Abstract
Background
Application of electrocautery to a metal guidewire is used by some operators to perform transseptal puncture (TSP). Commercially available dedicated radiofrequency (RF) guidewires may represent a better alternative. This study compares the safety and effectiveness of electrified guidewires to a dedicated RF wire.
Methods
TSP was performed on freshly excised porcine hearts using an electrified 0.014″ or 0.032″ guidewire under various power settings and was compared to TSP using a dedicated RF wire with 5 W power (0.035″ VersaCross RF System, Baylis Medical). The primary endpoint was the number of attempts required to achieve TSP. Secondary endpoints included the rate of TSP failure, TSP consistency, the effect of the distance between the tip of the guidewire and the tip of the dilator, and effect of RF power output level. Qualitative secondary endpoints included tissue puncture defect appearance, thermal damage to the TSP guidewire or dilator, and tissue temperature using thermal imaging.
Results
The RF wire required on average 1.10 ± 0.47 attempts to cross the septum. The 0.014″ electrified guidewire required an overall mean of 2.17 ± 2.36 attempts (2.0 times as many as the RF wire; p < .01), and the 0.032″ electrified guidewire required an overall mean of 3.90 ± 2.93 attempts (3.5 times as many as the RF wire; p < .01). Electrified guidewires had a higher rate of TSP failure, and caused larger defects and more tissue charring than the RF wire. Thermal analysis showed higher temperatures and a larger area of tissue heating with electrified guidewires than the RF wire.
Conclusion
Fewer RF applications were required to achieve TSP using a dedicated RF wire compared to an electrified guidewire. Smaller defects and lower tissue temperatures were also observed using the RF wire. Electrified guidewires required greater energy delivery and were associated with equipment damage and tissue charring, which may present a risk of thrombus, thermal injury, or scarring.
Keywords: atrial septal defect, diathermy, electrosurgery, interatrial septum, RF puncture, RF wire, transseptal puncture
1. INTRODUCTION
Transseptal puncture (TSP) is commonly performed using radiofrequency (RF) or mechanical needles to obtain left atrial (LA) access during procedures to treat heart rhythm disorders and structural heart disease. 1 Challenging TSP can occur due to anatomic variability, as well as in patients with a history of multiple ablations or congenital heart disease (CHD). 2 , 3 Performing TSP on fibrotic or aneurysmal septa using traditional mechanical needles can lead to lower success rates and a higher risk of injury. 4 In comparison, dedicated RF transseptal needles have been shown to improve the rate of successful LA cannulation, as well as reduce procedure time 5 and complications such as cardiac tamponade 5 and plastic particle embolization 6 even in cases of challenging anatomy. 4 , 7 , 8
While application of electrocautery to the proximal end of a mechanical transseptal needle has been used to facilitate catheterization of difficult septa, electrosurgical parameters have not been clearly defined or optimized for TSP, and there is variability between operators in power settings (20–50 W), RF application times (up to 11 s) and the number of energy applications required. 9 , 10 , 11 , 12 , 13 , 14 More importantly, electrifying a hollow needle can cause coring of cardiac tissue, presenting a risk of systemic embolization. 15 Use of electrocautery with standard metal guidewires has been proposed as an alternative to perform TSP 16 ; however, the safety and effectiveness of this approach are unknown. Standard guidewires are not optimized for electrosurgical use and have been associated with several complications during laparoscopic procedures including guidewire fracture, 17 , 18 stripping of the guidewire coating, 19 as well as electrical 20 and thermal injury 21 , 22 , 23 , 24 , 25 to patients. Recently, a dedicated RF transseptal wire (VersaCross Transseptal Solution, Baylis Medical) has been shown to be safe and effective at both performing TSP and reducing device exchanges to improve procedural efficiency. 26 , 27 The objective of this study was to compare the safety and effectiveness of electrified guidewires (EG) to a dedicated RF transseptal wire in an ex‐vivo porcine TSP model.
