Abstract
Patients with moderate to severe allergic rhinitis may benefit from subcutaneous immunotherapy (SCIT), despite the risk of systemic allergic reaction. Dupilumab is a fully human monoclonal antibody that blocks the shared receptor component for interleukin‐4 and interleukin‐13, key drivers of the type 2 inflammation seen in allergic rhinitis, thereby inhibiting their signaling. In the LIBERTY Grass AID trial (NCT03558997), 16 weeks of treatment with 300 mg of dupilumab every 2 weeks plus timothy grass (TG) SCIT did not reduce TG allergen challenge nasal symptom scores compared with SCIT only but did improve tolerability of SCIT up‐titration in patients with a history of grass pollen–induced seasonal allergic rhinitis. Here, we present the pharmacokinetics of functional serum dupilumab and concentration‐response relationships in 52 patients enrolled in this trial. Functional dupilumab concentrations and concentrations of TG‐specific IgE and IgG4 were assessed in blood samples collected from dupilumab‐only and SCIT + dupilumab–treated groups. Mean functional dupilumab concentrations were similar in both groups and reached a steady state of ≈70–80 mg/L at week 5. One week after the end of treatment, TG‐specific IgG4 concentrations were increased in the SCIT + dupilumab group, but not in the dupilumab‐only group, over the range of dupilumab concentrations evaluated, whereas no changes were seen for TG‐specific IgE concentrations. This study demonstrates that SCIT does not alter functional concentrations of serum dupilumab, and the impact of SCIT on TG‐specific immunoglobulins is not affected by functional dupilumab concentrations over the range studied, indicating that maximum response was achieved in all patients.
Keywords: concentration‐response relationship, dupilumab, grass allergy, pharmacokinetics, seasonal allergic rhinitis, subcutaneous immunotherapy
Allergic rhinitis is an IgE‐mediated disease leading to inflammation of the nasal mucosa and nasal symptoms such as rhinorrhea, itching, sneezing, and congestion 1 and has a considerable impact on patients’ quality of life. 2
Oral and intranasal antihistamines and intranasal corticosteroid treatment may alleviate symptoms in many but not all patients. 3 Patients with moderate‐to‐severe allergic rhinitis may benefit from subcutaneous immunotherapy (SCIT), in which they receive increasing allergen doses to modify the immunological response, resulting in allergen desensitization and symptom reduction. 4 However, serious systemic allergic reactions are a known risk, such that SCIT must be given in the clinic under medical supervision, with access to resuscitative equipment; the risk of systemic (allergic) reactions is highest in patients with asthma. 4 , 5 The crosslinking of FcεRI‐bound IgE by the allergen induces mast cell and basophil activation to release preformed histamine, leukotrienes, prostaglandins, and cytokines, which can transiently exacerbate allergic symptoms, resulting in limitations during SCIT dose escalation in some patients. 6 SCIT induces allergen‐specific antibodies, including IgG4, which is thought to have a protective effect against IgE‐mediated allergic symptoms by competing with IgE and blocking IgE‐mediated effector cell activation. 7 Increases in the serum IgG4‐to‐serum IgE ratio have been associated with a reduction of symptoms induced by nasal allergen provocation after 2 years of SCIT. 8 This ratio is thought to be more reflective of the competition for allergen binding between IgG4 and IgE and has shown a better correlation with clinical improvement. 7
The type 2 cytokines interleukin (IL)‐4 and IL‐13 play key roles in the pathophysiology of multiple (allergic) diseases, including allergic rhinitis. 9 Dupilumab, a fully human VelocImmune‐derived 10 , 11 monoclonal antibody, blocks the shared receptor component for IL‐4 and IL‐13, 12 , 13 thus inhibiting their signaling. Dupilumab has shown efficacy in several type 2 inflammatory diseases including atopic dermatitis, asthma, chronic rhinosinusitis with nasal polyps, and eosinophilic esophagitis. 14 , 15 , 16 , 17 , 18 These diseases often occur concurrently, with reported prevalence of allergic rhinitis in asthma patients of up to 90%. 19
Dupilumab has demonstrated efficacy in reducing allergic rhinitis symptoms in patients with asthma who have comorbid perennial allergic rhinitis. 20 , 21 In a phase 2a, multicenter, randomized, placebo‐controlled study in adults with grass pollen–induced seasonal allergic rhinitis (LIBERTY Grass AID; NCT03558997), treatment with SCIT + dupilumab for 16 weeks did not affect post–allergen challenge nasal symptom scores compared with SCIT alone, but did improve SCIT tolerability. 22
In this article, we present the concentration‐response and pharmacokinetics (PK) of functional dupilumab in the serum of patients enrolled in LIBERTY Grass AID.
