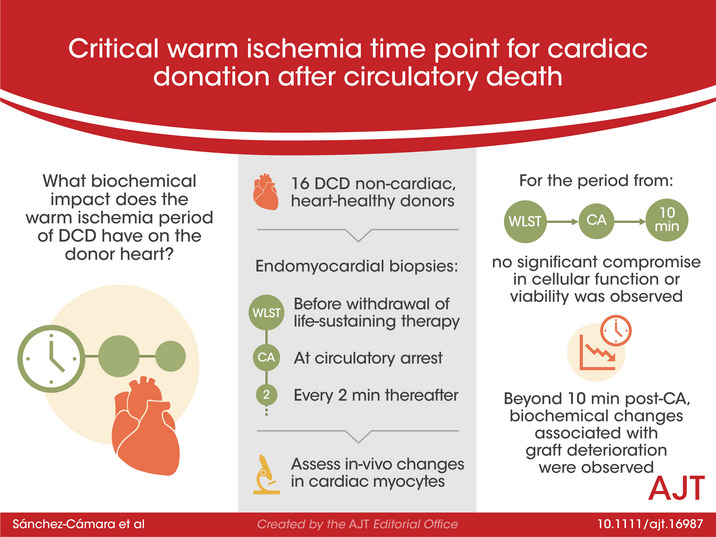
Abstract
Donation after circulatory death (DCD) represents a promising opportunity to overcome the relative shortage of donors for heart transplantation. However, the necessary period of warm ischemia is a concern. This study aims to determine the critical warm ischemia time based on in vivo biochemical changes. Sixteen DCD non‐cardiac donors, without cardiovascular disease, underwent serial endomyocardial biopsies immediately before withdrawal of life‐sustaining therapy (WLST), at circulatory arrest (CA) and every 2 min thereafter. Samples were processed into representative pools to assess calcium homeostasis, mitochondrial function and cellular viability. Compared to baseline, no significant deterioration was observed in any studied parameter at the time of CA (median: 9 min; IQR: 7–13 min; range: 4–19 min). Ten min after CA, phosphorylation of cAMP‐dependent protein kinase‐A on Thr197 and SERCA2 decreased markedly; and parallelly, mitochondrial complex II and IV activities decreased, and caspase 3/7 activity raised significantly. These results did not differ when donors with higher WLST to CA times (≥9 min) were analyzed separately. In human cardiomyocytes, the period from WLST to CA and the first 10 min after CA were not associated with a significant compromise in cellular function or viability. These findings may help to incorporate DCD into heart transplant programs.
Keywords: cardiac contractility, cardiac procurement, donation after circulatory death, normothermic regional perfusion
In‐vivo monitoring of changes in cardiac myocytes during donation after circulatory death shows that the time period from withdrawal of life‐sustaining therapy through ten minutes after cardiac arrest is not associated with significant compromise in cellular function or viability, supporting this duration of warm ischemia as safe for heart donation.

Abbreviations
- CA
circulatory arrest
- DBD
donation after brain death
- DCD
donation after circulatory death
- DPP
direct procurement with extra‐corporeal perfusion systems
- ECMO
extracorporeal membrane oxygenation
- ECP
extracorporeal perfusion
- EMB
endomyocardial biopsies
- HTx
heart transplantation
- LST
life‐sustaining therapy
- NRP
normothermic regional perfusion
- PKA
cAMP‐dependent protein kinase A
- PLN
phospholamban
- SERCA 2a
sarcoplasmic reticulum Ca2+‐ATPase
- SR
sarcoplasmic reticulum
- TA
thoraco‐abdominal
- WIT
warm ischemia time
- WLST
withdrawal of life‐sustaining therapy
1. INTRODUCTION
Heart transplantation remains the gold standard for treating end‐stage heart failure; it results in survival at least one year after transplantation in nearly 90% of cases. 1 However, there is a relative shortage of donation after brain death (DBD) compared to the growing number of potential cardiac recipients. Indeed, the need for a fully functional organ is the major limitation to expanding the heart pool by including suboptimal donors. 2
In this context, the possibility of expanding heart transplantation via donation after circulatory death (DCD) is promising. 3 In the last ten years, controlled DCD has increased the pool of abdominal organs and lungs, but this is not the case for hearts. Including DCD donors could increase the number of heart transplants by 15%–30%. 4 , 5 , 6 However, unlike conventional DBD, DCD organs undergo a period of warm ischemia during the withdrawal of life‐sustaining therapy (WLST) and between circulatory arrest (CA) and heart procurement. This raises concern about graft functionality and quality. 7 , 8 , 9 Seeing positive results from pioneering work in Australia and United Kingdom, several groups have published case series based on different protocols, which may include direct procurement using extra‐corporeal perfusion systems (DPP) or in‐situ normothermic regional perfusion (NRP). 10 , 11 , 12 , 13 , 14 In this context, it is of paramount importance to identify the time point when myocardial function decreases and the risk of graft dysfunction increases. 15 Determining this time point could greatly assist heart transplant teams by expanding the possibility of heart procurement from DCD. However, the time point associated with irreversible myocardial cell injury is currently unknown. 3 , 14 , 15
Therefore, the present study aims to determine the critical warm ischemia time (WIT) based on in‐vivo close monitoring of biochemical changes in cardiac myocytes during the process of DCD of abdominal organs and lungs. We focused on changes closely related to contractility and functional viability, including those implicated in the regulation of calcium homeostasis, mitochondrial energetics, and apoptotic cell death. 16 , 17 , 18
2. MATERIALS AND METHODS
2.1. Population and study design
This is a prospective single‐center study. The study protocol was approved by the local Clinical Research Ethics Committee (Exp 2016‐2‐13‐HCUVA). All consecutive DCD donors in type III asystole were screened during a 24‐month period from June 2017 to June 2019. Participants who met all the following inclusion criteria were included: age 18 to 75 years, no structural heart disease (assessed by echocardiography at the time of donation), and signed informed consent was provided by the legal representatives according to donor regulations.