2. DEVICE DESCRIPTION
The VersaCross RF transseptal system combines multiple steps (access, puncture, and exchange) using a single wire, thereby eliminating unnecessary device exchanges for LA catheterization (Figure 1). The VersaCross RF wire has a floppy J‐tip or pigtail distal end to allow direct venous access, a dedicated RF electrode for TSP, and a stiff 0.035″ shaft to support therapy sheath delivery, such as cryoballoon or LA appendage occlusion, and exchangeless repositioning to optimize puncture location. The wire is electrically insulated up to the discrete 0.7‐mm long dome‐shaped RF electrode, allowing for optimized current delivery with minimum power using the dedicated RF generator. Although both a J‐tip and pigtail configuration is available, a 7‐mm linear section immediately adjacent to the RF electrode delivers a straight puncture through the interatrial septum.
Figure 1.

The dedicated radiofrequency (RF) wire (VersaCross, Baylis Medical) has a stiff 0.035″ electrically insulated shaft and floppy tail with either a pigtail or J‐tip configuration, and a discrete RF electrode at the distal tip. In comparison, since standard 0.032” or 0.014″ guidewires are not electrically insulated, the “electrode” is created by manual exposure from the dilator
3. METHODS
3.1. Experimental model
An ex‐vivo porcine model was developed using hearts harvested the same day from swine weighing approximately 100 kg. Punctures were performed on the interatrial septum using the dedicated RF wire and corresponding generator (RFP‐100A, Baylis Medical), or by connecting a 0.014″ coronary guidewire (Astato, Asahi Intecc or BMW, Abbott Vascular) or a standard 0.032″ guidewire (Paragon, Integer) to an electrosurgical generator (ValleyLab, Medtronic). Several power settings (20, 35, and 50 W in cut mode) were used in each experiment utilizing EG. RF power was manually applied to the proximal end of the guidewire for approximately 2 s using an electrocautery pen to represent common clinical use, 9 , 11 , 28 while a predefined 1 s pulse mode setting (approximately 5 W) was delivered through the dedicated RF wire (Figure 2). The primary endpoint was the number of RF applications required for TSP. Secondary endpoints included TSP failure rate, TSP consistency, the effect of RF power output level, and effect of distance between the tip of the guidewire and the tip of the dilator. Additional qualitative secondary endpoints included tissue puncture collateral damage (e.g., tissue charring, defect size, puncture morphology homogeneity), damage to the TSP guidewire or dilator (e.g. melting, charring and deformation), and the area of tissue heating and peak temperature measured using thermal imaging (FLIR E60, FLIR Systems).
Figure 2.

Transseptal puncture was performed using a dedicated radiofrequency wire or different electrified guidewires under various power settings
3.2. Atrial septal puncture
Interatrial septa isolated from 36 porcine hearts were immersed in a room temperature bath of 0.9% sodium chloride solution and positioned on a cork sample holder (Figure 3A); buoyancy of the cork ring ensured a consistent force was applied to the septum using each wire. RF energy was delivered using the RF wire and EG under each power setting. Additional energy applications were delivered until the tip of the wire perforated the septum, or up to a maximum of eight attempts. Failure to puncture was defined as the inability to perforate the septum after eight RF applications. To model a range of conditions in clinical practice, multiple distances from the distal wire to the dilator tip were tested; 1 mm (i.e., underexposure of wire tip), 3 mm (i.e., ideal wire tip exposure), and 5 mm (i.e., overexposure of wire). The number of RF applications required to perforate the septum was recorded for each treatment condition. Each treatment condition was repeated at least six times. Inspection of puncture sites was performed immediately postpuncture under ×30–50 magnification using an optical microscope (VHX‐5000, Keyence) to confirm TSP success and qualitatively assess the defect site.
Figure 3.
