Abstract
Objectives
Functional movement disorders (FMD) refer to a heterogeneous group of manifestations incongruent with known neurological diseases. Functional neuroimaging studies in FMD indicate the overlap between cerebral regions in which abnormal activation occurs and those considered crucial for theory of mind (ToM), the ability to attribute mental states. The aim of this study was to explore whether FMD might be related to ToM disorders to the extent that they reduce the ability to make inferences about the mental states underlying motor behaviour during social interaction.
Materials & Methods
Eighteen subjects with FMD and 28 matched healthy controls (HC) were given a ToM battery. The severity of FMD was rated by the Simplified‐FMD Rating Scale (S‐FMDRS). Dissociative symptoms were evaluated by the Dissociative Experiences Scale (DES‐II).
Results
FMD scored worse than the HC in most ToM tasks: second‐order False Beliefs (p = .005), Faux‐Pas Recognition Test (p < .001) and Reading the Mind in the Eyes Test (p = .020); control questions elicited normal scores. The DES‐II indicated dissociative‐borderline psychopathology and negatively correlated with accuracy on the second‐order False Belief (Spearman's rho = −.444; p = .032); the positive correlation between DES‐II and severity of motor symptoms (S‐FMDRS) approached significance (Spearman's rho test = .392; p = .054). ToM disorders were not correlated with S‐FMDRS, due to the typical variability in FMD over time with regard to the severity of symptoms and the district of body involved.
Conclusions
Our results are consistent with the hypothesis that FMD are related to ToM deficits, and future studies are needed to define the specific nature of this relationship.
Keywords: dissociation, functional movement disorders, social cognition, theory of mind
1. INTRODUCTION
Functional movement disorders (FMD), once defined as ‘hysteria’ or ‘conversion disorders' (see Baizabal‐Carvallo et al. 1 for historical references and review), refer to clinically heterogeneous manifestations, incongruent with known neurological diseases. 2 In DSM‐5, 3 these manifestations fall into the general category of Somatic Symptoms and Related Disorders, which are mainly classified as Conversion Disorders or Functional Neurological Symptoms (FNS). 4
Despite being categorized separately from dissociative disorders, FNS might constitute one of the manifestations of dissociation, expression of loss of voluntary control over normal processes and functions (see Brown 5 for discussion).
The growing interest in FMD also derives from their position in the philosophical issue of mind‐brain dualism (see Newby et al. 6 for discussion), which, in the clinical perspective, refers to the boundary between psychiatric and neurological symptoms. 7
Functional neuroimaging studies in FMD show abnormal activation in regions crucial for the elaboration of certain aspects of social cognition, such as theory of mind (ToM), including the ventromedial prefrontal cortex, the cingulate gyrus, the right amygdala and the right temporoparietal junction. 8 , 9 , 10
ToM 11 , 12 defines the ability to attribute to our own and other individuals' mental states, that is beliefs, desires, intentions, emotions and knowledge, and to understand that other individuals' mental states are independent from ours. ToM allows understanding the motivations of others in generating actions and, ultimately, supports human social interaction. Two components of ToM have been identified, affective ToM, inferring others' emotions and feelings, and cognitive ToM, inferring others' beliefs and intentions, underlined by segregated neural substrates.
FMD might be mediated by the alteration of neural circuits that support a broad spectrum of constructs, such as attention, emotional processing, sense of agency and inference, 13 with the latter consisting in the process that generates beliefs about what is occurring within and outside the body. 14 Impaired ‘self‐agency’ in FMD is likely the most significant consequence of the mismatch between the expected and the actual sensory response. 15
It has been already hypothesized that ToM deficits may offer a unifying interpretation of the functional neurological disorder, 16 but the experimental evidence is scarce, limited to alexithymia (the inability to identify and describe emotional states). In one study, subjects with psychogenic non‐epileptic seizures (PNES) 17 , 18 had high prevalence of alexithymia and misinterpreted the emotional significance of others' actions, while being still able to recognize basic emotions by facial expressions. In a group of subjects with various types of functional motor symptoms, 19 alexithymia was more frequent than in organic movement disorders. The authors speculated that patients with alexithymia misinterpreted autonomic signs due to anxiety/panic as signs of organic illness.
In this observational study, we explored both cognitive and affective ToM in subjects with FMD compared with matched healthy controls (HC).
We hypothesized the FMD might be the consequence of ToM impairment; the altered prediction of the consequences of the movement could result in a mismatch between the top‐down expectation of the response and the actual response.