Methods
Study Design and Treatment
This study was conducted in accordance with the consensus ethics principles derived from international guidelines, including the 2013 Declaration of Helsinki and Council for International Organizations of Medical Sciences International Ethical Guidelines and applicable International Council for Harmonisation Good Clinical Practice Guidelines. All study documents were approved by an institutional review board/independent ethics committee before the study was initiated, and all participants signed an informed consent form before undertaking any study procedure.
LIBERTY Grass AID was a phase 2a, multicenter, randomized, double‐blind, placebo‐controlled, parallel‐group, 4‐arm study in adults with grass pollen–induced seasonal allergic rhinitis. The full study design has been previously reported. 22 Briefly, patients were randomized 1:1:1:1 to receive SCIT + dupilumab, dupilumab + SCIT placebo (dupilumab group), SCIT + dupilumab placebo (SCIT group), or SCIT placebo + dupilumab placebo (placebo group) for 16 weeks. Subcutaneous (SC) dupilumab 300 mg was administered every 2 weeks for a total of 16 weeks (following a dupilumab loading dose of 600 mg or placebo on day 1). Timothy grass (TG) SCIT extract or placebo was administered over 8 weeks using a rapidly escalating cluster protocol, followed by a maintenance regimen for the following 8 weeks, with a recommended SCIT target maintenance dose of 4000 bioequivalent allergy units. Analyses were performed in the 2 study groups receiving dupilumab treatment: the SCIT + dupilumab group and the dupilumab group. Functional dupilumab PK samples were collected at baseline before dosing and nasal allergen challenge and on days 36, 57, 92, 120, 141, and 169 (ie, at weeks 5, 8, 13, 17, 20, and 24) before dosing of dupilumab when dosing was on the same day. Participants were permitted to take oral antihistamines for allergic rhinitis symptoms as needed during the study, but not within 5 days before or during a visit for a nasal allergen challenge or skin testing.
Study Populations
Included patients were aged 18 to 55 years with a history of grass pollen–induced seasonal allergic rhinitis, had a positive TG allergen skin prick test, were positive for serum TG‐specific IgE (TG sIgE), and had a positive nasal allergen challenge. Baseline demographics and clinical characteristics have previously been reported 22 and are briefly summarized for the PK analysis set in Table 1. Patients were excluded if they had significant rhinitis or sinusitis outside of the grass pollen season.
Table 1.
Summary of Baseline Demographics
| Dupilumab (n = 26) | SCIT + Dupilumab (n = 26) | |
|---|---|---|
| Age, y | ||
| Mean (SD) | 40.3 (11.19) | 33.0 (10.58) |
| Sex, n (%) | ||
| Male | 10 (38.5) | 16 (61.5) |
| Female | 16 (61.5) | 10 (38.5) |
| Race, n (%) | ||
| White | 18 (69.2) | 21 (80.8) |
| Black or African American | 4 (15.4) | 2 (7.7) |
| Asian | 2 (7.7) | 2 (7.7) |
| Other | 2 (7.7) | 0 |
| Not reported | 0 | 1 (3.8) |
| Weight (kg) | ||
| Mean (SD) | 78.9 (16.89) | 79.0 (19.26) |
| Weight group, n (%) | ||
| <60 kg | 4 (15.4) | 2 (7.7) |
| ≥60–<100 kg | 20 (76.9) | 21 (80.8) |
| ≥100 kg | 2 (7.7) | 3 (11.5) |
| BMI, kg/m2 | ||
| Mean (SD) | 28.0 (6.64) | 26.6 (5.60) |
BMI, body mass index; SCIT, subcutaneous immunotherapy; SD, standard deviation.