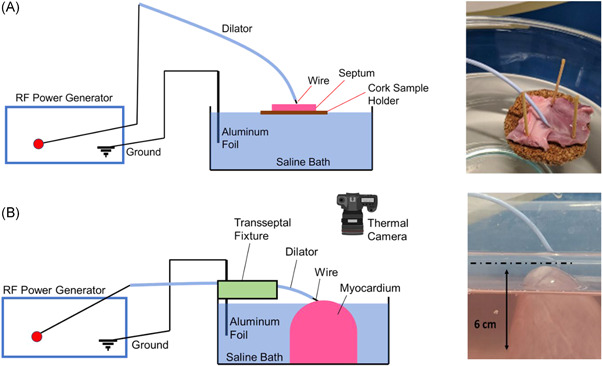
(A) Ex‐vivo septa were fixed onto a cork sample holder in a saline bath to perform transseptal puncture and to ensure consistent force application between experiments. (B) A thermal camera was placed above the myocardium that was immersed in a saline bath to capture the heat profile during radiofrequency delivery
3.3. Tissue thermal imaging
Approximately 6 cm of ventricular myocardium was isolated from the apex of porcine hearts and partially immersed in a room temperature saline bath (Figure 3B). Dilators were stabilized using a holding fixture to ensure consistent force against the tissue during RF application. The temperature was recorded using a thermal camera as RF was applied through each of the wires under different power settings and moderate (3 mm) exposure from the dilator tip. The color scale was normalized with respect to room temperature as the baseline (black).
3.4. Statistical analysis
The number of energy applications required to successfully puncture the septal tissue for each treatment condition was assessed as a mean with standard deviation (95% confidence interval). The paired t test was used to compare the number of applications. Levene's test was used to assess the homogeneity of variances. A probability value of <.05 was used to determine statistical significance. Analyses were performed using Excel software (Microsoft Office 10, Microsoft).
4. RESULTS
4.1. Tissue puncture effectiveness
The RF wire required on average 1.10 ± 0.47 attempts to cross the septum. The 0.014″ electrified guidewire required an overall mean of 2.17 ± 2.36 attempts (2.0 times as many as the RF wire; p < .01), and the 0.032″ electrified guidewire required an overall mean of 3.90 ± 2.93 attempts (3.5 times as many as the RF wire; p < .01, Figure 4A). Across power output levels, the 0.014″ electrified guidewire required a range of 1.2–2.6 times as many applications as the RF wire, and the 0.032″ electrified guidewire required 3.4–4.1 times as many attempts as the RF wire to perforate the septum (Figure 4B). The number of RF applications was significantly more consistent for the RF wire than both 0.014″ and 0.032″ electrified guidewire (p < .001, Figure 4B). Increase in the power applied to EG, from 20 to 50 W, did not significantly improve the consistency in TSP success. Across all power settings, the 0.014″ and 0.032″ EG failed to puncture the septum in 6% and 19% of tissues, respectively. The RF wire did not fail to perforate any of the tissue specimens.
Figure 4.

(A) Overall effectiveness of radiofrequency (RF) wire compared to electrified guidewires (EG) (B) 0.032″ EG requires significantly more RF applications and both EGs have more variability between tissue samples than the dedicated RF wire. (C) Transseptal puncture using the RF wire was 100% successful regardless of the distance between the wire tip and dilator tip, whereas the effectiveness of EG declined with distance. Data reported as mean attempts to perforate each sample, up to eight RF applications (significance shown for electrified guidewires versus RF wire. *p < .05, **p < .01, ***p < .0001 for means between each group; # p < .0001 for sample variance within each group)
While the dilator‐wire tip distance had no measurable effect on the rate of successful puncture using the RF wire, increasing the distance from 1 to 5 mm reduced TSP effectiveness for both 0.014″ and 0.032″ EG (Figure 4C). At 1 mm dilator‐wire tip distance, all wires perforated the septum on the first attempt. However, increasing the distance to 3 and 5 mm required more RF attempts and introduced variability in the TSP effectiveness, especially for electrified guidewires. At the maximum dilator‐wire tip distance tested (5 mm), the 0.014″ electrified guidewire required 5.8 ± 3.4 attempts for TSP and failed to puncture in 40% of the septa after 8 attempts, while the 0.032″ electrified guidewire failed to puncture in all septa after 8 attempts (Figure 4C).
4.2. Tissue puncture site comparison
There were visually larger defects with a greater amount of tissue charring, and nonhomogeneous morphology at puncture sites made by 0.014″ and 0.032″ EG compared to the RF wire (Figure 5). These qualitative differences between EG and RF wire septal defects were observed at all power settings and dilator‐wire tip distances. Within electrified guidewire groups, the size of puncture defects appeared larger, and more charring was observed with increasing power.
Figure 5.

