Abstract
Introduction
Hospital‐associated deconditioning (HAD) or post‐hospital syndrome is well recognized as reduced functional performance after an acute hospitalization. Recommendations for the management of HAD are still lacking, partly due to a poor understanding of the underlying processes. We aimed to review existing data on risk factors, pathophysiology, measurement tools, and potential interventions.
Materials and methods
We conducted a systematic review from bibliographical databases in English, Spanish and French with keywords such as ‘post‐hospitalization syndrome’ or ‘deconditioning’. We selected studies that included people aged 60 years or older. Three researchers independently selected articles and assessed their quality.
Results
From 4421 articles initially retrieved, we included 94 studies. Most were related to risk factors, trajectories and measures, and focused on the physical aspects of deconditioning. Risk factors for HAD included age, nutritional status, mobility, and pre‐admission functional status, but also cognitive impairment and depression. Regarding interventions, almost all studies were devoted to physical rehabilitation and environmental modifications. Only one study focused on cognitive stimulation.
Discussion
In the last decade, studies on HAD have mostly focused on the physical domain. However, neurological changes may also play a role in the pathophysiology of HAD. Beyond physical interventions, cognitive rehabilitation and neurological interventions should also be evaluated to improve deconditioning prevention and treatment in the hospital setting.
Keywords: deconditioning, hospital‐acquired functional decline, older persons, post‐hospitalization syndrome, vulnerability
Key points
Many studies have been devoted to HAD in the last decade, with some consistent findings.
Most studies have focused on the physical dimensions of HAD.
Few mechanistic and intervention studies have been conducted.
The cognitive and psychological dimensions of HAD remain understudied.
A consensus definition of HAD and a clear research agenda are needed.
1. INTRODUCTION
Hospital‐associated deconditioning (HAD) or post‐hospital syndrome is a state of poor functional performance after an acute hospitalization. 1 In a study of hospitalized community‐dwelling older people at 6 months after discharge, 43% needed continuing help with medications, 24% were still unable to walk a quarter of a mile, and 45% were still unable to drive. 2 The overall prevalence of HAD across studies has been estimated to be around 30%. 3 A cumulative meta‐analysis showed no significant change in prevalence over time, 2 despite significant changes in standards of care. HAD is a strong risk factor for mortality, re‐hospitalization, and institutionalization in the following year.4, 5 Moreover, HAD is associated with high costs; responsible for an estimated 58.5 Billion in healthcare spending in the United States in 2019, approximately 8.3% of total annual medical spending that year. 6 To improve overall quality of life and reduce healthcare spending, targeted prevention and intervention strategies are needed. 7
Numerous predictors of HAD have been described, associated with either patient‐related factors or aspects of the hospital setting.8, 9 Interventional studies on HAD are mainly focused on motor rehabilitation, and primarily carried out during the inpatient phase, with few studies centered on later recovery.10, 11 Hence, the optimal post‐acute rehabilitation program for patients with HAD is still unknown. 12 Moreover, recommendations for the management of HAD, focusing on prevention and guiding interventions, are still lacking. Our aim was to undertake a systematic review on HAD in the acute hospital setting and provide a practical overview aimed at frontline health professionals.
2. MATERIAL AND METHODS
We conducted a systematic review of existing literature in December 2020 following the Preferred Reporting Items for Systematic review and Meta‐Analysis (PRISMA) guidelines 13 and the PRISMA‐S (extension to the PRISMA Statement for Reporting Literature Searches in Systematic Reviews). 14
2.1. Selection criteria
We included original articles, theses or gray literature related to HAD (i.e., poor functional performance after an acute hospitalization) in people older than 60 years old, without any restriction on publication date. The exclusion criteria were: reviews, case‐reports, commentaries, conferences abstracts, qualitative studies, animal studies, participants younger than 60 years old, and studies focused on recovery from a specific pathology (e.g., stroke).
2.2. Selection strategy
The following bibliographical databases were searched: EMBASE, Medline, CINAHL, Web of Science, Bio Medical Central, Latin American and Caribbean Health Literature (Lilacs), Scientific Electronic Library online (Scielo), EM Premium for additional French articles, and Google Scholar for gray literature.
The following keywords were used: hospital‐associated decline, hospital‐associated deconditioning, functional decline, post‐hospital syndrome. The detailed search strategy is in Appendix 1 in the supporting Information.
After removing duplicates, the selection of articles was carried out in two stages. First, we reviewed article titles and abstracts. An independent three‐person review system was implemented and discrepancies were discussed until consensus was reached among the reviewers. The second stage consisted of reviewing the full text of the selected articles. The articles deemed to meet our inclusion criteria following this second stage full text review were included in the final sample.
2.3. Data extraction
We extracted key study characteristics including the authors' names, country, publication date, type of study, measures, participant characteristics and outcomes. We grouped studies according to their main focus: mechanisms, measures, interventions, trajectories and risk factors associated with HAD.
2.4. Risk of bias
Prior to conducting this systematic review, a formal protocol was developed and agreed by the research team. There were no deviations from the original protocol and all studies meeting our inclusion criteria were incorporated. However, publication bias remains a possible limitation because data from statistically significant studies are more likely to be published than those reporting negative findings. In addition, the quality of the evidence included was moderate, which may be a further source of bias in our review.
2.5. Quality assessment
The Strengthening the Reporting of Observational studies in Epidemiology (STROBE) guidelines were used to assess the quality of the observational studies, and the Consolidated Standards of Reporting Trials (CONSORT) for clinical trials. We considered studies to be of low methodological quality when they fulfilled less than 75% of the criteria.
3. RESULTS
Figure 1 shows the flowchart of included studies. Our initial search of the databases identified 4421 articles. After applying exclusion criteria, we reviewed the full text of 279 articles. This led to the final inclusion of 94 studies. We classified the included studies into five categories according to their focus: risk factors (42), trajectories (15), underlying mechanisms (12), potential measures or predictive tools (22), and intervention studies (13). Sixteen articles focused on multiple aspects (e.g., risk factors and mechanisms, or trajectories and risk factors).
FIGURE 1.
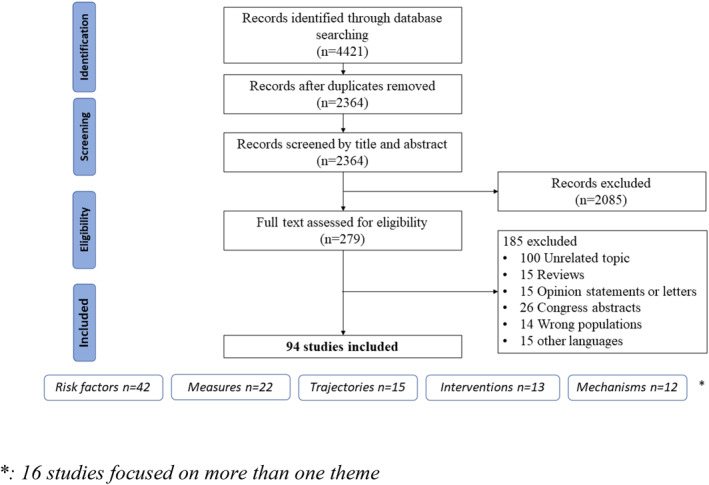
Preferred Reporting Items for Systematic review and Meta‐Analysis (PRISMA) Flow chart
3.1. Quality assessment
Half of included studies were considered to be of high methodological quality (see Appendix 2 in the Supporting Information S2). The geographical distribution of the studies is shown in Figure 2. Most studies were conducted in the United States and Europe.
FIGURE 2.
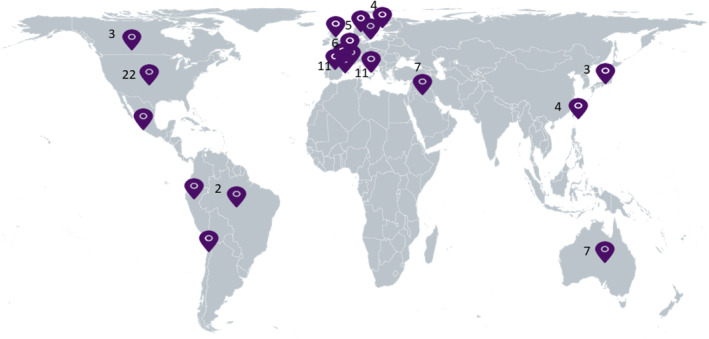
Geographic distribution of included articles
3.2. Risk factors
3.2.1. General characteristics
Forty‐two studies addressed HAD risk factors. The mean age was 75 years (ranging from 69 to 87 years). In general, studies included a higher proportion of women (56%–71%), with only four studies including a majority of men.
