Abstract
The cationic benzyl complex [(Me4TACD)Sr(CH2Ph)][A] (Me4TACD=1,4,7,10‐tetramethyltetraazacyclododecane; A=B(C6H3‐3,5‐Me2)4) reacted with two equivalents of phenylsilane to give the bridging hexahydridosilicate complex [(Me4TACD)2Sr2(thf)4(μ‐κ3 : κ3‐SiH6)][A]2 (3 a). Rapid phenyl exchange between phenylsilane molecules is assumed to generate monosilane SiH4 that is trapped by two strontium hydride cations [(Me4TACD)SrH(thf) x ]+. Complex 3 a decomposed in THF at room temperature to give the terminal silanide complex [(Me4TACD)Sr(SiH3)(thf)2][A], with release of H2. Upon reaction with a weak Brønsted acid, CO2, and 1,3,5,7‐cyclooctatetraene SiH4 was released. The reaction of a 1 : 2 mixture of cationic benzyl and neutral dibenzyl complex with phenylsilane gave the trinuclear silanide complex [(Me4TACD)3Sr3(μ2‐H)3(μ3‐SiH3)2][A], while nOctSiH3 led to the trinuclear (n‐octyl)pentahydridosilicate complex [(Me4TACD)3Sr3(μ2‐H)3(μ3‐SiH5 nOct)][A].
Keywords: alkaline earth metal, hydride complex, hydridosilicate, silanide, strontium
A dinuclear strontium hexahydridosilicate complex has been isolated. It can be regarded as the adduct of two cationic strontium hydride fragments [(Me4TACD)SrH(thf) x ]+ with SiH4.

Introduction
Hypercoordinate (“hypervalent”) silicates [SiX5]− and [SiX6]2− (X=H, halogen, alkyl) are of fundamental interest in understanding of structure and bonding [1] as well as in synthetic applications due to enhanced nucleophilicity of the substituent X. [2] For X=H, however, there is surprisingly little information in the literature. [3] While [SiH5]− has been studied in the gas phase, [4] only two examples of structurally characterized compounds with [SiH6]2− have been reported: Häussermann et al. obtained K2[SiH6] by high pressure solid state synthesis from a mixture of KH/Si/H2 or K4Si4/H2, [5] while Tilley et al. prepared the dinuclear ruthenium(II) complex [(PhBPPh 3)Ru]2[μ‐η4,η4‐SiH6] (PhBPPh 3=PhB(CH2PPh2)3) by substitution of a diarylsilane σ‐adduct at a ruthenium hydride. [6] In addition, a limited number of mixed hydrido silicates have been reported, including the structurally characterized dihydridotriphenylsilicate [Ph3SiH2]−[7] and a remarkably inert calix[4]pyrrole hydrido silicate. [8] The ability of tetracoordinate silicon to expand its coordination number is also important in alkaline‐earth metal catalysis involving hydrosilanes, where stepwise nucleophilic substitution via a hypercoordinate silicate may take place instead of classical σ‐bond metathesis. [9] Furthermore, in the context of understanding dihydrogen uptake and release in the hydrogen storage material K4Si4/KSiH3, [10] it is of interest to study the relationship between low valent systems SiH3 −, SiH2, [11] and [Si4]4− and hypercoordinate hydridosilicates [SiH5]− and [SiH6]2− as hydride adducts of monosilane SiH4. Here we show that the previously reported reactive hydrido cation of strontium [(Me4TACD)SrH(thf) x ]+ (Me4TACD=1,4,7,10‐tetramethyltetraazacyclododecane) [12] forms a hexahydridosilicate complex [(Me4TACD)2Sr2(thf)4(μ‐κ3 : κ3‐SiH6)][B(C6H3‐3,5‐Me2)4]2 (3 a) containing a bridging [SiH6]2− anion. Isolation of 3 a allowed the study of its structure, bonding, and reactivity.
Results and Discussion
When a yellow solution of [(Me4TACD)Sr(CH2Ph)][B(C6H3‐3,5‐Me2)4] (2 a) [12] in THF‐d 8 was reacted with excess PhSiH3 at room temperature, immediate discoloration was observed. Upon work‐up, [(Me4TACD)2Sr2(thf)4(μ‐κ3 : κ3‐SiH6)][B(C6H3‐3,5‐Me2)4]2 (3 a) containing a bridging hexahydridosilicate [SiH6]2− anion between two strontium centers was isolated (Scheme 1). σ‐Bond metathesis of the benzyl complex with PhSiH3 to give the reactive intermediate [(Me4TACD)SrH(thf) x ]+ and Ph(PhCH2)SiH2 can be inferred as the first step. Fast redistribution[ 9b , 13 ] of PhSiH3 in the presence of [(Me4TACD)SrH(thf) x ]+ further gives Ph2(PhCH2)SiH and SiH4. The latter reacts with two equiv. of [(Me4TACD)SrH(thf) x ]+ to give 3 a, although low temperature monitoring of the reaction mixture remained inconclusive. The 1H and 29Si NMR spectra of the high‐boiling residue of the reaction mixture indicated the formation of secondary and tertiary silanes R x R′ySiH(4‐x‐y) (R=PhCH2, x=0, 1 or 2; R′=Ph, y=0, 1, or 2; x+y=1, 2, or 3). When 3 a was heated in the presence of an excess of PhSiH3 the formation of Ph2SiH2 and SiH4 was observed but no Ph3SiH. Similar redistribution to SiH4 was observed in the synthesis of [(BDI)CaH]2 (BDI=CH[C(CH3)NDipp]2, Dipp=2,6‐diisopropylphenyl). [14]
Scheme 1.
