Abstract
An in vitro intestinal tissue model was developed for the investigation of bacterial association in the pig small intestine under different dietary regimes. In preliminary experiments, jejunal and ileal tissue was taken from Danish Landrace pigs fed standard diet and inoculated with either Salmonella or nonpathogenic Escherichia coli strains. Higher numbers of salmonellae associated with the ileal tissues, but the numbers did not reach significance. Hence, jejunal sections were inoculated with nonpathogenic E. coli and ileal sections were inoculated with salmonellae in the presence of mannose or commercial nondigestible oligosaccharides (NDO) at 2.5%. There was a significant decrease in E. coli associated with the jejunum in the presence of mannose (P < 0.05). Furthermore, in pigs fed a diet supplemented with commercial NDO at 4% there was a significant reduction in the numbers of E. coli in jejunal organ cultures of pigs fed the FOS diet (P < 0.05). There was a reduction, though not a significant one, in the association of Salmonella sp. to the ileal sections of pigs fed the commercial FOS diet. The feeding of commercial GOS or its addition to organ cultures did not affect E. coli or Salmonella numbers.
With the rise in antibiotic resistance and the subsequent removal of antibiotics from pig feed there is a need to identify alternatives which can reduce incidence of Salmonella enterica and pathogenic Escherichia coli in pig herds. However, appropriate models are needed. In the case of the pig, the problem is exacerbated by the lack of epithelial cell lines, their inadequacy in situations where feed constituents can induce a plethora of effects on the gastrointestinal tract, and the difficulties in maintaining mucosal integrity in organ culture models (4). In vivo loop models are available (4), but it is not always feasible to carry out combined feed and infections trials. Hence, for the study of complex bacterium diet-host interactions in the compartmentalized gut, we describe a simple intestine organ culture model. Tissue can be taken from pigs fed alternative diets and challenged with different bacterial species, and their effects on bacterial association can be measured. In the present study we looked at the effects of feeding a commercially available FOS and GOS on nonpathogenic E. coli and Salmonella in the jejunum and ileum intestinal segments of pigs.
It has been proposed that nondigestible oligosaccharides (NDO) can be utilized preferentially by lactobacilli and bifidobacterial species (12). This leads to the production of lactic acid and an increase in short-chain fatty acid (SCFA) production, resulting in a lower pH in the large intestine and may prevent the establishment of Salmonella (11). However, FOS and GOS could also affect Salmonella and E. coli in the small intestine in a number of ways, directly by the inhibition of bacterial binding sites or indirectly by altering the morphology of the small intestinal epithelium punctuated by SCFA production (9).
Natural infection of pigs with Salmonella serovar Typhimurium is associated with enteric disease, which occurs in pigs from weaning to about 4 months old (7). Enteric lesions can be seen in the ileum accompanied by villous atrophy and spread to extraintestinal sites. A number of E. coli types have been implicated in postweaning diarrhea in pigs, including enterotoxigenic E. coli (ETEC) and verotoxigenic and enteropathogenic E. coli (18). Previous studies have shown that E. coli regarded as being enteropathogenic in pigs has been shown to cause similar signs in ligated intestine in pigs (8, 10). We studied the effects of commercial FOS and GOS on Salmonella serovar Typhimurium and nonpathogenic E. coli in a pig intestinal organ culture model in vitro.
MATERIALS AND METHODS
Experimental design.
We investigated the association of Salmonella serovar Typhimurium S986 and E. coli K-36 to both the proximal jejunum and the distal ileum taken from five pigs fed standard Danish pig feed (13). On the basis of these studies, ileal and jejunal tissues were taken from a further five pigs. The ileal tissue was inoculated with Salmonella, and the jejunal tissue was inoculated with E. coli. Mannose (2.5%) or commercial FOS or GOS at 2.5% were added. Having established the model, intestinal tissue was then taken from 30 pigs (three groups, 10 animals per group) fed a diet supplemented with commercial FOS or GOS at 4% or without (control), and these tissues were inoculated with either E. coli or Salmonella.
Preparation of inoculum.