The DCD procedure followed the institutional protocol and national legislation in Spain (Organic Law 3/2018, December 5, on the Protection of Personal Data and Guarantee of Digital Rights, BOE No. 294). CA is defined as circulatory cessation with mechanical asystole (loss of femoral pulse). A 5‐min observational “stand‐off” period is legally required before death may be certified. In all DCD donors, endomyocardial biopsies (EMB) were performed immediately before WLST, at the time of CA, and every 2 min thereafter for the next 30 min. The collection of EMB did not interfere with the DCD process.
2.2. Collection of myocardial samples
EMB samples were collected in the operating room by skilled personnel familiar with the procedure. A long sheath 7‐Fr 43 cm guide catheter was inserted through the internal jugular vein and positioned at the apex of the right ventricle before mechanical ventilation support was removed. To ensure proper positioning of the catheter, pressure curves and direct visualization via echocardiography were used to guide the entire procedure. A 50‐cm biopsy forceps clamp (Cardinal Health, Inc.) was used to obtain EMB samples, preferentially from the right ventricular septum. The samples were immediately placed in cryotubes and immersed in liquid nitrogen in a portable container for subsequent biobanking (Biobank Network of the Region of Murcia, BIOBANC‐MUR, registered on the Registro Nacional de Biobancos under registration number B.0000859. BIOBANC‐MUR is supported by the Instituto de Salud Carlos III, PT20/00109).
2.3. Preparation of tissue samples
The samples were prepared in a cold room at 4ºC. Frozen samples of human heart tissue were pooled at intervals of 5 min to ensure a sufficient volume of myocardium for the planned experiments. The samples were homogenized in liquid nitrogen using a pestle and mortar in a lysis buffer (20 mM Tris‐HCl pH 8.0, 150 mM NaCl, 2 mM EDTA, 2 mM EGTA, 10% [v/v] glycerol, 0.1% [w/v] SDS, 1% [v/v] NP‐40, and 1% [w/v] sodium deoxycholate), supplemented with protease inhibitors and phosphatase inhibitors. The homogenate was incubated on ice for 20 min and centrifuged at 16 100g for 20 min at 4°C. The supernatant was subjected to a colorimetric bicinchoninic acid (BCA) protein assay to quantify the protein concentration.
2.4. Analysis of calcium homeostasis, mitochondrial function, and cellular death
2.4.1. Calcium homeostasis
SDS‐Polyacrylamide gel electrophoresis (PAGE) and western blots were used to evaluate the phosphorylation of cAMP‐dependent protein kinase A (PKA) and phospholamban (PLN), two proteins related to intracellular calcium transients. The human heart lysates (20 μg) were separated via SDS‐PAGE, transferred to a PVDF membrane, and probed overnight with primary antibodies for GADPH (Merck, 8795,1:5000), Phospho‐Thr197/PKA (Cell‐Signaling Technology, 4781, 1:1000), PKA (Cell‐Signaling Technology, 4782,1:1000), and phospho‐Ser16/PLN (Badrilla, A010‐12AP, 1:3000). Bands were visualized using enhanced chemiluminescent ECL (Amersham ECLTM Primer Western Blotting Detection Reagent (GE Healthcare) (RPN2232) in a ChemiDoc XRS+system using Image Lab software from Bio‐Rad Laboratories.
2.4.2. Mitochondrial function
Electron transport chain activity, including the activity of succinate dehydrogenase (complex II; cat. no. ab109908) and of cytochrome c oxidase (complex IV; cat. no. ab109909) were analyzed using commercial kits (Abcam) according to the manufacturer's instructions. The samples were prepared according to the instructions and placed in an anti‐Complex II or IV monoclonal antibody‐coated 96‐well plate. Absorbance was measured at OD600 nm in kinetic mode for 60 min at 1‐min intervals. The slope obtained for each sample was used to evaluate enzymatic activity, which is presented as a comparison to baseline conditions (before circulatory arrest).
