Abstract
Objectives
Omicron appears to lead to a milder illness for patients compared with previous COVID-19 variants. However, not all infected with Omicron would describe their illness as mild. In this study, we investigate the experienced severity and symptoms of the Omicron variant.
Methods
We conducted a nationwide cross-sectional study, including 5036 individuals of all ages, consisting of reverse transcription-polymerase chain reaction confirmed SARS-CoV-2 cases from 1 January to 31 January 2022 (n = 4506) and a control group without SARS-COV-2 infection in December 2021 or January 2022 (n = 530). Omicron was dominant during this period. Cases were asked about their acute symptoms and answered a web-based questionnaire 10–30 days after their positive test while controls were asked about symptoms during the past week.
Results
Among cases, 97% reported at least one symptom during the acute phase compared with 79% of controls. Just over half the cases assessed their illness as asymptomatic or mild, whereas 46% assessed their illness as moderate or severe. Children reported fewer symptoms and less severe illnesses than adults (P <0.001). The largest risk differences (RDs) between adult cases and controls due to symptoms were observed for fever (RD = 60.6%, confidence interval [CI] 57.4–63.6), fatigue (RD = 49.6%, CI 44.1–54.7), and chills (RD = 48.8%, CI 43.8–53.2).
Conclusion
Most of those infected with Omicron experience symptoms, and the Omicron variant appears to lead to less severe disease. However, this does not mean that all the infected experience an Omicron infection as mild. The unprecedented rate of Omicron infections worldwide leads to urgent questions about the rate of long COVID after Omicron infections.
Keywords: Omicron; COVID-19; Clinical characteristics, Faroe Islands; Survey
Introduction
The Omicron (B.1.1.529) variant of SARS-CoV-2 has led to extraordinary rates of COVID-19 worldwide. Omicron hosts a striking number of mutations in its spike gene. Early reports have provided evidence for extensive immune escape and reduced vaccine effectiveness, leading to a higher transmission rate (Cao et al., 2022; Planas et al., 2022; Rössler et al., 2022).
The Omicron variant has primarily been described as mild in the media (Leonhardt, 2022). Although infection levels have surged worldwide, health care systems in many countries have not been overwhelmed in the same way as in previous waves. However, most countries have high immunization either through previous infection or vaccination, which likely affects the resulting burden on the health care sector from the Omicron wave. Preliminary evidence suggests that Omicron causes less severe disease with a predominance of milder clinical manifestations such as rhinorrhea, sneezing, sore throat, and headache (Iacobucci, 2021; Lippi et al., 2022). Data regarding the clinical manifestation of the Omicron variant is rapidly increasing but is still preliminary and limited to a few reports and studies (Iacobucci, 2021; Li et al., 2022; Lippi et al., 2022; Maisa et al., 2022; Menni et al., 2022; Raju et al., 2022; Tiecco et al., 2022).
Studies have established that even mild SARS-COV-2 infections can lead to long COVID, also previously reported in the Faroes (Petersen et al., 2021a). However, this was when previous variants were circulating. Thus, one urgent question is how the risk of long COVID is after infection with the Omicron variant? It is still too early to predict, but the potential implications are enormous as Omicron has infected an unprecedented number of people worldwide. If many of the infected develop long COVID, millions could be burdened with debilitating symptoms for months.
The Faroe Islands is an island nation in the North Atlantic Ocean, with a population of ∼53,700. The vaccination status on 28 January 2022, was that 76.1% were fully vaccinated, and 38.2% were triple-vaccinated (The Government of the Faroe Islands, 2022). The first Omicron case in the Faroes was identified on 8 December 2021. After this first case, a massive surge roamed the islands. From December 2021 to February 2022, 58% of the population had a confirmed SARS-COV-2 infection (The Government of the Faroe Islands, 2022). However, this surge has not led to an unmanageable increase in hospitalization and deaths, albeit an increase in hospitalizations and deaths was observed going into February 2022 (Supplementary Figure 1). The regulations in the Faroe Islands in January 2022 were mainly quarantine of close contact and restrictions on public gatherings. However, from 1 January 2022, the regulations were lifted stepwise, particularly the quarantine rules, and on 28 February 2022, all restrictions were lifted.
Even if we know the hospitalization rate for Omicron is lower than with, e.g., the Delta variant, we still know little about the course of the illness. To address this issue and to investigate how those infected with Omicron experience their illness, we conducted a nationwide online survey, inviting all confirmed SARS-CoV-2 infected cases during January 2022 in the Faroe Islands, when almost all cases were of the Omicron variant. Further, the same survey was undertaken in a control group of individuals without SARS-CoV-2 infection in December 2021 and January 2022. This survey is planned to be repeated bi-monthly for six months to rapidly report the potential risk of long COVID after infection with the Omicron variant.
Methods
Data collection
In this nationwide cross-sectional survey in the Faroe Islands, all SARS-COV-2 confirmed cases, including children, from 1 January to 31 January 2022, received an email from the Chief Medical Officer's office on our behalf with an invitation to participate and a link to the survey.
A control group, defined as individuals without SARS-CoV-2 in December 2021 and January 2022, was invited from participants in two former seroprevalence studies conducted in April 2020 (Petersen et al., 2020) and November 2020 (Petersen et al., 2022), with 1075 and 960 participants, respectively, from which we had email addresses for the most. We did not have information about their COVID-19 status; only those not infected with SARS-COV-2 in December 2021 or January 2022 were eligible to participate.