Synthesis of hydridosilicate complexes 3 a,b and thermal decomposition to silanides 4 a,b.
Compound 3 a, albeit crystalline, did not meet the requirements for reliably elucidating the hydrogen atom geometry by single crystal X‐ray diffraction. Solid 3 a contained seven non‐coordinated and less well‐defined THF molecules in the asymmetric unit (see Supporting Information). More suitable crystals of [(Me4TACD)2Sr2(thf)4(μ‐κ3 : κ3‐SiH6)][B(C6H4‐4‐nBu)4]2 (3 b) were obtained using a related anion [B(C6H4‐4‐nBu)4]− starting from [(Me4TACD)Sr(CH2Ph)][B(C6H4‐4‐nBu)4] (2 b). 3 b crystallizes as a dinuclear complex with the [SiH6]2− bridging between two strontium ions; the silicon atom resides on a crystallographic center of inversion (Figure 1a). Each metal cation is coordinated by two THF ligands in addition to the κ4‐Me4TACD macrocycle. The quality of the diffraction data allowed the unambiguous assignment of local electron density maxima to the hydridic atom sites (Figure 1b) and free refinement of their positional parameters and individual isotropic displacement parameters. The bridging hydridosilicate ligand shows octahedral coordination about silicon, with the Si−H bond lengths in the range of 1.51(2) to 1.588(19) Å. Silicon and alkaline earth cations connect to opposite triangular faces of the octahedron; Sr−H bonds between 2.39(2) and 2.44(2) Å are equidistant within error. The hydride polyhedron about silicon is slightly elongated in the direction of Sr⋅⋅⋅Sr vector. The intra‐cation separation between the strontium sites amounts to 6.1140(4) Å; a similar distance of 6.0919(9) Å was found from the less precise structural study on 3 a, suggesting an analogous bonding situation in both crystalline solids. The Si−H bond lengths in 3 a are considerably shorter than the Si−H bond lengths observed in [(PhBPPh 3)Ru]2[μ‐η4,η4‐SiH6] (1.69 to 1.79 Å). [6] For the computationally optimized structure of K2SiH6, Si−H bond lengths of 1.62 Å were reported. [5]
Figure 1.
a) Displacement ellipsoid plot (30 % probability) of the dinuclear cation in the crystal structure of 3 b; only H atoms in the hydridosilicate are shown. [28] b) Difference Fourier map before including the three symmetrically independent H atoms of the hydridosilicate into the structure model. Green contour lines correspond to positive electron density and have been drawn at 0.1 e Å−3 intervals. c) Cutout of the 1H–29Si HMBC NMR spectrum of 3 b at −40 °C.
DFT calculations (B3PW91 functional) including dispersion corrections were carried out to describe the bonding in complex 3 a. The optimized structure is in fair agreement with the experimental one (see Supporting Information) with the Sr−H bonds well reproduced (2.42 Å). The bond lengths around silicon were found to be complicated to reproduce computationally (1.59 and 1.62 Å compared with 1.51 and 1.59 Å experimentally). This is probably due to a Basis Set Superposition Error (BSSE), since large basis sets had to be used to describe the hypervalent structure of silicon. However, the maximum deviation is 0.08 Å and therefore bonding analysis was carried out using Natural Bonding Orbital (NBO) method (see Supporting Information). The six Si−H bonds were found to be strongly polarized toward hydrogen (72 to 74 %) and involve overlap between an spd hybrid atomic orbital (18 % s, 50 % p, 32 % d) on silicon and a 1 s orbital of hydrogen. This is in line with average computed Si−H Wiberg Bond Indices (WBIs) of 0.6. At the second order, the Si−H bonds appear to be delocalized toward strontium in line with the formation of 3 center‐2 electron (3c–2e) bonds explaining the WBI value of 0.15 for Sr−H as well as WBI of 0.18 for Sr−Si.
