Abstract
Abstract
Insulin infusion increases skeletal muscle microvascular blood flow (MBF) in healthy people but is impaired during insulin resistance. However, we have shown that eliciting insulin secretion via oral glucose loading in healthy people impairs muscle MBF, whilst others have demonstrated intravenous glucose infusion stimulates MBF. We aimed to show that the route of glucose administration (oral versus intravenous) influences muscle MBF, and explore potential gut‐derived hormones that may explain these divergent responses. Ten healthy individuals underwent a 120 min oral glucose tolerance test (OGTT; 75 g glucose) and on a subsequent occasion an intravenous glucose tolerance test (IVGTT, bypassing the gut) matched for similar blood glucose excursions. Femoral artery and thigh muscle microvascular (contrast‐enhanced ultrasound) haemodynamics were measured at baseline and during the OGTT/IVGTT. Plasma insulin, C‐peptide, glucagon, non‐esterified fatty acids and a range of gut‐derived hormones and incretins (gastric inhibitory polypeptide (GIP) and glucagon‐like peptide‐1(GLP‐1)) were measured at baseline and throughout the OGTT/IVGTT. The IVGTT increased whereas the OGTT impaired MBF (1.3‐fold versus 0.5‐fold from baseline, respectively, P = 0.0006). The impairment in MBF during the OGTT occurred despite producing 2.8‐fold higher plasma insulin concentrations (P = 0.0001). The change in MBF from baseline (ΔMBF) negatively correlated with ΔGIP concentrations (r = −0.665, P < 0.0001). The natural log ratio of incretins GLP‐1:GIP was positively associated with ΔMBF (r = 0.658, P < 0.0001), suggesting they have opposing actions on the microvasculature. Postprandial hyperglycaemia per se does not acutely determine opposing microvascular responses between OGTT and IVGTT. Incretins may play a role in modulating skeletal muscle MBF in humans.
Key points
Insulin or mixed nutrient meals stimulate skeletal muscle microvascular blood flow (MBF) to aid in the delivery of nutrients; however, this vascular effect is lost during insulin resistance.
Food/drinks containing large glucose loads impair MBF in healthy people; however, this impairment is not observed when glucose is infused intravenously (bypassing the gut).
We investigated skeletal muscle MBF responses to a 75 g oral glucose tolerance test and intravenous glucose infusion and aimed to identify potential gut hormones responsible for glucose‐mediated changes in MBF.
Despite similar blood glucose concentrations, orally ingested glucose impaired, whereas intravenously infused glucose augmented, skeletal muscle MBF. The incretin gastric inhibitory polypeptide was negatively associated with MBF, suggestive of an incretin‐mediated MBF response to oral glucose ingestion.
This work provides new insight into why diets high in glucose may be detrimental to vascular health and provides new avenues for novel treatment strategies targeting microvascular dysfunction.
Keywords: cardiovascular physiology, gastrointestinal physiology, hyperglycaemia, insulin, skeletal muscle
Abstract figure legend Oral versus intravenous glucose loading produces opposing effects on the skeletal muscle microvasculature. The release of glucose‐dependent insulinotropic polypeptide (GIP) may be involved in the impaired microvascular responses to oral glucose loading.
Introduction
Skeletal muscle microvascular blood flow (MBF) increases in response to insulin infusion (hyperinsulinaemic euglycaemic clamp) (Coggins et al. 2001; Eggleston et al. 2007; Jahn et al. 2016) or a mixed‐nutrient meal (Vincent et al. 2006; Keske et al. 2009; Russell et al. 2018). This increase in skeletal muscle MBF promotes nutrient and hormone delivery to the myocyte (reviewed in (Clark, 2008; Wagenmakers, 2016)) and contributes to 40–50% of insulin‐stimulated skeletal muscle glucose disposal (Vincent et al. 2003; Vincent et al. 2004). This favourable vascular response is blunted in individuals that are obese (Clerk et al. 2006; Keske et al. 2009; Meijer et al. 2015; Wang et al. 2020), have metabolic syndrome (Jahn et al. 2016), have a parent with type 2 diabetes (Russell et al. 2022) or themselves have type 2 diabetes (Emanuel et al. 2018), positioning the skeletal muscle microvasculature as an important physiological tissue for human metabolism.
Insulin infusion (hyperinsulinaemic euglycaemic clamp) is commonly used to mimic postprandial metabolic and vascular responses (DeFronzo et al. 1979; Baron & Brechtel, 1993). However, a growing body of work suggests that the true postprandial state (following the ingestion of food/beverages) is more complex than intravenous infusions (Roberts‐Thomson et al. 2020), with dynamic changes in blood glucose, amino acids and insulin occurring, which are largely absent during insulin infusion (Spiller et al. 1987; Vincent et al. 2006; Roberts‐Thomson et al. 2020). One important variation between these conditions is the route via which glucose/nutrients are administered (orally versus intravenously). Oral administration of glucose elicits a significantly greater insulin response than an equivalent dose administered intravenously (Elrick et al. 1964). The ‘incretin effect’ refers to the secretion of the insulin‐promoting gut hormones glucagon‐like peptide‐1 (GLP‐1) and gastric inhibitory polypeptide (GIP) from the K‐cells of the small intestine following nutrient or food ingestion (Baggio & Drucker, 2007). In addition to their insulinotropic properties, there is evidence of their vasoactivity (Baggio & Drucker, 2007; Sjøberg et al. 2013; Chai et al. 2014; Subaran et al. 2014; Tan et al. 2018; Wang et al. 2020). GLP‐1 infusion has been demonstrated to increase microvascular perfusion in the skeletal muscle of animals (Sjøberg et al. 2013; Chai et al. 2014) and humans (Sjøberg et al. 2013; Subaran et al. 2014; Tan et al. 2018; Wang et al. 2020), supporting GLP‐1 as a key regulator of postprandial vascular function and metabolism. However, the effects of other glucoregulatory gut hormones such as GIP, peptide YY (PYY) and ghrelin, on skeletal muscle MBF in the postprandial state are not known.
Although it is well accepted that insulin stimulates muscle MBF, the ingestion of 50–75 g of glucose (on its own or in combination with a mixed nutrient meal) either impairs (Russell et al. 2018; Parker et al. 2020) or does not alter (Tobin et al. 2010) MBF in skeletal muscle of healthy individuals, despite physiological increases in plasma insulin levels. There are well‐known mechanisms that may explain why acute hyperglycaemia impairs vascular function, such as impaired nitric oxide (NO) signalling in the endothelium and augmented production of endothelium‐derived vasoconstrictors such as prostanoids (Tesfamariam et al. 1990; Renaudin et al. 1998; De Nigris et al. 2015). Therefore, it can be hypothesised that the extent of the glucose excursion in the circulation is linked to the extent of the impairment in MBF in skeletal muscle (Russell et al. 2018; Parker et al. 2020). However, others have demonstrated that when glucose is administered intravenously into healthy humans (Horton et al. 2020) or non‐human primates (Chadderdon et al. 2016, 2012), skeletal muscle MBF increases. As such, the route of glucose administration (i.e. glucose challenge with/without gut involvement), rather than hyperglycaemia in the circulation itself, may be the key factor behind divergent skeletal muscle MBF responses. The aim of the current study is to compare the vascular effects of glucose when administered orally versus intravenously when matched for blood glucose excursions in healthy humans. It was hypothesised that intravenously infused glucose would stimulate skeletal muscle MBF whereas oral glucose ingestion would impair MBF. Furthermore, it was hypothesised that increases in circulating gut‐derived hormones would be linked to oral glucose‐induced impairments in skeletal muscle MBF.
Parts of this study were published in abstract form at the 80th American Diabetes Association Scientific Meeting, June 2020.
