Abstract
Objectives
To identify differences in the clinical and epidemiologic characteristics of patients during the first and second waves of the COVID-19 pandemic at the EsSalud Lambayeque health care network, Peru.
Methods
An analytical cross-sectional study of 53,912 patients enrolled during the first and second waves of COVID-19 was conducted. Cluster analysis based on clustering large applications (CLARA) was applied to clinical-epidemiologic data presented at the time of care. The two pandemic waves were compared using clinical-epidemiologic data from epidemiologic surveillance.
Results
Cluster analysis identified four COVID-19 groups with a characteristic pattern. Cluster 1 included the largest number of participants in both waves, and the participants were predominantly female. Cluster 2 included patients with gastrointestinal, respiratory, and systemic symptoms. Cluster 3 was the “severe” cluster, characterized by older adults and patients with dyspnea or comorbidities (cardiovascular, diabetes, obesity). Cluster 4 included asymptomatic, pregnant, and less severe patients. We found differences in all clinical-epidemiologic characteristics according to the cluster to which they belonged.
Conclusion
Using cluster analysis, we identified characteristic patterns in each group. Respiratory, gastrointestinal, dyspnea, anosmia, and ageusia symptoms were higher in the second COVID-19 wave than the first COVID-19 wave.
Keywords: COVID-19, Cluster analysis, Coronavirus infection, Pandemic wave, Symptoms, Peru
Introduction
COVID-19 caused by SARS-CoV-2 began in late 2019 in Wuhan City, China, and spread rapidly worldwide (Guo et al., 2020). As of March 2022, the World Health Organization reported approximately 483 million confirmed cases and around 6 million deaths from this disease (WHO, 2021). Likewise, Peru, one of the countries most affected by COVID-19, reported nearly 3 million confirmed cases and 212,157 deaths (WHO, 2021), reporting the highest mortality rate (652.58 deaths per 100,000 population) worldwide (Mortality Analyses, 2021).
Differences in the clinical-epidemiologic profiles of individuals with COVID-19 were observed. This variation can be attributed to some important characteristics, such as a country's preparedness level, socioeconomic status, variant type, and vaccination coverage, among others (Zhang et al., 2022). For example, in South Africa, during the Omicron wave, it was seen that infection rates were higher than previous epidemic waves, but were not as severe (Jassat et al., 2022). In England, during the first and second pandemic waves, there was a decrease in the mortality rate between 2020 and 2021. However, this change was not noticeable for older people and people with comorbidities, such as for ethnic minorities (Gray et al., 2021). In India, there was a significantly higher mortality and complication rate in the second wave, with more casesbeing observed among those who are young and those without comorbidities (Kumar et al., 2021). There was also an outbreak of mucormycosis during the second wave associated with the virus variant and glycemic dysregulation (Arora et al., 2021). A similar situation was observed in Bangladesh, as mortality was more common among elder patients and those with comorbidities (Hossain et al., 2021). In a more regional context, Brazil reported data related to the Gamma variant of concern, which mostly affected younger people without comorbidities during the second pandemic wave compared to the first outbreak.
In Peru, the first wave of COVID-19 began in March 2020, peaking in August and ending in December 2020 (WHO, 2021), while the second wave began in January 2021 and culminated in June 2021, with the maximum number of infections and deaths being recorded in April (WHO, 2021). Lambayeque was one of the regions that was most affected by COVID-19 (Díaz-Vélez et al.,), and it ranked in the highest quartile of mortality and surpassing even the national death rate, with a regional mortality rate of 612.2 deaths (CDC Peru, 2021). The first wave of COVID-19 began in Lambayeque in March 2020, reached its peak in May, and ended in December 2020, while the second wave started in January and ended in June 2021 (CDC Peru, 2021).
The clinical presentation of COVID-19 includes cough, nasal congestion, fever, chest pain, and shortness of breath (Griffin et al., 2021; Jiang et al., 2020), varying according to the level of clinical classification (mild, moderate, severe, and critical) (Instituto de Evaluación de Tecnologías en Salud e Investigación, 2021). Previous studies have described clinical differences between the first and second waves, particularly in terms of age groups, symptomatology, and disease severity (Area et al., 2021; Friston et al., 2020; Iftimie et al., 2021; Mocanu et al., 2021; Mollinedo-Gajate et al., 2021; Salyer et al., 2021; Soriano et al., 2021; Vinceti et al., 2021).
In Peru, the second wave presented a more significant acceleration in infections, deaths, and bed occupancy in the intensive care units and intermediate care units, affecting mainly the elder and pregnant women (Huatuco-Hernández et al., 2021). The emergence of the second wave of COVID-19 may have been associated with the circulation of new variants with a higher virulence, transmissibility, and lethality, such as the British variant B.1.1.7 and Brazilian variant P.1, as reported by the National Institute of Health after performing genomic sequencing (CDC-Perú, 2021).
There is no documented evidence on the clinical-epidemiologic profile for the patients treated in the two waves of SARS-CoV-2 infection in Peru, much less at the regional level. In addition, there is little genomic surveillance at the local level to identify the sequence of the viral genome to identify clinical patterns according to the main SARS-CoV-2 strains, and, thus, provide an approximation of the clinical-epidemiologic differences of both waves, which could represent an effective tool in public health decision-making for the prevention and control of COVID-19 in the Lambayeque region. This study aimed to describe the main clinical and epidemiologic differences between the first and second waves of COVID-19 in the Lambayeque region, Peru, by analyzing the clinical follow-up records of the patients treated in hospitals of the EsSalud Lambayeque health care network. (Díaz-Vélez et al., 2021).
Methods
Study design
An analytical cross-sectional study was carried out to determine the clinical and epidemiologic differences among the patients treated for COVID-19 in institutions belonging to the EsSalud health network in Lambayeque, during the first and second waves of the COVID-19 pandemic.
Population and sample
The population consisted of patients treated for COVID-19 in institutions belonging to EsSalud from March 2020 to September 2021. The sample was made up of 53,912 patients with a suspected COVID-19 infection recorded in the Notification System of the Ministry of Health (NotiWebMINSA). A non-probabilistic census-type sampling was applied. Confirmed or suspected COVID-19 patients, new or continuing EsSalud user patients, and individuals who had been treated and notified in the NotiWeb System in EsSalud Lambayeque service networks during the aforementioned period were included. Patients with incomplete clinical records and those with absent clinical records for the variables of interest were excluded.
Instruments and variables
The study variables were collected, analyzed, and divided into three sections: general data of the notification and of the patient, the date of notification, and the classification of the case. General epidemiological variables included sex, age, and categorized age (children: 0-11 years, adolescents: 12-17 years, young: 18-20 years, adult: 30-59 years, and the older adults: 60 years and older). Clinical variables were subclassified into: (i) clinical manifestations with which the case type was recorded according to severity, date of symptom onset if the patient was isolated, and when they were isolated; (ii) symptoms- the presentation of cough, sore throat, nasal congestion, shortness of breath, fever, chills, malaise, diarrhea, nausea/vomiting, headache, anosmia, ageusia, ear pain, irritability/confusion, muscle pain, chest pain, abdominal pain, and joint pain, (iii) recording of clinical signs, body temperature, presence of pharyngeal exudate, conjunctival injection, seizure, coma, dyspnea/tachypnea; and lastly, d) comorbidities including pregnancy, postpartum period (<6 weeks), presence of cardiovascular disease, diabetes, liver disease, neurological/neuromuscular disease, obesity, immunodeficiency, kidney disease, liver damage, chronic lung disease, and cancer.