(A) Schematic layout showing multiple punctures made on the same septum using each wire under different dilator‐wire tip distances and electrified guidewire power settings, respectively: (B) 1 mm and 35 W, (C) 3 mm and 20 W, (D) 3 mm and 35 W, (E) 3 mm and 50 W, and (F) 5 mm and 35 W. The dedicated wire in all experiments delivered a preset power of 5 W (scale bar = 1 mm).
4.3. Guidewire and dilator damage
Qualitative inspection of wires and dilators before and after TSP revealed varying levels of equipment damage with the use of electrified guidewires (Figure 6). Shorter dilator‐wire tip distances led to visually greater wire and dilator damage. While positioning the wire tip 1 mm from the dilator improved the TSP effectiveness of electrified guidewires (Figure 4B), this created significant equipment damage including melting of the 0.014″ electrified guidewire, deformation, and charring of the 0.32″ electrified guidewire, as well as melting and charring of the respective dilators (Figure 6). Using moderate dilator‐wire tip distance (3 mm), increasing the power applied to electrified guidewires increased the level of equipment damage (Figure S1). In comparison, there was no sign of charring, melting, or deformation using the dedicated RF wire at all dilator‐wire tip distances (Figure S1).
Figure 6.

Representative images of the dedicated radiofrequency (RF) wire system, electrified guidewires (EG) and respective dilators using an optical microscope (A) before transseptal puncture and (B) after transseptal puncture. The dedicated RF wire system showed no damage. In both 0.014″ and 0.032″ EG setups, the metal wire tip was melted and/or deformed, and the respective dilator showed signs of melting and charring. Images show representative devices at moderate power levels and 1 mm distance between the dilator tip and wire tip, corresponding to the highest EG transseptal puncture effectiveness (scale bar = 1 mm)
4.4. Tissue puncture thermal impact
During RF application, heat distribution was visually larger in the case of EG compared to the RF wire by thermal imaging (Figure 7; Video S1). Peak temperature at the core of the heated area exceeded 100°C using EG compared to approximately 30°C using the RF wire, indicating a greater rise temperature increase relative to baseline (room temperature). The larger heating area and higher peak temperature at the core were observed at all power settings when EG were used relative to the RF wire.
Figure 7.

Thermal camera images of radiofrequency delivery using the 0.035″ dedicated RF wire, 0.014″ and 0.032″ electrified guidewires (EG; 20, 35, and 50 W delivered power). Temperature recordings were normalized to ambient room temperature (black), indicating an approximately 80°C temperature rise with electrified guidewires versus 10°C with the RF wire
5. DISCUSSION
Tissue puncture using RF energy relies on the transfer of alternating current to the target tissue to generate localized heating and tissue vaporization. Successful crossing requires focal delivery of current. The current density (rate of charge transfer per unit area) is dependent on the amount of delivered power and electrode size. Off‐label application of electrocautery to metal guidewires during atrial septal puncture may lead to unintended damage to the dilator or guidewire itself with potential adverse clinical consequences. First, EG have an active electrode that is dependent on manual exposure of the wire from dilator (i.e., dilator‐wire tip distance), which introduces uncertainty in the exact current density and, therefore, TSP effectiveness and consistency between cases. This may explain why more power was needed in the present study when using EG compared to a dedicated RF wire. Additionally, EG are typically coated with fluoropolymers and epoxy‐based hydrophilic coatings (such as polytetrafluoroethylene) for lubricity and electrical insulation, which has been suggested to be a source of current leakage along the entire length of the wire. 29 , 30 This not only impacts the effective current density and TSP effectiveness, but also poses electrosurgical safety risks for patients 31 , 32 , 33 that can, otherwise, be mitigated by minimizing power 34 , 35 and using dedicated insulated tools. 36 , 37
The present study compared the safety and effectiveness of a dedicated RF wire to EG using a range of power settings and dilator‐wire tip distances. The RF wire with preset average power of 5 W demonstrated 100% success at TSP. While higher power with EG was expected to correlate with increased current density and tissue vaporization, EG had lower effectiveness and greater variability in TSP success between samples than the dedicated RF wire. Increasing the power applied to EG from 20 to 50 W did not translate into a substantial improvement on TSP effectiveness, suggesting the current leakage along the length of the guidewire may reduce the effectiveness of RF delivery. The distance between the dilator and wire tip did not significantly impact TSP using the dedicated RF wire but appeared to have an inverse effect on TSP success using EG. However, a small dilator‐wire tip length also correlated with damage to both the wire and dilator tip, presenting a risk of particle liberation and embolization. At the maximum dilator‐wire distance tested (5 mm), the 0.014″ EG required significantly more RF applications than the RF wire, and the 0.032″ guidewires failed to perforate the septum. The discrete active electrode and insulated shaft of the dedicated RF wire may explain why TSP effectiveness was not sensitive to the distance between the dilator and wire, and why no damage was observed on the RF wire or its dedicated dilator.