2.4.3. Cellular death assay
Caspase 3/7 activation was measured using luminescent assays (Caspase‐Glo® 3/7, Promega). First, 100 µg of each human cardiac lysate were placed into 96‐well plates, and 100 μl of Caspase‐Glo® 3/7 reagent was added to each well. The samples were then mixed for 30 s. After 30 min of incubation at room temperature, the luminescence of each sample was measured using the Synergy‐HT multi‐detection plate reader (BioTek). Caspase activity was measured using luminescence raw values to obtain a relative‐to‐control value. Final caspase activity was calculated by averaging three replicates from two independent experiments.
2.5. Statistical analysis
The variables are described using mean ± standard error or frequency (%), as appropriate.
The evolution of each biomarker over time is represented in spaghetti plots (each line shows one patient). Means with standard errors are shown as points with error bars for each time period. A linear mixed model was fitted for each variable (Table S1), considering time as categorical covariate (fixed effects) as well as the intercept, which varies from patient to patient (random effects). Same model was used for the sensitivity analysis, where time from WLST to CA (above or below the median time, 9 min) was included as covariate. The estimates are highlighted (***) from the first significant one in each plot. Data analyses and graphics were performed using the statistical software R, version 4.0.
3. RESULTS
3.1. Characteristics of the DCD population
From a total of 56 DCD donors who were handled during the study period, 26 subjects did not meet the inclusion criteria. Of the 30 DCD donors who met the inclusion criteria, 14 subjects were excluded for other reasons: either family refusal (n = 2) or logistical or technical problems (n = 12). The final sample population comprised 16 DCD donors. The characteristics of the DCD donor cohort are shown in Table 1. For most DCD donors, the cause of WLST was intracranial hemorrhage. The median time from WLST to CA was 9 min (25th‐75th percentile: 7–13 min; range: 4–19 min). Hypoperfusion (systolic blood pressure <60 mmHg) occurred at a median of 5.5 min before CA.
TABLE 1.
Clinical characteristics of study DCD cohort
| Variable | n = 16 |
|---|---|
| Female | 4 (25.0) |
| Age, years | 63.3 ± 11.7 |
| Body mass index | 27.0 ± 4.2 |
| Body surface | 1.9 ± 0.2 |
| Cause of WLST | |
| Intracranial hemorrhage | 10 (61.4) |
| Ischemic stroke | 2 (12.5) |
| Traumatic brain injury | 1 (6.2) |
| Hypoxic brain injury | 3 (18.6) |
| Length of LST, days | 9.0 (4.3–11.5) |
| Mechanical ventilation, n (%) | 16 (100) |
| Catecholamines, n (%) | 8 (50) |
| WLST to CA, min | 9.0 (7.0–13.0) |
| SBP<60 mmHg to CA, min | 5.5 (3.8–7.0) |
| Myocardial samples per patient, n | 30.5 (24.8–35.0) |
Data are expressed as n = number (%), median (25th‐75th percentiles), and mean ± standard deviation.
Abbreviations: CA, circulatory arrest; LST, life‐support therapies; min, minutes; WLST, withdrawal of life‐sustaining therapy .
3.2. Cardiac contractility
The viability of cardiac contractility was evaluated based on PKA activation (Figure 1). The phosphorylation of PKA at CA (0–5 min) and within the first 10 min after CA (5–10 min) was similar to the baseline (before WLST). The phosphorylation of PKA on its activating residue Thr197 (panel A) significantly decreased 10 min after CA (p < .001). The phosphorylation of PLN at Ser16 site (panel B), a regulated protein of SERCA 2a, was similar. Western blots probed with a specific antibody demonstrated that PLN phosphorylation at Ser16 (p‐Ser16PLN) in cardiac tissue decreased markedly 10 min after CA compared to the control assay (supplemental material). Similarly, total monomeric PLN (total PLNm) in the cardiac tissue increased starting 10 min after CA (Supplemental Figure S1).
FIGURE 1.