3 a and 3 b are unstable in THF‐d 8 above 0 °C (see below) and were therefore characterized by low temperature NMR studies. The hydridosilicate ligand gives a broad resonance in the 1H NMR spectrum above 0 °C that becomes a sharp singlet at −20 °C and below. At −40 °C resonances are observable in the 29Si–1H HSQC and 29Si‐1H HMBC spectra that could not be detected above this temperature. Despite numerous attempts, direct measurements such as DEPT20 or DEPT45 experiments did not show any 29Si resonances. In the 29Si‐1H HMBC spectrum, the [SiH6]2− anion gives rise to a doublet at δ=172.6 ppm with a coupling constant of 1 J SiH=118 Hz (Figure 1c). In comparison, the 29Si resonance at δ=162 ppm of [(PhBPPh 3)Ru]2[μ‐η4,η4‐SiH6] is slightly upfield shifted and the observed coupling constant was 1 J SiH=74.5 Hz. [6] These findings as well as the shorter Si−H bond lengths in 3 b indicate that the [SiH6]2− shows an expectedly more ionic bonding to the strontium cations as opposed to [(PhBPPh 3)Ru]2[μ‐η4,η4‐SiH6] with stronger ruthenium‐hydride interactions. [6] Characteristic Si−H stretching frequencies were observed for 3 a at ν=1717 cm−1 in the ATR‐IR spectrum and ν=1792 cm−1 in the Raman spectrum. These wavenumbers are similar to those observed in K2SiH6 (ν=1739 cm−1, Raman) and [(PhBPPh 3)Ru]2[μ‐η4,η4‐SiH6] (ν=1746 cm−1, IR in Nujol). The deuterated isotopomer 3 a‐d 6 , prepared analogously from 2 a and PhSiD3, shows an Si−D stretching absorption in the ATR‐IR spectrum at ν=1247 cm−1 (νH/νD=1.44). The Si−H vibrational frequency as well as the 29Si NMR chemical shift were also obtained computationally. A stretching band with strong IR intensity at ν=1710 cm−1 was calculated, which is in good agreement with the observed experimental frequency. The 29Si NMR chemical shift was computed to be δ=208 ppm, with an overestimation of 10 %. This is in line with errors reported in the literature. [15]
When a solution of 3 a, b in THF‐d 8 was left standing above 0 °C, H2 evolved and in the 1H NMR spectrum the broad resonance of [SiH6]2− gave way to a new sharp signal at δ=5.88 ppm over the course of 4 h, in agreement with the chemical shift for the hydride ligand in [(Me4TACD)SrH(thf) x ]+. [12] No resonance for SiH4 was detected in the reaction mixture as shown by 1H NMR spectra, 29Si–1H HSQC, and 29Si–1H HMBC measurements at 23 °C. Cooling the decomposition mixture of 3 b to −40 °C showed a single resonance at δ=−130.8 ppm, in the region expected for a silanide anion SiH3 − coordinated to an electropositive metal (see below).[ 3c , 16 ]
These observations were supported by X‐ray diffraction analysis of colorless single crystals, which grew from the decomposition mixture of 3 a and identified as the terminal silanide complex [(Me4TACD)Sr(SiH3)(thf)2][B(C6H3‐3,5‐Me2)4] (4 a) (Figure 2). After an initial structure model was established, the most disagreeable diffraction intensities were consistently stronger than their calculated counterparts. The metric of the unit cell of 4 a allowed approximate non‐crystallographic rotation about the [0 1 −1] direction as twin law, and the presence of many partially overlapped intensities necessarily limits the precision of the diffraction experiment. Despite these limitations, local electron density maxima associated with the hydrogen atoms of a disordered terminal SiH3 moiety could be identified (see for further details in the Supporting Information). Based on a search in the CSD, [17] the hydrogen sites in this group were constrained to a distance Si−H=1.38 Å (see Supporting Information).
Figure 2.
Displacement ellipsoid plot (30 % probability) of the cationic complex with a terminal silanide ligand in 4 a; only H atoms in the majority conformer of the SiH3 group are shown. [28]
While there are several examples of structurally characterized alkali metal,[ 3c , 16 ] lanthanide, [18] and transition metal complexes [19] containing the SiH3 − anion, 4 a appears to be the first structurally authenticated complex with the SiH3 − anion coordinated to an alkaline earth metal.