We used the web-based application Research Electronic Data Capture tools hosted at Department of Occpulational Medicine and Public Health to create the online questionnaires and collect and manage data (Harris et al., 2019). The participants received a link to the questionnaire that was filled out online. The questionnaire included self-assessed severity of their illness (no symptoms, mild, moderate, severe) and a prespecified list of symptoms (n = 23) during their illness, rating the symptoms as mild, moderate, or severe. There were additional questions on the duration of symptoms, education, employment, smoking habits, height, weight, and selected chronic diseases (asthma, heart disease, carnitine transporter deficiency, inflammatory bowel disease, hypertension, hypercholesterolemia, chronic obstructive pulmonary disease, type 2 diabetes mellitus), medication, self-assessed health, date of positive polymerase chain reaction (PCR) test, previous SARS-COV-2 infection, and vaccination status and dates. Control subjects were asked if they had experienced any of the prespecified symptoms during the last week before the survey and the previously mentioned baseline characteristics. Data were collected from 17 January 2021 to 1 March 2022.
This study is registered at ClinicalTrials.gov (ClinicalTrials.gov Identifier: NCT05234294). It is conducted per the Data Protection Act, and all participants provided written consent.
Partial sequencing of the SARS-CoV-2 spike protein
To determine the distribution of the SARS-CoV-2 variants Delta, Omicron BA.1, and Omicron BA.2 throughout the study period, 405 randomly selected SARS-CoV-2 samples were subjected to partial sequencing of the spike receptor-binding domain (RBD). The spike RBD was amplified with SuperScript IV One-Step reversed transcription-PCR System (Invitrogen) and subjected to sanger sequenced using the BigDye™ Terminator v1.1 Cycle Sequencing Kit (Applied Biosystems) (Helmsdal et al., 2022).
Statistics
We present descriptive results with mean and SDs, median and 5%–95% percentile, or numbers and percentages. We used the χ2 test for categorical variables and Mann-Whitney U/Kruskal-Wallis test for continuous variables.
We compared the prevalence of symptoms among cases and controls using parametric g-computation on logistic regression to estimate the crude risk differences (RDs) for acute symptoms with 95% confidence intervals (CI). As very few controls were children, we restricted the RD analysis to adults. We adjusted for age, sex, body mass index (BMI), smoking (ever/never), chronic disease from the questionnaire (yes/no), vaccination status (no/yes, two or three vaccinations), and previous COVID-19 infection (yes/no). We obtained the 95% CIs through random bootstrap resampling with 1000 iterations. We used the R-packages “riskCommunicator” (McQuade, 2022) and “Forester” (Boyes, 2021) for modeling and generation of forest plots, respectively.
Using logistic regression, we investigated potential predictors of experiencing one or more symptoms during the acute phase (no symptoms vs. one or more symptoms), including sex, age, smoking, BMI, medication use, chronic diseases, vaccination status, and previously infected.
All analyses were performed on the whole population and stratified into children (<16 years) and adults (≥ 16 years). We set the cutoff at 16 years because those younger were offered the vaccine later than the rest of the population. We performed the analyses using R version 3.6.2 and IBM SPSS version 25.
Results
Participants
We invited all ∼14,600 individuals diagnosed with SARS-COV-2 in January 2022 in the Faroe Islands to complete the online survey. Some email addresses were missing or invalid; thus, the exact number of invitations is unknown. A total of 4552 participants answered the questionnaire, of which 32 did not provide an email address, and 14 had tested positive either in December 2021 or February 2022 and were excluded. Thus, the final sample was 4506 (∼31% participation rate), 2767 (61%) women, and 1739 (39%) men. The proportion of children younger than 16 years was 25% (n = 1133). Characteristics of the study populations are listed in Table 1 .
Table 1.
Demographic and clinical characteristics of Faroese individuals infected with Omicron in January 2022 (n = 4506) and control subjects (n = 530).
| Cases |
Controls |
|||||
|---|---|---|---|---|---|---|
| All | Adultsa | Childrenb | All | Adultsa | Childrenb | |
| Total (n) | 4506 | 3373 | 1133 | 530 | 515 | 14 |
|
Age (years), median (5%–95% percentile) |
34.2 (5.1 – 64.0) |
41.0 (18.9 – 66.0) |
9.2 (1.8–15.1) |
54.5 (20.9–74.4) |
54.8 (25.0–74.6) |
8.8 (3.9–14.7) |
| Age groups, n (%) | ||||||
| 0 - 9 | 656 (14.6) | - | 656 (14.6) | 7 (1.3) | - | 7 (1.3) |
| 10 – 15 | 477 (10.6) | - | 477 (10.6) | 7 (1.3) | - | 7 (1.3) |
| 16 – 34 | 1186 (26.3) | 1186 (26.3) | - | 76 (14.3) | 76 (14.3) | - |
| 35 – 49 | 1214 (26.9) | 1214 (26.9) | - | 131 (24.7) | 131 (24.7) | - |
| 50 – 66 | 830 (18.4) | 830 (18.4) | - | 205 (38.7) | 205 (38.7) | - |
| 67+ | 143 (3.2) | 143 (3.2) | - | 104 (19.7) | 104 (19.7) | - |
| Sex, n (%) | ||||||
| Women | 2767 (61.4) | 2175 (64.5) | 592 (52.3) | 244 (46.0) | 240 (46.6) | 4 (28.6) |