3.2.2. Methodological aspects
There were 20 cohort studies (prospective and retrospective) and 20 cross‐sectional studies. Two studies used secondary data from previous prospective cohort designs (post‐hoc and secondary analysis), and one was designed as a longitudinal survey. The sample size was greater than 1000 participants in 8 studies, and between 100 and 700 in 34 studies. The main statistical techniques employed for the data analysis were bivariate comparisons and multivariate logistic regression.
In 22 studies, the endpoint was the degree of basic activities of daily living (ADL) impairment at discharge. Ten studies evaluated performance in instrumental ADLs (IADLs) after discharge; 8 studies evaluated cognitive performance and 2 studies evaluated institutionalization rate.
3.2.3. Main outcomes
Eighteen studies mentioned sociodemographic factors5, 8, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30; 10 mentioned factors related to the hospital stay15, 16, 25, 30, 31, 32, 33, 34, 35, 36; 24 mentioned comorbidities as risk factors8, 9, 15, 16, 23, 24, 25, 26, 30, 32, 33, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48; and 10 discussed factors related to physiological reserve.9, 26, 30, 32, 37, 39, 40, 49, 50, 51 The individual factors identified are summarized in Figure 3.
FIGURE 3.
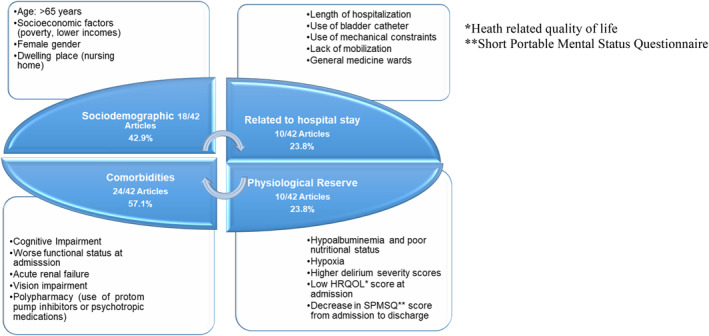
Risk factors associated with hospital‐associated deconditioning
3.3. Trajectories
3.3.1. General characteristics
Fifteen studies discussed HAD trajectories. The age across studies ranged from 67 to 80 years. Gender was almost equally distributed, with a slight majority of women (from 45% to 64%). One study was only focused on older community dwelling women. 52
3.3.2. Methodological aspects
All studies either had a prospective cohort design or reported a retrospective analysis from secondary data. Based on the STROBE checklist, one third of these studies were considered to be of high quality (see Appendix 2 in the Supporting Information S2). Sample size ranged from 104 to 2963 participants. Each study was conducted in a single site although many included different hospital areas/wards. The statistical techniques most frequently employed for data analysis were bivariate comparisons and multivariate logistic regression. The assessment time was highly heterogeneous, ranging from immediately following admission, to discharge, to 3 months or more than 3 years following discharge.
3.3.3. Main outcomes
One study reported a 30% risk of functional decline after discharge; 18% when measured by ADL at 3 months after discharge, and 40% when measured by IADL. 53 Despite the heterogeneity of measurement tools and assessment times, the results were similar over time. 52 Helvik and collaborators evaluated disability at 1‐year follow up, finding a higher rate of readmission and 30% of patients in whom disability had increased at discharge. 34 Those who recovered to their previous status seemed to need up to 3 months to do so.
A study performed in Spain showed that 57.7% of patients had functional decline in the first 24 h after admission and 32.6% at discharge. 54 The rate of readmission was 30% in the following 3 months. Another study showed that the loss of function before hospital was the main factor associated with HAD. 27 Similarly, a study from Asia reported a similar frequency of functional decline of 32% 6 months after discharge. 55
Another study found any hospitalization to be associated with decreased gait speed (−0.04 m/s) and higher odds of new limitations in mobility or ADLs. 56 Multiple hospitalizations within a year were associated with gait speed decline (−0.06 m/s) and greater odds of new limitations in mobility or ADLs. Rehospitalization was the main risk factor for functional decline in this study. One further study provided an original approach to the relationship between intervening events and nine possible transitions 57 with illnesses and injuries leading to hospitalization strongly associated with worsening functional ability for nearly all transitions between states of no disability, mild disability, and severe disability.
3.4. Mechanisms
3.4.1. General characteristics
Twelve papers addressed HAD mechanisms. All studies but one were published after 2010. The age across studies ranged from 55 to 87 years old. Women were more represented in these studies (from 60% to 75%). Two studies recruited participants from two different countries.58, 59
3.4.2. Methodological aspects
All studies were observational. More than half were considered of high quality (Supporting Information S2). The sample size ranged from 12 to 2963 participants.
3.4.3. Main outcomes
An overview of mechanisms from selected studies is summarized in Figure 4, which suggests the combined effects of hospitalization‐related factors and individual vulnerability factors.
FIGURE 4.
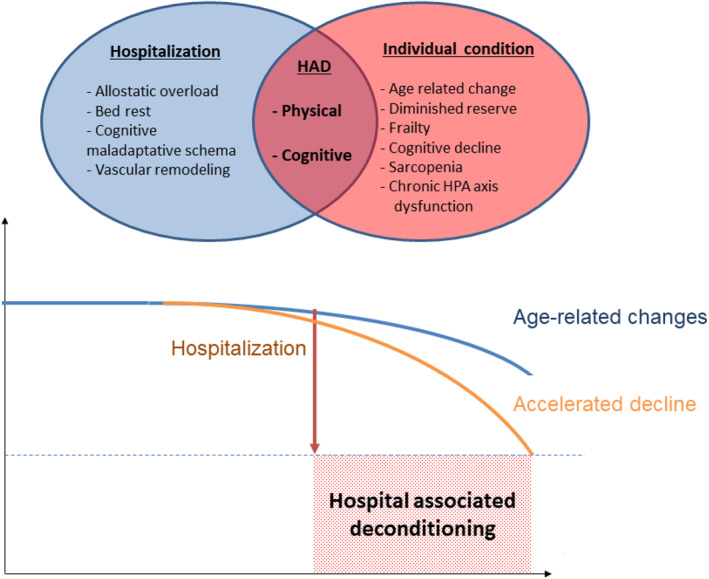
Mechanisms of hospital‐associated deconditioning: combination of hospitalization factors and individual vulnerability. HAD, Hospital‐associated deconditioning; HPA, hypothalamic‐pituitary‐adrenal
3.4.4. Mechanisms related to the hospitalization
3.4.4.1. Allostatic overload
Potential deconditioning factors included frequent sleep disruption, frightening sounds, painful stimuli, mobility restrictions, and poor nutrition, all of which could be deleterious on allostasis. 56 Allostasis is based on a balance between the hypothalamic‐pituitary‐adrenal (HPA) axis and the autonomic nervous system. 60 The notion of repetitive stressors might also explain why the length of hospitalization is often cited among risk factors predisposing to HAD.54, 61
3.4.4.2. Bed rest
Bed rest was cited as one of the most frequent deconditioning factors during hospitalization. Some studies commented that patients' mobility could be greatly reduced because of medical devices, such as urinary catheters and continuous intravenous infusions. Some commented that patients may also be hesitant to leave the room to avoid missing a diagnostic procedure or an unscheduled physician's visit.
In an observational study, 24‐h accelerometer data were collected from admission to discharge. 62 The median time spent walking was only 7 min per day. Time spent walking was higher in the group with better balance and walking score at admission. The authors reported that older patients were highly inactive during hospitalization due to their own condition, but hospitalization prolonged their inactive time, potentially leading to deconditioning.
One pilot study with a small sample of patients evidenced the considerable amount of time that patients spent being alone, 63 when they were not physically nor cognitively stimulated. In this light, the deconditioning effects of bedrest could be explained from both the physical and cognitive perspectives.
3.4.4.3. Maladaptive cognitive perception
An early study noted that 36% of hospitalized older patients had high levels of anxiety and depression 64 ; and 1 month after discharge, half of them remained anxious and depressed. The nosocomial‐based psychological distress may lead to a vicious circle to maladaptive cognitive schema. This schema does not follow a unique trajectory. In a prospective study, four cognitive change patterns were observed: the worsening then improving group; the low continuous group; the start high and decline group; and the start low and decline group. 65
3.4.5. Mechanisms related to the person
The baseline homeostatic capacity and overall physiological reserve of a patient may be a factor in an individual patient's susceptibility to deconditioning.
