Abstract
Streptococcus oralis is the predominant aciduric nonmutans streptococcus isolated from the human dentition, but the role of this organism in the initiation and progression of dental caries has yet to be established. To identify proteins that are differentially expressed by S. oralis growing under conditions of low pH, soluble cellular proteins extracted from bacteria grown in batch culture at pH 5.2 or 7.0 were analyzed by two-dimensional (2-D) gel electrophoresis. Thirty-nine proteins had altered expression at low pH; these were excised, digested with trypsin using an in-gel protocol, and further analyzed by peptide mass fingerprinting using matrix-assisted laser desorption ionization mass spectrometry. The resulting fingerprints were compared with the genomic database for Streptococcus pneumoniae, an organism that is phylogenetically closely related to S. oralis, and putative functions for the majority of these proteins were determined on the basis of functional homology. Twenty-eight proteins were up-regulated following growth at pH 5.2; these included enzymes of the glycolytic pathway (glyceraldehyde-3-phosphate dehydrogenase and lactate dehydrogenase), the polypeptide chains comprising ATP synthase, and proteins that are considered to play a role in the general stress response of bacteria, including the 60-kDa chaperone, Hsp33, and superoxide dismutase, and three distinct ABC transporters. These data identify, for the first time, gene products that may be important in the survival and proliferation of nonmutans aciduric S. oralis under conditions of low pH that are likely to be encountered by this organism in vivo.
Dental caries is one of the most common causes of tooth loss in the developed world, constituting a substantial economic burden. Following the intake of dietary carbohydrate, acidogenic and aciduric bacteria that form part of the oral biofilm ferment freely metabolizable sugars, producing acid which, when the pH reaches a critical level of below approximately 5.2, results in the demineralization of the tooth. Thus, acidogenicity and aciduricity, the abilities to produce acid and to grow under conditions of low pH, respectively, are considered important virulence determinants for bacteria associated with the initiation and progression of caries. Adaptation to growth at low pH is an essential characteristic for any organism occupying a niche within a caries-prone site or a carious lesion. The acidogenic bacteria which are most closely associated with the initiation and progression of dental caries are mutans streptococci (Streptococcus mutans and Streptococcus sobrinus), lactobacilli, and possibly Actinomyces species, but the role of other bacteria in the progression of the disease has recently been investigated, and a number of studies have highlighted the pathogenic potential of nonmutans streptococci (NMS). Streptococcus oralis, a member of the mitis group of viridans streptococci, which includes Streptococcus pneumoniae, forms a significant proportion of the aciduric microflora of plaque (5), while in other investigations NMS, including S. oralis, were found to exhibit acid-induced tolerance of low pH and acidogenicity (33, 38, 40, 42, 43). Collectively, these latter studies demonstrated that the NMS were heterogeneous with respect to acidogenicity; more recently, using repetitive extragenic palindromic PCR, it was shown that distinct aciduric subpopulations of S. oralis were present in dental plaque (1). In light of these findings, it was suggested that the role of NMS in the caries process and the central role previously assigned to mutans streptococci require reevaluation.
The methodologies employed to investigate the response of streptococci to acid stress are diverse, but many rely on conventional biochemical techniques for the measurement of particular enzymatic activities or on molecular approaches. Insertional mutagenesis techniques have been used to generate acid-sensitive mutants of S. mutans, and characterization of these isolates demonstrated that several genes, including ffh, a homologue to the Bacillus subtilis ylxM-ffh gene, and homologues to the diacylglycerol kinase and Era proteins of Escherichia coli were important in the acid stress response of the organism (11, 12, 45). In addition, increased activity of H+-ATPase in Streptococcus sanguis, Streptococcus gordonii, S. oralis, Streptococcus mitis, and S. mutans (40) has been shown to play a role in adaptation to low-pH conditions. In alternative approaches to the investigation of the low-pH response of S. mutans using antisense RNA strategies, the importance of a membrane-associated GTP-binding protein to the physiology of the organism was demonstrated (2, 36).
These studies have investigated the role of single proteins in the response of streptococci to exposure to low pH. The resolving power of two-dimensional (2-D) polyacrylamide gel electrophoresis (PAGE) facilitates the monitoring of expression of many hundreds of proteins simultaneously, however, and in a recent study Svensater et al. (39) generated 2-D maps of S. mutans proteins that were differentially regulated in response to various environmental stresses. That study demonstrated enhanced synthesis of 64 proteins on exposure of the organism to low-pH conditions, 25 of which were described as acid specific, and decreased synthesis was observed of a further 49 proteins. The identity of these proteins was not reported. We are interested in the stress responses and mechanisms by which S. oralis grows in acidic conditions. We used 2-D PAGE to separate soluble cellular proteins extracted from an aciduric S. oralis isolate grown at pH 5.2 or 7.0 in batch culture and generated 2-D maps of proteins with pIs in the range of 4 to 7 for each of the conditions. Proteins which were differentially expressed were excised, digested with trypsin using an in-gel protocol, and analyzed by matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF-MS) to generate a distinctive peptide mass fingerprint which is considered sufficiently discriminating to allow the unique identification of unknown proteins (29). Putative functions for the excised S. oralis proteins were determined by comparing the peptide mass fingerprints against the annotated genomic database of the sequenced strain of S. pneumoniae (http://igweb.integratedgenomics.com/IGwit/). These investigations provided identifications for soluble cellular proteins from S. oralis that were differentially expressed during growth at low pH and give an insight into the mechanisms by which an aciduric NMS isolate responds to growth in acidic conditions.
MATERIALS AND METHODS
Bacterial isolate and culture conditions.
S. oralis strain 176N, an isolate obtained during a study of the aciduricity of NMS and cultured from interproximal plaque of a 10- to 12-year old child on media buffered at pH 5.0, was used throughout this study. Cultures were maintained on Columbia agar (Oxoid Ltd., Basingstoke, Hampshire, United Kingdom) supplemented with 5% (vol/vol) defibrinated horse blood (TCS Microbiology, Buckingham, United Kingdom), and inoculated plates were incubated anaerobically (MK3 anaerobic workstation; Don Whitley Scientific Ltd., Shipley, West Yorkshire, United Kingdom) in an atmosphere of 10% CO2, 10% H2, and 80% N2 at 37°C for 24 to 48 h.
Growth parameters in liquid media were determined by monitoring A620 in a 96-well plate-reading spectrophotometer at 37°C (iEMS; Labsystems, Life Sciences International, Hampshire, United Kingdom) using Biolink software (version 5.0x; Labsystems). Suspensions of cells were prepared in 50 mM sodium phosphate buffer (pH 7.5) to an A620 of 1.5 and used as inocula for cultures. Bacterial growth curves were determined for growth in 200-μl volumes of brain heart infusion (BHI; Oxoid) at pH 7.0 and of BHI buffered to pH 5.2 by the addition of 0.2 M disodium hydrogen orthophosphate and 0.1 M citric acid (each at a final concentration of approximately 20 mM) and inoculated with 5% (vol/vol) bacterial suspension. These growth experiments were performed in triplicate. Cultures for protein extraction were routinely grown statically in 10-ml volumes of BHI at pH 7.0 and 5.2, inoculated with 5% (vol/vol) bacterial suspension prepared in 50 mM sodium phosphate buffer (pH 7.4) as described above, and incubated aerobically at 37°C until the mid-exponential phase of growth. The pH of cultures was determined, and glucose concentrations in media were measured using Sigma kit no. 510-A according to the manufacturer's instructions.
Extraction of cellular proteins for 2-D PAGE.