Methods
General
This study was approved by the Deakin University Human Research Ethics Committee (2019‐062) and conformed to the standards set by the Declaration of Helsinki. All participants provided written informed consent. Participants were included in the study if they were aged between 18 and 50 years, were normal to overweight (BMI range: 18.5–30.0 kg/m2) and normotensive (seated brachial blood pressure: <140/90 mmHg). Individuals were excluded if they were outside the age and BMI range, had a first degree relative or >1 grandparent with diagnosed type 2 diabetes, hypertensive (blood pressure: >140/90 mmHg), current smoker, pregnant or lactating, or had any personal history of cardiovascular disease, malignancy, diabetes, liver disease, pulmonary disease, arthritis or musculoskeletal disease. Based on an a priori sample size calculation, it was estimated that 13 participants would be required to detect a two‐fold difference in MBF between the oral glucose tolerance test (OGTT) and intravenous glucose tolerance test (IVGTT) (SD = 115%, effect size = 0.87, α = 0.05, power = 80%) based on previous work (Russell et al. 2018). Ten participants completed the study between September 2019 and March 2020 prior to COVID‐19‐related clinic closures.
Participant screening visit
All participants attended a brief screening visit at the testing facility where body weight, height and blood pressure were assessed, and a medical questionnaire completed, to confirm eligibility. Participants were scheduled for two clinical testing visits between 1 and 4 weeks apart. In order to match blood glucose excursions between the testing visits, the OGTT was performed on the first visit and the variable rate IVGTT on the second clinical testing visit. This design prohibited treatment randomisation and blinding. Ten participants completed both clinical testing visits of the study (OGTT and IVGTT).
Clinical testing visits
Participants were fasted overnight for 12 h and refrained from exercise and alcohol for 48 h prior to each clinical testing visit. A catheter was placed into the antecubital vein of one arm for blood draws and microbubble infusion for the OGTT testing visit, and an additional catheter placed into the antecubital vein of the alternate arm for the variable rate IVGTT.
OGTT
Participants consumed a 75 g glucose drink (Fronine, Carbotest, Thermo Fisher Scientific, Scoresby, Australia). Venous blood was sampled at 0, 5, 10, 15, 20, 30, 40, 50, 60, 80, 90, 100, 110 and 120 min following glucose ingestion.
Variable rate IVGTT
Participants underwent a variable rate intravenous glucose infusion (glucose 10% w/v solution, Baxter Healthcare, Brunswick, Australia) to mimic the blood glucose excursions elicited by the OGTT. Venous blood was sampled at the same time points as the OGTT. The glucose infusion rate was adjusted accordingly over the 120 min time course to match the glucose levels observed during the OGTT for each participant (Table 1).
Table 1.
Glucose infusion rates (10% glucose w/v) over the 120 min IVGTT
| Time (minutes) | Infusion rate (ml/min) |
|---|---|
| 0 | 1.5 ± 1.7 |
| 5 | 1.3 ± 1.5 |
| 10 | 3.3 ± 2.0 |
| 15 | 4.4 ± 1.4 |
| 20 | 5.0 ± 1.6 |
| 30 | 4.5 ± 1.4 |
| 40 | 2.9 ± 2.4 |
| 50 | 2.5 ± 3.3 |
| 60 | 1.7 ± 2.0 |
| 70 | 2.1 ± 2.1 |
| 80 | 2.3 ± 2.4 |
| 90 | 1.6 ± 2.3 |
| 100 | 1.3 ± 2.4 |
| 110 | 1.5 ± 2.5 |
| 120 | 0.0 ± 0.0 |
| Total glucose infused (g) | 29.9 ± 17.6 |
Data are expressed as means ± SD for n = 10 participants.
Skeletal muscle microvascular perfusion
Contrast‐enhanced ultrasound was used to measure skeletal muscle microvascular perfusion in the quadriceps of the right leg. A linear array (L9‐3) ultrasound transducer interfaced with an ultrasound machine (iU22; Philips Healthcare, Melbourne, Australia) was used as described previously (Russell et al. 2018; Parker et al. 2020). An intravenous contrast agent containing echogenic microbubbles (DEFINITY®, Lantheus Medical Imagining, Keilor Park, Australia) was diluted into saline solution (1 ml into 30 ml saline), and continuously infused (2.0–2.6 ml/min, based on the participant's body weight and the degree of tissue opacification) for skeletal muscle imaging. Once the systemic concentration of microbubbles reached steady state (∼5 min), a high‐energy pulse of ultrasound (mechanical index 1.3) was transmitted to destroy microbubbles in the selected region of imaging (vastus lateralis). Rate of reperfusion (mechanical index 0.11) into the microvasculature of the muscle was measured in real‐time at baseline and repeated at 60 and 120 min during the OGTT/IVGTT. Three data captures of 45 s were collected at each time point and averaged together and analysed using QLAB software (Philips Healthcare, Melbourne, Australia). All images were background subtracted (0.5 s image) to eliminate signal from larger fast filling blood vessels and tissue per se. Background‐subtracted acoustic intensity vs. time was fitted to the function: y = A(1 − e–β( t−t b)), where y is the acoustic intensity at time t, t b is the background time, A is the plateau of acoustic‐intensity (microvascular blood volume; MBV) and β is the rate constant (a measure of microvascular refilling rate), as previously described (Russell et al. 2017, 2018). MBF was calculated using A × β. Image analysis was performed identically at baseline, 60 and 120 min post‐OGTT/IVGTT.
Superficial femoral and large artery haemodynamics
Diameter, blood velocity and flow measurements of the superficial femoral artery were performed using a linear array (L12‐5) ultrasound transducer interfaced with an ultrasound machine (iU22; Philips Healthcare, Melbourne, Australia). Femoral artery diameter was measured in triplicate at the same phase of the cardiac cycle (R‐wave, based on the QRS complex) using 2D imaging of the longitudinal artery. Femoral artery velocity was assessed using pulse‐wave Doppler quantified by an automated tracing software and averaged over ∼10 heartbeats. Femoral artery blood flow (ml/min) was calculated using πr 2 × mean velocity × 60, where r is radius (cm) and mean velocity measured in cm/s.
All participants were fitted with a Mobil‐O‐Graph monitor validated to measure central and brachial blood pressure, heart rate, mean arterial pressure, augmentation index and pulse wave velocity (I.E.M., Stolberg, Germany). Measures were performed in triplicate at baseline, 60 and 120 min during the OGTT/IVGTT.
Blood sampling and plasma analysis
A fasting blood sample was taken during clinic visit 1 and sent to a nationally accredited pathology laboratory (Australian Clinical Labs, Burwood, Australia) for analysis of clinical chemistry including fasting glucose, glycosylated haemoglobin (HbA1c), total cholesterol, low‐density lipoprotein cholesterol (LDL), high‐density lipoprotein cholesterol (HDL), and triglycerides. Blood glucose was measured throughout the time course of the OGTT and IVGTT clinical testing visits using an automated radiometer analysis system (ABL800 FLEX; Radiometer Medical, Copenhagen, Denmark). Blood sampled throughout the time course of the OGTT/IVGTT was collected in BD 800 Vacutainers (Becton Dickinson, Mulgrave, Australia) containing protease, esterase and dipeptidyl peptidase‐4 inhibitors for preservation of GLP‐1, GIP and other gut‐derived hormones.
Plasma insulin, C‐peptide and active GLP‐1 were determined using an enzyme‐linked immunosorbent assay (ELISA) (ALPCO Diagnostics, Windham, NH, USA). Plasma non‐esterified fatty acids (NEFA) levels were determined using an enzymatic colorimetric assay (Wako Pure Chemical Industries, Osaka, Japan). Plasma total GLP‐1, total GIP, active ghrelin, PYY and glucagon were determined using a Milliplex multiplex assay (Millipore Human Metabolic Hormone Panel, Merck, Bayswater, Australia).
Statistical analysis
All statistical analysis was undertaken using GraphPad Prism (version 8.0, GraphPad Software, La Jolla, CA, USA) except for correlations, which were performed using SigmaPlot (Systat Software, San Jose, CA, USA). All data are expressed as means ± SD. Two‐way repeated measures ANOVA was used to compare multiple means with time (at baseline and throughout the 120 min OGTT/IVGTT time course) and condition (OGTT versus IVGTT) as within‐subject factors. Mixed model analyses were used for statistical testing of central and peripheral haemodynamic measures due to missing data for one participant. All data that were not normally distributed were transformed using natural log. Significant interaction and main effects were explored post hoc using Fisher's least significant difference test. Associations between variables (gut‐derived hormones) and muscle MBV, β and MBF were studied with the use of Pearson's correlations. Correlations were performed on changes in MBV, β and MBF versus changes in active GLP‐1, total GLP‐1, total GIP, natural log total GLP:GIP, ghrelin and PYY. Data were initially correlated with combined time‐point data from the OGTT and IVGTT (both 60 and 120 min). If a statistically significant correlation with an r‐value >0.4 was found, correlations were then performed on individual time points (60 and 120 min). All statistical analysis was conducted at 95% level of significance (P < 0.05).