Analyses
Changes in functional serum dupilumab concentrations over time were analyzed in the PK analysis set, which consisted of all randomized patients who received any study drug and who had at least one nonmissing dupilumab drug concentration result following the first dupilumab dose. Concentrations of functional dupilumab in serum were measured using a validated enzyme‐linked immunosorbent assay 23 with dupilumab as the assay standard and IL‐4Rα as capture reagent. Functional dupilumab was defined with either 1 or 2 unoccupied binding sites. The assay did not detect dupilumab when both binding sites were occupied by soluble IL‐4Rα or when at least 1 site was bound to membrane‐bound IL‐4Rα. The analyte recovery (percent) of the validated assay ranged from 96% to 105% for nonzero standards and quality control (QC) samples. The coefficient of variation (percent) for the nonzero standards and QC samples was ≤9%, indicating that the assay performed consistently with good precision and accuracy. The lower limit of quantification (LLOQ) was 0.078 mg/L. TG serum IgG4 (sIgG4) and serum IgE (sIgE) were measured in serum samples on the ImmunoCAP platform (Thermo Fisher Scientific, Waltham, Massachusetts). The intra‐assay precision for TG sIgG4 (high QC [HQC]) ranged from 1.28% to 11.83% and the interassay precision was 14.03%. The intra‐assay precision for TG sIgG4 (low QC [LQC]) ranged from 3.85% to 10.35% and the interassay precision was 10.87%. The LLOQ for TG sIgG4 was 0.15 μg/mL. The intra‐assay precision for the TG sIgE (HQC) ranged from 0.61% to 7.30%, while the interassay precision for the HQC was 10.78%. The intra‐assay precision for TG sIgE (LQC) ranged from 1.51% to 9.05%, while the interassay precision for the LQC was 5.41%. The LLOQ for TG sIgE was 0.10 kUA/L. The intra‐assay precision for TG‐sIgG (HQC) was 3.00% to 9.19% and the interassay precision was 7.86%. The intra‐assay precision for TG sIgG (LQC) was 1.27% to 8.73% and the interassay precision was 8.39%. The LLOQ for TG sIgG was 0.15 μg/mL.
Descriptive statistics of functional dupilumab concentrations were presented using the PK analysis set along with plots of mean (± standard deviation [SD]) concentrations in serum by nominal sampling time. Concentration‐response relationships between functional dupilumab concentrations and changes in TG sIgG4, TG sIgE, and TG sIgG4‐to‐sIgE ratios at week 17 were evaluated in an exploratory fashion using the concentration‐response analysis set (CRAS). The CRAS consisted of all patients included in the PK analysis set who had at least 1 nonmissing baseline and 1 nonmissing postdose response measurement for TG sIgG4 or TG sIgE, as applicable for each concentration‐response assessment. In the concentration‐response analyses, a last observation carried forward method was applied to the functional dupilumab concentration data or response of biomarkers at postbaseline time points. Changes from baseline were not imputed when baseline values were missing.
No formal statistical hypotheses were tested. Descriptive analyses were conducted using SAS version 9.4 (SAS Institute, Cary, North Carolina).
Results
Patient Population
A total of 103 patients were included in the study, of whom 52 were included in the PK and CRASs. Baseline demographics were similar among groups (Table 1). The mean age was 40.3 (±11.19 SD) years for the dupilumab group and 33.0 (±10.58 SD) for the SCIT + dupilumab group. Between 38.5% and 61.5% of patients were men. Of all serum samples collected from dupilumab‐treated patients, 76.6% had quantifiable functional dupilumab concentrations, 136 of 175 (77.7%) from the SCIT + dupilumab group and 132 of 175 (75.4%) from the dupilumab group.
Functional Dupilumab Concentrations
Mean concentrations of functional dupilumab were comparable between the SCIT + dupilumab and dupilumab groups (Figure 1 and Table 2) and appeared to be at steady state by week 5. Similar concentrations of functional dupilumab were observed in the presence and absence of SCIT. After treatment cessation, mean dupilumab concentrations declined from ≈80 mg/L at week 17 to 16 mg/L at week 24 in both groups.