Salmonella serovar Typhimurium S986 has been characterized in previous studies (21). In order to distinguish the E. coli inoculum from indigenous strains, a spontaneous nalidixic acid-resistant strain generated from E. coli O9:K36:H19 was used. Salmonella and E. coli were retrieved as required from cultures stored at −80°C, streaked on MacConkey agar plates, and incubated at 37°C for 16 h. Bacteria were transferred from MacConkey agar (Merck 105410) by a sweep (three colonies) to 8 ml of Luria-Bertini (LB) broth (Trypticase [Merck], 10 g/liter; yeast extract [Merck], 5 g/liter; NaCl, 5 g/liter; pH 7.5) and incubated for 3 h at 37°C. This culture (3 h) was used to inoculate 10-ml volumes of LB broth and incubated statically for 16 h at 37°C. The culture was centrifuged (1,500 × g, 15 min at 4°C), washed twice in phosphate-buffered saline (PBS; pH 7.2), and the pellet was resuspended in 10 ml of Dulbecco modified Eagle medium (DMEM) (Gibco) to give an inoculum of 5 × 108 CFU/ml.
Organ culture preparation.
Five pigs Danish (Landrace Yorkshire crossbred) 4 weeks after weaning (18 to 20 kg) fed standard Danish pig diet were chosen at random from the high health status herd at the Danish Institute of Agricultural Science, Foulum, Denmark. The pigs were killed with a lethal injection of pentobarbital sodium (200 g/liter). Animal experimentation and care of experimental animals complied with the regulations of the Danish Ministry of Justice (Law no. 726 [December 1993]). Immediately after slaughter, the abdominal wall was opened by a midline incision, and the gastrointestinal tract was removed. Lengths of small intestinal tissue were taken with 5-cm spaces between the segments. Three lengths (11 cm each) were taken aseptically from the ileum (30 cm from the ileocecal valve), and a further three lengths (11 cm each) were taken from the mid-jejunum, immersed in DMEM (Gibco), and kept on ice. Mesenteric membrane and fat tissue was carefully removed from the segments.
Two pieces of polyethylene tubing (Siltube, Eurpharm; inner diameter of 6 mm) were inserted a distance of 10 mm into either end of the tissue segment, and a suture was applied (USP 3; Kruuse) to keep the tubing in place. The tissue was washed through with 100 ml of PBS (pH 7.2) using a FillMaster pump (Type 311; Delta Scientific Medical) (flow rate of 7.7 cm/s) to remove the lumen content. A 21-gauge needle was then connected to the open end of the tubing, 10 ml of DMEM alone (control) or DMEM containing either nonpathogenic E. coli or Salmonella serovar Typhimurium S986 was inoculated, and the segment was sealed using Teflon plugs (5-cm inner diameter). The organ culture was immersed in DMEM in a 300-ml infusion bottle in a shaking water bath (150 rpm) at 37°C in a 10% CO2 atmosphere.
After 60 min, the tissue was removed from the infusion bottle, and the tissue was washed through with 100 ml of PBS. The tissues were weighed and homogenized on ice with a Janke-Kunkel Ultra-Turrax T25 homogenizer (NL) at 20,000 rpm on ice for 20 s in PBS plus Triton X-100 (1%). A 10-fold dilution series was prepared in triplicate from the homogenates to a final dilution of 10−6. Lactose and non-lactose fermenters were enumerated on MacConkey Agar, salmonellae were isolated on Brilliant Phenol Lysine Sucrose agar (Merck 1.10747), and E. coli was isolated on LB agar (tryptone [Merck], 10 g/liter; yeast extract [Merck], 5 g/liter; NaCl, 5 g/liter; agar [Merck], 15 g/liter; pH 7.5) with nalidixic acid at 25 μg/ml. Plates were incubated for 16 h at 37°C. Specific antisera (Behring, Marburg, Germany) and phenotypic tests (Biolog, Inc.) confirmed the presence of Salmonella.
Oligosaccharides and mannose studies.
To test the effects of mannose (2.5%) and NDO (2.5%) on the association of Salmonella and E. coli in vitro, four segments of jejunum and ileum were used from each of five pigs (4 weeks after weaning). The FOS product, Raftiline rST (Orafti), was a mixture of oligo- and polysaccharide (90 to 94%) extracted from chicory root and also contained some glucose and fructose (0 to 4%) and sucrose (4 to 8%). The average degree of polymerization (DP) of the FOS fraction was 10 to 12. The GOS product, Elix (Borcula Whey Products), contained GOS (60%), lactose (20%), glucose and galactose (20%), and the GOS fraction composed of saccharides with DP valves of 2 (33%), 3 (39%), 4 (18%), 5 (7%), and 6 to 8 (3%). Jejunal sections were inoculated with E. coli plus mannose, FOS, or GOS or with E. coli alone. Ileal sections were inoculated with Salmonella plus mannose, FOS, or GOS or with Salmonella alone.