2. MATERIALS AND METHODS
2.1. Participants
Participants were recruited among the consecutive patients referred to the Movement Disorders Outpatient Clinic of the Fondazione Policlinico Gemelli IRCCS University Hospital from January 2019 to January 2021.
Inclusion criteria were as follows: 1. definite clinical diagnosis of FMD, according to the Gupta and Lang criteria; 20 2. age ≥18 years; and 3. capacity to understand and sign an informed consent.
Exclusion criteria were as follows: 1. cognitive impairment or psychiatric condition that might interfere with the ability to perform the tasks included in the assessment. A concomitant neurological disorder (i.e., Parkinson's disease) was not considered an exclusion criterion when positive criteria of FMD were consistent with a clinically definite diagnosis. 21
Healthy control individuals (HC) (age‐, education‐ and gender‐matched) were selected based on the following criteria: absence of clinical signs of mental deterioration (CDR = 0); negative history for internal pathologies, neurological and psychiatric disorders or traumatic brain injuries. All selected subjects were administered the Mini‐Mental State Examination (MMSE) and were included in the control groups only if their adjusted score was above the cut‐off (23.80). Due to the time required to administer the entire battery for ToM assessment, each healthy subject underwent a limited number of tests. The study was approved by the Local Ethics Committee and was performed following the ethical standards laid down in the 1964 Declaration of Helsinki.
2.2. Methods
2.2.1. Neuropsychological testing
Subjects with FMD preliminarily received a neuropsychological test battery, which included the MMSE, immediate and delayed free recall of words, Raven's Coloured Progressive Matrices, Phonological Verbal Fluency, the Stroop test and Ekman's recognition of emotions from facial expressions.
2.2.2. ToM assessment
Two tasks exploring ToM, False Belief stories 22 , 23 and Faux‐Pas recognition task, 23 , 24 , 25 (translated and adapted by GA, unpublished materials) and the Reading the Mind in the Eyes Test (RMET 21 , 26 ) were given to patients and HC in two sessions for 45 min each.
The first‐order 23 , 27 and second‐order False Belief stories 22 , 23 are aimed at exploring the cognitive components of ToM. The task requires understanding that a person's belief or representation of an event might contrast with the real event. First‐order False Beliefs require attributing to another person false beliefs about a real event; second‐order False Beliefs require attributing false beliefs to another person based on what other people think about this person's thoughts. Four first‐order and two second‐order False Belief stories were presented to the participants. Each story was read aloud by the examiner while presenting the vignettes illustrating the story. Afterwards, the participant was requested to answer different questions about: (1) predicting the behaviour of the protagonist of the story on the basis of the existing information, mental states and actions of the protagonist (‘false belief’ question); (2) recalling information provided by the examiner during stimulus presentation (‘memory’ question); (3) factual details concerning the vignette (‘reality’ question) and, only for the second‐order False Beliefs, (4) question about possible events that could occur on the basis of the given information (‘inference’ question).
Faux‐Pas recognition task 23 , 24 , 25 is aimed at exploring both cognitive and affective ToM; in order to realize that someone made a gaffe, the participant has to infer that someone produced the gaffe unintentionally (cognitive component) and that someone may have been resentful or offended by what was said (affective component). Stories (five with and five without faux‐pas) were read aloud to the subjects. At the end of each story, the subject was asked five questions: faux‐pas recognition question (‘Did someone say something he/she shouldn't have?’); (2) reality question, which assesses understanding of the gaffe (‘Who said something he/she shouldn't have said?’); (3) inference question, which requires a representation of the listener's mental state (‘Why shouldn't he have said that?’); (4) faux‐pas motivation question, which requires representation of the mental state of the speaker (‘Why would he/she say that?’); and (5) control question, which asks the subject for details about the story. Further details on False Belief stories and Faux‐Pas recognition task are reported in Appendix S1.
Reading the Mind in the Eyes Test (RMET). 26 The Italian version of the RMET 28 was used to explore the affective component of ToM. It consists of photographs of the eye region of the face of an actor or actress, each presented on a slide. The participant is requested to point to the word denoting the mental state expressed by the eyes by selecting the response out of four adjectives that denote different complex mental states displayed on a vertical array below each photograph.