Figure 1.
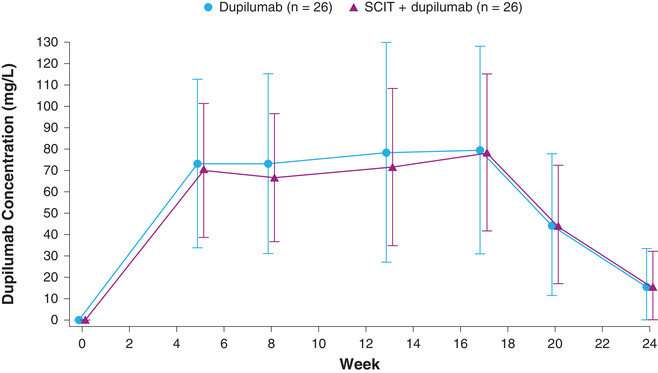
Mean (± SD) concentrations of functional dupilumab in serum by nominal time. Concentrations below the LLOQ were set to 0; screening was at −28 days but is presented as week 0. LLOQ, lower limit of quantification; SCIT, subcutaneous immunotherapy; SD, standard deviation.
Table 2.
Descriptive Statistics for Functional Serum Dupilumab Concentration at Each Time Point
| Dupilumab | SCIT + dupilumab | |||||
|---|---|---|---|---|---|---|
| Week | n | Mean (SD) | Median | n | Mean (SD) | Median |
| 0 | 26 | 0 (0) | 0 | 26 | 0 (0) | 0 |
| 5 | 26 | 73.2 (39.4) | 64.4 | 26 | 70.1 (31.2) | 74.4 |
| 8 | 26 | 73.2 (42.1) | 72.4 | 25 | 66.6 (30.0) | 68.7 |
| 13 | 24 | 78.3 (51.3) | 70.3 | 25 | 71.7 (36.8) | 70.7 |
| 17 | 24 | 79.4 (48.7) | 74.5 | 23 | 78.4 (36.8) | 87.2 |
| 20 | 24 | 44.6 (33.2) | 43.4 | 24 | 44.6 (27.7) | 42.0 |
| 24 | 19 | 15.5 (18.1) | 8.20 | 20 | 15.8 (16.3) | 12.3 |
SCIT, subcutaneous immunotherapy; SD, standard deviation.
Concentration‐Response Relationships
Changes from baseline in TG sIgG4 were observed in the SCIT + dupilumab at week 17, but not in the dupilumab group (Figure 2a), whereas no changes in TG sIgE were seen in either the SCIT + dupilumab or dupilumab groups (Figure 2b). In the SCIT + dupilumab, increases in TG sIgG4 from baseline were seen over the entire concentration range studied. Change from baseline in the TG sIgG4‐to‐TG sIgE ratio was greater in the SCIT + dupilumab group compared with the dupilumab group and constant over the entire concentration range (Figure 2c).
Figure 2.