Procedures and techniques
Two databases were used in this study: (i) the clinical follow-up database of the “Oficina de Inteligencia Sanitaria de Red Prestacional Lambayeque-EsSalud” and (ii) the epidemiological notification file database of the NotiWeb Epidemiological Surveillance System of the National Center for Epidemiology, Prevention and Control of Diseases, Peru.
The NotiWeb database was exported and matched with the clinical follow-up database using an identity document as the identifier code. A quality control process was then carried out to identify inconsistent data, and out-of-range and/or incomplete values. Subsequently, a variable called “type of pandemic wave” was created, in which the clinical-epidemiologic characteristics of the patients were grouped according to the period of each pandemic wave. The first wave was defined between the months of March 2020 to December 2020, and the second wave was between January 2021 to September 2021. This made it possible to identify the clinical pattern of each wave and identify the clinical-epidemiologic differences between each time period. Finally, the data were statistically analyzed.
Statistical analysis
The data were analyzed using the statistical program Stata v16.0 (StataCorp LP, College Station, Texas, USA).
For descriptive analysis of numerical variables, the best measure of central tendency and dispersion was calculated, and, for categorical variables, the absolute and relative frequencies were estimated. In the bivariate analysis, chi-square test was used after evaluating the assumption of expected frequencies. Fisher's exact test (categorical variables) was also used to compare the categorical clinical-epidemiologic variables between patients treated in the first and second waves. For numerical variables (age, length of hospitalization), the Student's t-test was used after evaluating the assumption of normal distribution and homoscedasticity. The Mann-Whitney U test was also used. P-values <0.05 were considered statistically significant.
In addition, an epidemiological curve was constructed based on the date of onset of symptoms of the first and second pandemic waves. The database was collapsed according to the date of onset of symptoms, and the variable weekly moving average of cases was created. Subsequently, case trends of each pandemic wave were estimated, and cumulative daily curves and weekly moving averages were plotted.
Clustering large applications (CLARA) was used as a clustering method to identify the groups of patients with similar clinical characteristics. This method is an option for large data because it works with a resampling scheme by taking smaller random subsamples and polling them to propose the results (Kassambara, 2017) and we used CLARA as implemented by the function clara of “cluster” package in R version 4.1.2. The silhouette method was used to determine the optimal number of clusters while accounting for epidemiological considerations.
Ethical aspects
The research protocol was approved by the ethics committee of the Almanzor Aguinaga Asenjo National Hospital with registration code No. CIE-RPL 047-SET-2021. In addition, the research protocol was registered in the repository of health research projects of the National Institute of Health-Peru (PRISA-INS). The study was carried out following the ethical principles of the Declaration of Helsinki. The confidentiality of the clinical records of the patients selected for the investigation was preserved. Anonymized codes were used to manage and analyze the information, and only the study investigators had access to the data.
Results
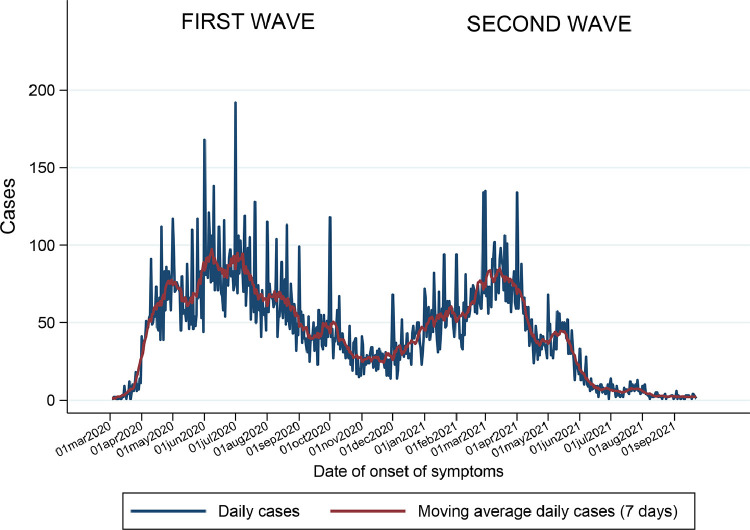
Between March 2020 and September 2021, two heterogeneous waves of COVID-19–positive cases were recorded. The first wave peaked in August 2020, followed by a decrease in the number of confirmed cases. In December 2020, economic reactivation in Peru began, meaning that there were no longer restrictions on access to public spaces and normal activities. This coincided with the onset of the second wave of the pandemic in January 2021, which peaked in April 2021. The peak of the second pandemic wave was slightly lower than that recorded for the first wave. The epidemiologic curve of confirmed cases in the Lambayeque health care network continued to decrease. The weekly moving average was much lower than that observed during the peak of the first and second pandemic waves (Figure 1 ).
Figure 1.
Epidemiological curve of confirmed COVID-19 cases in the Lambayeque health care network during the first and second waves according to the date of symptom onset.
Table 1 shows the epidemiologic and clinical characteristics of patients enrolled during the first and second waves of the COVID-19 pandemic in the Lambayeque health care network. Compared to the first wave, the second wave was characterized by patients with a higher mean age (47.92 vs 44.87) and a higher proportion of older adults (31.2% vs 25.8%) and males (50.3% vs 43.7%). Regarding signs and symptoms, a higher frequency of cough (56.3 vs 44.9%), nasal congestion (26.4% vs. 18.5%), respiratory distress (29.5% vs 25.3%), diarrhea (20.9% vs 17.3%), headache (39.0% vs 36.7%), and dyspnea (5.7% vs 3.6%) was observed during the second wave compared to the first. In addition, compared to the first wave, the second wave had a higher proportion of patients with cardiovascular disease (14.4% vs 11.0%), diabetes (7.4% vs 6.1%), cancer (1.7% vs 1.1%) and obesity (5.4% vs 0.6%). In contrast, there was a higher frequency of lung disease in the first wave compared than the second (1.0% vs 0.7%).
Table 1.