Results
Participant characteristics
Participant characteristics and anthropometrics are reported in Table 2. Participants were aged between 20 and 47 years (29 ± 8 years). Participants were apparently healthy with normal fasting blood glucose (<6.5 mmol/l), HbA1C (<6.0% and <42 mmol/mol), plasma insulin levels (<174 pmol/l) and seated brachial blood pressure (<140/90 mmHg).
Table 2.
Participant characteristics
| Mean ± SD | Range | |
|---|---|---|
| Age (years) | 29 ± 8 | 20–47 |
| Sex (M/F) | 4/6 | — |
| Weight (kg) | 71.0 ± 14.4 | 53.4– 98.9 |
| Height (cm) | 172.7 ± 10.3 | 158.2–189.0 |
| BMI (kg/m2) | 23.6 ± 3.0 | 20.6–29.4 |
| Fasting plasma glucose (mmol/l) | 4.5 ± 0.3 | 4.0–5.1 |
| HbA1c (%) | 5.2 ± 0.3 | 4.8–5.6 |
| HbA1c (mmol/mol) | 33.2 ± 3.0 | 29.0–38.0 |
| Fasting plasma insulin (pmol/l) | 34.6 ± 11.6 | 18.1–59.1 |
| Fasting plasma lipids | ||
| Total cholesterol (mmol/l) | 4.3 ± 1.4 | 2.6–7.5 |
| LDL (mmol/l) | 2.4 ± 1.2 | 1.1–5.1 |
| HDL (mmol/l) | 1.5 ± 0.3 | 1.1–1.9 |
| Triglycerides (mmol/l) | 0.9 ± 0.3 | 0.7–1.4 |
| Resting brachial blood pressure | ||
| SBP (mmHg) | 114 ± 9 | 104–130 |
| DBP (mmHg) | 74 ± 9 | 60–87 |
Data are expressed as means ± SD and range for n = 10 participants. BMI, body mass index; DBP, diastolic blood pressure; HDL, high‐density lipoprotein cholesterol; LDL, low‐density lipoprotein cholesterol; SBP, systolic blood pressure.
Metabolic responses
Blood glucose levels increased significantly at 15–120 min during the OGTT (P = 0.024 to 0.001, respectively) and at 20–120 min during the IVGTT (P = 0.003 to 0.018, respectively, Fig. 1A ). The glucose area under the time curve (AUC) was similar between the OGTT and IVGTT (799.3 ± 132.4 versus 804.2 ± 123.3 mmol/l×120 min, P = 0.703, Fig. 1B ).
Figure 1. Metabolic effects of an OGTT and IVGTT.
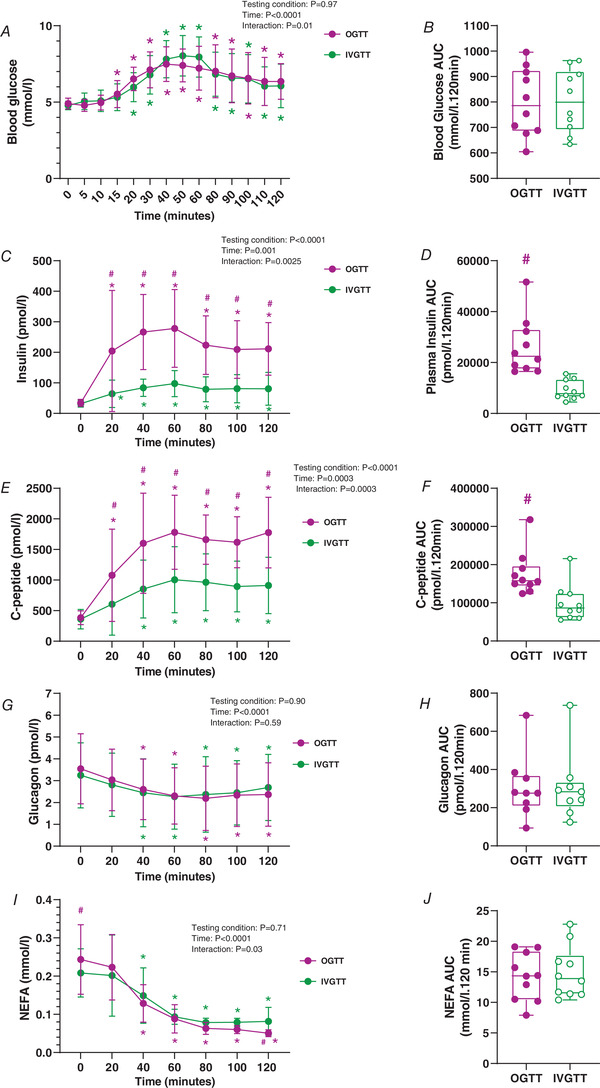
Graphs show OGTT (magenta) and IVGTT (green). A and B, 2 h blood glucose time course (A) and blood glucose area under the time curve (AUC) (B). C and D, 2 h plasma insulin time course (C) and plasma insulin AUC (D). E and F, 2 h plasma C‐peptide time course (E) and plasma C‐peptide AUC (F). G and H, 2 h plasma glucagon time course (G) and plasma glucagon AUC (H). I and J, 2 h plasma non‐esterified fatty acid (NEFA) time course (I) and plasma NEFA AUC (J). For visual clarity, time‐course data are expressed as line graphs with means ± SD. Bar graphs are presented as box and whisker plots with individual data points. The box represents the interquartile range alongside the median (line). The whiskers represent the minimum and maximum range of data for n = 10 participants. * P < 0.05 versus 0 min, #P < 0.05 versus IVGTT for the same time point (refer to Results for exact P‐values). [Colour figure can be viewed at wileyonlinelibrary.com]
Despite plasma insulin concentrations increasing over time during both conditions, the OGTT was significantly higher compared to the IVGTT from 20 to 120 min (P = 0.0196 to 0.0001, respectively, Fig. 1C ). Plasma insulin AUC was significantly higher during the OGTT compared to the IVGTT (26094.7 ± 11087.9 versus 9237.0 ± 3757.5 pmol/l×120 min, P = 0.0001, Fig. 1D ). These effects were similar for plasma C‐peptide (Fig. 1E and F ).
Plasma glucagon decreased significantly at 40–120 min compared to 0 min irrespective of the treatment (OGTT: P = 0.0189 to 0.0203; IVGTT: P = 0.0119 to 0.0298, respectively, Fig. 1G ). There were no differences in plasma glucagon AUC between treatments (P = 0.9211, Fig. 1H ).
Plasma NEFA concentrations decreased at 40–120 min compared to 0 min during both treatments (OGTT: P < 0.0001 to <0.0001; IVGTT: P = 0.0002 to <0.0001, respectively, Fig. 1I ). Plasma NEFA AUC was similar between treatments (P = 0.6569, Fig. 1J ).
Skeletal muscle microvascular measures
There were no interactions (P = 0.09) or effect of treatment (P = 0.61) or time (P = 0.64) for skeletal muscle MBV (Fig. 2A ) or ΔMBV (P = 0.44, 0.30 and 0.73, respectively) for both groups (Fig. 2B ).
Figure 2. Skeletal muscle microvascular responses to an OGTT and IVGTT.