Perforation of the atrial septum using a dedicated RF system has been previously shown to create a similar extent of tissue injury and healing as mechanical needle puncture. 38 In the present study, EG under all conditions led to larger tissue defects, evidence of tissue charring, and irregular morphology compared to the purpose‐built RF system, suggesting a greater extent of tissue injury. Tissue charring has been correlated with thermal injury, destructive degeneration with amorphous or necrotic tissue, 21 , 39 and thrombus formation. 40 Disruption of collagen and fibrous structures impact tissue elasticity 21 and may present a risk of tearing or persistent atrial septal defects. 41
The use of higher power presents greater risks of electrosurgical injury 34 , 35 , 42 and thermal damage, 43 as well as thrombus 44 and coagulum formation. 14 , 45 Localized temperature measurements demonstrated higher core temperature and a visually larger area of tissue heating with the use of EG, as compared to the dedicated RF wire, which is consistent with the observed larger puncture defects, tissue charring, and equipment deformation.
Overall findings suggest EG create larger defects and are more sensitive to the distance between the tip of the wire and the tip of the dilator, which may be difficult to assess and control precisely in a clinical setting, thereby, causing uncertainty in TSP success and inconsistency between cases. Although the RF wire has a larger shaft diameter (0.035″) compared to the EG used in this study (0.014″ and 0.032″), the size of septal defects appeared to be smaller.
5.1. Study limitations
This benchtop model was designed to simulate TSP in clinical practice while allowing a direct measurement of TSP effectiveness and examination of tissue defects using ex‐vivo swine tissues. However, it did not account for factors such as blood flow and body temperature, which may affect tissue quality, heat dissipation, and puncture site response. Application of diathermy to guidewires in this study was manually approximated to 2 s to mimic typical clinical use. This may have resulted in greater energy delivery than the 1 s output used with the dedicated RF wire. RF energy was selected based on default settings for the dedicated system (i.e., 5 W) or clinically relevant powers for standard guidewires (i.e., 20, 35, or 50 W); while this was not a head‐to‐head comparison using the same settings, it is expected that applying 5 W RF to a guidewire would yield low or no success based on the results of this study. Histological examination of punctured tissues was not performed. Further in vivo studies are needed to confirm the observations from the present model as well as assess cost implications. A prior study comparing a dedicated RF needle system with a mechanical needle for TSP found that the overall cost of performing a TSP was lower with the RF needle despite higher equipment costs. 46
6. CONCLUSION
A dedicated RF wire had greater success and consistency in achieving TSP compared to electrified metal guidewires, which required higher power, were more dependent on the distance between the wire tip and dilator tip, and required more RF applications to perforate tissues. Use of EG for TSP was associated with damage to the guidewire and dilator and may present electrosurgical hazards, as well as risks of thrombus, thermal injury, and tissue scarring. Further studies are needed to identify the clinical implications of these findings and assess cost considerations related to the routine use of dedicated RF wires for TSP.
Supporting information
Supporting information.
Supporting information.
ACKNOWLEDGMENTS
The authors wish to acknowledge support from Saja Al‐Dujaili, PhD, for her assistance in this study.
Wasserlauf J, Knight BP. Comparing the safety and effectiveness of dedicated radiofrequency transseptal wires to electrified metal guidewires. J Cardiovasc Electrophysiol. 2022;33:371‐379. 10.1111/jce.15341
Disclosures: Equipment and technical expertise for this study were provided by Baylis Medical. Dr. Knight served as a paid consultant to Baylis Medical but was not directly compensated for his efforts related to this study or manuscript preparation.