Clinical-epidemiological characteristics of patients with COVID-19 during the first and second waves.
| Variables |
Pandemic wave |
|||
|---|---|---|---|---|
|
First wave (n = 36938) |
Second wave (n = 16974) |
|||
| N | % | n | % | |
| Age (years)a | 44.87 ± 20.5 | 47.92 ± 20.8 | ||
| Age (categorized) | ||||
| Child (0-11) | 1859 | 5 | 626 | 3.7 |
| Adolescent (12-17) | 1451 | 3.9 | 535 | 3.2 |
| Young (18-29) | 5570 | 15.1 | 2431 | 14.3 |
| Adult (30-59) | 18539 | 50.2 | 8092 | 47.7 |
| Older adult (60-) | 9519 | 25.8 | 5290 | 31.2 |
| Gender | ||||
| Female | 20803 | 56.3 | 8444 | 49.8 |
| Male | 16135 | 43.7 | 8530 | 50.3. |
| Clinical characteristics | ||||
| General malaise | 16721 | 45.3 | 12044 | 71.0 |
| Cough | 16578 | 44.9 | 9556 | 56.3 |
| Sore throat | 14003 | 37.9 | 7205 | 42.5 |
| Fever | 12162 | 32.9 | 6215 | 36.6 |
| Headache | 11640 | 31.5 | 6219 | 36.6 |
| Nasal congestion | 6817 | 18.5 | 4473 | 26.4 |
| Muscle pain | 6185 | 16.7 | 3826 | 22.5 |
| Respiratory distress | 6569 | 17.8 | 3586 | 21.1 |
| Diarrhea | 5089 | 13.8 | 3096 | 18.2 |
| Chills | 521 | 1.4 | 1787 | 10.5 |
| Chest pain | 3923 | 10.6 | 1738 | 10.2 |
| Nausea | 2274 | 6.2 | 1257 | 7.4 |
| Anosmia | 334 | 0.9 | 1158 | 6.8 |
| Ageusia | 290 | 0.8 | 977 | 5.8 |
| Dyspnea | 1314 | 3.6 | 972 | 5.7 |
| Abdominal pain | 1082 | 2.9 | 596 | 3.5 |
| Ear pain | 48 | 0.1 | 204 | 1.2 |
| Pharyngeal exudate | 343 | 0.9 | 104 | 0.6 |
| Irritability | 298 | 0.8 | 79 | 0.5 |
| Conjunctival injection | 108 | 0.3 | 44 | 0.3 |
| Seizure | 8 | 0.0 | 2 | 0.0 |
| Comorbidities | ||||
| Cardiovascular disease | 4078 | 11.0 | 2445 | 14.4 |
| Diabetes | 2236 | 6.1 | 1251 | 7.4 |
| HIV | 42 | 0.1 | 12 | 0.1 |
| Kidney disease | 364 | 1.0 | 215 | 1.3 |
| Lung disease | 371 | 1.0 | 110 | 0.7 |
| Cancer | 399 | 1.1 | 281 | 1.7 |
| Obesity | 208 | 0.6 | 921 | 5.4 |
| Pregnancy | 1208 | 3.3 | 574 | 3.4 |
Mean and SD
Cluster analysis
From a purely statistical perspective, four clusters were determined to be the optimal number of clusters (Supplementary Figure 1). The population was categorized into these four clusters, and comparative tables and figures were made to characterize the patterns. The characteristics of the participants of the four clusters are shown in Table 2 . All the clinical-epidemiologic characteristics varied among the groups (P-values < 0.005).
Table 2.
Characteristics of the participants according to clusters.
| Characteristics | Overall N = 53,912a |
1 n = 16,832a |
2 n = 14,284a |
3 n = 11,680a |
4 n = 11,116a |
P-valueb |
|---|---|---|---|---|---|---|
| COVID-19 pandemic wave | <0.001 | |||||
| First wave | 36,938 (68.5%) | 10,700 (63.6%) | 8,805 (61.6%) | 6,816 (58.4%) | 10,617 (95.5%) | |
| Second wave | 16,974 (31.5%) | 6,132 (36.4%) | 5,479 (38.4%) | 4,864 (41.6%) | 499 (4.5%) | |
| Year | <0.001 | |||||
| 2020 | 36,938 (68.5%) | 10,700 (63.6%) | 8,805 (61.6%) | 6,816 (58.4%) | 10,617 (95.5%) | |
| 2021 | 16,974 (31.5%) | 6,132 (36.4%) | 5,479 (38.4%) | 4,864 (41.6%) | 499 (4.5%) | |
| Gender | <0.001 | |||||
| Female | 29,247 (54.2%) | 11,311 (67.2%) | 7,775 (54.4%) | 3,436 (29.4%) | 6,725 (60.5%) | |
| Male | 24,665 (45.8%) | 5,521 (32.8%) | 6,509 (45.6%) | 8,244 (70.6%) | 4,391 (39.5%) | |
| Age (years) | <0.001 | |||||
| Mean (SD) | 45.8 (20.6) | 38.9 (18.7) | 42.7 (18.1) | 63.6 (16.3) | 41.7 (20.0) | |
| Median (IQR) | 45.0 (31.0, 61.0) | 38.0 (26.0, 52.0) | 42.0 (29.0, 56.0) | 65.0 (53.0, 76.0) | 39.0 (28.0, 56.0) | |
| Range | 0.0, 103.0 | 0.0, 100.0 | 0.0, 103.0 | 0.3, 103.0 | 0.0, 101.0 | |
| Age group | <0.001 | |||||