Cells were collected at the middle of exponential phase by centrifugation at 2,400 × g, washed in 0.9% NaCl, and immediately resuspended in 25 mM Tris HCl (pH 7.5) containing 0.01% lysozyme, 0.026% phenylmethylsulfonyl fluoride, 0.005% chloramphenicol (added to inhibit further protein synthesis), and 18% sucrose and incubated for 15 min at 37°C as described by Giard et al. (10). The bacteria were pelleted by centrifugation, resuspended in 0.3% sodium dodecyl sulfate (SDS)–0.3% dithiothreitol (DTT)–50 mM Tris HCl (pH 7.5), incubated for 5 min at 100°C, vortexed, and centrifuged at 11,600 × g. The resulting supernatant was incubated at 4°C for 15 min following addition of 24 μl of 0.5 M Tris HCl (pH 7.5) containing 0.5% MgCl2 (anhydrous) and 4 U of endonuclease. Protein was precipitated by addition of 4 volumes of ice-cold acetone and collected by centrifugation (15,000 × g) at 4°C for 10 min. The resulting pellet was resuspended in 7 M deionized urea–2 M thiourea–2% Tergitol NP-40–62 mM DTT, to which was added 2% pH 3 to 10 carrier ampholytes (Bio-Rad Laboratories Ltd., Hemel Hempstead, Hertfordshire, United Kingdom).
Analysis of cellular proteins by 2-D PAGE.
Immobilized Pharmalyte gradient (IPG) 17-cm pH 4 to 7 linear strips were rehydrated under active conditions in a Protean IEF Cell (Bio-Rad) with a total cellular protein load of approximately 200 μg and focused for 60,000 to 65,000 V · h. Focused IPG strips were equilibrated in 16.5 mM Tris HCl (pH 8) containing 6 M urea, 30% (vol/vol) glycerol, 2% SDS, and 1% DTT for 15 min and subsequently for 15 min in the same buffer containing 2.5% iodoacetamide prior to loading onto second-dimension SDS-polyacrylamide 12 to 14% gradient gels (200 by 160 by 1.5 mm). The strips were embedded on the top of the SDS-gels by using 1% molten agarose in electrophoresis buffer (250 mM glycine, 25 mM Tris, 0.1% SDS). SDS-PAGE was carried out using a Protean II XL Cell (Bio-Rad). Proteins were visualized following staining with colloidal Coomassie brilliant blue (Sigma Chemical Company, Poole, Dorset, United Kingdom) as described by Neuhoff et al. (26). The Mr of individual protein spots was determined by comparison with low-molecular-weight markers run at the acidic end of the IPG strip, and pI was deduced from the linearity of the IPG strips. Gels were scanned at 300 dots/in. (Epson GT9600; Epson UK Ltd., Hemel Hempstead, Hertfordshire, United Kingdom), and spot detection was performed with the Phoretix 2D Advanced software (version 5.01; Phoretix, Newcastle upon Tyne, United Kingdom). A total of three independent cultures for each growth condition was processed. After localization of the protein spots and definition of their boundaries, we selected a reference gel against which each of the other gel images was matched. The proteins subjected to further analysis were visible in at least two of the gels of any one growth condition. The integrated optical density within the boundary of individual spots, the spot volume, was expressed as a percentage of the total integrated optical density. Where proteins occurred as distinct isoforms, the sum of the spot volumes for each was used. Proteins were considered to be differentially expressed if the mean percentage spot volume for an individual protein was up- or down-regulated 1.5-fold or greater. Significant differences in protein expression levels were determined by Student's t test with a set value of P ≤ 0.05. Those proteins whose expression was up- or down-regulated by 1.5-fold or greater were excised from gels and identified by peptide mass fingerprinting.
In-gel proteolytic digestion of resolved proteins.
Proteins were excised from the gel, diced finely, washed in 100 mM ammonium bicarbonate (NH4HCO3), dehydrated in acetonitrile (ACN), and dried in a vacuum centrifuge (SpeedVac Plus SC110A; Savant, Farmingdale, N.Y.) essentially as described by Shevchenko et al. (37). A minimal volume of 100 mM NH4HCO3 containing 10 mM DTT was added, and the gel fragments were incubated for 60 min at 56°C and then for 30 min in the dark in 55 mM iodoacetamide in 100 mM NH4HCO3. The gel pieces were washed with 100 mM NH4HCO3, dehydrated in ACN, and dried completely in the vacuum centrifuge. The gel pieces were reswollen in 50 mM NH4HCO3 containing trypsin (sequencing grade, modified; 13 ng/μl; Promega, Southampton, Hampshire, United Kingdom) and incubated at 37°C overnight.
MALDI-TOF MS.
Peptide extract (10 μl) was applied to a ZipTip (Millipore Ltd., Watford, Hertfordshire, United Kingdom) which has C18 resin fixed at its end and rinsed according to the manufacturer's instructions in 0.1% trifluoroacetic acid (TFA; high-pressure liquid chromatography grade; Perbio Science UK Ltd., Chester, United Kingdom); purified and concentrated peptides were eluted in 3 μl of 1:1 ACN–0.1% TFA. A 0.5-μl volume of the concentrated peptide-containing sample was mixed with 0.5 μl of a saturated solution of α-cyano-4-hydroxycinnamic acid (99% purity: Sigma-Aldrich, Gillingham, Dorset, United Kingdom) in 70% ACN–0.033% TFA on the mass spectrometer sample plate. Mass spectra were obtained on a Voyager Elite (PE Biosystems) with delayed extraction in reflector mode. Samples were irradiated with a nitrogen laser giving a 337-nm output with 3-ns pulse width, and molecular ions were accelerated at a potential of 20 kV. The laser intensity used was maintained at 1,000 U. All spectra were obtained as 256 shot averages. MALDI spectra were calibrated by close external calibration using a peptide mixture (PE Biosystems) containing des-argl-bradykinin (monoisotopic mass, 904.4681 Da), angiotensin 1 (monoisotopic mass, 1,296.6853 Da), and glul-fibrinopeptide B (monoisotopic mass, 1,570.6774 Da) on GRAMS software (PE Biosystems), labeling monoisotopic mass peaks.
Protein identification by peptide mass fingerprinting.
Current practices for the identification of proteins from 2-D gels frequently involve interrogation of the genomic sequence data available for a particular species. For those species for which these data are not yet available, protein identification may be more problematic if analyses are restricted by comparison with a limited number of individually sequenced genes deposited in nonredundant nucleic acid or protein sequence databases. It may be possible, however, to identify proteins on the basis of their homology with those derived from another closely related species or genus for which genomic sequence data are available.
There are currently only 25 partial and complete S. oralis genes present in the National Center for Biotechnology Information nonredundant database (http: //www.ncbi.nlm.nih.gov/), and the genome of this organism is not currently undergoing sequencing. S. oralis is, however, phylogenetically closely related to S. pneumoniae, with 16S rRNA genes exhibiting over 99% similarity (3, 19). As S. oralis and S. pneumoniae are phylogenetically similar, we have used the WIT S. pneumoniae annotated genomic database comprising 1,844 open reading frames (ORFs) (http://igweb.integratedgenomics.com/IGwit/) in combination with peptide mass fingerprinting to identify proteins expressed by S. oralis. This database assigns putative functions to the gene products of each ORF based on sequence homology and is curator managed (27).
To determine the suitability of the S. pneumoniae genomic data for the identification of cellular proteins from S. oralis, an in silico investigation was conducted. Translated sequences for the 25 complete and partial S. oralis genes were subjected to theoretical digestion with trypsin (http://expasy.cbr.nrc.ca/tools /peptide-mass.html), and a peptide mass fingerprint was generated. Theoretical peptides with a mass of greater than 3,200 Da were excluded from the search functions since peptides with higher masses are rarely observed in routine peptide mass fingerprinting. These data were submitted to MS-Fit, using the experimental search parameters outlined below.
MS-Fit (University of California San Francisco Mass Spectrometry Facility; http://prospector.ucsf.edu/ucsfhtml3.4/msfit.htm), installed locally, was used to identify proteins from peptide mass fingerprints by comparing the peptide masses from an individual spot with theoretical tryptic digests of translated genomic sequence data. All searches were performed against the S. pneumoniae nonredundant sequence database, the mass accuracy was ≤200 ppm, and scoring for missed cleavage sites was set at 0. Putative functions were assigned on the basis of the identity of the first candidate protein in the returned list. This protein always possesses the highest MOWSE (molecular weight search) score, a probability measure for the peptide mass fingerprint arising as a result of being a homologue of a given protein and which uses search-scoring algorithms developed from the observed distribution frequency of peptides in a nonredundant protein sequence database (reference 29 and references therein). In this study, putative functions were assigned to excised proteins if a high MOWSE score was recorded, usually at least three peptides matched, >10% coverage of the protein was obtained, and the protein under consideration had similar observed and theoretical Mr and pI values.