DATA AVAILABILITY STATEMENT
Reasonable requests for shared data will be considered by the authors on a case‐by‐case basis.
REFERENCES
- 1. Alkhouli M, Rihal CS, Holmes DR. Transseptal techniques for emerging structural heart interventions. JACC Cardiovasc Interv. 2016;9:2465‐2480. [DOI] [PubMed] [Google Scholar]
- 2. Szegedi N, Széplaki G, Herczeg S, et al. Repeat procedure is a new independent predictor of complications of atrial fibrillation ablation. EP Europace. 2019;21:732‐737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gaziano TA, Bitton A, Anand S, Abrahams‐Gessel S, Murphy A. Growing epidemic of coronary heart disease in low‐ and middle‐income countries. Curr Probl Cardiol. 2010;35:72‐115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jauvert G, Grimard C, Lazarus A, Alonso C. Comparison of a radiofrequency powered flexible needle with a classic rigid brockenbrough needle for transseptal punctures in terms of safety and efficacy. Heart, Lung Circ. 2015;24(24):173‐178. [DOI] [PubMed] [Google Scholar]
- 5. Winkle RA, Mead RH, Engel G, Patrawala RA. The use of a radiofrequency needle improves the safety and efficacy of transseptal puncture for atrial fibrillation ablation. Heart Rhythm. 2011;8:1411‐1415. [DOI] [PubMed] [Google Scholar]
- 6. Yoshida S, Suzuki T, Yoshida Y, et al. Feasibility and safety of transseptal puncture procedures for radiofrequency catheter ablation in small children weighing below 30 kg: single‐centre experience. EP Europace. 2015;18:1581‐1586. [DOI] [PubMed] [Google Scholar]
- 7. Hsu JC, Badhwar N, Gerstenfeld EP, et al. Randomized trial of conventional transseptal needle versus radiofrequency energy needle puncture for left atrial access (the TRAVERSE‐LA Study). J Am Heart Assoc. 2013;2:e000428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fromentin S, Sarrazin J‐F, Champagne J, et al. Prospective comparison between conventional transseptal puncture and transseptal needle puncture with radiofrequency energy. J Interv Card Electrophysiol. 2011;31(31):237‐242. [DOI] [PubMed] [Google Scholar]
- 9. Elayi CS, Gurley JC, Sessa DI, Kakavand TG, B. Surgical electrocautery facilitated transseptal puncture in children. Pacing Clin Electrophysiol. 2011;34:827‐831. [DOI] [PubMed] [Google Scholar]
- 10. Rogers JH, Stripe BR, Singh GD, Boyd WD, Fan D, Smith TWR. Initial clinical experience with the FlexPoint Steerable Transseptal Needle in left‐sided structural heart procedures. Catheter Cardiovasc Interv. 2018;92:792‐796. [DOI] [PubMed] [Google Scholar]
- 11. Bidart C, Vaseghi M, Cesario DA, et al. Radiofrequency current delivery via transseptal needle to facilitate septal puncture. Heart Rhythm. 2007;4:1573‐1576. [DOI] [PubMed] [Google Scholar]
- 12. Knecht S, Jaïs P, Nault I, et al. Radiofrequency puncture of the fossa ovalis for resistant transseptal access. Circ Arrhythm Electrophysiol. 2008;1:169‐174. [DOI] [PubMed] [Google Scholar]
- 13. Abed HS, Alasady M, Lau DH, Lim HS, Sanders P. Approach to the difficult transseptal: diathermy facilitated left atrial access. Heart, Lung Circ. 2012;21:108‐112. [DOI] [PubMed] [Google Scholar]
- 14. Gowda ST, Qureshi AM, Turner D, et al. Transseptal puncture using surgical electrocautery in children and adults with and without complex congenital heart disease. Catheter Cardiovasc Interv. 2017;90:E46‐E54. [DOI] [PubMed] [Google Scholar]
- 15. Greenstein E, Passman R, Lin AC, Knight BP. Incidence of tissue coring during transseptal catheterization when using electrocautery and a standard transseptal needle. Circ Arrhythm Electrophysiol. 2012;5:341‐344. [DOI] [PubMed] [Google Scholar]