2.2.3. The dissociative experiences scale
The Dissociative Experiences Scale (DES) 29 is a self‐reported instrument for measuring dissociation in normal and clinical populations. The current scale (DES‐II) is a 28‐item self‐report questionnaire in which subjects are asked to circle the percentage of times (by 10s) they have the specific experience from 0 to 100. 30 The mean of all item scores ranges from 0 to 100 and is called the total DES score, with a cut‐off score of 30 for psychopathology. For the purposes of our research, we used the Italian translation of the DES‐II. 31
2.2.4. Assessment of the severity of FMD
The severity of FMD was assessed using the simplified‐Functional Movement Disorders Rating Scales (S‐FMDRS). 32 The presence of abnormal movements in seven body regions (face and tongue; head and neck; left upper limb and shoulder; right upper limb and shoulder; trunk and abdomen; left lower limb; and right lower limb) is rated. In each body region, symptom severity is evaluated from 0 (none) to 3 (severe) and duration from 0 (none) to 3 (the symptom is evident continuously). Severity and duration of gait and speech disorders are also rated. All severity and duration scores were added to yield a total score (range 0–54).
2.3. Statistical analyses
Statistical analyses were carried out using SPSS (version 24) and Jamovi (version 1.6) (https://www.jamovi.org) software. Summary statistics are expressed as means and standard deviations (SD) or frequencies and percentages. Comparisons between the score obtained in each task by patients' group and the HC group that performed that task were evaluated by parametric (t tests) or non‐parametric (chi‐squared, Mann‐Whitney U) tests as appropriate. The Shapiro‐Wilk test was adopted to test the normal distribution of each numerical variable.
Pearson's r or Spearman's rho coefficients (depending on the distribution of the variable) were adopted for correlational analyses.
Effect sizes were expressed using the rank biserial correlation (rb) when performing non‐parametric between‐groups comparisons with the Mann‐Whitney U test and interpreted as follows: 0.1–0.3 as a small effect; 0.3–0.5 as an intermediate effect; and 0.5 and higher as a strong effect. 33 The statistical threshold was set at p < .05.
3. RESULTS
3.1. Demographic and clinical data of subjects with FMD and HC
Eighteen patients with FMD and 28 HC were included in the experimental groups. According to the set of tests administered, two groups of HC were obtained, both matched for age and education with the FMD group (FMD vs. first group—age: Student's t test (30) = .66; p = .516; education: Mann‐Whitney U test (30) = 92.00; p = .197; FMD vs. second group—age: Student's t test (30) = .13; p = .896; education: Mann‐Whitney U test (30) = 86.00; p = .124). The first group were given the Italian version of the RMET 28 and the Faux‐Pas task; 23 , 24 , 25 the second group were given the first‐ and second‐order False Belief task. 22 , 23 From here on, we refer to both groups as HC.
FMD presentation included gait disorders, dystonia, tremor and parkinsonism. The mean severity of FMD, measured by S‐FMDRS, was 12.06 ± 7.25. Neuropsychological performances were within the normal range (see Appendix S1).
Demographic data of FMD are reported in Table 1. The clinical description of the 18 FMD subjects is reported in Table 2. Additional details are available in Appendix S1.
TABLE 1.
Demographical data and performance in ToM battery of FMD and HC a
| HC | FMD | Statistical comparisons | ||||||
|---|---|---|---|---|---|---|---|---|
| Sample 1 [N =14] | Sample 2 [N =14] | [N =18] | HC sample 1 vs. FMD | HC sample 2 vs. FMD | ||||
| Age (years) | 64.86 | (10.98) | 61.93 | (12.68) | 61.17 | (18.58) | 0.516 b | 0.896 b |
| Education (years) | 11.71 | (2.78) | 11.64 | (1.74) | (9.94) | (3.17) | 0.197 c | .124 c |
| Sex M/F [N, %] | 7 (50.0%) | 7 (50.0%) | 7 (50.0%) | 7 (50.0%) | 5 (27.8%) | 13 (72.2%) | 0.198 d | .198 d |
| RMET—correct responses (0–36) | 25.71 | (2.16) | 21.83 | (5.77) | .020 c | |||
| Faux‐pas | ||||||||
| Recognition questions (0–5) | 2.21 | (1.42) | 0.28 | (.58) | <.001 c | |||
| Control stories questions (0–5) | 4.36 | (.63) | 3.89 | (1.23) | .452 c | |||
| Control questions (0–5) | 4.86 | (.36) | 4.56 | (.78) | .217 c | |||
| Control questions of control stories (0–5) | 4.71 | (.47) | 4.61 | (.78) | .981 c | |||
| I ORDER FALSE BELIEF | ||||||||
| False Belief stories (0–4) | 3.36 | (.75) | 3.22 | (.65) | .518 c | |||
| Reality questions (0–4) | 3.50 | (.76) | 3.78 | (.73) | .127 c | |||
| Memory questions (0–4) | 3.79 | (.43) | 3.61 | (.70) | .598 c | |||
| II ORDER FALSE BELIEF | ||||||||
| False Belief stories (0–5) | 3.50 | (1.23) | 2.28 | (.90) | .005 c | |||
| Reality questions (0–5) | 4.86 | (.36) | 4.06 | (.87) | .003 c | |||
| Memory questions (0–5) | 3.86 | (.77) | 3.39 | (1.09) | .222 c | |||
| Interference questions (0–5) | 3.86 | (1.35) | 3.83 | (.99) | .721 c | |||
Abbreviations: FMD, patients with functional movement disorders; SD, standard deviation; yrs, years.