RESULTS
Growth of S. oralis at low pH.
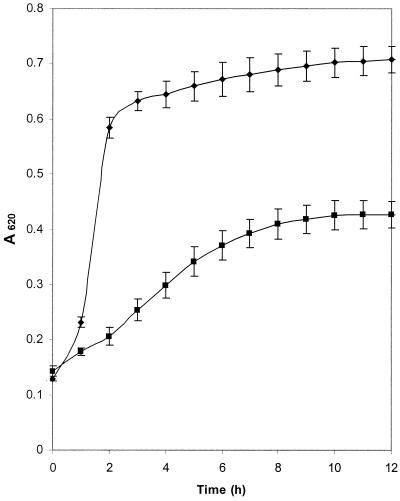
To demonstrate the aciduricity of the isolate under investigation, S. oralis strain 176N was cultured in 200-μl volumes of nutrient-rich media and nutrient-rich media adjusted to pH 5.2. No appreciable lag phase was observed for growth at either pH (Fig. 1); during the exponential phase of growth, doubling times were 4.6 and 1.1 h for pH 5.2 and 7.0, respectively. At the middle of exponential phase, the pH of each culture was 4.88 ± 0.03 and 6.42 ± 0.04 for media initially adjusted to pH 5.2 and 7.0, respectively, and glucose remained in the media. The A620 (±standard deviation) during stationary phase differed for the two cultures, being 0.727 ± 0.024 for pH 7.0 media and 0.455 ± 0.026 for cultures buffered at pH 5.2, and all of the glucose had been utilized.
FIG. 1.
Growth of S. oralis strain 176N at pH 5.2 and 7.0. S. oralis was cultured in nutrient-rich media buffered to pH 5.2 (■) or 7.0 (⧫). Growth was monitored by determination of A620, and error bars indicate standard deviation of the mean of experiments carried out in triplicate.
Resolution of soluble cellular proteins of S. oralis by 2-D PAGE.
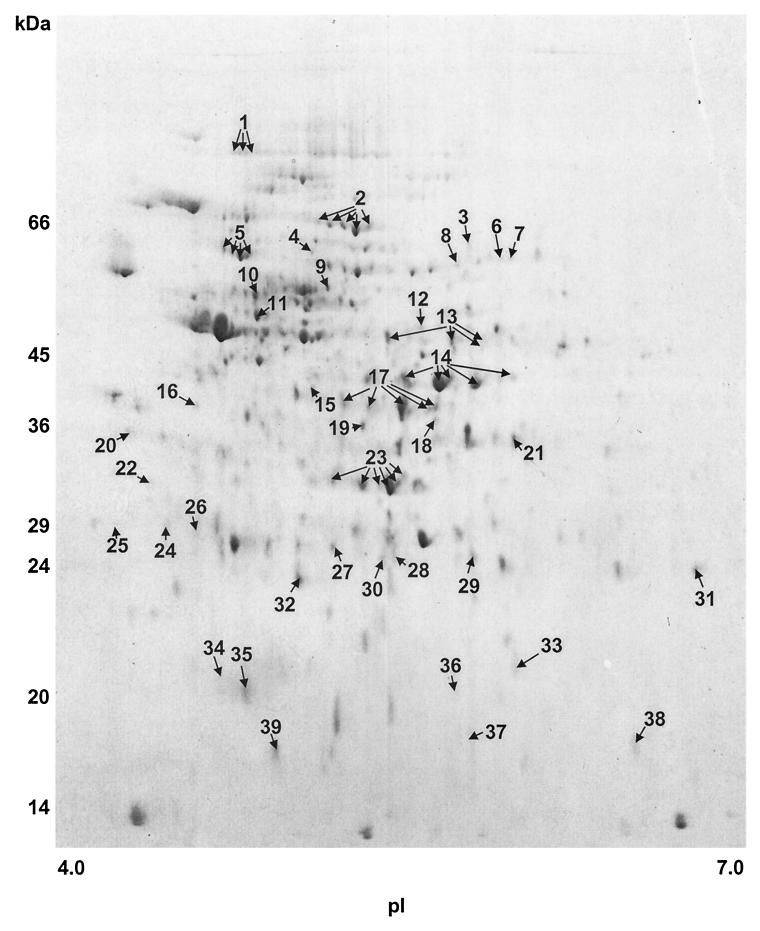
Soluble cellular proteins extracted from cells of S. oralis cultured at pH 5.2 were separated by 2-D PAGE; this resulted in a map in which 477 (median) well-resolved proteins were detected in the pI range of 4 to 7 when gels were stained with colloidal Coomassie brilliant blue (Fig. 2). This compared with 430 (median) proteins detectable for S. oralis cells grown to mid-exponential phase at pH 7.0 (data not shown). Comparison of the two maps revealed that 39 proteins had altered levels of expression when S. oralis was grown at low pH at a lower rate of growth. These were excised from gels and subjected to further analysis by peptide mass fingerprinting in order to assign putative functions to the proteins.
FIG. 2.
2-D PAGE analysis of cellular proteins of S. oralis cultured at pH 5.2. Extracted proteins were separated by isoelectric focusing in the pI range of 4 to 7 in the first dimension and gradient (12 to 14%) SDS-PAGE in the second dimension. Resolved proteins were visualized following staining with colloidal Coomassie brilliant blue. Spot numbering indicates those proteins with altered expression at pH 5.2 compared with those extracted from cells cultured at pH 7.0. Proteins for which different isoforms were observed are indicated by multiple arrows.
In silico identification of sequenced S. oralis genes using the S. pneumoniae genomic database.
We used the existing 25 partial and complete S. oralis sequences deposited in the National Center for Biotechnology Information nonredundant database, generated the translated amino acid sequences, and conducted theoretical digestions with trypsin as protease. The masses of the peptides resulting from these theoretical digestions were submitted to MS-Fit and compared with the S. pneumoniae genomic database (Table 1). Although many of the deposited sequences for S. oralis were short and incomplete, in all cases a high degree of similarity was apparent since all of the theoretical tryptic digests of translated partial and complete S. oralis gene sequences could be assigned to the same putative functions using MS-Fit by comparison with the S. pneumoniae database. These observations support the use of the S. pneumoniae database to identify S. oralis proteins by peptide mass fingerprinting. Two S. oralis genes, gtfR and rgg, were not present in the S. pneumoniae database and may be unique to S. oralis.
TABLE 1.