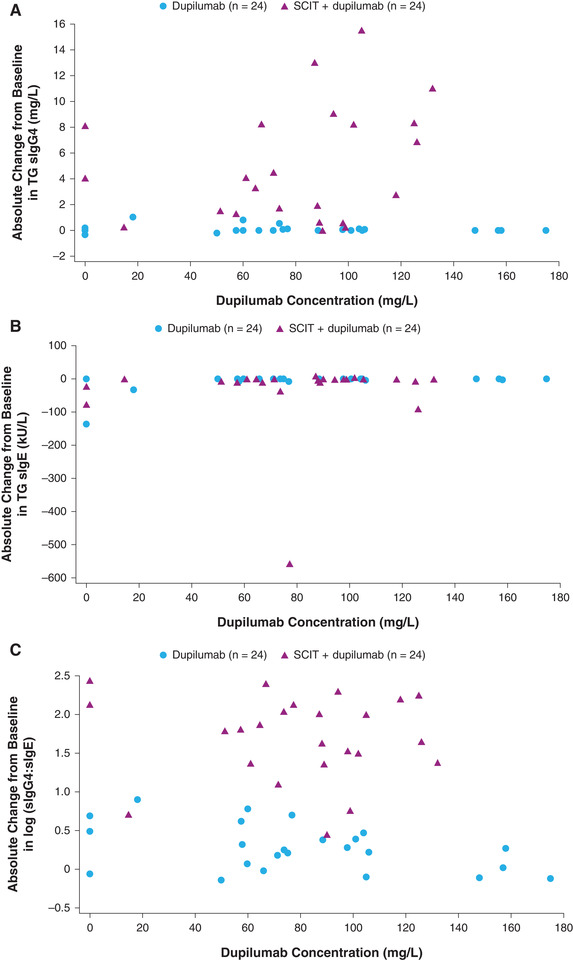
(a) Change from baseline in serum TG sIgG4 versus individual concentrations of functional dupilumab at week 17 (LOCF). (b) Change from baseline in TG sIgE versus individual concentrations of functional dupilumab at week 17 (LOCF). (c) Change from baseline in log‐transformed value of serum TG sIgG4‐to‐sIgE ratio vs individual concentrations of functional dupilumab at week 17 (LOCF). LOCF was used for missing values; concentrations below the LLOQ were set to 0. LLOQ, lower limit of quantification; LOCF, last observation carried forward; SCIT, subcutaneous immunotherapy; sIgE, serum IgE; TG, timothy grass.
Discussion
In this PK and concentration‐response study of dupilumab in patients with seasonal allergic rhinitis, concentrations of functional dupilumab were consistent between participants administered dupilumab and participants coadministered dupilumab and SCIT. Steady‐state functional dupilumab concentrations were achieved by week 5. The dupilumab group showed a slightly greater variability in concentrations compared with the SCIT + dupilumab group, as a few patients administered with dupilumab presented concentrations >2‐fold the mean concentration during the study due to low body weight (about 1 SD lower than the mean body weight). Body weight has been reported to be a significant covariate of population PK of dupilumab in healthy subjects and adult patients with atopic dermatitis. 24 The mean steady‐state concentration (≈70‐80 mg/L) achieved by a 300‐mg regimen was consistent with previous reports. 23
Coadministration of dupilumab and SCIT increased TG‐specific IgG4 concentrations compared with the administration of dupilumab only. Effects of SCIT on TG sIgG4 were constant over the entire range of evaluated dupilumab concentrations, indicating that the 300‐mg every 2 weeks regimen achieved the maximum response in all patients. While allergen‐specific SCIT is associated with elevated levels of allergen‐specific IgG4, 25 dupilumab does not seem to impact sIgG4. Therefore, dupilumab concentrations close to 0 should not impact sIgG4, suggesting that the size of the constant response has reached the maximum response for 300 mg every 2 weeks.
We found a greater change from baseline in the TG sIgG4‐to‐TG sIgE ratio at week 17 in the SCIT + dupilumab group compared with the dupilumab group over the concentration range studied. Comparing the SCIT + dupilumab group with the SCIT‐only group, 22 SCIT + dupilumab did not block the SCIT‐induced rise in TG sIgG4. Importantly, co‐administration of SCIT + dupilumab suppressed the SCIT‐related increase in TG sIgE to levels comparable to administration of dupilumab only, and this effect was not concentration‐dependent over the concentration range studied. Although immunogenicity was out of scope, antidrug antibody–positive response was observed in only 1 patient with a low titer, which excludes immunogenicity as a potential confounder from the concentration‐response relationships. Evidence suggests that IL‐4 plays a key role in isotype switching in B cells from IgM to IgE, 26 , 27 and mouse studies have shown that IL‐4 increases the proportion of B cells that secrete IgE compared with IgM or IgG1. 28 As dupilumab blocks the IL‐4 receptor, secretion of IgE is expected to be reduced in dupilumab recipients. Indeed, previous findings show that dupilumab suppresses (specific) IgEs across disease indications. 29 , 30 , 31 The difference in IgG4‐to‐IgE ratio between dupilumab and SCIT + dupilumab is therefore likely driven by SCIT‐related increases in TG sIgG4 production, and not by enhanced immunoglobulin class switching upon addition of SCIT to dupilumab. However, the difference in IgG4‐to‐IgE ratio between the SCIT + dupilumab and SCIT groups 22 is driven by dupilumab‐induced suppression of IgE levels.