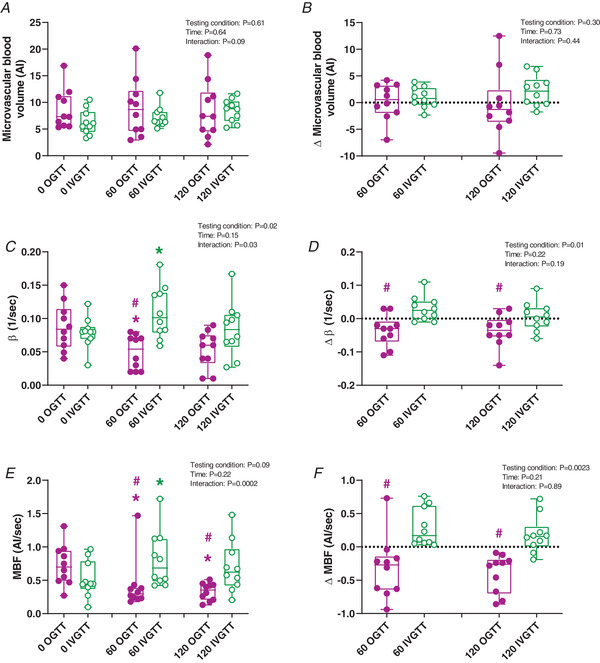
Graphs show OGTT (magenta) and IVGTT (green). A and B, skeletal muscle microvascular blood volume (MBV) at 0, 60 and 120 min (A), and ΔMBV at 60 and 120 min (B) from baseline. C and D, skeletal muscle microvascular β at 0, 60 and 120 min (C), and Δβ at 60 and 120 min (D) from baseline. E and F, skeletal muscle microvascular blood flow (MBF) at 0, 60 and 120 min (E), and ΔMBF at 60 and 120 min (F) from baseline. Bar graphs are presented as box and whisker plots with individual data points. The box represents the interquartile range alongside the median (line). The whiskers represent the minimum and maximum range of data for n = 10 participants. * P < 0.05 versus 0 min, #P < 0.05 versus IVGTT for the same time point (refer to Results for exact P‐values). AI, Acoustic Intensity. [Colour figure can be viewed at wileyonlinelibrary.com]
Skeletal muscle β (microvascular flow velocity) significantly decreased at 60 min compared to 0 min during the OGTT (P = 0.0321); however, it was significantly higher at 60 min during the IVGTT (P = 0.0223). These effects were absent at 120 min (OGTT: P = 0.0806; IVGTT: P = 0.9622, Fig. 2C ). However, the Δβ was significantly lower during the OGTT compared to the IVGTT at both 60 (P = 0.0073) and 120 min (P = 0.0498, Fig. 2D ).
MBF was lower at 60 and 120 min during the OGTT compared to 0 min (P = 0.0077 and 0.0009, respectively). In contrast, MBF was higher at 60 min compared to 0 min during the IVGTT (P = 0.0133); however, it was unchanged at 120 min (P = 0.1667, Fig. 2E ). Skeletal muscle MBF was lower during the OGTT compared to the IVGTT at both 60 and 120 min (P = 0.0163 and 0.0085, respectively). ΔMBF was lower at both 60 and 120 min during the OGTT compared to the IVGTT (P = 0.0158 and 0.0005, respectively, Fig. 2F ).
Large artery haemodynamic responses
No changes in superficial femoral artery diameter, velocity or blood flow were detected (data not shown).
Central and peripheral haemodynamic measures at baseline, 60 and 120 min during the OGTT and IVGTT are reported in Table 3. No interactions were noted for central blood pressure (central systolic blood pressure: P = 0.8945, central diastolic blood pressure: P = 0.3529), brachial blood pressure (brachial systolic blood pressure: P = 0.3870; brachial diastolic blood pressure: P = 0.4330), heart rate (P = 0.7069), augmentation index (P = 0.1395), pulse wave velocity (P = 0.7059) or mean arterial pressure (P = 0.7206). Due to equipment malfunction, Mobil‐O‐Graph data from one participant were lost.
Table 3.
Central and peripheral haemodynamics following the OGTT and IVGTT
| bSys BP (mmHg) | bDias BP (mmHg) | cSys BP (mmHg) | cDias BP (mmHg) | HR (BPM) | Aug Index (adjusted 75 BPM) | MAP (mmHg) | PWV (m/s) | |
|---|---|---|---|---|---|---|---|---|
| OGTT | ||||||||
| 0 min | 114 ± 9 | 74 ± 9 | 105 ± 9 | 75 ± 9 | 61 ± 7 | 13 ± 7 | 93 ± 9 | 5 ± 1 |
| 60 min | 115 ± 12 | 71 ± 5 | 105 ± 11 | 72 ± 6 | 59 ± 9 | 18 ± 15 | 91 ± 8 | 5 ± 1 |
| 120 min | 113 ± 11 | 71 ± 9 | 102 ± 9 | 72 ± 10 | 61 ± 10 | 9 ± 7 | 91 ± 9 | 5 ± 1 |
| IVGTT | ||||||||
| 0 min | 119 ± 12 | 76 ± 11 | 107 ± 14 | 74 ± 10 | 64 ± 7 | 6 ± 8 | 97 ± 12 | 5 ± 1 |
| 60 min | 118 ± 17 | 73 ± 11 | 105 ± 14 | 69 ± 7 | 61 ± 9 | 9 ± 12 | 94 ± 12 | 5 ± 1 |
| 120 min | 113 ± 11 | 78 ± 10 | 102 ± 8 | 75 ± 8 | 60 ± 10 | 9 ± 9 | 94 ± 10 | 5 ± 1 |
Table shows haemodynamic measures at 0, 60 and 120 min during an OGTT and IVGTT. Data are expressed as means ± SD. n = 9 participants. Aug Index, augmentation index; bDias BP, brachial diastolic blood pressure; bSys BP, brachial systolic blood pressure; cDias BP, central diastolic blood pressure; cSys BP, central systolic blood pressure; HR, heart rate; MAP, mean arterial pressure; PWV, pulse wave velocity.
Plasma incretin responses
Plasma incretin responses to the OGTT and IVGTT are reported in Fig. 3. Active GLP‐1 increased at 20–60 min (P < 0.0001 to 0.0003, respectively) and 100–120 min (P = 0.0106 to 0.0005, respectively) during the OGTT, compared to 0 min. Active GLP‐1 did not change during the IVGTT compared to 0 min except for a small decrease at 80 min (P = 0.0348, Fig. 3A ). Active GLP‐1 was significantly higher during the OGTT compared to IVGTT at 20–80 min (P < 0.0001 to <0.0001, respectively) and at 120 min (P = 0.0049, Fig. 3A ). As such, Δ active GLP‐1 was significantly higher from 20–80 min during the OGTT compared to IVGTT (P < 0.0001 to 0.0043, respectively, Fig. 3B ).
Figure 3. Plasma incretin responses to an OGTT and IVGTT.
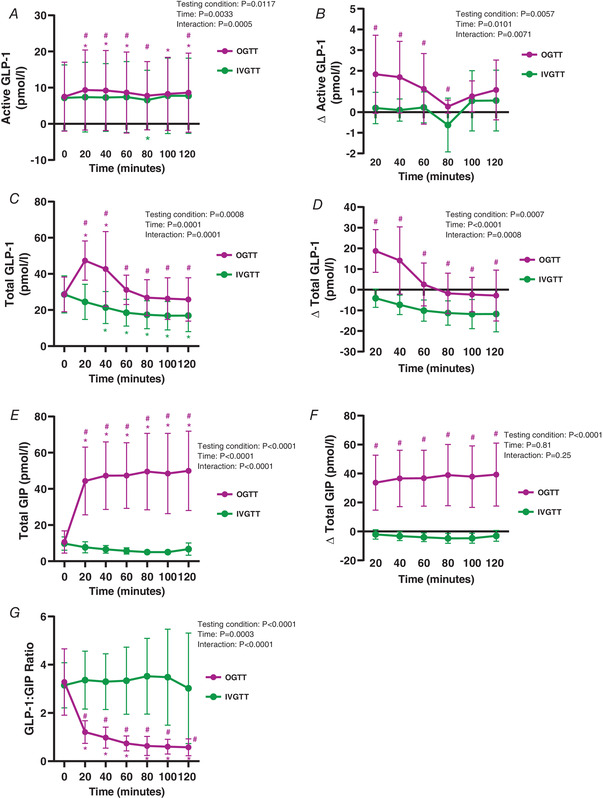
Graphs show OGTT (magenta) and IVGTT (green). A and B, 2 h active GLP‐1 time course (A) and Δ active GLP‐1 time course (B). C and D, 2 h total GLP‐1 time course (C) and Δ total GLP‐1 time course (D). E and F, 2 h total GIP time course (E) and Δ total GIP time course (F). G, 2 h GLP‐1:GIP ratio time course. For visual clarity, time‐course data are expressed as line graphs with means ± SD for n = 10 participants. * P < 0.05 versus 0 min, #P < 0.05 versus IVGTT for the same time point (refer to Results for exact P‐values). [Colour figure can be viewed at wileyonlinelibrary.com]
Total plasma GLP‐1 increased at 20 and 40 min from 0 min during the OGTT (P < 0.0001 for both). In contrast, total GLP‐1 decreased from 40–120 min during the IVGTT compared to 0 min (P < 0.0001 to <0.0001, respectively, Fig. 3C ). Total GLP‐1 levels were significantly higher at all time points during the OGTT, compared to IVGTT (P < 0.0001 to 0.0020, respectively, Fig. 3C ). The OGTT elicited a larger Δ total GLP‐1 at all time points compared to the IVGTT (P < 0.0001 to 0.0024, Fig. 3D ).