In vivo feeding trials.
Thirty pigs, 4 weeks old, obtained from the herd at The Research Centre Foulum were distributed among three experimental groups (10 pigs per group), allowing for equal distribution for litter and sex. The piglets were fed a semisynthetic control diet or modified control diet (wheat, 20%; cornstarch, 49.1%; cellulose, 4%; fish meal, 10.8%; casein, 10.8%; animal fat, 3%; CaCo3, 1%; CaHPO4 · 2H2O, 0.6%; NaCl, 0.3%; vitamin-mineral mixture, 0.2%; Cr2O3, 0.2%) containing commercial FOS or GOS at 4%. They were housed two and two in isolated pens. After 2 weeks on experiment one piglet from each pen was taken, and the remaining piglets were housed individually for the remaining 2 weeks of experiment (L. L. Mikkelsen, M. Jakobsen, and B. B. Jensen, unpublished results).
Each commercial oligosaccharide product (FOS and GOS) was included at a 4% level in the semisynthetic diet; the actual concentration of GOS was 2.4%. The oligosaccharide products were added at the expense of 2% cornstarch and 2% cellulose in the control diet. The piglets were fed the experimental diets ad libitum for 4 weeks. Sections of jejunum and ileum were taken from pigs fed on the FOS, GOS, and control diets as described above. Segments were inoculated with E. coli (jejunum) and Salmonella (ileum).
Hemagglutination activity.
To detect type 1 fimbrial expression by E. coli and Salmonella hemagglutination assays were performed as described previously (22). To confirm mannose sensitivity, PBS was replaced with 3% (wt/vol) d-mannose (Sigma) in PBS. To test sensitivity to oligosaccharides, FOS and GOS replaced the mannose at 2.5%.
Bacterial growth in DMEM with carbohydrates (2.5%).
DMEM (Gibco) was seeded with either E. coli Nalr (O9:K36:H19) or Salmonella serovar Typhimurium S986. Salmonella and E. coli were retrieved from cultures stored at −80°C, plated on MacConkey agar plates, and incubated overnight at 37°C. Bacteria were prepared as described above except that, after centrifugation and washing, tubes containing 8 ml of LB broth were seeded with 100 μl of the bacterial suspension and incubated statically at 37°C for 7 h in a water bath. Turbidity was measured at 1-h intervals at 650 nm using a spectrophotometer (Ultrospec II 4050; LKB, Cambridge, United Kingdom). Stock solutions were prepared of mannose (Merck), glucose (Merck), FOS, and GOS. The final concentration was 2.5%.
Statistics.
Data were analyzed by unpaired t test, and multiple comparisons were done by using the Tukey test using the Instat statistical package (GraphPad Software, San Diego, Calif.). The results were expressed as the mean ±. The standard deviation.
RESULTS
Association of E. coli K36Nalr and serovar Typhimurium S986 with the jejunum and ileum.
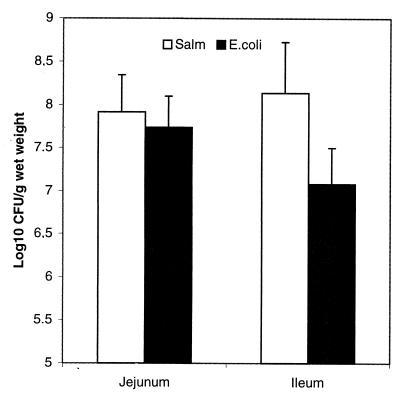
Similar numbers of Salmonella and E. coli were recovered from the jejunum, whereas higher numbers of salmonellae were recovered from the ileum (Fig. 1).
FIG. 1.
Jejunal and ileal tissue taken from five pigs fed a standard diet. Jejunal tissue and ileal tissue was inoculated with either E. coli or Salmonella and incubated for 1 h at 37°C. The numbers of E. coli and the number of salmonellae that had associated with the tissue were counted (n = 10, where n denotes the number of organ cultures).
Effects of mannose and NDO (2.5%) on bacterial association with organ cultures.