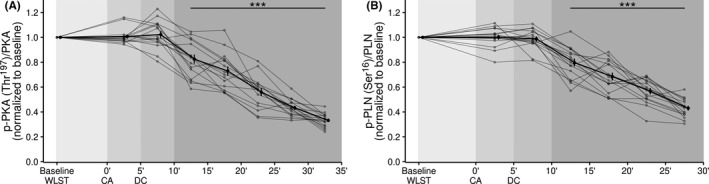
Temporal changes to protein kinase and phospholamban state. (A) PKA phosphorylation levels relative to total PKA expressed myocardium. (B) PLN phosphorylation levels relative to total PLN expressed myocardium. Data are shown as mean ± SE. ***p < .001, compared to baseline for that patient. CA, circulatory arrest; DC, death certification; PKA, protein kinase A; PLN, phospholamban; Ser, Serine; Thr, threonine; WLST, withdrawal of life‐sustaining therapy
3.3. Mitochondrial function
The activities of complex II and complex IV in the mitochondrial electron transport chain were evaluated to measure mitochondrial dysfunction. As shown in Figure 2, mitochondrial complex II and IV activities remained unchanged at the time of CA compared to baseline. CA resulted in a time‐dependent decrease in complex II activity; this decrease became significant 10 min after CA (panel A). Similar results were obtained for complex IV activity (panel B).
FIGURE 2.
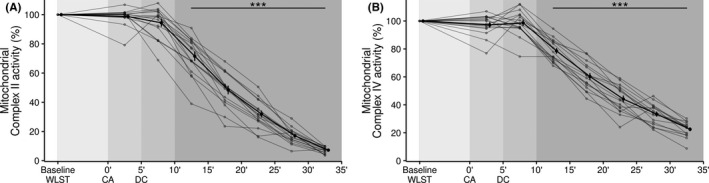
Temporal changes in mitochondrial complexes II and IV. (A) Complex II activity. (B) Complex IV activity. Data are shown as mean ± SE. ***p < .001, compared to baseline. CA, circulatory arrest; DC, death certification; WLST, withdrawal of life‐sustaining therapy
3.4. Apoptotic cell death
Activation of the apoptotic death program was assessed based on caspase 3/7 activity. As shown in Figure 3, the activity of both caspases remained unchanged at the time of CA (compared to WLST) and increased significantly 11 to 15 min after CA.
FIGURE 3.
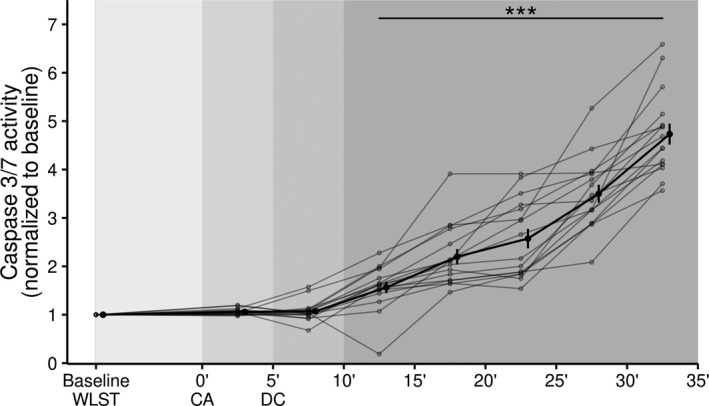
Temporal changes in caspase 3 and 7 activity as a marker of apoptotic death. Data are shown as mean ± SE. ***p < .001, compared with baseline. CA, circulatory arrest; DC, death certification; WLST, withdrawal of life‐sustaining therapy
3.5. Sensitivity analysis based on differences in WLST to CA time
We performed a sensitivity analysis by analyzing the samples from donors whose times from WLST to CA were above and below the median time (9 min) separately (Supplementary Table S1). In this analysis, we found no differences between subjects with shorter (4–8 min) and longer (9–19 min) times in terms of contractility, mitochondrial function, or apoptotic cell death (Figure 4).
FIGURE 4.
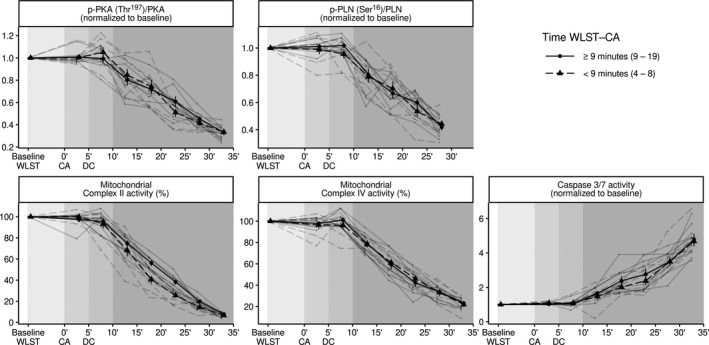
Sensitivity analysis using WLST to CA times below (circle) and above (triangle) the median value (9 min) for all studied parameters
4. DISCUSSION
The results of this study suggest that myocardial contractility and cellular viability are significantly compromised 10 min after CA. The WLST period before CA did not affect the processes examined here. These findings are consistent for calcium homeostasis, mitochondrial integrity, and apoptotic cellular death. Therefore, the present findings suggest that, in human cardiomyocytes, the period from WLST to CA and the first 10 min after CA are not associated with a significant compromise in cellular function or viability. Beyond this window of time, the risk of graft dysfunction might increase significantly.
