Abstract
The role of the β‐adrenoceptors (β‐ARs) in hypoxia‐driven diseases has gained visibility after the demonstration that propranolol promotes the regression of infantile hemangiomas and ameliorates the signs of retinopathy of prematurity (ROP). Besides the role of β2‐ARs, preclinical studies in ROP have also revealed that β3‐ARs are upregulated by hypoxia and that they are possibly involved in retinal angiogenesis. In a sort of figurative round trip, peculiarities typical of ROP, where hypoxia drives retinal neovascularization, have been then translated to cancer, a disease equally characterized by hypoxia‐driven angiogenesis. In this step, investigating the role of β3‐ARs has taken advantage of the assumption that cancer growth uses a set of strategies in common with embryo development. The possibility that hypoxic induction of β3‐ARs may represent one of the mechanisms through which primarily embryo (and then cancer, as an astute imitator) adapts to grow in an otherwise hostile environment, has grown evidence. In both cancer and embryo, β3‐ARs exert similar functions by exploiting a metabolic shift known as the Warburg effect, by acquiring resistance against xenobiotics, and by inducing a local immune tolerance. An additional potential role of β3‐AR as a marker of stemness has been suggested by the finding that its antagonism induces cancer cell differentiation evoking that β3‐ARs may help cancer to grow in a nonhospital environment, a strategy also exploited by embryos. From cancer, the round trip goes back to neonatal diseases for which new possible interpretative keys and potential pharmacological perspectives have been suggested.
Keywords: oxygen sensing receptor, oxygen‐related prematurity diseases, treatment approach, vessel proliferation/regression, β‐adrenergic system
Abbreviations
- CNS
central nervous system
- DA
ductus arteriosus
- eNOS
endothelial isoform of NOS
- HIF‐1
hypoxia‐inducible factor‐1
- IH
infantile hemangioma
- iNOS
nitric oxide synthase
- MDSC
myeloid‐derived suppressor cell
- NA
noradrenaline
- NK
natural killer
- NO
nitric oxide
- OIR
oxygen‐induced retinopathy
- P‐gp
P‐glycoprotein
- ROP
retinopathy of prematurity
- Treg
regulatory T cell
- UCP‐2
uncoupling protein‐2
- VEGF
vascular endothelial growth factor
- β‐AR
β‐adrenoceptor
1. INTRODUCTION
The present review is aimed at describing the scientific journey followed by a research group formed by physiologists and neonatologists who have extensively studied the β‐adrenergic system and the specific role of some β‐adrenoceptors (β‐ARs), over more than 10 years, in a scenario that crosses different medical disciplines and globally defined as hypoxia‐related. The travel started from the discovery of some biological peculiarities typical of a neonatal disease, where hypoxia drives retinal neovascularization. Such peculiarities were then translated to the oncology field, an area apparently very distant from the starting point, but equally characterized by hypoxia‐driven angiogenesis. In this virtual journey, different models were used, although following a “fil rouge” connecting all of them. Here, we will try to explain why β3‐AR, one of the three β‐ARs, is, in our opinion, this “fil rouge.” Thanks to the transition to the cancer model, the functional role of β3‐ARs became progressively clearer. β3‐ARs appeared to participate not only in cancer progression but also in cancer stemness and dedifferentiation, which appears as regression toward cells showing embryonal competences. Progressively, many of the primary mechanisms underlying the β3‐AR role found a logical sense if interpreted in light of embryo development. The upregulation of β3‐ARs in a hypoxic environment might represent a characteristic that justifies similar functions in both cancer progression and embryo growth. However, the demonstration that β3‐ARs actively participate in processes essential to embryo well‐being has thrown new light on the pathophysiological mechanisms of the main diseases related to prematurity and new therapeutic perspectives seem to emerge. A journey started from neonatal diseases and extended to cancer, comes back, like a round‐trip, to the starting point.
In recent years, the interest in the role played by the β‐adrenergic system in many physiopathological conditions has progressively grown. The fortuitous demonstration that the progression of infantile hemangiomas (IHs) was effectively counteracted by treatment with propranolol (a nonselective β1‐ and β2‐ARs antagonist), 1 paved the way to uncover the involvement of β2‐ARs in neovascular processes mediated by vascular endothelial growth factor (VEGF). This finding also suggested exploring the role of this receptor in additional neonatal diseases, characterized by some pathogenetic similarities with IH, such as retinopathy of prematurity (ROP). 2 The studies performed in a mouse model of ROP provided important and original contributions, the most intriguing of which was the characterization of the proangiogenic effect of another β‐AR, the β3‐AR, 3 , 4 , 5 , 6 , 7 which until then had been studied in different contexts. 8 , 9 , 10
The involvement of the β‐adrenergic system in IH, together with the growing interest around the effect of stress on cancer progression, 11 , 12 prompted numerous research groups to investigate the relationships between catecholamines and cancer growth and vascularization, and the possible anticancer activity of β‐blockers. A considerable amount of clinical and experimental data show β2‐AR as the β‐AR subtype predominantly involved in mediating the effects of catecholamines in cancer. 4 , 12 , 13 Nevertheless, on the basis of the results obtained in ROP investigations, a series of studies were planned to verify the potential involvement of β3‐ARs in cancer growth. This shifting focus actually marked the beginning of the journey, starting from neonatal diseases (IHs and ROP) characterized by hypoxia‐induced neovascularization and landing to cancer, an apparently different disease with a similar sustained vascularization favored by a hypoxic environment.
Although the data available at the beginning of the journey were still scarce and fragmented, the presence of β3‐ARs in different hypoxic scenarios together with their upregulation in response to hypoxia prompted us to hypothesize that β3‐ARs might play a possible common role in different pathologies. But, at the meantime, the involvement of β3‐ARs in promoting embryo competences in cancer cells together with their functional role in germ cells, in preimplantation embryos, and in the first week of embryogenesis, suggested the idea that the primary function of β3‐ARs would have been to promote embryo proliferation, vascularization, metabolic adaptation, immune tolerance, all functions that are reactivated in cancer through the intervention of β3‐ARs. Following this idea, the attention was focused on the similarities between the mechanisms exploited by cancer to grow in a nonhospital environment and the strategies adopted by embryos to develop within the maternal womb, which might be explained, at least in part, by similar functions fulfilled by β3‐ARs.
This new awareness regarding the β3‐AR role marked the beginning of the return journey. What was discovered in cancer, found a new significance if interpreted in light of embryo protection from a potentially hostile environment. Many of the apparently harmful functions performed by β3‐ARs in cancer appeared to be beneficial during intrauterine life. Consequently, in line with this thinking, most of the problems related to premature birth might be possibly attributable to the lack of protective effects of β3‐AR during the last weeks of intrauterine life.
This journey, which began in search of possible therapies for neonatal diseases, after stopping in the world of cancer now is going back to the starting point to exploit new opportunities offered by cancer research in counteracting neonatal diseases.
2. THE FIRST STEP: FROM IH TO ROP
2.1. Role of β2‐AR
The demonstration that propranolol, a nonselective β‐AR antagonist, blocking both β1‐ and β2‐ARs, induces the regression of a significant percentage of IHs 14 derives from the original observation that propranolol, administered for the cardiac implication to an infant suffering from IH, serendipitously leads to the regression of the hemangioma. 1 Although not completely understood, the therapeutic efficacy of propranolol is probably due to a mix of actions that includes vasoconstriction, decreased expression of angiogenic factors, mainly VEGF, and apoptosis of capillary endothelial cells. 15
IHs are the most common pediatric vascular tumors, which start within the first few months of life, show a phase of endothelial cell proliferation that lasts on 6–18 months and slowly involutes. 16 Little is known about the pathogenesis of IHs, but local hypoxia is considered as a stimulus triggering vascular proliferation, which would represent a homeostatic response of the hypoxic tissue to the increased production of cytokines such as VEGF; however, this possibility is presently debated. 17 Results obtained with propranolol in children suffering from IHs and a study demonstrating that the selective β2‐AR blocker ICI‐118,551 triggers apoptosis in hemangioblastoma cells 18 indicate that the β adrenergic system, and β2‐ARs in particular, are involved in the pathogenetic mechanisms underlying IHs. In light of these findings, propranolol administration has become the treatment of choice for IHs, although its success rate is about 60%–70%, 14 suggesting that β1‐ and β2‐ARs are not the only players involved in triggering the proliferation of endothelial cells.
The antiangiogenic effects of propranolol aroused interest regarding the possible involvement of the β‐adrenergic system in the pathogenesis of additional neonatal diseases characterized by hypoxia‐induced angiogenic processes. In this respect, ROP, a blinding disease affecting premature newborns, 19 is similarly characterized by hypoxia‐induced neovascularization that follows a first ischemic phase. The evidence that (i) surgical specimens of proliferating IHs are characterized by increased levels of hypoxia‐inducible factor 1 (HIF‐1), 20 a transcription factor responsible for adaptive changes to hypoxia including an increased expression of VEGF, (ii) the notion that both HIF‐1 and VEGF play a main role in ROP 21 and (iii) the finding that IHs are associated with the occurrence and severity of ROP 22 points to common pathogenetic mechanisms shared by IHs and ROP. 4 Therefore, the first step in the virtual journey from neonatal diseases to cancer was to hypothesize that treatment with propranolol would efficiently counteract ROP progression. The efficacy of this approach was first demonstrated in the mouse model of oxygen‐induced retinopathy (OIR), 3 a worldwide model extensively used to mimic ROP. In the OIR model, during the seventh day of life, newborn pups of the C57BL/6J mouse strain are exposed for 5 days to hyperoxia, which downregulates the production of proangiogenic factors and promotes a wide vaso‐obliteration around the optic nerve head (hyperoxic or ischemic phase). Then, newborn pups are returned to a normoxic environment for further 5 days. In this period, the avascular retina becomes markedly hypoxic thus promoting the upregulation of proangiogenic factors and the induction of severe neovascularization (hypoxic or proliferative phase). This vascular sprout mimics the tumultuous vascularization observed in human ROP, which can induce retinal tears or detachments. 23 In the OIR model, during the shift from the ischemic to the proliferative phase, an increase in retinal levels of noradrenaline (NA) was observed, 24 indicating overstimulation of the β‐adrenergic system and suggesting that β‐AR blockade might counteract hypoxia‐associated retinal damage. In this respect, the administration of propranolol, either systemically or as eye drops, during the hypoxic phase reduced retinal neovascularization by preventing HIF‐1 activation and VEGF upregulation. 3 , 25 In particular, 2% propranolol eye drops provided the retina with an amount of drug similar to that obtained after its subcutaneous injection at 20 mg/kg, suggesting that topical application might represent an alternative delivery route, potentially devoid of risks and side effects. 25 In addition, propranolol not only exerted antiangiogenic effects but also counteracted visual dysfunction that characterizes the OIR model by protecting retinal cells through autophagy stimulation and apoptosis inhibition. 26 Both antiangiogenic and neuroprotective effects of propranolol are likely to be mediated by β2‐ARs, as its effects were mimicked by the selective β2‐AR antagonist ICI‐118,551, but not by the β1‐AR antagonist atenolol. 27
