Abstract
Background
Empiric strategies for secondary prevention in cryptogenic stroke and cryptogenic TIA are lacking. The best therapy to prevent recurrence depends on the cause of stroke. Attempting a correct diagnosis is therefore the fundamental goal of stroke treatment. Further investigation into the source of embolism if suspected, and determination of the etiology, even if demanding, is the needed prerequisite for optimal secondary prevention and risk reduction.
Aims
This paper discusses evaluation and treatment of cryptogenic stroke in light of recent years’ clinical trials results and developments in cardiology and neuroradiology. No ethical approval was needed for this work.
Results
Cardioembolism due to paroxysmal atrial fibrillation, patent foramen ovale, or cardiomyopathy; occult atherosclerosis from unstable plaques and hypercoagulable conditions seem to be the most common underlying causes to be revealed by further investigations. Treatment of these conditions can reduce the stroke recurrence significantly.
Conclusions
An individual approach and targeted diagnostics using advanced medical technologies in selected patients, who may benefit from a tailored treatment regimen, can help reveal a probable cause in the majority of strokes and TIAs previously classified as cryptogenic.
Keywords: acta neurologica scandinavica ‐ PROOF, atrial fibrillation, cryptogenic stroke, guidelines, patent foramen ovale, secondary prevention, TIA
1. INTRODUCTION
The etiology of approximately one third of ischemic strokes remains undetermined, resulting in their classification as cryptogenic. 1 One sixth meet the definition of embolic stroke of undetermined source (ESUS). 2 The category cryptogenic is heterogeneous and includes cases with unknown etiology, where two or more competing causes are possible, or where the investigation is incomplete. ESUS occurs in the absence of lacunar infarcts, significant stenosis in pre‐ and cerebral arteries, or cardiac conditions with high risk of embolism, such as atrial fibrillation. Treatment is challenging: it is unknown whether standard secondary prevention provides sufficient protection against recurrence in all cases, while an empiric strategy of routinely administered anticoagulation appears to be both harmful and not effective. 3 , 4 Currently, single antiplatelet therapy, after a 3 weeks course of dual antiplatelet therapy in the acute phase, is the recommended long‐term secondary stroke prevention. Further investigation into the possible source of embolism and determination of stroke and TIA etiology is therefore still a prerequisite for optimal secondary prevention and risk reduction. While the neurological community is yet awaiting pertinent guidelines for cryptogenic stroke evaluation and treatment, diagnostic work‐up is left to the discretion of the treating physician and local availability, varying considerably. In light of recent years’ clinical trials results and developments in cardiology and neuroradiology, investigations should be targeted at identifying those high‐risk conditions that may affect changes in stroke management. Modern stroke units applying evidence‐based medicine should be central in raising the diagnostic quality in a quest to reduce the proportion of cryptogenic strokes to a minimum.
2. OVERVIEW OF THE EVALUATION OF STROKE AND TIA
Even though ischemic stroke and TIA are heterogeneous in origin, initial evaluation is to a certain extent standardized and involves a transthoracic echocardiogram, imaging of the cervical and intracranial arteries, a 12‐lead ECG, and at least 24 h of continuous heart‐rhythm monitoring (Table 1). While consensus exists surrounding standard etiologic work‐up, there is little agreement on more advanced investigations for the causes of cryptogenic stroke. However, a more extensive evaluation with an individualized approach will often clarify cases where the etiology is not revealed initially (Table 2). How comprehensive the evaluation needs to be, in each individual case, is determined by findings from the initial investigations and an overall assessment of risk factors and probable mechanism in different age groups (Figure 1). Developments in medical technologies have changed the landscape of stroke evaluation and substantially reduced the number of strokes and TIA initially diagnosed as cryptogenic. 5 , 6 Cardioembolism due to atrial fibrillation (AF), patent foramen ovale (PFO), or cardiopathy; hypercoagulable conditions or occult atherosclerosis from unstable plaques seem to be the most common causes identified by additional investigations. 5 , 6
TABLE 1.