Comparison of amino acid identities between sequenced or partially sequenced S. oralis genes (http://www.ncbi.nlm.nih.gov/) and ORFs of the S. pneumoniae sequenced genome (http://igweb.integratedgenomics.com/IGwit/)
| S. oralis gene | No. of amino acids coded by gene sequence | Identities (S. pneumoniae/ S. oralis) | S. pneumoniae ORF (MOWSE score)a |
|---|---|---|---|
| gyrB | 103 (p)b | 103/103 | 1293 (5.2e + 002) |
| gyrA | 99 (p) | 90/92 | 1630 (2.8e + 003) |
| parE | 69 (p) | 68/69 | 1042 (7.4e + 002) |
| parC | 61 (p) | 59/61 | 1043 (5.2e + 002) |
| atpB | 127 (p) | 109/127 | 0889 (8.6e + 001) |
| AAA26957.1 | 166 (p) | 149/166 | 0115 (0.9e + 001) |
| recP | 113 (p) | 110/113 | 1552 (1.3e + 003) |
| xpt | 131 (p) | 119/131 | 0402 (9.7e + 004) |
| hexB | 117 (p) | 104/117 | 1205 (7.4e + 001) |
| ddl | 147 (p) | 54/56 | 0122 (5.7e + 001) |
| sodA | 145 (p) | 115/132 | 0961 (1.9e + 003) |
| rpoB | 170 (p) | 168/170 | 0016 (7.9e + 006) |
| rpoB | 290 (p) | 280/290 | 0016 (1.5e + 006) |
| psaA | 309 | 281/309 | 0197 (2.3e + 006) |
| comD | 441 | 302/365 | 0257 (1.9e + 012) |
| comE | 210 | 207/210 | 0256 (6.0e + 008) |
| cpn60 | 184 (p) | 168/184 | 1512 (7.7e + 005) |
| pox | 591 | 559/591 | 0904 (9.2e + 013) |
| iga | 1,837 | 1,139/1,802 | 0097 (3.3e + 016) |
| pbpX | 666 | 565/666 | 0342 (9.7e + 009) |
| pbp2A | 477 | 434/477 | 0120 (5.8e + 008) |
| ponA | 637 | 526/637 | 0114 (2.3e + 006) |
| EF-Tu | 398 | 364/398 | 1501 (3.1e + 015) |
MS-Fit (Protein Prospector; http://prospector.ucsf.edu/) was used to calculate MOWSE scores. Theoretical tryptic digests of S. oralis amino acid sequences were generated, and peptides with Mr of <3,200 were searched against the S. pneumoniae genomic database, simulating data obtained using MALDI-TOF MS. A minimum of three peptides was required to match.
(p), partially sequenced.
Identification of S. oralis proteins with altered expression as a response to growth at low pH.
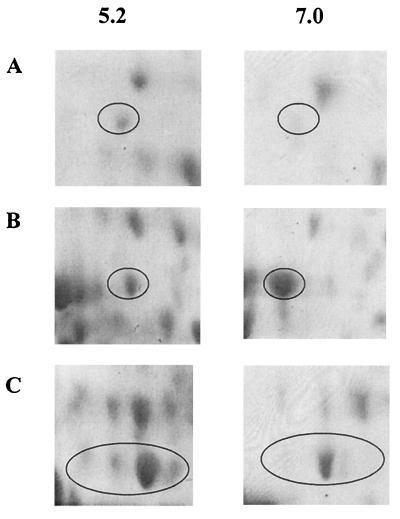
Of the 39 S. oralis proteins with altered expression following growth at pH 5.2 or 7.0, 28 were up-regulated at low pH (13 of these significantly up-regulated; P < 0.05) and 11 were down-regulated (6 of these significantly down-regulated; P < 0.05). Figure 3 shows the expanded regions of the pH 7.0 and pH 5.2 gels in which an ATP-binding cassette (ABC) transporter and the ATP synthase alpha and beta chains are resolved. Examination of these data indicate that expression of these proteins was up-regulated during growth at pH 5.2, and consideration of the corresponding spot volumes as a percentage of the total spot volume (Table 2) upholds these findings since all three proteins were significantly up-regulated (P < 0.05).
FIG. 3.
Representative S. oralis proteins up-regulated as a response to growth at low pH. S. oralis was cultured at pH 5.2 or 7.0, and cellular proteins were resolved by 2-D PAGE. Proteins were identified by peptide mass fingerprinting and comparison with the S. pneumoniae genomic database. (A) ABC transporter; (B) ATP synthase alpha chain; (C) ATP synthase beta chain.
TABLE 2.
S. oralis whole cell proteins up- or down-regulated following growth at pH 5.2
| Functional category | Spot no.a | Homologous proteinb | RPNc | Coveraged | Observed migratione
|
Theoretical migrationf
|
Peptides matchedg | MOWSE scoreh | Mean spot vol ratioi | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| pI | Mr | pI | Mr | ||||||||
| Cellular processes and stress proteins | 5 | 60-kDa chaperonin | 01512 | 21 | 4.8 | 60.9 | 4.8 | 57.1 | 10 | 3.04e + 005 | 2.78j |
| 16 | Hsp33 protein (redox-regulated chaperone) | 00083 | 16 | 4.6 | 38.1 | 4.7 | 31.7 | 3 | 1.45e + 002 | 2.98j | |
| 20 | Cell division protein Div IVA | 00205 | 16 | 4.4 | 36.0 | 4.6 | 30.1 | 3 | 4.43e + 002 | 2.16j | |
| 32 | Superoxide dismutase | 00961 | 45 | 5.0 | 23.0 | 5.1 | 18.9 | 6 | 2.62e + 005 | 2.03j | |
| Central and intermediary metabolism | 1 | Formate acetyltransferase 1 | 01016 | 26 | 4.8 | 84.4 | 5.1 | 87.8 | 10 | 3.59e + 007 | 23.46j |
| 2 | Pyruvate oxidase | 00904 | 25 | 5.1 | 65.9 | 5.1 | 65.3 | 13 | 8.38e + 008 | 2.11j | |
| 13 | NADP-specific glutamate dehydrogenase | 00804 | 31 | 5.7 | 47.5 | 5.3 | 46.7 | 12 | 5.03e + 007 | 6.24j | |
| 14 | Glyceraldehyde-3-phosphate dehydrogenase | 01440 | 30 | 5.5 | 41.7 | 5.1 | 31.5 | 3 | 1.46e + 003 | 2.01j | |
| 17 | l-Lactate dehydrogenase | 01637 | 21 | 5.4 | 37.5 | 5.1 | 35.4 | 5 | 3.6e + 003 | 1.70j | |
| 23 | Fructose bisphosphate aldolase | 00514 | 16 | 5.4 | 31.0 | 5.0 | 31.4 | 5 | 1.29e + 003 | 0.69j | |
| 30 | Acetoin utilization AcuB protein | 00165 | 26 | 5.3 | 24.0 | 5.1 | 24.1 | 4 | 4.37e + 004 | 2.20 | |
| 36 | Galactose-6-phosphate isomerase LacB subunit | 01826 | 32 | 5.6 | 15.0 | 5.5 | 19.0 | 5 | 1.18e + 004 | 0.28j | |
| ATP synthase | 9 | ATP synthase alpha chain | 00891 | 14 | 5.1 | 55.0 | 5.0 | 54.6 | 8 | 3.68e + 005 | 3.08j |
| 11 | ATP synthase beta chain | 00893 | 35 | 4.9 | 51.0 | 4.8 | 50.9 | 15 | 1.30e + 012 | 2.21j | |
| 19 | ATP synthase gamma chain | 00892 | 13 | 5.3 | 36.1 | 5.1 | 32.3 | 4 | 4.86e + 002 | 2.17 | |
| 33 | ATP synthase delta chain | 00890 | 18 | 5.8 | 17.0 | 7.8 | 20.5 | 4 | 2.38e + 003 | 15.00 | |
| 38 | ATP synthase epsilon chain | 00894 | 12 | 6.3 | 12.0 | 5.9 | 15.6 | 3 | 2.20e + 002 | 2.62 | |
| Biosynthesis of cofactors or prosthetic groups | 3 | Formate-tetrahydrofolate ligase | 00990 | 11 | 5.7 | 62.1 | 5.3 | 59.6 | 4 | 2.34e + 003 | 1.82 |
| 31 | Oxygen-insensitive NAD(P)H nitroreductase/dihydropteridine reductase | 01415 | 21 | 6.5 | 23.5 | 6.0 | 22.3 | 5 | 1.73e + 003 | 3.19 | |
| Transport and binding proteins | 4 | ABC transporter | 00827 | 30 | 5.8 | 58.5 | 4.9 | 52.7 | 13 | 1.76e + 010 | 2.04j |
| 24 | Hypothetical ABC transporter ATP-binding protein | 00823 | 14 | 4.5 | 29.1 | 4.7 | 28.4 | 6 | 3.77e + 005 | 2.13 | |
| 29 | Hypothetical ABC transporter ATP-binding protein (S. pyogenes) | 00525 | 9 | 5.7 | 25.0 | 7.1 | 43.9 | 4 | 1.16e + 003 | 3.14j | |
| 35 | EIIA componenent of MTL PTS | 00030 | 35 | 4.8 | 14.0 | 4.9 | 16.1 | 3 | 7.14e + 002 | 1.52 | |
| Purines, pyrimidines, nucleosides, and nucleotides | 6 | Nicotinate phosphoribosyltransferase | 00471 | 18 | 5.7 | 57.0 | 5.1 | 55.1 | 10 | 7.82e + 006 | 1.65 |
| 27 | Adenylate kinase | 00304 | 27 | 5.3 | 25.1 | 5.0 | 23.7 | 5 | 8.6e + 004 | 0.45 | |
| Replication | 12 | RecA protein | 01487 | 24 | 5.5 | 57.4 | 4.8 | 36.1 | 8 | 6.62e + 005 | 0.39j |
| Translation | 18 | Polyribonucleotide nucleotidyltransferase | 00502 | 9 | 5.6 | 37.0 | 5.0 | 81.0 | 5 | 7.37e + 003 | 0.54j |
| 28 | Polypeptide deformylase | 00945 | 16 | 5.4 | 25.0 | 5.1 | 22.7 | 4 | 2.68e + 004 | 2.07 | |
| Cell envelope | 15 | Membrane TMPC precursor | 01034 | 22 | 5.1 | 40.1 | 5.4 | 36.7 | 6 | 4.72e + 004 | 3.25 |
| Other | 10 | Succinyl-diaminopimelate desuccinylase | 01416 | 17 | 4.9 | 54.0 | 4.8 | 50.8 | 6 | 1.21e + 003 | 1.84 |
| 25 | Phosphoprotein phosphatase 2C | 00665 | 13 | 4.4 | 24.5 | 4.5 | 27.1 | 4 | 1.3e + 003 | 0.25j | |
| Unknown function | 7 | ORF 1374 | 01374 | 11 | 5.8 | 57.1 | 5.5 | 55.1 | 3 | 3.04e + 001 | 3.50 |
Refers to the proteins labeled in Fig. 2.