3.4.5.1. Age‐related physiologic changes
Aging is associated with a decline in organ function in all body systems. 66 Organ‐system decline leads to a reduction in overall functional reserve and limits an individual's capacity to respond to acute stressors. The changes are both structural and functional. In the central nervous system, brain volume decreases, as well as blood brain barrier permeability. In the body composition, structure changes with fatty infiltrations, muscle loss and strength loss, and function changes with a decline of balance and postural control. 67 Aging is also associated with higher basal cortisol levels, progressive loss of hypothalamic sensitivity through loss of glucocorticoid receptors in the central nervous system, decreased diurnal variation of cortisol, and a blunted negative feedback at the level of the HPA axis.
3.4.5.2. Frailty and geriatric syndromes
Although predictable changes to physiologic function occur with aging, age alone is not a sufficient predictor of a patient's risk for HAD. 57 Frailty is a state of reduced physiologic reserve beyond what would be expected with normal aging. Frailty accentuated the association of hospitalization with functional decline. 57 During a hospitalization, geriatric syndromes (e.g., falls, incontinence, pressure ulcers) range from 8% to 50%. 26 People with at least one geriatric syndrome were three times less likely to make a complete functional recovery.
3.4.5.3. Sarcopenia
Sarcopenia is defined as reduced skeletal muscle mass and/or function. Sarcopenic individuals are at risk for HAD because skeletal muscle is an important reservoir of aminoacids during periods of stress and hospitalization often leads to reduced nutritional intake. 40 Malnourished patients had a lower Barthel Index score than those at risk for malnutrition. Chronic disease‐related malnutrition, oral intake, and parenteral nutrition accentuated this association.
3.4.5.4. Cognitive decline
The serum level of the calcium binding protein S100B among older patients hospitalized for hip fracture did not differ between participants with and without delirium. 68 Hence, previous cognitive decline seemed to be a more preponderant factor for delirium than potential cerebral damage caused by peri‐operative stress. 69 The CSF biomarkers for Ab1–42, tau, and hyperphosphorylated tau were not significantly different in participants who did and did not develop delirium. 70 In contrast, prior cognitive decline was significantly related to delirium.
3.4.5.5. Chronic pre‐operative HPA axis dysfunction
Similarly to the preponderant role of a premorbid cognitive impairment, Manou‐Stathopoulou et al. proposed a shifting paradigm for the peri‐operative stress to the importance of a pre‐operative chronic stress. 71 This could be partly explained by a chronic HPA axis dysfunction in older people, which may be accentuated among frail older people. Inflammation biomarkers were higher in participants with delirium, but were not related to prior cognitive impairment. 59 From the same cohort, anticholinergic activity was also measured, regardless of their premorbid cognitive state, 58 but there was no significant relationship between CSF anticholinergic level and delirium.
3.5. Measures
3.5.1. General characteristics
Twenty‐two papers addressed HAD measures. Five were conducted in Belgium72, 73, 74, 75, 76 and four in Italy.77, 78, 79, 80 Most papers were published between 2010 and 2020 although some early works were published in the 1990s in the United States. 81 The mean age was 80 years old. In 17 studies, there was a majority of women.
3.5.2. Methodological aspects
Twenty‐one studies were observational prospective cohort studies and only one was a cross‐sectional study. 82 The smallest sample size was 70 and the largest 746.83, 84 Only 7 out of 22 studies achieved high quality score (Appendix 2 in Supporting Information S2).
Thirteen studies focused on the evaluation of multiple clinical predictors of HAD.29, 73, 74, 75, 78, 80, 81, 83, 85, 86, 87 These studies aimed to determine if certain assessment tools, such as the Short Physical Performance Battery (SPPB), 77 the MNA 79 or SHERPA, 76 among others, were useful to identify patients at risk of HAD. Eight studies tested the predictive power of different biological biomarkers, from blood tests to muscle strength.72, 82, 84, 88, 89, 90, 91 Only one study focused on cognitive function as a predictor of HAD. 51
There was great variability regarding outcomes. Many studies included some measure of functional status based on ADL or IADL. Some studies included death as an outcome, as well as rehospitalization, length of stay or incidence of delirium. Time for evaluation of functional decline was also very heterogeneous. Later evaluations tended to be based on patient recall or telephone interviews.
3.5.3. Main outcomes
Most predictive tools evaluated were able to predict functional decline during hospitalization. Among biomarkers, levels of IL‐6 and IGF1 on admission, combined with SHERPA score could predict disability 3 months after discharge. 72 Dipstick proteinuria also seemed to be a good biomarker of future HAD. 82 Dynapenia, balance, low handgrip strength and general muscular strength were also reported to be predictors of functional decline after hospitalization.88, 89 Interestingly, handgrip strength and general muscular power seemed to be predictors of HAD only in men. 92 The SHERPA score (Score Hospitalier d'Evaluation du Risque de Perte d'Autonomie), first published ten years ago 73 was re‐evaluated in one of the studies which found no predictive ability. 76 The Blaylock Risk Assessment Screening Score (BRASS) index, on the other hand, was useful to predict post‐hospitalization outcome. 80 Decline in SPMSQ during hospitalization also seemed able to predict decline 3 months after discharge. 51 Finally, walking less than 900 steps during hospitalization could also predict functional decline. 91 Many of the reviewed papers found clinical characteristics that, combined, may suggest a higher risk of functional decline. These characteristics included age, gender, number of comorbidities and functional status before admission and at the time of discharge, among others. One of the studies built a new screening tool (HARP) based on these characteristics. 81 Figure 5 provides a summary of the predictive tools for HAD identified through the literature review.
FIGURE 5.
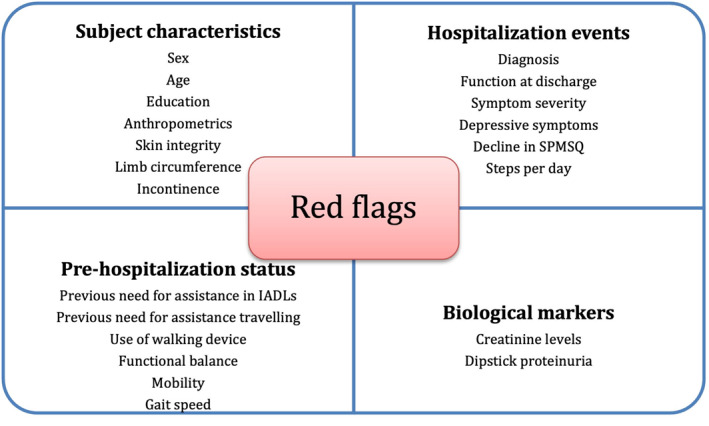
Predictive tools or clinical features for HAD
3.6. Interventions
3.6.1. General characteristics
Thirteen studies addressed interventions for HAD. Most studies were conducted in Australia, the United States of America or Spain after 2010. The mean age was 82 years old and only two studies included more men than women.
3.6.2. Methodological aspects
Most studies were designed as clinical trials or post‐hoc analysis of other datasets. Two of the designs were described as quasi‐experimental93, 94 and one described an intervention outside a clinical trial. 95 The focus of the studies was on multicomponent interventions94, 96, 97 and physical therapy and exercise. 93 One study combined physical exercise and testosterone administration. 98 There was one study evaluating the effect of occupational therapy. 95 Four studies were experimental single‐blind randomized controlled trials (three multicenter and 1 single‐center).98, 99, 100, 101 The fifth study by Cohen et al. was quasi‐experimental with a two‐group pre‐ and post‐design. 102
One of the studies focused on cognitive function measured by the 6‐m dual‐task Gait Velocity Test (GVT) and MMSE. 99 The most frequently used endpoint was ADL. Other endpoints utilized were death, 103 changes in cognitive and mood status, quality of life, handgrip strength and readmission at 3 months after discharge. 12 The main statistical techniques employed for data analyses were bivariate comparisons, multivariate logistic regression and linear mixed models. Studies were generally of good quality (Appendix 2 in Supporting Information S2).
3.6.3. Main outcomes
Four studies reported that interventions were effective. In the WALK‐FOR intervention, decline in basic ADLs occurred in 33% of subjects in the control group versus 23% in the intervention group. 102 Martinez‐Velilla et al. found that treatment in a comprehensive geriatric assessment unit was independently associated with lower risk of decline in ADLs, compared to a general medical unit. 100 On the other hand, Timmer et al. found that activities pacing in addition to typical occupational therapy during inpatient rehabilitation did not show benefits in the post‐hospitalization management of daily activities after returning home. 10 However, two studies found no effect of this kind of intervention on HAD23, 94 and one RCT even found that subjects in the intervention group had a worse outcome.