Algorithm for standard stroke and TIA evaluation
| Type of evaluation | Examinations | Purpose and clinical comments |
|---|---|---|
| Stroke topography | CT and MRI of the brain |
To confirm the diagnosis of ischemic stroke and exclude stroke mimics To exclude lacunar infarctions |
| MRI superior to CT in detecting acute infarctions, essential in detecting clinically evident and subclinical strokes, small lesions and lesions in the brain stem and cerebellum that may be important to characterize the stroke mechanism | ||
| Infarct location, volume, and multiplicity (single territory vs. multi‐territory lesions) | ||
| Neurovascular evaluation | CTA/MRA of pre‐ and cerebral vessels |
Extracranial end intracranial vascular survey to exclude proximal occlusive atherosclerosis, dissection (MRI with fat‐suppressed images), and cerebral venous sinus thrombosis |
| Carotid duplex ultrasound | ||
| Cardiac evaluation | ECG | To rule out concomitant cardiac ischemia and screen for cardiac arrhythmias |
| 24–72 h telemetry or Holter monitoring | If no arrhythmia detected with preliminary monitoring | |
| TTE, TEE | To detect major‐risk cardioembolism sources 1 | |
| TTE for ventricular imaging, used first in patients with coronary artery disease, congestive heart failure, or other ventricular disease evident from history or ECG | ||
| TTE superior in detecting aortic arch atheroma and cardiac shunt (bubble test), visualization of the left arterial appendage and left atrium; in case of unrevealed TTE results | ||
| Cardiac biomarkers (troponin I or T, BNP, NT‐proBNP) | May predict underlying cardiac condition | |
| Screening for vascular risk factors and hypercoagulable states | Patient's history | Previous TIA or stroke, history of MI, angina, claudication, carotid bruit, venous thrombosis, migraine with aura |
| Smoking and alcohol abuse, family history Complications during pregnancy 2 | ||
| BP measurements | Hypertension | |
| Fasting glucose, HbA1c | Diabetes mellitus | |
| BMI | Overweight | |
| Lipid profile | Dyslipidemia | |
| Blood tests for thrombophilia in patients <50 years old | Atrial and venous hypercoagulability 3 |
Abbreviations: BMI, Body mass index; BNP, Brain natriuretic peptide; BP, Blood pressure; CT, Computed tomography; CTA/MRA, Angiography; ECG, Electrocardiogram; HbA1c, Glycated hemoglobin; MI, Myocardial infarction; MRI, Magnetic resonance tomography; NT‐proBNP, N‐Terminal pro‐b‐type natriuretic peptide; TEE, Transesophageal echocardiography; TTE, Transthoracic echocardiography.
Major‐risk cardioembolism sources: mechanical prosthetic valve, mitral stenosis with atrial fibrillation, atrial fibrillation/atrial flutter, sick sinus syndrome, myocardial infarction <4 weeks, left ventricular thrombus, dilated cardiomyopathy, akinetic left ventricular segment, left ventricular ejection fraction<30%, left atrial/atrial appendage thrombus, atrial myxoma and other cardiac tumors, infective endocarditis.
Pregnancy complications: hypertension, diabetes mellitus, preeclampsia/eclampsia, spontaneous miscarriages, venous thrombosis.
Arterial and venous hypercoagulability screening: d‐dimer, erythrocyte sedimentation rate, lupus anticoagulant, anticardiolipin and ß‐2 glycoprotein antibodies, antithrombin III activity, protein C and S functional, prothrombin 20210a mutation, Factor V Leiden gene mutation. Thrombophilia tests may be falsely abnormal in acute phase and testing should be delayed for several weeks and when a patient is off anticoagulation. Initially, positive antiphospholipid antibody result needs to be confirmed three months later.
TABLE 2.