The suppressive effect of dupilumab on IgE concentrations in the SCIT + dupilumab group is in line with findings in the primary study. Although we did not find evidence for dupilumab‐related improvements in nasal symptom scores after an allergen challenge, the LIBERTY Grass AID study showed that the addition of dupilumab improved patient tolerability of SCIT. 22 A higher proportion of SCIT + dupilumab–treated patients achieved the SCIT maintenance dose compared with patients who received SCIT alone, and a lower proportion of SCIT + dupilumab–treated patients required epinephrine rescue treatment compared with SCIT alone. 22 Additionally, there were significantly fewer withdrawals in the SCIT + dupilumab group vs SCIT alone and, whereas the majority of SCIT‐group withdrawals were due to SCIT‐related intolerability, no SCIT tolerability–associated discontinuations were seen in the SCIT + dupilumab group. Taken together, this suggests that dupilumab successfully reduced exacerbations of allergic symptoms during SCIT treatment. This improved tolerance of SCIT may be attributed to the suppression of the SCIT‐induced increase in sIgE by dupilumab treatment which, along with the SCIT‐induced increase in sIgG4, leads to a higher sIgG4‐to‐sIgE ratio. This increased sIgG4‐to‐sIgE ratio is potentially an important contributor to improved tolerability of SCIT + dupilumab combination therapy.
A benefit of these analyses is that they were performed with data from a randomized, double‐blind, placebo‐controlled clinical study, which strengthens the validity of the results. The advantage of using the clinical exposure‐response data from a randomized controlled study is that both measured and unmeasured patient characteristics and covariates at baseline are likely to be balanced between patient cohorts. This leads to reducing the effect of unobserved confounders in concentration‐response analyses. This proof‐of‐concept study was not specifically designed for the assessment of PK or concentration‐response over a wide range of doses or concentrations. However, the steady‐state drug exposure in this study was comparable to other clinical studies in adult patients with atopic dermatitis, asthma, and chronic rhinosinusitis with nasal polyps at a given dosing regimen (300 mg every 2 weeks).
Conclusions
In this study, functional concentrations of dupilumab were consistent between participants coadministered administered dupilumab and participants coadministered SCIT + dupilumab; a steady state for functional dupilumab concentrations was achieved by week 5 after treatment initiation. Coadministration of dupilumab and SCIT increased TG‐specific IgG4 concentrations and the TG sIgG4‐to‐sIgE ratio (but not overall TG sIgE) compared to administration of dupilumab only. These effects were held as constant without an upward or downward trend over the entire range of evaluated dupilumab concentrations, indicating that the 300‐mg every 2 weeks regimen achieved maximum response in all patients.
Conflicts of Interest
M.A.K., Y.F., C.‐H.L., C.Q.W., A.R.R., M.P.O., M.R., and J.D.D. are employees and shareholders of Regeneron Pharmaceuticals, Inc. C.X. is an employee and may hold stock and/or stock options in Sanofi.
Author Contributions
M.A.K., Y.F., A.R.R., M.P.O., M.R., and J.D.D. made substantial contributions to the conception and design of the work. M.A.K. and Y.F. contributed to the acquisition of the data. M.A.K., Y.F., C.‐H.L., C.X., C.Q.W., A.R.R., M.P.O., M.R., and J.D.D. contributed to the analysis and interpretation of the data. M.A.K., Y.F., and M.P.O. drafted the article, and all authors revised it critically for important intellectual content. All authors approved the final version and are accountable for all aspects of the work.
Funding
This research was sponsored by Sanofi and Regeneron Pharmaceuticals, Inc. ClinicalTrials.gov Identifier: NCT03558997.
Acknowledgments
Medical writing/editorial assistance was provided by Corinne S. Wilson, PhD, of Excerpta Medica, and was funded by Sanofi Genzyme and Regeneron Pharmaceuticals, Inc., according to the Good Publication Practice guideline. The authors acknowledge Rakesh Padigala and Hong Yan of Regeneron Pharmaceuticals, Inc., for their programming support.