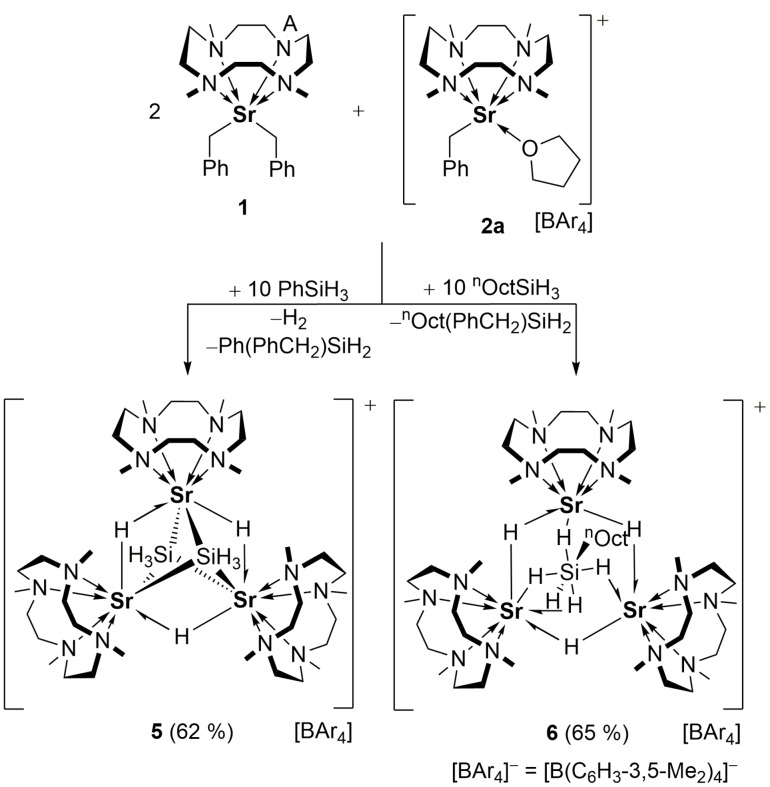
When one equivalent of 2 a were reacted with PhSiH3 in the presence of two equivalents of the neutral benzyl 1, the trinuclear cluster [(Me4TACD)3Sr3(μ‐H)3(μ3‐SiH3)2][B(C6H3‐3,5‐Me2)4] (5) was isolated in 62 % yield (Scheme 2). H2 gas evolution during the formation of 5 suggests that [(Me4TACD)Sr(SiH3)(thf) x ]+ is formed by reductive elimination of H2 from an undetected intermediate [(Me4TACD)Sr(SiH5)(thf) x ]+, by analogy to the formation of 4 from 3.
Scheme 2.
Synthesis of hydride‐silanide cluster 5 and hydride‐n‐octylhydridosilicate cluster 6.
Cluster 5 can formally be regarded as a combination of two units of [(Me4TACD)Sr(SiH3)H] and one unit of [(Me4TACD)SrH]+ or of two units of [(Me4TACD)Sr(SiH3)]+ and one unit of [(Me4TACD)SrH3]−, thus as a derivative of [(Me4TACD)3Sr3H5]+.
According to the single crystal diffraction analysis, the cation in 5 contains a triangular strontium core of distorted D 3h‐symmetry with three Sr⋅⋅⋅Sr edges between 4.0968(4) and 4.1707(5) Å bridged by H atoms. The Sr3 plane is capped by two SiH3 units on each side (Figure 3).
Figure 3.
Displacement ellipsoid plots (30 % probability) of the trinuclear cation in crystals of 5 (left) and 6 (right). Only hydride ligands and H atoms of SiH3 − or [nOctSiH5]2− ligands are shown. [28]
As for complex 3 b, DFT calculations were also carried out for complex 5 (see Supporting Information). At the NBO level, Sr−H as well as Sr−Si bonds were found. Both bonds are strongly polarized toward either H (85 %) or Si (88–90 %). At the second order, the Sr−H bonds are found to delocalize onto the adjacent Sr in line with the formation of 3c–2e Sr−H−Sr bonds. The Sr−H bonds involve an overlap between an spd hybrid atomic orbital on strontium and the 1 s orbital of hydrogen. A similar situation is found for the Sr−Si bonds formed by the overlap of an spd hybrid orbital (9 % s, 40 % p, 51 % d) on strontium and an sp hybrid orbital (60 % s, 40 % p) on silicon. This bonding situation is in line with the computed Sr−H and Sr−Si WBI of 0.35 and 0.30, respectively.
In the 1H NMR spectrum of 5 the hydride ligands appear at δ=5.86 ppm and the SiH3 − ligands at δ=2.07 ppm, both in the expected chemical shift ranges for these ligands.[ 3c , 12 , 16 , 20 ] Additionally, spin‐spin coupling (3 J HH=1.7 Hz) between the hydride and SiH3 − ligands in 5 was observed. 5 is relatively stable in THF‐d 8 solution and no decomposition was observed at room temperature within 48 h, in contrast to the related Me4TACD‐supported strontium hydride complexes 3, 6 and [(Me4TACD)3Sr3(μ‐H)4(thf)][B(C6H3‐3,5‐Me2)4]2. [12] In the ATR‐IR spectrum the Si−H stretching absorption of 5 was observed at ν=1923 cm−1. The deuterated isotopomer 5‐ d 9 prepared analogously to 5 using PhSiD 3 , shows a resonance for Sr−D at δ=5.95 ppm and for SiD3 − at δ=2.01 ppm in the 2H NMR spectrum.
The observation that the in situ generated [(Me4TACD)SrH(thf) x ]+ fragment strongly interacts with silanes prompted us to use nOctSiH3 as a hydride source to avoid aryl group redistribution at the silicon center. When two equiv. of 1 and one equiv. of 2 a were reacted with nOctSiH3, instantaneous reaction was observed. Single‐crystal structure analysis of the reaction product revealed formation of the trinuclear cluster [(Me4TACD)3Sr3(μ‐H)3(μ3‐SiH5 nOct)][B(C6H3‐3,5‐Me2)4] (6). The intermolecular Sr⋅⋅⋅Sr distances between 4.0908(4) Å and 4.1294(4) Å in 6 can be compared with those in 5, but in 6 the Sr3 triangle is bridged by a hypercoordinate [nOctSiH5]2− unit. Considering the latter as an adduct of two hydrides to nOctSiH3, complex 6 can be regarded as made up by two neutral [(Me4TACD)SrH2] fragments, one cationic [(Me4TACD)SrH]+ moiety and one nOctSiH3 molecule. To the best of our knowledge, an (alkyl)pentahydridosilicate has so far not been reported.