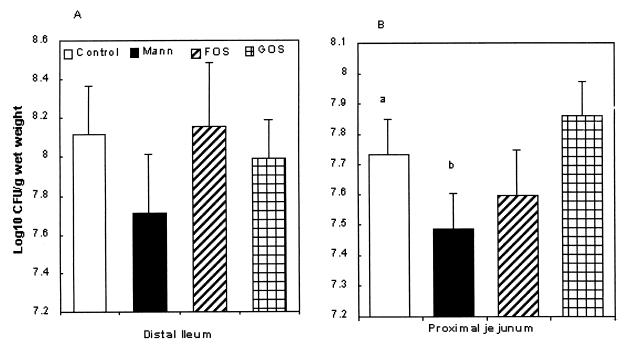
In ileal tissues challenged with Salmonella (Fig. 2A), there was a decrease in association with the addition of FOS, but the levels did not reach significance. In experiments with E. coli and added sugars at 2.5% in jejunal tissue (Fig. 2B), there was a significant reduction in association of E. coli with the addition of mannose compared with controls (no mannose) and tissue challenged with GOS (P < 0.05). There was a reduction in the association of E. coli with FOS and an increase with the addition of GOS, although neither was significant. Overall, the level of association of Salmonella with ileal tissue was higher than the level of association of E. coli with jejunum.
FIG. 2.
(A) Number of salmonellae (log10 CFU/g/[wet weight]) associated with ileal tissue (STM S986) after 1 h of incubation in the presence of carbohydrates (2.5%) (n = 10, where n denotes the number of organ cultures). (B) Number of E. coli (log10CFU/g [wet weight]) associated with jejunal tissue after 1 h in the presence or absence (control) of mannose or NDO's at 2.5% (n = 10).The letters “a” and “b” denote a significant difference (P < 0.05).
Effects of in vivo feeding of commercial FOS and GOS on association to intestinal organ cultures in vitro.
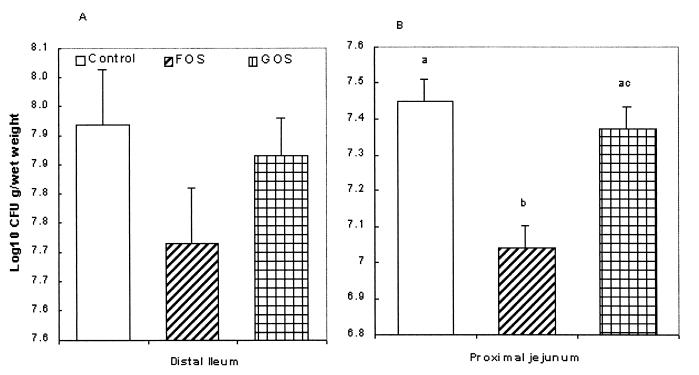
In the ileal cultures taken from the treated pigs there was a reduction in the association of Salmonella with the tissue from the FOS fed pigs, but the levels did not reach significance (Fig. 3A). In the jejunal organ cultures taken from piglets fed 4% FOS there was a significant reduction in the numbers of E. coli associating with the jejunal tissue (P < 0.05) (Fig. 3B). GOS showed no effect on Salmonella or E. coli numbers.
FIG. 3.
(A) Number of salmonellae (log10 CFU/g [wet weight]) in the tissue after 1 h of incubation associated with distal ileal tissue taken from pigs fed a diet with or without (control) supplementation with commercial NDO at 4%. (n = 20. where n denotes the number of organ cultures). (B) Number of E. coli (log10 CFU/g [wet weight]) in the tissue after 1 h of incubation associated with distal ileal tissue taken from pigs fed a diet with or without (control) the supplementation of NDO at 4% (n = 20). The letters “a”, “b”, and “c” denote a significant difference (P < 0.05).
Growth of E. coli and Salmonella in DMEM supplemented with carbohydrates and oligosaccharides.
Both E. coli and Salmonella exhibited limited growth in DMEM despite supplementation with carbohydrate (2.5%) (Table 1). Neither strain grew in DMEM or DMEM supplemented with FOS.
TABLE 1.
Growth of bacteria in DMEMa
| Substrate | Growth (OD650 nm) of:
|
|
|---|---|---|
| E. coli K-36 | STM S986 | |
| Glucose | 1.03 | 0.77 |
| GOS | 0.88 | 0.32 |
| Mannose | 0.84 | 0.71 |
| FOS | 0.09 | 0.10 |
| Control | 0.08 | 0.07 |
E. coli and Salmonella were grown for up to 8 h in DMEM supplemented with carbohydrates or NDO at 2.5%. The turbidity of E. coli and Salmonella serovar Typhimurium (STM) was measured at 650 nm (n = 3).