Algorithm for advanced stroke and TIA evaluation
| Type of evaluation | Examination | Purpose and clinical comments |
|---|---|---|
| Extended vascular evaluation | High‐resolution MRA or three‐dimensioned/contrast ultrasound of pre‐ and cerebral vessels | Intensified seek for artery‐to artery embolism (aortic arch atheroma, mild stenosis of a relevant artery, or significant stenosis of non‐relevant artery); plaque extension into small perforators or other non‐atherosclerotic inflammatory or non‐inflammatory arteriopathies |
| and | ||
|
CTA or MRA of the aorta Transcranial Doppler monitoring for emboli |
cardiogenic embolism (minor‐risk cardioembolism sources 1 ) | |
| Vasculitis test/autoimmune evaluationCSF examination Brain biopsy | May in selected cases lead to confirmation of suspected vasculitis as well as identification of other uncommon diagnoses | |
| Extended cardiac evaluation | Prolonged rhythm monitoring >72 h up to 1 year: long‐term | Intensified seek for undiagnosed AF and other transient arrhythmias |
| noninvasive 2 or invasive monitoring (ICM) | Risk‐factor and biomarker‐based predictive scores 3 | |
| CT and MRI of the heart | Intensified seek for minor‐risk cardioembolism sources 1 and paradoxical venous embolism | |
| To determine several cardiovascular parameters (left ventricular mass, left atrial volume, identifying cardiac shunt, scaring, or fibrosis in the myocardium) | ||
| In case TEE cannot be tolerated | ||
| Genetic testing |
Mitochondrial diseases Monogenic disease (CADASIL) Fabry's disease and other genetic causes |
To exclude other uncommon causes of brain ischemia |
| Work‐up for occult cancer |
Physical examination and patient's history CT thorax, abdomen, pelvis Mammography PET‐CT, Diagnostic biomarkers |
Age‐ and sex‐appropriate screening Adenocarcinomas, lung and pancreatic cancer types most frequent |
Abbreviations: AF, Atrial fibrillation; CADASIL, Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy, PET‐CT, Positron emission tomography CT; CSF, Cerebrospinal fluid; CT, Computed tomography; CTA, Computed tomography angiography; ICM, Insertable cardiac monitor; MRA Magnetic resonance tomography angiography; MRI, Magnetic resonance tomography; TEE, Transesophageal echocardiography.
Minor‐risk cardioembolic source: mitral valve prolapse, mitral annular calcification, aortic valve stenosis, calcific aortic valve, atrial high‐rate episodes, atrial appendage stasis with reduced flow velocities or spontaneous echodensities, atrial septal aneurysm, Chiari network, moderate systolic or diastolic dysfunction of left ventricle, endomyocardial fibrosis, patent foramen ovale, atrial septal defect.
Noninvasive monitoring strategies: mobile cardiac telemetry, patch monitor, event recorder, external loop recorder.
AF predictors: older age, hypertension, left ventricle hypertrophy, heart failure, coronary artery disease, left atrial enlargement, alcohol abuse, large vessel occlusion, multi‐territory and cortical lesions, chronic brain infarctions/leukoaraiosis, atrial premature beats, prolonged PR interval on ECG, P‐wave terminal force in lead V1.
FIGURE 1.