In the 1H NMR spectrum of 6 in THF‐d 8 the hydride and [nOctSiH5]2− ligands give a broad resonance (δ=5.66 ppm) at room temperature. In agreement with C s‐symmetry, the two resonances should give a pattern of five signals in a 2 : 2 : 2 : 1 : 1 ratio. Although below −40 °C the resonances sharpen, a complicated pattern of resonances appears at −80 °C, suggesting a less symmetric structure resulting from frozen fluxionality of the hydride ligands. A 29Si‐1H HSQC NMR measurement at −80 °C gave a 29Si resonance at δ=112.4 ppm. It appears that the exchange of hydride ligands is too fast above this temperature to obtain 2D NMR spectra. As in the case of 3 a, DEPT20 and DEPT45 measurements were unsuccessful. In the ATR‐IR spectrum of 6 a characteristic Si−H stretching frequency was observed at ν=1703 cm−1. DFT calculations were carried out on complex 6 (see Supporting Information). NBO analysis indicates the presence of Si−H and Sr−H bonds, which like complexes 3 and 5, are strongly polarized toward hydrogen (73 to 85 %). The Sr−H bonds are found at the second order donor–acceptor level to be delocalized onto the adjacent strontium atom, in line with the presence of 3c–2e Sr−H−Sr bonds as found in complex 5. The associated Sr−H WBI are 0.35 in line with the Sr−H WBI found for complex 5. The Si−H bonds are also found to be slightly delocalized toward strontium but the Si−H WBI are around 0.5, the values for Sr−H are approximately 0.1. The latter delocalization leads to a Sr−Si WBI of 0.1–0.18. These values are similar to those found for complex 3 b. The bonding situation in complex 6 can be considered a superposition of the bonding situation found in complexes 3 b and 5.
To explore the nature of the [SiH6]2− anion in 3 a, the reactivity toward a weak Brønsted acid, CO2 as an electrophile, and 1,3,5,7‐cyclooctatetraene (COT) as a mild oxidant was investigated (Scheme 3). Reactivity studies were carried out with 3 a rather than 3 b due to the more straightforward synthesis of 3 a, and easier isolation of the products with the [B(C6H3‐3,5‐Me2)4]− anion. Thus 3 a reacted with two equivalents of the weak Brønsted acid [NEt3H][B(C6H3‐3,5‐Me2)4] to release both H2 and SiH4 and cleanly gave the dication [(Me4TACD)Sr(thf)2][B(C6H3‐3,5‐Me2)4]2 (7), [12] which was identified in the 1H NMR spectrum of the protonolysis mixture in THF‐d 8. With CO2 (1 bar), 3 a quickly reacted at 0 °C to give the dinuclear formate complex [(Me4TACD)2Sr2(μ‐OCHO)2][B(C6H3‐3,5‐Me2)4]2 (8) with concomitant release of SiH4. 8 was isolated and fully characterized including by single crystal diffraction analysis. The NMR spectra show the characteristic resonances for the formate ligand at δ(1H)=8.47 ppm and δ(13C)=169.3 ppm, slightly downfield shifted compared to its isostructural calcium homolog (δ(1H)=8.37 ppm and δ(13C)=167.4 ppm). [21] 3 a reacted with 1,3,5,7‐cyclooctatetraene under release of H2 and SiH4 to give the dinuclear complex [(Me4TACD)2Sr2(μ,η8 : η8‐COT)][B(C6H3‐3,5‐Me2)4]2 (9). The cyclooctatetraenediyl ligand gives rise to resonances at δ(1H)=6.46 ppm and δ(13C)=92.6 ppm in the NMR spectra. Heavier alkaline earth metals containing the cyclooctatetraenediyl ligand are well‐known in the literature. [22] The calcium hydride complex [(BDI)CaH]2 has been reported by Hill et al. to form a bridging COT complex with release of H2. [23]
Scheme 3.
Reactivity of hydridosilicate complex 3 a.
When compared with the thermolysis of 3 a, which releases H2 but no SiH4, it appears that external reagents follow a distinct pathway that leads to fast dissociation of 3 a into two [(Me4TACD)SrH(thf) x ]+ and SiH4. Thus, the reactivity pattern of 3 a is best rationalized by regarding the complex as two SiH4‐masked nucleophilic hydride cations [(Me4TACD)SrH]+.
