats. J Food Biochem. 2004;28:61–73. [Google Scholar]
- 14. Daskaya-Dikmen C, Yucetepe A, Karbancioglu-Guler F, Daskaya H, Ozcelik B. Angiotensin-I-Converting enzyme (ACE)-Inhibitory peptides from plants. Nutrients. 2017;9 doi: 10.3390/nu9040316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Losurdo L, Quintieri L, Caputo L, Gallerani R, Mayo B, De Leo F. Cloning and expression of synthetic genes encoding angiotensin-I converting enzyme (ACE)-inhibitory bioactive peptides in Bifidobacterium pseudocatenulatum. FEMS Microbiol Lett. 2013;340:24–32. doi: 10.1111/1574-6968.12068. [DOI] [PubMed] [Google Scholar]
- 16. Romanos MA, Scorer CA, Clare JJ. Foreign gene expression in yeast: a review. Yeast. 1992;8:423–88. doi: 10.1002/yea.320080602. [DOI] [PubMed] [Google Scholar]
- 17. Jovanovic P, Jeremic S, Djokic L, Savic V, Radivojevic J, Maslak V, et al. Chemoselective biocatalytic reduction of conjugated nitroalkenes: new application for an Escherichia coli BL21(DE3) expression strain. Enzyme Microb Technol. 2014;60:16–23. doi: 10.1016/j.enzmictec.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 18. Scorer CA, Clare JJ, McCombie WR, Romanos MA, Sreekrishna K. Rapid selection using G418 of high copy number transformants of Pichia pastoris for high-level foreign gene expression. Biotechnology (N Y) 1994;12:181–4. doi: 10.1038/nbt0294-181. [DOI] [PubMed] [Google Scholar]
- 19.Sambrook J, Fritsch EFEF, Maniatis T, Laboratory CSH, Sambrook J, Fritsch EF, Maniatis T. Molecular cloning : a laboratory manual. 2nd ed. Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 20. Wu J, Ding X. Characterization of inhibition and stability of soy-protein-derived angiotensin I-converting enzyme inhibitory peptides. Food Res Int. 2002;35:367–75. [Google Scholar]
- 21. Macart M, Gerbaut L. An improvement of the Coomassie Blue dye binding method allowing an equal sensitivity to various proteins: application to cerebrospinal fluid. Clin Chim Acta. 1982;122:93–101. doi: 10.1016/0009-8981(82)90100-0. [DOI] [PubMed] [Google Scholar]
- 22. Asoodeh A, Homayouni-Tabrizi M, Shabestarian H, Emtenani S, Emtenani S. Biochemical characterization of a novel antioxidant and angiotensin I-converting enzyme inhibitory peptide from Struthio camelus egg white protein hydrolysis. J Food Drug Anal. 2016;24:332–42. doi: 10.1016/j.jfda.2015.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li Y, Wang B, Zhang H, Wang Z, Zhu S, Ma H. High-level expression of angiotensin converting enzyme inhibitory peptide Tuna AI as tandem multimer in Escherichia coli BL21 (DE3) Process Biochem. 2015;50:545–52. [Google Scholar]
- 24. Kim SK, Byun HG, Park PJ, Shahidi F. Angiotensin I converting enzyme inhibitory peptides purified from bovine skin gelatin hydrolysate. J Agric Food Chem. 2001;49:2992–7. doi: 10.1021/jf001119u. [DOI] [PubMed] [Google Scholar]
- 25. Matsui T, Li CH, Osajima Y. Preparation and characterization of novel bioactive peptides responsible for angiotensin I-converting enzyme inhibition from wheat germ. J Pept Sci. 1999;5:289–97. doi: 10.1002/(SICI)1099-1387(199907)5:7<289::AID-PSC196>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 26. Matsumura N, Fujii M, Takeda Y, Sugita K, Shimizu T. Angiotensin I-converting enzyme inhibitory peptides derived from bonito bowels autolysate. Biosci Biotechnol Biochem. 1993;57:695–7. doi: 10.1271/bbb.57.695. [DOI] [PubMed] [Google Scholar]
- 27. Yokoyama K, Chiba H, Yoshikawa M. Peptide inhibitors for angiotensin I-Converting enzyme from thermolysin digest of dried bonitot. Biosci Biotechnol Biochem. 1992;56:1541–5. doi: 10.1271/bbb.56.1541. [DOI] [PubMed] [Google Scholar]
- 28. Saito Y, Wanezaki K, Kawato A, Imayasu S. Structure and activity of angiotensin I converting enzyme inhibitory peptides from sake and sake lees. Biosci Biotechnol Biochem. 1994;58:1767–71. doi: 10.1271/bbb.58.1767. [DOI] [PubMed] [Google Scholar]
- 29. Suetsuna K, Chen JR. Identification of antihypertensive peptides from peptic digest of two microalgae, Chlorella vulgaris and Spirulina platensis. Mar Biotechnol (NY) 2001;3:305–9. doi: 10.1007/s10126-001-0012-7. [DOI] [PubMed] [Google Scholar]