| 0-4 | 1,098 (2.0%) | 521 (3.1%) | 129 (0.9%) | 11 (0.1%) | 437 (3.9%) | |
| 5-9 | 927 (1.7%) | 540 (3.2%) | 224 (1.6%) | 9 (0.1%) | 154 (1.4%) | |
| 10-14 | 1,278 (2.4%) | 671 (4.0%) | 391 (2.7%) | 10 (0.1%) | 206 (1.9%) | |
| 15-17 | 1,168 (2.2%) | 519 (3.1%) | 359 (2.5%) | 30 (0.3%) | 260 (2.3%) | |
| 18-29 | 8,001 (14.8%) | 3,132 (18.6%) | 2,549 (17.8%) | 238 (2.0%) | 2,082 (18.7%) | |
| 30-59 | 26,631 (49.4%) | 8,865 (52.7%) | 7,891 (55.2%) | 4,144 (35.5%) | 5,731 (51.6%) | |
| 60-79 | 11,747 (21.8%) | 2,347 (13.9%) | 2,405 (16.8%) | 5,154 (44.1%) | 1,841 (16.6%) | |
| 80-103 | 3,062 (5.7%) | 237 (1.4%) | 336 (2.4%) | 2,084 (17.8%) | 405 (3.6%) | |
| Case type | <0.001 | |||||
| Asymptomatic | 11,157 (20.7%) | 278 (1.7%) | 159 (1.1%) | 122 (1.0%) | 10,598 (95.3%) | |
| Symptomatic | 42,755 (79.3%) | 16,554 (98.3%) | 14,125 (98.9%) | 11,558 (99.0%) | 518 (4.7%) |
n (%)
Pearson's Chi-square test; Kruskal-Wallis's rank sum test; Fisher's exact test
IQR = Interquartile range
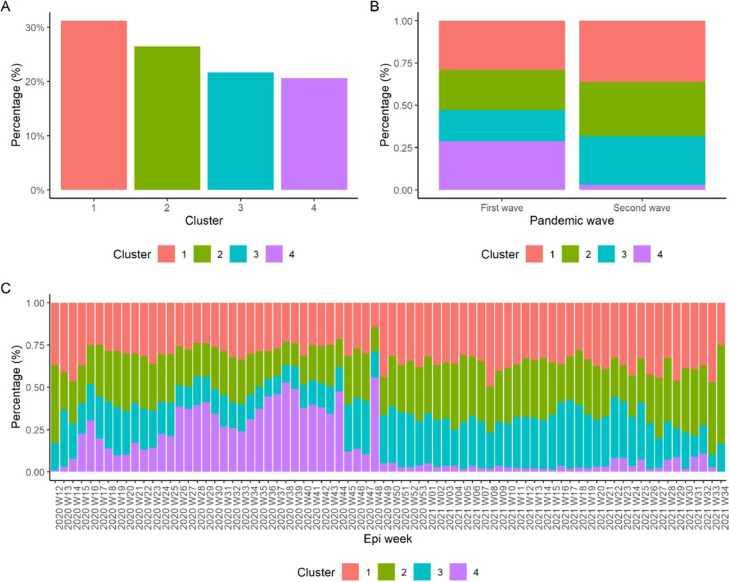
Figure 2a shows that cluster 1 was the most frequent cluster (N = 16,832; 31.2%), while the least frequent was cluster 4 (N = 11,116; 20.6%). Figure 2b shows the distribution of clusters according to pandemic waves. Cluster 1 predominated in the first (N = 10,700; 29%) and second waves (N = 6132; 36.1%). Cluster 4 was the second most frequent in the first wave (N = 10,617; 28.7%) and the least frequent in the second wave (N = 499; 2.9%). There was a statistically significant difference in the cluster distribution between the two pandemic waves (P < 0.001). Figure 2c shows the distribution of individuals by cluster according to epidemiological weeks (EW). In EW 12-2020, clusters 1 (0.4%) and 2 (0.6%) were the most frequent. However, a progressive increase in cluster 4 was observed between EW 13 to 25, with cluster 4 being predominant between EW 26 to SE 44 and finally, less frequent between EW 45 to 53 in 2020. In addition, cluster 3 increased and became more frequent in the second wave of 2021, while clusters 1 and 2 fluctuated, but seemed to be the most stable over time.
Figure 2.
Cluster analysis according to pandemic wave and EW. EW, epidemiological week
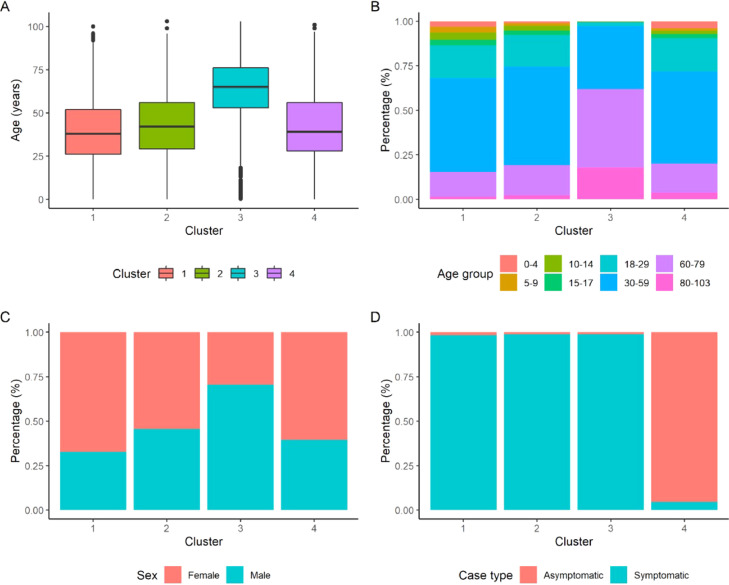
Figure 3a shows the age distribution according to cluster, with cluster 3 having a greater age distribution. Figure 3b presents the distribution of the age groups. The age group between 30 and 59 years of age was the most predominant in clusters 1 (52.7%), 2 (55.2%), and 4 (51.6%), while in cluster 3, the age group comprising patients between 60 and 79 years of age predominated (44.1%). Figure 3c shows that the female sex was more frequent in clusters 1 (67.2%), 2 (54.4%), and 4 (60.5%), while male sex was predominant in cluster 3 (70.6%). In Figure 3, clusters 1 (98.4%), 2 (98.9%), and 3 (99%) comprised predominantly of symptomatic patients, while cluster 4 was characterized by asymptomatic patients (95.3%).
Figure 3.
Clinical characteristics of the participants according to cluster of affiliation (i)
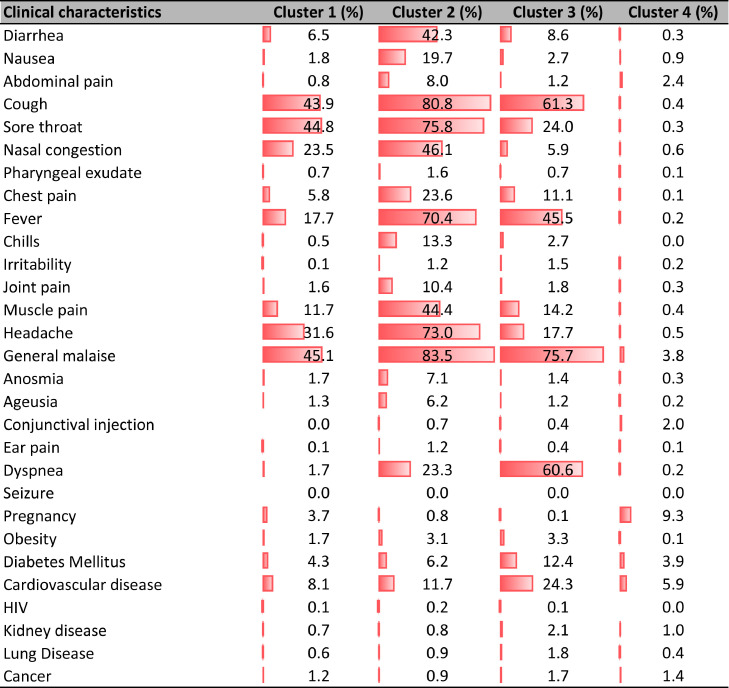
Patients in cluster 3 presented with the most severe clinical characteristics. Dyspnea was more predominant in cluster 3 (60.6%) compared to the other clusters, and chest pain was more frequent in cluster 2 (23.6%). The frequency of patients with cardiovascular disease, diabetes, and obesity was higher in cluster 3 compared to the remaining groups (Figure 4 ).
Figure 4.
Clinical characteristics of the participants according to cluster of affiliation (ii).