Hearts from DBD are not exposed to warm ischemia because of the controlled organ‐retrieval process. The main concern associated with the use of hearts from controlled DCD, or even suboptimal donors who undergo uncontrolled circulatory death followed by cardiac resuscitation, is exposure to warm ischemia and the subsequent risk of graft failure. During controlled DCD, heart grafts undergo two necessary insults. The first occurs after WLST due to the progression to severe hypoxia and hypoperfusion, and the second occurs after CA and includes the mandatory 5‐min stand‐off period to confirm permanent cessation of circulation and to certify death. Therefore, the length of time after CA when graft procurement may still be considered safe is key to the success of heart transplantations from DCD donors.
The present study has demonstrated that, from a biochemical perspective, the period of warm ischemia associated with the WLST before CA occurs is not directly associated with detrimental changes in myocardial functionality. In a porcine model, Iyer et al. described hemodynamic changes accompanied by a rapid fall in systemic pH, increases in blood lactate and troponin T, and right atrial and ventricular distension. Those findings suggest that the period of warm ischemia due to WLST may result in irreversible myocardial injury. 19 Our study is the first to evaluate this question in a human model of DCD donation. Despite progressive hemodynamic, respiratory, and metabolic deterioration, our study suggests that the period from WLST to CA is not associated with a significant negative impact on cardiomyocyte functionality or viability. These findings indirectly agree with the reversibility of cardiac function in patients who are resuscitated after a limited period of cardiac arrest. Indeed, several retrospective series have showed excellent results for cardiac transplantation following a period of cardiac arrest in the organ donor (mean: 15 min). 20 , 21 In our population, the median time from WLST to CA was 9 min (range: 4–19 min); this aligns with previous reports by Chew et al. 22 (10 ± 4 min) and Messer et al. 23 (median: 7 min) and is shorter than other small series (range: 8–22 min). 10 , 12 , 24 However, we recognize that times in our cohort were remarkably uniform and shorter than those reported in unselected DCD populations. Indeed, series from UK and Netherlands, including general DCD donors, reported median times from WLST to CA of 36 min and 20 min respectively, ranging from few minutes to hours. 25 , 26 Due to the variability of these times, we performed a sensitivity analysis by analyzing samples from donors with larger and shorter WLST to CA times separately; the results were similar to those obtained when all subjects were considered together. Therefore, these results suggest that warm ischemia begins at CA, and that the idea that functional ischemia begins when systolic blood pressure falls below 50 mmHg may be fabricated. However, these findings may not apply to DCD donors with longer times from WLST to CA (>20 min).
In our study, the main driver for cardiomyocyte loss of function and viability was the period after CA. In a previous study conducted in Australia in which a series of 23 DCD transplants were performed using a DPP approach, 35% of cases required immediate ECMO support due to primary graft failure. 22 In that study, only a longer time from CA to cardioplegia was significantly associated with a higher need for ECMO support (15 ± 3 min vs. 12 ± 2 min, p = 0.002). Therefore, the warm ischemic injury after CA seems to be the major determinant of the risk of graft failure following transplantation.
Several strategies have been proposed to recover heart function and to minimize this risk of graft failure. 14 After CA, an observation period of 5 min without circulation is required in most countries to confirm death. Immediately after death is certified, one of two technical approaches is used to procure the doner heart. The DPP approach involves direct organ procurement, administration of cardioplegia, and ex‐situ heart perfusion. The thoraco‐abdominal NRP strategy (TA‐NRP) involves cannulation to ensure extracorporeal‐driven circulation and in‐situ reanimation of the heart. 23 The Papworth group compares these strategies and finds a median interval of 22 min (range: 21–25) from CA to blood perfusion for DPP and a median interval of 14 min (11–15 min) for TA‐NRP (p < .001). 23 However, the baseline characteristics of the two groups had important differences, such that no conclusion of the impact of different times on clinical outcome could be made. 23 The successful use of TA‐NRP has also been reported in isolated cases in Belgium and Spain, including two cases at our center. 12 , 13 In these protocols, the time from CA to blood reperfusion is around ten minutes (5 min stand‐off period and 5 min for canulation and reperfusion), which is shorter than the time from CA to extra‐corporeal perfusion. Our study aligns with previous findings from 14 centers and more than 150 transplants suggesting that the time after CA is the key component in warm ischemia. 3 Our findings imply that, ideally, the sum of the 5‐min stand‐off period and the time to restore circulation should not exceed a total of 10 min. The use of the TA‐NRP technique, as well as future improvements to this technique, may help reduce this time from CA to restored circulation. However, not all jurisdictions permit the use of TA‐NRP; in such cases, DPP is the only alternative. In these contexts, pharmacological post‐conditioning with modified flush solutions may increase ischemia tolerance. 27
This study has some limitations, particularly due to the use of indirect biochemical measures of contractility and cellular viability. The evidence suggests that PLN/SERCA2a regulatome plays a fundamental role in the effective control of cardiac contractility. 17 , 28 Hence, efficient PLN phosphorylation is an important requirement for the proper management of calcium cycling and myocardial contractility. 29 Mitochondrial organelles play a central role in cardiomyocyte survival and death pathways. 30 , 31 , 32 Apoptotic cell death is the primary form of myocardial damage after CA. In response to the ischemic insult, pro‐apoptotic proteins are released from the intermembrane space of mitochondria into the cytosol; this process culminates in the activation of caspases and the degradation of cellular components. 33 This process requires permeabilization of the mitochondrial outer membrane, an event that is considered the “point of no return” during cell death. 32 Therefore, complexes II and IV have emerged as important factors in the induction of cell death. 34 , 35 After permeabilization of the mitochondrial outer membrane, the cell is doomed to die. The study of systemic abnormalities, metabolic and/or inflammatory, could contribute complementary information to the myocardial findings presented here. Unfortunately, the study design did not include the collection of blood samples.