Sample 1 HC completed the Reading the Mind in the Eyes Test and Faux‐Pas tasks; sample 2 HC completed the False Belief stories. Means, standard deviations (SD) and percentages are reported.
Independent‐sample t test.
Mann‐Whitney U test.
Chi‐squared test.
TABLE 2.
Detailed clinical features of subjects with FMD.
| Case | Age/Sex | Duration of illness (years) | Clinical presentation of FMD | Neurological/psychiatric comorbidity | S‐FMDRS Total Score (Part 3, Item 3) |
|---|---|---|---|---|---|
| 1 | 33/F | 11 | Upper limb dystonia | Depression | 26 |
| 2 | 52/F | 13 | Gait disorder, tremor | Depression | 14 |
| 3 | 54/F | 2 | Parkinsonism, dystonia, tremor | Anxiety | 21 |
| 4 | 64/M | 1 | Hemiballism tremor | Parkinson's disease | 12 |
| 5 | 44/F | 5 | Dystonia | Speech apraxia, cognitive dysfunction | 21 |
| 6 | 72/F | 8 | Dystonia | Depression | 14 |
| 7 | 85/M | 2 | Gait disorder, parkinsonism | Cognitive dysfunction, depression | 5 |
| 8 | 88/M | 7 | Limb dystonia, tremor, facial dyskinesia | ‐ | 10 |
| 9 | 78/F | 3 | gait disorder, postural instability | Anxiety | 4 |
| 10 | 71/F | 12 | Parkinsonism, postural instability | Anxiety, depression | 6 |
| 11 | 78/F | 2 | Tics, platysmal contraction | Anxiety | 6 |
| 12 | 29/M | 18 | Oromandibular dystonia, tremor | ‐ | 10 |
| 13 | 48/F | 8 | Stuttering, oromandibular dystonia | Meningioma, anxiety | 2 |
| 14 | 79/F | 2 | Upper limb dystonia | Speech apraxia | 12 |
| 15 | 70/F | 2 | Tremor | Anxiety, Parkinson's disease | 9 |
| 16 | 47/F | 1 | Lower limb dystonia | Anxiety, depression | 10 |
| 17 | 38/F | 1 | Motor sensory hemisyndrome | ‐ | 18 |
| 18 | 83/F | 1 | Dystonia | Depression | 9 |
Abbreviations: F, female; FMD, patients with functional movement disorders; M, male; S‐FMDRS, Simplified‐FMD Rating Scale.
3.2. Task exploring ToM abilities
No differences emerged between FMD subjects and HC in the first‐order False Belief task, but in second‐order False Beliefs and in the Faux‐Pas, FMD subjects performed worse than HC, but obtained normal scores in most questions devised as control condition. In particular, FMD subjects performed worse than HC on the second‐order False Beliefs (Mann‐Whitney U test (30) = 54.50; p = .005; rb = .57; see Table 1 and Figure 1) and in reality questions (Mann‐Whitney U test (30) = 56.00; p = .003; rb = .56; see Figure 1). No differences emerged in the memory and inference questions (all ps > 0.1). On the faux‐pas task, FMD subjects performed significantly worse than HC (Mann‐Whitney U test (30) = 23.00; p < .001; rb = .82; see Table 1 and Figure 2), but no difference emerged in any of the control questions (all ps > 0.1). Finally, in the RMET FMD subjects obtained significantly lower scores than HC (Mann‐Whitney U test (30) = 64.50; p = .020; rb = .49; see Table 1 and Figure 3).
FIGURE 1.