John D. Davis is a fellow of the American College of Clinical Pharmacology.
ClinicalTrials.gov Identifier: NCT03558997
References
- 1. Varshney J, Varshney H. Allergic rhinitis: an overview. Indian J Otolaryngol Head Neck Surg. 2015;67(2):143‐149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fineman SM. The burden of allergic rhinitis: beyond dollars and cents. Ann Allergy Asthma Immunol. 2002;88(4 suppl 1):2‐7. [DOI] [PubMed] [Google Scholar]
- 3. Seidman MD, Gurgel RK, Lin SY, et al. Clinical practice guideline: allergic rhinitis. Otolaryngol Head Neck Surg. 2015;152(suppl 1):S1‐S43. [DOI] [PubMed] [Google Scholar]
- 4. Durham SR, Penagos M. Sublingual or subcutaneous immunotherapy for allergic rhinitis? J Allergy Clin Immunol. 2016;137(2):339‐349.e10. [DOI] [PubMed] [Google Scholar]
- 5. Amin HS, Liss GM, Bernstein DI. Evaluation of near‐fatal reactions to allergen immunotherapy injections. J Allergy Clin Immunol. 2006;117(1):169‐175. [DOI] [PubMed] [Google Scholar]
- 6. Kubo M. Mast cells and basophils in allergic inflammation. Curr Opin Immunol. 2018;54:74‐79. [DOI] [PubMed] [Google Scholar]
- 7. Shamji MH, Kappen J, Abubakar‐Waziri H, et al. Nasal allergen‐neutralizing IgG4 antibodies block IgE‐mediated responses: novel biomarker of subcutaneous grass pollen immunotherapy. J Allergy Clin Immunol. 2019;143(3):1067‐1076. [DOI] [PubMed] [Google Scholar]
- 8. James LK, Shamji MH, Walker SM, et al. Long‐term tolerance after allergen immunotherapy is accompanied by selective persistence of blocking antibodies. J Allergy Clin Immunol. 2011;127(2):509‐516.e1‐5. [DOI] [PubMed] [Google Scholar]
- 9. Gandhi NA, Pirozzi G, Graham NMH. Commonality of the IL‐4/IL‐13 pathway in atopic diseases. Expert Rev Clin Immunol. 2017;13(5):425‐437. [DOI] [PubMed] [Google Scholar]
- 10. Macdonald LE, Karow M, Stevens S, et al. Precise and in situ genetic humanization of 6 Mb of mouse immunoglobulin genes. Proc Natl Acad Sci U S A. 2014;111(14):5147‐5152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Murphy AJ, Macdonald LE, Stevens S, et al. Mice with megabase humanization of their immunoglobulin genes generate antibodies as efficiently as normal mice. Proc Natl Acad Sci U S A. 2014;111(14):5153‐5158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gandhi NA, Bennett BL, Graham NMH, et al. Targeting key proximal drivers of type 2 inflammation in disease. Nat Rev Drug Discov. 2016;15(1):35‐50. [DOI] [PubMed] [Google Scholar]
- 13. Le Floc'h A, Allinne J, Nagashima K, et al. Dual blockade of IL‐4 and IL‐13 with dupilumab, an IL‐4Rα antibody, is required to broadly inhibit type 2 inflammation. Allergy. 2020;75(5):1188‐1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Simpson EL, Bieber T, Guttman‐Yassky E, et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375(24):2335‐2348. [DOI] [PubMed] [Google Scholar]
- 15. Blauvelt A, de Bruin‐Weller M, Gooderham M, et al. Long‐term management of moderate‐to‐severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1‐year, randomised, double‐blinded, placebo‐controlled, phase 3 trial. Lancet 2017;389(10086):2287‐2303. [DOI] [PubMed] [Google Scholar]
- 16. Castro M, Corren J, Pavord ID, et al. Dupilumab efficacy and safety in moderate‐to‐severe uncontrolled asthma. N Engl J Med. 2018;378(26):2486‐2496. [DOI] [PubMed] [Google Scholar]