Another potential limitation of this study is that uncontrolled characteristics of donor and donation procedures, such as age or pharmacological interventions, might influence the observed time course. Indeed, the studied population comprised DCD non‐cardiac donors who were older than conventional cardiac DCD donors. However, it was not possible to study samples from cardiac DCD donors, and the expected impact of older age, if any, would be earlier myocardial dysfunction than that expected in younger donors. This study also did not evaluate the recovery effect of reperfusion or the impact of hypothermic reperfusion or other protective measures. Therefore, our study may suggest time points when the risk of primary graft dysfunction becomes significantly higher, but it cannot identify specific time points when reversibility is probable or when return is no longer possible. Moreover, although we did not find any differences between subjects with longer and shorter periods between WLST and CA, we cannot exclude the possibility that a longer period from WLST to CA could impact these results. However, the consistent findings across all studied parameters support the temporal trends identified here.
Defining this critical time interval may enable the use of DCD as a source of heart grafts. This could improve the logistics of DCD donation and increase the heart donor pool. In addition, these findings might be extended to non‐controlled DCD donation. Our results suggest that cardiac resuscitation within the first 10 min after CA may ensure the viability of the organ. This is also relevant for expanding the pool of suboptimal resuscitated donors in the context of DBD donation.
In conclusion, following WLST, myocardial contractility and cellular viability seem to be preserved for the first 10 min after CA. Beyond this warm ischemia time point, graft function may be significantly compromised. These findings may help transplant teams and facilitate the expansion of cardiac transplantation from DCD donors.
DISCLOSURE
The authors of thismanuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
AUTHOR CONTRIBUTIONS
MCAL and AL conceived the work, generated the experimental data, and wrote and edited the manuscript. AL is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the analysis. SSC, MRV, RJR, JFGP, JAH, and EP were in charge of the DCD protocol design and procedures and of sample collection. FS helped design the experimental procedures and contributed to the discussion of the results. DAPF helped with the analysis, helped write the discussion, and edited the manuscript. AHV performed the statistical analysis of the data.
Supporting information
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by a grant from the Mutua Madrileña (XIV Convocatoria de Ayudas a la Investigación en Salud, 2017). Dr. Lax is a Ramon and Cajal Researcher in the Department of Medicine, University of Murcia. The authors thank Dr. Antonio Parrado from the Genomics Core Facility at the Biomedical Research Institute Virgen de la Arrixaca (IMIB‐Arrixaca) of Murcia (Spain) for allowing us to use the ChemiDoc Imaging System.
Sánchez‐Cámara S, Asensio‐López MC, Royo‐Villanova M, et al. Critical warm ischemia time point for cardiac donation after circulatory death. Am J Transplant. 2022;22:1321–1328. doi: 10.1111/ajt.16987
Silvia Sánchez‐Cámara and Mari C. Asensio‐López contributed equally.
Contributor Information
Antonio Lax, Email: alax@um.es.