Putative functions were assigned on the basis of the presence of homologues in the WIT database for S. pneumoniae. MTL PTS, mannitol phosphotransferase system.
RPN indicates the number assigned to each ORF in the WIT database for S. pneumoniae.
The percentage amino acid coverage (peptides observed/theoretical from sequence data).
Calculated from data presented in Fig. 2.
As given in the WIT database for S. pneumoniae.
Number of peptides observed in mass spectra contributing to MOWSE score.
As calculated by MS-Fit using peptide mass fingerprint data.
Ratio of integrated optical density for each protein when derived from cells cultured at pH 5.2 or 7.0 (mean of results from three gels for each growth condition).
Significantly different (P < 0.05) as calculated by Student's t test.
Polypeptides with altered expression which may be considered stress response proteins, namely, the 60-kDa chaperonin (RPN01512), Hsp33 (RPN00083), and superoxide dismutase (RPN00961), were up-regulated at low pH. All five individual proteins comprising the ATP synthase complex, otherwise known as H+-ATPase, were up-regulated as a response to growth under acidic conditions, as were a number of key glycolytic enzymes including glyceraldehyde-3-phosphate dehydrogenase (RPN01440) and lactate dehydrogenase (RPN01637), with a 23-fold increase in the amount of formate acetyltransferase (RPN01016). Conversely, fructose bisphosphate aldolase (RPN00514), a regulatory glycolytic enzyme, was down-regulated at low pH. Other homologues functioning in central and intermediary metabolism with increased expression at pH 5.2 included pyruvate oxidase (RPN00904), a glutamate dehydrogenase (RPN00804), and an acetoin utilization protein (RPN00165). Galactose-6-phosphate isomerase (RPN01826), an enzyme essential for the metabolism of galactose and lactose, was down-regulated at this pH.
Transport and binding proteins were represented among the up-regulated proteins in the cellular extracts of S. oralis (Table 2), with three homologues to distinct ABC transporters (RPN00827, RPN00823, and RPN00525) and the EIIA component of the mannitol phosphotransferase system (RPN00030). Formate-tetrahydrofolate ligase (RPN00990) and a protein with homology to a nitroreductase/dihydropteridine reductase (RPN01415), both enzymes associated with cofactor biosynthesis, were also up-regulated at this pH, as were nicotinate phosphoribosyltransferase (RPN00471) and adenylate kinase (RPN00304), proteins of nucleotide metabolism. Proteins associated with replication also exhibited differential regulation, the RecA protein (RPN01487) being decreased at low pH. Homologues of translation-associated proteins exhibited differential expression, with polyribonucleotide nucleotidyltransferase (RPN00502) and polypeptide deformylase (RPN00945) being significantly down- and up-regulated, respectively, at low pH. One cell envelope homologue, a membrane TMPC precursor (RPN01034), was detected in increased amounts in cells cultured at pH 5.2. We also observed down-regulation of a phosphatase acting on phosphoproteins (RPN00665) at this pH.
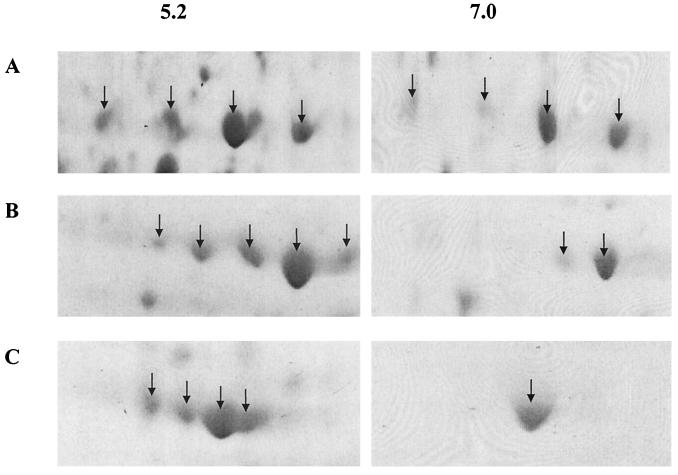
A number of proteins detected on 2-D gels of S. oralis cellular extracts were detectable in multiple forms (Fig. 2). The modulation of these isoforms, in terms of both number of polypeptides and the relative expression of each form, by growth under conditions of different pH is demonstrated using the examples of glyceraldehyde-3-phosphate dehydrogenase (RPN01440), pyruvate oxidase (RPN00904), and the 60-kDa chaperonin (RPN01512) (Fig. 4), where the individual isoforms are characterized by differences in observed mass and/or pI values. In total, seven of the differentially expressed proteins exhibited isoforms: 60-kDa chaperonin (RPN01512), formate acetyltransferase (RPN01016), pyruvate oxidase (RPN00904), NADP-specific glutamate dehydrogenase (RPN00804), fructose bisphosphate aldolase (RPN00514), glyceraldehyde-3-phosphate dehydrogenase (RPN01440), and lactate dehydrogenase (RPN01637), these having four, three, five, four, six, five, and five forms, respectively, when proteins were extracted from cells cultured at pH 5.2.
FIG. 4.
Isoforms of representative S. oralis proteins. Cellular proteins derived from cells cultured at pH 5.2 or pH 7.0 were resolved by 2-D PAGE. (A) Glyceraldehyde-3-phosphate dehydrogenase; (B) pyruvate oxidase; (C) 60-kDa chaperonin. Individual isoforms were identified by peptide mass fingerprinting and comparison with the S. pneumoniae genomic database.
Putative functions for 8 of the 39 S. oralis proteins that exhibited altered levels of expression at low pH remained unidentified. Seven of these proteins produced peptide mass fingerprint data, but the data failed to achieve a match with the S. pneumoniae genomic database according to our stringent search criteria; these proteins corresponded to proteins 8, 21, 22, 26, 34, 37, and 39 shown in Fig. 2. Only one protein matched an ORF (ORF 1374) extant in the database for which no putative function was assigned due to a lack of homologues in other nonredundant sequence databases (Table 2).