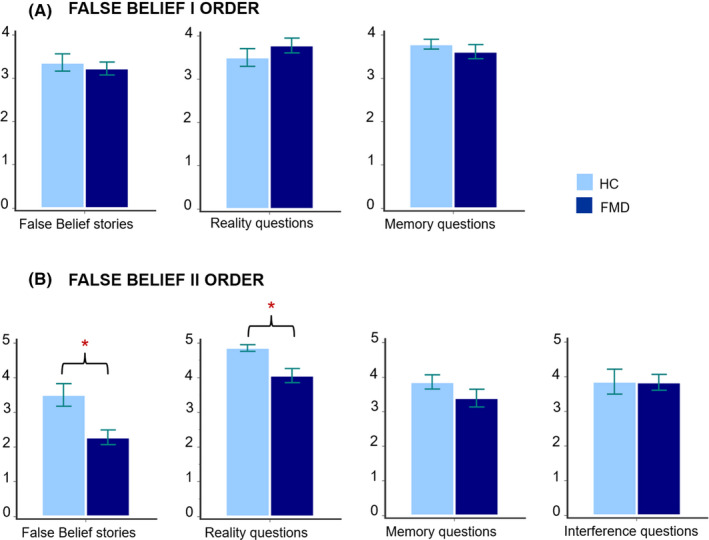
Performance of FMD and HC on False Belief tasks (Mann‐Whitney U test). Panel A: First‐order False Beliefs; Panel B: second‐order False Beliefs. *Statistical significance at p < .05. FMD, patients with functional movement disorders; HC, healthy controls
FIGURE 2.

Performance of FMD and HC on Faux‐Pas tasks (Mann‐Whitney U test). **Statistical significance at p < .001. FMD, patients with functional movement disorders; HC, healthy controls
FIGURE 3.

Performance of FMD and HC on RMET (Mann‐Whitney U test). *Statistical significance at p < .05. FMD, patients with functional movement disorders; HC, healthy controls; RMET, Reading the Mind in the Eyes Test
3.3. DES‐II Questionnaire
The mean score of the FMD group was 20.00 ± 21.50 (range 1.79–72.14), which is indicative of dissociative/borderline psychopathology. Four of 18 subjects scored >30 (the threshold indicative of dissociative symptoms), and three scored between 20 and 30 (borderline psychopathology).
3.4. Correlational analyses
Correlation between the severity of ToM disorders and severity of motor symptoms (S‐FMDRS) . No correlation emerged between any measure of ToM ability and S‐FMDRS (all ps > .1).
Correlation between the severity of ToM disorders and severity of dissociative symptoms (DES‐II questionnaire) . Accuracy on the second‐order False Belief was negatively correlated with the DES‐II (Spearman's rho = −.444; p = .032). No other significant correlation emerged (all ps > .1).
Correlation between the severity of motor symptoms (S‐FMDRS) and dissociative disorders (DES‐II questionnaire) . The correlational analysis between the S‐FMDRS and the DES‐II approached significance (Spearman's rho test = .392; p = .054). In addition, based on the median values of the distribution of S‐FMDRS scores, FMD subjects were split into two groups, with less severe (8 subjects) and more severe (10 subjects) movement disorders. Subjects with more severe motor symptoms performed worse than subjects with less severe motor symptoms on the DES‐II questionnaire (28.86 ± 25.18 vs. 8.93 ± 7.46; Mann‐Whitney U test = 16.00; p = .037, rb = .60).
Correlation between the severity of ToM disorders and neuropsychological tasks . The RMET was directly correlated with the verbal fluency score (Pearson's r = .493; p = .038); the first‐order False Belief test was directly correlated with the time score of the Stroop interference test (time) (Spearman's rho = .598; p = .011).
4. DISCUSSION
FMD subjects obtained pathological scores in most tasks of the ToM battery, evaluating both the cognitive and the affective components, and some of them had dissociative symptoms.
We did not observe correlation between severity of ToM impairment and functional motor disturbance. This is, indeed, not surprising, due to the instability of the scores obtained with the S‐FMDRS. As acknowledged by the authors who devised the scale, which is currently the only available tool to rate this type of disorders, 32 test‐retest reliability could not be confirmed, due to the inconsistency and fluctuation of symptoms that are characteristics and diagnostic criteria of FMD.
Despite the lack of statistical correlation, we believe that the disturbance of the ToM of our subjects can be considered a possible cause of the functional motor manifestation. First, ToM deficits were genuine. In fact, our subjects obtained normal scores in most of the ‘control questions’ in the False Beliefs and Faux‐Pas tasks, questions devised precisely to disentangle specific deficits of ToM from cognitive disorders in language and memory domains to which pathological scores could be otherwise attributed. The significant impairment in the RMET also confirms ToM deficits, mostly in the light of the normal performance the subjects obtained in Ekman's emotion recognition task. This pattern of results confirms that FMD subjects do not fail in the recognition of facial emotions per se but have specific difficulty in attributing emotional states, which is one of the characteristics of social cognition deficit.