Total GIP concentrations increased at all time points during the OGTT compared to 0 min (all P < 0.0001), with levels significantly greater than the IVGTT at all time points (all P < 0.0001, Fig. 3E ). Total GIP remained unchanged during the time course of the IVGTT. This was also reflected by a higher Δ total GIP during the OGTT than the IVGTT at all time points (all P < 0.0001, Fig. 3F ).
The ratio of total GLP‐1:GIP was significantly lower during the OGTT at all time points, compared to 0 min (all P < 0.0001). Furthermore, this ratio was lower during the OGTT compared to the IVGTT at all time points (P < 0.0001, Fig. 3G ). The GLP‐1:GIP ratio remained unchanged during the time course of the IVGTT.
Other gut hormone responses
Gut hormone responses to the OGTT and IVGTT are shown in Table 4. Active ghrelin was lower at 20 and 40 min during the OGTT (P = 0.0281 and 0.0160, respectively), compared to the IVGTT. A small but significant increase in plasma PYY was observed at 20 min during the OGTT compared to 0 min (P = 0.0278), but not at any other time point. Plasma PYY was significantly lower at 60 and 120 min compared to 0 min during the IVGTT (P = 0.0397 and 0.0143, respectively). PYY levels were significantly higher at 20 (P = 0.0103), 40 (P = 0.0190), and 60 min (P = 0.0250) during the OGTT compared to IVGTT (Table 4). ΔPYY was significantly higher at all time points during the OGTT compared to the IVGTT (Table 4).
Table 4.
Gut hormone responses to an OGTT and IVGTT
| Time (min) | |||||||
|---|---|---|---|---|---|---|---|
| 0 | 20 | 40 | 60 | 80 | 100 | 120 | |
| Active ghrelin (pmol/l) | |||||||
| OGTT | 13.7 ± 12.3 | 8.8 ± 6.7 # | 6.8 ± 3.8 # | 9.1 ± 7.4 | 9.2 ± 7.1 | 11.6 ± 9.6 | 11.3 ± 7.7 |
| OGTT – Δ from 0 min | — | −4.9 ± 8.2 | −6.9 ± 9.3 | −4.7 ± 9.7 | −4.5 ± 7.2 | −2.1 ± 5.0 | −2.5 ± 6.3 |
| IVGTT | 13.4 ± 9.9 | 14.8 ± 11.1 | 15.8 ± 10.9 | 10.3 ± 8.2 | 11.7 ± 6.7 | 16.3 ± 9.0 | 17.3 ± 10.3 |
| IVGTT – Δ from 0 min | — | 1.4 ± 9.1 | 2.4 ± 7.0 | −3.0 ± 10.2 | −1.7 ± 5.7 | 2.9 ± 6.4 | 3.9 ± 8.9 |
| PYY (pmol/l) | |||||||
| OGTT | 24.5 ± 18.3 | 26.7 ± 17.5 *# | 25.1 ± 17.2 # | 24.9 ± 17.8 # | 23.8 ± 18.1 | 24.7 ± 18.0 | 23.7 ± 18.6 |
| OGTT – Δ from 0 min | — | 2.1 ± 2.2 # | 0.6 ± 2.2 # | 0.4 ± 2.4 # | −0.7 ± 4.0 # | 0.2 ± 2.2 # | −0.8 ± 3.0 # |
| IVGTT | 22.9 ± 18.7 | 20.7 ± 19.6 | 20.3 ± 19.8 | 20.3 ± 20.0 * | 19.8 ± 19.9 | 20.2 ± 20.5 | 19.9 ± 20.0 * |
| IVGTT – Δ from 0 min | — | −2.2 ± 3.1 | −2.7 ± 3.3 | −2.6 ± 2.9 | −3.1 ± 4.1 | −2.7 ± 3.3 | −3.1 ± 2.7 |
Hormone levels over 120 min and Δ from baseline. Data are expressed as means ± SD for n = 10 participants.
P < 0.05 versus 0 min, # P < 0.05 versus IVGTT for the same time point (refer to Results for exact P‐values). PYY, peptide YY.
Associations between gut‐derived hormones and skeletal muscle microvascular blood flow
Figure 4 shows significant associations between changes in microvascular responses and changes in plasma gut‐derived hormones during the OGTT and IVGTT with an r‐value >0.4. ΔMBF was negatively associated with ΔGIP (r = −0.665; P < 0.0001) and this relationship was stronger at 120 min (r = −0.788; P < 0.0001) compared to 60 min (r = −0.566; P = 0.00934, Fig. 4A ). These effects were also similar for Δβ with ΔGIP (Fig. 4B ).
Figure 4. Associations between skeletal muscle microvascular haemodynamics and plasma gut‐derived hormone concentrations.
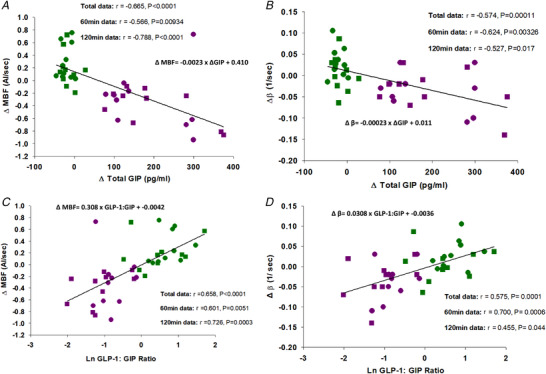
Graphs show OGTT (magenta), IVGTT (green), 60 min (circles) and 120 min (squares) data. Changes (Δ) in MBF versus Δ total GIP (A), Δβ versus Δ total GIP (B), ΔMBF versus natural log total GLP‐1:GIP (C) and Δβ versus natural log total GLP‐1:GIP (D). Continuous line and equation represent polynomial linear line of best fit for total data set. [Colour figure can be viewed at wileyonlinelibrary.com]
In contrast, ΔMBF was positively associated with natural log GLP‐1:GIP ratio (r = 0.658; P < 0.0001) and this relationship was stronger at 120 min (r = 0.726; P = 0.0003) compared to 60 min (r = 0.601; P = 0.0051, Fig. 4C ). These effects were similar for Δβ and natural log GLP‐1:GIP ratio (Fig. 4D ).
There were no associations between MBV and any gut hormone measured in this study. There were no associations between MBF and active GLP‐1, total GLP‐1, ghrelin or PYY.
Discussion
This is the first study to directly compare the peripheral macro‐ and microvascular effects of oral versus intravenous glucose loading in healthy humans. We demonstrate that (1) skeletal muscle MBF decreases with an OGTT and increases with an IVGTT when matched for similar blood glucose excursions; (2) these opposing vascular actions are not related to hyperglycaemia per se; (3) plasma levels of the incretin hormone GIP are strongly associated with impaired microvascular responses in skeletal muscle, whereas (4) plasma PYY and ghrelin are not associated with MBF. These findings suggest a novel incretin‐mediated mechanism regulating skeletal muscle MBF responses following oral glucose loading.