4. DISCUSSION
In this systematic review, we scoped the existing research on HAD in the acute hospital setting. The most frequently addressed topics were risk factors, measures, and trajectories. Interventions and mechanisms were much less frequently reported. The heterogeneity of our findings reflects the lack of a standard HAD definition, as well as a lack of consensus on the timeframe for evaluating premorbid factors and outcomes measured.
HAD leads to a significant increase in disability and dependency after hospitalization. Although deconditioning can happen at any age, the complex physiological changes that follow a period of inactivity are particularly frequent and potentially harmful in older people.104, 105
Risk factors seemed to be a common field of study in HAD. Despite the large sample size, most studies had a cross‐sectional design, making it inappropriate to draw conclusions regarding causality. In our review, we grouped risk factors into four categories (social, comorbidities, features of hospital stay and physiological reserve), and within each category some factors are clinically modifiable, and some are not. Potential benefits of our evidence synthesis are that an overall awareness of these risks may facilitate more individualized clinical assessments; and from a research perspective, allow future intervention studies to tackle specific aspects whilst controlling for others.
Overtime, the trajectory of functional decline remained roughly similar. Most studies showed an early hospital readmission rate of around 30%. Others found a very early functional decline that started just after admission. One particularly important and convergent finding from these studies is that recovery, if it happens, may take several months.
In our review, only a few studies focused on evaluating pathophysiological mechanisms. The impact on allostasis exerted by the multiple stressors experienced during hospitalization may cause an increase in total cortisol concentration, serum norepinephrine, and immune‐suppressing inflammatory cytokines such as IL‐10 and Transforming Growth Factor β. 106 These modifications may in turn affect multiple organs, including cardiovascular system, immune system, cognition, and lead to overall functional decline. 106 During an acute hospitalization, older adults spend approximately 83% of their hospital stay in bed and 12% of their time in a chair.105, 107 This review extensively outlined the different physiological changes triggered by bed rest. 108 The hemostatic system shifted toward hypocoagulability during bed rest, 109 modified postural control, altered joint coordination, and loss of balance. Muscle strength may decrease due to changes in the functional properties of the central nervous system, such as a loss of proprioceptive and cutaneous sensations. 108 Young healthy participants in a simulated prolonged bed rest situation showed modified sympathetic neural pathways, with longer central synaptic delay. 110
Chang et al. framed three propositions in a nosocomial based psychological stress model for hospitalization 111 : (1) a prolonged increased inflammatory response and a sustained upregulation; (2) fear, anxiety and an elevated sense of vulnerability, distress and helplessness, (3) the combination of the increased physiologic demands and psychological stress contributing to a transient state of physical and psychological deconditioning, and a vicious circle to maladaptive cognitive schema. However, as Wilson et al. extensively reviewed, hospital‐acquired sarcopenia may have more complex pathophysiological mechanisms, including complex interactions between aging, immunosenescence, frailty and sarcopenia. 112
Our findings suggest that HAD and frailty are intimately related, affecting a similar range of body systems (musculoskeletal, respiratory, urinary), daily functions (sleep cycle, mobility) and cognitive abilities. 113
Possibly due to the multifactorial pathophysiology of HAD, a comprehensive geriatric assessment unit seemed to be more effective than a conventional acute medical hospitalization unit in its prevention. This would suggest the need to implement interdisciplinary working and multidisciplinary geriatric assessment in all hospital departments.
Regarding interventions, studies were scarce and highlighted the need for more research. A recent meta‐analysis focused on different interventions to reduce HAD. 114 The authors showed that enhanced inpatient programs reduced the risk of declining ADL ability by 4% at discharge, reduced nursing home residence by 8% at one to 3 months post‐discharge and reduced 1‐month mortality by 23%. However, the level of evidence remains low and it is important to design robust clinical trials with clear target populations.
4.1. Review limitations and strengths
While we reviewed studies in three common languages, review articles published in some other major languages such as German or Chinese, were not included. As in any review, the limitations imposed by the search terms may have led to the exclusion of some relevant studies. However, we used a variety of sources and databases from non‐English speaking areas as well. Our review also has the advantage of addressing overall aspects of HAD, whereas existing reviews have generally focused on just one aspect of HAD.113, 114 The aim of this review was to be all‐encompassing, to narratively outline the current evidence and highlight the main research gaps.
5. CONCLUSIONS
This systematic review identifies important steps in the clinical management of HAD and keys areas for future research. A consensus definition of HAD and a clear research agenda are urgently needed. The current variability on risk assessments, body systems evaluated, time frames and outcomes mean that there is still ample scope for future key advances in the understanding and management of this severe condition, which is often overlooked during hospitalization and may have devastating consequences, both for individuals and health and social care systems. Until now, studies on interventions have mainly focused on musculoskeletal aspects of the condition and physical rehabilitation. We suggest a more holistic clinical and research focus including cognitive and psychological aspects, as well as hospital structure and systems modifications.
As part of a roadmap for clinical practice and future research, we propose that HAD in older people should be screened as a functional decline from pre‐admission baseline until at least 1 year after discharge. We also propose the objective capture of function at the point of admission and on discharge, in order to be able to model the magnitude of acute illness‐related functional loss and the amount of functional recovery during hospitalization. We propose that the measurement of function does not only include ADLs/mobility/disability but also cognitive and mood‐related variables (e.g. exhaustion, anxiety, depression, psychological wellbeing).
In terms of optimal care planning to reduce HAD, prevention may be the most important step. It would be important to be able to systematically and swiftly identify patients at high‐risk with a reliable tool. In addition, hospitalization‐related stress should be reduced to a minimum. Simple measures could be implemented such as promoting improved nutrition, improving the hospital environment, improving communication with staff and loved ones, and promoting activities and arts/creative engagement.
Allied health therapies should be involved as soon as possible for high‐risk patients as part of a comprehensive geriatric assessment, which may also include nurses, social workers, and psychiatry of old age, as needed. Care plans should be focused on an individual's goals and preferences and clearly communicated, to empower patients, promote autonomy and reduce anxiety and uncertainty.
Clinical units caring for vulnerable older people should have access to research funding and expertise and have enough time to engage in complex research. Mixed quantitative‐qualitative methodologies with robust patient and public involvement (PPI) strategies would be particularly rewarding in this area. Research efforts should be focused on complex, multifaceted interventions including mobility and cognitive stimulation programs.
CONFLICT OF INTEREST
Yaohua Chen discloses no conflicts. Arianna Almirall‐Sánchez discloses no conflicts. David Mockler discloses no conflicts. Emily Adrion discloses no conflicts. Clara Domínguez‐Vivero discloses no conflicts. Román Romero‐Ortuño discloses no conflicts.
AUTHOR CONTRIBUTIONS
Yaohua Chen, Arianna Almirall‐Sánchez and Clara Domínguez‐Vivero designed, acquired, analyzed, and interpreted the data, performed the literature search, drafted and revised the manuscript. Emily Adrion revised the manuscript. David Mockler designed and performed the literature search. Román Romero‐Ortuño designed the study and revised the manuscript.
Supporting information
Supporting Information S1
Supporting Information S2
ACKNOWLEDGMENTS
We would like to acknowledge the support from the Global Brain Health Institute (GBHI). The first authors are Atlantic Fellows for Equity in Brain Health and the last author (RRO) is member of Faculty at the Global Brain Health Institute (GBHI) in Trinity College Dublin. GBHI is funded by The Atlantic Philanthropies https://www.gbhi.org/about. RRO is also funded by Science Foundation Ireland under Grant numbers 18/FRL/6188 and 20/COV/8493.
Open access funding provided by IReL.
Chen Y, Almirall‐Sánchez A, Mockler D, Adrion E, Domínguez‐Vivero C, Romero‐Ortuño R. Hospital‐associated deconditioning: not only physical, but also cognitive. Int J Geriatr Psychiatry. 2022;37(3):1‐13. 10.1002/gps.5687
Yaohua Chen, Arianna Almirall‐Sánchez and Clara Domínguez‐Vivero are contributed equally to this article.
DATA AVAILABILITY STATEMENT
Due to the nature of this review, the data sharing statement is not applicable.