DISCUSSION
Growth of S. oralis strain 176N at pH 7.0 and 5.2 demonstrated that the isolate was aciduric, with no lag period before the initiation of the exponential growth phase for either pH. These and other data (1) confirm earlier investigations suggesting that the role of NMS in the caries process may need to be reassessed and that viridans streptococci other than S. mutans may contribute to the lowering of pH associated with enamel demineralization (33, 38, 42, 43). In addition, given that NMS including S. oralis are pioneer bacteria, acid production by these species may cause a shift in plaque pH such that colonization by mutans streptococci is facilitated (40). In light of these observations, we undertook a study to investigate proteins that may have relevance to the ability of S. oralis to survive and proliferate under conditions of low pH since bacterial growth is likely to play a role in caries initiation and/or progression under conditions of carbohydrate flux and cyclical changes in the pH of plaque. In contrast, many earlier investigations have instead focused on the response of caries-associated streptococci to immediate or short-term acid shock and have determined whether this response is adaptive (12, 13, 16, 38, 39).
Protein expression was determined using batch-grown cells in media at pH 7.0 and media adjusted to pH 5.2. The growth rates were not identical, and the pH of the media during growth was not constant, parameters which may be controlled by conducting experiments under continuous culture conditions. The overwhelming difference between the growth conditions was the initial pH of the media, however, although it maybe that the difference in growth rate had an effect on the expression of some of the proteins described here and that biofilm or continuous culture grown cells will also express different proteins. Numerous studies have used the high resolving power of 2-D electrophoresis to monitor changes in protein expression during the adaptation of microorganisms to environmental stress (4, 22, 28, 30, 41, 44). We have used this technique to investigate the regulation of gene product expression of S. oralis grown under conditions of differing pH and used peptide mass fingerprinting to identify those proteins with altered expression. While the entire proteome of S. oralis is not represented in our 2-D maps, and low-abundance proteins in the pl and Mr range studied will not be detected, we have demonstrated that 39 proteins were differentially expressed during growth at low pH. Using radiolabeling techniques in conjunction with one-dimensional electrophoresis to monitor protein expression by S. oralis ATCC 10557, a weak responder to acid shock, Hamilton and Svensater (13) demonstrated that six proteins were up-regulated on exposure to low pH and with masses consistent with those expected for the expression of heat shock proteins and components of a proton-translocating ATPase. We have shown that the S. pneumoniae genome is suitable for investigating the proteome of S. oralis, since theoretical tryptic digests of translated sequences of the 25 partial or complete S. oralis genes resulted in the identification of 23 proteins (92%) when analyzed against the S. pneumoniae genome. Preliminary investigations demonstrated that these in silico investigations could be extrapolated to the identification of many other S. oralis proteins, as 207 of 250 (83%) of those excised from gels were assigned putative functions (J. C. Wilkins, unpublished data).
The regulatory mechanisms governing the response of oral streptococci to acid adaptation are, as far as is known, markedly different from those employed by gram-negative bacteria (31). S. sanguis protects against acid killing via the arginine deiminase system, while Streptococcus salivarius responds to low pH by degrading urea to ammonia. Our data provide no evidence for equivalent systems up-regulated during growth of aciduric S. oralis at pH 5.2, but a glutamate dehydrogenase was up-regulated. ATP synthase, H+-ATPase, facilitates the physical extrusion and active efflux of H+ ions and plays a role in the response of bacteria to low external pH. In E. coli, F1F0 ATPase is synthesized irrespective of the environmental conditions (17), whereas in Enterococcus hirae environmental acidification of the cytoplasm leads to increased synthesis (21). For the aciduric S. oralis isolate investigated here, all of the subunits comprising the ATP synthase complex were expressed in greater amounts when bacteria were cultured at pH 5.2. These data confirm the findings of other investigators who suggested that acid adaptation in both S. mutans and NMS involves the induction of H+-ATPase and that ATPase in oral streptococci is transcriptionally regulated (31, 40). Induction of the ATPase, especially if the active complex has a low pH optimum, confers a competitive advantage during growth of an aciduric organism in dental plaque (31). In response to low pH, adaptive changes which are important in the maintaining the activity of membrane ATPases also occur in bacterial membranes. Insertional inactivation of ffh, which plays a role in the localization of membrane proteins, results in acid sensitivity (12). Decrease in membrane permeability to prevent the ingress of H+ has been demonstrated in E. coli. In response to pH changes, the synthesis of outer membrane proteins and a change in membrane lipid composition have been observed (35). Two-dimensional electrophoresis has been used as a powerful resolving technique to separate proteins, but it has been reported that there is an apparent bias toward soluble proteins on 2-D reference maps (24, 34). The use of stronger reagents for membrane solubilization and the introduction of fractionation steps prior to analysis by 2-D electrophoresis have been applied to increase the number of membrane proteins detected for E. coli. Using the methodology described in the present study, we still detected a number of cell wall and membrane-associated proteins, including ABC transporters and components of the ATPase complex. The lack of representation of the subunits of the F0 component of the F-ATPase, which are integral membrane proteins, could be attributed to lack of solubilization, despite the relatively aggressive extraction methods used here.
Salmonella (23) and S. mutans (39) in response to acid stress synthesize proteins which provide protection to a variety of other stresses. Svensater et al. (39) demonstrated enhanced synthesis of 64 unidentified proteins, 25 of which were acid specific. We identified the 60-kDa chaperonin (the GroEL homologue), Hsp33 (a redox-regulated chaperonin), and superoxide dismutase as proteins that were up-regulated by S. oralis in response to growth at pH 5.2. The chaperonins play a key role in the maturation of synthesized proteins and are pivotal in the degradation or refolding of denatured proteins (9, 14). Thus, chaperonins which interact with the glycolytic enzymes at low pH may increase their stability in the presence of an acid challenge (31). The superoxide dismutase of S. oralis is a homologue of the manganese-containing enzyme found in S. pneumoniae, where it is up-regulated by oxidative stress and may play a role in virulence (46). In Lactococcus lactis the manganese-containing superoxide dismutase homologue was induced under low-pH conditions (32), while in B. subtilis it is expressed as a general stress response protein (4).
Several glycolytic enzymes were up-regulated at pH 5.2; if this is reflected by increased glycolytic flux, the resulting increase in ATP production may support increased H+ extrusion under acidic conditions. Increased expression of both formate-pyruvate lyase and lactate dehydrogenase, key enzymes in the formation of metabolic end products by streptococci, suggests that the regulation of both by S. oralis may be in part a response to environmental pH. Earlier investigations of mutans streptococci and NMS demonstrated that the ratios of metabolic end products, predominantly lactate, formate, and acetate, were governed by the amounts of these two enzymes and their relative pH optima (15).
Three individual forms of putative ABC transporters were up-regulated by S. oralis at low pH. ABC transporters form a superfamily of diverse membrane proteins which utilize the energy derived from ATP hydrolysis to fuel the transport of solutes across the cell membrane (18). While the precise role of these proteins in the response to acid stress in streptococci has yet to be elucidated, an ABC transporter with homology to genes found in both Bacillus licheniformis and Staphylococcus epidermidis, which both function to confer resistance to antibiotics, made a significant contribution to the ability of S. mutans to grow at low pH (7). Enzymes involved in pathways of cofactor biosynthesis were also up-regulated by S. oralis at low pH. Formate-tetrahydrofolate ligase, which is required for the synthesis of a range of metabolites including purines, histidine, and formyl tRNA-Met, is of importance for growth of S. mutans at pH 5.0, and a mutant in which this gene had been inactivated was demonstrated to be both nutritionally and acid sensitive (6).