The hypothesis of some relation between ToM deficits and FMD might also find some indirect confirmation in other results; that is, the correlations between the dissociative disorder (DES‐II) and the severity of deficit in ToM tasks on the one hand, and between dissociative disorders and severity of FMD on the other (despite the limitations of the S‐FMDRS we have mentioned).
Social cognition disorders such as ToM and alexithymia have been associated with dissociative symptoms. 30 Our results are consistent with this evidence; the subjects with higher score on the DES‐II questionnaire were more impaired in the second‐order False Beliefs. At the same time, the relationship between social cognition disorder and conversion disorders is reported, 32 , 33 , 34 but only a few experimental works consider a specific expression of conversion disturbances such as FMD. 18 Our study confirms this relationship: the deficits that emerged in the second‐order False Belief test were significantly correlated with the severity of functional motor symptoms, understood as conversion disorders. Overall, these data contribute to highlight the relationship between disorders of social cognition, dissociative symptoms and FMD, and suggest that a coherent interpretation of FMD should take into consideration also these complex relationships.
Our study also shows that ToM (RMET and False Beliefs) and executive tasks (word fluency and Stroop time) are significantly related. The two skills seem to share some neural substrates in prefrontal regions. 34 The relationship between ToM and executive abilities is still an open question and deserves consideration. The two skills seem to share some neural substrates in prefrontal regions. 34 Much of the literature, especially in the field of autism, assumes, however, that the ToM represents a cognitive system that can be dissociated from other cognitive abilities, 35 thus excluding that the ToM deficit might be a by‐product of the executive disorder.
ToM deficits have also been documented in ‘organic’ movement disorders, such as parkinsonian syndromes. 36 The documentation of social cognition deficits in both parkinsonian patients and subjects with FMD is of great interest because they draw additional attention to the role of motor behaviour in social interaction and communication 37 and place the basal ganglia at the interface between neurological and psychiatric diseases (see Newby et al. 6 for discussion).
The main limitation of our work is the relatively low number of subjects examined and their heterogeneity. In future research, particular attention should be paid to the district of body involved in the FMD and the typology. It would be also appropriate to apply statistical models to control confounding factors such as demographic data, or comorbidities, primarily psychiatric, or the use of psychotropic drugs. Equally important would be considering anamnestic data that could be predictive of the type of manifestation and the affected body district.
Another important limitation of our study is that the tasks adopted are able to confirm in a general sense the presence of a disorder of ToM, but are not able to distinguish the impairment in predicting the sensorial consequences of one's own motor behaviour (e.g., in the lack of the ‘sense of self‐agency’) or the consequence of incorrect inferences about one's own emotional state, 18 from the impairment in predicting other individuals' motor behaviour due to inability to attribute mental states for example, through the facial and body expressions. We can hypothesize that this inability would lead to a mismatch between the response we expect and the other's actual motor response, triggering, on our part, an anomalous motor behaviour. This hypothesis, however, needs experimental support in future studies.
CONFLICT OF INTEREST
The authors declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/ane.13585.
Supporting information
Supplementary Material
ACKNOWLEDGEMENT
We wish to thank all the patients and healthy controls who participated in the study. Open Access Funding provided by Universita Cattolica del Sacro Cuore within the CRUI‐CARE Agreement. [Correction added on 16 May 2022, after first online publication: CRUI funding statement has been added.]
Silveri MC, Di Tella S, Lo Monaco MR, et al. Theory of mind: A clue for the interpretation of functional movement disorders. Acta Neurol Scand. 2022;145:571–578. doi: 10.1111/ane.13585
Funding information
This manuscript received no funding
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author [S.D.T], upon reasonable request.