The effects of acute hyperglycaemia on impaired vascular actions have been investigated in endothelial cell culture/in vitro models (Tesfamariam et al. 1990; De Nigris et al. 2015), animal models (Renaudin et al. 1998; 1999) and humans (Giugliano et al. 1997; Williams et al. 1998). We previously proposed that the extent of hyperglycaemia following nutrient ingestion is directly related to skeletal muscle MBF responses (Russell et al. 2018), and a greater postprandial glucose AUC is associated with a greater decrease in muscle MBF (Parker et al. 2020). However, prior work shows intravenously infused glucose (alone or in combination with insulin) increases skeletal muscle microvascular responses in healthy non‐human primates (Chadderdon et al. 2012) and humans (Horton et al. 2020). We now provide evidence that the route of glucose administration (glucose loading with/without involvement of the gut), and not the presence of hyperglycaemia in the blood, is the key determinant of divergent responses in muscle MBF between oral and intravenous glucose loading. Despite being matched for similar blood glucose concentrations, MBF is blunted during orally ingested glucose (OGTT) but stimulated during intravenously administered glucose (IVGTT). We postulate that glucose ingestion stimulates incretin secretion from the gut and this inhibits vasodilatation leading to impaired skeletal muscle MBF. We do not know why the OGTT would restrict flow to skeletal muscle in healthy individuals; however, blood flow redistribution (and therefore altered nutrient disposal) to other insulin sensitive tissues is a possibility. We (Hu et al. 2018) and others (Tobin et al. 2010) have shown that oral glucose loading stimulates adipose tissue MBF in healthy people, which provides one potential explanation. Taken together, our study demonstrates that the route of glucose administration is a key determinant of postprandial MBF responses and provides a novel mechanistic explanation for the divergent MBF responses of oral versus intravenously administered glucose.
As expected, we observed marked differences in plasma insulin concentrations between challenges. Although the ‘incretin effect’ provides an explanation for the differences in insulin concentrations (Kazakos, 2011), the greater levels of plasma insulin (a vasodilator in healthy humans) following the OGTT did not result in augmented MBF. These data provide compelling evidence of alternative factors in the regulation of MBF following oral glucose loading. However, measuring the skeletal muscle interstitial glucose and insulin concentrations to determine the impact of MBF on their delivery to the myocyte, and the rates of glucose disposal under experimental conditions where both glucose and insulin excursions are matched are important to follow up.
Considering the previously reported role of incretins (specifically GLP‐1) in regulating skeletal muscle MBF (Sjøberg et al. 2013; Subaran et al. 2014; Tan et al. 2018), we investigated whether incretins, or other gut‐derived factors, could explain the divergent muscle MBF responses observed between oral and intravenous glucose loading. GLP‐1 has been reported to recruit capillaries in the skeletal muscle of animals (Sjøberg et al. 2013; Chai et al. 2014) and humans (Sjøberg et al. 2013; Subaran et al. 2014; Tan et al. 2018). Our data show a significant but transient increase in total and active plasma GLP‐1 during the OGTT. However, neither active nor total GLP‐1 concentrations were associated with changes in muscle MBF. The potential vasodilatory actions of GLP‐1 may have been ‘masked’ by the higher GIP concentrations during the OGTT, which we now hypothesise to be vasoconstrictive in skeletal muscle. In the current study, we report a strong negative association between plasma GIP concentrations and muscle MBF. Whilst GIP has been shown to play an active role in augmenting blood flow and microvascular responses in adipose tissue (Asmar et al. 2019, 2016), the vascular actions of GIP in skeletal muscle have not previously been investigated. Although our findings of GIP impairing MBF may at first appear to be contradictory (Asmar et al. 2019, 2016), the regulation of blood flow in adipose tissue is believed to be largely dominated by adrenergic mechanisms, which differs from that of skeletal muscle (Frayn & Karpe, 2014; Asmar et al. 2019). As such, we then assessed the relationship between the ratio of GLP‐1:GIP and muscle MBF and demonstrated a strong positive relationship. Although this is not a causal relationship, it does highlight that the ratio of these incretins may also play an important role in postprandial skeletal muscle MBF. Follow‐up studies to determine the direct effect of GIP alone, and in combination with GLP‐1, are now required. Taken together, these data are suggestive of a novel incretin‐mediated mechanism to skeletal muscle MBF in response to oral glucose loading.
Elevated GIP levels have been implicated in obesity and glucose intolerance in humans (Creutzfeldt et al. 1978; Góralska et al. 2020). Interestingly, mice lacking the GIP receptor are protected against high fat diet‐induced obesity and insulin resistance (Miyawaki et al. 2002). In addition to GLP‐1 and GIP receptors being located in the pancreas (Dillon et al. 1993; Gremlich et al. 1995), they are also located on vascular endothelial cells (Lim et al. 2017). GLP‐1 is associated with NO production during hyperglycaemia in cultured endothelial cells (Lim et al. 2017) and stimulates skeletal muscle MBF in healthy rats via a NO synthase‐dependent pathway (Dong et al. 2013). The mechanism by which GIP impairs skeletal muscle MBF is unknown; however, there is evidence that it stimulates release of endothelin‐1 (ET‐1; a potent vasoconstrictor) in cultured arterial endothelial cells (Ding et al. 2004). This may provide some potential clues to the mechanism of GIP vasoconstriction given that ET‐1 opposes insulin‐stimulated MBF in rats (Ross et al. 2007), which is NO‐dependent (Vincent et al. 2004). This mechanism is speculative, but if ET‐1 is involved, the co‐infusion of an ET‐1 receptor antagonist with GIP in humans could help answer this.
In the current study, plasma ghrelin and PYY were examined as potential gut‐derived links to muscle MBF. Whilst we are not aware of any associations between PYY and skeletal muscle MBF, there is some evidence for PYY in the increase of blood flow and reduction of vascular resistance in parts of the brain (Tuor et al. 1988). Moreover, some positive associations have been drawn between ghrelin and increased perfusion of central organs following sepsis (Wu et al. 2005) and ischaemia–reperfusion injury (Bukowczan et al. 2015). However, we did not detect any associations between PYY or ghrelin and skeletal muscle MBF in our experimental protocol, and therefore propose that ghrelin and PYY are unlikely to be involved in the impaired MBF response during the OGTT.
Several limitations were identified in this study. First, to closely match blood glucose levels between the conditions, the OGTT had to be performed first and thus randomisation of the testing conditions was unable to be performed. Therefore, we cannot rule out an order effect of the testing conditions. Second, the measurement of gut‐derived hormones in plasma was a targeted approach, and therefore we cannot rule out the involvement of other (or unknown) gut‐derived factors which may have contributed to our observed blood flow effects. Third, we acknowledge that our participants were a healthy and young population and therefore cannot assume that MBF and gut hormone responses would be comparable in overweight individuals or those with type 2 diabetes. Finally, our study exclusively used contrast‐enhanced ultrasound to measure microvascular responses in skeletal muscle. The use of other non‐invasive microvascular perfusion techniques in parallel with contrast‐enhanced ultrasound would corroborate our findings.
In conclusion, we have directly shown for the first time that an OGTT leads to decreased skeletal muscle MBF in healthy people, whereas a variable rate IVGTT matched for blood glucose excursions leads to increased skeletal muscle MBF. We uncovered novel associations between plasma GIP concentrations and GLP‐1:GIP ratio and MBF responses, suggesting that glucose ingestion leads to secretion of GIP from the gut, which may promote vasoconstriction in the skeletal muscle microvasculature. Further work focused on the direct effects of a GIP infusion on the microvasculature is required to better understand this relationship.
Additional information
Competing interests
None.
Author contributions
K.M.R‐T. and M.A.K. were responsible for the conception and design of the research. K.M.R‐T. and M.A.K. performed all experiments. L.P., A.C.B. and P.A.D.G. assisted in the data collection. K.M.R‐T. performed all statistical analyses. All authors interpreted the data. K.M.R‐T. drafted the manuscript and M.A.K. provided first edits; all authors revised the manuscript. All authors have read and approved the final version of this manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
L.P. is supported by a NHMRC & National Heart Foundation Fellowship (APP1157930). This work was supported by the Diabetes Australia Research Program (Y21G‐KESM) and the Deakin University Institute for Physical Activity and Nutrition (2020‐2021) seed funds.
Supporting information
Peer Review History
Statistical Summary Document
Acknowledgement
Open access publishing facilitated by Deakin University, as part of the Wiley – Deakin University agreement via the Council of Australian University Librarians.