| Men | 1739 (38.6) | 1198 (35.5) | 541 (47.8) | 286 (54.0) | 275 (53.4) | 10 (71.4) |
| Ever smoker, n (%)d | ||||||
| Yes | 1678 (32.7) | 1673 (50.3) | 5 (0.44) | 312 (59.3) | 311 (60.9) | 0 (0) |
| No | 2782 (62.4) | 1654 (49.7) | 1128 (99.56) | 214 (40.7) | 200 (39.1) | 14 (100) |
|
Body mass index (kg/m2)e median (5%–95%) |
24.4 (15.2–32.0) |
25.9 (20.3–35.9) |
17.2 (13.5–24.8) |
26.8 (20.3–35.6) |
26.9 (21.1–35.5) |
17.1 (14.2–39.0) |
| Medication, n (%)f | ||||||
| Yes | 1010 (22.7) | 955 (28.6) | 55 (4.9) | 258 (49.1) | 256 (50.1) | 2 (14.3) |
| No | 3443 (77.3) | 2384 (71.4) | 1059 (95.1) | 268 (51.0) | 255 (49.9) | 12 (85.7) |
| Self-reported diseases, n (%)c | ||||||
| Yes | 1336 (29.7) | 1236 (36.6) | 100 (8.8) | 267 (50.4) | 265 (51.5) | 2 (14.3) |
| No | 3170 (70.4) | 2137 (63.4) | 1033 (91.2) | 263 (49.6) | 250 (48.5) | 12 (85.7) |
| Vaccine status,n (%) | ||||||
| None | 1151 (25.5) | 244 (7.2) | 907 (80.1) | 29 (5.5) | 21 (4.1) | 8 (57.1) |
| One | 62 (1.4) | 31 (0.9) | 31 (2.7) | 5 (0.9) | 3 (0.6) | 2 (14.3) |
| Two | 1933 (42.9) | 1740 (51.6) | 193 (17.0) | 113 (21.3) | 108 (21.0) | 4 (28.6) |
| Three | 1360 (30.2) | 1358 (40.3) | 2 (0.2) | 383 (72.3) | 383 (74.4) | 0 (0) |
| COVID previously, n (%)g | ||||||
| Yes | 201 (4.5) | 140 (4.2) | 61 (5.4) | 39 (7.4) | 36 (7.0) | 3 (21.4) |
| No | 4289 (95.5) | 3218 (95.8) | 1071 (94.6) | 491 (92.6) | 479 (93.0) | 11 (78.6) |
| Days since, median (5%–95% percentile) | 111 (35–695) | 135 (65–696) | 97 (67–507) | 123 (80–496) | 114 (79–496) | 129 (89–133) |
|
Duration of acute disease (days)h median (5%–95% percentile) |
3 (0–8) | 3 (0–9) | 2 (0–6) | NA | NA | NA |
| Days from infection to response, median (5%–95% percentile) | 18 (10–30) | 18 (10–29) | 18 (10–32) | NA | NA | NA |
| Days from last vaccination to infection, median (5%–95% percentile) | 109 (9–218) | 111 (9–220) | 85 (9–144) | NA | NA | NA |
≥16 years
<16 years
asthma, heart disease, carnitine transporter deficiency, inflammatory bowel disease, hypertension, hypercholesterolemia, chronic obstructive pulmonary disease, type 2 diabetes
Data missing:
n=46 (cases), n=4 (controls)
n=127 (cases), n=11 (controls)
n=53 (cases), n=19 (controls)
n=16 (cases)
n=1864 (this question was not in the survey from start)
Email addresses were available from ∼1700 controls from two previous seroprevalence studies (Petersen et al, 2020; Petersen et al, 2022). An unknown number of these were not eligible as they had been infected with SARS-CoV-2 in December 2021 and January 2022. In total, 567 individuals answered the questionnaire, but 37 were excluded because they had COVID-19 in December 2021 and January 2022, which led to a final control group of 530 (∼31%) individuals, 286 (52%) women, and 244 (48%) men.
The cases and controls differ significantly in all characteristics (P <0.001) except for days since the previous infection with SARS-CoV-2 (P >0.05). The difference was also observed when stratifying into adults (≥16 years) and children (<16 years).
Sequencing
The partial sequencing of the SARS-CoV-2 spike protein confirmed our assumption that most cases were infected with Omicron. Of the 405 positive samples genotyped in January 2022, 72% (n = 292) were Omicron BA.1, 24% (n = 97) were Omicron BA.2. In contrast, only 1% (n = 5) were Delta, and 3% (n = 11) were inconclusive (Supplementary Figure 2).
Reported symptoms during illness
Among cases, 97% reported at least one symptom during the acute phase with a median (5%–95% percentile) of 10 symptoms (1–19) compared with 79% of control subjects reporting a median of three symptoms (0–13) (Table 2 ).
Table 2.
Symptoms during the acute phase in Faroese individuals infected with Omicron in January 2022 and adult controls the week before answering the survey.
| All cases | Adults casesa | Children casesb | Adult controla | |
|---|---|---|---|---|
| Total (n) | 4506 | 3373 | 1133 | 516 |
| Symptoms, n (%)c | ||||
| Yes | 4361 (96.9) | 3303 (98.0) | 1058 (93.5) | 401 (78.8) |
| No | 142 (3.2) | 68 (2.0) | 74 (6.5) | 108 (21.2) |
| Number of symptomsc | ||||
| Median (5%–95% percentile) | 10 (1–19) | 11 (2–20) | 8 (0–16) | 3 (0–13) |
| Number of symptoms, n (%)c | ||||
| 0 | 142 (3.2) | 68 (2.0) | 74 (6.5) | 108 (21.2) |
| 1–2 | 277 (6.2) | 137 (4.1) | 140 (12.4) | 104 (20.4) |
| 3–5 | 544 (12.1) | 340 (10.1) | 204 (18.0) | 128 (25.1) |
| 6–8 | 711 (15.8) | 494 (14.7) | 217 (19.2) | 85 (16.7) |
| 9–12 | 1225 (27.2) | 944 (28.0) | 281 (24.8) | 56 (11.0) |
| 13–15 | 795 (17.7) | 659 (19.5) | 136 (12.0) | 12 (2.4) |
| ≥16 | 809 (18.0) | 729 (21.6) | 80 (7.1) | 16 (3.1) |
| Assessed severity of symptoms, n (%)c,e | ||||
| Mild | 4147 (92.0) | 3178 (94.2) | 969 (85.5) | 376 (73.9) |
| Moderate | 3798 (84.3) | 2934 (87.0) | 864 (76.3) | 199 (39.1) |
| Severe | 2825 (62.7) | 2260 (67.0) | 565 (49.9) | 76 (14.9) |
| Overall assessed severity of illness, n (%)d | ||||
| Asymptomatic | 354 (7.9) | 213 (6.3) | 141 (12.4) | NA |
| Mild course | 2041 (45.4) | 1465 (43.5) | 576 (50.8) | NA |
| Moderate course | 1520 (33.8) | 1203 (35.7) | 317 (28.0) | NA |
| Severe course | 571 (12.7) | 473 (14.1) | 98 (8.7) | NA |
| Don't know | 13 (0.3) | 12 (0.4) | 1 (0.1) | NA |
≥16 years
<16 years;
Data missing:
n = 3 (cases), n = 8 (controls)
n = 7 (cases).