REFERENCES
- 1. Baizabal‐Carvallo JF, Hallett M, Jankovic J. Pathogenesis and pathophysiology of functional (psychogenic) movement disorders. Neurobiology of Disease. 2019;127:32‐44. doi: 10.1016/j.nbd.2019.02.013 [DOI] [PubMed] [Google Scholar]
- 2. Carson A, Lehn A. Epidemiology. Handb Clin Neurol. 2016;139:47‐60. doi: 10.1016/b978-0-12-801772-2.00005-9 [DOI] [PubMed] [Google Scholar]
- 3. American Psychiatric Association . Diagnostic and statistical manual of mental disorders. Section II. Diagnostic Criteria and Codes. Somatic Symptom and Related Disorders 5th ed. American Psychiatric Association; 2013:309. http://repository.poltekkes‐kaltim.ac.id/657/1/Diagnostic%20and%20statistical%20manual%20of%20mental%20disorders%20_%20DSM‐5%20%28%20PDFDrive.com%20%29.pdf [Google Scholar]
- 4. Gilmour GS, Nielsen G, Teodoro T, et al. Management of functional neurological disorder. J Neurol. 2020;267(7):2164‐2172. doi: 10.1007/s00415-020-09772-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brown RJ. Dissociation and functional neurologic disorders. Handb Clin Neurol. 2016;139:85‐94. doi: 10.1016/B978-0-12-801772-2.00008-4 [DOI] [PubMed] [Google Scholar]
- 6. Newby R, Alty J, Kempster P. Functional dystonia and the borderland between neurology and psychiatry: new concepts. Mov Disord. 2016;31(12):1777‐1784. doi: 10.1002/mds.26805 [DOI] [PubMed] [Google Scholar]
- 7. Edwards MJ, Bhatia KP. Functional (psychogenic) movement disorders: merging mind and brain. Lancet Neurol. 2012;11(3):250‐260. doi: 10.1016/s1474-4422(11)70310-6 [DOI] [PubMed] [Google Scholar]
- 8. Carrington SJ, Bailey AJ. Are there theory of mind regions in the brain? A review of the neuroimaging literature. Hum Brain Mapp. 2009;30(8):2313‐2335. doi: 10.1002/hbm.20671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Frith CD, Frith U. The neural basis of mentalizing. Neuron. 2006;50(4):531‐534. doi: 10.1016/j.neuron.2006.05.001 [DOI] [PubMed] [Google Scholar]
- 10. Gallagher HL, Frith CD. Functional imaging of ‘theory of mind'. Trends Cogn Sci. 2003;7(2):77‐83. doi: 10.1016/s1364-6613(02)00025-6 [DOI] [PubMed] [Google Scholar]
- 11. Baron‐Cohen S. Mindblindness: An Essay on Autism and Theory of Mind. The MIT Press; 1995. [Google Scholar]
- 12. Premack D, Woodruff G. Does the chimpanzee have a theory of mind? Behav Brain Sci. 1978;1(4):515‐526. doi: 10.1017/S0140525X00076512 [DOI] [Google Scholar]
- 13. Drane DL, Fani N, Hallett M, Khalsa SS, Perez DL, Roberts NA. A framework for understanding the pathophysiology of functional neurological disorder. CNS Spectr. 2021;26(6):555‐561. doi: 10.1017/s1092852920001789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Teufel C, Fletcher PC. Forms of prediction in the nervous system. Nat Rev Neurosci. 2020;21(4):231‐242. doi: 10.1038/s41583-020-0275-5 [DOI] [PubMed] [Google Scholar]
- 15. Maurer CW, LaFaver K, Ameli R, Epstein SA, Hallett M, Horovitz SG. Impaired self‐agency in functional movement disorders: a resting‐state fMRI study. Neurology. 2016;87(6):564‐570. doi: 10.1212/wnl.0000000000002940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Espay AJ, Maloney T, Vannest J, et al. Impaired emotion processing in functional (psychogenic) tremor: a functional magnetic resonance imaging study. Neuroimage Clin. 2018;17:179‐187. doi: 10.1016/j.nicl.2017.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schönenberg M, Jusyte A, Höhnle N, et al. Theory of mind abilities in patients with psychogenic nonepileptic seizures. Epilepsy Behav E&B. 2015;53:20‐24. doi: 10.1016/j.yebeh.2015.09.036 [DOI] [PubMed] [Google Scholar]
- 18. Bewley J, Murphy PN, Mallows J, Baker GA. Does alexithymia differentiate between patients with nonepileptic seizures, patients with epilepsy, and nonpatient controls? Epilepsy Behav E&B. 2005;7(3):430‐437. doi: 10.1016/j.yebeh.2005.06.006 [DOI] [PubMed] [Google Scholar]
- 19. Demartini B, Petrochilos P, Ricciardi L, Price G, Edwards MJ, Joyce E. The role of alexithymia in the development of functional motor symptoms (conversion disorder). J Neurol Neurosurg Psychiatry. 2014;85(10):1132‐1137. doi: 10.1136/jnnp-2013-307203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gupta A, Lang AE. Psychogenic movement disorders. Curr Opin Neurol. 2009;22(4):430‐436. doi: 10.1097/WCO.0b013e32832dc169 [DOI] [PubMed] [Google Scholar]