On the basis of these preclinical studies, the efficacy and the safety of propranolol were tested in human preterm infants with ROP. The efficacy of propranolol administered after ROP diagnosis, either systemically per os 28 , 29 , 30 , 31 or topically by eye drops was demonstrated in a series of clinical trials. 32 , 33 In particular, in a study using oral propranolol, administered with the same posology as in IH‐suffering infants, the drug was demonstrated to be effective in reducing ROP progression but not sufficiently safe, 28 although additional studies did not observe any side effects of propranolol. 29 , 30 , 31 When propranolol was given as eye micro‐drops, no safety concerns were raised, with propranolol that was effective in slowing down ROP progression when administered at 0.2%, but not at 0.1%. 32 , 33 Interestingly, as outlined by two different meta‐analyses of randomized controlled trials, the efficacy of propranolol in reducing the progression of ROP is about 60%, 34 , 35 an effect comparable to that observed against IHs. 14 Therefore, in ROP as in IHs, the presence of a similar percentage of nonresponders suggests that players additional to β1‐ and β2‐ARs may be involved in the pathogenesis of both diseases. This hypothesis is strengthened by the experimental evidence that propranolol failed to counteract retinopathy when the OIR model was set up in the 129/S6 mouse strain, 36 a strain with a higher susceptibility to OIR than the C57BL/6J strain. 37 A possible explanation of the different response to propranolol in the two mouse strains may lay in the predominant hypoxia‐induced expression of β3‐AR, 36 the third member of the β‐AR family, which might explain the predisposition of the 129/S6 strain to develop more aggressive neovascularization and, therefore, its resistance to β1‐ and β2‐AR blockade. 38
2.2. Putative role of β3‐AR
β3‐AR is the last β‐AR discovered. Although it was clear from its pharmacological profile that the β‐AR family should contain a third member, the existence of β3‐AR was accepted only when it was cloned in 1989. 39 β3‐AR was then finally recognized as a member of the β‐AR family in 1994. 40 Like the other β‐ARs, β3‐ARs are members of the G protein‐coupled receptor family and share 50% sequence homology with β1‐ARs and 40% sequence homology with β2‐ARs, with main differences in the third intracellular loop and C‐terminal tail. At variance of β1‐ and β2‐ARs, β3‐ARs lack the phosphorylation sites in the third intracellular loop and C‐terminal tail, which render β3‐ARs quite resistant to agonist‐induced desensitization, a finding that points to β3‐ARs as interesting therapeutic targets for chronic treatments. 8
β3‐AR expression has been demonstrated in several organs and tissues, and the functional role of β3‐ARs seems to be tissue‐dependent. In this respect, the adipose tissue and the cardiovascular system have been of paramount importance to investigate the role of β3‐ARs. 41
In rodent adipose tissue, β3‐ARs are abundantly expressed, 42 while in humans, their expression is much lower. 43 , 44 , 45 In both human and rodent adipocytes, β3‐AR activation stimulates lipolysis and thermogenesis, 42 , 46 thus increasing energy expenditure and suggesting β3‐AR agonism as a promising approach for treating metabolic diseases. β3‐AR‐induced lipolysis is mediated by the inducible form of the nitric oxide synthase (iNOS) and the consequent increase in the levels of nitric oxide (NO). 47
In the cardiovascular system, the expression of β3‐ARs has been evidenced in the second half of the ‘90s, when their transcripts have been found to be expressed in the human ventricle. 48 In the following years, experimental evidence demonstrates the localization of β3‐ARs to heart 49 and to endothelial cells of cardiac vessels in which their expression is upregulated in diseased conditions. 50 , 51 , 52 From a functional point of view, β3‐AR agonism induces negative inotropic effects in the myocardium and vasodilation of blood vessels. 8 , 53 An effect of β3‐AR agonism against the deleterious effects of catecholamine overdrive has also been evidenced as β3‐AR stimulation results in anti‐hypertrophic heart remodeling 54 , 55 and anti‐fibrotic effects in heart ischemia. 56 At variance with β1‐ or the β2‐AR coupling to adenylate cyclase through Gs proteins in the heart, β3‐ARs couple to guanylate cyclase through Gi proteins. 48 In particular, in the human ventricle, negative inotropic effects of β3‐AR activation are mediated through NO production by the endothelial isoform of NOS (eNOS) 57 that together with the neuronal isoform of NOS leads to increased production of NO ultimately triggering negative inotropy in the heart. 58 The growing interest in the role of β3‐AR in the cardiovascular system prompted clinical trials aimed to determine whether β3‐AR agonism may be of therapeutic importance to counteract cardiovascular pathologies. 8
In addition to its vasodilatory effect, increased NO production following β3‐AR activation seems to exert a proangiogenic action as demonstrated in a model of limb ischemia induced by diabetes. 59 Moreover, β3‐AR agonism induces revascularization through the activation of eNOS/cGMP pathway 60 indicating β3‐AR involvement in angiogenic processes and suggesting that β3‐AR blockade may help in preventing pathologic angiogenesis. In this respect, β3‐AR upregulation has been demonstrated in IHs during the early proliferative phase 61 although β3‐AR overexpression seems to increase during the involution of IHs, which is compatible with the tissue transition from proliferating vascular endothelial cells to a fatty residuum. 62 The expression of β3‐ARs appears to be lower in biopsies from IH patients responding to propranolol than in biopsies from nonresponders, suggesting a possible correlation between β3‐AR levels and the reduced responsiveness to propranolol. 63
β3‐AR expression is restricted in tissues of adult healthy humans, as evidenced by a large‐scale RNA sequence analysis. 64 A similarly restricted expression has also been evidenced in rodents. 42 , 65 , 66 However, in several pathologic states, the expression of β3‐ARs is consistently increased indicating a β3‐AR potential role in diseases. In particular, β3‐AR upregulation can be observed in hypoxia‐ and/or hypoperfusion‐driven diseases, and the possible role of hypoxia in inducing β3‐AR expression has been recently discussed. 67
In the OIR model, studies on β3‐AR expression have given promising results that unleashed experimental works aimed at investigating the functional role of β3‐ARs in hypoxia‐driven retinal angiogenesis. Although both β1 and β2‐ARs were unaffected by low oxygen tension, a remarkable increase in β3‐AR expression was assessed in the endothelium of engorged vascular tufts that are formed in response to hypoxia in the inner capillary network (Figure 1). 3 β3‐AR localization to the endothelium of proliferating retinal vessels is supported by the finding that β3‐ARs are expressed by retinal endothelial cells in which their agonism promote cell migration and proliferation. 68 Additional pharmacological evidence suggests that β3‐ARs are localized to retinal vessels where they appear to regulate the diameter of arterioles. 69
Figure 1.
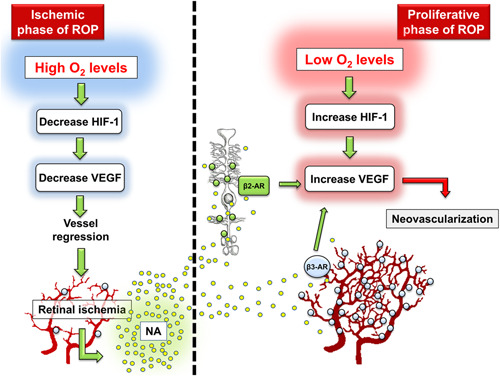
Involvement of the β‐adrenergic system in the pathogenesis of retinopathy of prematurity (ROP). Premature oxygen exposition of preterm newborns induces retinal vascular regression, secondary to the downregulation of both hypoxia‐inducible factor‐1 (HIF‐1) and vascular‐endothelial growth factor (VEGF). This progressive ischemia is responsible for the shift towards a retina that becomes progressively hypoxic and induces noradrenaline (NA) overload. During the proliferative phase, hypoxia induces HIF‐1 and VEGF upregulation thus promoting retinal neovascularization. NA, diffused into the retina, activates β2‐adrenoceptors (β2‐ARs) expressed by Müller cells and β3‐ARs localized to endothelial cells of engorged retinal tufts both contributing to VEGF production [Color figure can be viewed at wileyonlinelibrary.com]
Although the studies in both IH specimens and the OIR model did not allow us to speculate about the functional role of β3‐ARs, their upregulation in proliferating vessels suggested that they might participate in the angiogenic processes. This hypothesis was supported by several experimental findings. In particular, that hypoxia triggers catecholamine production 70 , 71 is confirmed by the finding that NA levels were increased by about twofold in the retina of OIR mice. 10 Considering that the affinity of β3‐ARs for NA is about seven‐fold higher than that of β2‐ARs, 72 it is plausible that in the hypoxic retina, in which both NA and β3‐AR levels were increased, NA was able to stimulate angiogenesis not only through β2‐ARs (whose levels were unaffected by hypoxia) but also via β3‐ARs. In addition, the finding that 129/S6 OIR mice did not respond to propranolol possibly because of their impressive hypoxia‐induced increase in β3‐AR expression (more than 10‐fold) 36 suggested the possibility that, in the 129/S6 strain, the angiogenic response might be driven by β3‐ARs thus resulting resistant to β1/β2‐AR blockade. 38 In this respect, the possibility that, in certain conditions, β3‐ARs might exert a proangiogenic action overcoming that of β2‐ARs was also suggested by some findings in β1/β2‐AR knockout mice that underwent OIR. 6 In these mice, lack of β1‐ and β2‐ARs rendered them resistant to OIR, which was unable to induce retinal neovascularization. However, if β3‐ARs were activated, retinal angiogenesis was more potently induced than in wild‐type mice, recruiting molecular mechanisms that involve the iNOS‐induced production of NO. These findings suggested that β3‐ARs are normally underactivated but, when adequately stimulated, they become fully active and capable to replace β2‐ARs in sustaining the angiogenic drive. In addition, in retinal explants, hypoxia‐induced upregulation of VEGF was blunted by β3‐AR blockade or silencing, while it was increased by β3‐AR agonism, 5 thus suggesting a direct role of β3‐ARs in regulating the production of proangiogenic factors in the hypoxic retina. Taken together, although not conclusive, these pieces of literature pointed to the possibility that β3‐ARs may fulfill a proangiogenic role in response to hypoxia thus opening the question of whether β3‐ARs may play a similar function in additional diseases such as cancer in which hypoxia exerts a paramount role.
3. FROM THE STUDY OF NEONATAL DISEASES TO CANCER
3.1. β3‐AR role in cancer growth and vascularization
The finding that β‐AR blockade exerts antiangiogenic activity against IHs and OIR/ROP, 1 , 2 , 3 , 4 , 5 , 6 , 7 together with the close relationship between stress‐induced catecholamine release and cancer growth 11 , 12 prompted many research groups to evaluate the possible benefits of β‐blockers in the prevention or reduction of cancer progression. Several studies, in fact, have demonstrated that stress (related to surgery or psychosocial factors including depression) accelerates cancer progression and reduces overall survival. 73 The relationship between stress and cancer progression has been explained by the activation of the sympathetic nervous system and the accumulation of stress‐related mediators such as catecholamines in the cancer microenvironment 11 indicating β‐ARs as major players of stress‐induced cancer‐related pathways. 74 Several epidemiological studies have explored the possible impact of β‐blockers on cancer‐related mortality or malignancy prognosis, but their results are not univocal. 75 , 76 However, clinical and experimental data demonstrate that nonselective β‐AR blockers (active against β1‐ and β2‐ARs) provide greater protection against cancer than selective β1‐AR blockers in inhibiting cancer burden and dissemination. 77 In addition, the relationship between the stress and the immune system has been focused mainly on the role of β2‐ARs, 78 although an increased expression of the three β‐ARs has been observed in cancer models as, for instance, splenic T‐lymphocytes of mice that underwent stress exposure. 79 In more recent years, alongside numerous clinical studies, experimental investigations have been performed in vitro and in vivo to better clarify the role of the β adrenergic system in cancer growth, and most of these studies have emphasized the central role of β2‐ARs. 12
Possible involvement of β3‐ARs in tumor progression has only been reported sporadically, in colon cancer, 80 leukemia cells, 81 vascular, 61 and breast cancer. 82 However, these studies have laid the ground for additional investigations aimed at evaluating the putative role of β3‐ARs in cancer. The first in vitro and in vivo experiments were aimed to demonstrate the presence of β3‐ARs in B16F10 melanoma cells and then to explore their possible contribution to cancer growth and vascularization. In vitro, β3‐ARs were localized for the first time to melanoma cells where they were overexpressed in response to hypoxia and regulated VEGF production. 83 In addition, β3‐AR blockade or silencing was found to prevent hypoxia‐induced VEGF accumulation and to reduce cancer cell proliferation by inducing cancer cell apoptosis. Similarly, in mice bearing B16F10 melanoma cells, β3‐AR antagonism reduced tumor growth and vascularization through the activation of apoptotic processes. 83 In analogy with what was observed in retinal explants in which β3‐AR antagonism displayed antiangiogenic effects through NO intermediation, in B16F10 cells, β3‐AR blockade prevented melanoma cell proliferation by decreasing the iNOS‐induced production of NO, while β3‐AR agonism increased melanoma cell proliferation by activating iNOS. 84 Further evidence from β1/2‐AR knockout mice bearing melanoma demonstrated that the intratumor levels of NA were significantly higher than in wild‐type mice suggesting that β3‐ARs might be predominantly activated in the presence of catecholamine overdrive. 85 In addition, the efficacy of β3‐AR antagonism was more pronounced in β1/2‐AR knockout than in wild‐type mice, with a significant reduction in cancer growth and vascularization, indicating that, when present, β1‐ and β2‐ARs may at least in part mask β3‐AR activity, and suggesting β‐AR redundancy in mediating catecholamine‐induced cancer growth.