Conclusion
The highly reactive strontium hydride cations [(Me4TACD)SrH(thf) x ]+ enable stabilization of hexahydridosilicate, silanide and (n‐alkyl)pentahydridosilicate anions. The highly nucleophilic character of the strontium hydride allows these rare silicon anions to be isolated. When compared to lighter alkaline earth metal congeners magnesium and calcium, strontium with its large ionic radius (ionic radii for c.n.=6: 0.86 Å for Mg, 1.14 Å for Ca, 1.32 Å for Sr), [24] and higher electropositivity (EN=1.30 for Mg, 1.00 for Ca, 0.95 for Sr) [25] exhibits more polar interactions and pronounced ligand lability. As a result, we have previously observed that the strontium hydride cation [(Me4TACD)SrH(thf) x ]+ readily undergoes redistribution (Schlenk equilibrium) in THF to give [(Me4TACD)Sr]2+ and [(Me4TACD)SrH2] (isolable as the trinuclear tetrahydride dication [(Me4TACD)3Sr3H4]2+). [12] In a mixed calcium–strontium system, this high lability and nucleophilicity results in hydride transfer from strontium to calcium. [12] The results reported here further underline the potential for highly reactive complexes of the electropositive heavy alkaline earth metals to exhibit unusual reactivity patterns,[ 14 , 20e , 26 ] some aspects of which have recently been ascribed to non‐negligible contributions of d‐orbitals in metal–ligand bonding. [27]
The isolation of thermally sensitive hexahydridosilicate 3 occurred due to the fast redistribution of phenylsilane catalyzed by [(Me4TACD)SrH(thf) x ]+ to give monosilane. When the more inert n‐alkyl hydrosilane nOctSiH3 was present, [(Me4TACD)3Sr3H3]3+ core stabilized an alkyl‐substituted pentahydridosilicate [nOctSiH5]2−. While reactions of 3 with a Brønsted acid, an electrophile such as CO2, and a mild oxidant all reflect the inherent reactivity of [(Me4TACD)SrH(thf) x ]+, thermal decomposition gave the terminal silanide 4, which may have formed by reductive elimination of H2 from the hypothetical pentahydridosilicate intermediate [(Me4TACD)Sr(SiH5)]+. In the context of hydrogen storage systems such as K4Si4/KSiH3, [10] it would be interesting to further explore the reductive elimination of H2 from these hydrogen‐rich silicon compounds.
Conflict of interest
The authors declare no conflict of interest.
1.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information
Supporting Information
Supporting Information
Acknowledgements
We thank the Deutsche Forschungsgemeinschaft for financial support, Prof. A. Filippou for help with vibrational spectra, Dr. G. Fink for NMR spectroscopic measurements, F. Ritter and Dr. L. J. Morris for X‐ray diffraction measurements. L.M. is member of the Institut Universitaire de France and thanks the Alexander von Humboldt foundation for a scholarship. CalMip is acknowledged for a generous grant of computing time. Open Access funding enabled and organized by Projekt DEAL.
T. Höllerhage, P. Ghana, T. P. Spaniol, A. Carpentier, L. Maron, U. Englert, J. Okuda, Angew. Chem. Int. Ed. 2022, 61, e202115379; Angew. Chem. 2022, 134, e202115379.
In memory of Robert H. Grubbs
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.
- 1a. Noury S., Silvi B., Gillespie R. J., Inorg. Chem. 2002, 41, 2164–2172; [DOI] [PubMed] [Google Scholar]
- 1b. Pierrefixe S. C. A. H., Bickelhaupt F. M., Struct. Chem. 2007, 18, 813–819; [Google Scholar]
- 1c. Dunning T. H., Xu L. T., Thompson J. V. K., J. Phys. Chem. A 2021, 125, 7414–7424; [DOI] [PubMed] [Google Scholar]
- 1d. Wilhite D. L., Spialter L., J. Am. Chem. Soc. 1973, 95, 2100–2104; [Google Scholar]
- 1e. Couzijn E. P. A., Ehlers A. W., Schakel M., Lammertsma K., J. Am. Chem. Soc. 2006, 128, 13634–13639. [DOI] [PubMed] [Google Scholar]
- 2.
- 2a. Chuit C., Corriu R. J. P., Reye C., Young J. C., Chem. Rev. 1993, 93, 1371–1448; [Google Scholar]
- 2b. Rendler S., Oestreich M., Synthesis 2005, 1727–1747; [Google Scholar]
- 2c. Nakao Y., Hiyama T., Chem. Soc. Rev. 2011, 40, 4893–4901; [DOI] [PubMed] [Google Scholar]
- 2d. Sugiyama A., Ohnishi Y.-y., Nakaoka M., Nakao Y., Sato H., Sakaki S., Nakao Y., Hiyama T., J. Am. Chem. Soc. 2008, 130, 12975–12985; [DOI] [PubMed] [Google Scholar]
- 2e. Kikushima K., Grellier M., Ohashi M., Ogoshi S., Angew. Chem. Int. Ed. 2017, 56, 16191–16196; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2017, 129, 16409–16414. [Google Scholar]
- 3.