Biography
Katherine M. Roberts‐Thomson is a PhD candidate at the School of Exercise and Nutrition Sciences, Deakin University, Australia. Her PhD is focused on the impact of glucose on skeletal muscle vascular function in healthy and insulin‐resistant populations. She received her Masters of nutrition and dietetics from the University of Sydney in 2014 and works as an accredited practising dietitian in the dietary management of type 2 diabetes. She is passionate about the translation of clinical research into dietary practice and management of chronic diseases.

Edited by: Kim Barrett & Bettina Mittendorfer
Linked articles: This article is highlighted in a Perspective article by Tamariz‐Ellemann et al. and a Journal Club article by Cohen & Wilkinson. To read these articles, visit https://doi.org/10.1113/JP282843 and https://doi.org/10.1113/JP282904.
The peer review history is available in the Supporting Information section of this article (https://doi.org/10.1113/JP282428#support‐information‐section).
This is an Editor's Choice article from the 1 April 2022 issue.
Data availability statement
Data generated for the current study are available on reasonable request from the corresponding author in the form of excel spreadsheets.
References
- Asmar M, Asmar A, Simonsen L, Dela F, Holst JJ & Bülow J (2019). GIP‐induced vasodilation in human adipose tissue involves capillary recruitment. Endocr Connect 8, 806–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asmar M, Simonsen L, Asmar A, Holst JJ, Dela F & Bülow J (2016). Insulin plays a permissive role for the vasoactive effect of GIP regulating adipose tissue metabolism in humans. J Clin Endocrinol Metab 101, 3155–3162. [DOI] [PubMed] [Google Scholar]
- Baggio LL & Drucker DJ (2007). Biology of incretins: GLP‐1 and GIP. Gastroenterology 132, 2131–2157. [DOI] [PubMed] [Google Scholar]
- Baron AD & Brechtel G (1993). Insulin differentially regulates systemic and skeletal muscle vascular resistance. Am J Physiol Endocrinol Metab 265, E61–E67. [DOI] [PubMed] [Google Scholar]
- Bukowczan J, Warzecha Z, Ceranowicz P, Kusnierz‐Cabala B, Tomaszewska R & Dembinski A (2015). Therapeutic effect of ghrelin in the course of ischemia/reperfusion‐induced acute pancreatitis. Curr Pharm Des 21, 2284–2290. [DOI] [PubMed] [Google Scholar]
- Chadderdon SM, Belcik JT, Bader L, Kievit P, Grove KL & Lindner JR (2016). Vasoconstrictor eicosanoids and impaired microvascular function in inactive and insulin‐resistant primates. Int J Obes 40, 1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadderdon SM, Belcik JT, Smith E, Pranger L, Kievit P, Grove KL & Lindner JR (2012). Activity restriction, impaired capillary function, and the development of insulin resistance in lean primates. Am J Physiol Endocrinol Metab 303, E607–E613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai W, Zhang X, Barrett EJ & Liu Z (2014). Glucagon‐like peptide 1 recruits muscle microvasculature and improves insulin's metabolic action in the presence of insulin resistance. Diabetes 63, 2788–2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark MG (2008). Impaired microvascular perfusion: a consequence of vascular dysfunction and a potential cause of insulin resistance in muscle. Am J Physiol Endocrinol Metab 295, E732–E750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerk LH, Vincent MA, Jahn LA, Liu Z, Lindner JR & Barrett EJ (2006). Obesity blunts insulin‐mediated microvascular recruitment in human forearm muscle. Diabetes 55, 1436–1442. [DOI] [PubMed] [Google Scholar]
- Coggins M, Lindner J, Rattigan S, Jahn L, Fasy E, Kaul S & Barrett E (2001). Physiologic hyperinsulinemia enhances human skeletal muscle perfusion by capillary recruitment. Diabetes 50, 2682–2690. [DOI] [PubMed] [Google Scholar]
- Creutzfeldt W, Ebert R, Willms B, Frerichs H & Brown J (1978). Gastric inhibitory polypeptide (GIP) and insulin in obesity: increased response to stimulation and defective feedback control of serum levels. Diabetologia 14, 15–24. [DOI] [PubMed] [Google Scholar]
- DeFronzo RA, Tobin JD & Andres R (1979). Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol Endocrinol Metab 237, E214. [DOI] [PubMed] [Google Scholar]
- De Nigris V, Pujadas G, La Sala L, Testa R, Genovese S & Ceriello A (2015). Short‐term high glucose exposure impairs insulin signaling in endothelial cells. Cardiovasc Diabetol 14, 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon JS, Tanizawa Y, Wheeler M, Leng X‐H, Ligon BB, Rabin D, Yoo‐Warren H, Permutt M & Boyd A 3rd (1993). Cloning and functional expression of the human glucagon‐like peptide‐1 (GLP‐1) receptor. Endocrinology 133, 1907–1910. [DOI] [PubMed] [Google Scholar]
- Ding K‐H, Zhong Q, Xu J & Isales CM (2004). Glucose‐dependent insulinotropic peptide: differential effects on hepatic artery vs. portal vein endothelial cells. Am J Physiol Endocrinol Metab 286, E773–E779. [DOI] [PubMed] [Google Scholar]
- Dong Z, Chai W, Wang W, Zhao L, Fu Z, Cao W & Liu Z (2013). Protein kinase A mediates glucagon‐like peptide 1‐induced nitric oxide production and muscle microvascular recruitment. Am J Physiol Endocrinol Metab 304, E222–E228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggleston EM, Jahn LA & Barrett EJ (2007). Hyperinsulinemia rapidly increases human muscle microvascular perfusion but fails to increase muscle insulin clearance: evidence that a saturable process mediates muscle insulin uptake. Diabetes 56, 2958–2963. [DOI] [PubMed] [Google Scholar]
- Elrick H, Stimmler L, Hlad C Jr & Arai Y (1964). Plasma insulin response to oral and intravenous glucose administration. J Clin Endocrinol Metab 24, 1076–1082. [DOI] [PubMed] [Google Scholar]
- Emanuel AL, de Clercq NC, Koopen AM, van Poelgeest E, Serlie MJ, van Raalte DH, Kramer MH, Nieuwdorp M, Eringa EC & Serné EH (2018). Iloprost infusion prevents the insulin‐induced reduction in skeletal muscle microvascular blood volume but does not enhance peripheral glucose uptake in type 2 diabetic patients. Diabetes Obes Metab 20, 2523–2531. [DOI] [PubMed] [Google Scholar]
- Frayn K & Karpe F (2014). Regulation of human subcutaneous adipose tissue blood flow. Int J Obes 38, 1019–1026. [DOI] [PubMed] [Google Scholar]
- Giugliano D, Marfella R, Coppola L, Verrazzo G, Acampora R, Giunta R, Nappo F, Lucarelli C & D'Onofrio F (1997). Vascular effects of acute hyperglycemia in humans are reversed by L‐arginine: evidence for reduced availability of nitric oxide during hyperglycemia. Circulation 95, 1783–1790. [DOI] [PubMed] [Google Scholar]
- Góralska J, Raźny U, Polus A, Dziewońska A, Gruca A, Zdzienicka A, Dembińska‐Kieć A, Solnica B, Micek A & Kapusta M (2020). Enhanced GIP secretion in obesity is associated with biochemical alteration and miRNA contribution to the development of liver steatosis. Nutrients 12, 476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gremlich S, Porret A, Cherif D, Vionnet N, Froguel P & Thorens B (1995). Cloning, functional expression, and chromosomal localization of the human pancreatic islet glucose‐dependent insulinotropic polypeptide receptor. Diabetes 44, 1202–1208. [DOI] [PubMed] [Google Scholar]
- Horton WB, Jahn LA, Hartline LM, Aylor KW, Patrie JT & Barrett EJ (2020). Hyperglycemia does not inhibit insulin's effects on microvascular perfusion in healthy humans: a randomized crossover study. Am J Physiol Endocrinol Metab 319, E753–E762. [DOI] [PubMed] [Google Scholar]