3.2. β3‐AR role in cancer microenvironment
A large amount of experimental data associated with epidemiological studies have suggested β2‐AR as the β‐AR subtype predominantly involved in mediating the effects of catecholamines in melanoma models, 86 although β3‐ARs appear to be highly expressed in human melanoma samples. 87 In addition, in human samples of melanocytic lesions, β3‐ARs were found to be expressed in the tumor microenvironment where their expression was directly correlated with melanoma malignancy. 88 Furthermore, β3‐AR expression by cancer‐associated fibroblasts, endothelial progenitor cells, mesenchymal stem cells, and monocytes was upregulated by either 1% oxygen concentration, which is usually adopted to reproduce intratumor hypoxia or the combination of hypoxia with glucose withdrawal that mimics intratumor ischemic environment. Moreover, β3‐ARs were demonstrated to be actively involved in the NA‐driven recruitment of circulating stromal cell precursors. In particular, in human dermal fibroblasts, NA stimulation induced melanoma cell motility, while, in a capillary morphogenesis assay, mesenchymal stem cells increased their ability to form vessel‐like structures. Both these activities were significantly reverted by β3‐AR antagonism suggesting for the first time a role for β3‐AR in cancer vasculogenesis. 88 Additionally, in human melanoma cells, catecholaminergic stimulus increased the expression of stemness markers, and the ability to form melanospheres, both activities that were reverted by β3‐AR antagonism suggesting a relationship between β3‐ARs, the degree of stemness, and cancer cell dedifferentiation. 88 The modulation of dedifferentiation processes by β3‐AR is in line with the finding that hypoxia plays a key role in promoting the loss of differentiated gene expression and the shift towards a stem cell‐like phenotype. 89
The expression of both β2‐ and β3‐ARs in stromal cells, and their involvement in tumor development and metastatic dissemination was confirmed in a study where prostate cancer growth was severely compromised in mice chemically sympathectomized and in double β2/β3‐AR knockout mice indicating a possible synergistic effect between different β‐ARs. 90
4. β3‐AR AS A COMMON PLAYER IN CANCER AND EMBRYO
4.1. Analogies between cancer growth and embryo development
Recently, a number of studies have revealed significant similarities between cancer growth and embryo development. 91 The proliferative activity of cancer cells, as well as the invasive ability of trophoblast cells, show evident analogies with similar skills. 92 Cellular mechanisms used by invasive placental cells during implantation are identical to those employed by cancer cells to invade and spread within the body and metastasize. 93 In addition, trophoblast cells as well as cancer cells, actively modulate the host immune response to escape from the mother's or the patient immune system by inducing a similar immune tolerance. 94 Finally, a plethora of proteins exclusively expressed during fetal development are detected in cancer patients and commonly used as oncofetal biomarkers to obtain an early diagnosis or prognostic indications for a wide variety of cancers, suggesting that cancer may reactivate typical embryonal competences. 95 , 96
In this respect, β3‐AR role in neonatal diseases or in cancer might be interpreted in light of the analogies between embryo development and cancer growth. In particular, the correlation between β3‐AR upregulation and hypoxia, the relationship between β3‐AR activation and the induction of stemness markers as well as the role played by β3‐ARs in the assembly of the cancer microenvironment, are highly suggestive of a possible involvement of β3‐AR in apparently different events. In particular, the awareness that the cancer microenvironment protects and promotes cancer growth with efficiency and care similar to those offered by the placenta for embryos prompted us to evaluate the possibility that β3‐ARs could perform similar functions in cancer and embryo. In both scenarios, β3‐AR is likely to represent a common link between hypoxia, cell proliferation, and vascularization. 4
Data in the literature provided suggestive elements for imagining a possible role of β3‐ARs in embryo. (i) Catecholamines play a significant role in the first week of embryogenesis, as demonstrated by the high embryo‐lethality observed in mice lacking tyrosine hydroxylase, the rate‐limiting enzyme in catecholamine biosynthesis 97 ; (ii) β3‐ARs are expressed in mammalian oocytes 98 , 99 and spermatozoa, in which they induce motility 100 ; (iii) β3‐ARs are also expressed in preimplantation embryos, 98 , 99 during the early stages of embryogenesis, 101 in embryonal tissues and in the placenta 102 , 103 ; (iv) β3‐ARs are upregulated in human pregnant myometrium where they represent the predominant β‐AR subtype and participate in the inhibition of spontaneous contractions. 104 , 105 In addition, β3‐AR agonism induces a moderate vasodilation in the human umbilical artery ring, suggesting that β3‐ARs may exert a role in regulating the fetoplacental circulation. 106 These data, although still fragmentary, suggest a possible role of β3‐ARs in fecundation, embryo implant, and growth.
Cancer growth and embryo development share many similarities in terms of molecular mechanisms to sustain their progression. 4 , 107 , 108 , 109 In addition to the striking similarities between invasive trophoblast cells and proliferating cancer cells, their common ability to induce vasculogenic mimicry represents one of the most intriguing similarities. 93 , 107 The term “vasculogenic mimicry” refers to the mechanism by which hypoxia promotes cancer cell transformation to endothelial cell‐like structures, able to organize themselves into vascular‐like structures, 110 a phenomenon that is similar to what observed when trophoblasts invade maternal spiral arteries to acquire specific vascular phenotype. 111
Both cancer and embryo usually grow in a similar hypoxic and acidotic environment 112 , 113 due, at least in part, to their peculiar metabolism. In particular, cancer and embryo share a metabolic shift from mitochondrial oxidative phosphorylation to aerobic glycolytic metabolism, independent of the environmental oxygen level, the so‐called Warburg effect. 114 This is a metabolic shift towards glycolysis that is activated even in the presence of oxygen. 115 This shift allows an increase in the synthesis of nucleic acid through the activation of the pentose phosphate pathway, which is convenient for highly proliferating cells. 116 , 117 In addition, the Warburg effect increases the production of nicotinamide adenine dinucleotide phosphate, the main defense for cancer and embryo towards oxidative stress. 118 , 119 Moreover, the Warburg effect induces the production of elevated levels of lactic acid that is exported outside the cells and favors the nesting of proliferating cells in the surrounding tissue. 120 , 121 , 122
Both cancer and embryo share common mechanisms able to protect them from exposure to potentially toxic xenobiotics. In this respect, some specific efflux transporters, widely exploited by cancer cells, 123 are extensively expressed in the placenta. 124 Among the different mechanisms capable of protecting cancer and embryo from the action of toxic substances, glutathione deserves to be mentioned. Glutathione facilitates excretion of xenobiotic and endogenous compounds through different mechanisms, the most efficient of which is the formation of glutathione S‐conjugates catalyzed by glutathione S‐transferase. 125 The crucial role of glutathione S‐conjugates explains not only why glutathione is precociously expressed during the embryonal life, 126 but also why, unfortunately, in cancer patients its formation increases the rate of conjugation and detoxification of chemotherapy agents, thus decreasing their effectiveness. 127
Finally, in both cancer and embryo, the host environment (the oncologic patient and the mother, respectively) actively modulates the immune response to tolerate a “foreign body.” 107 Several mechanisms and different immune subpopulations are commonly involved in the promotion of immune tolerance. Among these, regulatory T cells (Tregs) and myeloid‐derived suppressor cells (MDSCs) are recruited and activated in cancer or at the fetal–maternal interface 128 , 129 , 130 , 131 to suppress the activity of cytotoxic T cells and natural killer (NK) cells.
The expression of β3‐ARs in cancer and embryo, their upregulation under hypoxia 3 , 5 , 83 , 88 , 132 and the assumption that cancer and embryo rely on the hypoxic environment, 112 , 113 legitimated the hypothesis that β3‐AR overexpression might represent one of the mechanisms by which embryo originally (and cancer subsequently) adapts their growth in an otherwise hostile environment. 4
To verify the putative common role played by β3‐ARs in cancer and embryo, the possibility that β3‐AR‐based strategies primarily predisposed to favor embryo development were also adopted by cancer was investigated.
4.2. β3‐ARs and Warburg effect
Limiting oxidative stress preserves embryo (and, unfortunately, cancer) from oxidative stress‐induced cell death, 133 while the reduction of extracellular pH promotes the disaggregation of surrounding tissues and facilitates the infiltration of trophoblast or cancer cells. 134 , 135 , 136
Following early studies demonstrating that β3‐AR antagonism exerts antiproliferative effects in cancer, 83 , 88 a subsequent study addressed the possible role of β3‐ARs in the metabolic shift in cancer and embryo. In both cases, β3‐AR agonism induced an accelerated glycolysis, as confirmed by the increased glucose uptake and lactate export, in line with Warburg effect induction. 137 In particular, β3‐AR activation upregulated the expression of proteins that play a critical role in the Warburg effect. In addition, in both cancer and embryonic stem cells, β3‐AR agonism increased the expression of uncoupling protein‐2 (UCP‐2), a protein that uncouples the activity of the respiratory chain from ATP synthesis, thus favoring the Warburg effect, as also demonstrated in leukemia cells. 138 Moreover, β3‐AR agonism reduced oxidative stress in both cancer and embryo, an effect that was abolished by the β3‐AR blockade. 137 Overall, these findings demonstrate that β3‐ARs have a prominent role in activating the Warburg effect, thus inducing a metabolic advantage for cancer and embryo cells.
4.3. β3‐ARs and chemoresistance
Chemoresistance is an important challenge in cancer treatment and plays a major role in determining the clinical outcome of cancer patients by promoting recurrence, metastasis and by increasing the mortality risk. Chemoresistance is often a mix of multiple factors, including the deregulated expression/activity of membrane transporters involved in drug efflux. 139 In this respect, hypoxia is known to promote chemoresistance by enhancing the expression of drug transporters in several malignancies. 140 , 141 , 142 , 143 Considering that β3‐AR agonism increases glutathione synthesis 144 and that glutathione plays an important role in xenobiotic detoxication in both cancer 127 and embryo, 126 the possibility exists that β3‐ARs may play an active role in promoting chemoresistance.
β3‐AR role in chemoresistance was demonstrated in human myeloid leukemia cell lines, in particular in leukemia cells resistant to doxorubicin, a chemotherapy drug. 145 In chemoresistant cells, both β3‐ARs and the membrane transporter P‐glycoprotein (P‐gp), which is involved in drug efflux, 146 increased in their expression. In addition, β3‐AR blockade reinstated the sensitivity to doxorubicin, likely through downregulating P‐gp levels. Moreover, β3‐AR blockade reduced the expression of UCP‐2 and HIF‐1 suggesting a strict link between hypoxia, Warburg effect, and chemoresistance. 145
4.4. β3‐ARs and immune tolerance
Immune tolerance is one of the main mechanisms that preserve life and that is shared by cancer and embryo. 147 , 148 In fact, cancer develops several mechanisms to evade effective immune surveillance and similar mechanisms are used by the fetus to develop in the uterine environment. 149 , 150 In this respect, hypoxia is considered one of the most important regulators of cancer immune escape 151 through the activation of Treg cells 152 or the increased resistance of cancer cells to cytotoxic T lymphocytes. 153 As pan β‐AR agonism has been reported to promote immune suppression in metastatic melanoma patients, 154 a possible role of β3‐ARs was investigated.