Participants were asked to rate severity of any symptom separately, thus numbers in the column add up to more than the total number of participants.
Stratifying according to children and adults, 7% (n = 74) of the children infected in January 2022 were asymptomatic, whereas only 2% (n = 68) of the adults were asymptomatic. However, when asked to rate the severity of the illness, 12% (n = 141) of the children and 6% (n = 213) of the adults reported no symptoms, although they reported at least one symptom when asked explicitly (Table 2, Supplementary Figure 3). Overall, we observed a marked difference between infected children and adults, as 63% of children reported no or mild symptoms compared with 50% among the adults (P <0.001). Likewise, only 9% of children reported a severe illness compared with 14% of adults (P <0.001) (Supplementary Figure 3).
Among children younger than 10 years, 91% reported one or more symptoms, whereas 97% of children in the age group 10–15 years reported at least one symptom (P <0.001). The youngest children also reported significantly fewer symptoms compared with the age group 10–15 years (median of 6 vs. 9, P <0.001), and the overall experienced illness was milder among the youngest, with a higher proportion reporting no symptoms and mild illness (P <0.001) (Supplementary Table 1).
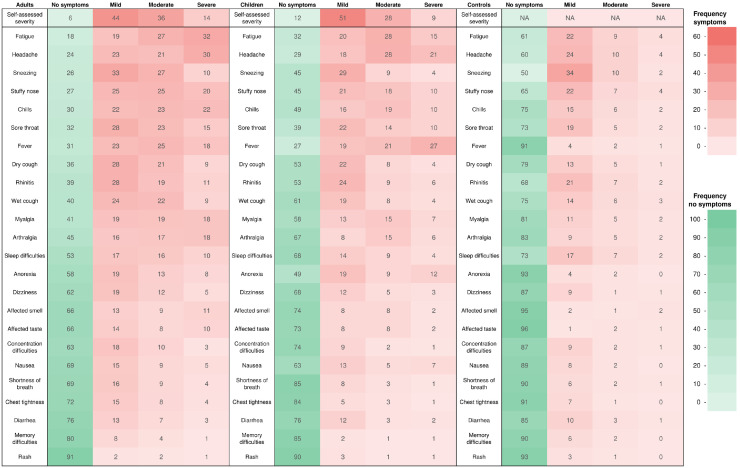
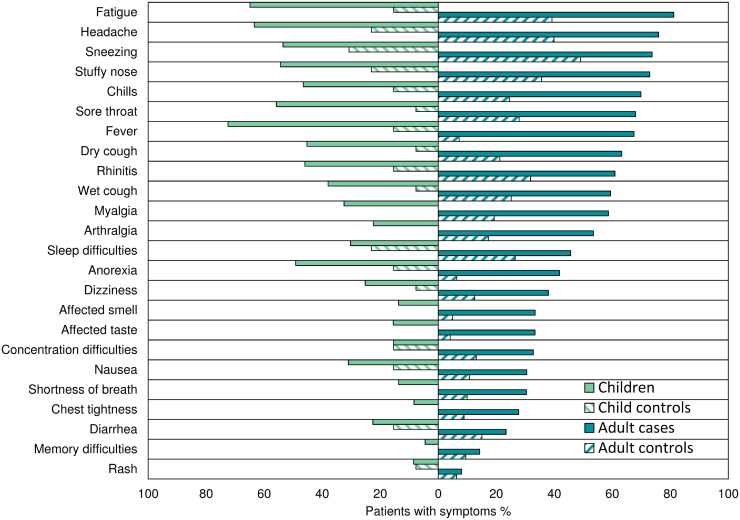
The most common symptoms among adult cases were fatigue (81%), headache (76%), sneezing (74%), and stuffy nose (73%), whereas sneezing (49%), headache (40%), fatigue (39%), and stuffy nose (36%) were the most prevalent among adult controls. Among infected children, fever (72%), fatigue (65%), headache (63%), and sore throat (56%) were the most prevalent symptoms, whereas sneezing (31%), headache (23%), and stuffy nose (23%) were the most prevalent among the few children in the control group (Figs. 1 and 2 ). Children reported significantly fewer symptoms than adults (median 8 vs. 11) (P <0.001). However, they reported fever (72% vs. 68%, P = 0.006) and reduced appetite (49% vs. 42%, P <0.001) significantly more often. All other symptoms were reported significantly more often in adult cases (P <0.001), except for diarrhea (P = 0.7), nausea (P = 0.2), and rash (P = 0.6), which were equally uncommon for both groups (Figs. 1 and 2).
Fig. 1.
Heat map of symptoms and severity during infection with the Omicron variant (%) stratified in SARS-CoV-2 infected adults (≥16 years), SARS-CoV-2 infected children (<16 years), and adult controls (≥16 years).
Fig. 2.
Prevalence of symptoms during infection with the Omicron variant (%) stratified in SARS-CoV-2 infected adults and controls (≥16 years) and SARS-CoV-2 infected children and controls (<16 years).