Hemagglutination activity.
Both the E. coli and the Salmonella strains used in this study exhibited hemagglutination activity when grown for 48 h under static conditions. FOS or GOS at 2.5% did not inhibit the hemagglutination activity of either strain.
DISCUSSION
It has been previously shown that neither Salmonella nor E. coli utilizes FOS as a sole carbohydrate source (17, 3), and this has been confirmed here. Salmonella and E. coli did not grow in DMEM except with the addition of carbohydrates. Neither Salmonella nor E. coli appeared to utilize FOS. E. coli appeared to utilize GOS more than Salmonella. Although the inclusion of FOS did not cause a significant reduction in Salmonella numbers in the organ cultures, the results from pigs fed a standard diet suggest that mannose and FOS will reduce association of E. coli in the jejunum, with the reduction reaching significance with the addition of mannose. This finding is in agreement with previous studies wherein mannose has been shown to reduce the adherence of E. coli in the urinary tract of mice (2) and of Salmonella in chickens (24). In the pigs from the feeding trial, the inclusion of FOS in the diet reduced the association of Salmonella but not significantly. However, jejunal organ cultures from the FOS-fed pigs showed a significant reduction in the recovery of E. coli from the tissue, and this is in agreement with previous work wherein FOS has been shown to reduce E. coli numbers associated with intestinal bacterial overgrowth in dogs (28).
Salmonella and E. coli have different binding patterns in the gut. It is generally accepted that the ileum is the main site of invasion in pigs, whereas in pathogenic E. coli the sites of association can differ. For instance, ETEC strains expressing F18ac associate throughout the small intestine (19), whereas ETEC strains expressing K88 associate more with the ileum (20), and in the present study we have shown that nonpathogenic E. coli strains associate in a pattern similar to that of F18ac-expressing strains. In previous studies pathogenic E. coli has been shown to be associated with the proximal small intestine (16); however, other authors have shown a greater association with the ileum, but this was probably due to the strain of E. coli involved (1). In the present study as in previous studies, salmonellae were recovered predominantly from the distal small intestine (29).
Few studies have investigated the effects of NDO in the pig small intestine. Previous work has concentrated on the effects of NDO in the large intestine, namely, their proliferative effects on different bacterial species, e.g., bifidobacteria (25). In the present study FOS was successful at reducing the numbers of E. coli in jejunal sections. Previous work has shown that mannose can specifically inhibit type 1 fimbria-mediated association of E. coli (23) and Salmonella (14). Since microbial fermentation of NDO is limited in the small intestine, the present findings are more likely due to the direct action of FOS on the gut.
In this present study GOS did not affect the association of E. coli or Salmonella in the culture model, suggesting that it may have a different mode of action from that of FOS or that it was present in insufficient amounts. The commercial GOS used in the feeding trial was 60% pure, corresponding to approximately 2.4% in the final feed. The utilization of GOS by a number of enteric bacteria has been extensively studied (26). The effects of GOS differed from FOS and β-galacto-oligosaccharides (TOS) in germ-free rats since it did not cause changes in the major bacterial groups studied (5). However, it was shown that GOS modified numerous glycolytic activities with an increase in β-galactosidase and α-glycoside activities. These activities can improve the fermentation of resistant starch and lactose, leading to SCFA and lactic acid production. These are a source of energy for the tissue (15) and have been shown to affect the association of Salmonella with Hep-2 cells (6). Neither commercial formulation of GOS or FOS had an effect on the association of Salmonella serovar Typhimurium with intestinal organ cultures. However, the commercial FOS fed in the diet significantly reduced the recovery of E. coli from jejunum tissue in vitro. We propose the porcine intestinal organ culture model for the study of the effects of feed components on commensal microflora and opportunistic pathogens such as Salmonella.
ACKNOWLEDGMENTS
This work was supported by the Danish Ministry of Agriculture, Food, and Fisheries, The Research Secretariat; The National Committee for Pig Breeding, Health, and Production; the Federation of Danish Pig Producers and Slaughterhouses; and the Scientific Academy.
We thank Trine Poulsen for excellent assistance in the practical aspects of the work, the staff of the intensive stable (Foulum) for the qualified care of the animals, and Ole Højberg for helpful discussions.