REFERENCES
- 1. Covinsky KE, Pierluissi E, Johnston CB. Hospitalization‐associated disability: “She was probably able to ambulate, but I’m not sure”. JAMA. 2011;306(16):1782‐1793. 10.1001/jama.2011.1556 [DOI] [PubMed] [Google Scholar]
- 2. Dharmarajan K, Han L, Gahbauer EA, Leo‐Summers LS, Gill TM. Disability and recovery after hospitalization for medical illness among community‐living older persons: a prospective cohort study. J Am Geriatr Soc. 2020;68(3):486‐495. 10.1111/jgs.16350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Loyd C, Markland AD, Zhang Y, et al. Prevalence of hospital‐associated disability in older adults: a meta‐analysis. J Am Med Dir Assoc. 2020;21(4):455‐461.e5. 10.1016/j.jamda.2019.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Van Rijn M, Buurman BM, Macneil Vroomen JL, et al. Changes in the in‐hospital mortality and 30‐day post‐discharge mortality in acutely admitted older patients: retrospective observational study. Age Ageing. 2016;45(1):41‐47. 10.1093/ageing/afv165 [DOI] [PubMed] [Google Scholar]
- 5. Hoyer EH, Needham DM, Atanelov L, Knox B, Friedman M, Brotman DJ. Association of impaired functional status at hospital discharge and subsequent rehospitalization. J Hosp Med. 2014;9(5):277‐282. 10.1002/jhm.215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. American Hospital Association . Fact Sheet: Post‐Acute Care. 2019. https://www.aha.org/system/files/media/file/2019/07/fact-sheet-post-acute-care-0719.pdf [Google Scholar]
- 7. Mudge AM, Kasper K, Clair A, et al. Recurrent readmissions in medical patients: a prospective study. J Hosp Med. 2011;6(2):61‐67. 10.1002/jhm.811 [DOI] [PubMed] [Google Scholar]
- 8. Palese A, Gonella S, Moreale R, et al. Hospital‐acquired functional decline in older patients cared for in acute medical wards and predictors: findings from a multicentre longitudinal study. Geriatr Nurs. 2016;37(3):192‐199. 10.1016/j.gerinurse.2016.01.001 [DOI] [PubMed] [Google Scholar]
- 9. Hartley P, Gibbins N, Saunders A, et al. The association between cognitive impairment and functional outcome in hospitalised older patients: a systematic review and meta‐analysis. Age Ageing. 2017. Published online January 23. 10.1093/ageing/afx007 [DOI] [PubMed] [Google Scholar]
- 10. Timmer AJ, Unsworth CA, Browne M. Occupational therapy and activity pacing with hospital‐associated deconditioned older adults: a randomised controlled trial. Disabil Rehabil. 2020;42(12):1727‐1735. 10.1080/09638288.2018.1535630 [DOI] [PubMed] [Google Scholar]
- 11. Mudge AM, Banks MD, Barnett AG, et al. CHERISH (collaboration for hospitalised elders reducing the impact of stays in hospital): protocol for a multi‐site improvement program to reduce geriatric syndromes in older inpatients. BMC Geriatr. 2017;17(1):11. 10.1186/s12877-016-0399-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kortebein P. Rehabilitation for hospital‐associated deconditioning. Am J Phys Med Rehabil. 2009;88(1):66‐77. 10.1097/PHM.0b013e3181838f70 [DOI] [PubMed] [Google Scholar]
- 13. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting Items for systematic reviews and meta‐analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006‐1012. 10.1016/j.jclinepi.2009.06.005 [DOI] [PubMed] [Google Scholar]
- 14. Rethlefsen ML, Kirtley S, Waffenschmidt S, et al. PRISMA‐S: an extension to the PRISMA statement for reporting literature Searches in systematic reviews. Syst Rev. 2021;10(1):39. 10.1186/s13643-020-01542-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Osuna‐Pozo CM, Ortiz‐Alonso J, Vidán M, Ferreira G, Serra‐Rexach JA. Revisión sobre el deterioro funcional en el anciano asociado al ingreso por enfermedad aguda. Rev Esp Geriatr Gerontol. 2014;49(2):77‐89. 10.1016/j.regg.2013.08.001 [DOI] [PubMed] [Google Scholar]
- 16. Vidán Astiz MT, García ES, Armesto MA, et al. Deterioro funcional durante la hospitalización en ancianos. Beneficios del ingreso en el servicio de geriatría. Rev Esp Geriatr Gerontol. 2008;43(3):133‐138. 10.1016/S0211-139X(08)71172-7 [DOI] [PubMed] [Google Scholar]
- 17. Bootsma AMJ, Buurman BM, Geerlings SE, de Rooij SE. Urinary incontinence and indwelling urinary catheters in acutely admitted elderly patients: relationship with mortality, institutionalization, and functional decline. J Am Med Dir Assoc. 2013;14(2):147.e7‐147.e12. 10.1016/j.jamda.2012.11.002 [DOI] [PubMed] [Google Scholar]
- 18. Callen BL, Mahoney JE, Grieves CB, Wells TJ, Enloe M. Frequency of hallway ambulation by hospitalized older adults on medical units of an academic hospital. Geriatr Nurs. 2004;25(4):212‐217. 10.1016/j.gerinurse.2004.06.016 [DOI] [PubMed] [Google Scholar]
- 19. Hsieh SJ, Madahar P, Hope AA, Zapata J, Gong MN. Clinical deterioration in older adults with delirium during early hospitalisation: a prospective cohort study. BMJ Open. 2015;5(9):e007496. 10.1136/bmjopen-2014-007496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pedone C, Ercolani S, Catani M, et al. Elderly patients with cognitive impairment have a high risk for functional decline during hospitalization: the GIFA study. J Gerontol Ser A Biol Sci Med Sci. 2005;60(12):1576‐1580. 10.1093/gerona/60.12.1576 [DOI] [PubMed] [Google Scholar]
- 21. Sánchez‐Rodríguez D, Marco E, Miralles R, et al. Sarcopenia, physical rehabilitation and functional outcomes of patients in a subacute geriatric care unit. Arch Gerontol Geriatr. 2014;59(1):39‐43. 10.1016/j.archger.2014.02.009 [DOI] [PubMed] [Google Scholar]
- 22. Sánchez E, Vidán MT, Serra JA, Fernández‐Avilés F, Bueno H. Prevalence of geriatric syndromes and impact on clinical and functional outcomes in older patients with acute cardiac diseases. Heart. 2011;97(19):1602‐1606. 10.1136/hrt.2011.227504 [DOI] [PubMed] [Google Scholar]
- 23. Basic D, Ní Chróinín D, Conforti D, Shanley C. Predictors on admission of functional decline among older patients hospitalised for acute care: a prospective observational study. Australas J Ageing. 2017;36(4):E57‐E63. 10.1111/ajag.12458 [DOI] [PubMed] [Google Scholar]
- 24. Beddoes‐Ley L, Khaw D, Duke M, Botti M. A profile of four patterns of vulnerability to functional decline in older general medicine patients in Victoria, Australia: a cross sectional survey. BMC Geriatr. 2016;16(1):1‐12. 10.1186/s12877-016-0323-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Condorhuamán‐Alvarado PY, Menéndez‐Colino R, Mauleón‐Ladrero C, Díez‐Sebastián J, Alarcón T, González‐Montalvo JI. Factores predictores de pérdida funcional al alta en ancianos hospitalizados por enfermedad aguda. Rev Esp Geriatr Gerontol. 2017;52(5):253‐256. 10.1016/j.regg.2017.03.006 [DOI] [PubMed] [Google Scholar]
- 26. Dasgupta M, Brymer C. Poor functional recovery after delirium is associated with other geriatric syndromes and additional illnesses. Int Psychogeriatr. 2015;27(5):793‐802. 10.1017/S1041610214002658 [DOI] [PubMed] [Google Scholar]
- 27. Fimognari FL, Pierantozzi A, De Alfieri W, et al. The severity of acute illness and functional trajectories in hospitalized older medical patients. J Gerontol Ser A Biol Sci Med Sci. 2017;72(1):102‐108. 10.1093/gerona/glw096 [DOI] [PubMed] [Google Scholar]
- 28. Holroyd S, Snustad DG, Chalifoux ZL. Attitudes of older adults’ on being told the diagnosis of Alzheimer’s disease. J Am Geriatr Soc. 1996;44(4):400‐403. [DOI] [PubMed] [Google Scholar]
- 29. Hoogerduijn JG, Buurman BM, Korevaar JC, Grobbee DE, De rooij SE, Schuurmans MJ. The prediction of functional decline in older hospitalised patients. Age Ageing. 2012;41(3):381‐387. 10.1093/ageing/afs015 [DOI] [PubMed] [Google Scholar]