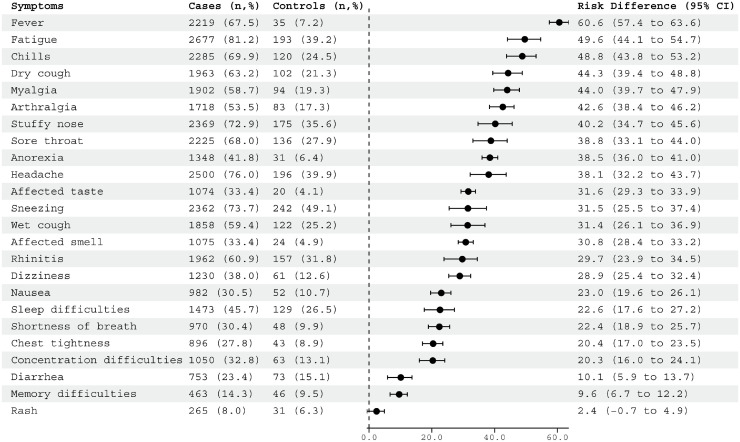
We calculated adjusted RD comparing cases to controls to address concurrent winter respiratory viruses circulating, as only reporting crude rates could overestimate the symptoms caused by Omicron. As very few controls were children (n = 14), we restricted the RD analysis to adults. The largest RD between cases and controls was observed for fever (RD = 61.3%, CI 58.5–64.0%), chills (RD = 45.4%, CI 41.4–49.4%), and dry cough (RD = 42.6%, CI 38.4–46.5%) (Supplementary Figure 4) but after adjustment, there was a change in the order of these symptoms. The largest RD persisted for fever (RD = 60.6%, CI 57.4–63.6%) and chills (RD = 48.8, 43.8–53.2%), whereas fatigue also entered the top three after adjustment (RD = 49.6%, CI 44.1–54.7%) (Fig. 3 ).
Fig. 3.
Adjusted risk differencea of symptoms during illness or previous week, comparing SARS-CoV-2 infected adults (≥16 years) and adult controls (≥16 years).
(aRisk difference with 95% confidence intervals was adjusted for age, sex, BMI, smoking, co-morbidity, vaccination (yes/no), and previous COVID-19 infection)
BMI = body mass index.
We investigated potential predictors of experiencing one or more symptoms during the acute phase compared with no symptoms among adult cases. We found that women were 2.7 times more likely to experience symptoms (odds ratio [OR] = 2.7, 95% CI 1.6–4.5), and people who reported chronic diseases were 2.8 times more likely to experience symptoms (OR = 2.8, 95% CI 1.3–5.9). Age was also a significant predictor of symptoms occurrence (P <0.002), whereas smoking (P = 0.2), BMI (P = 0.4), medication use (P = 0.6), vaccination status (P = 0.9), and previous infection with SARS-COV-2 (P = 0.08) were not associated with experiencing symptoms.
We asked the participants to rate their experienced illness (no symptoms, mild, moderate, and severe). The number of symptoms among adult cases was significantly associated with self-assessed illness severity. Those who reported an asymptomatic illness had a median of two symptoms compared with 9, 13, and 17 symptoms in those who reported a mild, moderate, and severe illness, respectively (P <0.001). The pairwise binary logistic regression comparing asymptomatic adult cases with the other groups showed that those who rated their illness as severe were more often women (OR = 3.4, 95% CI 2.3–4.9), ever smokers (OR = 1.5, 95% CI 1.2–2.9), people who reported having chronic diseases (OR = 1.8, 95% CI 1.2–2.9), and individuals without previous COVID-19 (OR = 3.6, 95% CI 1.5–8.7). Age was also significantly associated with experiencing more severe illness (P <0.001). Compared with the oldest age group, age groups 16–34 years and 35–49 years were more likely to experience severe illness (OR = 5.5, 95% CI 1.8–16.2 and 4.9, 95% CI 1.7–14.4). We saw similar results regarding moderate illness, as they were more often women (OR = 2.2, 95% CI 1.6–3.0) and individuals without previous COVID-19 (OR = 3.4, 95% CI 1.7–6.5). Age was also significantly associated with experiencing moderate illness (P <0.001), and compared with the oldest age group, age groups 16–34 years and 35–49 years were more likely to experience moderate illness (OR = 2.3, 95% CI 1.1–4.9 and 2.4, 95% CI 1.1–5.0) (Supplementary Figure 3). However, when we compared asymptomatic cases with those reporting a mild illness, only sex was significantly associated with severity, with an OR of 1.7 for women (95% CI 1.3–2.3).
We looked more closely at the association between vaccination status and experienced illness and reported symptoms. There was no difference in the proportion of asymptomatic in nonvaccinated, double-vaccinated, or triple-vaccinated cases (P = 0.1). However, the triple-vaccinated and unvaccinated cases rated their overall illness similarly (P = 0.8). In contrast, double-vaccinated cases rated their illness more severely than the two other groups (P <0.001) (Supplementary Figure 5 and Supplementary Table 2). Double-vaccinated cases had a significantly higher median number of symptoms than unvaccinated and triple-vaccinated cases (P <0.001). In contrast, we observed no difference between unvaccinated and triple-vaccinated cases (P = 0.9). Unvaccinated adults were more likely to have had COVID-19 previously (P <0.001). However, the associations between vaccination and self-assessed severity of the illness did not change by excluding those with previous SARS-CoV-2. Of note, no child <10 years was fully vaccinated (1%, n = 5 were vaccinated once), whereas 40% (n = 195) of those between 10 and 15 years were fully vaccinated (6%, n = 26 were vaccinated once) (Supplementary Figure 6).
Discussion
We conducted a nationwide survey during a massive surge of cases in the Faroe Islands, where partial sequencing showed that almost all SARS-COV-2 cases were of the Omicron variant during the study period. Over half of the patients reported that they either experienced no symptoms (8%) or a mild (45%) course of illness. In contrast, the others rated their illness as moderate (34%) or severe (13%), suggesting that Omicron is not experienced as mild for everyone.