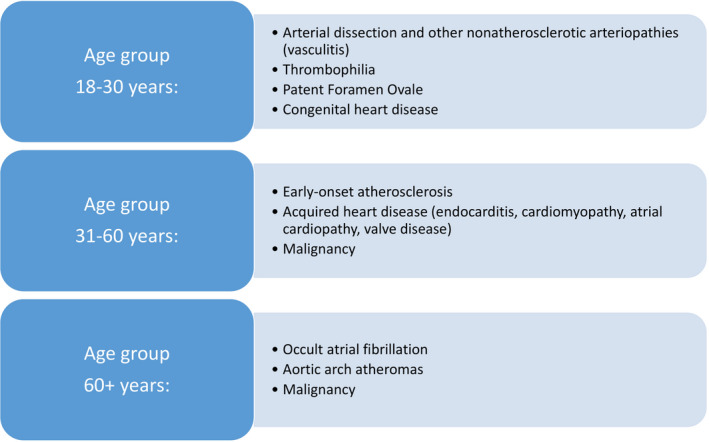
Most common causes of cryptogenic stroke and TIA according to age 2 , 5 , 6
3. OCCULT ATRIAL FIBRILLATION—LOOK LONGER AND USE MORE SOPHISTICATED MONITORING
In patients with underlying AF secondary prevention with antiplatelet drugs is insufficient, thus detection of the arrhythmia has therapeutic implications for patient care. As numerous studies have shown episodes of underlying AF in a significant proportion of cryptogenic stroke patients, there is consensus that further investigation of cardiac rhythm is important. Embolic‐appearing infarcts, multi‐territory localization, older age, and signs of atrial cardiopathy (pathological ECG changes, enlargement of left atrium, and elevated cardiac biomarkers) indicate a need of extended rhythm monitoring. 7 All eligible patients should undergo sufficient monitoring for underlying AF. Initially, whenever possible, continuous ECG monitoring for at least 72 h is recommended (Class IB level of evidence; LoE). 8 Prolonged rhythm monitoring for about 30 days to rule out AF as reasonable for patients with cryptogenic stroke and TIA has been recommended since 2014 (Class IIA LoE). Insertable cardiac monitors (ICMs) are the tools with highest sensitivity and specificity for identifying AF in patients with cryptogenic stroke, significantly more effective than any of the intermittent monitoring strategies. Depending on the definition of episode duration, monitoring duration, and the amount and type of screening performed before device implantation, the incidence of AF detected by ICMs ranges from 16% to 34%. 9 This benefit must be balanced against larger costs due to the ICMs use, so more personalized strategies are needed. Identifying new biomarkers to help selecting patients with the highest possibility of detecting AF by prolonged monitoring may be a useful tool. 10 As the recent LOOP study failed to show a significant reduction in strokes in high‐risk patients with AF detected by ICM, 11 careful evaluation of the significance of short and subclinical AF episodes is still crucial. The LOOP study findings might also imply that not only AF itself but the more complex atrial cardiopathy contributes to the risk of cardioembolic stroke.
4. PATENT FORAMEN OVALE AND ITS CAUSAL RELATION TO STROKE
PFO, a common condition in the healthy population, was identified as an independent risk factor for cryptogenic stroke, particularly in young adults with an otherwise unexplained cerebrovascular event. 5 , 6 However, stroke and TIA patients with detected PFO should be carefully evaluated to determine whether the PFO may have been responsible for the index stroke or should be considered as incidental finding. PFO and atrial septal abnormalities are considered to cause strokes secondary to paradoxical embolism from the peripheral venous system or embolization from thrombi formed within the atrial septum as a result of transient atrial arrhythmias. 5 , 6 Embolic‐appearing brain infarcts in younger patients with symptom onset associated with Valsalva's maneuver should raise a clear suspicion of underlying PFO and lead to additional investigation for patients ≤60 years of age. Among elderly patients with many vascular risk factors, evaluation may not be considered as PFO is probably less likely to cause the stroke. 12 Transesophageal echocardiography with agitated saline (“bubble”) contrast for visualizing right‐to‐left shunting through the septum is the gold standard for PFO detection. 13 Secondary prevention with antiplatelet agents and anticoagulation are likely to be equally effective, with antiplatelet therapy being the first choice. Anticoagulation is recommended for infarct recurrence and for venous embolism. According to recent randomized clinical trials, percutaneous closure of PFO in younger patients (≤ 60 years) with cryptogenic stroke decreased the risk of stroke recurrence, in contrast to earlier trials that showed no benefit. 13 Treatment strategies should be determined via patient involvement and shared medical decision‐making. Trials on PFO closure in older patients are ongoing.