- 30. Ocampo‐Chaparro JM, Mosquera‐Jiménez JI, Davis AS, Reyes‐Ortiz CA. Functional impairment associated with cognitive impairment in hospitalised elderly. Rev Esp Geriatr Gerontol. 2018;53(1):19‐22. 10.1016/j.regg.2017.05.007 [DOI] [PubMed] [Google Scholar]
- 31. Arora VM, Plein C, Chen S, Siddique J, Sachs GA, Meltzer DO. Relationship between quality of care and functional decline in hospitalized vulnerable elders. Med Care. 2009;47(8):895‐901. 10.1097/MLR.0b013e3181a7e3ec [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Isaia G, Maero B, Gatti A, et al. Risk factors of functional decline during hospitalization in the oldest old. Aging Clin Exp Res. 2009;21(6):453‐457. 10.1007/BF03327448 [DOI] [PubMed] [Google Scholar]
- 33. Zisberg A, Shadmi E, Gur‐Yaish N, Tonkikh O, Sinoff G. Hospital‐associated functional decline: the role of hospitalization processes beyond individual risk factors. J Am Geriatr Soc. 2015;63(1):55‐62. 10.1111/jgs.13193 [DOI] [PubMed] [Google Scholar]
- 34. Helvik A‐S, Selbæk G, Engedal K. Functional decline in older adults one year after hospitalization. Arch Gerontol Geriatr. 2013;57(3):305‐310. 10.1016/j.archger.2013.05.008 [DOI] [PubMed] [Google Scholar]
- 35. Friedman SM, Mendelson DA, Bingham KW, McCann RM. Hazards of hospitalization: residence prior to admission predicts outcomes. Gerontologist. 2008;48(4):537‐541. 10.1093/geront/48.4.537 [DOI] [PubMed] [Google Scholar]
- 36. Isaia G, Bo M, Aimonino N, et al. Functional decline two weeks before hospitalization in an elderly population. Aging Clin Exp Res. 2010;22(4):352‐355. 10.1007/BF03324939 [DOI] [PubMed] [Google Scholar]
- 37. Corsonello A, Maggio M, Fusco S, et al. Proton pump inhibitors and functional decline in older adults discharged from acute care hospitals. J Am Geriatr Soc. 2014;62(6):1110‐1115. 10.1111/jgs.12826 [DOI] [PubMed] [Google Scholar]
- 38. Fabbietti P, Ruggiero C, Sganga F, et al. Effects of hyperpolypharmacy and potentially inappropriate medications (PIMs) on functional decline in older patients discharged from acute care hospitals. Arch Gerontol Geriatr. 2018;77:158‐162. 10.1016/j.archger.2018.05.007 [DOI] [PubMed] [Google Scholar]
- 39. Mudge AM, O’Rourke P, Denaro CP. Timing and risk factors for functional changes associated with medical hospitalization in older patients. J Gerontol Ser A Biol Sci Med Sci. 2010;65(8):866‐872. 10.1093/gerona/glq069 [DOI] [PubMed] [Google Scholar]
- 40. Wakabayashi H, Sashika H. Malnutrition is associated with poor rehabilitation outcome in elderly inpatients with hospital‐asociated deconditioning: a prospective cohort study. J Rehabil Med. 2014;46(3):277‐282. 10.2340/16501977-1258 [DOI] [PubMed] [Google Scholar]
- 41. Zisberg A, Shadmi E, Sinoff G, Gur‐Yaish N, Srulovici E, Admi H. Low mobility during hospitalization and functional decline in older adults. J Am Geriatr Soc. 2011;59(2):266‐273. 10.1111/j.1532-5415.2010.03276.x [DOI] [PubMed] [Google Scholar]
- 42. Robinson TN, Wallace JI, Wu DS, et al. Accumulated frailty characteristics predict postoperative discharge institutionalization in the geriatric patient. J Am Coll Surg. 2011;213(1):37‐42. 10.1016/j.jamcollsurg.2011.01.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shinohara T, Tsuchida N, Yamane T, Shindo K, Otani T, Ishii D. Association between patients’ state upon admission and decline in activities of daily living. J Phys Ther Sci. 2019;31(10):813‐818. 10.1589/jpts.31.813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Shiraishi A, Yoshimura Y, Wakabayashi H, Tsuji Y. Poor oral status is associated with rehabilitation outcome in older people. Geriatr Gerontol Int. 2017;17(4):598‐604. 10.1111/ggi.12763 [DOI] [PubMed] [Google Scholar]
- 45. Stelmokas J, Rochette AD, Hogikyan R, et al. Influence of cognition on length of stay and rehospitalization in older veterans admitted for post‐acute care. J Appl Gerontol. 2020;39(6):609‐617. 10.1177/0733464819853989 [DOI] [PubMed] [Google Scholar]
- 46. Ticinesi A, Nouvenne A, Prati B, et al. Profiling the hospital‐dependent patient in a large academic hospital: observational study. Eur J Intern Med. 2019;64:41‐47. 10.1016/j.ejim.2019.02.013 [DOI] [PubMed] [Google Scholar]
- 47. Zaslavsky O, Zisberg A, Shadmi E. Impact of functional change before and during hospitalization on functional recovery 1 month following hospitalization. J Gerontol A Biol Sci Med Sci. 2015;70(3):381‐386. 10.1093/gerona/glu168 [DOI] [PubMed] [Google Scholar]
- 48. Traissac T, Videau M‐N, Bourdil M‐J, Bourdel‐Marchasson I, Salles N. The short mean length of stay of post‐emergency geriatric units is associated with the rate of early readmission in frail elderly. Aging Clin Exp Res. 2011;23(3):217‐222. 10.1007/BF03324963 [DOI] [PubMed] [Google Scholar]
- 49. Parlevliet JL, MacNeil‐Vroomen J, Buurman BM, De Rooij SE, Bosmans JE. Health‐related quality of life at admission is associated with postdischarge mortality, functional decline, and institutionalization in acutely hospitalized older medical patients. J Am Geriatr Soc. 2016;64(4):761‐768. 10.1111/jgs.14050 [DOI] [PubMed] [Google Scholar]
- 50. Quinlan N, Rudolph JL. Postoperative delirium and functional decline after noncardiac surgery. J Am Geriatr Soc. 2011;59(Suppl 2):301‐S304. 10.1111/j.1532-5415.2011.03679.x [DOI] [PubMed] [Google Scholar]
- 51. Zisberg A, Sinoff G, Agmon M, Tonkikh O, Gur‐Yaish N, Shadmi E. Even a small change can make a big difference: the case of in‐hospital cognitive decline and new IADL dependency. Age Ageing. 2016;45(4):500‐504. 10.1093/ageing/afw063 [DOI] [PubMed] [Google Scholar]
- 52. Boyd CM, Ricks M, Fried LP, et al. Functional decline and recovery of activities of daily living in hospitalized, disabled older women: the women’s health and aging study. J Am Geriatr Soc. 2009;57(10):1757‐1766. 10.1111/j.1532-5415.2009.02455.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sager MA, Franke T, Inouye SK, et al. Functional outcomes of acute medical illness and hospitalization in older persons. Arch Intern Med. 1996;156(6):645‐652. http://www.ncbi.nlm.nih.gov/pubmed/8629876 [PubMed] [Google Scholar]
- 54. Córcoles‐Jiménez MP, Villada‐Munera A, Del Egido‐Fernández MÁ, et al. Recovery of activities of daily living among older people one year after hip fracture. Clin Nurs Res. 2015;24(6):604‐623. 10.1177/1054773815573261 [DOI] [PubMed] [Google Scholar]
- 55. Chen CC‐H, Wang C, Huang G‐H. Functional trajectory 6 months posthospitalization. Nurs Res. 2008;57(2):93‐100. 10.1097/01.nnr.0000313485.18670.e2 [DOI] [PubMed] [Google Scholar]
- 56. Duan‐Porter W, Vo TN, Ullman K, et al. Hospitalization‐associated change in gait speed and risk of functional limitations for older adults. J Gerontol Ser A Biol Sci Med Sci. 2019;74(10):1657‐1663. 10.1093/gerona/glz027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Gill TM, Allore HG, Gahbauer EA, Murphy TE. Change in disability after hospitalization or restricted activity in older persons. JAMA. 2010;304(17):1919. 10.1001/jama.2010.1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Watne LO, Hall RJ, Molden E, et al. Anticholinergic activity in cerebrospinal fluid and serum in individuals with hip fracture with and without delirium. J Am Geriatr Soc. 2014;62(1):94‐102. 10.1111/jgs.12612 [DOI] [PubMed] [Google Scholar]
- 59. Neerland BE, Hall RJ, Seljeflot I, et al. Associations between delirium and preoperative cerebrospinal fluid C‐reactive protein, interleukin‐6, and interleukin‐6 receptor in individuals with acute hip fracture. J Am Geriatr Soc. 2016;64(7):1456‐1463. 10.1111/jgs.14238 [DOI] [PubMed] [Google Scholar]