Discussion
Clinical-epidemiological variation in the first and second waves of the pandemic
In the second wave of the COVID-19 pandemic, there was a greater number of older adult patients and a lower frequency of children and adolescents. These results are similar to those reported by Soriano et al. (2021) in a medical center in Madrid, Spain, in which a slightly higher, albeit not statistically significant, mean age was observed in the second wave compared to the first wave. However, these findings differ from those reported in similar studies conducted in China (Fan et al., 2021), Japan (Saito et al., 2021), and Italy (Vinceti et al., 2021). In a hospital in Spain, younger patients were predominant in the second wave (Iftimie et al., 2021). In Sudan, fewer elder patients with COVID-19 were reported in the third wave compared to the second wave (23.9% vs 76.1%), although there were no significant differences (Abd El-Raheem et al., 2021). In Peru, younger people, especially children, may not have been severely affected by the pandemic during the second wave, because during the first outbreak there were concerns about the increasing rates of infection. This situation was possibly because of the fear of the contagion after cases of multisystemic inflammatory syndrome in children were reported (Yáñez et al., 2020).
There was a higher frequency of male patients in the second wave compared to the first wave. This result is similar to that described in a systematic review, in which a higher incidence of COVID-19 was found in males (Li et al., 2020). However, our finding is in contrast to that described in a similar study conducted in a university clinic in Madrid, Spain, in which differences were very small and not statistically significant (47.7% vs 46.2%) (Soriano et al., 2021). Another study in Spain identified a higher frequency of males in the second wave compared to the first wave, particularly in hospitalized patients with severe SARS-CoV-2 infection (70.8% vs 64.5%) (Mollinedo-Gajate et al., 2021). In Sudan, no significant differences were observed based on sex during the pandemic waves (Abd El-Raheem et al., 2021).
There were more patients with chronic diseases in the second wave compared to the first wave. This finding is similar to that reported in Spain, in which a higher frequency of patients with cardiovascular diseases was observed, whereas the number of diabetic patients decreased, although no significant differences were found in the analysis (Iftimie et al., 2021). However, our results differ from those reported in Japan, in which a lower prevalence of patients with cardiovascular, cerebrovascular, respiratory, renal, diabetic, and obese comorbidities was observed in the second wave compared to the first wave (Saito et al., 2021). Our findings are also contrary to those in Sudan, in which the frequency of patients with comorbidities markedly reduced in the third wave compared to the second wave of the COVID-19 pandemic (Abd El-Raheem et al., 2021).
In addition, we found that there were more patients with cancer and neoplastic diseases in the second wave compared with the first wave, albeit without significant differences, similar to what was reported in Spain. (Iftimie et al., 2021). In contrast, these results are contrary to the findings of Saito et al. (2021) in Japan, who reported a greater number of patients with neoplasms in the first wave of the COVID-19 pandemic.
In relation to the clinical manifestations of COVID-19, we found a significant increase in the frequency of cough, nasal congestion, respiratory distress, fever, malaise and diarrhea, headache, muscle pain, and abdominal pain in the second wave compared to the first wave. In contrast, dyspnea increased by 3% in the second wave, as opposed to the fewer number of patients with fever, dyspnea, cough, and diarrhea reported by Simona et al.. However, only cough showed significant differences between each wave of the pandemic (Iftimie et al., 2021).
Cluster analysis based on CLARA
In this study, the four clusters evolved over time, according to EW. Cluster 3 was the most severe cluster, showing an increase in the number of patients during the second pandemic wave, which could be explained by the low availability of medical oxygen in the Peruvian territory during both the first and second waves of COVID-19 (Fraser, 2020; Herrera‐Añazco et al., 2021; Schwalb and Seas, 2021). In addition, cluster 2 remained constant over time, whereas cluster 4 predominated in the first pandemic wave and then significantly declined.
According to the CLARA analysis, we identified four distinct clusters. Cluster 1 comprised mostly of female patients and was characterized as the most frequent cluster during the two pandemic waves. Cluster 2 was characterized by gastrointestinal, respiratory, and systemic symptoms and had the second highest incidence of dyspnea. Cluster 3 was characterized by dyspnea, which was the most severe clinical feature in our analysis. Cluster 4 was mainly characterized by asymptomatic presentation, with a higher frequency of pregnant women and fewer cases of dyspnea.
We found significant differences in age among the four clusters. This is important because multiple studies have identified older age as a factor associated with greater severity of COVID-19 (Klaiber et al., 2021; Palmieri et al., 2020). We observed significant differences among the groups based on sex. This is consistent with what was reported in a study conducted in China, in which a cluster analysis based on factor analysis of mixed data was performed, and no differences in age and sex were found among the three clusters (Han et al., 2021). This evaluation is relevant to determine the impact of age and sex on mortality in patients with COVID-19 (Wenham et al., 2020).
Cluster 1 did not show a characteristic clinical pattern in comparison with the other groups evaluated. This cluster included the largest number of individuals with the highest proportion of females and was the most predominant in the two pandemic waves evaluated in this study. With respect to age, this cluster was characterized by the highest proportion of children and adolescents between 5 and 17 years of age, as well as young people and adults. These results are similar to those described in a cluster study of COVID-19 patients by a French group. Their cluster 3 was made up of young patients with gastrointestinal symptoms and had the highest survival rate (96.5%) (Bondeelle et al., 2021).
Cluster 2 presented a high frequency of patients with risk factors for COVID-19, including cardiovascular disease, diabetes, obesity, and pulmonary disease, and was only surpassed by the “severe” cluster 3. These results partially coincide with a cluster study conducted in France that showed a higher proportion of systemic, respiratory, and gastrointestinal symptoms in cluster 3, characterized by the highest survival rate in onco-hematology patients (Bondeelle et al., 2021). Our findings also partially coincide with a study performed in China, in which cough was predominant in group B, classified as the “intermediate-severe” group. However, the presence of respiratory, gastrointestinal, and systemic symptoms was higher in the group A or “severe” group (Han et al., 2021). Our results coincide with those described in a similar study conducted in Spain, in which cluster 4 was characterized by diarrhea, vomiting, and abdominal pain, while cluster 3 was characterized by headache, sore throat, and arthromyalgia (Rubio-Rivas et al., 2020). Finally, cluster 2 of our study presented the highest frequency of anosmia and ageusia, similar to that described mainly in cluster 2 in a Spanish cluster analysis study (Rubio-Rivas et al., 2020).
Cluster 3 was characterized by dyspnea, the most severe sign among the four groups, and thus, this cluster was described as the “severe” group. This cluster is similar to what was described in an onco-hematological hospital in France, in which a cluster analysis was performed in COVID-19 patients where the majority of patients presented with dyspnea (mainly in cluster 3 [88%]) and this manifestation differed among clusters, making it important to address as a serious prognostic factor (Bondeelle et al., 2021). In a study performed in China, the second highest proportion of dyspnea was found in patients in cluster A with greater severity (35.2%) (31). This finding is also consistent with that described in a cluster analysis of just over 12,000 patients hospitalized for COVID-19 in Spain, in which cluster 1 accounted for the highest in-hospital mortality, and was characterized by being the largest cluster and comprising the triad of fever, cough and dyspnea (34).