- 21. Tinazzi M, Geroin C, Erro R, et al. Functional motor disorders associated with other neurological diseases: beyond the boundaries of “organic” neurology. Eur J Neurol. 2021;28(5):1752‐1758. doi: 10.1111/ene.14674 [DOI] [PubMed] [Google Scholar]
- 22. Rowe AD, Bullock PR, Polkey CE, Morris RG. "Theory of mind" impairments and their relationship to executive functioning following frontal lobe excisions. Brain. 2001;124(Pt 3):600‐616. doi: 10.1093/brain/124.3.600 [DOI] [PubMed] [Google Scholar]
- 23. Stone VE, Baron‐Cohen S, Knight RT. Frontal lobe contributions to theory of mind. J Cogn Neurosci. 1998;10(5):640‐656. doi: 10.1162/089892998562942 [DOI] [PubMed] [Google Scholar]
- 24. Baron‐Cohen S, O'Riordan M, Stone V, Jones R, Plaisted K. Recognition of faux pas by normally developing children and children with Asperger syndrome or high‐functioning autism. J Autism Dev Disord. 1999;29(5):407‐418. doi: 10.1023/a:1023035012436 [DOI] [PubMed] [Google Scholar]
- 25. Shamay‐Tsoory SG, Tomer R, Berger BD, Aharon‐Peretz J. Characterization of empathy deficits following prefrontal brain damage: the role of the right ventromedial prefrontal cortex. J Cogn Neurosci. 2003;15(3):324‐337. doi: 10.1162/089892903321593063 [DOI] [PubMed] [Google Scholar]
- 26. Baron‐Cohen S, Wheelwright S, Hill J, Raste Y, Plumb I. The "Reading the Mind in the Eyes" Test revised version: a study with normal adults, and adults with Asperger syndrome or high‐functioning autism. J Child Psychol Psychiatry. 2001;42(2):241‐251. doi: 10.1111/1469-7610.00715 [DOI] [PubMed] [Google Scholar]
- 27. Wimmer H, Perner J. Beliefs about beliefs: representation and constraining function of wrong beliefs in young children's understanding of deception. Cognition. 1983;13(1):103‐128. doi: 10.1016/0010-0277(83)90004-5 [DOI] [PubMed] [Google Scholar]
- 28. Serafin M, Surian L. Il Test degli Occhi: uno strumento per valutare la" teoria della mente". Giornale Italiano Di Psicologia. 2004;31(4):839‐862. doi: 10.1421/18849 [DOI] [Google Scholar]
- 29. Bernstein EM, Putnam FW. Development, reliability, and validity of a dissociation scale. J Nerv Ment Dis. 1986;174(12):727‐735. doi: 10.1097/00005053-198612000-00004 [DOI] [PubMed] [Google Scholar]
- 30. Carlson EB & Putnam FW. An update on the dissociative experiences scale. Dissociation. 1993;6:16‐27. [Google Scholar]
- 31. Schimmenti A. Dissociative experiences and dissociative minds: exploring a nomological network of dissociative functioning. J Trauma Dissociation. 2016;17(3):338‐361. doi: 10.1080/15299732.2015.1108948 [DOI] [PubMed] [Google Scholar]
- 32. Nielsen G, Ricciardi L, Meppelink AM, Holt K, Teodoro T, Edwards M. A simplified version of the psychogenic movement disorders rating scale: the simplified functional movement disorders rating scale (S‐FMDRS). Mov Disord Clin Pract. 2017;4(5):710‐716. doi: 10.1002/mdc3.12475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cohen J. Statistical power analysis for the behavioral sciences. Vol 3. 2nd ed. Erlbaum; 1988:79‐80. [Google Scholar]
- 34. Bishop DV. Annotation: autism, executive functions and theory of mind: a neuropsychological perspective. J Child Psychol Psychiatry. 1993;34(3):279‐293. doi: 10.1111/j.1469-7610.1993.tb00992.x [DOI] [PubMed] [Google Scholar]
- 35. Happé FG. An advanced test of theory of mind: understanding of story characters’ thoughts and feelings by able autistic, mentally handicapped, and normal children and adults. J Autism Dev Disord. 1994;24(2):129‐154. doi: 10.1007/BF02172093 [DOI] [PubMed] [Google Scholar]
- 36. Poletti M, Enrici I, Bonuccelli U, Adenzato M. Theory of Mind in Parkinson's disease. Behav Brain Res. 2011;219(2):342‐350. doi: 10.1016/j.bbr.2011.01.010 [DOI] [PubMed] [Google Scholar]
- 37. Blakemore SJ, Decety J. From the perception of action to the understanding of intention. Nat Rev Neurosci. 2001;2(8):561‐567. doi: 10.1038/35086023 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
The data that support the findings of this study are available from the corresponding author [S.D.T], upon reasonable request.