5. OCCULT MALIGNANCY—AN UNCOMMON BUT IMPORTANT RISK FACTOR
Work‐up for uncommon risk factors should include evaluation for underlying occult malignancy, which can lead directly or indirectly to stroke and TIA. In cancer patients, the stroke risk is higher due to a state of hypercoagulability, embolism from marantic endocarditis, and tumor as well as elevated risk of AF and atherosclerosis. Occult malignancy should be suspected in patients with otherwise unexplained multi‐territory stroke, in the elderly, and if cancer‐suspected symptoms are present. 14 High D‐dimer, low hemoglobin, and smoking may be some of the predictors of active cancer. Secondary prevention using anticoagulants and cancer treatment reduces the risk of stroke recurrence. 14
6. NONSTENOTIC PLAQUES IN PRECEREBRAL ARTERIES AND AORTIC ARCH ATHEROMATOSIS—MARKERS OF SYSTEMIC ATHEROSCLEROSIS
Atherosclerotic disease with <50% vessel stenosis in precerebral arteries or plaques in the aortic arch and thoracic aorta is now increasingly being considered as potential cause of cryptogenic stroke and TIA. 1 , 6 , 15 Lipid‐rich parts of the atheromatous deposits may embolize, and the irregular surface of complex plaques with ulcerations, hemorrhages, or rupture of the fibrous cap may give rise to thrombus formation and subsequent thromboembolic stroke. 15 In the absence of other plausible causes, additional examinations of the vessel wall in the aorta and precerebral arteries (using CT, MRI, ultrasonography with and without contrast medium, microemboli signal detection, and 18 F‐fluorodeoxyglucose positron emission tomography) may contribute to the identification of atheroembolic stroke etiology.
7. DISCUSSION
Trials have demonstrated that one‐size treatment does not fit all cryptogenic stroke patients, as there is a wide range of possible underlying causes in this patients group, highlighting the importance of further systemic and evidence‐based evaluation. Before the diagnosis of cryptogenic stroke and TIA is made, an adequate cardiac and neurovascular examination as well as screening for relevant underlying causes should be performed (Table 2). A significant proportion of patients with stroke and TIA that, according to standard assessment, is classified as cryptogenic may have their etiology revealed by supplementary examinations with a more personalized approach (Figure 2). Extended use of imaging diagnostics and advanced medical technologies, such as insertable rhythm monitoring devices and high‐resolution imaging techniques, contributes to the detection of etiology in many patients. Patients with underlying conditions associated with a high risk of recurrence will benefit the most from such an examination.
FIGURE 2.
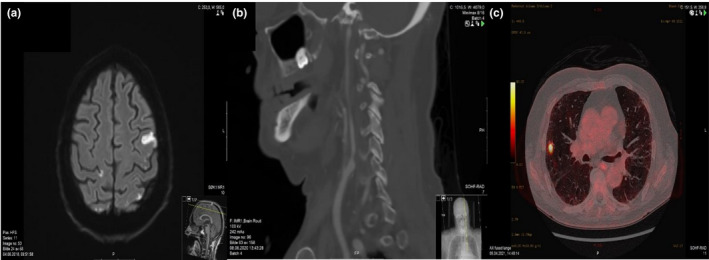
Cryptogenic stroke end TIA patients evaluated in The Nordic Atrial Fibrillation and Stroke (NORFIB) study 10 : MRI showing multi‐territory diffusion changes in patient with occult atrial fibrillation (A), Computed tomography angiogram (CTA) showing nonstenotic unstable plaque in the left internal carotid artery (B), Positron emission tomography (PET) showing occult malignancy in the right lung detected a few months after a cryptogenic stroke (C)
8. CONCLUSION
A unified diagnostic strategy for cryptogenic stroke that is both evidence‐based and cost‐effective has not yet been defined. Targeted selection and judicious use of appropriate tests (“precision medicine”) in the work‐up of cryptogenic strokes are still crucial and may be even more relevant in the future. We hope that the methodological approach discussed and proposed in this article can be a benefit for clinicians evaluating stroke and TIA patients while waiting for guidelines for the evaluation and treatment of cryptogenic stroke.
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/ane.13590.
ACKNOWLEDGMENTS
None.
Ratajczak‐Tretel B, Lambert AT, Atar D, Aamodt AH. Cryptogenic stroke and TIA: Suggested diagnostic approach while waiting for evaluation and treatment guidelines. Acta Neurol Scand. 2022;145:641–646. doi: 10.1111/ane.13590
Funding information
This work had no external funding. BRT and ATL are recipients of a PhD grants from the South‐Eastern Norway Regional Health Authority.