- 60. McEwen BS, Gianaros PJ. Stress‐ and allostasis‐induced brain plasticity. Annu Rev Med. 2011;62(1):431‐445. 10.1146/annurev-med-052209-100430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wong RY, Miller WC. Adverse outcomes following hospitalization in acutely ill older patients. BMC Geriatr. 2008;8:1‐9. 10.1186/1471-2318-8-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Villumsen M, Jorgensen MG, Andreasen J, Rathleff MS, Mølgaard CM. Very low levels of physical activity in older patients during hospitalization at an acute geriatric ward: a prospective cohort study. J Aging Phys Act. 2015;23(4):542‐549. 10.1123/japa.2014-0115 [DOI] [PubMed] [Google Scholar]
- 63. Belala N, Maier C, Heldmann P, Schwenk M, Becker C. A pilot observational study to analyze (in)activity and reasons for sedentary behavior of cognitively impaired geriatric acute inpatients. Z Gerontol Geriatr. 2019;52(Suppl 4):273‐281. 10.1007/s00391-019-01644-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Walker IV FB, Novack DH, Kaiser DL, Knight A, Oblinger P. Anxiety and depression among medical and surgical patients nearing hospital discharge. J Gen Intern Med. 1987;2(2):99‐101. 10.1007/BF02596305 [DOI] [PubMed] [Google Scholar]
- 65. Chen CCH, Chiu MJ, Chen SP, Cheng CM, Huang GH. Patterns of cognitive change in elderly patients during and 6 months after hospitalisation: a prospective cohort study. Int J Nurs Stud. 2011;48(3):338‐346. 10.1016/j.ijnurstu.2010.03.011 [DOI] [PubMed] [Google Scholar]
- 66. Khan KT, Hemati K, Donovan AL. Geriatric physiology and the frailty syndrome. Anesthesiol Clin. 2019;37(3):453‐474. 10.1016/j.anclin.2019.04.006 [DOI] [PubMed] [Google Scholar]
- 67. Brioche T, Pagano AF, Py G, Chopard A. Muscle wasting and aging: experimental models, fatty infiltrations, and prevention. Mol Aspects Med. 2016;50:56‐87. 10.1016/j.mam.2016.04.006 [DOI] [PubMed] [Google Scholar]
- 68. Beishuizen SJE, Scholtens RM, van Munster BC, de Rooij SE. Unraveling the relationship between delirium, brain damage, and subsequent cognitive decline in a cohort of individuals undergoing surgery for hip fracture. J Am Geriatr Soc. 2017;65(1):130‐136. 10.1111/jgs.14470 [DOI] [PubMed] [Google Scholar]
- 69. Hallgren J, Fransson EI, Reynolds CA, Finkel D, Pedersen NL, Dahl Aslan AK. Cognitive trajectories in relation to hospitalization among older Swedish adults. Arch Gerontol Geriatr. 2018;74(May 2017):9‐14. 10.1016/j.archger.2017.09.002 [DOI] [PubMed] [Google Scholar]
- 70. Witlox J, Kalisvaart KJ, De Jonghe JFM, et al. Cerebrospinal fluid β‐amyloid and tau are not associated with risk of delirium: a prospective cohort study in older adults with hip fracture. J Am Geriatr Soc. 2011;59(7):1260‐1267. 10.1111/j.1532-5415.2011.03482.x [DOI] [PubMed] [Google Scholar]
- 71. Manou‐Stathopoulou V, Korbonits M, Ackland GL. Redefining the perioperative stress response: a narrative review. Br J Anaesth. 2019;123(5):570‐583. 10.1016/j.bja.2019.08.011 [DOI] [PubMed] [Google Scholar]
- 72. de Saint‐Hubert M, Jamart J, Morrhaye G, et al. Serum IL‐6 and IGF‐1 improve clinical prediction of functional decline after hospitalization in older patients. Aging Clin Exp Res. 2011;23(2):106‐111. 10.3275/7028 [DOI] [PubMed] [Google Scholar]
- 73. Cornette P, Swine C, Malhomme B, Gillet J‐B, Meert P, D’Hoore W. Early evaluation of the risk of functional decline following hospitalization of older patients: development of a predictive tool. Eur J Public Health. 2006;16(2):203‐208. 10.1093/eurpub/cki054 [DOI] [PubMed] [Google Scholar]
- 74. Van Grootven B, Jeuris A, Jonckers M, et al. Predicting hospitalisation‐associated functional decline in older patients admitted to a cardiac care unit with cardiovascular disease: a prospective cohort study. BMC Geriatr. 2020;20(1):112. 10.1186/s12877-020-01510-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Deschodt M, Wellens NIH, Braes T, et al. Prediction of functional decline in older hospitalized patients: a comparative multicenter study of three screening tools. Aging Clin Exp Res. 2011;23(5‐6):421‐426. 10.1007/BF03325237 [DOI] [PubMed] [Google Scholar]
- 76. De Brauwer I, Cornette P, Boland B, Verschuren F, D’Hoore W. Can we predict functional decline in hospitalized older people admitted through the emergency department? Reanalysis of a predictive tool ten years after its conception. BMC Geriatr. 2017;17(1):1‐7. 10.1186/s12877-017-0498-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Corsonello A, Lattanzio F, Pedone C, et al. Prognostic significance of the short physical performance Battery in older patients discharged from acute care hospitals. Rejuvenation Res. 2012;15(1):41‐48. 10.1089/rej.2011.1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Dagani J, Ferrari C, Boero ME, et al. A prospective, multidimensional follow‐up study of a geriatric hospitalised population: predictors of discharge and well‐being. Aging Clin Exp Res. 2013;25(6):691‐701. 10.1007/s40520-013-0153-3 [DOI] [PubMed] [Google Scholar]
- 79. Salvi F, Giorgi R, Grilli A, et al. Mini Nutritional Assessment (short form) and functional decline in older patients admitted to an acute medical ward. Aging Clin Exp Res. 2008;20(4):322‐328. 10.1007/BF03324863 [DOI] [PubMed] [Google Scholar]
- 80. Guido D, Perna S, Peroni G, Guerriero F, Rondanelli M. A comorbidity prognostic effect on post‐hospitalization outcome in a geriatric rehabilitation setting: the pivotal role of functionality, assessed by mediation model, and association with the Brass index. Aging Clin Exp Res. 2015;27(6):849‐856. 10.1007/s40520-015-0360-1 [DOI] [PubMed] [Google Scholar]
- 81. Sager MA, Rudberg MA, Jalaluddin M, et al. Hospital admission risk profile (HARP): identifying older patients at risk for functional decline following acute medical illness and hospitalization. J Am Geriatr Soc. 1996;44(3):251‐257. 10.1111/j.1532-5415.1996.tb00910.x [DOI] [PubMed] [Google Scholar]
- 82. Chao C Ter, Tsai H Bin, Chiang CK, Huang JW, Hung KY. Dipstick proteinuria level is significantly associated with pre‐morbid and in‐hospital functional status among hospitalized older adults: a preliminary study. Sci Rep. 2017;7(February):1‐9. 10.1038/srep42030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Avelino‐Silva TJ, Farfel JM, Curiati JAE, Amaral JRG, Campora F, Jacob‐Filho W. Comprehensive geriatric assessment predicts mortality and adverse outcomes in hospitalized older adults. BMC Geriatr. 2014;14:129. 10.1186/1471-2318-14-129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Hartley P, Romero‐Ortuno R, Wellwood I, Deaton C. Changes in muscle strength and physical function in older patients during and after hospitalisation: a prospective repeated‐measures cohort study. Age Ageing. 2021;50(1):153‐160. 10.1093/ageing/afaa103 [DOI] [PubMed] [Google Scholar]
- 85. Barnes DE, Mehta KM, Boscardin WJ, et al. Prediction of recovery, dependence or death in elders who become disabled during hospitalization. J Gen Intern Med. 2013;28(2):261‐268. 10.1007/s11606-012-2226-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Abizanda Soler P, León Ortiz M, Romero Rizos L, et al. La pérdida funcional al ingreso, principal variable explicativa de discapacidad y mortalidad al alta y al mes en ancianos hospitalizados. Rev Esp Geriatr Gerontol. 2007;42(4):201‐211. 10.1016/S0211-139X(07)73552-7 [DOI] [Google Scholar]