In two complementary studies, the immune tolerant role of β3‐ARs was evaluated in cancer and during pregnancy. The first study was addressed to study the possible relationship between β3‐ARs and cancer immune tolerance. 155 In particular, the ability of immune cells to kill cancer cells was found to significantly increase when both immune and cancer cells were pre‐exposed to the β3‐AR blockade. In addition, in a mouse model of melanoma, β3‐AR blockade or β3‐AR silencing reduced tumor growth by triggering the switch from an immune‐suppressive to an immune‐competent tumor microenvironment, thus supporting the hypothesis that β3‐ARs play a role in promoting cancer immune tolerance. 155
In a second study, the role of β3‐ARs in the maintenance of prenatal immune tolerance was investigated. 156 In pregnant mice, β3‐AR blockade increased the number and the cytotoxicity of NK cells, while attenuating the infiltration of MDSCs and Treg cells in the mouse placenta. A similar phenomenon was observed in the decidua where maternal β3‐AR blockade induced a surge of immune‐competent CD8 cells, concomitant with a reduction of immune tolerant decidual NK cells, indicating that β3‐ARs might guarantee a halo of immune tolerance within the fetal–maternal interface by modulating the same immune cells as in cancer. 156
Globally, these results suggest the possibility that hypoxia may promote the biological shift towards a tolerant immunophenotype through the upregulation of β3‐ARs, and that this trick may be used by both embryo and cancer to create an aura of immune tolerance in a previous immune‐competent environment. In this light, the cancer microenvironment may be considered a sort of “tumor placenta” and cancer as a disease that exploits the same tricks allowing the embryo to grow, by reactivating fetal competences through the intervention of β3‐ARs.
4.5. β3‐ARs and differentiation
Stem cells self‐renew and differentiate in response to factors that control stem cell fate; in this context, a key factor controlling stem cell phenotype is oxygen, with hypoxia participating in maintaining the undifferentiated state, while increasing oxygen tension promoting stem cell differentiation. During hypoxia, HIF‐1 leads to the transcription of stemness and proliferation markers, upregulates the expression of proteins and enzymes that drive the Warburg shift, reduces the activity of mitochondria, and induces angiogenic processes through the production of VEGF, all of which are metabolic activities involved in maintain cancer cell stemness. 157
As the upregulation of hypoxia‐sensing proteins is crucial to prevent cell differentiation, β3‐AR role in the differentiation process became of crucial interest if one considers this receptor as a sort of marker of stemness. Among β‐ARs, β1‐AR activation with metoprolol has been found to increase migration and proliferation of cardiac stem cells by stimulating the retention of an undifferentiated profile. 158 The additional finding that β3‐ARs are gradually downregulated during embryogenesis, 101 in concomitance with increasing oxygen tension, suggests the possibility that a progressive reduction of β3‐AR expression may be linked to cell differentiation. To substantiate the hypothesis that β3‐ARs are involved in the maintenance of cell stemness, a series of studies on cancer cell differentiation were performed.
In a mouse model of melanoma, the pharmacological blockade of β3‐ARs reduced the expression of cancer stem cell markers and induced a differentiated phenotype of hematopoietic and mesenchymal stem cells. 159 In particular, hematopoietic progenitor cells, differentiated in a hematopoietic niche, have been shown to develop within the tumor mass. In addition, trans‐differentiation from mesenchymal stem cells to preadipocytes has been also demonstrated, 159 in line with previous data from hemangioma stem cells treated with propranolol. 160
In mice bearing Neuro2A neuroblastoma cells, either β3‐AR blockade or β3‐AR silencing inhibited the growth of neuroblastoma and its progression. In particular, as in the melanoma model, β3‐AR blockade reduced the expression of stemness markers, but increased the expression of differentiation markers. In addition, neuroblastoma cell differentiation following β3‐AR antagonism has been substantiated by increased neurite formation. 161
These findings together are indicative of a relationship between β3‐ARs and the undifferentiated state of cancer, suggesting the possibility that similar relationships might also exist in the embryo. In this perspective, the antagonism of β3‐ARs might represent a new therapeutic approach to counteract the proliferation of cancer, its metabolic shift, chemoresistance, immune tolerance and to promote its differentiation (Figure 2).
Figure 2.
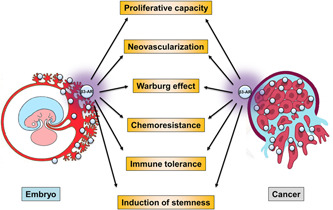
Similarities in the role of β3‐AR in promoting cancer growth and embryo development. β3‐ARs increase cell proliferative capacity thanks to their antiapoptotic action and stimulate neovascularization that is necessary to cancer growth and placentation. In addition, β3‐ARs ensure the metabolic shift that guarantees a proliferative advantage to cancer and embryo, chemoresistance and immune tolerance, all functions indispensable for cancer growth and embryo development. Finally, hypoxia and β3‐ARs may act jointly to orient cancer cells and embryo towards an undifferentiated state [Color figure can be viewed at wileyonlinelibrary.com]
5. FUTURE PERSPECTIVES FOR NEONATAL DISEASES
5.1. Return journey to neonatal diseases
The journey, which started from neonatal diseases and landed to cancer, was at the beginning focused to evaluate a possible role of β3‐ARs in neovascularization processes induced by hypoxia. However, studies performed in cancer demonstrated the pleiotropic roles of β3‐ARs, which appear to be involved in common mechanisms to favor similar skills in cancer and embryo.
The awareness that β3‐ARs support cancer growth by reactivating embryonal competences, suggested that β3‐ARs might play a relevant role during intrauterine life. This awareness “de facto” represented the start of the return journey. While in the outward journey the interest was focused on the possible role of β3‐AR upregulation in the hypoxic environment and consequently on the possible advantage in β3‐AR antagonism, in the return journey the attention was specularly addressed towards the physiologic function of β3‐ARs during the intrauterine life, and the possible advantage in their agonism, especially for preterm newborns presumably deprived, at least in part, of β3‐AR function.
In this respect, the expression of β3‐ARs might be favored by the hypoxic intrauterine environment where β3‐ARs may contribute to embryonal and fetal development, for example by promoting prenatal vascular mimicry or maintaining specific embryonal or fetal peculiarities.
Embryo growth takes place in a physiologically hypoxic environment 113 and hypoxia is essential to promote embryo development. 162 Under hypoxia, as within the maternal womb, HIF‐1 regulates the expression of a series of proangiogenic factors, that are determinant to support vascular mimicry. 163 In the meantime, low fetal oxygen tension sustains the patency of the ductus arteriosus (DA), an extracardiac fetal shunt connecting the main pulmonary trunk to the descending aorta to divert most of the right ventricular blood output away from the vasoconstricted pulmonary circulation to the systemic circulation. 164 At birth, newborns are suddenly exposed to a significantly more oxygenated atmosphere if compared with the intrauterine environment. After exposition to oxygen‐rich environment, HIF‐1 is rapidly hydroxylated by oxygen‐sensitive prolyl hydroxylase domain‐containing proteins, ubiquitinated by the von Hippen–Lindau protein and, finally, degraded by the proteasome, inducing the downregulation of proangiogenic factors and the stop of vascular mimicry. 165 , 166 At the same time, at birth, neonatal oxygen tension rapidly increases inducing a prompt DA closure within 24–72 h. 167
In term newborns, in which the vascularization processes had already been completed in utero, the exposition to relative hyperoxia reverts the patency of DA and promotes its closure. 168 Considering that β3‐ARs are upregulated by hypoxia and induce vasodilatation through the NO pathway, it was plausible to hypothesize participation of β3‐ARs in caliber modulation of DA.
In a recent study, 169 β3‐ARs were found to be highly expressed in mouse DA during intrauterine life, whereas they were less expressed in DA harvested from newborns early after delivery. With the aim to demonstrate that β3‐ARs expressed by fetal DA participated in the maintenance of DA patency, pregnant dams were given acute or chronic administration of β3‐AR antagonists, previously demonstrated to cross the placental barrier. While acute administration of the antagonist at the lowest dose did not reduce the lumen size in fetuses, the highest dose induced severe constriction and increased wall thickness. 169 Overall, this study supports the hypothesis that β3‐ARs are involved in the maintenance of DA patency, suggesting the possibility that they may participate in intrauterine functions, which ensure fetal well‐being. In addition, β3‐AR levels were found to downregulate during the shift from hypoxic to a relatively hyperoxic environment, an observation that is in line with findings from peripheral blood mononuclear cells where β3‐ARs, upregulated by hypoxia, were significantly downregulated after re‐exposition to normoxia. 169
Therefore, as in the outward journey in which the association between hypoxia and β3‐ARs upregulation suggested to explore a possible involvement of β3‐ARs in neovascularization, in the return journey, the possible association between relative hyperoxia and β3‐AR downregulation might legitimate to explore the hypothesis of an involvement of β3‐AR reduced expression in vascular regression.
In preterm newborns, the vascularization processes are still incomplete and the vascular regression after a precocious exposition to a relative hyperoxic environment constitutes the anatomical substrate of the main diseases related to prematurity. The putative demonstration that vascular regression might be mediated by β3‐AR downregulation, might open the perspective for a new interpretation of prematurity‐related diseases and shed new light on possible therapeutic opportunities.
5.2. Perspectives for preterm newborns
As in term newborns, in preterm newborns, the exposure to a relative hyperoxia at birth induces a sharp reduction in HIF‐1 levels 166 and, therefore, a collapse of both HIF‐1‐dependent and placenta‐dependent proangiogenic factors. 170 However, in preterm newborns, the abrupt transition from intra‐ to extra‐uterine life produces a sudden arrest of vascularization (if not even an apparent regression) in districts with immature vascularity. Vascularization arrest is more pronounced the more premature the newborn is and the greater is its need for oxygen supplementation. 171 Around these poorly vascularized scenarios, the foundations are laid for the development of the main diseases related to prematurity. It is well‐known, for example, that premature exposure to a relatively hyperoxic environment induces the partial and temporary regression of the still incomplete retinal vascularization that is responsible for the first ischemic phase of ROP. 172 A similar phenomenon occurs in the lungs, where high oxygen levels induce alveolar hypoplasia and abnormal vascular organization, 173 typical events of the so‐called “new” bronchopulmonary dysplasia of a preterm newborn. 174 However, in preterm newborns, especially of very low gestational age, retina and lungs are not the only districts with immature vascularity. In fact, the vascularization of additional districts including the central nervous system (CNS) or the gut requires a physiologically hypoxic environment rich in HIF‐1. 175 , 176 In this respect, human autoptic studies demonstrate that the vascularity of the CNS white matter from 16 to 32 weeks of gestational age is rudimental. 177 Therefore, vascular immaturity at birth and vascular regression after exposure to a relative hyperoxia might explain why other typical ischemic diseases such as periventricular leukomalacia in CNS or necrotizing enterocolitis in the gut electively affect premature infants. The demonstration that in these scenarios vascular responses to relative hyperoxia are coupled to β3‐AR downregulation might pave the way for novel pharmacological attempts aimed at counteracting the ischemic effect of early exposure to oxygen in preterm newborns. In this respect, β3‐AR agonism might ensure normal vascularization processes despite the exposition to relative hyperoxia and might represent a novel therapeutic opportunity for preterm newborns (Figure 3).
Figure 3.
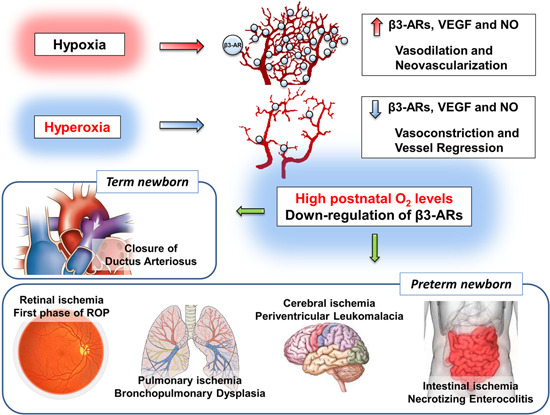
Possible involvement of β‐adrenoceptors (β3‐ARs) in the pathogenesis of prematurity‐related diseases. β3‐AR upregulation under hypoxia causes vascular endothelial growth factor (VEGF)‐induced neovessel proliferation and vasodilation through the nitric oxide (NO) pathway. Conversely, exposure to normoxia (sensed as relative hyperoxia) determines vasoconstriction and vessel regression possibly through the downregulation of β3‐ARs. In the ductus arteriosus, in particular, β3‐ARs are downregulated after birth thus favoring its closure. The exposure to relative hyperoxia stops vascularization or even induces vascular regression, anatomical conditions common to the main pathologies related to prematurity. Whether vascular regression would be mediated by β3‐AR downregulation, then the perspective exists that β3‐AR activation might prevent disease progression [Color figure can be viewed at wileyonlinelibrary.com]
Before concluding this intriguing round‐trip, started from the study of some specific neonatal diseases, passed through cancer, and now directed again to prematurity‐related diseases, there are still some essential stops. The next stop will be to evaluate β3‐AR involvement in vascularization processes during the intrauterine life in experimental models. Then, whether relative hyperoxia after birth induces severe neonatal diseases through β3‐AR downregulation and whether β3‐AR activation might prevent disease onset, will be also studied. This study will be applied to verify the possibility of targeting β3‐ARs to prevent the ischemic phase of ROP, the vascular regression responsible for bronchopulmonary dysplasia, the cerebral or intestinal ischemia, responsible for periventricular leukomalacia and necrotizing enterocolitis, respectively.