- 3a. Prince P. D., Bearpark M. J., McGrady G. S., Steed J. W., Dalton Trans. 2008, 271–282; [DOI] [PubMed] [Google Scholar]
- 3b. Jochmann P., Davin J. P., Spaniol T. P., Maron L., Okuda J., Angew. Chem. Int. Ed. 2012, 51, 4452–4455; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2012, 124, 4528–4531; [Google Scholar]
- 3c. Schuhknecht D., Leich V., Spaniol T. P., Douair I., Maron L., Okuda J., Chem. Eur. J. 2020, 26, 2821–2825; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3d. Schuhknecht D., Leich V., Spaniol T. P., Okuda J., Chem. Eur. J. 2018, 24, 13424–13427; [DOI] [PubMed] [Google Scholar]
- 3e. Zhou J., Chu J., Zhang Y., Yang G., Leng X., Chen Y., Angew. Chem. Int. Ed. 2013, 52, 4243–4246; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2013, 125, 4337–4340. [Google Scholar]
- 4.
- 4a. Hajdasz D. J., Squires R. R., J. Am. Chem. Soc. 1986, 108, 3139–3140; [Google Scholar]
- 4b. Hajdasz D. J., Ho Y., Squires R. R., J. Am. Chem. Soc. 1994, 116, 10751–10760. [Google Scholar]
- 5. Puhakainen K., Benson D., Nylén J., Konar S., Stoyanov E., Leinenweber K., Häussermann U., Angew. Chem. Int. Ed. 2012, 51, 3156–3160; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2012, 124, 3210–3214. [Google Scholar]
- 6. Lipke M. C., Tilley T. D., Angew. Chem. Int. Ed. 2012, 51, 11115–11121; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2012, 124, 11277–11283. [Google Scholar]
- 7. Bearpark M. J., McGrady G. S., Prince P. D., Steed J. W., J. Am. Chem. Soc. 2001, 123, 7736–7737. [DOI] [PubMed] [Google Scholar]
- 8. Ebner F., Greb L., J. Am. Chem. Soc. 2018, 140, 17409–17412. [DOI] [PubMed] [Google Scholar]
- 9.
- 9a. Buch F., Brettar J., Harder S., Angew. Chem. Int. Ed. 2006, 45, 2807–2811; [DOI] [PubMed] [Google Scholar]
- 9b. Schuhknecht D., Spaniol T. P., Maron L., Okuda J., Angew. Chem. Int. Ed. 2020, 59, 310–314; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2020, 132, 317–322; [Google Scholar]
- 9c. Dunne J. F., Neal S. R., Engelkemier J., Ellern A., Sadow A. D., J. Am. Chem. Soc. 2011, 133, 16782–16785. [DOI] [PubMed] [Google Scholar]
- 10.
- 10a. Janot R., Tang W. S., Clémençon D., Chotard J. N., J. Mater. Chem. A 2016, 4, 19045–19052; [Google Scholar]
- 10b. Chotard J.-N., Tang W. S., Raybaud P., Janot R., Chem. Eur. J. 2011, 17, 12302–12309. [DOI] [PubMed] [Google Scholar]
- 11. Protchenko A. V., Birjkumar K. H., Dange D., Schwarz A. D., Vidovic D., Jones C., Kaltsoyannis N., Mountford P., Aldridge S., J. Am. Chem. Soc. 2012, 134, 6500–6503. [DOI] [PubMed] [Google Scholar]
- 12. Höllerhage T., Carpentier A., Spaniol T. P., Maron L., Englert U., Okuda J., Chem. Commun. 2021, 57, 6316–6319. [DOI] [PubMed] [Google Scholar]
- 13.
- 13a. Castillo I., Tilley T. D., J. Am. Chem. Soc. 2001, 123, 10526–10534; [DOI] [PubMed] [Google Scholar]
- 13b. Perrin L., Maron L., Eisenstein O., Tilley T. D., Organometallics 2009, 28, 3767–3775; [Google Scholar]
- 13c. Liu X., Xiang L., Louyriac E., Maron L., Leng X., Chen Y., J. Am. Chem. Soc. 2019, 141, 138–142; [DOI] [PubMed] [Google Scholar]
- 13d. Liu Z., Shi X., Cheng J., Dalton Trans. 2020, 49, 8340–8346; [DOI] [PubMed] [Google Scholar]
- 13e. Li T., McCabe K. N., Maron L., Leng X., Chen Y., ACS Catal. 2021, 11, 6348–6356. [Google Scholar]
- 14. Wilson A. S. S., Hill M. S., Mahon M. F., Dinoi C., Maron L., Science 2017, 358, 1168. [DOI] [PubMed] [Google Scholar]
- 15. del Rosal I., Maron L., Poteau R., Jolibois F., Dalton Trans. 2008, 3959–3970. [DOI] [PubMed] [Google Scholar]
- 16. Wolstenholme D. J., Prince P. D., McGrady G. S., Landry M. J., Steed J. W., Inorg. Chem. 2011, 50, 11222–11227. [DOI] [PubMed] [Google Scholar]
- 17. Groom C. R., Bruno I. J., Lightfoot M. P., Ward S. C., Acta Crystallogr. Sect. B 2016, 72, 171–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Radu N. S., Hollander F. J., Tilley T. D., Rheingold A. L., Chem. Commun. 1996, 2459–2460. [Google Scholar]
- 19.