- 87. Bell SP, Schnelle J, Nwosu SK, et al. Development of a multivariable model to predict vulnerability in older American patients hospitalised with cardiovascular disease. BMJ Open. 2015;5(8):e008122. 10.1136/bmjopen-2015-008122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Chen L, Lee WJ, Xu Y, et al. Dynapenia and poor balance predict post‐discharge functional decline and mortality of older adults. Int J Gerontol. 2019;13(2):129‐133. 10.6890/IJGE.201906_13(2).0006 [DOI] [Google Scholar]
- 89. García‐Peña C, García‐Fabela LC, Gutiérrez‐Robledo LM, García‐González JJ, Arango‐Lopera VE, Pérez‐Zepeda MU. Handgrip strength predicts functional decline at discharge in hospitalized male elderly: a hospital cohort study. PLoS One. 2013;8(7):e69849. Bayer A, ed. 10.1371/journal.pone.0069849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Aarden JJ, van der Schaaf M, van der Esch M, et al. Muscle strength is longitudinally associated with mobility among older adults after acute hospitalization: the hospital‐ADL study. PLoS One. 2019;14(7). In: Kamolz L‐P, ed. 10.1371/journal.pone.0219041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Agmon M, Zisberg A, Tonkikh O, Sinoff G, Shadmi E. Anxiety symptoms during hospitalization of elderly are associated with increased risk of post‐discharge falls. Int psychogeriatr. 2016;28(6):951‐958. 10.1017/S1041610215002306 [DOI] [PubMed] [Google Scholar]
- 92. García‐Peña C, García‐Fabela LC, Gutiérrez‐Robledo LM, García‐González JJ, Arango‐Lopera VE, Pérez‐Zepeda MU. Handgrip strength predicts functional decline at discharge in hospitalized male elderly: a hospital cohort study. PLoS One. 2013;8(7):e69849. 10.1371/journal.pone.0069849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Cohen Y, Zisberg A, Chayat Y, et al. Walking for better outcomes and recovery: the effect of WALK‐FOR in preventing hospital‐associated functional decline among older adults. J Gerontol Ser A. 2019;74(10):1664‐1670. 10.1093/gerona/glz025 [DOI] [PubMed] [Google Scholar]
- 94. Heeren P, Devriendt E, Fieuws S, et al. Unplanned readmission prevention by a geriatric emergency network for transitional care (URGENT): a prospective before‐after study. BMC Geriatr. 2019;19(1):215. 10.1186/s12877-019-1233-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Canon MBF, do CoutoTV. Uma proposta de atuação da Terapia Ocupacional junto a idosos hospitalizados. Cad Ter Ocup da UFSCar. 2014;22(2):373‐382. 10.4322/cto.2014.057 [DOI] [Google Scholar]
- 96. Provencher V, Clemson L, Wales K, et al. Supporting at‐risk older adults transitioning from hospital to home: who benefits from an evidence‐based patient‐centered discharge planning intervention? Post‐hoc analysis from a randomized trial. BMC Geriatr. 2020;20(1):84. 10.1186/s12877-020-1494-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Severinsen KD, Tufton A, Hannan E, Schwind JS, Schmucker D, Cutler A. Evaluating outcomes from an integrated health service for older patients. Ochsner J. 2015;15(4):423‐428. http://www.ncbi.nlm.nih.gov/pubmed/26730227 [PMC free article] [PubMed] [Google Scholar]
- 98. Deer RR, Dickinson JM, Baillargeon J, Fisher SR, Raji M, Volpi E. A phase I randomized clinical trial of evidence‐based, pragmatic interventions to improve functional recovery after hospitalization in geriatric patients. J Gerontol Ser A. 2019;74(10):1628‐1636. 10.1093/gerona/glz084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Sáez de Asteasu ML, Martínez‐Velilla N, Zambom‐Ferraresi F, et al. Assessing the impact of physical exercise on cognitive function in older medical patients during acute hospitalization: Secondary analysis of a randomized trial. PLOS Med. 2019;16(7):e1002852. 10.1371/journal.pmed.1002852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Martínez‐Velilla N, Casas‐Herrero A, Zambom‐Ferraresi F, et al. Effect of exercise intervention on functional decline in very elderly patients during acute hospitalization. JAMA Intern Med. 2019;179(1):28. 10.1001/jamainternmed.2018.4869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Stevens‐Lapsley JE, Loyd BJ, Falvey JR, et al. Progressive multi‐component home‐based physical therapy for deconditioned older adults following acute hospitalization: a pilot randomized controlled trial. Clin Rehabil. 2016;30(8):776‐785. 10.1177/0269215515603219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Cohen Y, Zisberg A, Chayat Y, et al. Walking for better outcomes and recovery: the effect of WALK‐FOR in preventing hospital‐associated functional decline among older adults. J Gerontol A Biol Sci Med Sci. 2019;74(10):1664‐1670. 10.1093/gerona/glz025 [DOI] [PubMed] [Google Scholar]
- 103. Chodos AH, Kushel MB, Greysen SR, et al. Hospitalization‐associated disability in adults admitted to a safety‐net hospital. J Gen Intern Med. 2015;30(12):1765‐1772. 10.1007/s11606-015-3395-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. van Vliet M, Huisman M, Deeg DJH. Decreasing hospital length of stay: effects on daily functioning in older adults. J Am Geriatr Soc. 2017;65(6):1214‐1221. 10.1111/jgs.14767 [DOI] [PubMed] [Google Scholar]
- 105. Falvey JR, Mangione KK, Stevens‐Lapsley JE. Rethinking hospital‐associated deconditioning: proposed paradigm shift. Phys Ther. 2015;95(9):1307‐1315. 10.2522/ptj.20140511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Goldwater DS, Dharmarajan K, McEwan BS, Krumholz HM. Is posthospital syndrome a result of hospitalization‐induced allostatic overload? J Hosp Med. 2018;13(5):1‐9. 10.12788/jhm.2986 [DOI] [PubMed] [Google Scholar]
- 107. Hartley P, Keevil VL, Westgate K, et al. Using accelerometers to measure physical activity in older patients admitted to hospital. Curr Gerontol Geriatr Res. 2018;2018:1‐9. 10.1155/2018/3280240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Canu M‐H, Fourneau J, Coq J‐O, et al. Interplay between hypoactivity, muscle properties and motor command: how to escape the vicious deconditioning circle? Ann Phys Rehabil Med. 2019;62(2):122‐127. 10.1016/j.rehab.2018.09.009 [DOI] [PubMed] [Google Scholar]
- 109. Waha JE, Goswami N, Schlagenhauf A, et al. Effects of exercise and nutrition on the coagulation system during bedrest immobilization. Medicine Baltim. 2015;94(38):e1555. 10.1097/MD.0000000000001555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Klassen SA, De Abreu S, Greaves DK, et al. Long‐duration bed rest modifies sympathetic neural recruitment strategies in male and female participants. J Appl Physiol. 2018;124(3):769‐779. 10.1152/japplphysiol.00640.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Chang BP. Can hospitalization be hazardous to your health? A nosocomial based stress model for hospitalization. Gen Hosp Psychiatr. 2019;60:83‐89. 10.1016/j.genhosppsych.2019.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Wilson D, Jackson T, Sapey E, Lord JM. Frailty and sarcopenia: the potential role of an aged immune system. Ageing Res Rev. 2017;36:1‐10. 10.1016/j.arr.2017.01.006 [DOI] [PubMed] [Google Scholar]
- 113. Gordon S, Grimmer KA, Barras S. Assessment for incipient hospital‐acquired deconditioning in acute hospital settings: a systematic literature review. J Rehabil Med. 2019;51(6):397‐404. 10.2340/16501977-2546 [DOI] [PubMed] [Google Scholar]
- 114. Smith TO, Sreekanta A, Walkeden S, Penhale B, Hanson S. Interventions for reducing hospital‐associated deconditioning: a systematic review and meta‐analysis. Arch Gerontol Geriatr. 2020; 90:104176. 10.1016/j.archger.2020.104176 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information S1
Supporting Information S2
Data Availability Statement
Due to the nature of this review, the data sharing statement is not applicable.