Cluster 3 was dominated by the largest number of individuals with comorbidities, similar to what was described by Bondeelle et al. (2021) who identified a higher proportion of comorbidities in patients grouped in cluster 2, characterized by the lowest survival rate (50.6%). Moreover, our findings align with those of a study carried out in the United States following a cluster analysis that found >50% of patients with the lowest proportion of SARS-CoV-2 positivity were without comorbidities, concluding that the presenting comorbidities represent a risk factor for severe COVID-19 (35).
In addition, cluster 3 consisted of older adult male patients, which is consistent with a study performed in France, in which cluster 2 was made up of older adult patients who required oxygen therapy and had alterations in the C-reactive protein levels (Bondeelle et al., 2021). This is also similar to a study by Liang Han et al. who found that their more severe cluster A was characterized by a higher mean age and proportion of male patients (31). We also found a high frequency of dyspnea in cluster 2. However, we observed a lower frequency of dyspnea in clusters 1 (1.7%) and 4 (<1%), which could therefore represent the groups with less severity in the clinical evolution of these patients.
Cluster 4 included the highest number of pregnant women and children ranging in age from 0 to 4 years old, which could explain the fact that this group was also characterized by the predominance of being asymptomatic (Figure 3d). The number of clinical manifestations in these populations have been reported to be lower than other groups (Allotey et al., 2020; Mehta et al., 2020; Yanes-Lane et al., 2020). In addition, this cluster had the lowest number of individuals with severe systemic and gastrointestinal diseases. However, this cluster had the second highest number of patients with renal disease and cancer, surpassed only by cluster 4. This is similar to what was found in a French study, in which only 4% of the patients in cluster 1 had an acute renal failure (Bondeelle et al., 2021; Cholankeril et al., 2020; Pan et al., 2020; Tian et al., 2020; Zhang et al., 2020).
Public health relevance
This epidemiologic analysis of the COVID-19 pandemic in the Lambayeque region is relevant for public health decision-making at the regional level, especially at the level of the health care networks of the social health insurance of our country since it provides a better understanding of the clinical-epidemiologic behavior of each wave of the pandemic. The decision to perform the cluster analysis was based on the importance of capturing clinical patterns at the population level and assessing how they evolve over time between the two pandemic waves. Another reason for deciding on this analysis based on the clinical manifestations was of the large gap in the care of moderate, severe, and critical COVID-19 patients in Peru. Indeed, an interesting proportion of patients were managed at home, and there was a high proportion of oligoasymptomatic patients who probably did not agree to undergo a confirmatory test for COVID-19 because of the fragmentation of the health system caused by the health emergency long before the pandemic.
Limitations and strengths
This research has some limitations. First, there may be potential information bias because the clinical-epidemiological variables were not obtained from the clinical histories of the patients selected for the present study. In addition, it was not possible to obtain the measurement of biochemical parameters such as glucose, fibrinogen, and D-dimer (Mollinedo-Gajate et al., 2021). Nor was it possible to analyze the variation in pharmacological therapy (use of anticoagulants, corticosteroids, etc.) administered to patients with COVID-19 infection, (12) and, particularly in the cluster analysis, it was not possible to obtain measurements of severe outcomes such as hospitalizations and death in critical units, biochemical markers, and hospital stay, among others. Second, there may be a selection bias, given that our findings cannot be inferred to the entire study population of patients at the national level or patients present in the Lambayeque region during the first and second waves of the pandemic since the data were collected only from the patients enrolled in the EsSalud Lambayeque health care network. Third, due to the cross-sectional design of the study, it is not possible to attribute causality among the clinical and epidemiological characteristics that were associated with the first and second pandemic waves. Fourth, information on medication usage was unavailable, and therefore we could not determine the effect of medications on the development of the clinical-epidemiological features in each epidemic wave. More research should be performed using this data, especially on self-medication and other therapeutic alternatives used in Peru (Quispe-Cañari et al., 2021; Vasquez-Elera et al., 2022; Villena-Tejada et al., 2021). Nevertheless, this study analyzed a large and diverse sample of patients treated for COVID-19 in the health care networks of the Lambayeque region and the findings obtained to allow the development of future studies aimed at identifying the clinical-epidemiological variation of COVID-19 patients at local and regional levels, including diagnostic and therapeutic variables, to explain the most relevant factors between each pandemic wave.
Conclusions
In this study, differences in the clinical and epidemiological features of COVID-19 were found between the first and second epidemic waves in Peru. Older age, male sex, comorbidities, and presence of neoplastic disease were more common in the second than in the first outbreak. These characteristics were also related to an increased number of clinical manifestations that occurred during the second wave (featured by dyspnea but also other respiratory and gastrointestinal symptoms). According to the CLARA analysis, four distinct clusters were identified. Cluster 1 was characterized by being the most frequent of the two pandemic waves and was made up of the largest number of women. Cluster 2 was characterized by gastrointestinal, respiratory, and systemic symptoms and had the second highest incidence of dyspnea. Cluster 3 was characterized by a higher number of older adults with comorbidities, and dyspnea was the most severe clinical feature. Cluster 4 was mainly characterized by asymptomatic presentation, with a higher frequency of pregnant women and fewer cases of dyspnea. This clinical-epidemiological analysis provides a better understanding of the complex behavior of each epidemic wave and can inform future public health decision-making in local and regional health care networks.
Declaration of competing interest
The authors have no conflicts of interests to declare.
Funding
This study was supported by the Instituto de Evaluación de Tecnologías en Salud e Investigación (IETSI), EsSalud, Peru.
CRediT authorship contribution statement
Mario J. Valladares-Garrido: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. Virgilio E. Failoc-Rojas: Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing. Percy Soto-Becerra: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. Sandra Zeña-Ñañez: Investigation, Methodology, Writing – original draft, Writing – review & editing. J. Smith Torres-Roman: Investigation, Methodology, Writing – original draft, Writing – review & editing. Jorge L. Fernández-Mogollón: Investigation, Methodology, Writing – original draft, Writing – review & editing. Irina G. Colchado-Palacios: Investigation, Methodology, Writing – original draft, Writing – review & editing. Carlos E. Apolaya-Segura: Investigation, Methodology, Writing – original draft, Writing – review & editing. Jhoni A. Dávila-Gonzales: Investigation, Methodology, Writing – original draft, Writing – review & editing. Laura R. Arce-Villalobos: Investigation, Methodology, Writing – original draft, Writing – review & editing. Roxana del Pilar Neciosup-Puican: Investigation, Methodology, Writing – original draft, Writing – review & editing. Alexander G. Calvay-Requejo: Investigation, Methodology, Writing – original draft, Writing – review & editing. Jorge L. Maguiña: Conceptualization, Investigation, Methodology, Writing – original draft, Writing – review & editing. Moisés Apolaya-Segura: Conceptualization, Investigation, Methodology, Writing – original draft, Writing – review & editing. Cristian Díaz-Vélez: Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijid.2022.07.045.