Domingo A. Pascual‐Figal, Email: alax@um.es, Email: dpascual@um.es.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Politi P, Piccinelli M, Poli PF, et al. Ten years of “extended” life: quality of life among heart transplantation survivors. Transplantation. 2004;78(2):257‐263. doi: 10.1097/01.TP.0000133537.87951.F2 [DOI] [PubMed] [Google Scholar]
- 2. Kittleson MM, Kobashigawa JA. Cardiac transplantation: current outcomes and contemporary controversies. JACC Hear Fail. 2017;5(12):857‐868. doi: 10.1016/j.jchf.2017.08.021 [DOI] [PubMed] [Google Scholar]
- 3. Domínguez‐Gil B, Ascher N, Capron AM, et al. Expanding controlled donation after the circulatory determination of death: statement from an international collaborative. Intensive Care Med. 2021;47(3):265‐281. doi: 10.1007/s00134-020-06341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Noterdaeme T, Detry O, Hans M‐F, et al. What is the potential increase in the heart graft pool by cardiac donation after circulatory death? Transpl Int. 2013;26(1):61‐66. doi: 10.1111/j.1432-2277.2012.01575.x [DOI] [PubMed] [Google Scholar]
- 5. Messer S, Lannon J, Wong E, et al. The potential of transplanting hearts from donation after circulatory determined Death (DCD) donors within the United Kingdom. J Hear Lung Transplant. 2015;34(4):S275. doi: 10.1016/j.healun.2015.01.772 [DOI] [Google Scholar]
- 6. Macdonald P, Dhital K. Heart transplantation from donation‐after‐circulatory‐death (DCD) donors: back to the future‐Evolving trends in heart transplantation from DCD donors. J Hear Lung Transplant. 2019;38(6):599‐600. doi: 10.1016/j.healun.2019.03.010 [DOI] [PubMed] [Google Scholar]
- 7. Dhital KK, Chew HC, Macdonald PS. Donation after circulatory death heart transplantation. Curr Opin Organ Transplant. 2017;22(3):189‐197. doi: 10.1097/MOT.0000000000000419 [DOI] [PubMed] [Google Scholar]
- 8. Pettit SJ, Petrie MC. Transplantation of hearts donated after circulatory‐determined death: a song of fire and ice. Circ Hear Fail. 2019;12(4): doi: 10.1161/CIRCHEARTFAILURE.119.005991 [DOI] [PubMed] [Google Scholar]
- 9. Mehra MR. Challenges, diligence, and a breakthrough in donation after circulatory death in heart transplantation. J Hear Lung Transplant. 2017;36(12):1319‐1321. doi: 10.1016/j.healun.2017.10.022 [DOI] [PubMed] [Google Scholar]
- 10. Dhital KK, Iyer A, Connellan M, et al. Adult heart transplantation with distant procurement and ex‐vivo preservation of donor hearts after circulatory death: a case series. Lancet. 2015;385(9987):2585‐2591. doi: 10.1016/S0140-6736(15)60038-1 [DOI] [PubMed] [Google Scholar]
- 11. Messer S, Page A, Axell R, et al. Outcome after heart transplantation from donation after circulatory‐determined death donors. J Hear Lung Transplant. 2017;36(12):1311‐1318. doi: 10.1016/j.healun.2017.10.021 [DOI] [PubMed] [Google Scholar]
- 12. Tchana‐Sato V, Ledoux D, Detry O, et al. Successful clinical transplantation of hearts donated after circulatory death using normothermic regional perfusion. J Hear Lung Transplant. 2019;38(6):593‐598. doi: 10.1016/j.healun.2019.02.015 [DOI] [PubMed] [Google Scholar]
- 13. Miñambres E, Royo‐Villanova M, Pérez‐Redondo M, et al. Spanish experience with heart transplants from controlled donation after the circulatory determination of death using thoraco‐abdominal normothermic regional perfusion and cold storage. Am J Transplant. 2021;21(4):1597‐1602. doi: 10.1111/ajt.16446 [DOI] [PubMed] [Google Scholar]
- 14. Anguela‐Calvet L, Moreno‐Gonzalez G, Sbraga F, Gonzalez‐Costello J, Tsui S, Oliver‐Juan E. Heart donation from donors after controlled circulatory death. Transplantation. 2020;105(7):1482‐1491. doi: 10.1097/tp.0000000000003545 [DOI] [PubMed] [Google Scholar]
- 15. Niederberger P, Farine E, Raillard M, et al. Heart transplantation with donation after circulatory death: what have we learned from preclinical studies? Circ Hear Fail. 2019;12(4):5517. doi: 10.1161/CIRCHEARTFAILURE.118.005517 [DOI] [PubMed] [Google Scholar]
- 16. Traaseth NJ, Thomas DD, Veglia G. Effects of Ser16 phosphorylation on the allosteric transitions of phospholamban/Ca2+‐ATPase complex. J Mol Biol. 2006;358(4):1041‐1050. doi: 10.1016/j.jmb.2006.02.047 [DOI] [PubMed] [Google Scholar]