REFERENCES
- 1.Arbuckle J B R. Observations on the association of pathogenic Escherichia coli with the small intestinal villi of pigs. Res Vet Sci. 1976;20:233–236. [PubMed] [Google Scholar]
- 2.Aronson M, Medalia O, Schori L, Mirelman D, Sharon H, Ofek I. Prevention of colonisation of the urinary tract of mice with Escherichia coli by blocking of bacterial adherence with methy α-d-mannopyranoside. J Infect Dis. 1979;139:329–332. doi: 10.1093/infdis/139.3.329. [DOI] [PubMed] [Google Scholar]
- 3.Bailey J S, Blankenship L C, Cox N A. Effect of fructooligosaccharide on Salmonella colonization of the chicken intestine. Poultry Sci. 1991;70:2433–2438. doi: 10.3382/ps.0702433. [DOI] [PubMed] [Google Scholar]
- 4.Bolton A J, Osborne M P, Wallis T S, Stephen J. Interaction of Salmonella cholerasuis, Salmonella dublin and Salmonella typhimurium with porcine and bovine terminal ileum in vivo. Microbiology. 1999;145:2431–2441. doi: 10.1099/00221287-145-9-2431. [DOI] [PubMed] [Google Scholar]
- 5.Djouzi B Z, Andrieux C. Compared effects of three oligosaccharides on metabolism of intestinal microflora in rats inoculated with a human faecal flora. Br J Nutr. 1997;78:313–324. doi: 10.1079/bjn19970149. [DOI] [PubMed] [Google Scholar]
- 6.Durant J A, Lowry V K, Nisbet D J, Stanker L H, Corrier D E, Ricke S C. Short-chain fatty acids affect cell-association and invasion of Hep-2 cells by Salmonella typhimurium. J Environ Sci Health. 1999;B34:1083–1099. doi: 10.1080/03601239909373246. [DOI] [PubMed] [Google Scholar]
- 7.Fedorka-Cray P J, Gray J T, Wray C. Salmonella infections in pigs. In: Wray C, Wray A, editors. Salmonella in domestic animals. Oxon, United Kingdom: CABI Publishing; 2000. pp. 191–207. [Google Scholar]
- 8.Gyles C L, Barnum D A. Escherichia coli in ligated segments of pig intestine. J Pathol Bacteriol. 1967;94:189–194. doi: 10.1002/path.1700940124. [DOI] [PubMed] [Google Scholar]
- 9.Howard M D, Gordon D T, Pace L W, Garleb K A, Kerley M S. Effects of dietary supplementation with fructooligosaccharides on colonic microbiota populations and epithelial cell proliferation in neonatal pigs. J Pediatr Gastroenterol Nutr. 1995;21:297–303. doi: 10.1097/00005176-199510000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Jayappa H G, Goodnow R A, Geary S J. Role of Escherichia coli type 1 pilus in colonization of porcine ileum and its protective nature as a vaccine antigen in controlling colibacillosis. Infect Immun. 1985;48:350–354. doi: 10.1128/iai.48.2.350-354.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Juven B J, Meinersmann R J, Stern N J. Antagonistic effects of lactobacilli and pediococci to control intestinal colonization by human enteropathogens in live poultry. J Appl Bacteriol. 1991;70:95–103. doi: 10.1111/j.1365-2672.1991.tb04433.x. [DOI] [PubMed] [Google Scholar]
- 12.Kaplan H, Hutkins R W. Fermentation of fructoligosaccharides by lactic acid bacteria and bifidobacteria. Appl Environ Microbiol. 2000;66:2682–2684. doi: 10.1128/aem.66.6.2682-2684.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laerke H N, Jensen B B. d-Tagatose has low small intestinal digestibility but high fermentability in the large intestine of pigs. J Nutr. 1999;129:2231–2235. doi: 10.1093/jn/129.5.1002. [DOI] [PubMed] [Google Scholar]
- 14.Linquist B L, Lebenthal E, Lee P C, Stinson M W, Merrick J M. Adherence of Salmonella typhimurium to small-intestinal enterocytes of the rat. Infect Immun. 1987;55:3044–3050. doi: 10.1128/iai.55.12.3044-3050.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Macfarlane G T, Cummings J H. The colonic flora, fermentation and large bowel digestive function. In: Phillips T S F, Pemberton J H, Shorter R G, editors. The large intestine: physiology, pathophysiology and disease. New York, N.Y: Raven Press; 1991. pp. 51–92. [Google Scholar]