DATA AVAILABILITY STATEMENT
Data sharing not applicable – no new data generated.
REFERENCES
- 1. Ornello R, Degan D, Tiseo C, et al. Distribution and temporal trends from 1993 to 2015 of ischemic stroke subtypes: a systematic review and meta‐analysis. Stroke. 2018;49(4):814‐819. [DOI] [PubMed] [Google Scholar]
- 2. Hart RG, Catanese L, Perera KS, Ntaios G, Connolly SJ. Embolic stroke of undetermined source: a systematic review and clinical update. Stroke. 2017;48(4):867‐872. [DOI] [PubMed] [Google Scholar]
- 3. Hart RG, Sharma M, Mundl H, et al. Rivaroxaban for stroke prevention after embolic stroke of undetermined source. N Engl J Med. 2018;378:2191‐2201. [DOI] [PubMed] [Google Scholar]
- 4. Diener HC, Sacco RL, Easton JD, et al. RE‐SPECT ESUS steering committee and investigators. dabigatran for prevention of stroke after embolic stroke of undetermined source. N Engl J Med. 2019;380(20):1906‐1917. [DOI] [PubMed] [Google Scholar]
- 5. Yaghi S, Bernstein RA, Passman R, Okin PM, Furie KL. Cryptogenic stroke: research and practice. Circ Res. 2017;120:527‐540. [DOI] [PubMed] [Google Scholar]
- 6. Bang OY, Ovbiagele B, Kim JS. Evaluation of cryptogenic stroke with advanced diagnostic techniques. Stroke. 2014;45(4):1186‐1194. [DOI] [PubMed] [Google Scholar]
- 7. Favilla CG, Ingala E, Jara J, et al. Predictors of finding occult atrial fibrillation after cryptogenic stroke. Stroke. 2015;46:1210‐1215. [DOI] [PubMed] [Google Scholar]
- 8. Hindricks G, Potpara T, Dagres N, et al. ESC scientific document group, 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European association for cardio‐thoracic surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European society of cardiology (ESC) developed with the special contribution of the european heart rhythm association (EHRA) of the ESC. Eur Heart J. 2021;42(5):373‐498. [DOI] [PubMed] [Google Scholar]
- 9. Lu Y, Diao SS, Huang SJ, et al. Insertable cardiac monitors for detection of atrial fibrillation after cryptogenic stroke: a meta‐analysis. Neurol Sci. 2021;42(10):4139‐4148. [DOI] [PubMed] [Google Scholar]
- 10. Ratajczak‐Tretel B, Lambert AT, Aamodt AH, et al. Atrial fibrillation in cryptogenic stroke and transient ischaemic attack ‐ the nordic atrial fibrillation and stroke (NOR‐FIB) study: rationale and design. Eur Stroke J. 2019;4(2):172‐180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Svendsen JH, Diederichsen SZ, Højberg S, et al. Implantable loop recorder detection of atrial fibrillation to prevent stroke (The LOOP Study): a randomised controlled trial. Lancet. 2021;S0140‐6736(21):01698‐1706. [DOI] [PubMed] [Google Scholar]
- 12. Kent DM, Ruthazer R, Weimar C, et al. An index to identify stroke‐related vs incidental patent foramen ovale in cryptogenic stroke. Neurology. 2013;81(7):619‐625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Messé SR, Gronseth GS, Kent DM, et al. Practice advisory update summary: patent foramen ovale and secondary stroke prevention: report of the guideline subcommittee of the American academy of neurology. Neurology. 2020;94(20):876‐885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dardiotis E, Aloizou AM, Markoula S, et al. Cancer‐associated stroke: Pathophysiology, detection and management (Review). Int J Oncol. 2019;54(3):779‐796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bulwa Z, Gupta A. Embolic stroke of undetermined source: The role of the nonstenotic carotid plaque. J Neurol Sci. 2017;15(382):49‐52. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable – no new data generated.