- 17. Bachert C, Han JK, Desrosiers M, et al. Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS‐24 and LIBERTY NP SINUS‐52): results from two multicentre, randomised, double‐blind, placebo‐controlled, parallel‐group phase 3 trials. Lancet. 2019;394(10209):1638‐1650. [DOI] [PubMed] [Google Scholar]
- 18. Hirano I, Dellon ES, Hamilton JD, et al. Efficacy of dupilumab in a phase 2 randomized trial of adults with active eosinophilic esophagitis. Gastroenterology. 2020;158(1):111‐122.e10. [DOI] [PubMed] [Google Scholar]
- 19. Leynaert B, Neukirch F, Demoly P, Bousquet J. Epidemiologic evidence for asthma and rhinitis comorbidity. J Allergy Clin Immunol. 2000;106(suppl 5):S201‐S205. [DOI] [PubMed] [Google Scholar]
- 20. Weinstein SF, Katial R, Jayawardena S, et al. Efficacy and safety of dupilumab in perennial allergic rhinitis and comorbid asthma. J Allergy Clin Immunol. 2018;142(1):171–177.el. [DOI] [PubMed] [Google Scholar]
- 21. Busse WW, Maspero JF, Lu Y, et al. Efficacy of dupilumab on clinical outcomes in patients with asthma and perennial allergic rhinitis. Ann Allergy Asthma Immunol. 2020;125(5):565‐576.e1. [DOI] [PubMed] [Google Scholar]
- 22. Corren J, Saini SS, Gagnon R, et al. Short‐term subcutaneous allergy immunotherapy and dupilumab are well tolerated in allergic rhinitis: a randomized trial. J Asthma Allergy. 2021;14:1045‐1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kovalenko P, Davis JD, Li M, et al. Base and covariate population pharmacokinetic analyses of dupilumab using phase 3 data. Clin Pharmacol Drug Dev. 2020;9(6):756‐767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kovalenko P, DiCioccio AT, Davis JD, et al. Exploratory population PK analysis of dupilumab, a fully human monoclonal antibody against IL‐4Rα, in atopic dermatitis patients and normal volunteers. CPT Pharmacometrics Syst Pharmacol. 2016;5(11):617‐624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zidarn M, Košnik M, Šilar M, Bajrović N, Korošec P. Sustained effect of grass pollen subcutaneous immunotherapy on suppression of allergen‐specific basophil response; a real‐life, nonrandomized controlled study. Allergy. 2015;70(5):547‐555. [DOI] [PubMed] [Google Scholar]
- 26. Pène J, Rousset F, Brière F, et al. IgE production by normal human B cells induced by alloreactive T cell clones is mediated by IL‐4 and suppressed by IFN‐gamma. J Immunol. 1988;141(4):1218‐1224. [PubMed] [Google Scholar]
- 27. Poulsen LK, Hummelshoj L. Triggers of IgE class switching and allergy development. Ann Med. 2007;39(6):440‐456. [DOI] [PubMed] [Google Scholar]
- 28. Lebman DA, Coffman RL. Interleukin 4 causes isotype switching to IgE in T cell‐stimulated clonal B cell cultures. J Exp Med. 1988;168(3):853‐862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Corren J, Castro M, O'Riordan T, et al. Dupilumab efficacy in patients with uncontrolled, moderate‐to‐severe allergic asthma. J Allergy Clin Immunol Pract. 2020;8(2):516‐526. [DOI] [PubMed] [Google Scholar]
- 30. Guttman‐Yassky E, Bissonnette R, Ungar B, et al. Dupilumab progressively improves systemic and cutaneous abnormalities in patients with atopic dermatitis. J Allergy Clin Immunol. 2019;143(1):155‐172. [DOI] [PubMed] [Google Scholar]
- 31. Kariyawasam HH, James LK, Gane SB. Dupilumab: clinical efficacy of blocking IL‐4/IL‐13 signalling in chronic rhinosinusitis with nasal polyps. Drug Des Devel Ther. 2020;14:1757‐1769. [DOI] [PMC free article] [PubMed] [Google Scholar]