6. CONCLUSIONS
In conclusion, the findings from the studies here reported open new scenarios towards understanding the pathogenic mechanisms underlying diseases in which vascularization is affected by fluctuating oxygen levels. The perinatal period, characterized by the transition from a relatively hypoxic intrauterine environment to the normoxic extrauterine life, offers an extraordinary point of observation for future approaches in the treatment of oncologic and neonatal diseases.
Travelers usually dream of the next adventure even before concluding a trip. Unfortunately, drugs with specific antagonistic activity against β3‐ARs are not yet available for humans, and therefore the prospect of antitumor trials with selective β3‐AR blockers appears futuristic. On the contrary, if the current journey will end with the demonstration that in animal models exposed to hyperoxia, early treatment with β3‐ARs agonists may avoid vascular regression, the way will be opened for human trials with the objective to prevent a number of prematurity‐related diseases. In this perspective, the pharmaceutical armamentarium for β3‐AR agonism is relatively equipped and β3‐AR agonists are also being tested in repurposing trials for cardiovascular and metabolic conditions. 8 , 178 , 179 In addition, recently mirabegron, a selective β3‐AR agonist, has been demonstrated safe and well‐tolerated in children 180 , 181 and even approved for the pediatric population. 182
In this sense, we can at least begin to dream of the next trip.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
7.
ACKNOWLEDGMENTS
This study was supported by the Italian Ministry of Health (RF‐2011‐02351158); Meyer Foundation, A. Meyer Children's University Hospital, Florence, Italy; and Ente Cassa di Risparmio di Firenze, Italy.
Biographies
Luca Filippi, MD, PhD, a specialist in pediatrics, neonatology, anesthesiology and resuscitation, is an Associate Professor of Pediatrics at Pisa University, with specific experience in neonatal intensive care. He received PhD in Neonatal Research at Maastricht University (Netherlands). After the observation that propranolol is effective in the regression of infantile hemangiomas, he hypothesized that this drug might be effective also in reducing the progression of ROP. He coordinated several clinical trials with propranolol in newborns with ROP. He hypothesized that β3‐AR upregulation may represent the same strategy adopted by embryo and cancer to growth in a hostile environment.
Alessandro Pini, PhD, is an Associate Professor in Histology at the Department of Experimental and Clinical Medicine of the University of Florence (Italy). After graduating in Biology in 2005 at the University of Florence (Italy), he approached neurosciences at the Rita Levi Montalcini Center for Brain Repair (University of Turin). He obtained his PhD in Human Morphology and Morphogenesis at the University of Florence. His research expertise is focused on the morphological process occurring in tissue development and the pathophysiological mechanisms of tissue repair.
Maurizio Cammalleri, PhD, is a Researcher in Physiology at the Department of Biology of the University of Pisa. After graduating in Biology in 1998, his major interest was electrophysiology in which he gained specific experience. He received his PhD in Neurosciences at the University of Pisa. After a first experience in the field of neuronal plasticity in collaboration with Pietro Sanna and Floyd Bloom at the Scripps Research Institute of San Diego, his research activity was focused on functional studies in the CNS, including the retina. His experience in animal models of proliferative retinopathies was important to demonstrate the functional role of the β‐adrenergic system in the retina
Paola Bagnoli, PhD, former Full Professor of Physiology at the University of Pisa with a track record of retinal research for more than 30 years. She received her PhD in Neurophysiology at the Scuola Normale Superiore (Pisa) and completed her post‐doctoral training at the Brain Research Institute of Zurich (Switzerland). In the 80s, she approached immunohistochemistry at the Dept of Neurosciences of the State University of New York. Since her first paper in 2007 about the anti‐angiogenic role of somatostatin in models of hypoxia‐associated retinal diseases, she continued to investigate angiogenic mechanisms in the retina until to land the role of β‐adrenoceptors (β‐ARs) in the oxygen‐induced retinopathy model. Her work led to the discovery of the anti‐angiogenic role of propranolol afterward substantiated by clinical trials in preterm newborns. In the meantime, the intriguing role of β3‐ARs in the hypoxic retina popped out and moved her research to additional fields including cancer. She is currently visiting professor at the Dept. of Biology of Pisa University.
Massimo Dal Monte, PhD, is a Full Professor of Physiology at the University of Pisa. He received his PhD in Biochemistry at the University of Florence and the title of specialist in Biochemistry and Clinical Chemistry at the University of Pisa. His research interest is on the molecular mechanisms underlying hypoxia‐driven angiogenic processes. Focusing his interest first on the hypoxic retina in which he studied the involvement of the β‐adrenergic system in modulating pathways associated with neovascularization, he then studied the role of β‐adrenoceptors in melanoma growth, evidencing the prominent function of β3‐ARs in melanoma biology.
Filippi L, Pini A, Cammalleri M, Bagnoli P, Dal Monte M. β3‐Adrenoceptor, a novel player in the round‐trip from neonatal diseases to cancer: suggestive clues from embryo. Med Res Rev. 2022;42:1179‐1201. 10.1002/med.21874
7.1. DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Léauté‐Labrèze C, Dumas de la Roque E, Hubiche T, Boralevi F, Thambo JB, Taïeb A. Propranolol for severe hemangiomas of infancy. N Engl J Med. 2008;358(24):2649‐2651. [DOI] [PubMed] [Google Scholar]
- 2. Filippi L, Cavallaro G, Fiorini P, et al. Study protocol: safety and efficacy of propranolol in newborns with Retinopathy of Prematurity (PROP‐ROP): ISRCTN18523491. BMC Pediatr. 2010;10:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ristori C, Filippi L, Dal Monte M, et al. Role of the adrenergic system in a mouse model of oxygen‐induced retinopathy: antiangiogenic effects of beta‐adrenoreceptor blockade. Invest Ophthalmol Vis Sci. 2011;52(1):155‐170. [DOI] [PubMed] [Google Scholar]
- 4. Filippi L, Dal Monte M, Casini G, Daniotti M, Sereni F, Bagnoli P. Infantile hemangiomas, retinopathy of prematurity and cancer: a common pathogenetic role of the β‐adrenergic system. Med Res Rev. 2015;35(3):619‐652. [DOI] [PubMed] [Google Scholar]
- 5. Dal Monte M, Filippi L, Bagnoli P. Beta3‐adrenergic receptors modulate vascular endothelial growth factor release in response to hypoxia through the nitric oxide pathway in mouse retinal explants. Naunyn Schmiedebergs Arch Pharmacol. 2013;386(4):269‐278. [DOI] [PubMed] [Google Scholar]
- 6. Dal Monte M, Cammalleri M, Mattei E, Filippi L, Bagnoli P. Protective effects of β1/2 adrenergic receptor deletion in a model of oxygen‐induced retinopathy. Invest Ophthalmol Vis Sci. 2014;56(1):59‐73. [DOI] [PubMed] [Google Scholar]
- 7. Casini G, Dal Monte M, Fornaciari I, Filippi L, Bagnoli P. The β‐adrenergic system as a possible new target for pharmacologic treatment of neovascular retinal diseases. Prog Retin Eye Res. 2014;42:103‐129. [DOI] [PubMed] [Google Scholar]
- 8. Michel LYM, Farah C, Balligand JL. The beta3 adrenergic receptor in healthy and pathological cardiovascular tissues. Cells. 2020;9(12):2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Finlin BS, Memetimin H, Zhu B, et al. The β3‐adrenergic receptor agonist mirabegron improves glucose homeostasis in obese humans. J Clin Invest. 2020;130(5):2319‐2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Maryanovich M, Zahalka AH, Pierce H, et al. Adrenergic nerve degeneration in bone marrow drives aging of the hematopoietic stem cell niche. Nat Med. 2018;24(6):782‐791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lutgendorf SK, DeGeest K, Dahmoush L, et al. Social isolation is associated with elevated tumor norepinephrine in ovarian carcinoma patients. Brain Behav Immun. 2011;25(2):250‐255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cole SW, Sood AK. Molecular pathways: beta‐adrenergic signaling in cancer. Clin Cancer Res. 2012;18(5):1201‐1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Eckerling A, Ricon‐Becker I, Sorski L, Sandbank E, Ben‐Eliyahu S. Stress and cancer: mechanisms, significance and future directions. Nat Rev Cancer. 2021;21:105‐785. [DOI] [PubMed] [Google Scholar]
- 14. Léauté‐Labrèze C, Hoeger P, Mazereeuw‐Hautier J, et al. A randomized, controlled trial of oral propranolol in infantile hemangioma. N Engl J Med. 2015;372(8):735‐746. [DOI] [PubMed] [Google Scholar]
- 15. Sebaratnam D, Rodríguez Bandera AI, Wong LF, Wargon O. Infantile hemangioma. Part 2: management. J Am Acad Dermatol. 2021;S0190–9622(21):02352‐02355. [DOI] [PubMed] [Google Scholar]
- 16. Rodríguez Bandera AI, Sebaratnam DF, Wargon O, Wong LF. Infantile hemangioma. Part 1: epidemiology, pathogenesis, clinical presentation and assessment. J Am Acad Dermatol. 2021;S0190–9622(21):02351‐02353. [DOI] [PubMed] [Google Scholar]
- 17. de Jong S, Itinteang T, Withers AH, Davis PF, Tan ST. Does hypoxia play a role in infantile hemangioma? Arch Dermatol Res. 2016;308(4):219‐227. [DOI] [PubMed] [Google Scholar]
- 18. Cuesta AM, Albiñana V, Gallardo‐Vara E, et al. The β2‐adrenergic receptor antagonist ICI‐118,551 blocks the constitutively activated HIF signalling in hemangioblastomas from von Hippel‐Lindau disease. Sci Rep. 2019;9(1):10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liegl R, Hellström A, Smith LE. Retinopathy of prematurity: the need for prevention. Eye Brain. 2016;8:91‐102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kleinman ME, Greives MR, Churgin SS, et al. Hypoxia‐induced mediators of stem/progenitor cell trafficking are increased in children with hemangioma. Arterioscler Thromb Vasc Biol. 2007;27(12):2664‐2670. [DOI] [PubMed] [Google Scholar]
- 21. Chen J, Smith LE. Retinopathy of prematurity. Angiogenesis. 2007;10(2):133‐140. [DOI] [PubMed] [Google Scholar]
- 22. Praveen V, Vidavalur R, Rosenkrantz TS, Hussain N. Infantile hemangiomas and retinopathy of prematurity: possible association. Pediatrics. 2009;123(3):e484‐e489. [DOI] [PubMed] [Google Scholar]
- 23. Smith LE, Wesolowski E, McLellan A, et al. Oxygen‐induced retinopathy in the mouse. Invest Ophthalmol Vis Sci. 1994;35(1):101‐111. [PubMed] [Google Scholar]
- 24. Dal Monte M, Martini D, Latina V, Pavan B, Filippi L, Bagnoli P. Beta‐adrenoreceptor agonism influences retinal responses to hypoxia in a model of retinopathy of prematurity. Invest Ophthalmol Vis Sci. 2012;53(4):2181‐2192. [DOI] [PubMed] [Google Scholar]
- 25. Dal Monte M, Casini G, la Marca G, Isacchi B, Filippi L, Bagnoli P. Eye drop propranolol administration promotes the recovery of oxygen‐induced retinopathy in mice. Exp Eye Res. 2013;111:27‐35. [DOI] [PubMed] [Google Scholar]