- 16. Khan JM, Rogers T, Eng MH, Lederman RJ, Greenbaum AB. Guidewire electrosurgery‐assisted trans‐septal puncture. Catheter Cardiovasc Interv. 2018;91:1164‐1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Burdick J, Schmalz M, Geenen J. Guidewire fracture during endoscopic sphincterotomy. Endoscopy. 1993;25:251‐252. [DOI] [PubMed] [Google Scholar]
- 18. Fry LC, Linder JD, Mönkemüller KE. Cholangitis as a result of hydrophilic guidewire fracture. Gastrointest Endosc. 2002;56:943‐944. [DOI] [PubMed] [Google Scholar]
- 19. Adioui T, Tamzaourte M, Touibi Y, Rouibaa F, Aourarh A. Intrapancreatic guidewire outer coat stripping during endoscopic treatment of chronic pancreatitis: a rare complication. Presse medicale (Paris, France: 1983). 2016;46:240‐241 . [DOI] [PubMed] [Google Scholar]
- 20. Liu Q, Sun XB. Indirect electrical injuries from capacitive coupling: a rarely mentioned electrosurgical complication in monopolar laparoscopy. Acta Obstet Gynecol Scand. 2013;92:238‐241. [DOI] [PubMed] [Google Scholar]
- 21. Fiorelli A, Accardo M, Carelli E, et al. Harmonic technology versus neodymium‐doped yttrium aluminium garnet laser and electrocautery for lung metastasectomy: an experimental study. Interact Cardiovasc Thorac Surg. 2016;23:47‐56. [DOI] [PubMed] [Google Scholar]
- 22. Robinson TN, Jones EL, Dunn CL, et al. Separating the laparoscopic camera cord from the monopolar “Bovie” cord reduces unintended thermal injury from antenna coupling: a randomized controlled trial. Ann Surg. 2015;261:1056‐1060. [DOI] [PubMed] [Google Scholar]
- 23. Townsend NT, Jones EL, Overbey D, Dunne B, McHenry J, Robinson TN. Single‐incision laparoscopic surgery increases the risk of unintentional thermal injury from the monopolar “Bovie” instrument in comparison with traditional laparoscopy. Surg Endosc. 2017;31:3146‐3151. [DOI] [PubMed] [Google Scholar]
- 24. Townsend NT, Jones EL, Paniccia A, Vandervelde J, McHenry JR, Robinson TN. Antenna coupling explains unintended thermal injury caused by common operating room monitoring devices. Surg Laparosc Endosc Percutan Tech. 2015;25:111‐113. [DOI] [PubMed] [Google Scholar]
- 25. Smith TL, Smith JM. Electrosurgery in otolaryngology–head and neck surgery: principles, advances, and complications. Laryngoscope. 2001;111:769‐780. [DOI] [PubMed] [Google Scholar]
- 26. Sayah N, Simon F, Garceau P, et al. Initial clinical experience with VersaCross transseptal system for transcatheter mitral valve repair. Catheter Cardiovasc Interv. 2020;97:1230‐1234. [DOI] [PubMed] [Google Scholar]
- 27. Inohara T, Gilhofer T, Luong C, Tsang M, Saw J. VersaCross radiofrequency system reduces time to left atrial access versus conventional mechanical needle. J Interv Card Electrophysiol. 2021. 10.1007/s10840-020-00931-7 [DOI] [PubMed] [Google Scholar]
- 28. Capulzini L, Paparella G, Sorgente A, et al. Feasibility, safety, and outcome of a challenging transseptal puncture facilitated by radiofrequency energy delivery: a prospective single‐centre study. EP Europace. 2010;12:662‐667. [DOI] [PubMed] [Google Scholar]
- 29. Barlow DE. Endoscopic applications of electrosurgery: a review of basic principles. Gastrointest Endosc. 1982;28:73‐76. [DOI] [PubMed] [Google Scholar]
- 30. Montero PN, Robinson TN, Weaver JS, Stiegmann GV. Insulation failure in laparoscopic instruments. Surg Endosc. 2010;24(24):462‐465. [DOI] [PubMed] [Google Scholar]