The most prevalent symptoms in our study were upper respiratory tract symptoms, such as sneezing, stuffy nose, headache, and fatigue. These findings align with reports from Canada, France, India, and the UK (Li et al., 2022; Maisa et al., 2022; Menni et al., 2022; Raju et al., 2022). A Canadian study with 1063 Omicron cases during December 2021 reported that the most common symptoms were nasal congestion (73%), cough (65%), and headache (54%) (Li et al., 2022). The French study from November 2021 to January 2022 (n = 468) reported fatigue (57%), cough (52%), fever (48%), and headache (44%) as the most frequent symptoms (Maisa et al., 2022). The Indian study from December 2021 to January 2022 (n = 1175) reported fever (43%), body pain (23%), runny nose (22%), and cough (21%) as the most predominant symptoms (Raju et al., 2022). Finally, a study from the UK, only including vaccinated individuals, found that runny nose (77%), headache (75%), sore throat (71%), and sneezing (63%) were the most prevalent symptoms (Menni et al., 2022). The UK study also reported that Omicron led to milder disease than the Delta variant, with a shorter period of illness and fewer hospitalizations among vaccinated individuals (Menni et al., 2022). Our study showed that the clinical characteristics differed in children, especially the youngest children, who experienced fewer symptoms and less severe self-assessed illness. Although the Indian and French studies included children, they did not report specific information about children compared with adults. Our results are in line with previous variants' studies where symptoms appear rarer in children (Behnood et al., 2022; Blomberg et al., 2021). Although children experienced a milder illness, we found that people aged 16–49 years were more likely to report severe illness compared with older adults, which is contrary to what has been found with previous variants where age was associated with increased severity of disease (Romero Starke et al., 2021). Thus, this may indicate a difference between Omicron and the former variants, which should be explored in further studies. Another potential explanation for the discrepancy may be that we assess disease severity based on self-reported severity while many studies measure severity through hospitalization and/or intensive care unit (ICU) admissions, i.e., more severe cases. In addition to the studies mentioned previously, a few studies have assessed the severity of Omicron based on hospital admissions and ICU admissions without specifically addressing symptoms (Abdullah et al., 2022; Iuliano et al., 2022; Wolter et al., 2022).
Omicron displays potent immune escape characteristics, and the protection against infection with Omicron offered by both previous SARS-COV-2 infection and vaccination seems lower than with previous variants (Accorsi et al., 2022; Andrews et al., 2022; Pulliam et al., 2022). The lower vaccine effectiveness (VE) is seen as immunity wanes after vaccination or previous infection. However, even if VE against infection wanes quickly, VE against death and hospitalization remains high, especially for triple-vaccinated (Hansen et al., 2022). In our survey, vaccinated cases did not experience fewer symptoms or milder illness than unvaccinated cases, indicating that vaccination does not necessarily reduce the experienced severity of the illness caused by Omicron. Surprisingly, we found that those with two vaccinations rated their severity worse than the unvaccinated and those with three vaccinations. The median time from the last vaccination was 177 days, and the continued protection of the vaccine can have faded to some degree during this time. In addition, our definition of severe disease is based on self-reported severity, as mentioned previously, and not hospitalization or ICU admission, as in many studies (Feikin et al., 2022). Altarawneh et al. found that previous infection alone, vaccination alone, and a combination of vaccination and previous infection all showed strong effectiveness against severe, critical, or fatal COVID-19 because of Omicron. However, they found that the protection of vaccination against infection was negligible by six months after the second dose (Altarawneh et al., 2022). The few studies performed when Omicron was dominant do not report on the association between vaccination and symptoms (Li et al., 2022; Maisa et al., 2022; Menni et al., 2022; Raju et al., 2022) or include hospitalized or ICU admission cases (Feikin et al., 2022; Modes et al., 2022). Modes et al. (2022) found that among adults hospitalized with SARS-CoV-2 infection during an Omicron-predominant period, COVID-19 vaccination, particularly receiving a booster dose, was associated with a lower likelihood of ICU admission. There were very few hospitalizations in the Faroes, i.e., few severe cases. This, together with the definition of disease severity, may partly explain why we see that vaccination does not seem to influence the severity of the disease caused by Omicron. Our results are also contrary to studies with former variants where symptoms of COVID-19 were less common in vaccinated versus unvaccinated participants (Antonelli et al., 2022). However, our observation is also vulnerable to reporting bias, as those unvaccinated and triple-vaccinated cases might be more inclined to minimize their symptoms. In contrast, those potentially awaiting their booster vaccination could potentially be biased in the opposite direction.
The study's main strengths include the nationwide nature with broad representation in all age groups, the considerable sample size, and the inclusion of a control group. This makes it possible to compare acute symptoms among cases and the background population and reduces the likelihood of overestimating symptoms. All included cases have PCR-confirmed SARS-COV-2 caused most likely by the Omicron variant. This assumption is reasonable as partial sequencing of a representative subset of samples during January 2022 showed that almost all cases were infected with Omicron in January 2022. Further, we had information to stratify analyses according to important confounders such as vaccination status, former SARS-COV-2 infection, co-morbidities, BMI, and smoking.
The main limitation is the self-reporting of symptoms, which can lead to both under-estimation and over-estimation, and the relatively low participation rate may have led to selection bias which may affect generalizability. One could imagine a higher participation rate for those who experience symptoms or that people who did not experience symptoms were more likely to participate. Further, we do not have information about hospitalization among the participants. However, we have information on the total number of hospitalizations, which showed relatively few hospitalizations because of COVID-19 in January 2022. Further, around 25% of the infected participants were children, and for the youngest children, answers were given by their parents. The Chief Medical Officer's office sent the invitation, and it was impossible to send reminders to nonresponders or compare nonresponders with responders. Further, the control group and the cases differed significantly in all characteristics, especially with very few children among the controls. To circumvent this limitation, the RD analysis was performed, including only adults and other analyses stratified by children and adults. Still, we believe that including a nonperfect control group is better than not being able to consider the winter respiratory viruses circulating, which prevents from overestimating the symptoms.