- 17. Kranias EG, Hajjar RJ. Modulation of cardiac contractility by the phopholamban/SERCA2a regulatome. Circ Res. 2012;110(12):1646‐1660. doi: 10.1161/CIRCRESAHA.111.259754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lax A, Soler F, Fernández‐Belda F. Mitochondrial damage as death inducer in heart‐derived H9c2 cells: more than one way for an early demise. J Bioenerg Biomembr. 2009;41(4):369‐377. doi: 10.1007/s10863-009-9236-4 [DOI] [PubMed] [Google Scholar]
- 19. Iyer A, Chew HC, Gao L, et al. Pathophysiological trends during withdrawal of life support: implications for organ donation after circulatory death. Transplantation. 2016;100(12):2621‐2629. doi: 10.1097/TP.0000000000001396 [DOI] [PubMed] [Google Scholar]
- 20. Ali AA, Lim E, Thanikachalam M, et al. Cardiac arrest in the organ donor does not negatively influence recipient survival after heart transplantation. Eur J Cardiothorac Surg. 2007;31(5):930‐934. doi: 10.1016/J.EJCTS.2007.01.074 [DOI] [PubMed] [Google Scholar]
- 21. Galeone A, Varnous S, Lebreton G, et al. Impact of cardiac arrest resuscitated donors on heart transplant recipients’ outcome. J Thorac Cardiovasc Surg. 2017;153(3):622‐630. doi: 10.1016/J.JTCVS.2016.10.079 [DOI] [PubMed] [Google Scholar]
- 22. Chew HC, Iyer A, Connellan M, et al. Outcomes of donation after circulatory death heart transplantation in Australia. J Am Coll Cardiol. 2019;73(12):1447‐1459. doi: 10.1016/j.jacc.2018.12.067 [DOI] [PubMed] [Google Scholar]
- 23. Messer S, Cernic S, Page A, et al. A 5‐year single‐center early experience of heart transplantation from donation after circulatory‐determined death donors. J Hear Lung Transplant. 2020;39(12):1463‐1475. doi: 10.1016/j.healun.2020.10.001 [DOI] [PubMed] [Google Scholar]
- 24. Vandendriessche K, Tchana‐Sato V, Ledoux D, et al. Transplantation of donor hearts after circulatory death using normothermic regional perfusion and cold storage preservation. Eur J Cardio‐Thoracic Surg. 2021;60(4):813‐819. doi: 10.1093/ejcts/ezab139 [DOI] [PubMed] [Google Scholar]
- 25. Wind J, Snoeijs MGJ, Brugman CA, et al. Prediction of time of death after withdrawal of life‐sustaining treatment in potential donors after cardiac death. Crit Care Med. 2012;40(3):766‐769. doi: 10.1097/CCM.0b013e318232e2e7 [DOI] [PubMed] [Google Scholar]
- 26. Suntharalingam C, Sharples L, Dudley C, Bradley JA, Watson CJE. Time to cardiac death after withdrawal of life‐sustaining treatment in potential organ donors. Am J Transplant. 2009;9(9):2157‐2165. doi: 10.1111/j.1600-6143.2009.02758.x [DOI] [PubMed] [Google Scholar]
- 27. Iyer A, Gao L, Doyle A, et al. Increasing the tolerance of DCD hearts to warm ischemia by pharmacological postconditioning. Am J Transplant. 2014;14(8):1744‐1752. doi: 10.1111/ajt.12782 [DOI] [PubMed] [Google Scholar]
- 28. Periasamy M, Bhupathy P, Babu GJ. Regulation of sarcoplasmic reticulum Ca2+ ATPase pump expression and its relevance to cardiac muscle physiology and pathology. Cardiovasc Res. 2008;77(2):265‐273. doi: 10.1093/cvr/cvm056 [DOI] [PubMed] [Google Scholar]
- 29. Koss KL, Kranias EG. Phospholamban: a prominent regulator of myocardial contractility. Circ Res. 1996;79(6):1059‐1063. doi: 10.1161/01.RES.79.6.1059 [DOI] [PubMed] [Google Scholar]
- 30. Ricci JE, Waterhouse N, Green DR. Mitochondrial functions during cell death, a complex (I‐V) dilemma. Cell Death Differ. 2003;10(5):488‐492. doi: 10.1038/sj.cdd.4401225 [DOI] [PubMed] [Google Scholar]
- 31. Singh R, Letai A, Sarosiek K. Regulation of apoptosis in health and disease: the balancing act of BCL‐2 family proteins. Nat Rev Mol Cell Biol. 2019;20(3):175‐193. doi: 10.1038/s41580-018-0089-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Flores‐Romero H, Ros U, Garcia‐Saez AJ. Pore formation in regulated cell death. EMBO J. 2020;39(23): doi: 10.15252/embj.2020105753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lakhani SA, Masud A, Kuida K, et al. Caspases 3 and 7: key mediators of mitochondrial events of apoptosis. Science (80‐). 2006;311(5762):847‐851. doi: 10.1126/science.1115035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pfleger J, He M, Abdellatif M. Mitochondrial complex II is a source of the reserve respiratory capacity that is regulated by metabolic sensors and promotes cell survival. Cell Death Dis. 2015;6(7):e1835. doi: 10.1038/cddis.2015.202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Grimm S. Respiratory chain complex II as general sensor for apoptosis. Biochim Biophys Acta Bioenerg. 2013;1827(5):565‐572. doi: 10.1016/j.bbabio.2012.09.009 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