- Hu D, Remash D, Russell RD, Greenaway T, Rattigan S, Squibb KA, Jones G, Premilovac D, Richards SM & Keske MA (2018). Impairments in adipose tissue microcirculation in type 2 diabetes mellitus assessed by real‐time contrast‐enhanced ultrasound. Circ Cardiovasc Imaging 11, e007074. [DOI] [PubMed] [Google Scholar]
- Jahn LA, Hartline L, Rao N, Logan B, Kim JJ, Aylor K, Gan L‐M, Westergren HU & Barrett EJ (2016). Insulin enhances endothelial function throughout the arterial tree in healthy but not metabolic syndrome subjects. J Clin Endocrinol Metab 101, 1198–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazakos K (2011). Incretin effect: Glp‐1, Gip, Dpp4. Diabetes Res Clin Pract 93, S32–S36. [DOI] [PubMed] [Google Scholar]
- Keske MA, Clerk LH, Price WJ, Jahn LA & Barrett EJ (2009). Obesity blunts microvascular recruitment in human forearm muscle following a mixed meal. Diabetes Care 32, 1672–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim DM, Park KY, Hwang WM, Kim JY & Kim BJ (2017). Difference in protective effects of GIP and GLP‑1 on endothelial cells according to cyclic adenosine monophosphate response. Exp Ther Med 13, 2558–2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer RI, Serné EH, Korkmaz HI, van der Peet DL, de Boer MP, Niessen HW, van Hinsbergh VW, Yudkin JS, Smulders YM & Eringa EC (2015). Insulin‐induced changes in skeletal muscle microvascular perfusion are dependent upon perivascular adipose tissue in women. Diabetologia 58, 1907–1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyawaki K, Yamada Y, Ban N, Ihara Y, Tsukiyama K, Zhou H, Fujimoto S, Oku A, Tsuda K & Toyokuni S (2002). Inhibition of gastric inhibitory polypeptide signaling prevents obesity. Nat Med 8, 738–742. [DOI] [PubMed] [Google Scholar]
- Parker L, Morrison DJ, Betik AC, Roberts‐Thomson K, Kaur G, Wadley GD, Shaw CS & Keske MA (2020). High‐glucose mixed‐nutrient meal ingestion impairs skeletal muscle microvascular blood flow in healthy young men. Am J Physiol Endocrinol Metab 318, E1014–E1021. [DOI] [PubMed] [Google Scholar]
- Renaudin C, Michoud E, Lagarde M & Wiernsperger N (1999). Impaired microvascular responses to acute hyperglycemia in type I diabetic rats. J Diabetes Complications 13, 39–44. [DOI] [PubMed] [Google Scholar]
- Renaudin C, Michoud E, Rapin J, Lagarde M & Wiernsperger N (1998). Hyperglycaemia modifies the reaction of microvessels to insulin in rat skeletal muscle. Diabetologia 41, 26–33. [DOI] [PubMed] [Google Scholar]
- Roberts‐Thomson KM, Betik AC, Premilovac D, Rattigan S, Richards SM, Ross RM, Russell RD, Kaur G, Parker L & Keske MA (2020). Postprandial microvascular blood flow in skeletal muscle: Similarities and disparities to the hyperinsulinaemic‐euglycaemic clamp. Clin Exp Pharmacol Physiol 47, 725–737. [DOI] [PubMed] [Google Scholar]
- Ross R, Kolka C, Rattigan S & Clark M (2007). Acute blockade by endothelin‐1 of haemodynamic insulin action in rats. Diabetologia 50, 443–451. [DOI] [PubMed] [Google Scholar]
- Russell RD, Hu D, Greenaway T, Blackwood SJ, Dwyer RM, Sharman JE, Jones G, Squibb KA, Brown AA & Otahal P (2017). Skeletal muscle microvascular‐linked improvements in glycemic control from resistance training in individuals with type 2 diabetes. Diabetes Care 40, 1256–1263. [DOI] [PubMed] [Google Scholar]
- Russell RD, Hu D, Greenaway T, Sharman JE, Rattigan S, Richards SM & Keske MA (2018). Oral glucose challenge impairs skeletal muscle microvascular blood flow in healthy people. Am J Physiol Endocrinol Metab 315, E307–E315. [DOI] [PubMed] [Google Scholar]
- Russell RD, Roberts‐Thomson KM, Hu D, Greenaway T, Betik AC, Parker L, Premilovac D, Rattigan S, Richards SM, Wadley GD & Keske MA (2022) Impaired postprandial skeletal muscle vascular responses to a mixed meal challenge in normoyglycemic people with a parent with type 2 diabetes. Diabetologia 65, 216–225 [DOI] [PubMed] [Google Scholar]
- Sjøberg KA, Holst JJ, Rattigan S, Richter EA & Kiens B (2013). GLP‐1 increases microvascular recruitment but not glucose uptake in human and rat skeletal muscle. Am J Physiol Endocrinol Metab 306, E355–E362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiller GA, Jensen CD, Pattison T, Chuck CS, Whittam JH & Scala J (1987). Effect of protein dose on serum glucose and insulin response to sugars. Am J Clin Nutr 46, 474–480. [DOI] [PubMed] [Google Scholar]
- Subaran SC, Sauder MA, Chai W, Jahn LA, Fowler DE, Aylor KW, Basu A & Liu Z (2014). GLP‐1 at physiological concentrations recruits skeletal and cardiac muscle microvasculature in healthy humans. Clin Sci 127, 163–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan AW, Subaran SC, Sauder MA, Chai W, Jahn LA, Fowler DE, Patrie JT, Aylor KW, Basu A & Liu Z (2018). GLP‐1 and insulin recruit muscle microvasculature and dilate conduit artery individually but not additively in healthy humans. J Endocr Soc 2, 190–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesfamariam B, Brown ML, Deykin D & Cohen RA (1990). Elevated glucose promotes generation of endothelium‐derived vasoconstrictor prostanoids in rabbit aorta. J Clin Invest 85, 929–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin L, Simonsen L & Bülow J (2010). Real‐time contrast‐enhanced ultrasound determination of microvascular blood volume in abdominal subcutaneous adipose tissue in man. Evidence for adipose tissue capillary recruitment. Clin Physiol Funct Imaging 30, 447–452. [DOI] [PubMed] [Google Scholar]
- Tuor U, Kondysar M & Harding R (1988). Effect of angiotensin II and peptide YY on cerebral and circumventricular blood flow. Peptides 9, 141–149. [DOI] [PubMed] [Google Scholar]
- Vincent M, Barrett E, Lindner J, Clark M & Rattigan S (2003). Inhibiting NOS blocks microvascular recruitment and blunts muscle glucose uptake in response to insulin. Am J Physiol Endocrinol Metab 285, E123–E129. [DOI] [PubMed] [Google Scholar]
- Vincent MA, Clerk LH, Lindner JR, Klibanov AL, Clark MG, Rattigan S & Barrett EJ (2004). Microvascular recruitment is an early insulin effect that regulates skeletal muscle glucose uptake in vivo. Diabetes 53, 1418–1423. [DOI] [PubMed] [Google Scholar]
- Vincent MA, Clerk LH, Lindner JR, Price WJ, Jahn LA, Leong‐Poi H & Barrett EJ (2006). Mixed meal and light exercise each recruit muscle capillaries in healthy humans. Am J Physiol Endocrinol Metab 290, E1191–E1197. [DOI] [PubMed] [Google Scholar]
- Wagenmakers AJ (2016). Impact of physical activity, ageing, obesity and metabolic syndrome on muscle microvascular perfusion and endothelial metabolism. J Physiol 594, 2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Tan AW, Jahn LA, Hartline L, Patrie JT, Lin S, Barrett EJ, Aylor KW & Liu Z (2020). Vasodilatory actions of glucagon‐like peptide 1 are preserved in skeletal and cardiac muscle microvasculature but not in conduit artery in obese humans with vascular insulin resistance. Diabetes Care 43, 634–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SB, Goldfine AB, Timimi FK, Ting HH, Roddy M‐A, Simonson DC & Creager MA (1998). Acute hyperglycemia attenuates endothelium‐dependent vasodilation in humans in vivo. Circulation 97, 1695–1701. [DOI] [PubMed] [Google Scholar]
- Wu R, Dong W, Zhou M, Cui X, Hank Simms H & Wang P (2005). Ghrelin improves tissue perfusion in severe sepsis via downregulation of endothelin‐1. Cardiovasc Res 68, 318–326. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Peer Review History
Statistical Summary Document
Data Availability Statement
Data generated for the current study are available on reasonable request from the corresponding author in the form of excel spreadsheets.