In summary, our study substantiates previous suggestions that the Omicron SARS-CoV-2 variant is not mild for everyone. Therefore, we plan to repeat this survey bi-monthly for six months to quickly assess the potential risk of long COVID after infection with the Omicron variant.
Acknowledgments
Conflicts of interest
The authors have no competing interests to declare.
Funding source
The sequencing was funded by the special COVID-19 funding from the Faroese Research Council.
Ethical approval statement
The study is conducted in compliance with the Declaration of Helsinki, and all participants provide consent. Further, the study is conducted according to the Data Protection Act.
In the Faroe Islands, studies of this kind, i.e., only collection data, albeit sensitive data, are exempt from an IRB approval. The reason is that only biomedical research projects are covered by the jurisdiction of the Faroese Scientific Ethical committee. A link to the Act on Research Ethics Review of Health Research Projects can be found here: https://www.logir.fo/Anordning/961-af-15-07-2013-om-ikrafttraeden-for-Faeroerne-af-lov-om-videnskabsetisk-behandling-af-sundhedsvidenskabelige.
Acknowledgment
We thank the participants for their contribution.
Author contributions
MSP and MFK conceptualized and designed the study. SL, JLH, and LFM were responsible for identifying eligible participants and sending the invitation. MSP and EHE were responsible for collecting the data. NV, MMD, and DHC performed the genetic analyses. MSP and SK carried out the analysis. MSP and MFK drafted the manuscript. All authors reviewed and approved the final version. EHE and SK had access to the raw data from the survey and NV, MMD, and DHC for the sequencing data. MSP and MFK had final responsibility for the decision to submit for publication.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijid.2022.07.005.
Appendix. Supplementary materials
References
- Abdullah F, Myers J, Basu D, Tintinger G, Ueckermann V, Mathebula M, et al. Decreased severity of disease during the first global Omicron variant COVID-19 outbreak in a large hospital in Tshwane, South Africa. Int J Infect Dis. 2022;116:38–42. doi: 10.1016/j.ijid.2021.12.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Accorsi EK, Britton A, Fleming-Dutra KE, Smith ZR, Shang N, Derado G, et al. Association between 3 doses of mRNA COVID-19 vaccine and symptomatic infection caused by the SARS-CoV-2 Omicron and Delta variants. JAMA. 2022;327:639–651. doi: 10.1001/jama.2022.0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altarawneh HN, Chemaitelly H, Ayoub HH, Tang P, Hasan MR, Yassine HM, et al. Effects of previous infection and vaccination on symptomatic Omicron infections. N Engl J Med. 2022;387:21–34. doi: 10.1056/NEJMoa2203965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews N, Stowe J, Kirsebom F, Toffa S, Rickeard T, Gallagher E, et al. COVID-19 vaccine effectiveness against the Omicron (B.1.1.529) variant. N Engl J Med. 2022;386:1532–1546. doi: 10.1056/NEJMoa2119451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonelli M, Penfold RS, Merino J, Sudre CH, Molteni E, Berry S, et al. Risk factors and disease profile of post-vaccination SARS-CoV-2 infection in UK users of the COVID Symptom Study app: a prospective, community-based, nested, case-control study. The Lancet Infectious Diseases. 2022;22:43–55. doi: 10.1016/S1473-3099(21)00460-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behnood SA, Shafran R, Bennett SD, Zhang AXD, O'Mahoney LL, Stephenson TJ, et al. Persistent symptoms following SARS-CoV-2 infection amongst children and young people: a meta-analysis of controlled and uncontrolled studies. J Infect. 2022;84:158–170. doi: 10.1016/j.jinf.2021.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomberg B, Mohn KG, Brokstad KA, Zhou F, Linchausen DW, Hansen BA, et al. Long COVID in a prospective cohort of home-isolated patients. Nat Med. 2021;27:1607–1613. doi: 10.1038/s41591-021-01433-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyes R. Forester: an R package for creating publication-ready forest plot, https://github.com/rdboyes/forester, 2021 (accessed on 23 March 2022)
- Cao Y, Wang J, Jian F, Xiao T, Song W, Yisimayi A, et al. Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies. Nature. 2022;602:657–663. doi: 10.1038/s41586-021-04385-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feikin DR, Abu-Raddad LJ, Andrews N, Davies MA, Higdon MM, Orenstein WA, et al. Assessing vaccine effectiveness against severe COVID-19 disease caused by Omicron variant. Report from a meeting of the World Health Organization. Vaccine. 2022;40:3516–3527. doi: 10.1016/j.vaccine.2022.04.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, McLeod L, Delacqua G, Delacqua F, Kirby J, Duda SN, Consortium REDCap. The REDCap consortium: Building an international community of software partners. J Biomed Inform. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen CH, Schelde AB, Moustsen-Helm EHD, Krause TG, Mølbak K, et al. Vaccine effectiveness against infection and COVID-19-associated hospitalisation with the Omicron (B.1.1.529) variant after vaccination with the BNT162b2 or mRNA-1273 vaccine: a nationwide Danish cohort study. ResearchSquare. March 2022 doi: 10.21203/rs.3.rs-1486018/v1. accessed on 10 April 2022. [DOI] [Google Scholar]
- Helmsdal G, Hansen OK, Møller LF, Christiansen DH, Petersen MS, Kristiansen MF. Omicron outbreak at a private gathering in the Faroe Islands, infecting 21 of 33 triple-vaccinated healthcare workers. Clin Infect Dis. 2022:ciac089. doi: 10.1093/cid/ciac089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacobucci G. COVID-19: runny nose, headache, and fatigue are commonest symptoms of Omicron, early data show. BMJ. 2021;375:n3103. doi: 10.1136/bmj.n3103. [DOI] [PubMed] [Google Scholar]
- Leonhardt D. Omicron is milder. https://www.nytimes.com/2022/01/05/briefing/omicron-risk-milder-pandemic.html, 2022 (accessed 17 March 2022).