- 26. Cammalleri M, Locri F, Catalani E, et al. The beta‐adrenergic receptor blocker propranolol counteracts retinal dysfunction in a mouse model of oxygen induced retinopathy: restoring the balance between apoptosis and autophagy. Front Cell Neurosci. 2017;11:395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Martini D, Monte MD, Ristori C, et al. Antiangiogenic effects of β2‐adrenergic receptor blockade in a mouse model of oxygen‐induced retinopathy. J Neurochem. 2011;119(6):1317‐1329. [DOI] [PubMed] [Google Scholar]
- 28. Filippi L, Cavallaro G, Bagnoli P, et al. Oral propranolol for retinopathy of prematurity: risks, safety concerns, and perspectives. J Pediatr. 2013;163(6):1570‐1577. [DOI] [PubMed] [Google Scholar]
- 29. Makhoul IR, Peleg O, Miller B, et al. Oral propranolol versus placebo for retinopathy of prematurity: a pilot, randomised, double‐blind prospective study. Arch Dis Child. 2013;98(7):565‐567. [DOI] [PubMed] [Google Scholar]
- 30. Bancalari A, Schade R, Muñoz T, Lazcano C, Parada R, Peña R. Oral propranolol in early stages of retinopathy of prematurity. J Perinat Med. 2016;44(5):499‐503. [DOI] [PubMed] [Google Scholar]
- 31. Korkmaz L, Baştuğ O, Ozdemir A, et al. The efficacy of propranolol in retinopathy of prematurity and its correlation with the platelet mass index. Curr Eye Res. 2017;42(1):88‐97. [DOI] [PubMed] [Google Scholar]
- 32. Filippi L, Cavallaro G, Bagnoli P, et al. Propranolol 0.1% eye micro‐drops in newborns with retinopathy of prematurity: a pilot clinical trial. Pediatr Res. 2017;81(2):307‐314. [DOI] [PubMed] [Google Scholar]
- 33. Filippi L, Cavallaro G, Berti E, et al. Propranolol 0.2% eye micro‐drops for retinopathy of prematurity: a prospective phase IIB study. Front Pediatr. 2019;7:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kaempfen S, Neumann RP, Jost K, Schulzke SM. Beta‐blockers for prevention and treatment of retinopathy of prematurity in preterm infants. Cochrane Database Syst Rev. 2018. 2;3(3):CD011893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kong HB, Zheng GY, He BM, Zhang Y, Zhou Q. Clinical efficacy and safety of propranolol in the prevention and treatment of retinopathy of prematurity: a meta‐analysis of randomized controlled trials. Front Pediatr. 2021;9:631673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chen J, Joyal JS, Hatton CJ, et al. Propranolol inhibition of β‐adrenergic receptor does not suppress pathologic neovascularization in oxygen‐induced retinopathy. Invest Ophthalmol Vis Sci. 2012;53(6):2968‐2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chan CK, Pham LN, Zhou J, Spee C, Ryan SJ, Hinton DR. Differential expression of pro‐ and antiangiogenic factors in mouse strain‐dependent hypoxia‐induced retinal neovascularization. Lab Invest. 2005;85(6):721‐733. [DOI] [PubMed] [Google Scholar]
- 38. Filippi L, Dal Monte M, Bagnoli P. Different efficacy of propranolol in mice with oxygen‐induced retinopathy: could differential effects of propranolol be related to differences in mouse strains? Invest Ophthalmol Vis Sci. 2012. 30;53(11):7421‐7423. [DOI] [PubMed] [Google Scholar]
- 39. Emorine LJ, Marullo S, Briend‐Sutren MM, et al. Molecular characterization of the human beta 3‐adrenergic receptor. Science. 1989;245(4922):1118‐1121. [DOI] [PubMed] [Google Scholar]
- 40. Bylund DB, Eikenberg DC, Hieble JP, et al. International Union of Pharmacology nomenclature of adrenoceptors. Pharmacol Rev. 1994;46(2):121‐136. [PubMed] [Google Scholar]
- 41. Schena G, Caplan MJ. Everything you always wanted to know about β3‐AR * (* but were afraid to ask). Cells. 2019;8(4):357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nahmias C, Blin N, Elalouf JM, Mattei MG, Strosberg AD, Emorine LJ. Molecular characterization of the mouse beta 3‐adrenergic receptor: relationship with the atypical receptor of adipocytes. EMBO J. 1991;10(12):3721‐3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Krief S, Lönnqvist F, Raimbault S, et al. Tissue distribution of beta 3‐adrenergic receptor mRNA in man. J Clin Invest. 1993;91(1):344‐349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chamberlain PD, Jennings KH, Paul F, et al. The tissue distribution of the human beta3‐adrenoceptor studied using a monoclonal antibody: direct evidence of the beta3‐adrenoceptor in human adipose tissue, atrium and skeletal muscle. Int J Obes Relat Metab Disord. 1999;23(10):1057‐1065. [DOI] [PubMed] [Google Scholar]
- 45. De Matteis R, Arch JR, Petroni ML, Ferrari D, Cinti S, Stock MJ. Immunohistochemical identification of the beta(3)‐adrenoceptor in intact human adipocytes and ventricular myocardium: effect of obesity and treatment with ephedrine and caffeine. Int J Obes Relat Metab Disord. 2002;26(11):1442‐1450. [DOI] [PubMed] [Google Scholar]
- 46. Sennitt MV, Kaumann AJ, Molenaar P, et al. The contribution of classical (beta1/2‐) and atypical beta‐adrenoceptors to the stimulation of human white adipocyte lipolysis and right atrial appendage contraction by novel beta3‐adrenoceptor agonists of differing selectivities. J Pharmacol Exp Ther. 1998;285(3):1084‐1095. [PubMed] [Google Scholar]
- 47. Hodis J, Vaclavíková R, Farghali H. Beta‐3 agonist‐induced lipolysis and nitric oxide production: relationship to PPARgamma agonist/antagonist and AMP kinase modulation. Gen Physiol Biophys. 2011;30(1):90‐99. [DOI] [PubMed] [Google Scholar]
- 48. Gauthier C, Tavernier G, Charpentier F, Langin D, Le, Marec H. Functional beta3‐adrenoceptor in the human heart. J Clin Invest. 1996;98(2):556‐562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cannavo A, Koch WJ. Targeting β3‐adrenergic receptors in the heart: selective agonism and β‐blockade. J Cardiovasc Pharmacol. 2017;69(2):71‐78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cheng HJ, Zhang ZS, Onishi K, Ukai T, Sane DC, Cheng CP. Upregulation of functional beta(3)‐adrenergic receptor in the failing canine myocardium. Circ Res. 2001;89(7):599‐606. [DOI] [PubMed] [Google Scholar]
- 51. Moniotte S, Kobzik L, Feron O, Trochu JN, Gauthier C, Balligand JL. Upregulation of beta(3)‐adrenoceptors and altered contractile response to inotropic amines in human failing myocardium. Circulation. 2001;103(12):1649‐1655. [DOI] [PubMed] [Google Scholar]
- 52. Dessy C, Moniotte S, Ghisdal P, Havaux X, Noirhomme P, Balligand JL. Endothelial beta3‐adrenoceptors mediate vasorelaxation of human coronary microarteries through nitric oxide and endothelium‐dependent hyperpolarization. Circulation. 2004;110(8):948‐954. [DOI] [PubMed] [Google Scholar]
- 53. Dessy C, Balligand JL. Beta3‐adrenergic receptors in cardiac and vascular tissues emerging concepts and therapeutic perspectives. Adv Pharmacol. 2010;59:135‐163. [DOI] [PubMed] [Google Scholar]
- 54. Niu X, Watts VL, Cingolani OH, et al. Cardioprotective effect of beta‐3 adrenergic receptor agonism: role of neuronal nitric oxide synthase. J Am Coll Cardiol. 2012;59(22):1979‐1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Watts VL, Sepulveda FM, Cingolani OH, et al. Anti‐hypertrophic and anti‐oxidant effect of beta3‐adrenergic stimulation in myocytes requires differential neuronal NOS phosphorylation. J Mol Cell Cardiol. 2013;62:8‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Niu X, Zhao L, Li X, et al. β3‐Adrenoreceptor stimulation protects against myocardial infarction injury via eNOS and nNOS activation. PLOS One. 2014;9(6):e98713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Gauthier C, Leblais V, Kobzik L, et al. The negative inotropic effect of beta3‐adrenoceptor stimulation is mediated by activation of a nitric oxide synthase pathway in human ventricle. J Clin Invest. 1998;102(7):1377‐1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Balligand JL. Cardiac salvage by tweaking with beta‐3‐adrenergic receptors. Cardiovasc Res. 2016;111(2):128‐133. [DOI] [PubMed] [Google Scholar]
- 59. Bubb KJ, Ravindran D, Cartland SP, et al. β3 adrenergic receptor stimulation promotes reperfusion in ischemic limbs in a murine diabetic model. Front Pharmacol. 2021;12:666334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Salie R, Alsalhin AKH, Marais E, Lochner A. Cardioprotective effects of beta3‐adrenergic receptor (β3‐AR) pre‐, per‐, and post‐treatment in ischemia‐reperfusion. Cardiovasc Drugs Ther. 2019;33(2):163‐177. [DOI] [PubMed] [Google Scholar]
- 61. Chisholm KM, Chang KW, Truong MT, Kwok S, West RB, Heerema‐McKenney AE. β‐Adrenergic receptor expression in vascular tumors. Mod Pathol. 2012;25(11):1446‐1451. [DOI] [PubMed] [Google Scholar]
- 62. Phillips JD, Zhang H, Wei T, Richter GT. Expression of β‐adrenergic receptor subtypes in proliferative, involuted, and propranolol‐responsive infantile hemangiomas. JAMA Facial Plast Surg. 2017;19(2):102‐107. [DOI] [PubMed] [Google Scholar]
- 63. Bassi A, Filippeschi C, Oranges T, et al. Infantile hemangiomas β3‐adrenoceptor overexpression is associated with nonresponse to propranolol. Pediatr Res. Published online March 2, 2021. 10.1038/s41390-021-01385-x [DOI] [PubMed] [Google Scholar]
- 64. Uhlén M, Fagerberg L, Hallström BM, et al. Proteomics. Tissue‐based map of the human proteome. Science. 2015;347(6220):1260419. [DOI] [PubMed] [Google Scholar]
- 65. Evans BA, Papaioannou M, Bonazzi VR, Summers RJ. Expression of beta 3‐adrenoceptor mRNA in rat tissues. Br J Pharmacol. 1996;117(1):210‐216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Li X, Kim Y, Tsang EK, et al. The impact of rare variation on gene expression across tissues. Nature. 2017;550(7675):239‐243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Dal Monte M, Evans BA, Arioglu‐Inan E, Michel MC. Upregulation of β3‐adrenoceptors‐a general marker of and protective mechanism against hypoxia? Naunyn Schmiedebergs Arch Pharmacol. 2020;393(2):141‐146. [DOI] [PubMed] [Google Scholar]
- 68. Steinle JJ, Booz GW, Meininger CJ, Day JN, Granger HJ. Beta 3‐adrenergic receptors regulate retinal endothelial cell migration and proliferation. J Biol Chem. 2003;278(23):20681‐20686. [DOI] [PubMed] [Google Scholar]
- 69. Mori A, Miwa T, Sakamoto K, Nakahara T, Ishii K. Pharmacological evidence for the presence of functional beta(3)‐adrenoceptors in rat retinal blood vessels. Naunyn Schmiedebergs Arch Pharmacol. 2010;382(2):119‐126. [DOI] [PubMed] [Google Scholar]
- 70. Mulder AL, Golde JM, Goor AA, Giussani DA, Blanco CE. Developmental changes in plasma catecholamine concentrations during normoxia and acute hypoxia in the chick embryo. J Physiol. 2000;527(pt 3):593‐599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Borovsky V, Herman M, Dunphy G, Caplea A, Ely D. CO2 asphyxia increases plasma norepinephrine in rats via sympathetic nerves. Am J Physiol. 1998;274(1):R19‐R22. [DOI] [PubMed] [Google Scholar]
- 72. Hoffmann C, Leitz MR, Oberdorf‐Maass S, Lohse MJ, Klotz KN. Comparative pharmacology of human beta‐adrenergic receptor subtypes—characterization of stably transfected receptors in CHO cells. Naunyn Schmiedebergs Arch Pharmacol. 2004;369(2):151‐159. [DOI] [PubMed] [Google Scholar]
- 73. Antoni MH, Lutgendorf SK, Cole SW, et al. The influence of bio‐behavioural factors on tumour biology: pathways and mechanisms. Nat Rev Cancer. 2006;6(3):240‐248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Tang J, Li Z, Lu L, Cho CH. β‐Adrenergic system, a backstage manipulator regulating tumour progression and drug target in cancer therapy. Semin Cancer Biol. 2013;23(6 pt B):533‐542. [DOI] [PubMed] [Google Scholar]