- 16.McAllister J S, Kurtz H J, Short E C., Jr Changes in the intestinal flora of young pigs with postweaning diarrhea or edema disease. J Anim Sci. 1979;49:868–879. doi: 10.2527/jas1979.493868x. [DOI] [PubMed] [Google Scholar]
- 17.Mitsuoka T, Hidaka H, Eida T. Effect of fructo-oligosaccharides on intestinal microflora. Die Nahrung. 1987;31:427–436. doi: 10.1002/food.19870310528. [DOI] [PubMed] [Google Scholar]
- 18.Moxley R A, Duhamel G E. Comparative pathology of bacterial enteric diseases of swine. In: Paul P S, Francis D H, editors. Mechanisms in the pathogenesis of enteric diseases 2. New York, N.Y: Kluwer Academic/Plenum Publishers; 1999. pp. 83–100. [DOI] [PubMed] [Google Scholar]
- 19.Nagy B, Whipp S C, Imbrechts H, Bertschinger H U, Dean-Nystro E A, Casey T A, Salajka E. Biological relationship between F18ab and F18ac fimbriae of enterotoxigenic and verotoxigenic Escherichia coli from weaned pigs with oedema disease or diarrhoea. Microb Pathog. 1997;22:1–11. doi: 10.1006/mpat.1996.0085. [DOI] [PubMed] [Google Scholar]
- 20.Nagy B, Arp L H, Moon H W, Casey T A. Colonization of the small intestine of weaned pigs by enteropathogenic Escherichia coli that lack known colonization factors. Vet Pathol. 1992;29:239–246. doi: 10.1177/030098589202900308. [DOI] [PubMed] [Google Scholar]
- 21.Naughton P J, Grant G, Bardocz B, Pusztai A. Modulation of Salmonella infection by the lectins of Canavalia ensiformis (Con A) and Galanthus nivalis (GNA) in the rat model in vivo. J Appl Microbiol. 2000;88:720–727. doi: 10.1046/j.1365-2672.2000.01062.x. [DOI] [PubMed] [Google Scholar]
- 22.Naughton P J, Grant G, Bardocz S, Allen-Vercoe E, Woodward M J, Pusztai A. Expression of type 1 fimbriae (SEF 21) of Salmonella enterica serotype Enteritidis in the early colonisation of the rat intestine. J Med Microbiol. 2001;50:181–189. doi: 10.1099/0022-1317-50-2-191. [DOI] [PubMed] [Google Scholar]
- 23.Ofek I, Mirelman D, Sharon N. Adherence of Escherichia coli to human mucosal cells mediated by mannose receptors. Nature. 1977;265:623–625. doi: 10.1038/265623a0. [DOI] [PubMed] [Google Scholar]
- 24.Oyofo B, deLoach J R, Corrier D E, Norman J O, Ziprin R L, Mollenhauer H H. Prevention of Salmonella typhimurium colonization of broilers with d-mannose. Poultry Sci. 1989;68:1357–1360. doi: 10.3382/ps.0681357. [DOI] [PubMed] [Google Scholar]
- 25.Rao A V. Dose-response effects of inulin and oligofructose on intestinal bifidogenesis effects. J Nutr. 1999;129:1442S–1445S. doi: 10.1093/jn/129.7.1442S. [DOI] [PubMed] [Google Scholar]
- 26.Tanaka R, Takayama H, Morotomi M, Kuroshima T, Ueyama S, Matsumoto K, Kuroda A, Mutai M. Effects of administration of TOS and Bifidobacterium breve 4006 on the human fecal flora. Bifidobacteria Microflora. 1983;2:17–24. [Google Scholar]
- 27.Wilcock B P, Schwartz K J. Salmonellosis. In: Leman A D, Straw B E, Mengeling W L, D'Allaire S, Taylor D J, editors. Diseases of swine. 7th ed. London, United Kingdom: Iowa State University PressWolfe Publishing, Ltd.; 1992. pp. 570–583. [Google Scholar]
- 28.Willard M D, Simpson R B, Delles E K, Cohen N D, Fossum T W, Kolp D, Reinhart G. Effects of dietary supplementation of fructo-oligosaccharides on small intestinal bacteria overgrowth in dogs. Am J Vet Res. 1992;55:654–659. [PubMed] [Google Scholar]
- 29.Wood R L, Pospischil A, Rose R. Distribution of persistent Salmonella typhimurium infection in internal organs of swine. Am J Vet Res. 1989;50:1015–1021. [PubMed] [Google Scholar]