- Li A, Maier A, Carter M, Guan TH. Omicron and S-gene target failure cases in the highest COVID-19 case rate region in Canada-December 2021. J Med Virol. 2022;94:1784–1786. doi: 10.1002/jmv.27562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippi G, Nocini R, Henry BM. Analysis of online search trends suggests that SARS-CoV-2 Omicron (B.1.1.529) variant causes different symptoms. J Infect. 2022;84:e76–e77. doi: 10.1016/j.jinf.2022.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iuliano AD, Brunkard JM, Boehmer TK, Peterson E, Adjei S, Binder AM, et al. Trends in disease severity and health care utilization during the early Omicron variant period compared with previous SARS-CoV-2 high transmission periods - United States, December 2020–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:146–152. doi: 10.15585/mmwr.mm7104e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisa A, Spaccaferri G, Fournier L, Schaeffer J, Deniau J, Rolland P, et al. First cases of Omicron in France are exhibiting mild symptoms, November 2021–January 2022. Infect Dis Now. 2022;52:160–164. doi: 10.1016/j.idnow.2022.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuade JGM. riskCommunicator: G-computation to estimate interpretable epidemiological effects. R package version 1.1.1, https://cran.r-project.org/web/packages/riskCommunicator/riskCommunicator.pdf, 2022. (accessed on 23 March 2022)
- Menni C, Valdes AM, Polidori L, Antonelli M, Penamakuri S, Nogal A, et al. Symptom prevalence, duration, and risk of hospital admission in individuals infected with SARS-CoV-2 during periods of Omicron and Delta variant dominance: a prospective observational study from the ZOE COVID Study. Lancet. 2022;399:1618–1624. doi: 10.1016/S0140-6736(22)00327-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modes ME, Directo MP, Melgar M, Johnson LR, Yang H, Chaudhary P, et al. Clinical characteristics and outcomes among adults hospitalized with laboratory-confirmed SARS-CoV-2 infection during periods of B.1.617.2 (Delta) and B.1.1.529 (Omicron) variant predominance – One Hospital, California, July 15-September 23, 2021, and December 21, 2021-January 27, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:217–223. doi: 10.15585/mmwr.mm7106e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen MS, Kristiansen MF, Hanusson KD, Danielsen ME, Á Steig B, Gaini S, et al. Long COVID in the Faroe Islands: a longitudinal study among nonhospitalized patients. Clin Infect Dis. 2021;73:e4058–e4063. doi: 10.1093/cid/ciaa1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen MS, Strøm M, Christiansen DH, Fjallsbak JP, Eliasen EH, Johansen M, et al. Seroprevalence of SARS-CoV-2-Specific antibodies, Faroe Islands. Emerg Infect Dis. 2020;26:2761–2763. doi: 10.3201/eid2611.202736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen MS, Strøm M, Fjallsbak JP, Hansen JL, Larsen S, Eliasen EH, et al. Low seroprevalence among undetected COVID-19 cases, Faroe Islands, November 2020. Emerg Infect Dis. 2022;28:242–244. doi: 10.3201/eid2801.210917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planas D, Saunders N, Maes P, Guivel-Benhassine F, Planchais C, Buchrieser J, et al. Considerable escape of SARS-CoV-2 Omicron to antibody neutralization. Nature. 2022;602:671–675. doi: 10.1038/s41586-021-04389-z. [DOI] [PubMed] [Google Scholar]
- Pulliam JRC, van Schalkwyk C, Govender N, von Gottberg A, Cohen C, Groome MJ, et al. Increased risk of SARS-CoV-2 reinfection associated with emergence of Omicron in South Africa. Science. 2022;376:eabn4947. doi: 10.1126/science.abn4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raju MK, Thangaraj JWV, Selvavinayagam TS, Somasundaram A, Parthipan K, Sivadoss R, et al. Clinical profile of patients infected with suspected SARS-CoV-2 Omicron variant of concern, Tamil Nadu, India, December 2021–January 2022. Indian J Med Res. 2022;155:165–170. doi: 10.4103/ijmr.ijmr_312_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero Starke K, Reissig D, Petereit-Haack G, Schmauder S, Nienhaus A, Seidler A. The isolated effect of age on the risk of COVID-19 severe outcomes: a systematic review with meta-analysis. BMJ Glob Health. 2021;6 doi: 10.1136/bmjgh-2021-006434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rössler A, Riepler L, Bante D, von Laer D, Kimpel J. SARS-CoV-2 Omicron variant neutralization in serum from vaccinated and convalescent persons. N Engl J Med. 2022;386:698–700. doi: 10.1056/NEJMc2119236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Government of the Faroe Islands . Tórshavn; Faroe Islands: 2022. Corona statistics in the Faroe Islands.https://korona.fo/hagtol-koppseting?_l=fo [Google Scholar]
- Tiecco G, Storti S, Degli Antoni M, Focà E, Castelli F, Quiros-Roldan E. Omicron genetic and clinical peculiarities that may overturn SARS-CoV-2 pandemic: a literature review. Int J Mol Sci. 2022;23:1987. doi: 10.3390/ijms23041987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolter N, Jassat W, Walaza S, Welch R, Moultrie H, Groome M, et al. Early assessment of the clinical severity of the SARS-CoV-2 Omicron variant in South Africa: a data linkage study. Lancet. 2022;399:437–446. doi: 10.1016/S0140-6736(22)00017-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.