Several S. oralis proteins, including the 60-kDa chaperonin, existed in different isomeric forms, these being expressed differentially at low pH. That these isomeric forms arose from polypeptides with the same putative function was confirmed by peptide mass fingerprinting. The isoforms probably arose as a result of posttranslational modifications. Chaperonins may be acetylated and phosphorylated, and the GroEL homologue of Mycobacterium bovis has a noncovalently bound interaction with lipids (8). In L. lactis, glyceraldehyde-3-phosphate dehydrogenase existed as isoforms, and it was suggested that the isoform with lowest pI arose as a result of deamination or phosphorylation or by modification with a group resulting in a shift of the pI toward the acidic range of the 2-D map (20). Phosphorylation and dephosphorylation of proteins by the action of kinases and phosphatases, respectively, has long been recognized as a key mechanism by which functional activity may be regulated, and a number of such modifications that were originally thought to occur exclusively in eukaryotic systems have now been discovered in prokaryotes (reference 25 and references therein). Posttranslational modifications may result in the formation of these isoforms, and it is interesting to speculate on the role, if any, of the down-regulated phosphoprotein phosphatase in the different patterns of isomeric forms.
Taken together, our data clearly demonstrate that the expression of proteins from a number of functional categories is modulated as a result of culture of S. oralis at low pH. Some of these may be nonspecific stress response proteins, while others are key components of central and intermediary metabolism. The acid-specific stress response has yet to be determined, and the relevance of each up-regulated protein to the low-pH response of this aciduric isolate and its significance in the dental caries process have yet to be established. This proteomics-based approach to this biological problem has, however, revealed a number of targets for future gene inactivation or functional enzymology studies. These data would further facilitate the assessment of their role in aciduricity and any further pleiotropic effects.
ACKNOWLEDGMENT
This study was supported in part by a Ph.D. studentship awarded by the Pathological Society of Great Britain and Ireland.
REFERENCES
- 1.Alam S, Brailsford S R, Adams S, Allison C, Sheehy E, Zoitopoulos L, Kidd E A, Beighton D. Genotypic heterogeneity of Streptococcus oralis and distinct aciduric subpopulations in human dental plaque. Appl Environ Microbiol. 2000;66:3330–3336. doi: 10.1128/aem.66.8.3330-3336.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baev D, England R, Kuramitsu H K. Stress-induced membrane association of the Streptococcus mutans GTP-binding protein, an essential G protein, and investigation of its physiological role by utilizing an antisense RNA strategy. Infect Immun. 1999;67:4510–4516. doi: 10.1128/iai.67.9.4510-4516.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bentley R W, Leigh J A, Collins M D. Intrageneric structure of Streptococcus based on comparative analysis of small-subunit rRNA sequences. Int J Syst Bacteriol. 1991;41:487–494. doi: 10.1099/00207713-41-4-487. [DOI] [PubMed] [Google Scholar]
- 4.Bernhardt J, Volker U, Volker A, Antelmann H, Schmid R, Mach H, Hecker M. Specific and general stress proteins in Bacillus subtilis—a two-dimensional protein electrophoresis study. Microbiology. 1997;143:999–1017. doi: 10.1099/00221287-143-3-999. [DOI] [PubMed] [Google Scholar]
- 5.Brailsford S R, Byrne R W, Adams S, Zoitoppoulos L, Allison C, Beighton D. Investigation of the aciduric microflora of plaque. Caries Res. 1999;33:290. [Google Scholar]
- 6.Crowley P J, Gutierrez J A, Hillman J D, Bleiweis A S. Genetic and physiologic analysis of a formyl-tetrahydrofolate synthase mutant of Streptococcus mutans. J Bacteriol. 1997;179:1563–1572. doi: 10.1128/jb.179.5.1563-1572.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cvitkovitch D G, Gutierrez J A, Behari J, Youngman P J, Wetz J E, Crowley P J, Hillman J D, Brady L J, Bleiweis A S. Tn917-lac mutagenesis of Streptococcus mutans to identify environmentally regulated genes. FEMS Microbiol Lett. 2000;182:149–154. doi: 10.1111/j.1574-6968.2000.tb08889.x. [DOI] [PubMed] [Google Scholar]
- 8.de Bruyn J, Soetaert K, Buyssens P, Calonne I, de Coene J L, Gallet X, Brasseur R, Wattiez R, Falmagne P, Montrozier H, Laneelle M A, Daffe M. Evidence for specific and non-covalent binding of lipids to natural and recombinant Mycobacterium bovis BCG hsp60 proteins, and to the Escherichia coli homologue, GroEL. Microbiology. 2000;146:1513–1524. doi: 10.1099/00221287-146-7-1513. [DOI] [PubMed] [Google Scholar]
- 9.Georgopoulos C, Welch W J. Role of the major heat shock proteins as molecular chaperones. Annu Rev Cell Biol. 1993;9:601–631. doi: 10.1146/annurev.cb.09.110193.003125. [DOI] [PubMed] [Google Scholar]
- 10.Giard J-C, Hartke A, Flahaut S, Boutibonnes P, Auffray Y. Glucose starvation response in Enterococcus faecalis JH2–2: survival and protein analysis. Res Microbiol. 1997;148:27–35. doi: 10.1016/S0923-2508(97)81897-9. [DOI] [PubMed] [Google Scholar]
- 11.Gutierrez J A, Crowley P J, Brown D P, Hillman J D, Youngman P, Bleiweis A S. Insertional mutagenesis and recovery of interrupted genes of Streptococcus mutans by using transposon Tn917: preliminary characterization of mutants displaying acid sensitivity and nutritional requirements. J Bacteriol. 1996;178:4166–4175. doi: 10.1128/jb.178.14.4166-4175.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gutierrez J A, Crowley P J, Cvitkovitch D G, Brady L J, Hamilton I R, Hillman J D, Bleiweis A S. Streptococcus mutans ffh, a gene encoding a homologue of the 54kDa subunit of the signal recognition particle, is involved in resistance to acid stress. Microbiology. 1999;145:357–366. doi: 10.1099/13500872-145-2-357. [DOI] [PubMed] [Google Scholar]
- 13.Hamilton I R, Svensater G. Acid-regulated proteins induced by Streptococcus mutans and other oral bacteria during acid shock. Oral Microbiol Immunol. 1998;13:292–300. doi: 10.1111/j.1399-302x.1998.tb00710.x. [DOI] [PubMed] [Google Scholar]
- 14.Hartl F-U, Hlodan R, Langer T. Molecular chaperones in protein folding: the art of avoiding sticky situations. Trends Biochem Sci. 1994;19:20–25. doi: 10.1016/0968-0004(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 15.Iwami Y, Abbe K, Takahashi-Abbe S, Yamada T. Acid production by streptococci growing at low pH in a chemostat under anaerobic conditions. Oral Microbiol Immunol. 1992;7:304–308. doi: 10.1111/j.1399-302x.1992.tb00593.x. [DOI] [PubMed] [Google Scholar]
- 16.Jayaraman G C, Penders J E, Burne R A. Transcriptional analysis of the Streptococcus mutans hrcA, grpE and dnaK genes and regulation of expression in response to heat shock and environmental acidification. Mol Microbiol. 1997;25:329–341. doi: 10.1046/j.1365-2958.1997.4671835.x. [DOI] [PubMed] [Google Scholar]
- 17.Jensen P R, Westerhoff H V, Michelsen O. Excess capacity of H+ATPase and inverse respiratory control in Escherichia coli. EMBO J. 1993;12:1277–1282. doi: 10.1002/j.1460-2075.1993.tb05772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones P M, George A M. Subunit interactions in ABC transporters: towards a functional architecture. FEMS Microbiol Lett. 1999;179:187–202. doi: 10.1111/j.1574-6968.1999.tb08727.x. [DOI] [PubMed] [Google Scholar]