Appendix. Supplementary materials
References
- Abd El-Raheem GOH, Mohamed DSI, Yousif MAA, Elamin HES. Characteristics and severity of COVID-19 among Sudanese patients during the waves of the pandemic. Sci Afr. 2021;14:e01033. doi: 10.1016/j.sciaf.2021.e01033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allotey J, Stallings E, Bonet M, Yap M, Chatterjee S, Kew T, et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020;370:m3320. doi: 10.1136/bmj.m3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Area I, Lorenzo H, Marcos PJ, Nieto JJ. One year of the COVID-19 pandemic in Galicia: a global view of age-group statistics during three waves. Int J Environ Res Public Health. 2021;18:5104. doi: 10.3390/ijerph18105104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora R, Goel R, Khanam S, Kumar S, Shah S, Singh S, Chhabra M, Meher R, Khurana N, Sagar T, Kumar S, Garg S, Kumar J, Saxena S, Pant R. Rhino-orbito-cerebral-mucormycosis during the COVID-19 second wave in 2021 - a preliminary report from a single hospital. Clin Ophthalmol. 2021;15:3505–3514. doi: 10.2147/OPTH.S324977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondeelle L, Chevret S, Cassonnet S, Harel S, Denis B, de Castro N, et al. Profiles and outcomes in patients with COVID-19 admitted to wards of a French oncohematological hospital: A clustering approach. PLoS One. 2021;16 doi: 10.1371/journal.pone.0250569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cholankeril G, Podboy A, Aivaliotis VI, Tarlow B, Pham EA, Spencer SP, et al. High prevalence of concurrent gastrointestinal manifestations in patients with severe acute respiratory syndrome coronavirus 2: Early experience from California. Gastroenterology. 2020;159:775–777. doi: 10.1053/j.gastro.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC-Perú . Prevención y Control de Enfermedades; 2021. Boletín epidemiológico del Perú. Volumen 30-SE 18. Centro Nacional de Epidemiología.https://www.dge.gob.pe/portalnuevo/publicaciones/boletines-epidemiologicos/ (accessed dd month yyyy) [Google Scholar]
- Díaz-Vélez C, Urrunaga-Pastor D, Romero-Cerdán A, Peña-Sánchez ER, Fernández Mogollon JL, Cossio Chafloque JD, et al. Risk factors for mortality in hospitalized patients with COVID-19 from three hospitals in Peru: a retrospective cohort study. F1000Res. 2021;10:224. doi: 10.12688/f1000research.51474.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan G, Yang Z, Lin Q, Zhao S, Yang L, He D. Decreased Case Fatality Rate of COVID-19 in the second Wave: a study in 53 countries or regions. Transbound Emerg Dis. 2021;68:213–215. doi: 10.1111/tbed.13819. [DOI] [PubMed] [Google Scholar]
- Fraser B. COVID-19 strains remote regions of Peru. Lancet. 2020;395:1684. doi: 10.1016/S0140-6736(20)31236-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Parr T, Zeidman P, Razi A, Flandin G, Daunizeau J, et al. Second waves, social distancing, and the spread of COVID-19 across the USA. Wellcome Open Res. 2020;5:103. doi: 10.12688/wellcomeopenres.15986.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray WK, Navaratnam AV, Day J, Wendon J, Briggs TWR. COVID-19 hospital activity and in-hospital mortality during the first and second waves of the pandemic in England: an observational study. Thorax. 2021;0:1–8. doi: 10.1136/thoraxjnl-2021-218025. [DOI] [PubMed] [Google Scholar]
- Griffin DO, Brennan-Rieder D, Ngo B, Kory P, Confalonieri M, Shapiro L, et al. The importance of understanding the stages of COVID-19 in treatment and trials. AIDS Rev. 2021;23:40–47. doi: 10.24875/AIDSRev.200001261. [DOI] [PubMed] [Google Scholar]
- Guo YR, Cao QD, Hong ZS, Tan YY, Chen SD, Jin HJ, et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak - an update on the status. Mil Med Res. 2020;7:11. doi: 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han L, Shen P, Yan J, Huang Y, Ba X, Lin W, Wang H, Huang Y, Qin K, Wang Y, Chen Z, Tu S. Exploring the clinical characteristics of COVID-19 clusters identified using factor analysis of mixed data-based cluster analysis. Front Med (Lausanne) 2021;8 doi: 10.3389/fmed.2021.644724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera-Añazco P, Uyen-Cateriano A, Mezones-Holguin E, Taype-Rondan A, Mayta-Tristan P, Malaga G, et al. Some lessons that Peru did not learn before the second wave of COVID-19. Int J Health Plann Mgmt. 2021;36:995–998. doi: 10.1002/hpm.3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain MI, Parvin S, Islam MS, Alam MJ, Podder S, Datta R, et al. Demographic profile and outcome of patients admitted to a COVID dedicated hospital in Bangladesh during the second wave. Med (Baltim) 2021;100:e27281. doi: 10.1097/MD.0000000000027281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huatuco-Hernández JA, Paredes-Villanueva FJ, Quispe-Cuestas MI, Fiestas-Pflücker GA, Nuñez-Rodas M, Salazar–Cuba X, et al. Características maternas y resultados perinatales en mujeres peruanas infectadas con COVID-19: un estudio observacional y transversal. Rev Cuerpo Med HNAAA. 2021;14:344–351. [Google Scholar]
- Iftimie S, López-Azcona AF, Vallverdú I, Hernández-Flix S, de Febrer G, Parra S, Hernández-Aguilera A, Riu F, Joven J, Andreychuk N, Baiges-Gaya G, Ballester F, Benavent M, Burdeos J, Català A, Castañé È, Castañé H, Colom J, Feliu M, Gabaldó X, Garrido D, Garrido P, Gil J, Guelbenzu P, Lozano C, Marimon F, Pardo P, Pujol I, Rabassa A, Revuelta L, Ríos M, Rius-Gordillo N, Rodríguez-Tomàs E, Rojewski W, Roquer-Fanlo E, Sabaté N, Teixidó A, Vasco C, Camps J, Castro A, et al. First and second waves of coronavirus disease-19: a comparative study in hospitalized patients in Reus, Spain. PLoS One. 2021;16 doi: 10.1371/journal.pone.0248029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Instituto de Evaluación de Tecnologías en Salud e Investigación . 2021. Guía de Práctica Clínica para el Manejo de COVID-19: guía en Versión Extensa. Versión 1, mayo 2021. [Google Scholar]
- Jassat W, Abdool Karim SS, Mudara C, Welch R, Ozougwu L, Groome MJ, et al. Clinical severity of COVID-19 in patients admitted to hospital during the omicron wave in South Africa: a retrospective observational study. Lancet Glob Health. 2022;10:e961–e969. doi: 10.1016/S2214-109X(22)00114-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortality analyses. Johns Hopkins coronavirus resource center. https://coronavirus.jhu.edu/data/mortality, 2021 (accessed 15 June 2021).