- 19a. Mitzenheim C., Braun T., Angew. Chem. Int. Ed. 2013, 52, 8625–8628; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2013, 125, 8787–8790; [Google Scholar]
- 19b. Schmitzer S., Weis U., Kaeb H., Buchner W., Malisch W., Polzer T., Posset U., Kiefer W., Inorg. Chem. 1993, 32, 303–309; [Google Scholar]
- 19c. Zuzek A. A., Parkin G., J. Am. Chem. Soc. 2014, 136, 8177–8180; [DOI] [PubMed] [Google Scholar]
- 19d. Hao L., Chem. Commun. 1998, 1089–1090. [Google Scholar]
- 20.
- 20a. Maitland B., Wiesinger M., Langer J., Ballmann G., Pahl J., Elsen H., Färber C., Harder S., Angew. Chem. Int. Ed. 2017, 56, 11880–11884; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2017, 129, 12042–12046; [Google Scholar]
- 20b. Mukherjee D., Höllerhage T., Leich V., Spaniol T. P., Englert U., Maron L., Okuda J., J. Am. Chem. Soc. 2018, 140, 3403–3411; [DOI] [PubMed] [Google Scholar]
- 20c. de Bruin-Dickason C. N., Sutcliffe T., Alvarez Lamsfus C., Deacon G. B., Maron L., Jones C., Chem. Commun. 2018, 54, 786–789; [DOI] [PubMed] [Google Scholar]
- 20d. Shi X., Qin G., Wang Y., Zhao L., Liu Z., Cheng J., Angew. Chem. Int. Ed. 2019, 58, 4356–4360; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2019, 131, 4400–4404; [Google Scholar]
- 20e. Rösch B., Gentner T. X., Elsen H., Fischer C. A., Langer J., Wiesinger M., Harder S., Angew. Chem. Int. Ed. 2019, 58, 5396–5401; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2019, 131, 5450–5455; [Google Scholar]
- 20f. Martin J., Eyselein J., Langer J., Elsen H., Harder S., Chem. Commun. 2020, 56, 9178–9181. [DOI] [PubMed] [Google Scholar]
- 21. Schuhknecht D., Spaniol T. P., Yang Y., Maron L., Okuda J., Inorg. Chem. 2020, 59, 9406–9415. [DOI] [PubMed] [Google Scholar]
- 22.
- 22a. Hutchings D. S., Junk P. C., Patalinghug W. C., Raston C. L., White A. H., J. Chem. Soc. Chem. Commun. 1989, 973–974; [Google Scholar]
- 22b. He L., Cheng J., Wang T., Li C., Gong Z., Liu H., Zeng B.-B., Jiang H., Zhu W., Chem. Phys. Lett. 2008, 462, 45–48; [Google Scholar]
- 22c. Walter M. D., Wolmershäuser G., Sitzmann H., J. Am. Chem. Soc. 2005, 127, 17494–17503; [DOI] [PubMed] [Google Scholar]
- 22d. Sroor F. M., Vendier L., Etienne M., Dalton Trans. 2018, 47, 12587–12595. [DOI] [PubMed] [Google Scholar]
- 23. Hill M. S., Mahon M. F., Wilson A. S. S., Dinoi C., Maron L., Richards E., Chem. Commun. 2019, 55, 5732–5735. [DOI] [PubMed] [Google Scholar]
- 24. Shannon R., Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar]
- 25. Allred A. L., J. Inorg. Nucl. Chem. 1961, 17, 215–221. [Google Scholar]
- 26.
- 26a. Rösch B., Gentner T. X., Langer J., Färber C., Eyselein J., Zhao L., Ding C., Frenking G., Harder S., Science 2021, 371, 1125–1128; [DOI] [PubMed] [Google Scholar]
- 26b. Wiesinger M., Rösch B., Knüpfer C., Mai J., Langer J., Harder S., Eur. J. Inorg. Chem. 2021, 3731–3741. [Google Scholar]
- 27.
- 27a. Garcia L., Anker M. D., Mahon M. F., Maron L., Hill M. S., Dalton Trans. 2018, 47, 12684–12693; [DOI] [PubMed] [Google Scholar]
- 27b. Schuhknecht D., Spaniol T. P., Douair I., Maron L., Okuda J., Chem. Commun. 2019, 55, 14837–14839; [DOI] [PubMed] [Google Scholar]
- 27c. Stegner P., Färber C., Oetzel J., Siemeling U., Wiesinger M., Langer J., Pan S., Holzmann N., Frenking G., Albold U., Sarkar B., Harder S., Angew. Chem. Int. Ed. 2020, 59, 14615–14620; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2020, 132, 14723–14728. [Google Scholar]
- 28. Deposition Number(s) 2112938 (for 3 a), 2112939 (for 3 b), 2112940 (for 4 a), 2112941 (for 5), 2112942 (for 6), 2112943 (for 8), 2112944 (for 9) contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service www.ccdc.cam.ac.uk/structures.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information
Supporting Information
Supporting Information
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.