- 19.Kawamura Y, Hou X G, Sultana F, Miura H, Ezaki T. Determination of 16S rRNA sequences of Streptococcus mitis and Streptococcus gordonii and phylogenetic relationships among members of the genus Streptococcus. Int J Syst Bacteriol. 1995;45:406–408. doi: 10.1099/00207713-45-2-406. [DOI] [PubMed] [Google Scholar]
- 20.Kilstrup M, Jacobsen S, Hammer K, Vogensen F K. Induction of heat shock proteins DnaK, GroEL, and GroES by salt stress in Lactococcus lactis. Appl Environ Microbiol. 1997;63:1826–1837. doi: 10.1128/aem.63.5.1826-1837.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kobayashi H, Suzuki T, Kinoshita N, Unemoto T. Amplification of the Streptococcus faecalis proton-translocating ATPase by a decrease in cytoplasmic pH. J Bacteriol. 1984;158:1157–1160. doi: 10.1128/jb.158.3.1157-1160.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laplace J M, Thuault M, Hartke A, Boutibonnes P, Auffray Y. Sodium hypochlorite stress in Enterococcus faecalis: influence of antecedent growth conditions and induced proteins. Curr Microbiol. 1997;34:284–289. doi: 10.1007/s002849900183. [DOI] [PubMed] [Google Scholar]
- 23.Lee I S, Lin J, Hall H K, Bearson B, Foster J W. The stationary-phase sigma factor sigma S (RpoS) is required for a sustained acid tolerance response in virulent Salmonella typhimurium. Mol Microbiol. 1995;17:155–167. doi: 10.1111/j.1365-2958.1995.mmi_17010155.x. [DOI] [PubMed] [Google Scholar]
- 24.Molloy M P, Herbert B R, Walsh B J, Tyler M I, Traini M, Sanchez J C, Hochstrasser D F, Williams K L, Gooley A A. Extraction of membrane proteins by differential solubilization for separation using two-dimensional gel electrophoresis. Electrophoresis. 1998;19:837–844. doi: 10.1002/elps.1150190539. [DOI] [PubMed] [Google Scholar]
- 25.Mukhopadyay S, Kapatral V, Xu W, Chakrabarty A M. Characterization of a Hank's type serine/threonine kinase and serine/threonine phosphoprotein phosphatase in Pseudomonas aeruginosa. J Bacteriol. 1999;181:6615–6622. doi: 10.1128/jb.181.21.6615-6622.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neuhoff V, Arold N, Taube D, Ehrhardt W. Improved staining of proteins in polyacrylamide gels including isoelectric-focusing gels with clear background at nanogram sensitivity using Coomassie brilliant blue G250 and R250. Electrophoresis. 1988;9:255–262. doi: 10.1002/elps.1150090603. [DOI] [PubMed] [Google Scholar]
- 27.Overbeek R, Laresen N, Pusch G D, D'Souza M, Selkov E, Krypides N, Fonstein M, Maltsev N, Selkov E. WIT: integrated system for high-throughput genome sequence analysis and metabolic reconstruction. Nucleic Acids Res. 2000;28:123–125. doi: 10.1093/nar/28.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Panoff J-M, Corroler D, Thammavongs B, Boutibonnes P. Differentiation between cold shock proteins and cold acclimation proteins in a mesophilic gram-positive bacterium, Enterococcus faecalis JH2–2. J Bacteriol. 1997;179:4451–4454. doi: 10.1128/jb.179.13.4451-4454.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pappin D J C, Hojrup P, Bleasby A J. Rapid identification of proteins by peptide-mass fingerprinting. Curr Biol. 1993;3:327–332. doi: 10.1016/0960-9822(93)90195-t. [DOI] [PubMed] [Google Scholar]
- 30.Phan-Thanh L, Mahouin F. A proteomic approach to study the acid response in Listeria monocytogenes. Electrophoresis. 1999;20:2214–2224. doi: 10.1002/(SICI)1522-2683(19990801)20:11<2214::AID-ELPS2214>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 31.Quivey R G, Jr, Kuhnert W L, Hahn K. Adaptation of oral streptococci to low pH. Adv Microb Physiol. 2000;42:239–274. doi: 10.1016/s0065-2911(00)42004-7. [DOI] [PubMed] [Google Scholar]
- 32.Sanders J W, Leenhouts K J, Haandrikman A J, Venema G, Kok J. Stress response in Lactococcus lactis: cloning, expression analysis, and mutation of the lactococcal superoxide dismutase gene. J Bacteriol. 1995;177:5254–5260. doi: 10.1128/jb.177.18.5254-5260.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sansone C, van Houte J, Joshipura K, Kent R, Margolis H C. The association of mutans streptococci and non mutans streptococci capable of acidogenesis at a low pH with dental caries on enamel and root surfaces. J Dent Res. 1993;72:508–516. doi: 10.1177/00220345930720020701. [DOI] [PubMed] [Google Scholar]
- 34.Santoni V, Molloy M, Rabilloud T. Membrane proteins and proteomics: un amour impossible? Electrophoresis. 2000;21:1054–1070. doi: 10.1002/(SICI)1522-2683(20000401)21:6<1054::AID-ELPS1054>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 35.Sato M, Machida K, Arikado E, Saito H, Kakegawa T, Kobayashi H. Expression of outer membrane proteins in Escherichia coli growing at acid pH. Appl Environ Microbiol. 2000;66:943–947. doi: 10.1128/aem.66.3.943-947.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sato T, Wu J, Kurumitsu H. The sgp gene modulates stress responses of Streptococcus mutans: utilization of an antisense RNA strategy to investigate essential gene functions. FEMS Microbiol Lett. 1998;159:241–245. doi: 10.1111/j.1574-6968.1998.tb12867.x. [DOI] [PubMed] [Google Scholar]
- 37.Shevchenko A, Wilm M, Vorm O, Mann M. Mass spectrometric sequencing of proteins from silver-stained polyacrylamide gels. Anal Chem. 1996;68:850–858. doi: 10.1021/ac950914h. [DOI] [PubMed] [Google Scholar]
- 38.Svensater G, Larsson U B, Greif E C, Cvitovitch D G, Hamilton I R. Acid tolerance response and survival by oral bacteria. Oral Microbiol Immunol. 1997;12:266–273. doi: 10.1111/j.1399-302x.1997.tb00390.x. [DOI] [PubMed] [Google Scholar]
- 39.Svensater G, Sjogreen B, Hamilton I R. Multiple stress responses in Streptococcus mutans and the induction of general and stress-specific proteins. Microbiology. 2000;146:107–117. doi: 10.1099/00221287-146-1-107. [DOI] [PubMed] [Google Scholar]
- 40.Takahashi N, Yamada T. Acid-induced acid tolerance and acidogenicity of non-mutans streptococci. Oral Microbiol Immunol. 1999;14:43–48. doi: 10.1034/j.1399-302x.1999.140105.x. [DOI] [PubMed] [Google Scholar]
- 41.van Bogelen R A, Olson E R, Wanner B L, Neidhardt F C. Global analysis of proteins synthesized during phosphorus restriction in Escherichia coli. J Bacteriol. 1996;178:4344–4366. doi: 10.1128/jb.178.15.4344-4366.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Houte J, Sansone C, Joshipura K, Kent R. Mutans streptococci and non-mutans streptococci acidogenic at low pH, and in vitro acidogenic potential of dental plaque in two different areas of the human dentition. J Dent Res. 1991;70:1503–1507. doi: 10.1177/00220345910700120601. [DOI] [PubMed] [Google Scholar]
- 43.van Houte J, Lopman J, Kent R. The final pH of bacteria comprising the predominant flora on sound and carious human root and enamel surfaces. J Dent Res. 1996;75:1008–1014. doi: 10.1177/00220345960750040201. [DOI] [PubMed] [Google Scholar]
- 44.Vasseur C, Labadie J, Hebraud M. Differential protein expression by Pseudomonas fragi submitted to various stresses. Electrophoresis. 1999;20:2204–2213. doi: 10.1002/(SICI)1522-2683(19990801)20:11<2204::AID-ELPS2204>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 45.Yamashita Y, Takehara T, Kuramitsu H K. Molecular characterization of a Streptococcus mutans mutant altered in environmental stress responses. J Bacteriol. 1993;175:6220–6228. doi: 10.1128/jb.175.19.6220-6228.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yesilkaya H, Kadioglu A, Gingles N, Alexander J E, Mitchell T J, Paton P W. Role of manganese-containing superoxide dismutase in oxidative stress and virulence of Streptococcus pneumoniae. Infect Immun. 2000;68:2819–2826. doi: 10.1128/iai.68.5.2819-2826.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]