- Jiang F, Deng L, Zhang L, Cai Y, Cheung CW, Xia Z. Review of the clinical characteristics of coronavirus disease 2019 (COVID-19) J Gen Intern Med. 2020;35:1545–1549. doi: 10.1007/s11606-020-05762-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassambara A. STHDA; 2017. Practical guide to cluster analysis in R: unsupervised machine learning. [Google Scholar]
- Klaiber P, Wen JH, DeLongis A, Sin NL. The ups and downs of daily life during COVID-19: age differences in affect, stress, and positive events. J Gerontol B Psychol Sci Soc Sci. 2021;76:e30–e37. doi: 10.1093/geronb/gbaa096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar G, Mukherjee A, Sharma RK, Menon GR, Sahu D, Wig N, et al. Clinical profile of hospitalized COVID-19 patients in first & second wave of the pandemic: insights from an Indian registry based observational study. Indian J Med Res. 2021;153:619–628. doi: 10.4103/ijmr.ijmr_1628_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li LQ, Huang T, Wang YQ, Wang ZP, Liang Y, Huang TB, et al. COVID-19 patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol. 2020;92:577–583. doi: 10.1002/jmv.25757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta NS, Mytton OT, Mullins EWS, Fowler TA, Falconer CL, Murphy OB, et al. SARS-CoV-2 (COVID-19): what do we know about children? a systematic review. Clin Infect Dis. 2020;71:2469–2479. doi: 10.1093/cid/ciaa556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocanu A, Noja GG, Istodor AV, Moise G, Leretter M, Rusu LC, et al. Individual characteristics as prognostic factors of the evolution of hospitalized COVID-19 Romanian patients: a comparative observational study between the first and second waves based on Gaussian graphical models and structural equation modeling. J Clin Med. 2021;10:1958. doi: 10.3390/jcm10091958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollinedo-Gajate I, Villar-Álvarez F, Zambrano-Chacón M de los Á, Núñez-García L., de la Dueña-Muñoz L, López-Chang C, et al. First and second waves of coronavirus disease 2019 in Madrid, Spain: clinical characteristics and hematological risk factors associated with critical/fatal illness. Crit Care Explor. 2021;3:e0346. doi: 10.1097/CCE.0000000000000346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmieri L, Vanacore N, Donfrancesco C, Lo Noce C, Canevelli M, Punzo O, et al. Clinical characteristics of hospitalized individuals dying with COVID-19 by age group in Italy. J Gerontol A Biol Sci Med Sci. 2020;75:1796–1800. doi: 10.1093/gerona/glaa146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan L, Mu M, Yang P, Sun Y, Wang R, Yan J, et al. Clinical characteristics of COVID-19 patients with digestive symptoms in Hubei, China: a descriptive, cross-sectional, multicenter study. Am J Gastroenterol. 2020;115:766–773. doi: 10.14309/ajg.0000000000000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quispe-Cañari JF, Fidel-Rosales E, Manrique D, Mascaró-Zan J, Huamán-Castillón KM, Chamorro-Espinoza SE, et al. Self-medication practices during the COVID-19 pandemic among the adult population in Peru: a cross-sectional survey. Saudi Pharm J. 2021;29:1–11. doi: 10.1016/j.jsps.2020.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio-Rivas M, Corbella X, Mora-Luján JM, Loureiro-Amigo J, López Sampalo A, Yera Bergua C, et al. Predicting clinical outcome with phenotypic clusters in COVID-19 pneumonia: an analysis of 12,066 hospitalized patients from the Spanish registry semi–COVID-19. J Clin Med. 2020;9:E3488. doi: 10.3390/jcm9113488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito S, Asai Y, Matsunaga N, Hayakawa K, Terada M, Ohtsu H, et al. First and second COVID-19 waves in Japan: a comparison of disease severity and characteristics. J Infect. 2021;82:84–123. doi: 10.1016/j.jinf.2020.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salyer SJ, Maeda J, Sembuche S, Kebede Y, Tshangela A, Moussif M, et al. The first and second waves of the COVID-19 pandemic in Africa: a cross-sectional study. Lancet. 2021;397:1265–1275. doi: 10.1016/S0140-6736(21)00632-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwalb A, Seas C. The COVID-19 pandemic in Peru: what went wrong? Am J Trop Med Hyg. 2021;104:1176–1178. doi: 10.4269/ajtmh.20-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano V, Ganado-Pinilla P, Sanchez-Santos M, Gómez-Gallego F, Barreiro P, de Mendoza C, et al. Main differences between the first and second waves of COVID-19 in Madrid, Spain. Int J Infect Dis. 2021;105:374–376. doi: 10.1016/j.ijid.2021.02.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y, Rong L, Nian W, He Y. Review article: gastrointestinal features in COVID-19 and the possibility of faecal transmission. Aliment Pharmacol Ther. 2020;51:843–851. doi: 10.1111/apt.15731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasquez-Elera LE, Failoc-Rojas VE, Martinez-Rivera RN, Morocho-Alburqueque N, Temoche-Rivas MS, Valladares-Garrido MJ. Self-medication in hospitalized patients with COVID-19: a cross-sectional study in northern Peru. GERMS. 2022;12:46–53. doi: 10.18683/germs.2022.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villena-Tejada M, Vera-Ferchau I, Cardona-Rivero A, Zamalloa-Cornejo R, Quispe-Florez M, Frisancho-Triveño Z, et al. Use of medicinal plants for COVID-19 prevention and respiratory symptom treatment during the pandemic in Cusco, Peru: a cross-sectional survey. PLoS One. 2021;16 doi: 10.1371/journal.pone.0257165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinceti M, Filippini T, Rothman KJ, Di Federico S, Orsini N. SARS-CoV-2 infection incidence during the first and second COVID-19 waves in Italy. Environ Res. 2021;197 doi: 10.1016/j.envres.2021.111097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenham C, Smith J, Morgan R. COVID-19: the gendered impacts of the outbreak. Lancet. 2020;395:846–848. doi: 10.1016/S0140-6736(20)30526-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Coronavirus (COVID-19) Dashboard. https://covid19.who.int, 2021 (accessed 15 June 2021).
- Yanes-Lane M, Winters N, Fregonese F, Bastos M, Perlman-Arrow S, Campbell JR, et al. Proportion of asymptomatic infection among COVID-19 positive persons and their transmission potential: a systematic review and meta-analysis. PLoS One. 2020;15 doi: 10.1371/journal.pone.0241536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yáñez JA, Alvarez-Risco A, Delgado-Zegarra J. COVID-19 in Peru: from supervised walks for children to the first case of Kawasaki-like syndrome. BMJ. 2020;369:m2418. doi: 10.1136/bmj.m2418. [DOI] [PubMed] [Google Scholar]
- Zhang M, Liang Y, Yu D, Du B, Cheng W, Li L, et al. A systematic review of vaccine breakthrough infections by SARS-CoV-2 delta variant. Int J Biol Sci. 2022;18:889–900. doi: 10.7150/ijbs.68973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Tang C, Tian D, Hou X, Yang Y. Management of digestive disorders and procedures associated with COVID-19. Am J Gastroenterol. 2020;115:1153–1155. doi: 10.14309/ajg.0000000000000728. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.