- 75. Watkins JL, Thaker PH, Nick AM, et al. Clinical impact of selective and nonselective beta‐blockers on survival in patients with ovarian cancer. Cancer. 2015;121(19):3444‐3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Weberpals J, Jansen L, Carr PR, Hoffmeister M, Brenner H. Beta blockers and cancer prognosis—the role of immortal time bias: a systematic review and meta‐analysis. Cancer Treat Rev. 2016;47:1‐11. [DOI] [PubMed] [Google Scholar]
- 77. Barron TI, Connolly RM, Sharp L, Bennett K, Visvanathan K. Beta blockers and breast cancer mortality: a population‐based study. J Clin Oncol. 2011;29(19):2635‐2644. [DOI] [PubMed] [Google Scholar]
- 78. Eng JW, Kokolus KM, Reed CB, Hylander BL, Ma WW, Repasky EA. A nervous tumor microenvironment: the impact of adrenergic stress on cancer cells, immunosuppression, and immunotherapeutic response. Cancer Immunol Immunother. 2014;63(11):1115‐1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Laukova M, Vargovic P, Vlcek M, et al. Catecholamine production is differently regulated in splenic T‐ and B‐cells following stress exposure. Immunobiology. 2013;218(5):780‐789. [DOI] [PubMed] [Google Scholar]
- 80. Perrone MG, Notarnicola M, Caruso MG, Tutino V, Scilimati A. Upregulation of beta3‐adrenergic receptor mRNA in human colon cancer: a preliminary study. Oncology. 2008;75(3–4):224‐229. [DOI] [PubMed] [Google Scholar]
- 81. Lamkin DM, Sloan EK, Patel AJ, et al. Chronic stress enhances progression of acute lymphoblastic leukemia via β‐adrenergic signaling. Brain Behav Immun. 2012;26(4):635‐641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Montoya A, Amaya CN, Belmont A, et al. Use of non‐selective β‐blockers is associated with decreased tumor proliferative indices in early‐stage breast cancer. Oncotarget. 2017;8(4):6446‐6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Dal Monte M, Casini G, Filippi L, Nicchia GP, Svelto M, Bagnoli P. Functional involvement of β3‐adrenergic receptors in melanoma growth and vascularization. J Mol Med (Berl). 2013;91(12):1407‐1419. [DOI] [PubMed] [Google Scholar]
- 84. Dal Monte M, Fornaciari I, Nicchia GP, Svelto M, Casini G, Bagnoli P. β3‐adrenergic receptor activity modulates melanoma cell proliferation and survival through nitric oxide signaling. Naunyn Schmiedebergs Arch Pharmacol. 2014;387(6):533‐543. [DOI] [PubMed] [Google Scholar]
- 85. Sereni F, Dal Monte M, Filippi L, Bagnoli P. Role of host β1‐ and β2‐adrenergic receptors in a murine model of B16 melanoma: functional involvement of β3‐adrenergic receptors. Naunyn Schmiedebergs Arch Pharmacol. 2015;388(12):1317‐1331. [DOI] [PubMed] [Google Scholar]
- 86. Filippi L, Bruno G, Domazetovic V, Favre C, Calvani M. Current therapies and new targets to fight melanoma: a promising role for the β3‐adrenoreceptor. Cancers (Basel). 2020;12(6):1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Rains SL, Amaya CN, Bryan BA. Beta‐adrenergic receptors are expressed across diverse cancers. Oncoscience. 2017;4(7–8):95‐105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Calvani M, Pelon F, Comito G, et al. Norepinephrine promotes tumor microenvironment reactivity through β3‐adrenoreceptors during melanoma progression. Oncotarget. 2015;6(7):4615‐4632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Axelson H, Fredlund E, Ovenberger M, Landberg G, Påhlman S. Hypoxia‐induced dedifferentiation of tumor cells—a mechanism behind heterogeneity and aggressiveness of solid tumors. Semin Cell Dev Biol. 2005;16(4–5):554‐563. [DOI] [PubMed] [Google Scholar]
- 90. Magnon C, Hall SJ, Lin J, et al. Autonomic nerve development contributes to prostate cancer progression. Science. 2013;341(6142):1236361. [DOI] [PubMed] [Google Scholar]
- 91. Manzo G. Similarities between embryo development and cancer process suggest new strategies for research and therapy of tumors: a new point of view. Front Cell Dev Biol. 2019;7:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Soundararajan R, Rao AJ. Trophoblast 'pseudo‐tumorigenesis': significance and contributory factors. Reprod Biol Endocrinol. 2004;2:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Murray MJ, Lessey BA. Embryo implantation and tumor metastasis: common pathways of invasion and angiogenesis. Semin Reprod Endocrinol. 1999;17(3):275‐290. [DOI] [PubMed] [Google Scholar]
- 94. Ridolfi L, Petrini M, Fiammenghi L, Riccobon A, Ridolfi R. Human embryo immune escape mechanisms rediscovered by the tumor. Immunobiology. 2009;214(1):61‐76. [DOI] [PubMed] [Google Scholar]
- 95. Wepsic HT. Overview of oncofetal antigens in cancer. Ann Clin Lab Sci. 1983;13(4):261‐266. [PubMed] [Google Scholar]
- 96. Sidransky D. Emerging molecular markers of cancer. Nat Rev Cancer. 2002;2(3):210‐219. [DOI] [PubMed] [Google Scholar]
- 97. Slotkin TA, Auman JT, Seidler FJ. Ontogenesis of beta‐adrenoceptor signaling: implications for perinatal physiology and for fetal effects of tocolytic drugs. J Pharmacol Exp Ther. 2003;306(1):1‐7. [DOI] [PubMed] [Google Scholar]
- 98. Cikos S, Veselá J, Il'ková G, Rehák P, Czikková S, Koppel J. Expression of beta adrenergic receptors in mouse oocytes and preimplantation embryos. Mol Reprod Dev. 2005;71(2):145‐153. [DOI] [PubMed] [Google Scholar]
- 99. Čikoš Š, Czikková S, Chrenek P, et al. Expression of adrenergic receptors in bovine and rabbit oocytes and preimplantation embryos. Reprod Domest Anim. 2014;49(1):92‐100. [DOI] [PubMed] [Google Scholar]
- 100. Adeoya‐Osiguwa SA, Gibbons R, Fraser LR. Identification of functional alpha2‐ and beta‐adrenergic receptors in mammalian spermatozoa. Hum Reprod. 2006;21(6):1555‐1563. [DOI] [PubMed] [Google Scholar]
- 101. Fujinaga M, Scott JC. Gene expression of catecholamine synthesizing enzymes and beta adrenoceptor subtypes during rat embryogenesis. Neurosci Lett. 1997;231(2):108‐112. [DOI] [PubMed] [Google Scholar]
- 102. Resch BE, Ducza E, Gáspár R, Falkay G. Role of adrenergic receptor subtypes in the control of human placental blood vessels. Mol Reprod Dev. 2003;66(2):166‐171. [DOI] [PubMed] [Google Scholar]
- 103. Hynes PG, Friel AM, Smith TJ, Morrison JJ. Beta‐adrenoceptor subtype expression in human placenta and umbilical arteries in normal and preeclamptic pregnancies. Hypertens Pregnancy. 2008;27(2):169‐181. [DOI] [PubMed] [Google Scholar]
- 104. Rouget C, Bardou M, Breuiller‐Fouché M, et al. Beta3‐adrenoceptor is the predominant beta‐adrenoceptor subtype in human myometrium and its expression is up‐regulated in pregnancy. J Clin Endocrinol Metab. 2005;90(3):1644‐1650. [DOI] [PubMed] [Google Scholar]
- 105. Bardou M, Rouget C, Breuiller‐Fouché M, et al. Is the beta3‐adrenoceptor (ADRB3) a potential target for uterorelaxant drugs? BMC Pregnancy Childbirth. 2007;7(suppl 1):S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Dennedy MC, Houlihan DD, McMillan H, Morrison JJ. Beta2‐ and beta3‐adrenoreceptor agonists: human myometrial selectivity and effects on umbilical artery tone. Am J Obstet Gynecol. 2002;187(3):641‐647. [DOI] [PubMed] [Google Scholar]
- 107. Holtan SG, Creedon DJ, Haluska P, Markovic SN. Cancer and pregnancy: parallels in growth, invasion, and immune modulation and implications for cancer therapeutic agents. Mayo Clin Proc. 2009;84(11):985‐1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Ma Y, Zhang P, Wang F, Yang J, Yang Z, Qin H. The relationship between early embryo development and tumourigenesis. J Cell Mol Med. 2010;14(12):2697‐2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Costanzo V, Bardelli A, Siena S, Abrignani S. Exploring the links between cancer and placenta development. Open Biol. 2018;8(6):180081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Wei X, Chen Y, Jiang X, et al. Mechanisms of vasculogenic mimicry in hypoxic tumor microenvironments. Mol Cancer. 2021;20(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Rai A, Cross JC. Development of the hemochorial maternal vascular spaces in the placenta through endothelial and vasculogenic mimicry. Dev Biol. 2014;387(2):131‐141. [DOI] [PubMed] [Google Scholar]
- 112. Petrova V, Annicchiarico‐Petruzzelli M, Melino G, Amelio I. The hypoxic tumour microenvironment. Oncogenesis. 2018;7(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Jauniaux E, Watson A, Burton G. Evaluation of respiratory gases and acid‐base gradients in human fetal fluids and uteroplacental tissue between 7 and 16 weeks' gestation. Am J Obstet Gynecol. 2001;184(5):998‐1003. [DOI] [PubMed] [Google Scholar]
- 114. Smith DG, Sturmey RG. Parallels between embryo and cancer cell metabolism. Biochem Soc Trans. 2013;41(2):664‐669. [DOI] [PubMed] [Google Scholar]
- 115. Warburg O, Wind F, Negelein E. The metabolism of tumors in the body. J Gen Physiol. 1927;8(6):519‐530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Krisher RL, Prather RS. A role for the Warburg effect in preimplantation embryo development: metabolic modification to support rapid cell proliferation. Mol Reprod Dev. 2012;79(5):311‐320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324(5930):1029‐1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Liberti MV, Locasale JW. The Warburg effect: how does it benefit cancer cells? Trends Biochem Sci. 2016;41(3):211‐218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Guérin P, El Mouatassim S, Ménézo Y. Oxidative stress and protection against reactive oxygen species in the pre‐implantation embryo and its surroundings. Hum Reprod Update. 2001;7(2):175‐189. [DOI] [PubMed] [Google Scholar]
- 120. Redel BK, Brown AN, Spate LD, Whitworth KM, Green JA, Prather RS. Glycolysis in preimplantation development is partially controlled by the Warburg effect. Mol Reprod Dev. 2012;79(4):262‐271. [DOI] [PubMed] [Google Scholar]
- 121. Ma LN, Huang XB, Muyayalo KP, Mor G, Liao AH. Lactic acid: a novel signaling molecule in early pregnancy? Front Immunol. 2020;11:279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. San‐Millán I, Brooks GA. Reexamining cancer metabolism: lactate production for carcinogenesis could be the purpose and explanation of the Warburg effect. Carcinogenesis. 2017;38(2):119‐133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Longley DB, Johnston PG. Molecular mechanisms of drug resistance. J Pathol. 2005;205(2):275‐292. [DOI] [PubMed] [Google Scholar]
- 124. Mathias AA, Hitti J, Unadkat JD. P‐glycoprotein and breast cancer resistance protein expression in human placentae of various gestational ages. Am J Physiol Regul Integr Comp Physiol. 2005;289(4):R963‐R969. [DOI] [PubMed] [Google Scholar]
- 125. Wu G, Fang YZ, Yang S, Lupton JR, Turner ND. Glutathione metabolism and its implications for health. J Nutr. 2004;134(3):489‐492. [DOI] [PubMed] [Google Scholar]
- 126. Hansen JM, Harris C. Glutathione during embryonic development. Biochim Biophys Acta. 2015;1850(8):1527‐1542. [DOI] [PubMed] [Google Scholar]
- 127. Traverso N, Ricciarelli R, Nitti M, et al. Role of glutathione in cancer progression and chemoresistance. Oxid Med Cell Longev. 2013;2013:972913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133(5):775‐787. [DOI] [PubMed] [Google Scholar]