- 31. Wu M‐P, Ou C‐S, Chen S‐L, Yen EY, Rowbotham R. Complications and recommended practices for electrosurgery in laparoscopy. Am J Surg. 2000;179:67‐73. [DOI] [PubMed] [Google Scholar]
- 32. Tucker RD, Voyles CR. Laparoscopic electrosurgical complications and their prevention. AORN J. 1995;62:49‐71. [DOI] [PubMed] [Google Scholar]
- 33. Voyles CR, Tucker RD. Education and engineering solutions for potential problems with laparoscopic monopolar electrosurgery. Am J Surg. 1992;164:57‐62. [DOI] [PubMed] [Google Scholar]
- 34. Brill AI. Electrosurgery: principles and practice to reduce risk and maximize efficacy. obstetrics and gynecology. Clinics. 2011;38:687‐702. [DOI] [PubMed] [Google Scholar]
- 35. Odell RC. Surgical complications specific to monopolar electrosurgical energy: engineering changes that have made electrosurgery safer. J Minim Invasive Gynecol. 2013;20(20):288‐298. [DOI] [PubMed] [Google Scholar]
- 36. Demircin S, Aslan F, Karagoz YM, Atilgan M. Medicolegal aspects of surgical diathermy burns: a case report and review of the literature. Rom J Leg Med. 2013;21:173‐176. [Google Scholar]
- 37. Perantinides PG, Tsarouhas AP, Katzman VS. The medicolegal risks of thermal injury during laparoscopic monopolar electrosurgery. J Healthc Risk Manag. 1998;18:47‐55. [DOI] [PubMed] [Google Scholar]
- 38. Veldtman GR, Wilson GJ, Peirone A, et al. Radiofrequency perforation and conventional needle percutaneous transseptal left heart access: pathological features. Catheter Cardiovasc Interv. 2005;65:556‐563. [DOI] [PubMed] [Google Scholar]
- 39. Sawabata N, Nezu K, Tojo T, Kitamura S. In vitro comparison between argon beam coagulator and Nd:YAG laser in lung contraction therapy. Ann Thorac Surg. 1996;62(62):1485‐1488. [DOI] [PubMed] [Google Scholar]
- 40. Haines DE, Verow AF. Observations on electrode‐tissue interface temperature and effect on electrical impedance during radiofrequency ablation of ventricular myocardium. Circulation. 1990;82:1034‐1038. [DOI] [PubMed] [Google Scholar]
- 41. Eshcol J, Wimmer AP. Hemodynamically significant iatrogenic atrial septal defects after cryoballoon ablation. HeartRhythm Case Rep. 2018;5:17‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Taheri A, Mansoori P, Sandoval LF, Feldman SR, Pearce D, Williford PM. Electrosurgery: Part II. Technology, applications, and safety of electrosurgical devices. J Am Acad Dermatol. 2014;70:607.e601‐607.e612. [DOI] [PubMed] [Google Scholar]
- 43. Guy DJR, Boyd A, Thomas SP, Ross DL. Increasing power versus duration for radiofrequency ablation with a high superfusate flow. Pacing Clin Electrophysiol. 2003;26:1379‐1385. [DOI] [PubMed] [Google Scholar]
- 44. Khairy P, Chauvet P, Lehmann J, et al. Lower Incidence of thrombus formation with cryoenergy versus radiofrequency catheter ablation. Circulation. 2003;107:2045‐2050. [DOI] [PubMed] [Google Scholar]
- 45. ANFINSEN O‐G, GJESDAL K, BROSSTAD F, et al. The activation of platelet function, coagulation, and fibrinolysis during radiofrequency catheter ablation in heparinized patients. J Cardiovasc Electrophysiol. 1999;10:503‐512. [DOI] [PubMed] [Google Scholar]
- 46. Sanchez JM, Shah R, Kouassi Y, Chronowic M, Wilson L, Marcus GM. A cost‐effectiveness analysis comparing a conventional mechanical needle to a radiofrequency device for transseptal punctures. J Cardiovasc Electrophysiol. 2020;31:1672‐1677. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Supporting information.
Data Availability Statement
Reasonable requests for shared data will be considered by the authors on a case‐by‐case basis.