- 129. Pan PY, Wang GX, Yin B, et al. Reversion of immune tolerance in advanced malignancy: modulation of myeloid‐derived suppressor cell development by blockade of stem‐cell factor function. Blood. 2008;111(1):219‐228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Ahmadi M, Mohammadi M, Ali‐Hassanzadeh M, Zare M, Gharesi‐Fard B. MDSCs in pregnancy: critical players for a balanced immune system at the feto‐maternal interface. Cell Immunol. 2019;346:103990. [DOI] [PubMed] [Google Scholar]
- 131. Michaëlsson J, Mold JE, McCune JM, Nixon DF. Regulation of T cell responses in the developing human fetus. J Immunol. 2006;176(10):5741‐5748. [DOI] [PubMed] [Google Scholar]
- 132. Cheong HI, Asosingh K, Stephens OR, et al. Hypoxia sensing through β‐adrenergic receptors. JCI Insight. 2016;1(21):e90240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Karagenc L, Sertkaya Z, Ciray N, Ulug U, Bahçeci M. Impact of oxygen concentration on embryonic development of mouse zygotes. Reprod Biomed Online. 2004;9(4):409‐417. [DOI] [PubMed] [Google Scholar]
- 134. Gardner DK. Lactate production by the mammalian blastocyst: manipulating the microenvironment for uterine implantation and invasion? BioEssays. 2015;37(4):364‐371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Zuo RJ, Gu XW, Qi QR, et al. Warburg‐like glycolysis and lactate shuttle in mouse decidua during early pregnancy. J Biol Chem. 2015;290(35):21280‐21291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. de la Cruz‐López KG, Castro‐Muñoz LJ, Reyes‐Hernández DO, García‐Carrancá A, Manzo‐Merino J. Lactate in the regulation of tumor microenvironment and therapeutic approaches. Front Oncol. 2019;9:1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Calvani M, Cavallini L, Tondo A, et al. β3‐Adrenoreceptors control mitochondrial dormancy in melanoma and embryonic stem cells. Oxid Med Cell Longev. 2018;2018:6816508‐6816510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Samudio I, Fiegl M, McQueen T, Clise‐Dwyer K, Andreeff M. The Warburg effect in leukemia‐stroma cocultures is mediated by mitochondrial uncoupling associated with uncoupling protein 2 activation. Cancer Res. 2008;68(13):5198‐5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Wang X, Zhang H, Chen X. Drug resistance and combating drug resistance in cancer. Cancer Drug Resist. 2019;2:141‐160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Xie J, Li DW, Chen XW, Wang F, Dong P. Expression and significance of hypoxia‐inducible factor‐1α and MDR1/P‐glycoprotein in laryngeal carcinoma tissue and hypoxic Hep‐2 cells. Oncol Lett. 2013;6(1):232‐238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Ding Z, Yang L, Xie X, et al. Expression and significance of hypoxia‐inducible factor‐1 alpha and MDR1/P‐glycoprotein in human colon carcinoma tissue and cells. J Cancer Res Clin Oncol. 2010;136(11):1697‐1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Chen J, Ding Z, Peng Y, et al. HIF‐1α inhibition reverses multidrug resistance in colon cancer cells via downregulation of MDR1/P‐glycoprotein. PLOS One. 2014;9(6):e98882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Muz B, Kusdono HD, Azab F, et al. Tariquidar sensitizes multiple myeloma cells to proteasome inhibitors via reduction of hypoxia‐induced P‐gp‐mediated drug resistance. Leuk Lymphoma. 2017;58(12):2916‐2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Yoshioka Y, Kadoi H, Yamamuro A, Ishimaru Y, Maeda S. Noradrenaline increases intracellular glutathione in human astrocytoma U‐251 MG cells by inducing glutamate‐cysteine ligase protein via β3‐adrenoceptor stimulation. Eur J Pharmacol. 2016;772:51‐61. [DOI] [PubMed] [Google Scholar]
- 145. Calvani M, Dabraio A, Bruno G, et al. β3‐Adrenoreceptor blockade reduces hypoxic myeloid leukemic cells survival and chemoresistance. Int J Mol Sci. 2020;21(12):4210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Pan ST, Li ZL, He ZX, Qiu JX, Zhou SF. Molecular mechanisms for tumour resistance to chemotherapy. Clin Exp Pharmacol Physiol. 2016;43(8):723‐737. [DOI] [PubMed] [Google Scholar]
- 147. Arck PC, Hecher K. Fetomaternal immune cross‐talk and its consequences for maternal and offspring's health. Nat Med. 2013;19(5):548‐556. [DOI] [PubMed] [Google Scholar]
- 148. Barnet MB, Blinman P, Cooper W, Boyer MJ, Kao S, Goodnow CC. Understanding immune tolerance of cancer: re‐purposing insights from fetal allografts and microbes. BioEssays. 2018;40(8):e1800050. [DOI] [PubMed] [Google Scholar]
- 149. Lu B, Finn OJ. T‐cell death and cancer immune tolerance. Cell Death Differ. 2008;15(1):70‐79. [DOI] [PubMed] [Google Scholar]
- 150. Rackaityte E, Halkias J. Mechanisms of fetal T cell tolerance and immune regulation. Front Immunol. 2020;11:588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Vito A, El‐Sayes N, Mossman K. Hypoxia‐driven immune escape in the tumor microenvironment. Cells. 2020;9(4):992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Facciabene A, Peng X, Hagemann IS, et al. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and T(reg) cells. Nature. 2011;475(7355):226‐230. [DOI] [PubMed] [Google Scholar]
- 153. Barsoum IB, Koti M, Siemens DR, Graham CH. Mechanisms of hypoxia‐mediated immune escape in cancer. Cancer Res. 2014;74(24):7185‐7190. [DOI] [PubMed] [Google Scholar]
- 154. Kokolus KM, Zhang Y, Sivik JM, et al. Beta blocker use correlates with better overall survival in metastatic melanoma patients and improves the efficacy of immunotherapies in mice. Oncoimmunology. 2017;7(3):e1405205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Calvani M, Bruno G, Dal Monte M, et al. β3‐Adrenoceptor as a potential immuno‐suppressor agent in melanoma. Br J Pharmacol. 2019;176(14):2509‐2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Calvani M, Dabraio A, Subbiani A, et al. β3‐Adrenoceptors as putative regulator of immune tolerance in cancer and pregnancy. Front Immunol. 2020;11:2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Di Mattia M, Mauro A, Citeroni MR, et al. Insight into hypoxia stemness control. Cells. 2021;10(8):2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Saheera S, Potnuri AG, Nair RR. Modulation of cardiac stem cell characteristics by metoprolol in hypertensive heart disease. Hypertens Res. 2018;41(4):253‐262. [DOI] [PubMed] [Google Scholar]
- 159. Calvani M, Bruno G, Dabraio A, et al. β3‐Adrenoreceptor blockade induces stem cells differentiation in melanoma microenvironment. Int J Mol Sci. 2020;21(4):1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Ma X, Zhao T, Ouyang T, Xin S, Ma Y, Chang M. Propranolol enhanced adipogenesis instead of induction of apoptosis of hemangiomas stem cells. Int J Clin Exp Pathol. 2014;7(7):3809‐3817. [PMC free article] [PubMed] [Google Scholar]
- 161. Bruno G, Cencetti F, Pini A, et al. β3‐adrenoreceptor blockade reduces tumor growth and increases neuronal differentiation in neuroblastoma via SK2/S1P2 modulation. Oncogene. 2020;39(2):368‐384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Krock BL, Skuli N, Simon MC. Hypoxia‐induced angiogenesis: good and evil. Genes Cancer. 2011;2(12):1117‐1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Haigh JJ. Role of VEGF in organogenesis. Organogenesis. 2008;4(4):247‐256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Dice JE, Bhatia J. Patent ductus arteriosus: an overview. J Pediatr Pharmacol Ther. 2007;12(3):138‐146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Wang GL, Semenza GL. Characterization of hypoxia‐inducible factor 1 and regulation of DNA binding activity by hypoxia. J Biol Chem. 1993;268(29):21513‐21518. [PubMed] [Google Scholar]
- 166. Semenza GL. Hydroxylation of HIF‐1: oxygen sensing at the molecular level. Physiology (Bethesda). 2004;19:176‐182. [DOI] [PubMed] [Google Scholar]
- 167. Kovalcik V. The response of the isolated ductus arteriosus to oxygen and anoxia. J Physiol. 1963;169(1):185‐197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Coceani F, Baragatti B. Mechanisms for ductus arteriosus closure. Semin Perinatol. 2012;36(2):92‐97. [DOI] [PubMed] [Google Scholar]
- 169. Pini A, Fazi C, Nardini P, et al. Effect of beta 3 adrenoreceptor modulation on patency of the ductus arteriosus. Cells. 2020;9(12):2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Ahmed A, Dunk C, Ahmad S, Khaliq A. Regulation of placental vascular endothelial growth factor (VEGF) and placenta growth factor (PIGF) and soluble Flt‐1 by oxygen—a review. Placenta. 2000;21(suppl A):S16‐S24. [DOI] [PubMed] [Google Scholar]
- 171. Fraisl P, Mazzone M, Schmidt T, Carmeliet P. Regulation of angiogenesis by oxygen and metabolism. Dev Cell. 2009;16(2):167‐179. [DOI] [PubMed] [Google Scholar]
- 172. Smith LE, Hard AL, Hellström A. The biology of retinopathy of prematurity: how knowledge of pathogenesis guides treatment. Clin Perinatol. 2013;40(2):201‐214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Buczynski BW, Maduekwe ET, O'Reilly MA. The role of hyperoxia in the pathogenesis of experimental BPD. Semin Perinatol. 2013;37(2):69‐78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Abman SH. Bronchopulmonary dysplasia: “a vascular hypothesis”. Am J Respir Crit Care Med. 2001;164(10 pt 1):1755‐1756. [DOI] [PubMed] [Google Scholar]
- 175. Trollmann R, Gassmann M. The role of hypoxia‐inducible transcription factors in the hypoxic neonatal brain. Brain Dev. 2009;31(7):503‐509. [DOI] [PubMed] [Google Scholar]
- 176. Nankervis CA, Giannone PJ, Reber KM. The neonatal intestinal vasculature: contributing factors to necrotizing enterocolitis. Semin Perinatol. 2008;32(2):83‐91. [DOI] [PubMed] [Google Scholar]
- 177. Ballabh P, Braun A, Nedergaard M. Anatomic analysis of blood vessels in germinal matrix, cerebral cortex, and white matter in developing infants. Pediatr Res. 2004;56(1):117‐124. [DOI] [PubMed] [Google Scholar]
- 178. White WB, Siddiqui E, Tat T, Franks B, Schermer CR. Cardiovascular safety of mirabegron: analysis of an integrated clinical trial database of patients with overactive bladder syndrome. J Am Soc Hypertens. 2018;12(11):768‐778. [DOI] [PubMed] [Google Scholar]
- 179. Baskin AS, Linderman JD, Brychta RJ, et al. Regulation of human adipose tissue activation, gallbladder size, and bile acid metabolism by a β3‐adrenergic receptor agonist. Diabetes. 2018;67(10):2113‐2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Park JS, Lee YS, Lee CN, Kim SH, Kim SW, Han SW. Efficacy and safety of mirabegron, a β3‐adrenoceptor agonist, for treating neurogenic bladder in pediatric patients with spina bifida: a retrospective pilot study. World J Urol. 2019;37(8):1665‐1670. [DOI] [PubMed] [Google Scholar]
- 181. Rittig S, Baka‐Ostrowska M, Tøndel C, et al. The pharmacokinetics, safety, and tolerability of mirabegron in children and adolescents with neurogenic detrusor overactivity or idiopathic overactive bladder and development of a population pharmacokinetic model‐based pediatric dose estimation. J Pediatr Urol. 2020;16(1):31. [DOI] [PubMed] [Google Scholar]
- 182. Keam SJ. Mirabegron: pediatric first approval. Paediatr Drugs. 2021;23(4):411‐415. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


